Abstract
Human monoclonal antibodies (HuMAbs) prepared from patients with viral infections could provide information on human epitopes important for the development of vaccines as well as potential therapeutic applications. Through the fusion of peripheral blood mononuclear cells from a total of five influenza-vaccinated volunteers, with newly developed murine–human chimera fusion partner cells, named SPYMEG, we obtained 10 hybridoma clones stably producing anti-influenza virus antibodies: one for influenza A H1N1, four for influenza A H3N2 and five for influenza B. Surprisingly, most of the HuMAbs showed broad reactivity within subtype and four (two for H3N2 and two for B) showed broad neutralizing ability. Importantly, epitope mapping revealed that the two broad neutralizing antibodies to H3N2 derived from different donors recognized the same epitope located underneath the receptor-binding site of the hemagglutinin globular region that is highly conserved among H3N2 strains.
Keywords: Influenza virus, Human monoclonal antibody, Virus neutralization, Global epitope
Introduction
The technology of monoclonal antibody (MAbs) preparation is important to provide material for the characterization of unique epitopes and conformational structures as well as the development of rapid diagnostic kits. Further, acute infections could be more effectively prevented with a passive immune strategy, as evidenced by the successful treatment of healthcare workers infected with severe acute respiratory syndrome (SARS)-coronavirus by transfusion with convalescent phase plasma obtained from SARS patients [1] and successful protection against viral infection in a mouse model of SARS-coronavirus infection with human MAbs (HuMAbs) obtained from a patient’s memory cell repertoire [2]. Also, studies of Spanish flu that evaluated the effects of transfusion with influenza-convalescent blood products may offer insights regarding potential treatments for H5N1 infection [3]. Furthermore, it has been reported that an avian flu patient recovered following treatment with convalescent plasma from a donor [4]. Recently, there have been several trials using MAbs with neutralizing activity for the prevention of acute severe symptoms of influenza virus infection including H5N1 [5], [6], [7], [8]. Further, protective properties of MAbs directed at the stem region of hemagglutinin (HA) of influenza A virus H1N1 [9] and H5N1 [6], [7], [8] were demonstrated in mice. Based on these data, preparation of HuMAbs against viruses causing severe acute diseases that have potential therapeutic applications becomes of great importance.
Several factors affect the development of HuMAbs: the source of immune cells, such as human-derived memory B or plasma cells or murine cells; and the procedure to immortalize the immune cells, such as fusion with an appropriate partner cell line, EB virus-based transformation, molecular cloning of the immunoglobulin gene using phage display, and humanization of murine MAbs. Recently, a rapid cloning method for high-affinity HuMAbs against influenza virus was established using single-cell reverse transcriptase-polymerase chain reaction for the immunoglobulin variable regions. For that, single-sorted plasma cells secreting influenza-specific IgG+ derived from influenza-vaccinated healthy volunteers were used [10]. In this study, we employed a novel approach for the development of HuMAbs against influenza viruses using a newly prepared cell line, named SPYMEG, that was generated by fusion of SP2 myeloma cells of murine origin and MEG-01 human megakaryoblastic leukemia cells [11] as partner cells with high fusion efficiency with human lymphocytes (Kuhara, Personal Communication). Using peripheral blood mononuclear cells (PBMCs) from influenza-vaccinated volunteers, we developed several individual HuMAbs that showed broad neutralizing activity against human influenza A (H3N2) and B viruses.
Materials and methods
Volunteers. A total of five healthy volunteers who received influenza vaccination were selected (A, 28 year-old male; B, 35 year-old female; C, 28 year-old male; D, 57 year-old male; and E, 29 year-old female). Ten milliliters of blood were obtained from individual volunteer and the PBMCs were prepared by centrifugation through Ficoll Pack Plus (GE Healthcare, Uppsala, Sweden) for 40 min at 520g.
Vaccine. The 2006/2007 influenza vaccine containing A/New Caledonia/20/99, A/Hiroshima/52/05, and B/Malaysia/2506/04 was kindly provided by the Research Foundation for Microbial Diseases of Osaka University, Kagawa, Japan.
Viruses. One influenza A vaccine strain of H1N1 subtype (A/New Caledonia/20/99), five influenza A vaccine strains of H3N2 subtype (A/Aichi/2/68, A/Guizhou/54/89, A/Wyoming/2/03, A/New York/55/04 and A/Hiroshima/52/05) and four influenza B vaccine strains (B/Victoria/2/87, B/Mie/1/93, B/Shanghai/261/02 and B/Malaysia/2506/04) were used in this study. The A/Hiroshima/52/05 and B/Malaysia/2506/04 strains were kindly provided by the National Institute of Infectious Diseases, Tokyo, Japan. Viruses were propagated in Madin–Darby canine kidney (MDCK) cells and the culture fluids were stored at −80 °C. Infectivity was titrated on MDCK cells and expressed as infectious focus-forming units (FFU) per milliliter.
Fusion partner cell line, SPYMEG. For establishment of the SPYMEG cell line, cultured cells of mouse myeloma cell line, SP2/0-Ag14 (Riken Cell Bank: RCB0209) were fused with cells of human megakaryoblastic cell line, MEG-01 (JCRB Cell Bank: IFO050151) using polyethylene glycol #1500 (Roche Diagnostics, Mannheim, Germany). The fused cells were cultured in RPMI medium containing 10% fetal calf serum (FCS) in the presence of hypoxanthine–aminopterin–thymidine (HAT) for 5 days and further cultured in FCS-free RPMI medium for 3 days. The remaining cells were subsequently cultured with 10 μg/mL of 8-azaguanine for 10 days, after which limiting dilution was performed, generating the cell line designated SPYMEG. SPYMEG is non-secretor of human or murine immunoglobulin, 8-azaguanine-resistant and HAT-sensitive.
Cell fusion. The PBMCs were fused with SPYMEG cells at a ratio of 10:1 with polyethylene glycol #1500. Fused cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 15% FCS in 96-well microplates for 10–14 days in the presence of HAT. The first screening of the culture medium for antibodies specific to influenza viruses was performed by enzyme-linked immunosorbent assay (ELISA), as described below. Specific antibody-positive wells were next subjected to cell cloning by limiting dilution. The second screening was also performed by ELISA.
ELISA. The 96-well microplates coated with viral antigens were reacted with the culture medium of fused cells for 30 min at room temperature, followed by peroxidase-conjugated rabbit anti-human IgG (Medical and Biological Laboratories, Aichi, Japan).
Immunofluorescence assay. Monolayers of MDCK cells in 8-well chamber slides were mock-infected or infected with influenza A and B viruses. After 8 h of incubation, the infected cells were fixed with ethanol then reacted with the culture medium of individual hybridoma cell clones. As control antibodies, we used several murine MAbs, i.e., C43 to nucleoprotein (NP) of influenza A H3N2 virus [12], C179 to HA of H1N1 [12], F49 to HA of H3N2 (Okuno, unpublished), 9F3 to NP of influenza B virus [13], and 9E10 to HA of influenza B virus [14]. The cells were then reacted with fluorescein isothiocyanate (FITC)-conjugated rabbit anti-human or anti-mouse antibodies (Jackson ImmunoResearch, Cambridgeshire, UK).
Western blotting. Purified HA vaccine antigens in sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) sample buffer were subjected to electrophoresis in a 10% polyacrylamide gel. Proteins in the gel were blotted onto polyvinylidene difluoride membranes then incubated with the culture medium of individual hybridoma clones. After incubation with peroxidase-conjugated goat anti-human IgG (H + L) antibody (Jackson ImmunoResearch), the peroxidase reaction on the membrane was visualized using the ECL Western Blotting Detection System (GE Healthcare, Uppsala, Sweden).
Peroxidase and anti-peroxidase (PAP) staining. PAP staining was carried out as described previously [15]. Briefly, MDCK cells were infected at a multiplicity of infection of 0.1 with influenza virus and cultured for 6 h in FCS-free DMEM. The infected cells were fixed with ethanol and reacted with the culture medium of individual hybridoma cell clones. As controls, we used several murine MAbs as mentioned above. The cells were further incubated with rabbit anti-human or anti-mouse IgG antibody (Cappel), then with goat anti-rabbit IgG antibody (Cappel), and finally with PAP complex (Cappel).
Hemagglutinin-inhibition (HI) test. The HI test was carried out as described previously [12]. The culture medium of individual hybridoma clones was treated with receptor destroying enzyme (RDE). The results were expressed as the reciprocal of the highest dilution of the culture medium to show inhibition.
Virus neutralization (VN) test. The VN test was carried out as described previously [15]. Briefly, the culture media of individual hybridoma clones that were treated with RDE were diluted 1:8 with serum-free medium. The diluted culture medium of individual hybridoma clones (50 μL) was mixed with 100 FFU of influenza virus (50 μL), then applied to MDCK cells in a 96-well microplate. After culturing for 6 h, the cells were fixed with ethanol and stained with PAP as above. The data were expressed as percentage of infectivity.
Sequence analysis of HuMAb variable regions. Total RNA was extracted from 1 × 105 hybridoma cells and reverse transcription (RT) was performed at 42 °C for 90 min, using Human IgGH RT-primer (5′-TGGAGGGCACGGTCACCACGC-3′) and IgGL RT-Primer (5′-TTGTGACGGGCGAGCTCAGGC-3′) for kappa chain, and also using dT Primer for lambda chain. Next, we performed 5′ RACE PCR with the SMART™ RACE cDNA Amplification Kit (Clontech Laboratories, Mountain View, CA), using Human IgGH RACE-primer (5′-AAGGTGTGCACGCCGCTGGTC-3′), Human IgGL(kappa) RACE-primer (5′-GTGCTGCTGAGGCTGTAGGTG-3′) or Human IgGL(lambda) RACE-primer (5′-CCAYTGTCTTCTCCACRGTRCTCYC-3′ and 5′-TCAGAGGAGGRYGGGAACAGAGTG-3′) as the reverse primer and Universal primer mix as the forward primer, and then cloned the PCR products into the cloning plasmid pMD20-T (Takara Bio, Kyoto, Japan). Sequence analysis was performed with an ABI3730 Sequencer (Perkin Elmer, Waltham, MA), using the M13 primer RV or M13 primer M4 and the BigDye Terminators v3.1 Cycle Sequencing Kit (Perkin Elmer).
Epitope mapping. A total of 158 sets of 15-residue peptides (overlapping by 13 amino acids) spanning amino acid (aa) residues 1–329 of the HA1 region of A/Hiroshima/52/05 (JPT Peptide Technologies GmbH, Berlin, Germany) were subjected to binding activity assay using peptide microarrays [16], [17] with the HuMAbs obtained.
Ethics. All human materials were collected using protocols approved by the Institutional Review Boards of the Research Institute for Microbial Diseases, Osaka University.
Results
Development of HuMAbs
Ten milliliters of blood from five volunteers was obtained at various time points after influenza vaccination for fusion with SPYMEG cells. In 14 experiments, a total of 122 wells were positive for influenza virus-specific HuMAbs in the first screening. However, during subsequent cell cloning and expansion to a large-scale culture, most of the hybridoma cell clones lost the ability to produce HuMAbs. Eventually, 10 hybridoma clones were found to stably produce specific HuMAbs for at least 3 months (Table 1 ). The efficacy to detect the specific antibody-producing wells was higher with the PBMCs derived from vaccines at 2–3 weeks post-vaccination.
Table 1.
Development of HuMAbs to influenza A and B viruses.
| Volunteer (age, sex) | Sampling (weeks post-vaccination) | ELISA+ wellsa | Stable clone | Recognizingb virus | HIc | VNd |
|---|---|---|---|---|---|---|
| A (28, male) | 2 | 44 | A-1 | B | <2 | 0 |
| A-2 | H3 | <2 | 4.3 | |||
| 3 | 5 | |||||
| B (35, female) | 1 | 3 | ||||
| 2 | 10 | B-1 | H3 | <2 | 98.9 | |
| B-2 | H3 | <2 | 0 | |||
| B-3 | B | <2 | 31.7 | |||
| 3 | 1 | |||||
| 4 | 0 | |||||
| 4 | 1 | |||||
| C (28, male) | 1 | 4 | ||||
| 2 | 6 | |||||
| 3 | 5 | C-1 | B | <2 | 0 | |
| 4 | 11 | |||||
| 4 | 0 | |||||
| D (57, male) | 3.5 | 23 | D-1 | H3 | <2 | 80.5 |
| D-2 | B | <2 | 16.7 | |||
| E (29, female) | 3 | 9 | E-1 | H1 | <2 | 0 |
| E-2 | B | <2 | 37.8 | |||
The fused cells from all volunteers were seeded in four 96-well microplates (384 wells).
To determine the virus recognized by the HuMAbs, we performed ELISA and PAP staining with A/New Caledonia/20/99, A/Hiroshima/52/05, and B/Malaysia/2506/04 as H1, H3, and B viruses, respectively.
The HI test was performed with A/New Caledonia/20/99, A/Hiroshima/52/05, and B/Malaysia/2506/04 as H1, H3, and B viruses, respectively. The results are shown as the reciprocal of serial 2-fold dilutions of the culture supernatants containing HuMAbs.
The VN test was performed with A/New Caledonia/20/99, A/Hiroshima/52/05, and B/Malaysia/2506/04 as H1, H3, and B viruses, respectively. The results are shown as % inhibition.
We next determined the influenza virus type recognized by individual HuMAbs. First, an immunofluorescence analysis was carried out and the results were as follows: E-1 recognized H1N1; A-2, B-1, B-2 and D-1 recognized H3N2; and A-1, B-3, C-1, D-2 and E-2 recognized B viruses. Representative results of B-1 and D-1 to influenza A virus (H3N2) and B-3 and E-2 to influenza B virus are shown in Fig. 1 A. As control antibodies, we used several anti-influenza HA and NP murine MAbs. The staining pattern of infected MDCK cells obtained with our HuMAbs was very similar to that obtained with the anti-HA, but not anti-NP murine MAbs. PAP staining and ELISA of these HuMAbs gave the same results (Table 1). Five MAbs (E-1 to H1N1; B-1 and D-1 to H3N2; and A-1 and C-1 to B) showed distinct bands of HA protein on Western blotting, but the other MAbs showed little or no reactivity with any viral proteins including HA by this method, indicating their recognition of conformational epitope(s) (Fig. 1B). As a control antibody, we used 2000-fold dilution of donor B-derived plasma.
Fig. 1.
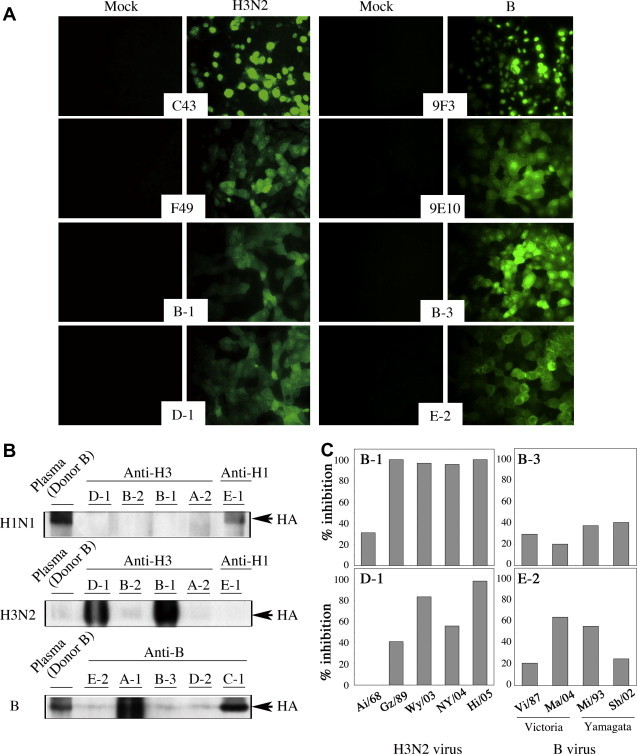
Broad reactivity of HuMAbs to influenza A and B viruses. (A) Specific immunostaining of influenza virus-infected MDCK cells was performed with HuMAbs B-1, D-1, B-3 and E-2. MDCK cells were mock-infected or infected with A/Hiroshima/52/05 (H3N2) and B/Malaysia/2506/04. As positive control, the following murine MAbs were used: C43 to NP and F49 to HA of H3N2; and 9F3 to NP and 9E10 to HA of B virus. (B) Viral protein recognized by HuMAbs was identified by Western blotting. The H1N1-, H3N2- and B-derived purified HA vaccine antigens were analyzed by SDS–PAGE, then immunoblotted with individual HuMAbs. As a positive control, a 2000-fold dilution of the plasma from vaccinated donor B was used. (C) Neutralizing activities of HuMAbs were evaluated with 100 FFU of influenza A H3N2 and B viruses. HuMAbs B-1 and D-1 for H3N2 and B-3 and E-2 for B, showing significant neutralizing activity against A/Hiroshima/52/05 (Hi/05; H3N2) and B/Malaysia/2506/04 (Ma/04; B), as shown in Table 2, were further examined for broad neutralizing activities against past vaccine strains: for H3N2, A/Aichi/2/68 (Ai/68), A/Guizhou/54/89 (Gz/89), A/Wyoming/2/03 (Wy/03), and A/New York/55/04 (NY/04); and for B, B/Victoria/2/87 (Vi/87), B/Mie/1/93 (Mi/93) and B/Shanghai/261/02 (Sh/02).
Broad neutralizing activity of HuMAbs
Anti-H3N2 and anti-B HuMAbs obtained in this study were next examined for cross-reactivity with several vaccine strains of influenza virus subtypes by PAP staining and VN testing. All the HuMAbs, except for D-2 weakly reacting with Sh/02, showed broad reactivity with strains within the same subtype on PAP staining (Table 2 ). Next, HuMAbs were examined for broad neutralizing activity against several H3N2 and B virus strains (Fig. 1C). The results of the VN test were similar to those of PAP straining. B-1 and D-1 showed strong neutralizing activity against H3N2 vaccine strains that were isolated during 1968–2005 and during 1989–2005, respectively. B-3 and E-2 showed mild neutralizing activity against B Victoria-group and Yamagata-group strains that were isolated during 1987–2004. There was no cross-reaction in these HuMAbs among H1N1 (data not shown), H3N2 and/or B viruses (Table 2). Interestingly, little or no HI activity was detected in the supernatant of hybridoma cell clones (Table 1), but a little HI activity was detected with the concentrated purified B-1 and D-1 (data not shown).
Table 2.
Cross-reactivity of HuMAbs to various isolates of influenza A and B viruses by PAP staining.
| HuMAb | Influenza A virus (H3N2)a |
Influenza B virusb |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Victoria-group |
Yamagata-group |
||||||||
| Ai/68 | Gz/89 | Wy/03 | NY/04 | Hi/05 | Vi/87 | Ma/04 | Mi/93 | Sh/02 | |
| A-2 | >128 | >128 | >128 | >128 | >128 | — | <2 | — | — |
| B-1 | 128 | >128 | >128 | >128 | >128 | — | <2 | — | — |
| B-2 | <2 | >128 | >128 | 128 | >128 | — | <2 | — | — |
| D-1 | <2 | >128 | >128 | >128 | >128 | — | <2 | — | — |
| A-1 | —c | — | — | — | <2 | >128 | >128 | >128 | >128 |
| B-3 | — | — | — | — | <2 | 32 | 8 | 32 | 32 |
| C-1 | — | — | — | — | <2 | 128 | 128 | >128 | >128 |
| D-2 | — | — | — | — | <2 | <2 | <2 | <2 | 2 |
| E-2 | — | — | — | — | <2 | >128 | >128 | >128 | >128 |
Ai/68 for A/Aichi/2/68; Gz/89 for A/Guizhou/54/89; Wy/03 for A/Wyoming/2/03; NY/04 for A/New York/55/04; and Hi/05 for A/Hiroshima/52/05.
Vi/87 for B/Victoria/2/87; B/Malaysia/2506/04; Mi/93 for B/Mie/1/93; and Sh/02 for B/Shanghai/261/02.
Not done.
Identification of HA epitopes of broadly neutralizing HuMAbs
Based on the results of the Western blotting analysis, HuMAbs B-1 and D-1 seemed to recognize sequential epitopes of HA. Using a series of overlapping peptides covering the entire sequence of the HA1 region, we tried to identify the neutralizing epitopes. Interestingly, both MAbs showed the same responses to the synthesized peptides, i.e., significant responses to 2 regions by 4 (aa 167–181, 169–183, 171–185 and 173–187) and 2 (aa 225–239 and 227–241) peptides, spanning the overlapping regions aa 173–181 and 227–239, respectively (Fig. 2 A). These regions are highly conserved, except for aa 173 and 227. These amino acids were located underneath the receptor-binding site of the HA globular region (Fig. 2B).
Fig. 2.
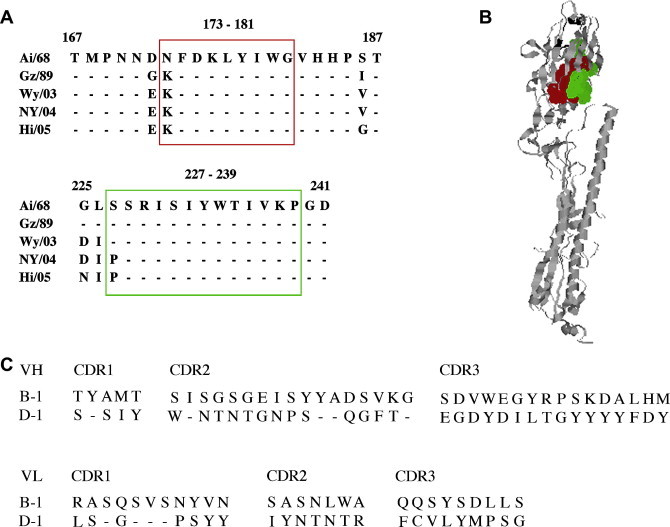
The antigenic regions of broadly neutralizing HuMAbs. (A) Based on the reactivity of B-1 and D-1 HuMAbs with a series of overlapping peptides covering sequences of the HA1 region, amino acid sequences from aa 167–187 and from aa 225–241 are shown. Common amino acid sequences reactive with HA1 peptides are aa 173–181 and from aa 227–239 that are boxed by red and green, respectively. (B) The monomeric structure of H3 HA is shown. The image was created with RasMol 2.6.4, and the HA structure was obtained from the Protein Data Bank (PDB Accession No. 2VIU). The positions of the binding sites are shown as red dots (aa 173–181) and green dots (aa 227–239). The receptor-binding sites are shown as black ribbons. (C) The deduced amino acid sequences of CDRs in the VH and VL of HuMAbs B-1 and D-1 are shown.
Sequence analysis of the IgG variable regions of heavy (VH) and light (VL) chains
Sequence analysis of the VH and VL region of HuMAbs B-1 and D-1 was performed. Further, amino acid sequences of complementarity-determining regions (CDRs) were deduced and compared (Fig. 2C). Although B-1 and D-1 recognized the same region in HA1, these HuMAbs had different amino acid residues in the CDRs.
Discussion
Two serotypes of influenza virus, influenza A (H1N1 and H3N2) and influenza B viruses are circulating predominantly with the H3N2 serotype and B viruses highly prevailed during the last 2 years in Japan [18], [19]. In fact, HuMAbs obtained from five Japanese volunteers given the 2007 vaccine mostly recognized H3N2 and B viruses. Those HuMAbs are broadly reactive with the past strains of H3N2 and B viruses, as revealed by PAP staining as well as the VN assay.
Nine of 10 HuMAbs obtained in this study showed cross-reactivity with a wide-range of past strains of influenza A H3N2 or B virus. In addition, cross-reactivity was also observed for most of them in the VN test. This result may suggest that since humans have history of exposure to influenza virus antigens by natural infection as well as influenza vaccination, the immune cells recognizing global epitopes in the influenza virus seem to quickly respond by vaccination and therefore the use of PBMCs few weeks post-vaccination for cell fusion may be advantageous for the preparation of a wide-range subtype-reactive MAbs like B-1 and D-1. Both HuMAbs recognized two separate regions (aa 173–181 and aa 227–239), located close to each other in the three dimensional structure of HA1, a novel human epitope. This result suggested that B-1 and D-1 recognized a conformational epitope. There have been several studies of HA epitopes of influenza A H3N2, using murine MAbs [20], [21], [22], [23], [24], [25], or rabbit [26], [27] and human [28] polyclonal antisera. Moreover, a mouse MAb recognizing a conformational epitope containing aa 186, 220, 229 and 230 was reported [23]. However, this epitope overlapped only at aa 229 and 230 with the epitope of B-1 and D-1. It is noteworthy to mention that the above two HuMAbs recognizing the same epitope with a highly conserved sequence were independently prepared from two volunteers, as confirmed from the different sequences of their CDRs.
In conclusion, the development of HuMAbs using PBMCs from volunteers who have been naturally exposed to influenza viruses or vaccinated, as well as from patients with influenza infection at a convalescent phase, would provide potential therapeutic application(s).
Acknowledgments
We are grateful to Drs. Takaaki Nakaya, Madiha S. Ibrahim, and Yohei Watanabe in our institute for valuable discussion and critical reading of the manuscript. This study was partly supported by the budget from Benesis Corporation as a collaboration with Osaka University.
References
- 1.Yeh K.M., Chiueh T.S., Siu L.K., Lin J.C., Chan P.K., Peng M.Y., Wan H.L., Chen J.H., Hu B.S., Perng C.L., Lu J.J., Chang F.Y. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J. Antimicrob. Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Traggial E., Becker S., Subbarao K., Kolesnikova L., Uematsu Y., Gismondo M.R., Murphy B.R., Rappuoli R., Lanzavecchia A. An efficient method to make human monoclonal antibodies from memory B cells: potent neutralization of SARS coronavirus. Nat. Med. 2004;10:871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Mata-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann. Intern. Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 4.Zhou B., Zhong N., Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N. Engl. J. Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 5.Hanson B.J., Boon A.C., Lim A.P., Webb A., Ooi E.E., Webby R.J. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir. Res. 2006;7:126. doi: 10.1186/1465-9921-7-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simmons C.P., Bernasconi N.L., Suguitan A.L., Mills K., Ward J.M., Chau N.V., Hien T.T., Sallusto F., Ha do Q., Farrar J., de Jong M.D., Lanzavecchia A., Subbarao K. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4:e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Throsby M., van den Brink E., Jongeneelen M., Poon L.L., Alard P., Cornelissen L., Bakker A., Cox F., van Deventer E., Guan Y., Cinatl J., ter Meulen J., Lasters I., Carsetti R., Peiris M., de Kruif J., Goudsmit J. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3:e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui J., Hwang W.C., Perez S., Wei G., Aird D., Chen L.M., Santelli E., Stec B., Cadwell G., Ali M., Wan H., Murakami A., Yammanuru A., Han T., Cox N.J., Bankston L.A., Donis R.O., Liddington R.C., Marasco W.A. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okuno Y., Matsumoto K., Isegawa Y., Ueda S. Protection against the mouse-adapted A/FM/1/47 strain of influenza A virus in mice by a monoclonal antibody with cross-neutralizing activity among H1 and H2 strains. J. Virol. 1994;68:517–520. doi: 10.1128/jvi.68.1.517-520.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wrammert J., Smith K., Miller J., Langley W.A., Kokko K., Larsen C., Zheng N.Y., Mays I., Garman L., Helms C., James J., Air G.M., Capra J.D., Ahmed R., Wilson P.C. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453:667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogura M., Morishima Y., Ohno R., Kato Y., Hirabayashi N., Nagura H., Saito H. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood. 1985;66:1384–1392. [PubMed] [Google Scholar]
- 12.Okuno Y., Isegawa Y., Sasao F., Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 1993;67:2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa N., Kubota R., Morikawa S., Nakagawa T., Baba K., Okuno Y. Characterization of new epidemic strains of influenza B virus by using neutralizing monoclonal antibodies. J. Med. Virol. 2001;65:745–750. doi: 10.1002/jmv.2099. [DOI] [PubMed] [Google Scholar]
- 14.Nakagawa N., Maeda A., Kase T., Kubota R., Okuno Y. Rapid detection and identification of two lineages of influenza B strains with monoclonal antibodies. J. Virol. Methods. 1999;79:113–120. doi: 10.1016/s0166-0934(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 15.Okuno Y., Tanaka K., Baba K., Maeda A., Kunita N., Ueda S. Rapid focus reduction neutralization test of influenza A and B viruses in microtiter system. J. Clin. Microbiol. 1990;28:1308–1313. doi: 10.1128/jcm.28.6.1308-1313.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reineke U., Kramer A., Schneider-Mergener J. Antigen sequence and library-based mapping of linear and discontinuous protein–protein-interaction sites by spot synthesis. Curr. Top. Microbiol. Immunol. 1999;243:23–36. doi: 10.1007/978-3-642-60142-2_2. [DOI] [PubMed] [Google Scholar]
- 17.Reineke U., Ivascu C., Schlief M., Landgraf C., Gericke S., Zahn G., Herzel H., Volkmer-Engert R., Schneider-Mergener J. Identification of distinct antibody epitopes and mimotopes from a peptide array of 5520 randomly generated sequences. Immunol. Methods. 2002;267:37–51. doi: 10.1016/s0022-1759(02)00139-4. [DOI] [PubMed] [Google Scholar]
- 18.National Institute of Infectious Diseases (NIID), Influenza 2005/06 season, Japan, Infectious Agents Surveillance Report (IASR), vol. 321, 2006, pp. 293–294 (in Japanese).
- 19.National Institute of Infectious Diseases (NIID), Influenza 2006/07 season, Japan, Infectious Agents Surveillance Report (IASR), vol. 333, 2007, pp. 311–313 (in Japanese).
- 20.Wiley D.C., Wilson I.A., Skehel J.J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289:373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- 21.Underwood P.A. Mapping of antigenic changes in the haemagglutinin of Hong Kong influenza (H3N2) strains using a large panel of monoclonal antibodies. J. Gen. Virol. 1982;62:153–169. doi: 10.1099/0022-1317-62-1-153. [DOI] [PubMed] [Google Scholar]
- 22.Wilson I.A., Niman H.L., Houghten R.A., Cherenson A.R., Connolly M.L., Lerner R.A. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- 23.Underwood P.A. An antigenic map of the haemagglutinin of the influenza Hong Kong subtype (H3N2), constructed using mouse monoclonal antibodies. Mol. Immunol. 1984;21:663–671. doi: 10.1016/0161-5890(84)90052-x. [DOI] [PubMed] [Google Scholar]
- 24.Kostolansky F., Varecková E., Betáková T., Mucha V., Russ G., Wharton S.A. The strong positive correlation between effective affinity and infectivity neutralization of highly cross-reactive monoclonal antibody IIB4, which recognizes antigenic site B on influenza A virus haemagglutinin. J. Gen. Virol. 2000;81:1727–1735. doi: 10.1099/0022-1317-81-7-1727. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima S., Nakajima K., Nobusawa E., Zhao J., Tanaka S., Fukuzawa K. Comparison of epitope structures of H3HAs through protein modeling of influenza A virus hemagglutinin: mechanism for selection of antigenic variants in the presence of a monoclonal antibody. Microbiol. Immunol. 2007;51:1179–1187. doi: 10.1111/j.1348-0421.2007.tb04013.x. [DOI] [PubMed] [Google Scholar]
- 26.Green N., Alexander H., Olson A., Alexander S., Shinnick T.M., Sutcliffe J.G., Lerner R.A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982;28:477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- 27.White J.M., Wilson I.A. Anti-peptide antibodies detect steps in a protein conformational change: low-pH activation of the influenza virus hemagglutinin. J. Cell Biol. 1987;105:2887–2896. doi: 10.1083/jcb.105.6.2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakajima S., Nobusawa E., Nakajima K. Variation in response among individuals to antigenic sites on the HA protein of human influenza virus may be responsible for the emergence of drift strains in the human population. Virology. 2000;274:220–231. doi: 10.1006/viro.2000.0453. [DOI] [PubMed] [Google Scholar]


