Abstract
Entry of SARS-CoV into a target cell is initiated by binding of the S1 domain of spike protein to a receptor, followed by conformational changes of the spike protein S2 domain, resulting in the formation of a six-helix bundle by the heptad-repeat (HR1 and HR2) regions. Our previous studies have demonstrated that peptides derived from HR2 region could inhibit SARS-CoV entry. However, synthesis of these peptides is at high cost. In this study, we designed two recombinant proteins, one containing two HR1 and one HR2 peptides (denoted HR121), and the other consisting of two HR2 and one HR1 peptides (designated HR212). These two proteins could be easily purified with the low cost of production, exhibiting high stability and potent inhibitory activity on entry of the HIV/SARS pseudoviruses with IC50 values of 4.13 and 0.95 μM, respectively. These features suggest that HR121 and HR212 can serve as potent inhibitors of SARS-CoV entry.
Keywords: Design, SARS-CoV, Protein inhibitors, Heptad-repeat regions, Spike protein, Pseudotyped virus
Severe acute respiratory syndrome (SARS) is a life-threatening form of atypical pneumonia [1], [2]. It has seriously threatened public health and socioeconomic stability. More than 8000 people were infected and 774 worldwide, mostly in Asia, were killed by the disease before it was brought under control in July between the winter and spring in 2002–2003 (WHO website: www.who.int). However, the current therapeutic agents for SARS-CoV infection are mainly hormone agents. Despite the success of these drugs in treating SARS, they also cause many sequelae, such as arthralgia [3]. Accordingly, new types of therapeutics are needed.
SARS is caused by a novel coronavirus, SARS-CoV [4], [5], [6], [7], [8], [9]. Coronaviruses, belonging to coronaviridae family, are enveloped positive-strand RNA viruses. The enveloped glycoproteins of the enveloped viruses initiate entry of viruses into their host cells by binding to cell surface receptors followed by conformation changes leading to membrane fusion and delivery of the genome in the cytoplasm [10], [11], [12], [13], [14]. As for SARS-CoV, the fusion process is mediated by the spike protein on the surface of the virus [15]. The spike protein is a typical class I viral fusion protein just like the transmembrane glycoprotein of many other enveloped viruses [14], [16]. It is composed of two subunits: S1, a surface subunit that binds to the host cell surface receptor; and S2, a transmembrane subunit that ultimately inserts into the host cell membrane and promotes the fusion events. The S2 domain of the spike protein contains two helical regions, one near the amino terminus (HR1) and the other near the carboxy terminus (HR2), both of which are well conserved. In the late stages of membrane fusion, these two regions interact with each other in an antiparallel manner to form a trimer-of-hairpins that shares significant similarity with the fusion core structure of MHV spike protein and other viral fusion proteins [17]. This facilitates the juxtaposition of the virus and cell membranes, leading to membrane fusion. Moreover, peptides derived from HR2 region of the spike protein have been found to have inhibitory activity just as those of retrovirus and paramyxovirus fusion protein [18], [19], [20], [21]. HR2 peptides likely inhibit formation of the trimer-of-hairpins in a dominant-negative manner by binding to HR1 region just like T-20 (Fig. 1 A). T-20 is the first inhibitor to be approved by the US Food and Drug Administration (FDA) and European Commission for the treatment of HIV infection in adults and children aged 6 years and older. However, one of the major limitations of T-20 is the difficulty of peptide synthesis, resulting in high cost of production and insufficiency of supplies [22]. Accordingly, it is necessary to find the analogs of these peptide inhibitors with the same fusion-inhibitory activity, but the production is at low cost.
Fig. 1.
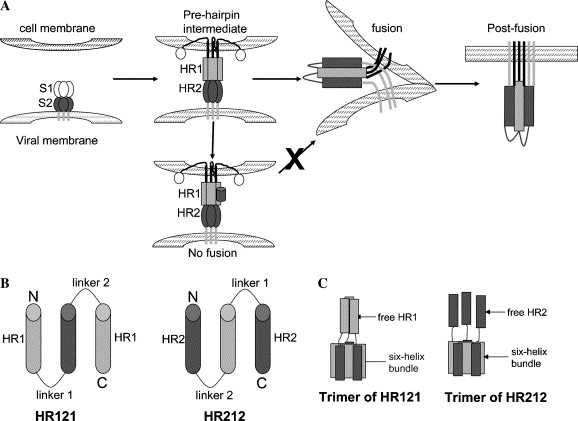
Target SARS-CoV membrane fusion. (A) A schematic model of SARS-CoV membrane fusion [21]. In the prehairpin intermediate, the central coiled-coil formed by three HR1s and three HR2s was exposed and could be targeted by exogenous HRs. In the absence of inhibitors, this transient state ultimately collapsed into a six-helix bundle that brought the HR1 and HR2 regions into close proximity, promoting membrane fusion. The entry inhibitors (black cylinder) targeting HR1 regions prevented the formation of the six-helix bundle. (B) HR121 contained two HR1 segments and one HR2 segment alternatively linked with the short Gly/Ser peptide sequences, while HR212 consisted of two HR2 segments and one HR1 segment. The sequences of HR1 and HR2 for constructing HR121 and HR212 were peptides N35 and C35, respectively; linker 1, GGSGG; and linker 2, SGGRGG. (C) Schematic models of the trimer of HR121 (left) and HR212 (right). Both HR121 and HR212 assembled into six-helix bundles with free HR1s or HR2s.
Recombinant proteins that contain the HR1 and HR2 segments of HIV-1 gp41 separated by a short, hydrophilic, six-residue linker (HR1-HR2, 2-helix) have been produced and shown to fold into thermally stable six-helix bundles [23]. We have also previously shown that 2-helix proteins from a number of paramyxovirus F proteins, such as NDV [24], Mumps virus [25], Measles virus [26], and Menangle virus [27], could also form typical six-helix bundles and be used successfully for crystallization. Based on our previous constructs, we designed two proteins, HR121 (HR1-HR2-HR1) and HR212 (HR2-HR1-HR2), analogous to HR1 and HR2, respectively. One more HR sequence was linked with 2-helix, which led to the formation of HR121 and HR212. In this study, we used a GST-fusion system to express HR121 and HR212 separately in an Escherichia coli (E. coli). HR121 and HR212 could be easily expressed and purified, exhibiting stable α-helical structure in solution and potent inhibitory activity on entry of the HIV/SARS pseudotyped virus.
Materials and methods
Plasmids and cell lines. pNL43LucE−R− (HIV-luc) and the codon optimized SARS-CoV S protein expression plasmid pcTSh (strain BJ01, GenBank Accession No. AY278488, a gift from Dr. Hongkui Deng) were utilized in the production of HIV/SARS pseudoviruses. 293T cells used in pseudotyped virus generation and Huh 7 cells used in cell–cell fusion assays were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum.
Gene construction. Learn Coil-VMF program [28] was used to predict the amino acid sequences of HR1 and HR2 regions. Genes encoding amino acids 898–1005 for HR1 (N108), 900–943 for HR1 (N44), 916–950 for HR1 (N35), 1149–1186 for HR2 (C38), and 1151–1185 for HR2 (C35) were amplified by PCR from pcTSh. Based on Tripet’s finding [29], we selected N35 and C35 to construct HR121 (N35-C35-N35) and HR212 (C35-N35-C35). HR121 consisted of two N35 segments and one C35 segment alternatively linked with the short peptide sequences, while HR212 consisted of two C35 segments and one N35 segment (Fig. 1B). The genes encoding the two proteins were then subcloned into E. coli expression vector pGEX-6P-1 by two restriction enzyme sites of EcoRI and XhoI. A stop codon was introduced before the XhoI site. This cloning strategy yielded glutathione S-transferase (GST) fusion proteins and the fusion proteins were named after GST-HR121 and GST-HR212, respectively. N108, N44, and C38 were subcloned into the BamHI/XhoI site of pET-30a and yielded the proteins with N-terminal 6× his-tag, thrombin, and enterokinase cleavage sites. The purified proteins were named after N108-30a, N44-30a, and C38-30a, respectively.
Protein expression and purification. The recombinant plasmids of pGEX-6p-1-HR121, pGEX-6p-1-HR212 and the plasmid pGEX-6p-1 itself were transformed into E. coil strain BL21 (DE3). Single colony from the respective transformation was grown at 37 °C in 2× YT to an optical density (OD) at 600 nm of 0.8–1.0 and then induced with 0.5 mM IPTG at 20 °C for 4 h. Bacterial cells were harvested and lysed by sonication in phosphate-buffered saline (PBS, 10 mM sodium phosphate, pH 7.3; 150 mM NaCl). Triton X-100 was then added to a final concentration of 1% and the lysate was incubated for 30 min on ice and subsequently clarified by centrifugation at 12,000g for 15 min at 4°C. The clarified supernatants were applied to Glutathione-Sepharose 4B affinity column (Pharmacia). The column was then washed with 10 bed volumes of PBS and eluted with reduced l-glutathione (15 mM). The HR121 and HR212 proteins were subsequently cleaved from the fusion proteins by GST-fusion rhino-virus 3C protease (GST-3C), kindly provided by Drs. K. Hudson and J. Heath, and were loaded on glutathione–Sepharose 4B affinity column again to remove GST and GST-3C. The samples were then purified by a Hiload Superdex G75 column (Pharmacia) running on Akta Explorer FPLC system (Amersham-Pharmacia). The fractions of the peak were collected and run on 12% SDS–PAGE. The peak molecular weight was estimated by comparison with the protein standards (Pharmacia) running on the same column. The clarified supernatants of N108, N44, and C38 were applied on Ni-chelated Sepharose affinity column (Pharmacia). The column was then washed by PBS over 10 column volumes and eluted with imidazole (500, 300, and 200 mM, respectively).
Chemical cross-linking. This was done according to the method described earlier [26]. Briefly, the gel-filtration purified HR121 and HR212 were dialyzed against cross-linking buffer (50 mM Hepes, pH 8.3; 100 mM NaCl) separately and concentrated to about 2 mg/ml by ultrafiltration (10 kDa cut-off). Proteins were cross-linked with ethylene glycol bis (succinimidyl succinate) (EGS, dissolved in DMSO) (Sigma). The reactions were incubated for 1 h on ice at concentrations of 0, 0.1, 0.2, 0.4, 0.8, and 1.2 mM EGS and stopped by 50 mM glycine. Cross-linked products were analyzed under reducing conditions on 12% SDS–PAGE.
CD spectroscopy. CD spectra were performed on a Jasco J-715 spectrophotometer with proteins in PBS. Wavelength spectra were recorded at 37 °C using a 0.1 cm path-length cuvette. The protein concentration used for this was 10 μg/ml.
GST pull-down assay. Excess N108-30a and C38-30a in classified bacterium supernatants were, respectively, mixed with GST-HR121 and GST-HR212. The mixtures were incubated for 1 h at room temperature before glutathione–Sepharose 4B affinity gel was added. The gel with the protein mixtures was then incubated with gentle agitation at room temperature for 30 min. The suspension was centrifuged at 500g for 5 min to sediment the gel with adsorbed fusion protein complexes. The column was then washed with 10 bed volumes of PBS and eluted with reduced l-glutathione. The eluted samples were analyzed by SDS–PAGE.
Inhibition of HIV/SARS pseudovirus entry by HR121 and HR212. HIV/SARS pseudovirus was produced as described by Deng [30]. pNL43LucE−R− and pcTSh were co-transfected into 293T cells. Forty eight hours later, HIV/SARS pseudovirus-containing supernatant was mixed with serially diluted protein. The virus/protein mixture was then transferred to 24-well plates seeded with Huh 7 cells. Three hours later, the medium was replaced. Following 2 days incubation at 37 °C, the cells were harvested in lysis buffer, analyzed for luciferase activity by the addition of luciferase substrate, and measured for 10 s in a TD-20/10 luminometer. The IC50 values were calculated by fitting the HR121 and HR212 titration data Langmuir function {normalized luciferase activity = 1/(1 + C/IC50)}.
Results and discussion
Design of HR121 and HR212
The trimeric core of the ectodomain of SARS-CoV S2, 2-helix, which has been solved crystallographically [17], comprises residues 900–948 (HR1) and 1145–1184 (HR2) of SARS-CoV S2 covalently linked to a 22-residue linker. The structure forms a thermostable trimer of hairpins in which HR1 and HR2 are entirely helical and arranged in a six-helix bundle. We have made use of the 2-helix core as a scaffold to link another HR1 to its C terminus and another HR2 to its N terminus, respectively (Fig. 1C). The rational for this design is that three HR1s or HR2s will be stabilized as a consequence of it being covalently linked to the S2 ectodomain core by a flexible linker. Then the stability of the designed HR212 and HR121 will be improved.
Both HR121 and HR212 were expressed as soluble stable proteins
In this study, to produce HR121 and HR212, we used the recombinant E. coli expression system which was able to provide protein products in large quantities at low cost. The designed two proteins were expressed as GST fusion proteins in soluble form and it was easy to elute GST-HR121 and GST-HR212 from the glutathione–Sepharose column by the normal elution buffer (15 mM reduced glutathione, 50 mM Tris–HCl, pH 8.0). Moreover, the two GST fusion proteins could be easily cleaved by GST-3C protease (Fig. 2 ). Namely, the free HR121 and HR212 were easily expressed and purified in large quantities at low cost.
Fig. 2.
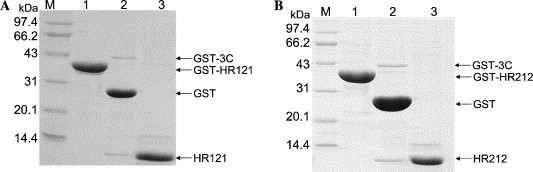
SDS–PAGE analysis of the purified proteins. Both HR121 (A) and HR212 (B) were expressed as GST fusion proteins and cleaved by GST-3C. Lane M, protein markers (in kDa); lane 1, GST fusion protein with HR121 or HR212; lane 2, the GST fusion proteins digested after GST-3C; and lane 3, the free HR121 and HR212.
The purified HR121 and HR212 were then analyzed in gel filtration for estimation of the molecular weight and the assembly character. The elution peaks of both HR121 and HR212 proteins were just after the peak position corresponding to 52 kDa, while the calculated molecular weights of HR121 and HR212 were 12.4 and 12.6 kDa, respectively, which indicates that both HR121 and HR212 could form oligomers, probably trimers (Figs. 3 A and B). Subsequently, chemical cross-linking demonstrated that HR121 and HR212 could indeed form trimer (Figs. 3C and D), although the monomer/dimer bands did not completely disappear even in high concentrations of the cross-linker. However, the density of the trimer band tended to get thicker with the gradual increase of the cross-linker concentrations. The molecular weight of the cross-linked trimer was around 38 kDa, close to the theoretical value. These gave strong evidence that both HR121 and HR212 could still assemble into six-helix bundle structures in PBS similar to that formed by 2-helix. However, the six-helix bundles formed by HR121 or HR212 also had three superfluous HR1s or HR2s, which were different from that formed by 2-helix.
Fig. 3.
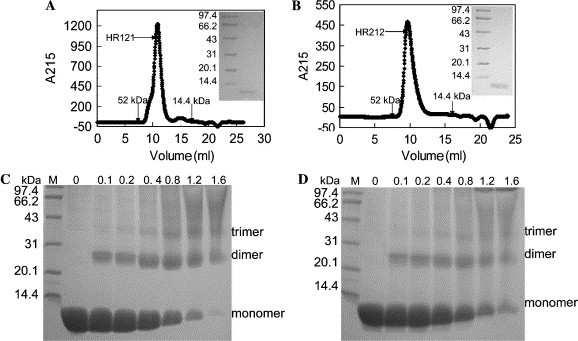
Gel filtration analysis and chemical cross-linking of the designed proteins. The protein samples were loaded on Superdex G75 column. The relative positions of the standard protein markers are indicated (kDa). (A,B) The gel filtration profiles of HR121 and HR212, respectively. The inset pictures are SDS–PAGE analysis of the sample from the peaks; left: protein markers (kDa); right: the proteins from the peaks. (C,D) Chemical cross-linking of HR121 and HR212, respectively. Protein markers are shown in lane M. The numbers 0, 0.1, 0.2, 0.4, 0.8, 1.2, and 1.6 indicate the concentrations of ethylene glycol bis(succinimidyl succinate) (EGS) in millimolar used. Bands corresponding to monomer, dimer, and trimer are also indicated.
Both HR121 and HR212 exhibited helical structure
The biophysical properties of the individual proteins concerned were tested by CD spectrometry as described under Materials and methods. HR121 and HR212 had a CD spectrum exhibiting double minima at 208 and 222 nm while N44-30a and C38-30a were unordered. These indicated that HR121 and HR212 showed a salient α-helix character (Figs. 4 A and B). Namely, the two proteins were more stable than single HR1 or HR2.
Fig. 4.
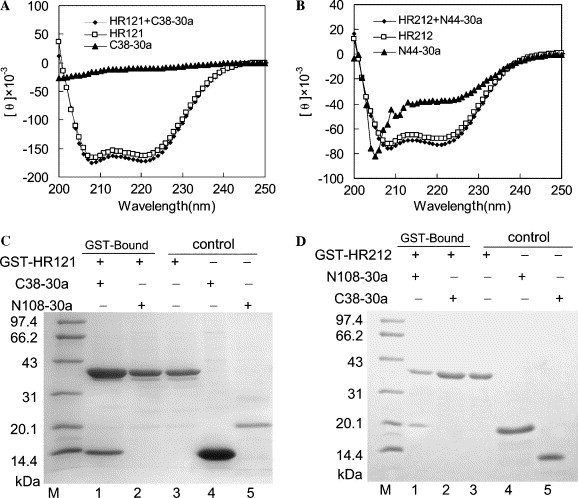
Analysis of the binding activities of HR121 and HR212 in vitro. (A,B) CD spectra of HR121 and HR212 before and after adding C38-30a and N44-30a. The ellipticities at 208 and 222 nm increased upon mixing HR121 and C38-30a (A), and mixing HR212 and N44-30a also increased the ellipticities at 208 and 222 nm (B). (C) The C38-30a was pulled down by GST-HR121. The results show that GST-HR121 could interact with C38-30a (lane 1), while GST-HR121 could not pull down N108-30a (lane 2); lane 3, purified GST-HR121; lane 4, purified C38-30a; lane 5, purified N108-30a; and lane M, protein markers (kDa). (D) GST-HR212 could interact with N108-30a (lane 1), while GST-HR212 could not pull down C38-30a (lane 2); lane 3, purified GST-HR212; lane 4, purified N108-30a; lane 5, purified C38-30a; and lane M, protein markers (kDa).
HR121 could bind to C38-30a in vitro and HR212 could pull down N108-30a
GST-HR121 bound to C38-30a, while it did not bind to N108-30a. GST-HR212 bound to N108-30a, while it did not pull down C38-30a (Figs. 4C and D). Moreover, GST-HR212 could pull down N44-30a (data not shown). These implied that GST as well as the fusion partner of 50 extra amino acids of N108-30a and C38-30a did not affect the binding activity of HR121 and HR212. The ellipticities at 208 and 222 nm increased upon mixing HR121 with C38-30a and they also increased upon mixing HR212 with N44-30a (Figs. 4A and B). The increase in ellipticity at 208 and 222 nm upon mixing indicated an interaction between the two proteins and the interaction induced a helical conformation. These results showed that HR121 was analogous to its monomer (HR1) that could bind to C38 and HR212 was analogous to HR2.
In this study, N108-30a had a strong tendency to aggregate. It was partially soluble and liable to precipitate in PBS but was soluble in clarified bacterium supernatants while N44-30a was soluble in both PBS and supernatant, but aggregated at high concentration. On the other hand, peptide N44 bound to HR2 region of the spike protein according to the crystal structure [17] and GST-HR212 could pull down N44-30a (data not shown). Due to these two reasons, we analyzed the spectra of N44-30a and the mixture of N44-30a and HR212, not the mixture of N108-30a and HR212 in CD spectra analysis.
However, both HR121 and HR212 were soluble in PBS and they did not aggregate even at high concentration. That is to say, HR121 and HR212 were more stable than their respective analogs, HR1 and HR2.
Both HR121 and HR212 could inhibit HIV/SARS pseudovirus mediated cell fusion
SARS-CoV has been forbidden to be used as experimental materials by the government, so HIV/SARS pseudovirus was used in the cell fusion assays. It is the safe, conformational mimetics of the SARS-CoV [19], [31], [32], [33]. Both HR121 and HR212 exhibited anti-viral activity (Fig. 5 ) with IC50 values 4.13 ± 0.43 and 0.95 ± 0.12 μM, respectively.
Fig. 5.
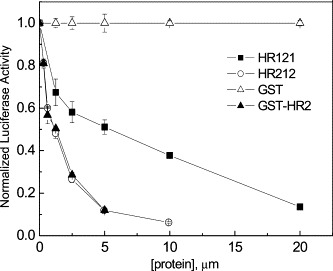
Titration of viral infectivity by HR121 (filled squares), HR212 (open circles), GST-HR2-38 (filled triangles), and GST (open triangles). The IC50 values of HR121, HR212, and GST-HR2-38 in this assay are 4.13 ± 0.43, 0.95 ± 0.12, and 1.02 ± 0.02 μM, respectively. GST protein itself was used as control and no inhibitions were observed. The data represent the means ± SEM of three separate experiments.
The GST-HR2-38 and HR1-30a constructed in previous study [20] were also tested for the inhibitory activity of HIV/SARS pseudovirus. The IC50 value of GST-HR2-38 was 1.02 μM approximate to that of HR212. However, HR1-30a did not have any effect on virus infection while HR121 (HR1 analog) had fusion inhibitory activity. It was proposed that formation of the bundles (Fig. 1C) was irreversible under physiological conditions. HR121 could reverse at physiological conditions to expose its HR2 segment. The exposed HR2 bound to viral HR1 region and prevented the formation of S2 six-helix bundle, thereby blocking fusion by the same mechanism as that of individual HR2 peptides [21].
Taking all the above into consideration, the designed proteins, HR121 and HR212, inhibited entry of the HIV/SARS pseudovirus and the inhibitory activity of HR212 was comparable to that of HR2. Moreover, the designed proteins were easily purified with the low cost of production and they were more stable than single HR1 or HR2. Accordingly, this design strategy should also be used in other enveloped viruses, especially HIV.
Acknowledgments
We are grateful to Dr. Shibo Jiang of York Blood New Center for critical reading of the manuscript and providing constructive comments and suggestions. This work was supported by grants from National Projects 973 (Grant Nos. G1999075602 and 2001CB510001).
Contributor Information
George F. Gao, Email: ggao66@yahoo.com.
Po Tien, Email: tienpo@sun.im.ac.cn.
References
- 1.Tsang K.W., Ho P.L., Ooi G.C., Yee W.K., Wang T., Chan-Yeung M., Lam W.K., Seto W.H., Yam L.Y., Cheung T.M., Wong P.C., Lam B., Ip M.S., Chan J., Yuen K.Y., Lai K.N. A cluster of cases of severe acute respiratory syndrome in Hong Kong. N. Engl. J. Med. 2003;348:1977–1985. doi: 10.1056/NEJMoa030666. [DOI] [PubMed] [Google Scholar]
- 2.Poutanen S.M., Low D.E., Henry B., Finkelstein S., Rose D., Green K., Tellier R., Draker R., Adachi D., Ayers M., Chan A.K., Skowronski D.M., Salit I., Simor A.E., Slutsky A.S., Doyle P.W., Krajden M., Petric M., Brunham R.C., McGeer A.J. Identification of severe acute respiratory syndrome in Canada. N. Engl. J. Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 3.Gao H.S., Wang S.X., Chen L.M., Li Y.M., Yuan S.L., Jiao Z.S., Hui W.L., Yang Z., Zhao Z.Y. Analysis of relation between the usage of corticosteroid in treatment and arthralgia as a sequela of SARS patients. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2004;16:277–280. [PubMed] [Google Scholar]
- 4.Eickmann M., Becker S., Klenk H.D., Doerr H.W., Stadler K., Censini S., Guidotti S., Masignani V., Scarselli M., Mora M., Donati C., Han J.H., Song H.C., Abrignani S., Covacci A., Rappuoli R. Phylogeny of the SARS coronavirus. Science. 2003;302:1504–1505. doi: 10.1126/science.302.5650.1504b. [DOI] [PubMed] [Google Scholar]
- 5.Peiris J.S., Lai S.T., Poon L.L., Guan Y., Yam L.Y., Lim W., Nicholls J., Yee W.K., Yan W.W., Cheung M.T., Cheng V.C., Chan K.H., Tsang D.N., Yung R.W., Ng T.K., Yuen K.Y. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., Rollin P.E., Dowell S.F., Ling A.E., Humphrey C.D., Shieh W.J., Guarner J., Paddock C.D., Rota P., Fields B., DeRisi J., Yang J.Y., Cox N., Hughes J.M., LeDuc J.W., Bellini W.J., Anderson L.J. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 7.Kuiken T., Fouchier R.A., Schutten M., Rimmelzwaan G.F., van Amerongen G., van Riel D., Laman J.D., de Jong T., van Doornum G., Lim W., Ling A.E., Chan P.K., Tam J.S., Zambon M.C., Gopal R., Drosten C., van der Werf S., Escriou N., Manuguerra J.C., Stohr K., Peiris J.S., Osterhaus A.D. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 9.Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen-Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 10.Eckert D.M., Kim P.S. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- 11.Weissenhorn W., Dessen A., Calder L.J., Harrison S.C., Skehel J.J., Wiley D.C. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 1999;16:3–9. doi: 10.1080/096876899294706. [DOI] [PubMed] [Google Scholar]
- 12.Skehel J.J., Wiley D.C. Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin. Annu. Rev. Biochem. 2000;69:531–569. doi: 10.1146/annurev.biochem.69.1.531. [DOI] [PubMed] [Google Scholar]
- 13.Bentz J. Membrane fusion mediated by coiled coils: a hypothesis. Biophys. J. 2000;78:886–900. doi: 10.1016/S0006-3495(00)76646-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bosch B.J., van der Zee R., de Haan C.A., Rottier P.J. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2003;77:8801–8811. doi: 10.1128/JVI.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang Z.Y., Huang Y., Ganesh L., Leung K., Kong W.P., Schwartz O., Subbarao K., Nabel G.J. pH-dependent entry of severe acute respiratory syndrome coronavirus is mediated by the spike glycoprotein and enhanced by dendritic cell transfer through DC-SIGN. J. Virol. 2004;78:5642–5650. doi: 10.1128/JVI.78.11.5642-5650.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallaher W.R., Ball J.M., Garry R.F., Griffin M.C., Montelaro R.C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res. Hum. Retroviruses. 1989;5:431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y., Lou Z., Liu Y., Pang H., Tien P., Gao G.F., Rao Z. Crystal structure of SARS-CoV spike protein fusion core. J. Biol. Chem. 2004;279:49414–49419. doi: 10.1074/jbc.M408782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bosch B.J., Martina B.E., Van Der Zee R., Lepault J., Haijema B.J., Versluis C., Heck A.J., De Groot R., Osterhaus A.D., Rottier P.J. Severe acute respiratory syndrome coronavirus (SARS-CoV) infection inhibition using spike protein heptad repeat-derived peptides. Proc. Natl. Acad. Sci. USA. 2004;101:8455–8460. doi: 10.1073/pnas.0400576101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuan K., Yi L., Chen J., Qu X., Qing T., Rao X., Jiang P., Hu J., Xiong Z., Nie Y., Shi X., Wang W., Ling C., Yin X., Fan K., Lai L., Ding M., Deng H. Suppression of SARS-CoV entry by peptides corresponding to heptad regions on spike glycoprotein. Biochem. Biophys. Res. Commun. 2004;319:746–752. doi: 10.1016/j.bbrc.2004.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu J., Xiao G., Xu Y., Yuan F., Zheng C., Liu Y., Yan H., Cole D.K., Bell J.I., Rao Z., Tien P., Gao G.F. Following the rule: formation of the 6-helix bundle of the fusion core from severe acute respiratory syndrome coronavirus spike protein and identification of potent peptide inhibitors. Biochem. Biophys. Res. Commun. 2004;319:283–288. doi: 10.1016/j.bbrc.2004.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu S., Xiao G., Chen Y., He Y., Niu J., Escalante C.R., Xiong H., Farmar J., Debnath A.K., Tien P., Jiang S. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet. 2004;363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leao J.C., Frezzini C., Porter S. Enfuvirtide: a new class of antiretroviral therapy for HIV infection. Oral Dis. 2004;10:327–329. doi: 10.1111/j.1601-0825.2004.01042.x. [DOI] [PubMed] [Google Scholar]
- 23.Markosyan R.M., Ma X., Lu M., Cohen F.S., Melikyan G.B. The mechanism of inhibition of HIV-1 env-mediated cell-cell fusion by recombinant cores of gp41 ectodomain. Virology. 2002;302:174–184. doi: 10.1006/viro.2002.1593. [DOI] [PubMed] [Google Scholar]
- 24.Li P.Y., Zhu J.Q., Wu B.L., Gao F., Tien P., Rao Z., Gao G.F. Crystallization and preliminary X-ray diffraction analysis of post-fusion six-helix bundle core structure from Newcastle disease virus F protein. Acta Crystallogr. D. 2003;59:1296–1298. doi: 10.1107/s0907444903009909. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y., Zhu J., Feng M.G., Tien P., Gao G.F. Six-helix bundle assembly and analysis of the central core of mumps virus fusion protein. Arch. Biochem. Biophys. 2004;421:143–148. doi: 10.1016/j.abb.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 26.Zhu J., Zhang C.W., Qi Y., Tien P., Gao G.F. The fusion protein core of measles virus forms stable coiled-coil trimer. Biochem. Biophys. Res. Commun. 2002;299:897–902. doi: 10.1016/s0006-291x(02)02761-4. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J.Q., Zhang C.W., Rao Z., Tien P., Gao G.F. Biochemical and biophysical analysis of heptad repeat regions from the fusion protein of Menangle virus, a newly emergent paramyxovirus. Arch. Virol. 2003;148:1301–1316. doi: 10.1007/s00705-003-0105-x. [DOI] [PubMed] [Google Scholar]
- 28.Singh M., Berger B., Kim P.S. LearnCoil-VMF: computational evidence for coiled-coil-like motifs in many viral membrane-fusion proteins. J. Mol. Biol. 1999;290:1031–1041. doi: 10.1006/jmbi.1999.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tripet B., Howard M.W., Jobling M., Holmes R.K., Holmes K.V., Hodges R.S. Structural characterization of the SARS-coronavirus spike S fusion protein core. J. Biol. Chem. 2004;279:20836–20849. doi: 10.1074/jbc.M400759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng H.K., Unutmaz D., KewalRamani V.N., Littman D.R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 31.Wang P., Chen J., Zheng A., Nie Y., Shi X., Wang W., Wang G., Luo M., Liu H., Tan L., Song X., Wang Z., Yin X., Qu X., Wang X., Qing T., Ding M., Deng H. Expression cloning of functional receptor used by SARS coronavirus. Biochem. Biophys. Res. Commun. 2004;315:439–444. doi: 10.1016/j.bbrc.2004.01.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang Y., Yang Z.Y., Kong W.P., Nabel G.J. Generation of synthetic severe acute respiratory syndrome coronavirus pseudoparticles: implications for assembly and vaccine production. J. Virol. 2004;78:12557–12565. doi: 10.1128/JVI.78.22.12557-12565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang H., Wang G., Li J., Nie Y., Shi X., Lian G., Wang W., Yin X., Zhao Y., Qu X., Ding M., Deng H. Identification of an antigenic determinant on the S2 domain of the severe acute respiratory syndrome coronavirus spike glycoprotein capable of inducing neutralizing antibodies. J. Virol. 2004;78:6938–6945. doi: 10.1128/JVI.78.13.6938-6945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


