Abstract
The Zika virus (ZIKV) is a mosquito-borne flavivirus that has reemerged as a serious public health problem around the world. Syndromes of infected people range from asymptomatic infections to severe neurological disorders, such as Guillain-Barré syndrome and microcephaly. Screening anti-ZIKV drugs derived from Chinese medicinal herbs is one method of identifying antiviral agents. In this paper, we report that (1) Cephalotaxine (CET), an alkaloid isolated from Cephalotaxus drupacea, was effective in inhibiting ZIKV activity in vitro (i.e., in Vero and A549 cell lines) and (2) the mechanisms which underlie these effects involve virucidal activity and a decrease in viral replication. Specifically, CET was found to decrease ZIKV RNA and viral protein expression, inhibit ZIKV replication, and inhibit ZIKV mRNA/protein production. We also determined that CET is effective in inhibiting dengue virus 1–4 (DENV1-4). Taken together, our findings indicate that CET could be an effective lead compound in the treatment of ZIKV and also suggest that further investigation and development of CET-derived drugs may lead to a new class of anti-Flavivirus medications.
Keywords: Antiviral, Cephalotaxine, Viral production, Replication, Dengue virus, Zika virus
Highlights
-
•
CET against ZIKV infection via inhibiting replication and stability.
-
•
CET was identified as a potent inhibitor of ZIKV infection.
-
•
CET as a candidate compound for potential ZIKV treatment.
1. Introduction
The Zika virus (ZIKV) is a mosquito-borne, enveloped RNA virus that belongs to the Flavivirus genus and the Flaviviridae family (a family to which the Hepatitis C virus (HCV], Dengue virus (DENV], West Nile virus (WNV], Yellow fever virus [YFV], and Japanese encephalitis virus [JEV] also belong.) ZIKV was first isolated from rhesus monkeys near the Zika Forest in Uganda in 1947 [1,2]. The viral genome of ZIKV encodes a polyprotein which consists of a capsid, a premembrane/membrane, an envelope, and seven nonstructural proteins: NS1, NS2A, NS2B, NS3, NS4A, NS4 B, and NS5 [3]. In recent years, ZIKV infection has become a serious and escalating threat to global health. For example, ZIKV spread rapidly in 2015 in at least 33 regions and countries in Central and South America, such as Brazil, becoming an epidemic that affected between 0.5 and 1.5 million people [[4], [5], [6], [7]]. ZIKV infection has been associated with several neurological complications, such as Guillain-Barré syndrome in adults and microcephaly in infants [8,9].
Prior to the outbreak which affected Brazil and other Central and South American countries, ZIKV infection was only considered to lead only to a mild disease. However, as the 2015 outbreak identified, when pregnant women are infected with ZIKV, their babies can be born with serious birth defects, such as fetal growth restrictions as well as neurological and ocular abnormalities. In some cases, ZIKV during pregnancy can even led to perinatal death [10]. ZIKV is also known to cause a benign febrile illness in approximately 18% of infected individuals. This illness leads to symptoms which are similar to those of other arbovirus infections, including DENV and the chikungunya virus (CHIKV). Specifically, these symptoms include fever, rash, joint pain, conjunctivitis, and less commonly, headaches, vomiting and jaundice [11]. Other clinical symptoms typical of ZIKV infection include fever, headaches, joint pain, conjunctivitis, and macular atrophy [12].
In February 2016, the World Health Organization (WHO) declared ZIKV to be a Public Health Emergency of International Concern (PHEIC) [13]. Strategies of fighting ZIKV infection include the development of vaccines and the screening of antiviral agents that inhibit different stages of the viral life cycle [14]. Unfortunately, no approved antiviral ZIKV agents are currently available. There is thus an urgent need to develop safe and effective antiviral agents against ZIKV and to elucidate their mechanisms. Doing so should help identify lead compounds which have the potential for further clinical development in the fight against ZIKV.
Cephalotaxine (CET), harringtonine (HT), homoharringtonine (HHT), isoharringtonine, and deoxyharringtoninea are alkaloids which can be isolated and purified from the Chinese coniferous tree Cephalotaxus hainanensis [15]. CET has shown promising antiviral activities against hepatitis B [16] and has also been found to have antileukemic activities [17]. Due to their wide range of effects, CET drugs are also believed to have great potential in the treatment of other diseases, including some cancers [18]. HT inhibits CHIKV replication by down-regulating viral protein expression, while HHT shows activity against HBV and the coronavirus. Previous research further determined that both HT and HHT are promising candidates for the treatment of diseases related to the varicella-zoster virus (VZV) [16,[19], [20], [21]]. Although such drugs have similar structures, their substituents vary widely, which may translate into differences in pharmacological activity [21]. Therefore, in the current study, we opted to investigate the potential anti-ZIKV activity of CET in Vero cells. Our results indicate that CET indeed possesses anti-ZIKV activity. Thus, we also opted to investigate the potential mechanisms which underlie the anti-ZIKV activity of CET. In so doing, we demonstrated that CET (1) disrupts the viral life cycle by preventing ZIKV from replicating and (2) exhibits virucidal activity against ZIKV. These findings suggest that CET has the potential to be developed as a therapeutic agent against ZIKV.
2. Materials and methods
2.1. Cell lines, viruses, and drugs
This study used Vero (African green monkey kidney cells; ATCC® CRL-1586TM) and A549 (Human lung carcinoma epithelial cells; ATCC® CCL-185™) cells, as these cells are more permissive to ZIKV (PRAVABC59, ATCC® VR-1843™) replication. Both Vero and A549 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 5% fetal bovine serum (FBS) at 37 °C and 5% CO2. Virus stock was determined using fluorescent focus unit assays and then stored at −80 °C until use. CET (purity ≥ 98%), purchased from a natural product manufacturer (ChemFaces, Wuhan Economic and Technological Development Zone, China), was dissolved in 100% dimethyl sulfoxide (DMSO) as a 20 mM stock solution and stored at −20 °C until use. At the time of use, the stock solution was diluted in DMEM until the desired working concentration was reached. Moreover, the volume of DMSO in each working concentration was adjusted so that the concentration of DMSO was the same for every solution, in order to avoid solvent interference among groups.
2.2. Cell viability assay
Cell viability profiles of CET-treated cells were assessed using the Cell Counting Kit 8 (DOJINDO Laboratories, Kumamoto, Japan) in accordance with the manufacturer’s protocol. Briefly, a monolayer of Vero cells was plated in 96-well microplates, and variable concentrations of CET were added to triplicate wells. Vero cells were then incubated at 37 °C for 48 h. At the end of the incubation period, the culture medium was discarded, and 100 μl of fresh medium containing 10 μl of CCK-8 solution was added to each well for 1 h. The optical densities of treated cells were then measured at 450 nm using a Bio-Tek Synergy Multi-Mode microplate reader, and values for treated cells were normalized with those of untreated cells [22].
2.3. Fluorescent focus units (FFU) assay
Confluent monolayers of Vero cells were seeded in 12-well microplates and infected with a serial dilution of virus medium. Following a 2 h infection period, the cells were overlaid with DMEM containing 1.5% methylcellulose and 2% FBS, and then incubated for two days. Viral fluorescence foci were visualized and counted using an inverted fluorescence microscope (Olympus CKX41).
2.4. Virus reduction assay
Confluent monolayers of Vero cells were plated in 12-well microplates and incubated for 16 h. Subsequently, 500 μl of DMEM containing 2% FBS, various concentrations of CET, and 400 foci forming units (FFUs) of ZIKV were added to the cells. Following incubation for another 2 h, cells were washed twice with phosphate buffered saline (PBS) and then further treated with CET for an additional 48 h. After another two-day incubation period, supernatants were harvested and the viral titers contained therein were determined using IFA. Relative quantifications of ZIKV RNA in Vero cells were determined using quantitative reverse transcription PCR (qRT-PCR).
2.5. qRT-PCR
The total RNA of cells was extracted 24 or 48 h after CET treatment using Trizol reagent (Ambion). Gene expression was then quantified using the One-Step qRT-PCR SYBR green kit (Bioman, cat no. QRP001) according to the manufacturer’s instructions. The primer sequences used for ZIKV, DENV, and β-actin are shown in Supplementary Table 1. Specifically, qRT-PCR was performed in 96-well plates and absorbance was measured using the Roche Lightcycler 480 (Roche Applied Science, Indianapolis, IN). Measurements were obtained in triplicate, and β-actin was used as an internal control. The relative quantification of gene expression was determined using the 2-△△CT method [23].
2.6. Immunofluorescence assay (IFA)
Infected cells were fixed using 4% paraformaldehyde for 1 h. After being washed three times with PBS, cells were stained with anti-flavivirus envelope antibodies (4G2) at room temperature for 2 h. After being washed once with PBS, cells were stained with Alexa Fluor 488-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc). After a final PBS wash, cells were observed using an inverted fluorescence microscope (Olympus CKX41) [23,24].
2.7. Time of addition assay
To determine which stage of the viral life cycle that CET acts upon, we compared its effects with full-duration treatment, co-treatment, pre-treatment, and post-treatment groups via a time of addition assay. For this, after confluent Vero cells were plated in 12-well microplates, indicated concentrations of CET were added to the medium at various time points as follows. (1) For the full-duration treatment group, CET was added throughout the infection period. (2) For the co-treatment group, CET was added at the initial point of ZIKV infection. (3) For the post-treatment group, CET was added after ZIKV infection. (4) For the pre-treatment group, CET was added 3 h before ZIKV infection. Following ZIKV infection, Vero cells in all treatment groups were incubated at 37 °C for 24 h, at which point the levels of intracellular viral RNA were compared with those of a vehicle group using qRT-PCR [22,23].
2.8. Western blot analysis
Intracellular viral envelope protein levels were measured by Western blot. Briefly, infected cell lysates treated with different concentrations of CET were harvested after 48 h of infection. Immunoblotting was performed using anti-flavivirus envelope antibodies 4G2 ascites (1:1000, produced in-house) and anti-β-actin (1:4000; Santa Cruz Biotechnology, Dallas, Texas, USA) as primary antibodies as well as anti-mouse or anti-human horseradish peroxidase-conjugated antibodies as secondary antibodies. Target proteins were detected using an ECL reagent, and images were obtained using a UVP chemiluminescence machine.
2.9. Virucidal assay
The cell-free anti-ZIKV activity of CET was investigated by incubating ZIKV suspensions containing 5 × 104 FFU with indicated concentrations of CET at 37 °C for 4 h. The treated supernatants were then diluted in DMEM and added to the confluent Vero cells. Viral titers were determined by counting viral fluorescence foci using the previously described IFA method [23].
2.10. Antiviral activity against other viruses
The antiviral effects of CET were further evaluated against DENV1 (Hawaii strain), DENV2 (16,681 strain), DENV3 (H87 strain), and DENV4 (H241 strain). Briefly, confluent Vero cells seeded in 12-well microplates were infected with FFUs of DENV1, DENV2, DENV3, or DENV4 and then incubated with indicated concentrations of CET for 48 h. Following this, dose-dependent viral RNA reduction assays were performed using qRT-PCR to determine whether CET was effective in inhibiting viral replication [23].
2.11. Statistical analysis
Statistical analysis was performed using GraphPad Prism software, and data were expressed as the mean ± standard deviation of triplicate experiments, The statistical significance of data was assessed using a two-tailed Student’s t-test, in which a p value of <0.05 was considered significant [[22], [23], [24]].
3. Results
3.1. CET inhibited ZIKV infection
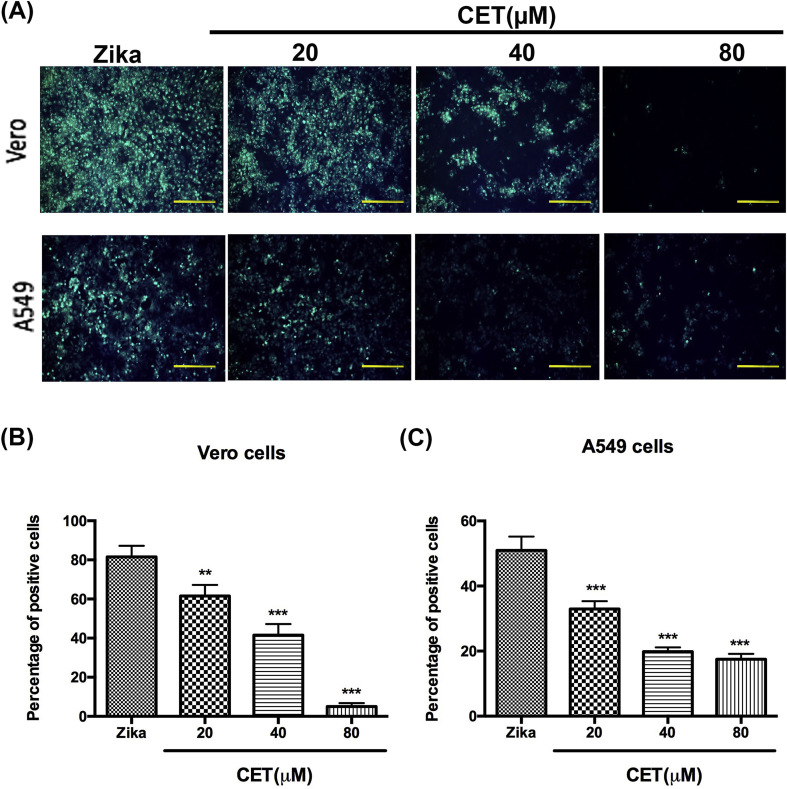
Earlier research found that CET was able to inhibit HBV infection [16]; however, the anti-viral effects of CET against other viruses have not been previously investigated. In this study, we first used Vero and A549 cells to investigate the anti-ZIKV ability of CET. For this, Vero cells and A549 cells were seeded in 12-well plates overnight and were then infected with ZIKV at 1500 FFU per well. After 48 h of incubation with ZIKV and indicated concentrations of CET, cells were fixed and stained by 4G2 antibodies, which react to all flaviviruses. Following quantification of our results, we demonstrated that CET from 20 to 100 μM significantly inhibited ZIKV infection in a dose-dependent manner (Fig. 1 A, B, and 1C).
Fig. 1.
CET inhibited ZIKV infection. The inhibition of CET in ZIKV-infected Vero cells was determined by IFA (A). Quantification of Vero cells (B) and A549 cells (C). The data shown here represent the mean and SD of triplicate experiments. Statistical significance was determined via a t-test compared with the ZIKV group: * indicates p < 0.05; ** indicates p < 0.01; and *** indicates p < 0.001.
3.2. CET inhibited ZIKV production
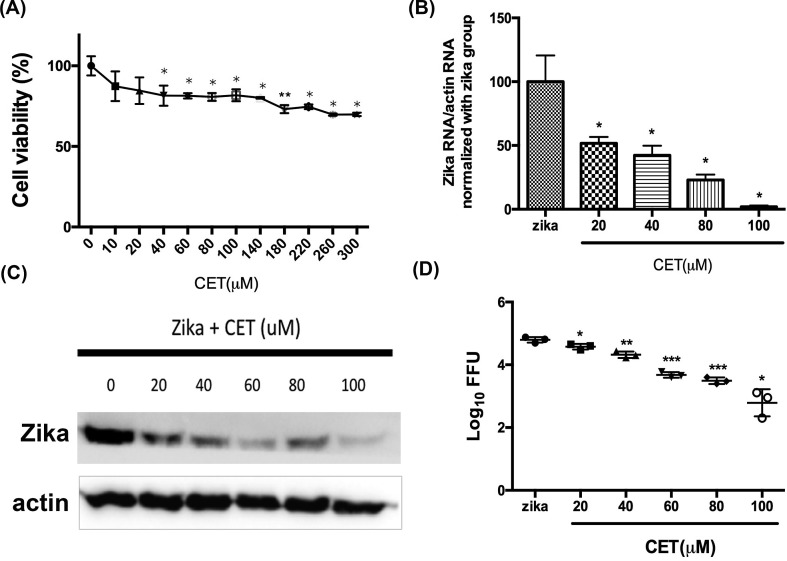
The viability of CET-treated Vero cells was determined using a CCK-8 assay, and a comparison of the results with a control group revealed that CET possesses mild cytotoxicity. The CC50 of CET though was found to be more than 300 μM (Fig. 2 A); however, as CET is an FDA-approved drug, we were not concerned with a lack of safety.
Fig. 2.
Dose-dependent anti-ZIKV production of CET. (A) The viability of CET-treated Vero cells was determined by CCK-8 assays. (B–D) The anti-ZIKV ability of CET was verified by measuring viral RNA levels, protein expression, and viral titers. (B) ZIKV RNA levels were analyzed by qRT-PCR. (C) ZIKV protein expression was detected by Western blotting. (D) Virus titers were assessed by FFU. The data shown here represent the mean and SD of triplicate experiments. Statistical significance was determined via a t-test compared with the ZIKV group: * indicates p < 0.05; ** indicates p < 0.01; and *** indicates p < 0.001.
To verify the anti-ZIKV ability and inhibition level of CET, qRT-PCR, Western blotting, and FFU assays were performed. For this, Vero cells were seeded in 12-well plates overnight, infected with 400 FFU ZIKV per well, and incubated with indicated concentrations of CET for 48 h before qRT-PCR, Western blotting, and FFU assays were employed. Results of qRT-PCR showed that CET inhibited ZIKV infection in a dose-dependent manner (Fig. 2B). In particular, a concentration of 100 μM CET was found to suppress approximately 98% of ZIKV RNA production. Furthermore, Vero cells infected with 1000 FFU ZIKV per well under indicated dosages of CET for 48 h incubation.
Results of Western blotting analysis revealed that ZIKV protein expression was significantly reduced under CET treatment (Fig. 2C). Results of FFU assays revealed that CET is able to decrease ZIKV progeny yield in a dose-dependent manner (Fig. 2D). Taken together, the above evidence indicates that CET is effective in suppressing ZIKV propagation.
3.3. CET may affect ZIKV RNA replication and stability
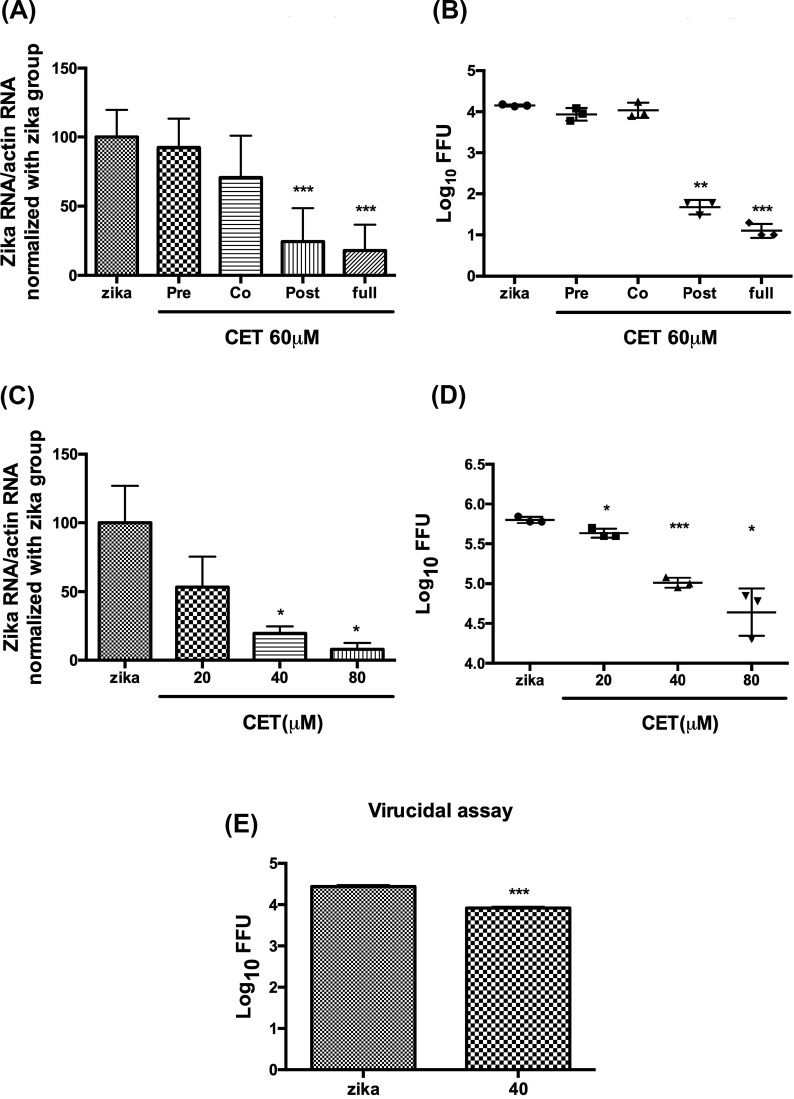
Our data indicated that CET is able to inhibit ZIKV infection by decreasing viral RNA, protein, and progeny yield. Therefore, to further investigate the mechanism which underlies the ability of CET to inhibit ZIKV, we performed a time of addition assay. For this, 60 μM CET was added to Vero cells in pre-treatment, co-treatment, post-treatment, and full-duration treatment groups. (Cells in all groups were infected with 8000 FFU ZIKV per well for 1 h) Following the end of the infection period, cells were washed two times and the medium was replaced. After 24 h incubation, cells were lysed for RNA analysis, and the supernatant was collected for FFU assays. Results of this analysis revealed that CET likely affects the late stage of ZIKV infection (Fig. 3 A and B), which means that the inhibitory effects of CET likely occur after virus entry. To investigate CET inhibition after ZIKV entry, Vero cells were infected with ZIKV, washed with PBS to remove viruses that had not infected the cells, and then treated with indicated concentrations of CET. Cell lysates and supernatants were collected after 24 h incubation for further analysis. Results showed that administering CET after the initial infection (i.e., the post-treatment group) suppressed viral RNA replication. In particular, when cells in the post-treatment group were administered 40 or 80 μM CET, ZIKV RNA levels were significantly reduced (Fig. 3C). FFU assays further revealed that the viral yield decreased in the post-treatment group when cells were administered 20, 40, or 80 μM CET (Fig. 3D). In addition, virucidal assays showed that CET affected virus stability. FFU assays, in which cells were incubated with supernatant containing 50,000 FFU ZIKV with or without 40 μM CET at room temperature for 6 h prior to detection, revealed that CET decreased the virus level after a 6 h incubation (Fig. 3E), further confirming that CET likely possesses the ability to influence virus stability.
Fig. 3.
Time of addition assay to investigate the effects of treating ZIKV-infected Vero cells with 60 μM CET. (A) RNA levels and (B) virus progeny production. The effects of administering CET after ZIKV infection (i.e., the post-treatment group) as revealed by (C) qRT-PCR and (D) FFU assay. (E) Results of a virucidal assay which assessed the effects of treatment with 40 μM CET. The data shown here represent the mean and SD of triplicate experiments. Statistical significance was determined via a t-test compared with the ZIKV group: * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
3.4. CET inhibited different subtypes of dengue virus
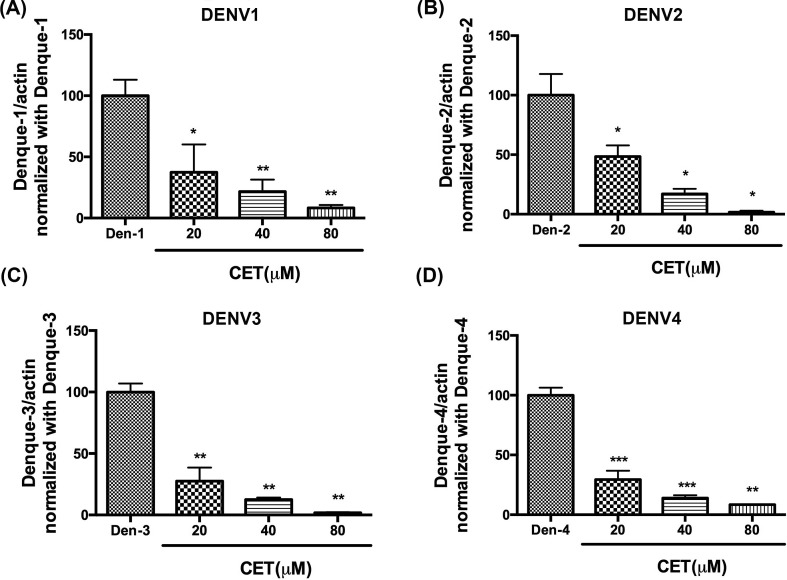
We further assessed the ability of CET to inhibit the dengue virus. For this, Vero cells were seeded in 12-well plates overnight and then inoculated with indicated concentrations of CET and 30,000 FFU DENV1 per well, 4000 FFU DENV2 per well, 10,000 FFU DENV3 per well, or 20,000 FFU DENV4 per well. After 48 h incubation, the RNA of treated cells was extracted and analyzed by qRT-PCR. Our results revealed that 20 μM–80 μM CET inhibited these four serotypes of dengue viruses in a dose-dependent manner (Fig. 4 ). Therefore, CET also has the potential to be used in the treatment of dengue virus infection.
Fig. 4.
Inhibition of CET activity in different serotypes of dengue virus. (A–D) Effects of CET treatment on DENV1-4. The anti-dengue ability of CET was determined using qRT-PCR. The data shown here represent the mean and SD. Statistical significance was determined via a t-test compared with each dengue virus group: * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001.
4. Discussion
The outbreak of ZIKV in Brazil in 2015 was one of most uncontrollable epidemics that the world has experienced. Since then, ZIKV has become a flavivirus of great medical concern. Nonetheless, to date, despite significant efforts to develop effective vaccines, no effective treatment for this emerging pathogen has been found. Therefore, identifying broad-spectrum antiviral drugs and effective clinical compounds is critical for the treatment of ZIKV patients and for the control of ZIKV outbreaks [25,26].
CET and its derived ester compounds (such as HT and HHT) have all been found to possess activity against leukemic cells [16,18]. The anti-tumor ability of these compounds is believed to arise from their inhibitory effects on protein synthesis [27]. Previous research also determined that CET and its ester derivatives have inhibitory effects against HBV [16], VZV and CHIKV [19,21]. However, this is the first study to demonstrate that CET inhibits ZIKV infection. Specifically, results of IFA revealed that CET treatment reduced viral infection in both Vero and A549 cells (Fig. 1).
Researchers who previously assessed the viability of HEPG2 cells and ETBr cells treated with CET found that CET is not cytotoxic and whereas they induced dose-dependent anti-HBV and anti-bovine viral diarrhoea virus (BVDV) as a surrogate for HCV effects, respectively [16]. Our results also demonstrated that CET possesses low cytotoxicity and does not affect cell viability (Fig. 2A). Moreover, viral RNA levels, protein expression levels, and viral titers (Fig. 2B, C, and 2D) in CET-treated Vero cells revealed that 20–80 μM CET led to >50–90% virus inhibition, consistent with previous findings.
Previous studies demonstrated that HT and HHT inhibit replication of CHIKV, VZV [19], and the Sindbis virus (SINV) [28]. HHT has further been shown to exert antiviral effects against coronavirus (CoV) [20] and HBV [16]. Both HT and HHT are able to inhibit the replication of both RNA and DNA viruses, which indicates that their antiviral activities are most likely due to cellular rather than viral factors and that cellular factors are essential for viral replication. CET esters have been shown to interfere with RNA translation by blocking the binding of aminoacyl-tRNA to acceptor sites on the large ribosomal subunit, thereby also blocking the subsequent formation of a peptide bond [21,27]. Moreover, HT has been found to limit the replication of CHIKV by inhibiting large ribosomal subunits and subsequently down-regulating the translation of viral proteins [19]. Similar to previous studies, results of our time of addition assay showed that CET affected the late stage of ZIKV infection (Fig. 3A and B).
We further sought to determine the possible mechanism by which CET influences ZIKV RNA replication. Previous research which investigated VZV determined that both HT and HHT likely employ other mechanisms in inhibiting ZIKV. For example, HT and HHT likely inhibit lytic gene expression and replication, which may in turn interfere with signal transduction pathways and transcription factors involved in VZV lytic gene expression. On the other hand, in herpesviruses (HHVs), HT and HHT are able to block cell cycle progression during the G1/S or G2/M transition, which in turn affects lytic gene expression and replication [29,30]. Thus, the mechanisms by which HT, HHT [21], and CET inhibit VZV replication may involve disrupting the cell cycle of infected cells.
In virology, RNA levels are indicative of virus replication (Fig. 3C), and FFU levels are indicative of virus release (Fig. 3D). In the current study, for the post-treatment group (i.e., the Vero cells that received CET after ZIKV-infection), the decreases we observed in viral replication and viral release were the same, which suggest that the primary mechanism by which CET inhibited ZIKV involved viral replication, not virus release. However, the time of addition assay showed that the viral yield of the full-duration treatment group was lower than that of the post-treatment group (by approximately 0.5 logs, Fig. 3B). This finding suggests that not only can CET disrupt viral replication, but it may also possess other anti-ZIKV activities. To determine this, we performed a virucidal assay of CET, the results of which confirmed that CET decreased viral stability, thereby reducing viral yield (Fig. 3E). In summary, our data indicate that CET is able to inhibit ZIKV infection by affecting both viral replication and viral stability.
Finally, we also studied the effects of CET on four different dengue virus serotypes and found that CET reduced DENV RNA levels in a Vero cell model, in which CET inhibited all four dengue virus serotypes in a dose-dependent manner (Fig. 4). Therefore, CET may also be useful in the treatment of dengue virus infections.
To conclude, our results indicate that CET is an effective antiviral agent against ZIKV. CET exerts anti-ZIKV activities through the inhibition of viral production and replication as well as through virucidal activity. The evidence reported here supports the further study of CET as a candidate compound for ZIKV treatment.
Declaration of Competing Interest
None.
Acknowledgements
The authors’ work was supported in part by grants from the Ministry of Science and Technology (MOST 107-2320-B-016-006- and MOST 108-2320-B-016 -011 -MY2), and the grants from Ministry of National Defense (MAB-108-061) at Taiwan.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrc.2019.12.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Dick G.W., Kitchen S.F., Haddow A.J. Zika virus. I. Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Espano E., Nam J.H., Song E.J., Song D., Lee C.K., Kim J.K. Lipophilic statins inhibit Zika virus production in Vero cells. Sci. Rep. 2019;9:11461. doi: 10.1038/s41598-019-47956-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuno G., Chang G.J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 2007;152:687–696. doi: 10.1007/s00705-006-0903-z. [DOI] [PubMed] [Google Scholar]
- 4.Petersen L.R., Jamieson D.J., Powers A.M., Honein M.A. Zika virus. N. Engl. J. Med. 2016;374:1552–1563. doi: 10.1056/NEJMra1602113. [DOI] [PubMed] [Google Scholar]
- 5.Hennessey M., Fischer M., Staples J.E. Zika virus spreads to new areas - region of the americas, may 2015-january 2016. MMWR Morb. Mortal. Wkly. Rep. 2016;65:55–58. doi: 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira M.G., Costa Mda C., de Oliveira W.K., Nunes M.L., Rodrigues L.C. The epidemic of Zika virus-related microcephaly in Brazil: detection, control, etiology, and future scenarios. Am. J. Public Health. 2016;106:601–605. doi: 10.2105/AJPH.2016.303113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faria N.R., Azevedo R., Kraemer M.U.G., Souza R., Cunha M.S., Hill S.C., Theze J., Bonsall M.B., Bowden T.A., Rissanen I., Rocco I.M., Nogueira J.S., Maeda A.Y., Vasami F., Macedo F.L.L., Suzuki A., Rodrigues S.G., Cruz A.C.R., Nunes B.T., Medeiros D.B.A., Rodrigues D.S.G., Queiroz A.L.N., da Silva E.V.P., Henriques D.F., da Rosa E.S.T., de Oliveira C.S., Martins L.C., Vasconcelos H.B., Casseb L.M.N., Simith D.B., Messina J.P., Abade L., Lourenco J., Alcantara L.C.J., de Lima M.M., Giovanetti M., Hay S.I., de Oliveira R.S., Lemos P.D.S., de Oliveira L.F., de Lima C.P.S., da Silva S.P., de Vasconcelos J.M., Franco L., Cardoso J.F., Vianez-Junior J., Mir D., Bello G., Delatorre E., Khan K., Creatore M., Coelho G.E., de Oliveira W.K., Tesh R., Pybus O.G., Nunes M.R.T., Vasconcelos P.F.C. Zika virus in the Americas: early epidemiological and genetic findings. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.L., Loe M.W.C., Lee R.C.H., Chu J.J.H. Antiviral activity of pinocembrin against Zika virus replication. Antivir. Res. 2019;167:13–24. doi: 10.1016/j.antiviral.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Chen J., Liang Y., Yi P., Xu L., Hawkins H.K., Rossi S.L., Soong L., Cai J., Menon R., Sun J. Outcomes of congenital Zika disease depend on timing of infection and maternal-fetal interferon action. Cell Rep. 2017;21:1588–1599. doi: 10.1016/j.celrep.2017.10.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chibueze E.C., Tirado V., Lopes K.D., Balogun O.O., Takemoto Y., Swa T., Dagvadorj A., Nagata C., Morisaki N., Menendez C., Ota E., Mori R., Oladapo O.T. Zika virus infection in pregnancy: a systematic review of disease course and complications. Reprod. Health. 2017;14:28. doi: 10.1186/s12978-017-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zammarchi L., Stella G., Mantella A., Bartolozzi D., Tappe D., Gunther S., Oestereich L., Cadar D., Munoz-Fontela C., Bartoloni A., Schmidt-Chanasit J. Zika virus infections imported to Italy: clinical, immunological and virological findings, and public health implications. J. Clin. Virol. 2015;63:32–35. doi: 10.1016/j.jcv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 12.Ventura C.V., Maia M., Bravo-Filho V., Gois A.L., Belfort R., Jr. Zika virus in Brazil and macular atrophy in a child with microcephaly. Lancet. 2016;387:228. doi: 10.1016/S0140-6736(16)00006-4. [DOI] [PubMed] [Google Scholar]
- 13.Zika virus infection: global update on epidemiology and potentially associated clinical manifestations. Wkly. Epidemiol. Rec. 2016;91:73–81. [PubMed] [Google Scholar]
- 14.Batista M.N., Braga A.C.S., Campos G.R.F., Souza M.M., Matos R.P.A., Lopes T.Z., Candido N.M., Lima M.L.D., Machado F.C., Andrade S.T.Q., Bittar C., Nogueira M.L., Carneiro B.M., Mariutti R.B., Arni R.K., Calmon M.F., Rahal P. Natural products isolated from oriental medicinal herbs inactivate Zika virus. Viruses. 2019;11 doi: 10.3390/v11010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu S., Wang J. Homoharringtonine and omacetaxine for myeloid hematological malignancies. J. Hematol. Oncol. 2014;7:2. doi: 10.1186/1756-8722-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero M.R., Serrano M.A., Efferth T., Alvarez M., Marin J.J. Effect of cantharidin, cephalotaxine and homoharringtonine on "in vitro" models of hepatitis B virus (HBV) and bovine viral diarrhoea virus (BVDV) replication. Planta Med. 2007;73:552–558. doi: 10.1055/s-2007-967184. [DOI] [PubMed] [Google Scholar]
- 17.Perard-Viret J., Quteishat L., Alsalim R., Royer J., Dumas F. Cephalotaxus alkaloids. Alkaloids Chem. Biol. 2017;78:205–352. doi: 10.1016/bs.alkal.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efferth T., Sauerbrey A., Halatsch M.E., Ross D.D., Gebhart E. Molecular modes of action of cephalotaxine and homoharringtonine from the coniferous tree Cephalotaxus hainanensis in human tumor cell lines. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003;367:56–67. doi: 10.1007/s00210-002-0632-0. [DOI] [PubMed] [Google Scholar]
- 19.Kaur P., Thiruchelvan M., Lee R.C., Chen H., Chen K.C., Ng M.L., Chu J.J. Inhibition of chikungunya virus replication by harringtonine, a novel antiviral that suppresses viral protein expression. Antimicrob. Agents Chemother. 2013;57:155–167. doi: 10.1128/AAC.01467-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao J., Forrest J.C., Zhang X. A screen of the NIH Clinical Collection small molecule library identifies potential anti-coronavirus drugs. Antivir. Res. 2015;114:1–10. doi: 10.1016/j.antiviral.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.E., Song Y.J. Anti-varicella-zoster virus activity of cephalotaxine esters in vitro. J. Microbiol. 2019;57:74–79. doi: 10.1007/s12275-019-8514-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho Y.J., Liu F.C., Yeh C.T., Yang C.M., Lin C.C., Lin T.Y., Hsieh P.S., Hu M.K., Gong Z., Lu J.W. Micafungin is a novel anti-viral agent of chikungunya virus through multiple mechanisms. Antivir. Res. 2018;159:134–142. doi: 10.1016/j.antiviral.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Ho Y.J., Lu J.W., Huang Y.L., Lai Z.Z. Palmatine inhibits Zika virus infection by disrupting virus binding, entry, and stability. Biochem. Biophys. Res. Commun. 2019;518:732–738. doi: 10.1016/j.bbrc.2019.08.120. [DOI] [PubMed] [Google Scholar]
- 24.Lu J.W., Hsieh P.S., Lin C.C., Hu M.K., Huang S.M., Wang Y.M., Liang C.Y., Gong Z., Ho Y.J. Synergistic effects of combination treatment using EGCG and suramin against the chikungunya virus. Biochem. Biophys. Res. Commun. 2017;491:595–602. doi: 10.1016/j.bbrc.2017.07.157. [DOI] [PubMed] [Google Scholar]
- 25.Adcock R.S., Chu Y.K., Golden J.E., Chung D.H. Evaluation of anti-Zika virus activities of broad-spectrum antivirals and NIH clinical collection compounds using a cell-based, high-throughput screen assay. Antivir. Res. 2017;138:47–56. doi: 10.1016/j.antiviral.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 26.Agbulos D.S., Barelli L., Giordano B.V., HunCurr F.F. Zika virus: quantification, propagation, detection, and storage. Curr. Protoc. Microbiol. 2016;43 doi: 10.1002/cpmc.19. 15D 14 11-15D. [DOI] [PubMed] [Google Scholar]
- 27.Fresno M., Jimenez A., Vazquez D. Inhibition of translation in eukaryotic systems by harringtonine. Eur. J. Biochem. 1977;72:323–330. doi: 10.1111/j.1432-1033.1977.tb11256.x. [DOI] [PubMed] [Google Scholar]
- 28.Jia K., Yuan Y., Liu W., Liu L., Qin Q., Yi M. Identification of inhibitory compounds against Singapore grouper iridovirus infection by cell viability-based screening assay and droplet digital PCR. Mar. Biotechnol. 2018;20:35–44. doi: 10.1007/s10126-017-9785-1. [DOI] [PubMed] [Google Scholar]
- 29.Huang M.T. Harringtonine, an inhibitor of initiation of protein biosynthesis. Mol. Pharmacol. 1975;11:511–519. [PubMed] [Google Scholar]
- 30.Zhou D.C., Zittoun R., Marie J.P. Homoharringtonine: an effective new natural product in cancer chemotherapy. Bull. Cancer. 1995;82:987–995. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.