Abstract
Poultry production is an important economic activity on inhabited islands of the Galápagos archipelago. There has been a recent surge in both small-scale backyard chickens and larger scale broiler production associated with growth in the human population and the tourist industry. With increased poultry production, concerns have been expressed about the increasing risk of transfer of disease from chickens to native Galápagos bird species that may have little resistance to introduced pathogens [Wikelski, M., Foufopoulos, J., Vargas, H., Snell, H., 2004. Galápagos birds and diseases: invasive pathogens as threats for island species. Ecology and Society 9(5). Available from: URL:http://www.ecologyandsociety.org/vol9/iss1/art5]. This study evaluates risks posed by chicken disease to endemic and native Galápagos bird species, based on empirical evidence of pathogens present in chickens on the islands and a literature review of effects of these pathogens in wild species. Pathogens identified in domestic chicken populations of immediate avian conservation concern are Newcastle disease, Mycoplasma gallisepticum, and the proventricular parasite Dispharynx sp. Newcastle disease (avian paramyxovirus-1) poses an imminent threat to Galápagos penguins (Spheniscus mendiculus), flightless cormorants (Phalacrocorax harrisi), and lava gulls (Larus fuliginosus), species with very small population sizes (less than 1500 animals each). Additionally, litter from broiler farms could affect ecological processes in local ecosystems. Improved poultry biosecurity measures are urgently needed on the Galápagos Islands for avian disease management, yet developing these strategies presents political, social, and economic challenges.
Keywords: Galápagos islands, Native birds, Avian conservation, Pathogens, Chickens, Gallus gallus, Disease risk
1. Introduction
The Galápagos archipelago, located approximately 1000 km west of continental Ecuador (Fig. 1 ), is renowned for its endemic flora and fauna whose study has greatly influenced modern evolutionary theory (Darwin, 1859, Grant and Grant, 2003). From the 19th century to the present, human activities, including the introduction of invasive animal and plant species, have negatively impacted Galápagos ecosystems (Snell et al., 2002, MacFarland and Cifuentes, 1996). In other island ecosystems, such as Hawaii, anthropogenic introduction of exotic vertebrate and invertebrate species is linked to the emergence of infectious disease (e.g., avian malaria and avian pox) and subsequent decline in many native bird species (Van Riper III et al., 1986, Atkinson et al., 1995).
Fig. 1.
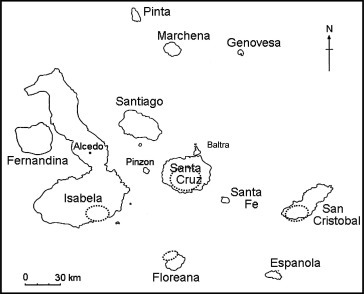
Map of the Galápagos Islands. Areas shown in dashed circles represent general locations of chicken farming activities.
Disease carried by any bird species introduced to the Galápagos Islands (chickens Gallus gallus, pigeons Columba livia, smooth-billed anis Crotophaga ani, Guinea fowl Numida meleagridis, and farmyard ducks of genus Cairina or Anas) may threaten its native avifauna, comprising 58 resident species (22 endemic and 36 native). However, little published information is available on diseases present in domestic, introduced, or native birds in the Galápagos archipelago (Wikelski et al., 2004). Chickens (G. gallus) and pigeons (C. livia) are the principal avian species introduced to the Galápagos by human colonists. Feral pigeons have inhabited Santa Cruz, San Cristobal, and Isabela islands. Current eradication efforts are rapidly reducing the numbers of introduced pigeons (Phillips et al., 2003).
In contrast, the numbers of domestic chickens are increasing in inhabited areas of the Galápagos. Domestic chickens are present on Santa Cruz, Isabela, San Cristobal, Floreana, and Baltra (Fig. 1). Three types of poultry farming are practiced on the Galápagos Islands: small-scale backyard meat chickens (1–40 birds per farm) (Fig. 2 ), small to medium-scale egg layers, and medium to relatively large-scale commercial broiler operations (2000–4000 birds) (Fig. 3 ). Currently, there are 23 broiler chicken farms on Santa Cruz, 6 on San Cristobal, and 4 on Isabela. In the past five to ten years, poultry production has intensified due to demand from the growing human population and tourist industry. Under Galápagos law, broiler chickens, brought to Galápagos at 1–5 days of age, must be unvaccinated and certified as healthy by approved aviculture facilities on the Ecuadorian mainland. Currently, no livestock vaccinations are permitted on the Galapagos Islands (David Cruz, SICGAL, personal communication). Feral populations of chickens exist on Santa Cruz, Isabela, San Cristobal, and Floreana. In addition to the risk of introducing disease into native Galapagos avifauna, waste from domestic poultry operations may have detrimental effects on local plant and animal communities in the Galapagos due to nutrient enrichment and water contamination.
Fig. 2.

Backyard chickens mingling with Darwin’s ground finches (Geospiza sp.) on Floreana Island, Galápagos.
Fig. 3.

Typical broiler pen, Isabela Island, Galápagos.
In 2001, the Saint Louis Zoo and the University of Missouri – St. Louis, in cooperation with the Galápagos National Park Service and the Charles Darwin Research Station, initiated an avian disease surveillance program in the Galápagos Islands. This monitoring serves to identify pathogens that pose a particular risk to native populations and helps to target certain pathogens for future disease surveillance. A similar approach to identifying high-risk pathogens has been performed for killer whales (Orcinus orca) (Gaydos et al., 2004). Here, we present results of poultry disease surveys from 2001 to 2003 in relation to their threat to native birds, discuss ecological threats of broiler aviculture and backyard chickens to Galápagos ecosystems, identify disease research priorities, and discuss potential strategies for the control and prevention of disease transmission to native birds.
2. Methods
2.1. Chicken disease identification
Between July of 2001 and September of 2003, 55 domestic chickens were collected from 6 premises on Santa Cruz and 45 were collected from 7 premises on San Cristobal. Birds were collected for avian necropsy workshops. In addition, blood was collected in November 2003 from 72 broiler chickens on four relatively large-scale (1000–3000 chickens) poultry farms and one medium-scale (50–100 chickens) backyard operation on San Cristobal.
After physical examination, a blood sample was taken from the ulnar vein. Fresh blood smears were prepared, fixed, and stained using a modified Wright-Giemsa staining technique (Diff Quick) and evaluated for the presence of hemoparasites (Antech Diagnostics- Chicago, Alsip IL 60903, USA). Blood was collected in lithium heparin tubes. Plasma aliquots were stored in liquid nitrogen in the field and transferred to −70 °C ultrafreezer before serologic testing. Plasma from birds collected between July 2001 and September 2003 was antibody tested for the following diseases (antibody testing method in parenthesis): influenza A/avian influenza (agar gel immunodiffusion – AGID); avian adenovirus group II/hemorrhagic enteritis of turkeys (AGID); avian paramyxovirus I/Newcastle disease (hemagglutination inhibition – HI); Pasteurella multocida (fowl cholera) (microscopic agglutination – MA); Mycoplasma gallisepticum (hemagglutination inhibition – HI); Salmonella typhimurium (tube agglutination) and Salmonella pullorum (tube agglutination) at the Veterinary Medical Diagnostic Laboratory, University of Missouri – Columbia, Columbia, MO 65205. Further aliquots were antibody tested for avian encephalomyelitis (AGID), avian adenovirus group-I (AGID), Marek’s disease virus (AGID), infectious bursal disease virus (AGID), avian paramyxoviruses 2 and 3 (HI), and avian reovirus (immunoflourescent antibody testing) at the National Veterinary Services Laboratory, Ames, IA 50010. Ten of these birds were tested for Chlamydophila psittaci by elementary body analysis at the Texas Veterinary Medical Diagnostic Laboratory, College Station, TX 77841. Of the samples collected in November 2003, blood smears were examined for hemoparasites. Serotests were performed for Newcastle disease, reovirus, infectious bronchitis virus, infectious bursal disease, Mycoplasma sp., and avian influenza using an IDEXX Enzyme-linked Immunosorbent Assay (ELISA) at LAFAVET Laboratories, Quito, Ecuador. Different diagnostic test methods were used for serology of the November 2003 samples as compared to the July 2001 and 2002 serotests. Variation in sample volume meant that not all tests were conducted on all birds.
The 100 birds collected for the necropsy workshops were euthanized humanely by intravenous injection of pentobarbital sodium euthanasia solution (Beuthanasia-D Special, Schering Plough Animal Health Corporation, Union, NJ 07083, USA). Complete post-mortem examinations (gross and microscopic examination) were performed on all birds except those from the November 2003 collections in San Cristobal, where only serum was taken. Representative samples of major organs were fixed in 10% neutral buffered formalin (fixed tissue samples were transferred to ethanol to facilitate international transportation). Tissues were routinely processed for light microscopic histopathology (hematoxylin and eosin staining) at the Veterinary Medical Diagnostic Laboratory, Columbia, MO 65205, USA. Subsequent samples were routinely processed at AXXIS and Astrid Rhon laboratories, Quito, Ecuador. Tissues from each bird were examined by a veterinary pathologist (T.W., N.G.) for microscopic lesions. In one bird, further paraffin sections were evaluated for Toxoplasma gondii antigen using polyclonal antibody 125P (GioGenex, San Ramon, CA 94583) and streptavidin, and 3-amino-9-ethylcarbazole (AEC) chromagen detection. Appropriate positive and negative controls were run simultaneously. Helminths and ectoparasites retrieved during necropsy were placed in 10% formalin and transferred to absolute ethanol or placed directly in ethanol for examination. Preserved specimens were identified by a parasitologist (M. Dailey). In some cases, parasites were only present in histologic sections and they were identified as morphological features allowed. Therefore, sample sizes for evaluation of prevalence of particular parasites differed between parasites.
3. Results
Based on results from July of 2001, 2002, and September of 2003, seropositivity was identified for the following pathogens (number positive/total examined, %): infectious bursal disease (22/58, 38%), M. gallisepticum (14/38, 37%), Avian adenovirus type I (46/57, 81%), Marek’s disease (10/23, 43%), avian encephalomyelitis (10/24, 42%), infectious bronchitis virus – Massachussetts strain (4/24, 17%), infectious bronchitis virus – Connecticut strain (2/24, 8%), and C. psittaci (1/10, 10%). Chickens from this sample pool were serologically negative for avian cholera, avian influenza, avian adenovirus II, Newcastle disease (Avian Paramyxovirus-1), avian paramyxovirus 2 and 3, S. pullorum, S. typhimurium, and infectious tenosynovitis virus (Table 1 ). In subsequent serologic tests of chickens from farms on San Cristobal Island in November 2003 using a different diagnostic laboratory and test than the previous samples, seroreactivity for Newcastle disease was detected on three out of five farms with 16 out of 72 birds positive (22.2%). Seropositivity for M. gallisepticum was detected in 5 out of 12 chickens on one farm from the November 2003 samples; the other farms were negative. Regarding other serologic tests from chicken farms from November 2003, seropositivity was detected for infectious bursal disease (30/72, 41.7%), infectious bronchitis virus (33/72, 45.8%), and infectious tenosynovitis virus (49/72, 68%). Antibodies for avian influenza were not detected in any of the broiler farms (Table 1).
Table 1.
Serology results for chickens collected in Santa Cruz and San Cristobal, Galápagos Islands from July 2001 to September 2003, and a November 2003 serosurvey from San Cristobal
| Disease or agent | Total seroreactive chickens positive/number tested | Seroreactive chickens by island |
||
|---|---|---|---|---|
| Santa Cruz July 2001–September 2003 | San Cristobal July 2001–September 2003 | San Cristobal November 2003 | ||
| Avian cholera | 0/23 | 0/16 | 0/7 | NA |
| Avian influenza | 0/133 | 0/35 | 0/26 | 0/72 |
| Avian adenovirus I | 46/57 (81%) | 28/33 (85%) | 18/24 (75%) | NA |
| Avian adenovirus II | 0/23 | 0/16 | 0/7 | NA |
| Newcastle disease/PMV1 | 16/134 (12%) | 0/35 | 0/27 | 16/72 (22%) |
| IBV | 39/96 (41%) | 0/16 | 6/8 (75%) | 33/72 (46%) |
| IBV-Massa | 4/24 (17%) | 0/16 | 4/8 (50%) | NA |
| IBV-Conna | 2/24 (8%) | 0/16 | 2/8 (25%) | NA |
| Avian encephalomyelitis | 10/24 (42%) | 3/16 (19%) | 7/8 (88%) | NA |
| Marek’s disease | 10/23 (43%) | 9/16 (56%) | 1/7 (14%) | NA |
| IBDb | 52/130(40%) | 13/33 (39%) | 9/25 (36%) | 30/72 (42%) |
| Paramyxovirus 2 | 0/55 | 0/32 | 0/23 | NA |
| Paramyxovirus 3 | 0/55 | 0/32 | 0/23 | NA |
| Infectious tenosynovitis virus (reovirus) | 49/96 (51%) | 0/16 | 0/8 | 49/72 (68%) |
| M. gallisepticum | 19/110 (17%) | 12/19 (63%) | 2/19 (11%) | 5/72 (7%) |
| S. pullorum | 0/38 | 0/19 | 0/19 | NA |
| S. typhimurium | 0/38 | 0/19 | 0/19 | NA |
| C. psittaci | 1/10 (10%) | 1/10 (10%) | Not tested | NA |
Infectious bronchitis virus – Massachusetts strain or Connecticut strain.
Infectious bursal disease.
On histopathology, fowlpox was identified in 6% (6/100) and Macrorhabdus sp., a fungal enteric pathogen (Tomaszewski et al., 2003), in 10.5% (6/57) of chickens examined. A systemic infection with T. gondii was also identified by histopathology and immunohistochemistry in the one chicken we tested.
No hemoparasites were detected on examination of blood smears. Parasites identified grossly or histologically include the following (number positive/total number examined, %infected): the conjunctival nematode Oxyspirura mansoni (41/98, 42%), gastrointestinal nematodes Capillaria sp. (22/88, 25%), Dispharynx sp. (4/88, 4.5%), Tetrameres sp. (28/100, 28%), Ascarida galli (14/100, 14%), Heterakis gallinae (21/88, 23.4%), cestodes Raillietina echinobothrida (24/88, 27.3%), Davaina proglottina (33/68, 48.5%), renal trematodes (7/88, 8%), intestinal flagellates (7/88, 8%), enteric coccidia (16/68, 23.5%), and T. gondii (1/88, 1.1%). Ectoparasites identified included lice (Phthiraptera) in 9% of birds and mites (Epidermoptes bilobatus) in 2 birds from San Cristobal.
Pathogens detected in this study, documented pathogenicity in wild birds, and native Galápagos species at risk are summarized in Table 1, Table 2 .
Table 2.
Pathogens in domestic chickens on San Cristobal and Santa Cruz Islands from 2001 to 2003, known pathogenicity to wild birds, and risk to Galápagos bird species
| Pathogen | Disease type | Wild bird species affected | Virulence | Citation | Galápagos bird species at risk |
|---|---|---|---|---|---|
| Avian paramyxovirus type I (Newcastle disease) | Virus | Double-crested cormorants, white pelicans, mallard ducks; Gulls, USA, and Canada | Moderate to high | Wobeser et al., 1993, Banerjee et al., 1994, Docherty and Friend, 1999 | Flightless cormorant, brown pelican, Galápagos penguin, lava gull, Galápagos finches mockingbirds, Galápagos pintail |
| Avian encephalomyelitis (picornavirus) | Virus | Pheasant, quail, turkeys | Unknown | Van Steenis, 1971, Calnek, 2003 | Unknown: antibodies reported in waved albatross (Padilla et al., 2003) |
| Infectious bursal disease (birnavirus) | Virus | Herring gull | High | http://w3.vet.cornell.edu/nst/ | Lava gull |
| Marek’s disease (herpesvirus) | Virus | Suggestive lesions of Marek’s disease in a great-horned owl | Unknown | Halliwell (1971) | Barn owl, short-eared owl, Galápagos penguin |
| Avian adenovirus type I | Virus | Turkeys, pigeons, mallard ducks, guineafowl, pheasants, geese | Unknown | Gerlach (1999) | Fightless cormorants, waved albatross (Padilla et al., 2003), boobies, white-cheeked pintail, lava gull, terns |
| Infectious bronchitis virus (coronavirus) | Virus | Racing pigeons | Moderate | Barr et al. (1988) | Galápagos doves |
| Fowlpox (poxvirus) | Virus | Galliformes | Low to high | Gerlach, 1999, Thiel et al., 2005 | Galápagos passerines (recombination with passerine strains possible, canarypox-like isolate identified) |
| Infectious tenosynovitis (reovirus) | Virus | Muscovy ducks | Low | Wobeser (1997) | White-cheeked pintail |
| M. gallisepticum (Mycoplasma sp.) | Bacteria | House finch, American goldfinch, house sparrow, many species of Passeriformes, Piciforms, Apodiformes, and Columbiformes, USA Wild turkeys, USA | Moderate to high | Davidson et al., 1982, Jessup et al., 1983, Friend, 1999, Hartup et al., 2001, Luttrell et al., 2001, Forrester and Spalding, 2003 | Darwin’s finches, mockingbirds Galápagos doves, dark-billed cuckoos, yellow warbler |
| Dispharynx sp. | Nematode parasite | Many passerines: Brown-headed cowbird, boat-tailed grackle, American crows, blue jay, northern mockingbird, Northern cardinal, Carolina wren, wild turkey | Moderate to high (depends on intensity of infection) | Rickard, 1985, Forrester and Spalding, 2003 | Dark-billed cuckoos, Darwin’s finches, mockingbirds, yellow warbler, Galápagos flycatcher, Vermillion flycatcher |
| Capillaria spp. | Nematode parasite | C. contorta and C. annulata (poultry capillaria): turkey, goose, pheasant, guinea fowl, peafowl, black-crowned night heron, double-crested cormorant | Low to moderate | McDougald, 2003, Forrester and Spalding, 2003 | Flightless cormorant, yellow-crowned night heron, lava heron |
| T. gondii | Protozoa | Members of Accipitiriformes, Anseriformes, Galliformes, Gruiformes, Charadriiformes, Columbiformes, Strigiformes, Passerifomes | Moderate to high | Dubey, 2002 | Any wild bird species in contact with feral or domestic cat feces |
| Megabacteria (Macrorhabdus sp.) | Fungus | Canaries | Moderate | Uyttebroek and Ducatelle, 1990, Lumeij, 1999, Tomaszewski et al., 2003 | Darwin’s finches, yellow warblers, mockingbirds, yellow-billed cuckoo, Galápagos and Vermillion flycatchers |
4. Discussion
4.1. Chicken pathogens found and their risk to native birds
Pathology and serology studies from 2001 to 2003 show evidence of a variety of viral, bacterial, and parasitic diseases in introduced chickens of the Galápagos Islands. Although some diseases are chicken-specific, many pathogens identified are not species-specific and pose an imminent threat to native avifauna. Seropositivity was detected for 11 of 18 chicken pathogens tested and eight of these pathogens were detected in over 15% of chickens tested. This relatively high pathogen prevalence may be due in part to sample bias. Most chickens in this study appeared clinically normal. However, eight of 172 chickens in this study were clinically ill and many of the samples collected in the July 2001 to September 2003 surveys included chickens from farms with a history of disease, thus potentially over-estimating the true prevalence of disease among the Galápagos domestic chicken population. Additionally, broiler chickens may be seropositive not due to previous or current infection, but due to vaccination of adults on the islands or of chicks purchased from broiler suppliers on the Ecuadorian continent, despite the prohibition on vaccination. Although potential sample bias may overestimate the true prevalence of pathogens in Galápagos chickens, we believe that, in the presence of uncertainty, slight over-estimation of disease prevalence is prudent when evaluating the potential of disease transmission from domestic poultry to Galápagos endemic avifauna, many of which have naturally small populations.
A large proportion of chickens from pooled samples from July 2001 to November 2003 were seroreactive for avian adenovirus I (81%), infectious bronchitis virus (41%), infectious tenosynovitis virus (51%), Marek’s disease (43%), avian encephalomyelitis (42%), and infectious bursal disease (40%). In Argentina, adenovirus antibody prevalence similar to that in our study was associated with poor hygienic conditions on commercial poultry farms (Gonzalez et al., 1978). The seroprevalence for infectious bronchitis virus in our study resembled that in backyard poultry operations in villages of the Yucatan Peninsula (56%) and may be a common cause of respiratory disease in Galápagos chickens as well (Gutierrez-Ruiz et al., 2000). Comparable serologic results were also seen for Avian encephalomyelitis presence in fancy chickens from Switzerland. In this study, relatively high pathogen prevalence was associated with close contact and group rearing of similar chicken age classes (Wunderwald and Hoop, 2002).
Our study shows serologic evidence of Newcastle Disease in chickens from San Cristobal Island. Newcastle disease shows high pathogenicity in many species that are related to Galápagos endemics. Newcastle disease causes high mortality in the double-crested cormorant Phalacrocorax auritis (Docherty and Friend, 1999, Banerjee et al., 1994, Wobeser et al., 1993), a congener of the flightless cormorant Phalacrocorax harrisi. In 1992 and 2000, disease outbreaks attributed to velogenic viscerotropic Newcastle disease caused the death of over 2000 and 500 chickens, respectively, on Santa Cruz Island. Disease eradication plans were implemented by selective culling (Ministerio de Agricultura y Ganadería, Dirección Provincial Agropecuaria de Galápagos). However, antibodies to Newcastle disease were not detected in Santa Cruz chickens tested in our study. Explanations for this finding include potential false negative serotests, low sample size, and local eradication of disease during the outbreak of 1999–2000. Newcastle disease antibodies were detected in broiler chickens from San Cristobal in November 2003 by enzyme-linked immunosorbent assay. Although the chickens tested appeared relatively normal, necropsy findings on abbatoir chickens from the seropositive flock are consistent with a Newcastle disease strain of low to moderate pathogenicity. Potential sources of Newcastle disease virus are domestic pigeons (Alexander, 2003), contaminated equipment (Alexander, 1988), illegal transport of cock-fighting birds between islands, migratory birds, or introduction of infected birds or poultry products from the Ecuadorian mainland.
Newcastle disease has the potential to cause high mortality and morbidity in wild and domestic birds. Gulls, pelicans, penguins, and cormorants are among the highly susceptible groups of wild birds (Docherty and Friend, 1999). Consequently, lava gulls (Larus fuliginosus), flightless cormorants (P. harrisi) and Galápagos penguins (Spheniscus mendiculus), endemic species with small population sizes (<1500 individuals), may be particularly susceptible to infection. A 1997 outbreak of a highly pathogenic strain of Newcastle disease caused nesting failure and more than 2000 deaths in a double-crested cormorant colony in the Salton Sea, southern California (Docherty and Friend, 1999). Clearly, Newcastle disease, present in Galápagos chickens, has the potential to cause severe decline or extinction of the flightless cormorant population. Continued surveys of domestic chickens and wild birds for Newcastle disease as well as the isolation, molecular characterization and pathogenicity typing of Newcastle disease strains present on the Galápagos Islands are necessary to prevent and manage the disease.
M. gallisepticum, serologically detected in many chickens, causes respiratory disease in birds (Klemen, 2003). Certain strains of M. gallisepticum can cause high morbidity and mortality in members of the Fringillidae, in particular house finches (Carpodacus mexicanus) and American goldfinches (Carduelis tristis) (Hartup et al., 2001). A variant of M. gallisepticum associated with domestic poultry or pen-reared wild turkeys (Luttrell et al., 2001) has caused severe conjunctivitis in house finches (C. mexicanus), resulting in population declines (Dhondt et al., 1998, Pillai et al., 2003). Although antibodies to M. gallisepticum were detected in two wild Galápagos passerines (Ricaurte, 1994), susceptibility to mycoplasmal infection of native Galápagos avifauna is unknown. Conjunctivitis, sinusitis, and rhinitis of undetermined etiology have been observed in finches and mockingbirds on Isabela and Santa Cruz (Jiménez and Gottdenker, personal observation). Studies are underway to identify if Mycoplasma sp. or other respiratory pathogen is involved in the etiology of this disease.
Fowlpox virus was confirmed in chickens by gross necropsy, histopathology, and molecular techniques (polymerase chain reaction and sequencing). Molecular sequencing data from avian pox lesions in domestic and wild birds from the Galápagos Islands indicate that the poxvirus present in chickens (fowlpox virus) differs greatly from that in wild passerines (Thiel et al., 2005). Although transmission of poxvirus from chickens to Galápagos passerines is unlikely, there is the possibility of recombination of chicken and passerine poxvirus strains, potentially altering virulence. Moreover, Thiel et al. (2005) detected significant recombination between ancestors of the strains found within Galápagos passerines and between these strains and Canarypox.
Serologic results indicate that poultry on both Santa Cruz and San Cristobal Islands have been exposed to infectious bursal disease (IBD). IBD is a Birnavirus that causes necrosis of the lymphoid tissues (Sivanandan and Maheswaran, 1980, Lukert and Saif, 2003) resulting in immunosuppression that allows the emergence of viral, bacterial, and fungal infections (Charlton, 2000). Relatively high antibody titers to IBD were detected in herring gulls in the Baltic Sea (Hollmen et al., 2000) and free-ranging Antarctic penguins (Pygoscelis adeliae and Aptenodyes forsteri) (Gardner et al., 1997). It remains unknown whether IBD is pathogenic to endemic birds such as lava gulls (L. fuliginosus) and Galápagos penguins (S. mendiculus). Regardless, a very pathogenic strain of IBD has recently invaded South America (Enzo and Yannick, 2003). If introduced to Galápagos chickens, this strain may cause proliferation of a variety of pathogens in domestic flocks that may spillover into wild bird populations. Furthermore, penguins and flightless cormorants may have contact with infected chicken products via increasing tourist and fishing activities in Western Galápagos and local migrants such as yellow warblers that may carry the disease from inhabited to uninhabited regions. Diseases such as infectious bursal disease as reported in Antarctic penguins (Gardner et al., 1997) could be transmitted to these populations by contact with chicken waste.
Although infectious bronchitis virus, identified serologically in 42.8% (33/77) chickens on San Cristobal, is considered to be relatively species-specific, seropositivity has been recorded in several wild bird species including prairie chickens Tympanuchus cupido (Peterson et al., 2002), rockhopper penguins Eudyptes chrysocome (Karesh et al., 1999), pheasants Phasianus colchicus, and pigeons C. livia (Barr et al., 1988). It is not known if infectious bronchitis virus is pathogenic in native Galápagos birds.
Positive serotiters to Marek’s disease, a herpesvirus, were identified in 43.5% (10/23) of the chickens necropsied. Antibodies to Marek’s disease had previously been observed in Galápagos chickens (Vargas and Snell, 1997). Experimental studies suggest that the only wild bird species susceptible to Marek’s disease would be in the Order Galliformes (Pradhan et al., 1985, Cho and Kenzy, 1975). There are no native galliform species in the Galápagos, therefore the risk of Marek’s disease is low. Indeed, a study of Marek’s disease on the Galápagos penguin in 1996 gave negative results (Miller et al., 2001).
Although many parasites identified in this study are chicken-specific, the nematodes O. mansoni, Capillaria sp., Dispharynx sp., and Tetrameres sp. are likely to affect a variety of avian species (Permin and Hansen, 1998). D. proglottina, identified in some chickens, may cause enteritis in wild birds (Permin and Hansen, 1998). Capillaria sp. present in chickens may infect a variety of avian species, causing high levels of mortality in heavy infections (Barnes, 1986). Many species of North American passerines are highly susceptible to infection with the proventricular nematode (Dispharynx sp.) (Rickard, 1985) (Table 2). In heavy infections, Dispharynx sp. can cause illness, particularly in juvenile birds (Rickard, 1985, Forrester and Spalding, 2003). A number of mortalities in Galápagos dark billed cuckoos were attributed to Dispharynx spiralis (Vargas and Bensted-Smith, 2000). Because Dispharynx has a large host range, poultry may not be the only source of potential infection for Galápagos endemics, as different species or subspecies of this parasite may have been present on the Galápagos before the introduction of chickens. Regardless, heavy infections of Dispharynx sp. in chickens, combined with an abundance of suitable intermediate hosts (e.g., isopods), may be a source of infection for native birds.
T. gondii, identified in one chicken, is an apicomplexan parasite capable of infecting a wide variety of animals, including wild birds (Work et al., 2000, Dubey, 2002) reptiles, and mammals. Definitive hosts are restricted to the family Felidae, particularly the domestic cat (Felis domesticus). Consumption of oocysts from cat feces is the typical route of exposure, although ingestion of undercooked infected meat products can result in infection (Tenter et al., 2000). This finding is significant, because it indicates the potential for toxoplasmal infections in wild Galápagos birds, as feral cats are abundant on inhabited islands.
4.2. Chicken pathogens not found but for which monitoring should continue
There was no serologic evidence for avian influenza in poultry populations of Santa Cruz and San Cristobal. Shorebirds, gulls, and waterfowl are natural hosts for avian influenza (Suarez, 2002). Many passerine species are susceptible to pathogenic infection with the chicken/Hong Kong H5N1 strain of avian influenza (Perkins and Swayne, 2003). Due to the emergence of avian influenza in the poultry industry and its worldwide threat to avian and human public health (Hatta and Kawaoka, 2002), it is important to continue surveillance for avian influenza on the Galápagos archipelago.
Protozoan hemoparasites such as Plasmodium, Haemoproteus or Leucocytozoon, were not detected in any chickens. However, continued monitoring of poultry and wild birds for these diseases is important, because black flies (Simuliidae), an intermediate host for leucocytozoonosis, and Culex quinquefasciatus, a vector of avian malaria and avian pox, have been introduced onto the Galápagos (Mouchet et al., 1995, Peck et al., 1998, Whiteman et al., in press). The introduction of C. quinquefasciatus on Hawaii is associated with the emergence of avian malaria and avian pox in wild bird populations (Van Riper III et al., 1986, Fonseca et al., 2000). C. quinquefasciatus is also a vector of arboviral diseases such as West Nile virus (Dohm et al., 2002). Four randomly chosen chickens from the necropsy group tested negative for antibodies to West Nile virus. Although there is currently no evidence of West Nile virus introduction into the Galápagos, domestic chickens may be a sentinel (Langevin et al., 2001) should this, or other viruses, expand their range to the Galápagos archipelago.
4.3. The chicken problem: Potential spillover of disease into wild bird populations
The most common types of poultry raising practices are small-scale backyard chickens and moderate to large-scale broiler production facilities. Backyard chickens are hatched and raised on the Galápagos Islands, while broiler chickens are transported to the archipelago at one day to one week of age and subsequently grown to market age (6 weeks). Both husbandry strategies present a threat to native and endemic Galápagos birds.
Backyard poultry often have higher macroparasite loads than broiler chickens and may thus provide a source for infection of wild birds attracted to available feed and water. Backyard chickens and finches often share feeding areas (Fig. 2). Outbreaks of respiratory disease are common in backyard chickens, and often rapidly spread to neighboring homes and farms (Gutierrez-Ruiz et al., 2000), in part due to their relative mobility. Illegal trade in fighting chickens from mainland Ecuador and between islands is also a potential mechanism for introduction of pathogens such as virulent strains of Newcastle disease into domestic broiler and backyard flocks. However, backyard chickens may be more resistant to infectious disease due to hybrid vigor.
Theoretically, chicks should arrive relatively healthy to the Galápagos, but in reality they may be incubating directly, neonatally, or transovarially transmitted infectious diseases. It is also difficult and expensive to monitor the certified aviculture companies on the Ecuadorian mainland for diseases that pose a threat to wild birds. Implementation of biosecurity protocols in broiler facilities that minimize contact with wild birds is relatively straightforward. Unfortunately, many broiler houses on the Galápagos do not follow strict biosecurity protocols and have inadequate broiler house construction, allowing for direct contact between some native bird species. Unlike backyard chickens living in relatively low densities, high densities in broiler farms may increase contact rates between infected individuals and provide a highly concentrated pathogen source, facilitating disease spread to wild birds and backyard chickens. Additionally, high concentrations of poultry in broiler houses may provide an ideal opportunity for recombination to occur between strains of a pathogen, potentially resulting in the emergence of a pathogen with increased virulence for other avian species.
4.4. Potential ecological disturbances of broiler farms in the Galápagos
In addition to introduced pathogens, intensified poultry production on the Galápagos Islands may cause local ecological disturbances. Most broiler farmers on the Galápagos apply used litter to their agricultural fields. In the Southeastern U.S., the application of broiler litter to pastures has been associated with environmental nitrogen contamination. Over-application of poultry litter to agricultural fields may cause leaching of nitrate into groundwater (Andres, 1995, Gordillo and Cabrera, 1997). Increased nitrogen load may alter plant community composition and successional dynamics (Tilman, 1987). Higher soil nitrogen levels may facilitate the growth of detrimental invasive species present in the Galápagos, such as blackberry (Rubus sp.). Additionally, a change in plant species composition or vegetation structure may also alter avian bird community composition (Wilson and Belcher, 1989) and nesting success (Remes, 2003). Improperly composted broiler litter may also facilitate the spread of potential bacterial, viral, and parasitic pathogens to wild birds.
Broiler farms also require large quantities of water, a limited resource in Galápagos. Water reservoirs at broiler farms could also provide breeding grounds for mosquitoes, and thus increase the potential of vector-borne disease transmission. Future research on the impact of Galápagos broiler farms on disease threats and ecosystem processes is important to natural resource management and environmental conservation. In the meantime, practices that minimize negative ecological impacts and disease spread from poultry litter (e.g., mandatory composting) should be implemented.
4.5. Poultry management strategies and wild bird conservation
Pigeons (C. livia) are being eradicated on the Galápagos Islands, in part due to their potential for disease transmission and public health threats. Chickens also harbor infectious diseases that pose a risk to native bird populations and human public health on the Galápagos Islands. However, eradication of all chickens on the Galápagos Islands is an economic, political, and social impossibility. The growing human population and burgeoning tourist industry of the Galápagos depend on chickens as a source of protein and/or income.
Improved health management of Galápagos chickens can prevent the introduction of potentially devastating infectious diseases. For example, Newcastle disease, historically and serologically present in Galápagos chickens, may cause high poultry mortality and decline or extinction in endangered native birds, such as the flightless cormorant. A policy decision needs to be made whether to eradicate or control Newcastle disease on the Galápagos. Vaccination of poultry flocks is controversial, because animal vaccination is prohibited in the Galápagos. Vaccines can control, but not eradicate Newcastle disease, and could result in transfer of Newcastle disease to wild bird species if modified-live or live vaccines are used. From the perspective of wild bird conservation, Newcastle disease eradication would be the best management strategy. Test and slaughter programs in order to eradicate Newcastle disease in Galápagos poultry should be considered. However, eradication strategies are politically and economically difficult because funds are needed to compensate farmers for the deaths of birds that test positive. Furthermore, disease eradication strategies have the potential to cause the avicultural sector to mistrust local disease control programs, hindering further disease reporting, investigation, and control. Before eradication plans are implemented, the strains, pathogenicity, and possible origins of Newcastle disease present in Galápagos poultry should be identified, and poultry producers must be educated regarding disease control strategies. In order to better evaluate the pathogenicity of disease on endemic Galápagos avifauna, ex situ experimental infection of endemic birds (e.g., ground finches) with pathogens isolated from Galápagos chickens would be ideal, but small population sizes preclude disease transmission studies in endemics such as the mangrove finch, medium tree finch, flightless cormorant, and Galápagos penguin.
Currently, avian disease surveillance programs in the Galápagos monitor wild bird populations for poultry-borne and other infectious diseases. Results of these studies will guide avian disease management policies on the Galápagos Islands. Further studies are also needed regarding the effects of broiler operations on local ecosystems. Regardless, eradication, prevention, and control of disease and ecological effects of poultry production on the Galápagos Islands rely on well designed, scientifically based government policies and local community participation. These policies must be flexible, vigilant (e.g., unexpected introduction of avian influenza), transparent to the general public, and quick to respond to emergency situations. Viable economic alternatives to high-intensity broiler production on the Galápagos are important to develop due to the ecological and disease threats they present. A contingency plan that will enable rapid response for the successful mitigation of disease outbreaks of Galápagos native birds and poultry is necessary and urgent. Such a plan should identify national and international collaborators and laboratories for diagnosis of pathogens, budget costs, establish operational agreements, and maintain permits to facilitate the action plan when abnormal mortality of native birds or poultry is encountered.
Acknowledgments
Funding for this research was provided by the E. Desmond Lee Collaborative between the Saint Louis Zoo and the University of Missouri – St. Louis. We thank the Galápagos National Park, the Charles Darwin Research Station, and SESA/SICGAL Galápagos for their support of this project. Also, we thank chicken farmers on Santa Cruz and San Cristobal for their collaboration, and the University of Missouri Columbia Veterinary Diagnostic Laboratory, Athens Veterinary Diagnostic Laboratory of the University of Georgia, National Veterinary Services Laboratory, Ames, Iowa, Texas Veterinary Medical Diagnostic Laboratory, LIDIVET laboratory, Quito, Ecuador, and Astrid Rhon Laboratory, Quito, Ecuador, for diagnostic support. We also thank Dr. James Gibbs, Dr. Mary Duncan, Noah Whiteman, Monica Soria, and the rest of the laboratory group of Dr. P.G. Parker for helpful comments on this paper.
References
- Alexander D.J. Newcastle disease. In: Barnes H.J., Fatly A.M., Glasson J.R., McDougal J.R., Shayne D.E., editors. Diseases of Poultry. 11th ed. Iowa State University Press; Ames, IO: 2003. pp. 64–87. [Google Scholar]
- Alexander D.J. Newcastle disease: methods of spread. In: Alexander D.J., editor. Newcastle’s Disease. Kluwer Academic Publishers; Boston, MA: 1988. pp. 256–272. [Google Scholar]
- Andres A.S. Nitrate loss via ground water flow, Coastal Sussex County, Delaware. In: Steel K., editor. Animal Waste and the Land–Water Interface. Lewis Publishing Company; New York: 1995. pp. 69–76. [Google Scholar]
- Atkinson C.T., Woods K.L., Dusek R.J., Sileo L., Iko W.M. Wildlife disease and conservation in Hawaii: pathogenicity of avian malaria (Plasmodium relictum) in experimentally infected ’I’iwi (Vestiaria coccinea) Parasitology. 1995;111:S59–S69. doi: 10.1017/s003118200007582x. [DOI] [PubMed] [Google Scholar]
- Banerjee M., Reed W.M., Fitzgerald S.D., Panigrahy B. Neurotropic velogenic Newcastle disease in cormorants in Michigan: pathology and virus characterization. Avian Diseases. 1994;38:873–878. [PubMed] [Google Scholar]
- Barr D.A., Reece R.L., O’Rourke D., Button C., Faragher J.T. Isolation of infectious bronchitis virus from a flock of racing pigeons. Australian Veterinary Journal. 1988;65(7):228. doi: 10.1111/j.1751-0813.1988.tb14468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes H.J. Parasites. In: Harrison G.J., Harrison L.R., editors. Clinical Avian Medicine and Surgery. W.B. Saunders; Philadelphia: 1986. pp. 472–485. [Google Scholar]
- Calnek B.W. Avian encephalomyelitis. In: Barnes H.J., Fatly A.M., Glasson J.R., McDougal J.R., Shayne D.E., editors. Diseases of Poultry. 11th ed. Iowa State University Press; Ames, IO: 2003. pp. 271–282. [Google Scholar]
- Charlton B.R. Association of Avian Pathologists; Pennsylvania: 2000. Avian Disease Manual. [Google Scholar]
- Cho B.R., Kenzy S.G. Virologic and serologic studies of zoo birds for Marek’s disease virus infection. Infection and Immunity. 1975;11:809–814. doi: 10.1128/iai.11.4.809-814.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwin C. Harvard University Press; Cambridge, MA: 1859. On the Origin of Species. A Facsimile of the First Edition (1975) with an Introduction by Ernst Mayr. 1975. [Google Scholar]
- Davidson W.R., Nettles V.F., Couvillion C.E., Yoder H.W. Infectious sinusitis in wild turkeys. Avian Diseases. 1982;26:402–405. [PubMed] [Google Scholar]
- Dhondt A.A., Tessaglia D.L., Slothower R.L. Epidemic mycoplasmal conjunctivitis in house finches from eastern North America. Journal of Wildlife Diseases. 1998;34:265–280. doi: 10.7589/0090-3558-34.2.265. [DOI] [PubMed] [Google Scholar]
- Docherty, D.E., Friend, M., 1999. Newcastle Disease. In: Friend, M., Franson, J.C. (Eds.), Field Manual of Wildlife Diseases: General Field Procedures and Diseases of Birds. USGS, Biological Researches Division Information and Technology Report 1999-001, pp. 175–179.
- Dohm D.J., Sardelis M.R., Turell M.J. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae) Journal of Medical Entomology. 2002;39:640–644. doi: 10.1603/0022-2585-39.4.640. [DOI] [PubMed] [Google Scholar]
- Dubey J.P. A review of toxoplasmosis in wild birds. Veterinary Parasitology. 2002;106(2):121–153. doi: 10.1016/s0304-4017(02)00034-1. [DOI] [PubMed] [Google Scholar]
- Enzo P., Yannick G. vvIBDV Ahora en Venezuela: país tras país, el virus hipervirulento de Gumboro está invadiendo el continente Americano. Avicultura Profesional. 2003;21(1/2):24–27. [Google Scholar]
- Fonseca D.M., LaPointe D.A., Fleischer R.C. Bottlenecks and multiple introductions: population genetics of the vector of avian malaria in Hawaii. Molecular Ecology. 2000;9:1803–1814. doi: 10.1046/j.1365-294x.2000.01070.x. [DOI] [PubMed] [Google Scholar]
- Forrester D.J., Spalding M.G. University of Florida Press; Gainesville, FL: 2003. Parasites and Diseases of Wild Birds in Florida. [Google Scholar]
- Friend, M., 1999. Mycoplasmosis. In: Friend, M., Franson, J.C. (Eds.), Field Manual of Wildlife Diseases; General Field Procedures and Diseases of Birds. USGS, Biological Researches Division Information and Technology Report 1999-001, pp. 115–119.
- Gardner H., Kerry K., Riddle M., Brouer S., Gleeson L. Poultry virus infection in Antarctic penguins. Nature. 1997;387:245. doi: 10.1038/387245a0. [DOI] [PubMed] [Google Scholar]
- Gaydos J.K., Balcomb K.C., III, Osborne R.W., Dierauf L. Evaluating potential infectious disease threats for southern resident killer whales, Orcinus orca: a model for endangered species. Biological Conservation. 2004;117:253–262. [Google Scholar]
- Gerlach H. Viruses. In: Ritchie B.W., Harrison G.J., Harrison L.R., editors. Avian Medicine: Principles and Application. Wingers Publishing Inc.; Lake Worth, FL: 1999. pp. 862–948. [Google Scholar]
- Gonzalez E.T., Schudel A.A., Etcheverrigaray M.E., Zabala Suarez J.E. Avian adenoviruses – distribution of infection in commercial flocks of fowls in Argentina. Avian Disease. 1978;22(4):787–789. [PubMed] [Google Scholar]
- Gordillo R.M., Cabrera M.L. Mineralizable nitrogen in broiler litter: I. Effect of selected litter chemical characteristics. Journal of Environmental Quality. 1997;26:1672–1679. [Google Scholar]
- Grant B.R., Grant P.R. What Darwin’s finches can teach us about the evolutionary origin and regulation of biodiversity. BioScience. 2003;53:965–975. [Google Scholar]
- Gutierrez-Ruiz E.J., Ramirez-Cruz G.T., Camara Gamboa E.I., Alexander D.J., Gough R.E. A serological survey for avian infectious bronchitis virus and Newcastle disease virus antibodies in backyard (free-range) village chickens in Mexico. Tropical Animal Health and Production. 2000;32(6):381–390. doi: 10.1023/A:1005281619260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell W.H. Lesions of Marek’s disease in a great-horned owl. Avian Diseases. 1971;15:49–55. [PubMed] [Google Scholar]
- Hartup B.K., Dhondt A.A., Sydenstricker K.V., Hochachka W.M., Kollias G.V. Host range and dynamics of mycoplasmal conjunctivitis among birds in North America. Journal of Wildlife Diseases. 2001;37:72–81. doi: 10.7589/0090-3558-37.1.72. [DOI] [PubMed] [Google Scholar]
- Hatta M., Kawaoka Y. The continued pandemic threat posed by avian influenza viruses in Hong Kong. Trends in Microbiology. 2002;10:340–344. doi: 10.1016/s0966-842x(02)02388-0. [DOI] [PubMed] [Google Scholar]
- Hollmen T., Franson J.C., Docherty D., Kilpi M., Hario M., Creekmore L., Petersen M. Infectious bursal disease virus antibodies in eider ducks and herring gulls. Condor. 2000;102:688–691. [Google Scholar]
- Jessup D.A., DaMassa A.G, Lewis R., Jones F.R. Mycoplasma gallisepticum infection in wild-type turkeys living in close contact with domestic fowl. Journal of the American Veterinary Medical Association. 1983;183:1245–1247. [PubMed] [Google Scholar]
- Karesh W.B., Uhart M.M., Frere E., Gandini P., Braselton W.E., Puche H., Cook R.A. Health evaluation of free-ranging rockhopper penguins (Eudyptes chrysocomes) in Argentina. Journal of Zoo and Wildlife Medicine. 1999;30:25–31. [PubMed] [Google Scholar]
- Klemen S.H. Mycoplasmosis. In: Barnes H.J., Fatly A.M., Glasson J.R., McDougal J.R., Shayne D.E., editors. Diseases of Poultry. 11th ed. Iowa State University Press; Ames, IO: 2003. pp. 86–87. [Google Scholar]
- Langevin S.A, Bunning M., Davis B., Komar N. Experimental infection of chickens as candidate sentinels for West Nile virus. Emerging Infectious Disease. 2001;7:726–729. doi: 10.3201/eid0704.010422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukert P.D., Saif Y.M. Infectious bursal disease. In: Barnes H.J., Fatly A.M., Glasson J.R., McDougal J.R., Shayne D.E., editors. Diseases of Poultry. 11th ed. Iowa State University Press; Ames, IO: 2003. pp. 719–774. [Google Scholar]
- Lumeij J.T. Gastroenterology. In: Ritchie B.W, Harrison G.J., Harrison L.R., editors. Avian Medicine: Principles and Application. Wingers Publishing, Inc.; Lake Worth, FL: 1999. pp. 482–521. [Google Scholar]
- Luttrell M.P., Stallknecht D.E., Kleven S.H., Kavanaugh D.M., Corn J.L., Fischer J.R. Mycoplasma gallisepticum in house finches (Carpodacus mexicanus) and other wild birds associated with poultry production facilities. Avian Diseases. 2001;45:321–329. [PubMed] [Google Scholar]
- MacFarland C., Cifuentes M. Case study: Galápagos, Ecuador. In: Dompka V., editor. American Association for the Advancement of Science; Washington, DC: 1996. pp. 135–188. (Human Population, Biodiversity and Protected Areas: Science and Policy Issues. Report of a Workshop, April 20–21, 1995). [Google Scholar]
- McDougald L.R. Internal Parasites. In: Barnes H.J., Fatly A.M., Glasson J.R., McDougal J.R., Shayne D.E., editors. Diseases of Poultry. 11th ed. Iowa State University Press; Ames, IO: 2003. pp. 931–971. [Google Scholar]
- Miller G.D., Hofkin B.V., Snell H., Hahn A., Miller R.D. Avian malaria and Marek’s Disease: potential threats to Galápagos Penguins (Spheniscus mendiculus) Marine Ornithology. 2001;29(1):43–46. [Google Scholar]
- Mouchet J., Giacomini T., Julvez J. Human diffusion of arthropod vectors throughout the world. Santé. 1995;5:293–298. [PubMed] [Google Scholar]
- Padilla L.R., Huyvaert K.P., Merkel J., Miller R.E., Parker P.G. Hematology, plasma chemistry, serology and Chlamydophila status of the waved albatross (Phoebastria irrorata) on the Galápagos Islands. Journal of Zoo and Wildlife Medicine. 2003;34(3):278–283. doi: 10.1638/02-076. [DOI] [PubMed] [Google Scholar]
- Peck S.B., Heraty J., Landry B., Sinclair B.J. Introduced insect fauna of an oceanic archipelago: The Galápagos Islands, Ecuador. American Entomologist. 1998;44:218–237. [Google Scholar]
- Perkins L.E, Swayne D.E. Varied pathogenicity of a Hong Kong-origin H5N1 avian influenza virus in four passerine species and budgerigars. Veterinary Pathology. 2003;40:14–24. doi: 10.1354/vp.40-1-14. [DOI] [PubMed] [Google Scholar]
- Permin A., Hansen J.W. Food and Agriculture Organization of the United Nations; Rome: 1998. The Epidemiology, Diagnosis, and Control of Poultry Parasites. [Google Scholar]
- Peterson M.J., Ferro P.J., Peterson M.N., Sullivan R.M., Toole B.E., Silvy N.J. Infectious disease survey of lesser prairie chickens in north Texas. Journal of Wildlife Diseases. 2002;38:834–839. doi: 10.7589/0090-3558-38.4.834. [DOI] [PubMed] [Google Scholar]
- Phillips R.B., Snell H.L., Vargas H. Feral rock doves in the Galápagos Islands: Biological and economic threats. Noticias de Galápagos. 2003;62:6–11. [Google Scholar]
- Pillai S.R., Mays H.L., Jr., Ley D.H., Luttrell P., Panangala V.S., Farmer K.L., Roberts S.R. Molecular variability of house finch Mycoplasma gallisepticum isolates as revealed by sequencing and restriction fragment length polymorphism analysis of the pvpA gene. Avian Diseases. 2003;47:640–648. doi: 10.1637/6095. [DOI] [PubMed] [Google Scholar]
- Pradhan H.K., Mohanty G.C., Mukit A. Marek’s disease in Japanese quails (Coturnix coturnix japonica): a study of natural cases. Avian Diseases. 1985;29:575–582. [PubMed] [Google Scholar]
- Remes V. Effects of exotic habitat on nesting success, territory density, and settlement pattern in the blackcap (Silvia atricapilla) Conservation Biology. 2003;17(4):1127–1133. [Google Scholar]
- Ricaurte B. La Avicultura en la provincia de Galápagos y sus implicaciones ecologicas. Avicultura Ecuatoriana. 1994;39:13–14. [Google Scholar]
- Rickard L.G. Proventricular lesions associated with natural and experimental infections of Dispharynx nasuta. Canadian Journal of Zoology. 1985;63:2663–2668. [Google Scholar]
- Sivanandan V., Maheswaran S.K. Immune profile of infectious bursal disease (IBD). Effect of IBD on pokeweed-mitogen-stimulated peripheral blood lymphocytes of chickens. Avian Diseases. 1980;24:734–742. [PubMed] [Google Scholar]
- Snell H.L., Tye A., Causton C.E., Bensted-Smith R. A Biodiversity vision for the Galápagos Islands. Charles Darwin Foundation and World Wildlife Fund; Puerto Ayora, Galápagos: 2002. Current status of and threats to the terrestrial biodiversity of Galápagos; pp. 30–47. [Google Scholar]
- Suarez D.L. Evolution of avian influenza viruses. Veterinary Microbiology. 2002;74:15–27. doi: 10.1016/s0378-1135(00)00161-9. [DOI] [PubMed] [Google Scholar]
- Tenter A.M., Heckeroth A.R., Weiss L.M. Toxoplasma gondii: from animals to humans. International Journal for Parasitology. 2000;30:1217–1258. doi: 10.1016/s0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel, T., Whiteman, N.K., Tirape, A., Maquero, M.I., Cedeno, V., Walsh, T., Jimenez, G., Parker, P.G., 2005. Characterization of Canarypox-like Viruses Infecting Endemic Birds in the Galápagos Islands. Journal of Wildlife Disease (in press). [DOI] [PubMed]
- Tilman D. Secondary succession and the pattern of plant dominance along experimental nitrogen gradients. Ecological Monographs. 1987;57:189–214. [Google Scholar]
- Tomaszewski E.K., Logan K.S., Snowden K.F., Kurtzman C.P., Phalen D.N. Phylogenetic analysis identifies the ‘megabacterium’ of birds as a novel anamorphic ascomycetous yeast, Macrorhabdus ornithogaster gen. Nov., sp. Nov. International Journal of Systematic and Evolutionary Microbiology. 2003;53:1201–1205. doi: 10.1099/ijs.0.02514-0. [DOI] [PubMed] [Google Scholar]
- Uyttebroek E., Ducatelle R. Megabacterium proventriculitis, a disease of cage birds. Vlaams Diegeneeskundig Tijdschrift. 1990;59:147–150. [Google Scholar]
- Van Riper C., III, Van Riper S.G., Goff M.L., Laird M. The epizootiology and ecological significance of malaria in Hawaiian land birds. Ecological Monographs. 1986;56:327–344. [Google Scholar]
- Van Steenis G. Survey of various avian species for neutralizing antibody and susceptibility to avian encephalomyelitis virus. Research in Veterinary Science. 1971;12:308–311. [PubMed] [Google Scholar]
- Vargas H., Bensted-Smith R. Past and present ornithology in Galápagos. In: Sitwell N., Baert L., Cuppois G., editors. Royal Belgian Institute of Natural Sciences; Brussels, Belgium: 2000. pp. 47–52. (Proceedings of the Symposium Science and Conservation in Galápagos). [Google Scholar]
- Vargas H., Snell H.L. The arrival of Marek’s disease to Galápagos. Noticias de Galápagos. 1997;58:4–5. [Google Scholar]
- Whiteman, N.K., Goodman, S.J., Sinclair, B.J., Walsh, T., Cunningham, A.A., Kramer, L.D., Parker, P.G., 2005. Establishment of the avian disease vector Culex quinquefasciatus Say 1823 (Diptera: Culicidae) on the Galápagos Islands, Ecuador. Ibis (in press).
- Wikelski M., Foufopoulos J., Vargas H., Snell H. Galápagos birds and diseases: invasive pathogens as threats for island species. Ecology and Society. 2004;9(5) Available from: < http://www.ecologyandsociety.org/vol9/iss1/art5>. [Google Scholar]
- Wilson S.D., Belcher J.W. Plant and bird communities of native prairie and introduced Eurasian vegetation in Manitoba, Canada. Conservation Biology. 1989;3:39–44. [Google Scholar]
- Wobeser G., Leighton F.A., Norman R., Myers D.J., Onderka D., Pybus M., Neufeld J.L., Fox G.A., Alexander D.G. Newcastle disease in wild water birds in western Canada, 1990. Canadian Veterinary Journal. 1993;34:353. [PMC free article] [PubMed] [Google Scholar]
- Wobeser G. Plenum Press; New York: 1997. Diseases of Wild Waterfowl. [Google Scholar]
- Work T.M., Massey J.G., Rideout B.A., Gardiner C.H., Ledig D., Dubey J.P. Toxoplasmosis in free-ranging Hawaiian crows (Corvus hawaiiensis) Journal of Wildlife Diseases. 2000;36:205–212. doi: 10.7589/0090-3558-36.2.205. [DOI] [PubMed] [Google Scholar]
- Wunderwald C., Hoop R.K. Serological monitoring of 40 Swiss fancy breed poultry flocks. Avian Pathology. 2002;31:157–162. doi: 10.1080/03079450120118649. [DOI] [PubMed] [Google Scholar]


