Abstract
The worldwide outbreak of the swine-origin 2009 H1N1 influenza A virus (IAV) and an increasing number of influenza cases caused by a highly pathogenic avian influenza (HPAI) H5N1 have accelerated the need to develop vaccines and antiviral agents against IAVs. Among various antivirals, neutralizing monoclonal antibodies (mAbs) are considered important passive therapeutics having an immediate effect against viral pathogens. Here we report a pseudovirus neutralization assay for rapid screening of neutralizing mAbs targeting hemagglutinin (HA) of H5N1 and H1N1 IAV. In this study, we generated six pseudoviruses with an HIV-1 backbone, respectively, expressing HA of four clades of H5N1 IAV and the 2009 epidemic H1N1 IAV. The resulting pseudoviruses were able to infect a variety of human and non-human cells, with 293T cells from human kidney as the most susceptible target cells. Using the established pseudovirus neutralization assay, we showed that three of ten selected mAbs specific to HA could potently neutralize infection of a pseudovirus bearing HA from the homologous IAV A/VietNam/1194/2004(H5N1) strain. This was highly consistent with the result of a microneutralization assay testing the same strain of a live IAV. Since the pseudovirus neutralization assay does not involve an infectious virus and can be performed without the requirement of a biosafety-3 laboratory, it may be applied for safe and rapid screening of neutralizing mAbs and antiviral agents targeting HA of IAVs.
Keywords: Influenza A virus, Hemagglutinin, Pseudovirus neutralization assay, Monoclonal antibodies
1. Introduction
The worldwide outbreak of the swine-origin H1N1 influenza A virus (IAV) poses an increasing threat of a future influenza epidemic potentially induced by the highly pathogenic avian influenza (HPAI) H5N1 virus. Since its re-emergence in 2003, the H5N1 IAV has caused 294 deaths among a total of 498 confirmed cases as of May 06, 2010 (http://www.who.int/csr/disease/avian_influenza/country/cases_table_2010_05_06/en/index.html) [1], [2], highlighting the significance of developing vaccines and antiviral agents against divergent IAVs.
Among all structural proteins of influenza viruses, hemagglutinin (HA) plays important roles in viral binding to receptors, virus entry and membrane fusion. During virus infection, HA first mediates virus attachment and subsequent entry into target cells [3], [4], [5]. Thus, HA protein is considered the primary target for inducing protective immunity against IAVs [6], [7], [8]. Indeed, antibodies specific for HA have been shown to inhibit and/or neutralize virus infection in vaccinated hosts and anti-HA monoclonal antibodies (mAbs) are regarded as one of the important passive therapeutics for treatment of influenza [9], [10], [11], [12]. It has also been shown that mAbs targeting HA glycoprotein may broadly neutralize homologous and heterologous strains with different clades of IAV infection and prevent formation of escape mutants [13], [14], [15], [16]. To identify more potent and specific neutralizing mAbs as immunotherapeutics for treatment of influenza, it is essential to develop an assay for rapid screening of neutralizing mAbs against HA of IAVs.
Currently, microneutralization and plaque reduction assays are mainly used to evaluate the neutralizing activity of antibodies [16], [17]; however, the infectious, replication-competent live IAVs and biosafety level 3 (BSL-3) facilities are generally required for performing these assays. Here we report the development of a safe and convenient neutralization assay for rapid screening neutralizing mAbs targeting HA of H5N1 and H1N1 IAV. We first generated a series of pseudoviruses bearing HAs of four clades of H5N1 IAV isolated from avians and humans, respectively, and the 2009 epidemic H1N1 IAV, and an HIV-1 backbone (pNL4-3.luc.RE). These pseudoviruses are single-cycle and replication-deficient viruses [18], [19], [20]. Then we used these pseudoviruses to establish a neutralizing assay in a BSL-2 laboratory, based on which we screened a panel of mAbs specific for the HA of an H5N1 virus and successfully identified three specific neutralizing mAbs, suggesting that this established assay is safe and efficient for screening neutralizing mAbs targeting HA of IAVs.
2. Materials and methods
2.1. Recombinant plasmid construction
The genes encoding corresponding HA of human H5N1 IAV isolates, including A/QH/59/05, A/Xinjiang/1/2006, A/Anhui/1/2005, A/Hong Kong/156/97 and A/VietNam/1194/2004 (Table 1 ), were amplified by RT-PCR and inserted into pcDNA3.1 vector (Invitrogen, Carlsbad, CA). The codon-optimized genes expressing HA of human A/California/06/2009(H1N1) (Table 1) were synthesized by Integrated DNA Technologies (Coralville, IA) and inserted into pcDNA3.1 vector as above. The constructed recombinant plasmids containing HA were, respectively, designated as QH-HA-, XJ-HA-, AH-HA-, HK97-HA-, 1194-HA- and H1N1-HA-pcDNA3.1 and confirmed for correct insertion by sequencing analysis.
Table 1.
Source of HA for generating pseudovirus [28].
| Pseudovirus | Subtype | Virus strain | Protein | Accession # | Clade | Host | Country |
|---|---|---|---|---|---|---|---|
| HK-HA | H5N1 | A/Hong Kong/156/97 | HA | AAC40508 | 0 | Human | Hong Kong |
| 1194-HA | H5N1 | A/VietNam/1194/2004 | HA | ABP51976 | 1 | Human | Vietnam |
| QH-HA | H5N1 | A/Qinghai/59/05 | HA | ABE68921 | 2.2 | Goose | China |
| XJ-HA | H5N1 | A/Xinjiang/1/2006 | HA | ACJ68614 | 2.2 | Human | China |
| AH-HA | H5N1 | A/Anhui/1/2005 | HA | ABD28180 | 2.3 | Human | China |
| H1N1-HA | H1N1 | A/California/06/2009 | HA | ACP41935 | N/A | Human | USA |
The protein information was retrieved from the GenBank database. N/A, not applicable.
2.2. Generation of pseudovirus bearing HA of IAVs and detection of pseudovirus titers
Generation of influenza pseudovirus was done as previously described with some modifications [21], [22]. Briefly, 293T cells (ATCC, Manassas, VA) were co-transfected with 10 μg plasmid encoding Env-defective, luciferase-expressing HIV-1 (pNL4-3.luc.RE) and 10 μg each of the 6 plasmids, including QH-HA-, XJ-HA-, AH-HA-, HK97-HA-, 1194-HA- and H1N1-HA-pcDNA3.1, respectively, into 100-mm culture dishes using calcium phosphate method. Cells were changed into fresh DMEM 8 h later, and exogenous bacterial neuraminidase (NA) from Vibrio cholerae (Sigma, St. Louis, MO) was added at the concentration of 4.8 μg/ml 24 and 48 h post-transfection. The transfection without adding NA was used as parallel controls. Supernatants containing HA pseudovirus with or without NA were harvested 72 h post-transfection, and used for single-cycle infection. The viral particles with corresponding HA were named as QH-HA, XJ-HA, AH-HA, HK-HA, 1194-HA, and H1N1-HA pseudoviruses, respectively (Table 1). The plasmids encoding vesicular stomatitis virus G protein (VSV-G), VSV-G-pcDNA3.1, and the pcDNA3.1 vector only were used to co-transfect with pNL4-3.luc.RE plasmid to generate VSV-G pseudovirus and pseudovirus without Env (Env−) as controls. For titration of the pseudovirus, 293T cells were infected with 100 μl of each pseudovirus at 2-fold dilution in 96-well culture plates, followed by detection of luciferase activity 72 h later. The titer of pseudovirus was expressed as relative luciferase unit (RLU).
2.3. ELISA for detection of p24
The p24 in produced pseudovirus was measured by ELISA. Briefly, 96-well ELISA plates were pre-coated with anti-HIV-1 p24 (clone NIH 183-H12, 5 μg/ml) overnight at 4 °C and blocked with 2% non-fat milk at 37 °C for 2 h. Lysed pseudoviruses were added to the plates and incubated at 37 °C for 1 h. After washes, HIV-IgG sera were added (1 μg/ml) and incubated at 37 °C for 1 h, followed by sequentially incubated with biotin-labeled goat anti-human IgG (1:10,000) and streptavidin-conjugated horseradish peroxidase (HRP) (1:10,000) at 37 °C for 1 h. The substrate 3,3′,5,5′-tetramethylbenzidine (TMB) (Zymed, Carlsbad, CA) was added to the plates, and the reaction was stopped by 1 N H2SO4. The absorbance at 450 nm (A450) was measured by ELISA plate reader (Tecan, San Jose, CA).
2.4. Western blot
HIV-1 p24 in the viral core and HA on the surface of the generated pseudoviruses were determined by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), followed by Western blot according to our previous protocols with some modifications [23]. Briefly, lysed pseudoviruses were resolved by 10–20% Tricine gel (Invitrogen), which were then transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA), and blocked in 5% non-fat milk overnight at 4 °C. The blots were, respectively, incubated with anti-HIV-1 p24 (clone NIH 183-H12) and anti-HA #8 mAb (produced below) at 1:1000 dilution. After three washes, the blots were incubated with HRP-conjugated goat anti-mouse IgG (1:5000, Zymed) for 1 h at room temperature. Signals were visualized with ECL Western blot Substrate Reagents and Amersham Hyperfilm (GE Healthcare, Piscataway, NJ).
2.5. Transduction of influenza pseudovirus in different cell lines
To detect cell tropism, 104 cells/well of 293T, Madin-Darby canine kidney (MDCK), A549, Chinese hamster ovary (CHO-K1), and Vero cells (ATCC) were, respectively, transduced with produced pseudoviruses. Fresh DMEM was added 24 h later, and luciferase activity was detected 72 h post-transduction and expressed as RLU.
2.6. Expression of rHA protein
The genes encoding full-length HA of IAV (A/VietNam/1194/2004(H5N1)) plus 6× His tag were amplified by PCR and inserted into the pAcGP67A Baculovirus Transfer Vector (BD Biosciences, San Jose, CA) to construct rHA-Bacul plasmid. The expression of rHA protein was conducted according to the manufacturer’s protocols and our previously described method [23]. Briefly, 2 μg rHA-Bacul plasmid was mixed with 0.5 μg BD BaculoGold linearized Baculovirus DNA and sit at room temperature for 5 min before addition of 1 ml BD BaculoGold Transfection Buffer B. The mixture was added to Sf9 insect cells (ATCC) pre-covered with 1 ml Transfection Buffer A, followed by incubation of the cells at 28 °C for 4 h and further culture of the cells in fresh SF-900 II SFM (Invitrogen) for 4 days. Supernatants were collected and infected cells with three more cycles for virus amplification. Cell culture supernatant from the 4th cycle was collected for purification of rHA protein using His columns (Promega, Madison, WI).
2.7. Mouse vaccination and mAb generation and screening
Five female BALB/c mice aged 4–6 weeks were subcutaneously vaccinated with purified rHA protein (20 μg/mouse) in the presence of Freund’s complete adjuvant (FCA, Sigma) and boosted twice with the same immunogen (10 μg/mouse) containing Freund’s incomplete adjuvant (FIA, Sigma) at 21-day intervals. The hybridoma technique for generation and screening of specific mAbs against HA protein was performed according to the standard protocol [22]. Briefly, vaccinated mice were sacrificed 10 days after the last boost, and the splenocytes were fused with mouse myeloma cells (SP2/0). The HA-specific mAb-screening positive hybridomas were screened by ELISA using rHA protein as the coating antigen. Positive cells were expanded, retested and subcloned to generate stable hybridoma cell lines. The mAbs were purified from culture supernatant using nProtein A Sepharose 4 Fast Flow (GE Healthcare).
2.8. HA pseudovirus neutralization assay
Neutralizing activity of mAbs against infection of HA pseudovirus was performed as follows. Briefly, pseudovirus-containing supernatants were incubated with serially diluted H5N1 HA mAbs at 37 °C for 1 h before adding to 293T cells pre-plated in 96-well culture plates (104/well). MAb 33G4 from severe acute respiratory syndrome coronavirus (SARS) [22] was used as the negative control. Twenty-four hours later, cells were re-fed with fresh medium, which was followed by lysing cells 72 h later using cell lysis buffer (Promega) and transferring the lysates into 96-well luminometer plates. Luciferase substrate (Promega) was added to the plates, and relative luciferase activity was determined in Ultra 384 luminometer (Tecan). The neutralization of HA pseudovirus was calculated [24] and presented as 50% neutralizing antibody titer (NT50).
2.9. Microneutralization assay
The titer of anti-influenza HA neutralizing activity of mAbs was determined by microneutralization assay as follows. Briefly, serial 2-fold dilution of mAbs was mixed with 100TCID50 of A/VietNam/1194/04(H5N1) and incubated at 37 °C for 2 h before adding to MDCK cells in quadruplicate. Medium was replaced with fresh DMEM 2 h later, and cell culture was continued for 72 h at 37 °C. Viral cytopathic effect (CPE) was observed daily and recorded on day 3 post-infection. The neutralizing antibody titer (NT50) was determined by the HA test and defined as the lowest dilution that was negative for HA when 50 μl of cell culture supernatant was incubated with equal volume of 0.5% turkey red blood cells (Lampire Biological Laboratories, Pipersville, PA) at room temperature for 30 min.
2.10. Statistical analysis
Values were presented as mean with standard deviation (SD). Statistical significance among different groups was calculated by Student’s t test using Stata statistical software. P values less than 0.05 were considered significant.
3. Results
3.1. Generation and characterization of HA pseudovirus carrying HA of IAVs
To verify whether these HA pseudoviruses contain the HIV-1 p24, we first used ELISA to detect HIV-1 p24 in the pseudovirus-containing supernatants. All six HA pseudoviruses, one VSV-G pseudovirus and one Env− pseudovirus contained about 50 ng/ml of HIV p24. Then, we did Western blot to further detect the reactivity of HA pseudovirus with mAbs specific for HIV-1 p24 and IAV HA, respectively. As shown in Fig. 1 , clear bands, respectively, corresponding to p24 and HA were observed in all six HA pseudoviruses, suggesting that the HA of influenza pseudovirus was incorporated into the HIV-1 viral particles.
Fig. 1.
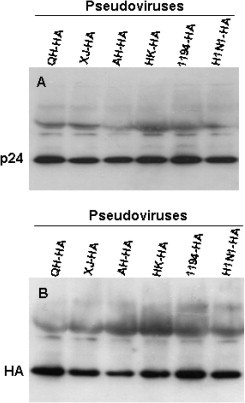
Western blot analysis of the generated HA pseudoviruses. Pseudoviruses (50 ng/ml p24) were, respectively, detected by mAbs (1:1000) specific for HIV-1 p24 (A) and IAV HA (#8, B).
Exogenous NA was added to 293T cells 24 and 48 h post-transfection to further compare the transduction activity of pseudovirus in the absence and presence of NA. Fig. 2 A indicates that HA pseudovirus containing NA induced a significantly higher level of infection, with an average 50-fold increase of RLU in contrast to pseudovirus without NA in transduced 293T cells. The highest infectivity was reached by QH-HA, a pseudovirus expressing the HA of a highly pathogenic H5N1 IAV in goose (Table 1). Since HA pseudoviruses containing NA may be able to improve the infection rate significantly, they were used for subsequent experiments to detect pseudovirus cell tropism and test neutralizating activity for HA mAbs.
Fig. 2.
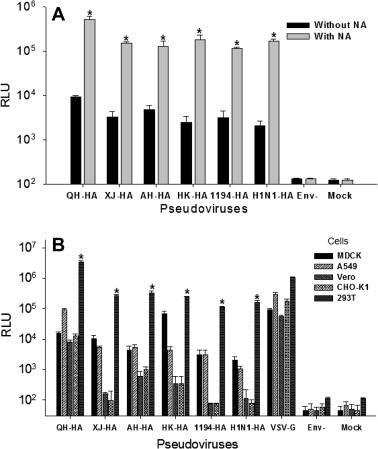
Infectivity and host cell tropism of HA pseudoviruses. (A) Comparison of infection rates of pseudoviruses with and without exogenous NA in 293T cells. * indicates P < 0.05 when comparing each pseudovirus with and without NA. (B) Detection of cell tropism of HA pseudoviruses in MDCK, A549, Vero, CHO-K1 and 293T cells. For each HA pseudovirus, * indicates P < 0.05 when comparing the infectivity in 293T cells and other cells. VSV-G pseudovirus was used as the positive control, and Env− pseudovirus and cells only (mock) were used as the negative control. Pseudoviruses (50 ng/ml p24) were used for infection of cells, and the data are expressed as the Mean RLU ± SD of 3 parallel wells in 96-well culture plates. The experiment was repeated three times and similar results were obtained.
3.2. HA pseudoviruses were able to infect a variety of cell lines and maintained significantly higher infectivity in human 293T cells than in other human and non-human cells
To detect cell tropism of the produced HA pseudovirus, cells from various tissues and different hosts, including MDCK (Dog kidney), A549 (Human lung), CHO-K1 (Chinese hamster ovary), Vero (African green monkey kidney) and 293T (Human kidney) cells were, respectively, transduced with six pseudoviruses containing HIV-1 p24 (50 ng/ml), and luciferase activity was detected 72 h post-infection. VSV-G pseudovirus, which has a broad host range capable of infecting multiple tissues in various hosts, and Env− pseudovirus were used as the positive and negative controls. As shown in Fig. 2B, all six HA pseudoviruses (QH-HA, XJ-HA, AH-HA, HK-HA, 1194-HA and H1N1-HA) could infect human A549 cells, and non-human cells, including MDCK, Vero and CHO-K1, with the infectivity from A549 and MDCK cells being relatively higher than that from Vero and CHO-K1 cells. Particularly, these pseudoviruses demonstrated a significantly higher level of infective ability in 293T cells than other cells, suggesting that the cells derived from human kidney may be more susceptible than those derived from other human tissues or non-human hosts to the HA pseudovirus infection. In addition, avian (goose)-origin pseudovirus (QH-HA) showed a higher affinity and infectivity than human-hosted pseudoviruses, such as XJ-HA, AH-HA, HK-HA, 1194-HA and H1N1-HA. As expected, the positive control, VSV-G pseudovirus, was able to infect all tested cell lines, while negative control, Env− pseudovirus was unable to infect any of these cell lines.
3.3. Established HA pseudovirus neutralization assay could quickly and effectively screen neutralizing activity of mAbs targeting HA of H5N1 virus
ELISA was used to initially screen mAbs generated by immunization of mice with rHA of A/VietNam/1194/2004(H5N1). As shown in Fig. 3 A, 20 mAbs were initially screened for HA-specific antibody responses, 10 of which with the highest antibody titer were selected to detect neutralizing activity against five pseudoviruses expressing HA of H5N1 IAVs (QH-HA, XJ-HA, AH-HA, HK-HA, 1194-HA), one pseudovirus bearing HA of 2009 epidemic H1N1 IAV (H1N1-HA) (Table 1), and the VSV-G pseudovirus in 293T cells since this cell line demonstrated the highest ability to support virus infection. As shown in Fig. 3B, among selected 10 mAbs specific to HA of A/VietNam/1194/2004(H5N1), 3 mAbs (#8, #9 and #17), particularly #8 mAb, neutralized the homologous strain of pseudotyped H5N1 (1194-HA) virus infection, while other HA-specific mAbs showed non-neutralizing activity against homologous and heterologous H5N1 (QH-HA, XJ-HA, AH-HA, HK-HA, 1194-HA) and 2009 epidemic H1N1 (H1N1-HA) pseudoviruses, as well as VSV-G pseudovirus. In addition, the unrelated control mAb 33G4, which is specific for SARS-CoV spike protein, could not neutralize any of the above pseudoviruses. These results indicate that the established pseudovirus neutralization assay is specific to HA, suggesting its potential application for quick and effective screening of mAbs targeting HA of IAVs.
Fig. 3.
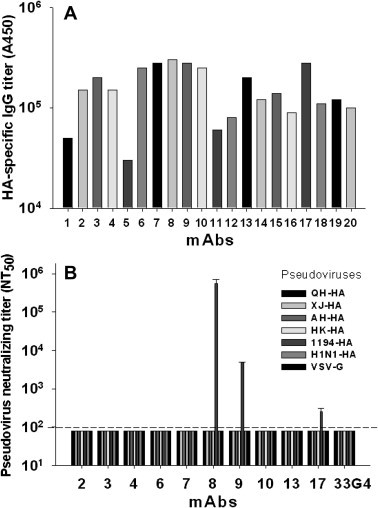
Antibody responses and neutralizing titer detection of mAbs targeting HA of A/VietNam/1194/2004(H5N1) by pseudovirus neutralization assay. (A) ELISA detection of IgG response of 20 screened mAbs specific to HA. The data are expressed as absorbance at 450 nm (A450). (B) Pseudovirus neutralization detection of HA-specific mAbs against infection of HA pseudovirus in 293T cells. Ten mAbs with the highest antibody titer were selected for screening neutralizing activity. 33G4 mAb and VSV-G were used as negative mAb and pseudovirus controls, respectively. The data are presented as Mean NT50 ± SD of 3 parallel wells of each dilution in 96-well culture plates. The dotted line indicates the detection limit. The experiment was repeated three times and similar results were obtained.
3.4. Established HA pseudovirus neutralization assay was highly correlated with microneutralization assay
The selected 10 mAbs were further tested for the neutralizing activity against a wild-type H5N1 virus (A/VietNam/1194/2004) using microneutralization assay, and the results were compared with those from pseudovirus neutralization assay based on the homologous H5N1 virus expressing HA of H5N1 (1194-HA). Table 2 shows that #8, #9 and #17 mAbs, especially #8 mAb, could inhibit infection of live H5N1 virus (A/VietNam/1194/2004), while other mAbs had no neutralizing activity against this virus in tested MDCK cells. Since the results correspond to those of the pseudovirus neutralizing assay based on the HA of the same virus type, the established pseudovirus neutralization assay was confirmed as a reliable method for testing the neutralizing activity of mAbs against HA of IAVs.
Table 2.
Correlation of neutralizing titers of mAbs targeting HA of A/VietNam/1194/2004(H5N1) determined by pseudovirus neutralization assay and microneutralization assay.
| No. of mAbs | NT50 for neutralizing infection by |
|
|---|---|---|
| 1194-HA pseudovirusa | A/VietNam/1194/2004(H5N1)b | |
| 2 | <1:100 | <1:100 |
| 3 | <1:100 | <1:100 |
| 4 | <1:100 | <1:100 |
| 6 | <1:100 | <1:100 |
| 7 | <1:100 | <1:100 |
| 8 | 1:5.5 × 105 | 1:1.3 × 103 |
| 9 | 1:4.9 × 103 | 1:6.4 × 102 |
| 10 | <1:100 | <1:100 |
| 13 | <1:100 | <1:100 |
| 17 | 1:2.5 × 102 | 1:100 |
The detection limit is 1:100.
Using pseudovirus neutralization assay.
Using microneutralization assay.
4. Discussion
In this study, six pseudoviruses were produced in the supernatant of 293T cells in the presence or absence of exogenous NA, respectively, expressing HA of a variety of four clades (clade 0, 1, 2.2, and 2.3) of HPAI H5N1 virus strains and the epidemic strain of 2009 H1N1 virus isolated from avian goose and humans (Table 1). Although packaged HA pseudoviruses without NA may infect target cells, the addition of exogenous NA to 293T cells co-transfected by HIV-1 (pNL4-3.luc.RE) and HA plasmid was shown to significantly increase the transduction efficiency (Fig. 2A), possibly because NA protein may help to remove sialic acid from the host cells to facilitate viral release [25], thus making it an indispensable component of HA-based IAV infection. These results are consistent with previous reports by Rong and colleagues [21]. The produced HA pseudoviruses were demonstrated to infect a number of non-human (MDCK, Vero and CHO-K1) and human (293T and A549) cells. Because the human 293T and A549 cells maintained higher transduction efficacy (Fig. 2B), it can be reasonably inferred that both the avian-origin H5N1 and the swine-origin H1N1 viruses isolated from avian (goose) and humans have a higher tendency to infect humans than other hosts, such as Chinese hamster and African green monkey. These results further highlight the importance of developing anti-IAV agents and vaccines for treatment and prevention of influenza.
A pseudovirus neutralization assay was further developed based on the produced HA pseudoviruses and used to screen neutralizing mAbs against HA of H5N1 virus (A/VietNam/1194/2004). Three of ten selected mAbs were able to neutralize infection by the pseudovirus carrying HA from the homologous H5N1 IAV, while none of them neutralized pseudoviruses expressing HA from heterologous IAVs and the unrelated VSV-G pseudovirus in the tested cell lines (Fig. 3B), the result of which is highly consistent with that from microneutralization assay (Table 2). These findings demonstrated that the established pseudovirus neutralization assay is highly specific and sensitive, thus making it especially suited to the quick screening of a large amount of mAbs in a short period of time. Compared with microneutralization assay and plaque reduction neutralization assay that require the handling of a live virus and the use of approved BSL-3 laboratory facilities, the established non-infectious virus-based pseudovirus neutralization assay is relatively safer and particularly useful when access to a BSL-3 laboratory is restricted.
Neutralizing antibodies targeting HA of IAVs play crucial roles in neutralizing virus infection, clearing virus and suppressing virus spread [26], [27], [28]. Thus, application of the pseudovirus neutralization assay may be extended to evaluate the efficacy of HA-based influenza vaccine candidates and antiviral agents targeting HA of IAVs. This neutralization method could also be optimized for detecting specific neutralizing activity of clinical patient samples, which may be useful for predicting prognosis of the disease. To conclude, the HA pseudovirus-based neutralizing assay can be applied to screen neutralizing mAbs and therapeutics targeting IAV HA, and to evaluate the HA-based IAV vaccines, as well as to detect neutralizing activity of clinical samples, making it possible to assess virus-neutralizing activities safely and rapidly without using live infectious influenza viruses.
Acknowledgments
This study was supported by the National High Technology R&D Program of China (863 Program, No. 2006AA02Z406), National Basic Research Program of China (973 Program, No. 2005CB523001), National Natural Science Foundation of China (30901371), and Mega-projects of Science Research for the 11th Five-Year Plan (2009ZX10004-4001).
References
- 1.Smith G.J., Vijaykrishna D., Bahl J. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 2.Neumann G., Chen H., Gao G.F. H5N1 influenza viruses: outbreaks and biological properties. Cell Res. 2010;20:51–61. doi: 10.1038/cr.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell R.J., Kerry P.S., Stevens D.J. Structure of influenza hemagglutinin in complex with an inhibitor of membrane fusion. Proc. Natl. Acad. Sci. USA. 2008;105:17736–17741. doi: 10.1073/pnas.0807142105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z.N., Lee B.J., Langley W.A. Length requirements for membrane fusion of influenza virus hemagglutinin peptide linkers to transmembrane or fusion peptide domains. J. Virol. 2008;82:6337–6348. doi: 10.1128/JVI.02576-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 6.Tompkins S.M., Lin Y., Leser G.P. Recombinant parainfluenza virus 5 (PIV5) expressing the influenza A virus hemagglutinin provides immunity in mice to influenza A virus challenge. Virology. 2007;362:139–150. doi: 10.1016/j.virol.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kodihalli S., Goto H., Kobasa D.L. DNA vaccine encoding hemagglutinin provides protective immunity against H5N1 influenza virus infection in mice. J. Virol. 1999;73:2094–2098. doi: 10.1128/jvi.73.3.2094-2098.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Treanor J.J., Schiff G.M., Hayden F.G. Safety and immunogenicity of a baculovirus-expressed hemagglutinin influenza vaccine: a randomized controlled trial. JAMA. 2007;297:1577–1582. doi: 10.1001/jama.297.14.1577. [DOI] [PubMed] [Google Scholar]
- 9.Lim A.P., Chan C.E., Wong S.K. Neutralizing human monoclonal antibody against H5N1 influenza HA selected from a Fab-phage display library. Virol. J. 2008;5:130. doi: 10.1186/1743-422X-5-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanson B.J., Boon A.C., Lim A.P. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir. Res. 2006;7:126. doi: 10.1186/1465-9921-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friesen R.H., Koudstaal W., Koldijk M.H. New class of monoclonal antibodies against severe influenza: prophylactic and therapeutic efficacy in ferrets. PLoS One. 2010;5:e9106. doi: 10.1371/journal.pone.0009106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez O., Tsibane T., Basler C.F. Neutralizing anti-influenza virus monoclonal antibodies: therapeutics and tools for discovery. Int. Rev. Immunol. 2009;28:69–92. doi: 10.1080/08830180802593540. [DOI] [PubMed] [Google Scholar]
- 13.Simmons C.P., Bernasconi N.L., Suguitan A.L. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4:e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prabakaran M., Prabhu N., He F. Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants. PLoS One. 2009;4:e5672. doi: 10.1371/journal.pone.0005672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maneewatch S., Thanongsaksrikul J., Songserm T. Human single-chain antibodies that neutralize homologous and heterologous strains and clades of influenza A virus subtype H5N1. Antivir. Ther. 2009;14:221–230. [PubMed] [Google Scholar]
- 16.Yoshida R., Igarashi M., Ozaki H. Cross-protective potential of a novel monoclonal antibody directed against antigenic site B of the hemagglutinin of influenza A viruses. PLoS Pathog. 2009;5:e1000350. doi: 10.1371/journal.ppat.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause J.C., Tumpey T.M., Huffman C.J. Naturally occurring human monoclonal antibodies neutralize both 1918 and 2009 pandemic influenza A (H1N1) viruses. J. Virol. 2010;84:3127–3130. doi: 10.1128/JVI.02184-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han D.P., Kim H.G., Kim Y.B. Development of a safe neutralization assay for SARS-CoV and characterization of S-glycoprotein. Virology. 2004;326:140–149. doi: 10.1016/j.virol.2004.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang S., Xiao L., Zhou H. Generation and characterization of an H5N1 avian influenza virus hemagglutinin glycoprotein pseudotyped lentivirus. J. Virol. Methods. 2008;154:99–103. doi: 10.1016/j.jviromet.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Alberini I., Del T.E., Fasolo A. Pseudoparticle neutralization is a reliable assay to measure immunity and cross-reactivity to H5N1 influenza viruses. Vaccine. 2009;27:5998–6003. doi: 10.1016/j.vaccine.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 21.Guo Y., Rumschlag-Booms E., Wang J. Analysis of hemagglutinin-mediated entry tropism of H5N1 avian influenza. Virol. J. 2009;6:39. doi: 10.1186/1743-422X-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y., Lu H., Siddiqui P. Receptor-binding domain of severe acute respiratory syndrome coronavirus spike protein contains multiple conformation-dependent epitopes that induce highly potent neutralizing antibodies. J. Immunol. 2005;174:4908–4915. doi: 10.4049/jimmunol.174.8.4908. [DOI] [PubMed] [Google Scholar]
- 23.Du L., Zhao G., Chan C.C. Recombinant receptor-binding domain of SARS-CoV spike protein expressed in mammalian, insect and E. coli cells elicits potent neutralizing antibody and protective immunity. Virology. 2009;393:144–150. doi: 10.1016/j.virol.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chou T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 25.Neumann G., Noda T., Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nayak B., Kumar S., DiNapoli J.M. Contributions of the avian influenza virus HA, NA, and M2 surface proteins to the induction of neutralizing antibodies and protective immunity. J. Virol. 2010;84:2408–2420. doi: 10.1128/JVI.02135-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia J.M., Pepin S., Lagarde N. Heterosubtype neutralizing responses to influenza A (H5N1) viruses are mediated by antibodies to virus haemagglutinin. PLoS One. 2009;4:e7918. doi: 10.1371/journal.pone.0007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun L., Lu X., Li C. Generation, characterization and epitope mapping of two neutralizing and protective human recombinant antibodies against influenza A H5N1 viruses. PLoS One. 2009;4:e5476. doi: 10.1371/journal.pone.0005476. [DOI] [PMC free article] [PubMed] [Google Scholar]


