Abstract
We report on chloroquine, a 4-amino-quinoline, as an effective inhibitor of the replication of the severe acute respiratory syndrome coronavirus (SARS-CoV) in vitro. Chloroquine is a clinically approved drug effective against malaria. We tested chloroquine phosphate for its antiviral potential against SARS-CoV-induced cytopathicity in Vero E6 cell culture. Results indicate that the IC50 of chloroquine for antiviral activity (8.8 ± 1.2 μM) was significantly lower than its cytostatic activity; CC50 (261.3 ± 14.5 μM), yielding a selectivity index of 30. The IC50 of chloroquine for inhibition of SARS-CoV in vitro approximates the plasma concentrations of chloroquine reached during treatment of acute malaria. Addition of chloroquine to infected cultures could be delayed for up to 5 h postinfection, without an important drop in antiviral activity. Chloroquine, an old antimalarial drug, may be considered for immediate use in the prevention and treatment of SARS-CoV infections.
Keywords: SARS-CoV, Severe acute respiratory syndrome, Coronavirus, Chloroquine, Antiviral activity
Severe acute respiratory syndrome (SARS) has recently emerged as a new highly contagious human disease with a major impact all over the world [1]. The global SARS epidemic started in the Guangdong Province in southern China, where several cases of atypical pneumonia of unknown etiology were reported at the end of November 2002. A novel member of the Coronaviridae family has been identified as the causative agent of SARS [2], [3], [4], [5], [6], [7]. Three other human coronaviruses (HCoV) OC43, 229E, and the recently characterized NL63 are important causes of upper respiratory tract illnesses. In late fall and winter they are responsible for approximately one-third of the common colds.
During the epidemic in 2003, treatment of SARS was empirical due to the limited understanding of this new disease. Protease inhibitors (lopinavir/ritonavir) in combination with ribavirin may be of benefit as antiviral therapy, when given in the early phase of the illness [8], [9]. The role of interferon and systemic steroids in preventing immune-mediated lung injury requires further investigation [10], [11].
Since the epidemic, a lot of effort has been put into antiviral research to find compounds effective against SARS-CoV. Glycyrrhizin (an active component of liquorice roots), niclosamide (an antihelminthic drug), nelfinavir (a human immunodeficiency deficiency virus (HIV) protease inhibitor), and SNAP (a nitric oxide donor) were reported to have an antiviral effect against SARS-CoV [12], [13], [14], [15].
Savarino et al. [16] hypothesized that chloroquine might be of some use for the clinical management of SARS. Chloroquine is known as an antimalarial agent and elicits also antiviral effects against several viruses including HIV type 1 (HIV-1) [17], [18], [19], hepatitis B virus [20], herpes simplex virus type 1 [21], and HCoV-229E [22]. The antiviral effects of chloroquine against HIV type 1 replication are currently being tested in clinical trials [16]. Besides a direct antiviral effect, chloroquine is endowed with immunomodulatory activity, suppressing the production and release of tumour necrosis factor and interleukin 6, which mediate the inflammatory complications of several viral diseases [16].
In this study, we evaluate chloroquine for its potential to protect against SARS-CoV infection in vitro.
Materials and methods
Cell culture and virus. The SARS-CoV Frankfurt 1 strain was kindly provided by Prof. Dr. H. F. Rabenau from the Johann Wolfgang Goethe University, Frankfurt, Germany. Vero E6 cells were propagated in minimal essential medium (MEM; Gibco, Life Technologies, Rockville, MD) supplemented with 10% fetal calf serum (FCS, Integro, Zaandam, The Netherlands), 1% l-glutamine (Gibco, Life Technologies, Rockville, MD), and 1.4 % sodium bicarbonate (Gibco, Life Technologies, Rockville, MD). Virus-infected cells were maintained at 37 °C in 5% CO2 in MEM supplemented with 2% FCS.
Compounds. We tested chloroquine phosphate (7-chloro-4-[[4-(diethylamino)-1-methylbutyl]amino]quinoline phosphate, Alpha pharma, Braine-l’Alleud, Belgium) and interferon β-1a (Avonex, Biogen) was used as a positive control.
Real-time quantitative RT-PCR. A real-time quantitative RT-PCR (Taqman) was designed in the putative nsp11 region in the replicase 1B domain of the SARS-associated coronavirus (SARS-CoV) genome forward primer (SARS-FP: 5′-CACCCGCGAAGAAGCTATTC-3′), MGB probe (SARS-TP: FAM 5′-TGCGTGGATTGGCTT-3′ NFQ-MGB), and reverse primer (SARS-RP: 5′-TTGCATGACAGCCCTCTACATC-3′). A 25 μl RT-PCR was carried out using 5 μl of extracted RNA or standard cRNA, 12.5 μl TaqMan One-Step RT-PCR Master Mix containing ROX as a passive reference (Applied Biosystems, Foster City, CA, USA), 900 nM forward and reverse primers, and 150 nM MGB probe. Amplification and detection were performed in an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, Foster City, CA, USA) under the following conditions: an initial reverse transcription at 48 °C for 30 min, followed by PCR activation at 95 °C for 10 min and 45 cycles of amplification (15 s at 95 °C and 1 min at 60 °C). During amplification, the ABI PRISM sequence detector monitored real-time PCR amplification by quantitative analysing fluorescence emissions. The reporter dye (FAM) signal was measured against the internal reference dye (ROX) signal to normalize for non-PCR-related fluorescence fluctuations occurring from well to well. The threshold cycle represented the refraction cycle number at which a positive amplification was measured, and was set at 10 times the standard deviation of the mean baseline emission calculated for PCR cycles 3–5.
Construction of cRNA standards. The TaqMan SARS-CoV forward primer was modified with a T7-promoter sequence at the 5′-end (SARS-FPT7 5′-TAATACGACTCACTATAGGGAGGCACCCGCGAAGAAGCTATTC- 3′). PCR products amplified with the modified primer pairs were quantified spectrophotometrically at 260 nm. Two hundred nanograms of PCR product was used for in vitro transcription (MEGAshortscript T7 kit, Ambion, Austin, TX, USA) performed at 37 °C for an overnight period in a 20 μl reaction mix containing 2 μl reaction buffer, 2 μl of each NTP, and 2 μl enzyme mix. The cDNAs were removed by digesting with 2 U of RNase-free DNase I for 15 min at 37 °C. The cRNAs were precipitated by adding 3 μl of 3 M NaOAc and 60 μl of 96% EtOH and a subsequent incubation at −20 °C for 30 min. After 15 min of centrifugation at 13,000 rpm, supernatant was removed and 500 μl of 70% EtOH was added. After another 5 min centrifugation at 13,000 rpm, supernatant was removed and the pellet was dissolved in 200 μl RNase free H2O (Sigma–Aldrich NV, Bornem, Belgium) and stored at −80 °C. Quantification of cRNAs was performed spectrophotometrically at 260 nm. The measurements of cRNA concentration were performed in duplicate and then converted to the molecule number [23].
Antiviral assay. Antiviral activity and cytotoxicity measurements were based on the viability of cells that had been infected or not infected with 100 CCID50 (50% cell culture infective doses) of the SARS-CoV in the presence of various concentrations of the test compounds. Three days after infection, the number of viable cells was quantified by a tetrazolium-based colorimetric method, in which the reduction of the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxy-phenyl)-2-(4-sulphophenyl)-2H-tetrazolium (MTS) dye (CellTiter 96 AQueous One Solution kit, Promega, The Netherlands) by cellular dehydrogenases to an insoluble coloured formazan was measured in a spectrophotometer (Multiskan EX, Thermo Labsystems, Belgium) at 492 nm [24], [25]. The selectivity index was determined as the ratio of the concentration of the compound that reduced cell viability to 50% (CC50 or 50% cytotoxic concentration) to the concentration of the compound needed to inhibit the viral cytopathic effect to 50% of the control value (IC50 or 50% inhibitory concentration). Interferon β is used as a positive control.
Time-of-addition assay. Subconfluent monolayers of Vero E6 cells in 96-well plates were infected with 100 CCID50 SARS-CoV. After 20 min of adsorption, cell monolayers were washed five times with MEM. Chloroquine was added at a concentration 12-fold above the IC50 (8.8 μM) in triplicate, at the time of infection or at different time points thereafter. Eight hours after infection (a time at which the first viral cycle has been completed), cell supernatants were collected, viral RNA was extracted, and the antiviral activity was determined by using the quantitative RT-PCR described above.
Virus yield assay. After incubation of the virus-infected Vero E6 cells with different concentrations of the test compounds, under the appropriate conditions, supernatants containing free viruses were subjected to quantitative RT-PCR. Virus titers were determined on day 1 and day 3 postinfection.
Results and discussion
In this study we report the in vitro antiviral activity of chloroquine against SARS-CoV Frankfurt 1 strain infection. Cytotoxicity in Vero E6 cells was measured in parallel with the antiviral activity. This experiment, done in quadruplicate, was repeated three times, and representative results are shown in Fig. 1 .
Fig. 1.
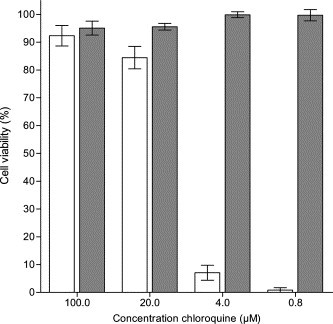
SARS-CoV-infected Vero E6 cells were incubated for three days in the presence of 0, 0.8, 4, 20, and 100 μM chloroquine. Data are mean values ± SE of four replicates. White bars indicate the effect of chloroquine on viability of cells infected with SARS-CoV. Black bars show the effect of chloroquine on viability of mock-infected cells. The concentration of chloroquine that results in 50% inhibition of the viral cytopathic effect, IC50, is 8.8 ± 1.2 μM. IFN β-1a was used as a positive control for the antiviral assay and yielded an IC50 of 2550 IU/mL (data not shown).
In the virus yield assay, where viral RNA was quantified one and three days postinfection, no significant replication was observed after one day when the cells were treated with 4 μM chloroquine. To inhibit virus replication by 99% three days postinfection, 16 μM chloroquine was needed (Fig. 2 ).
Fig. 2.
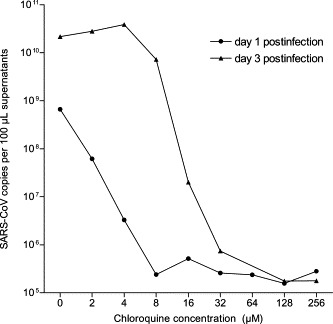
Dose–response of inhibitory effect of chloroquine on the virus yield. SARS-CoV-infected Vero E6 cells were incubated for one and three days in the presence of 0, 2, 4, 8, 16, 32, 64, 128, or 256 μM chloroquine. Cell supernatants were used for viral RNA extraction and subjected to real-time RT-PCR. cRNA standards were used for absolute quantification of the genome equivalents of SARS-CoV. These results are from a single experiment, representative of two independent experiments.
To obtain initial insight into the stage in the viral replication cycle at which chloroquine may exert its antiviral activity, a time-of-drug-addition assay was elaborated. Vero E6 cells were infected with 100 CCID50 SARS-CoV. One virus replication cycle takes 5–6 h [26]. We therefore quantitated the effect on viral replication at 8 h postinfection, i.e., a time point at which progeny virus in the supernatant is solely derived from the first replication cycle. The compound proved equally active when added during adsorption or at 1 h after infection. This indicates that the virus does likely not interfere with the early steps of viral replication, i.e., attachment or penetration. At later time points a gradual loss of the antiviral activity of chloroquine was noted. (Fig. 3 ).
Fig. 3.
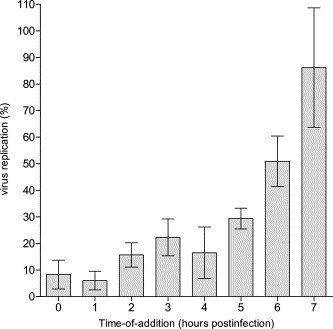
Time-of-drug-addition experiments performed using quantitative RT-PCR on viral RNA extracted from the cell supernatant. The data represent mean values ± SE for three separate experiments. Virus replication was calculated as percentage of SARS-CoV genome equivalents comparing treated with untreated infected cells.
The IC50 of chloroquine inhibition of SARS-CoV replication in Vero E6 cells, 8.8 μM, is below (1000-fold) the plasma concentrations of chloroquine that are reached in human plasma, following treatment with chloroquine (for acute malaria) at a dose of 25 mg/kg over three days [27]. The dose of chloroquine used for the treatment of rheumatoid arthritis (3.6 mg/kg) generates plasma chloroquine concentrations of 1–3 μM, which is in the same concentration range as the IC50 for inhibition of SARS-CoV [28].
Our results show that chloroquine inhibits the replication of SARS-CoV in Vero E6 cells. Since immunopathological factors may play a significant role in SARS-CoV, it will be of interest to further study whether chloroquine is also effective in terms of modulation of inflammatory responses to SARS-CoV infections.
Chloroquine is given prophylactically at a dose of 300 mg/week to people travelling to malaria endemic areas. If SARS re-emerges, chloroquine can be of great importance as prophylactic medication for people living in and travelling to the affected area. Chloroquine is ubiquitously available, of low cost, and easy to administer. It may be considered for immediate use in the prevention and treatment of SARS-CoV infections.
Acknowledgments
We thank the colleagues of the laboratory of Clinical and Epidemiological Virology, Department of Microbiology and Immunology, Rega Institute for Medical Research, University of Leuven, Belgium, for helpful comments and discussion. This work was supported by a fellowship of the Flemish Fonds voor Wetenschappelijk Onderzoek (FWO) to Leen Vijgen, and by FWO-Grant G.0288.01.
References
- 1.World Health Organization, 2003. Communicable Disease Surveillance and Response. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available from: <http://www.who.int/csr/sars/country/table2003_09_23/en/>. (revised 26 September 2003)
- 2.Drosten C., Gunther S., Preiser W., van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A., Berger A., Burguiere A.M., Cinatl J., Eickmann M., Escriou N., Grywna K., Kramme S., Manuguerra J.C., Muller S., Rickerts V., Sturmer M., Vieth S., Klenk H.D., Osterhaus A.D., Schmitz H., Doerr H.W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 3.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J., SARS Working Group A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 4.Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.-H., Tong S., Tamin A., Lowe L., Frace M., Derisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C.T., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D.M.E., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 7.Marra M.A., Jones S.J.M., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S.N., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D., Upton C., Roper R. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 8.Chan K.S., Lai S.T., Chu C.M., Tsui E., Tam C.Y., Wong M.M., Tse M.W., Que T.L., Peiris J.S., Sung J., Wong V.C., Yuen K.Y. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9:399–406. [PubMed] [Google Scholar]
- 9.Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y., HKU/UCH SARS study group Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cinatl J., Michaelis M., Scholz M., Doerr H.W. Role of interferons in the treatment of severe acute respiratory syndrome. Expert. Opin. Biol. Ther. 2004;4:827–836. doi: 10.1517/14712598.4.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhaori G. Antiviral treatment of SARS: can we draw any conclusions? CMAJ. 2003;169:1165–1166. [PMC free article] [PubMed] [Google Scholar]
- 12.Cinatl J., Morgenstern B., Bauer G., Chandra P., Rabenau H., Doerr H.W. Glycyrrhizin, an active component of liquorice roots, and replication of SARS-associated coronavirus. Lancet. 2003;361:2045–2046. doi: 10.1016/S0140-6736(03)13615-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C.J., Jan J.T., Chen C.M., Hsieh H.P., Hwang D.R., Liu H.W., Liu C.Y., Huang H.W., Chen S.C., Hong C.F., Lin R.K., Chao Y.S., Hsu J.T. Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 2004;48:2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamamoto N., Yang R., Yoshinaka Y., Amari S., Nakano T., Cinatl J., Rabenau H., Doerr H.W., Hunsmann G., Otaka A., Tamamura H., Fujii N., Yamamoto N. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem. Biophys. Res. Commun. 2004;318:719–725. doi: 10.1016/j.bbrc.2004.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keyaerts E., Vijgen L., Chen L., Maes P., Hedenstierna G., Van Ranst M. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai W.P., Nara P.L., Kung H.F., Oroszlan S. Inhibition of human immunodeficiency virus infectivity by chloroquine. AIDS Res. Hum. Retroviruses. 1990;6:481–489. doi: 10.1089/aid.1990.6.481. [DOI] [PubMed] [Google Scholar]
- 18.Pardridge W.M., Yang J., Diagne A. Chloroquine inhibits HIV-1 replication in human peripheral blood lymphocytes. Immunol. Lett. 1998;64:45–47. doi: 10.1016/s0165-2478(98)00096-0. [DOI] [PubMed] [Google Scholar]
- 19.Savarino A., Gennero L., Sperber K., Boelaert J.R. The anti-HIV-1 activity of chloroquine. J. Clin. Virol. 2001;20:131–135. doi: 10.1016/s1386-6532(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 20.Kouroumalis E.A., Koskinas J. Treatment of chronic active hepatitis B (CAH B) with chloroquine: a preliminary report. Ann. Acad. Med. Singapore. 1986;15:149–152. [PubMed] [Google Scholar]
- 21.Singh A.K., Sidhu G.S., Friedman R.M., Maheshwari R.K. Mechanism of enhancement of the antiviral action of interferon against herpes simplex virus-1 by chloroquine. J. Interferon Cytokine Res. 1996;16:725–731. doi: 10.1089/jir.1996.16.725. [DOI] [PubMed] [Google Scholar]
- 22.Blau D., Holmes K. Human Coronavirus HCoV-229E enters susceptible cells via the endocytic pathway. In: Lavi E., editor. The Nidoviruses, Coronaviruses and Arteriviruses. Kluwer; New York: 2001. pp. 193–197. [DOI] [PubMed] [Google Scholar]
- 23.Fronhoffs S., Totzke G., Stier S., Wernert N., Rothe M., Bruning T., Koch B., Sachinidis A., Vetter H., Ko Y. A method for the rapid construction of cRNA standard curves in quantitative real-time reverse transcription polymerase chain reaction. Mol. Cell. Probes. 2002;16:99–110. doi: 10.1006/mcpr.2002.0405. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels R., Balzarini J., Baba M., Snoeck R., Schols D., Herdewijn P., Desmyter J., De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J. Virol. Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 25.Goodwin C.J., Holt S.J., Downes S., Marshall N.J. Microculture tetrazolium assays: a comparison between two new tetrazolium salts, XTT and MTS. J. Immunol. Methods. 1995;179:95–103. doi: 10.1016/0022-1759(94)00277-4. [DOI] [PubMed] [Google Scholar]
- 26.Ng M.L., Tan S.H., See E.E., Ooi E.E., Ling A.E. Proliferative growth of SARS coronavirus in Vero E6 cells. J. Gen. Virol. 2003;84:3291–3303. doi: 10.1099/vir.0.19505-0. [DOI] [PubMed] [Google Scholar]
- 27.Charmot G., Coulaud J.P. Treatment of Plasmodium falciparum malaria in Africa (except cerebral malaria) Med. Trop. 1990;50:103–108. [PubMed] [Google Scholar]
- 28.Wollheim F.A., Hanson A., Laurell C. Chloroquine treatment in rheumatoid arthritis. Correlation of clinical response to plasma protein changes and chloroquine levels. Scand. J. Rheumatol. 1978;7:171–176. doi: 10.3109/03009747809095649. [DOI] [PubMed] [Google Scholar]


