Abstract
EGFR-mutant lung adenocarcinomas contain a subpopulation of cells that have undergone epithelial-to-mesenchymal transition and can grow independently of EGFR. To kill these cancer cells, we need a novel therapeutic approach other than EGFR inhibitors. If a molecule is specifically expressed on the cell surface of such EGFR-independent EGFR-mutant cancer cells, it can be a therapeutic target. We found that a mesenchymal EGFR-independent subline derived from HCC827 cells, an EGFR-mutant lung adenocarcinoma cell line, expressed angiotensin-converting enzyme 2 (ACE2) to a greater extent than its parental cells. ACE2 was also expressed at least partially in most of the primary EGFR-mutant lung adenocarcinomas examined, and the ACE2 expression level in the cancer cells was much higher than that in normal lung epithelial cells. In addition, we developed an anti-ACE2 mouse monoclonal antibody (mAb), termed H8R64, that was internalized by ACE2-expressing cells. If an antibody-drug conjugate consisting of a humanized mAb based on H8R64 and a potent anticancer drug were produced, it could be effective for the treatment of EGFR-mutant lung adenocarcinomas.
Keywords: EGFR-mutant lung adenocarcinoma, Tyrosine kinase inhibitors (TKIs), Epithelial-to-mesenchymal transition (EMT), Angiotensin-converting enzyme 2 (ACE2), Monoclonal antibody (mAb), Antibody-drug conjugate (ADC)
Highlights
-
•
A mesenchymal EGFR-mutant lung adenocarcinoma cell line expresses ACE2.
-
•
EGFR-mutant lung adenocarcinoma tissues contain cancer cells that express ACE2.
-
•
We developed an anti-ACE2 antibody that is internalized by ACE2-positive cells.
-
•
ACE2 is a potential therapeutic target for EGFR-mutant lung cancer.
1. Introduction
Lung adenocarcinomas with an activating mutation in the epidermal growth factor receptor (EGFR) gene are heavily dependent on EGFR signaling for survival and proliferation. Although many of these tumors initially respond well to EGFR tyrosine kinase inhibitors (TKIs), acquired resistance nearly always develops on average 1 year after the initiation of TKI therapy [1], [2]. In 5% or fewer patients with acquired resistance, the adenocarcinoma cells lose the original phenotype and display a mesenchymal phenotype instead, indicating that they have undergone epithelial to mesenchymal transition (EMT) [1], [2], [3]. We have previously demonstrated that three EGFR-mutant lung adenocarcinoma cell lines, HCC827, HCC4006, and H1975, have subpopulations of cells that have undergone EMT and can survive independently of EGFR signaling, although such cells retain the same EGFR mutation as their parental cells [4], [5]. Since these EGFR-independent EGFR-mutant cancer cells never undergo apoptosis through EGFR inhibition using TKIs, such cells appear to lie at the root of TKI resistance.
Development of a monoclonal antibody (mAb) that binds to a membranous protein of the mesenchymal EGFR-mutant cancer cells to be internalized by the cells is essential because antibody-drug conjugates (ADCs), developed through the conjugation of an anticancer agent to the mAb [6], could be a promising therapeutic option for TKI-resistant EGFR-mutant lung cancer. We previously developed an efficient screening method that can select mAbs internalized by target cells [7], [8]. In this screening system, a recombinant protein termed DT3C that consists of diphtheria toxin (DT) lacking the receptor-binding domain but containing the C1, C2, and C3 domains of streptococcal protein G (3C), plays a critical role. When this mAb-DT3C conjugate is internalized, it functions like an ADC and induces extensive cell death of the targeted cells [7], [8].
Angiotensin-converting enzyme 2 (ACE2) catalyzes the conversion of angiotensin II to angiotensin 1-7. Angiotensin II, the major effector molecule of the conventional renin-angiotensin system, is implicated in the pathogenesis of cardiovascular disorders, including hypertension, atherosclerosis, and myocardial infarction, whereas ACE2 and its product angiotensin 1-7 are thought to prevent the detrimental effects of angiotensin II [9]. In respiratory organs, ACE2 is the receptor for severe acute respiratory syndrome (SARS)-associated coronavirus (SARS-CoV) [10], [11]. However, ACE2 has also been shown to attenuate inflammation and/or acute lung injury caused by SARS-CoV infection, influenza virus infection, or other etiologies mainly through inhibition of angiotensin II-mediated NF-κB signaling [11], [12], [13], [14], [15], [16]. As expected from the fact that ACE2 acts as the receptor for SARS-CoV, it has been reported to be localized to the apical membrane of respiratory epithelial cells, including alveolar epithelia [10]. As far as we are aware, no studies are available on ACE2 expression in EGFR-mutant lung adenocarcinomas. In addition, ACE2 seems to be only expressed at low levels in normal lung and lung cancer tissues according to the Human Protein Atlas [17].
In this study, we demonstrated that a TKI-resistant subline derived from EGFR-mutant HCC827 cells express ACE2 at a much higher level than its parental cells, and that primary EGFR-mutant lung adenocarcinoma tissues contain cancer cells that express ACE2 to a greater degree than normal alveolar epithelial cells. In addition we developed a new anti-ACE2 mouse mAb (H8R64) that was internalized by target cells. If an ADC consisting of a humanized mAb based on H8R64 and a potent anticancer drug were produced, the new ADC could be effective for the treatment of EGFR-mutant lung adenocarcinomas containing TKI-resistant cells.
2. Materials and methods
The experimental procedures were approved by the Institutional Review Board at Sapporo Medical University.
2.1. Cells analyzed
Three EGFR-mutant lung adenocarcinoma cell lines, HCC827 (del E746-A750), HCC4006 (del L747-E749 + A750P), and H1975 (L858R + T790 M), were purchased from the American Type Culture Collection (Manassas, VA, USA). We previously cloned and extensively analyzed EGFR-TKI resistant sublines from these three EGFR-mutant cell lines: HCC827 gefitinib-resistant (GR) 2, HCC4006 GR3, and H1975 WZ4002-resistant (WR) 7 cells [4], [5]. Of note, gefitinib and WZ4002 are first- and third-generation EGFR TKIs, respectively [1], [2]. These cells and the mouse myeloma P3U1 cells (Japanese Collection of Research Bioresources Cell Bank, Osaka, Japan) were cultured in RPMI-1640 supplemented with 10% (v/v) Super Low IgG-FBS (Hyclone, Thermo Fisher Scientific Japan, Yokohama, Japan), and 1% (v/v) streptomycin-penicillin-glutamine solution (Thermo Fisher Scientific) at 37°C/5% CO2. We also examined primary human lung (HuL) 4–6 cells, which are normal human alveolar epithelial cells derived from three individuals and maintained in Bronchial Epithelial Cell Growth Medium (Lonza Japan, Tokyo, Japan) [18], for ACE2 expression. Written, informed consent was obtained from each patient [18].
2.2. Production and screening of hybridomas
We immunized a Balb/c mouse with HCC827 GR2 cells as described previously [7], [8]. Two days after the final injection of cells, the mouse was sacrificed, and 1.65 × 108 splenocytes were fused with 5.0 × 105 P3U1 cells using polyethylene glycol. When the hybridomas had grown to ∼50% confluence, the culture supernatant fluid, where polyclonal antibodies were produced, was tested for antibody internalization. The supernatant from each well and DT3C were incubated together in 96-well microplates at room temperature for 30 min to form Ab-DT3C conjugates [7]. HCC827 GR2 cells were then added to each well at a concentration of 1 × 104 cells/well and incubated for 72 h. The number of viable cells after treatment was then estimated using a WST-1 assay (Roche Diagnostics, Indianapolis, IN, USA). The hybridomas that induced extensive cell death were selected and cloned by limiting dilution.
2.3. Immunoprecipitation and mass spectrometry
We biotinylated the surfaces of HCC827 GR2 cells using a Biotinylation Kit (Thermo Fisher Scientific) and the cell membranes were then solubilized as described previously [8]. A mouse mAb termed H8R64, developed in this study, and a control mouse IgG2b (BD Biosciences, Tokyo, Japan) were used for immunoprecipitation. The following methods used were described previously [19].
2.4. Evaluating mAb internalization by cells
The H8R64 mAb and DT3C were incubated together at room temperature for 30 min to generate the mAb-DT3C conjugate. HCC827 GR2 cells were seeded and incubated in the presence of the mAb-DT3C conjugate for 72 h. The viability of cancer cells was assessed using a WST-1 assay. For efficient formation of the mAb-DT3C conjugate, we used the quantity of mAb required for sufficient formation of mAb-DT3C conjugates at each DT3C concentration tested. In theory, each conjugate consisted of one mAb molecule (150 kDa) and two DT3Cs (140 kDa) [7].
2.5. Primary EGFR-mutant lung adenocarcinoma tissues
Tumor tissues were obtained from five EGFR-mutant lung adenocarcinoma patients who were operated on at Sapporo Medical University Hospital between August 2009 and January 2014. The patient group included a man and four women, with a median age of 65 years (range, 54–79 years; Table 1 ). All tumor slides were stained with hematoxylin and eosin (H&E) and reviewed by one of the authors (YS) to verify the diagnosis. All of these tumors expressed EGFR protein at a high level by immunohistochemistry (data not shown). The EGFR mutations identified in each tumor are described in Table 1.
Table 1.
Clinicopathological characteristics of the lung adenocarcinoma patients.
| Pt. | Age/Sex | EGFR mutation | Allred scores of ACE2 |
|---|---|---|---|
| 1 | 65/F | L861Q | 0 |
| 2 | 79/M | L858R | 2 |
| 3 | 54/F | Ex19 del | 4 |
| 4 | 58/F | Ex19 del | 2 |
| 5 | 71/F | L858R | 6 |
∗Pt., patient; F, female; M, male; Ex, exon; del, deletion.
2.6. ACE2 immunohistochemistry
Immunohistochemical staining for ACE2 was performed on formalin-fixed, paraffin-embedded (FFPE) primary EGFR-mutant lung adenocarcinoma tissue sections. We selected a representative FFPE tissue block from each patient that contained non-cancerous and cancerous tissue to precisely evaluate ACE2 expression in both regions. Whole tissue sections were retrieved using Novocastra Epitope Retrieval Solution pH 9 (Leica Biosystems, Nußloch, Germany) at 100 °C for 20 min. An anti-human ACE2 mouse mAb (clone #171606, R&D Systems, Minneapolis, MN, USA) was used as a primary antibody at 5 μg/ml and immunohistochemical staining was conducted using a Leica BOND-MAX (Leica). We confirmed that immunohistochemistry for ACE2 expression as performed above detected strong membranous expression of ACE2 in small intestinal epithelia, a positive control [17] (data not shown). ACE2 membranous staining was scored based on intensity (0, none; 1, weak; 2, intermediate; 3, strong) and proportion of carcinoma cells expressing the molecule (0, none; 1, <1/100; 2, 1/100–1/10; 3, 1/10–1/3; 4, 1/3–2/3; 5, >2/3) using the Allred scoring method [20].
2.7. RNA interference assay
Cells (2 × 105) were plated in 6-well plates, grown for 24 h, and then transfected with AccuTarget Negative Control (NC) siRNA duplexes, or two AccuTarget Predesigned siRNA duplexes targeting ACE2 (#1001297 and #1001300, named ACE2 siRNA #1 and #2, respectively, in this study; Bioneer, Daejeon, Korea) using Lipofectamine RNAiMAX Reagent and OPTI-MEM I (Invitrogen, Thermo Fisher Scientific) according to the manufacturer's recommendations. Downregulation of the targeted gene's expression was verified by flow cytometer using a FACS-Calibur (BD Biosciences) with the H8R64 mAb.
2.8. Quantitative RT-PCR
Total cellular RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), and 1 μg of total RNA was reverse transcribed into cDNA using the ReverTra Ace qPCR RT Master Mix with gDNA Remover (Toyobo, Osaka, Japan) according to the manufacturer's instructions. Real-time PCR was performed using a combination of the QuantiTect Primer Assay (Qiagen) and THUNDERBIRD SYBR qPCR Mix (Toyobo) in a LightCycler Nano (Roche Applied Science). The amount of β-actin mRNA (Hs_ACTB_1_SG) in each sample was used to standardize the quantity of ACE2 mRNA (Hs_ACE2_1_SG). The relative ACE2 mRNA expression levels between HCC827 parental cells and GR2 cells were calculated by the difference of the threshold cycle (comparative ΔΔCT method) and presented as the average of triplicate experiments. We also conducted conventional RT-PCR for ACE2 expression using the same PCR primers.
2.9. Statistical analysis
Differences in cell viability between NC siRNA or ACE2 siRNA transfected HCC827 GR2 cells treated with the H8R64 mAb-DT3C conjugate were evaluated by paired t-tests. Differences in cell viability between HCC827 parental and GR2 cells treated with the same conjugate, and in expression levels of ACE2 mRNA between HCC827 parental and GR2 cells were evaluated by unpaired t-tests. A P-value less than 0.05 was considered significant. All statistical calculations were performed with JMP software (JMP for Windows version 7; SAS Institute Japan; Tokyo, Japan).
3. Results
3.1. Establishment of anti-ACE2 mAb H8R64
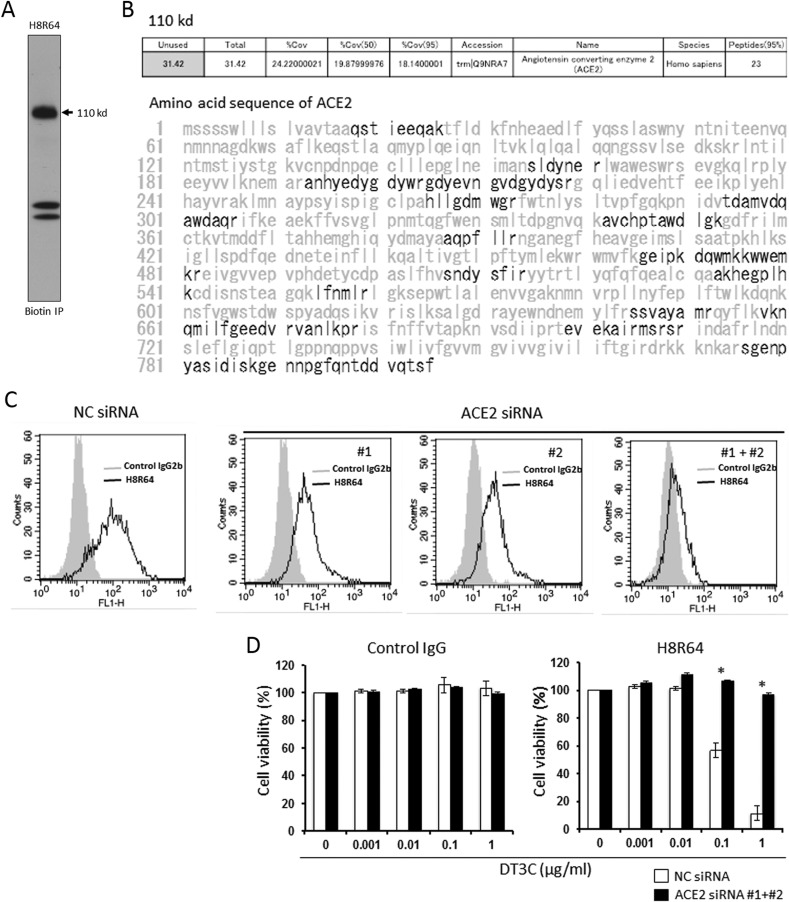
We cloned hybridomas from wells where Ab-DT3C conjugates had substantially decreased the viability of HCC827 GR2 cells based on the premise that cell viability in each well reflected the efficiency of internalization of Ab-DT3C conjugates into the cells. We consequently established a hybridoma secreting mAb H8R64 that recognized a protein of ∼110 kDa under reducing conditions (Fig. 1 A). Mass spectrometry identified ACE2 as a possible candidate of the 110 kDa molecule (Fig. 1B), and the expression of the target protein was clearly reduced by RNA interference for ACE2 (Fig. 1C), which collectively indicated that the mAb termed H8R64 bound to ACE2. In addition, although H8R64-DT3C conjugates substantially induced cell death in NC siRNA-transfected HCC827 GR2 cells as expected, the cells became markedly resistant to the treatment and survived when ACE2 was knocked down by siRNA (Fig. 1D).
Fig. 1.
Development of a new anti-ACE2 mouse mAb H8R64. (A) Lysates of HCC827 GR2 cells were immunoprecipitated with mAb H8R64. The band at 110 kDa clearly appears under reducing conditions. (B) Identification of the 110 kDa band as ACE2 using mass spectrometry. Boldface type indicates the sequence of the detected peptides. (C) Knockdown experiments using ACE2 siRNAs (10 nM each) confirm that the molecule mAb H8R64 recognizes is ACE2. (D) Viability of HCC827 GR2 cells that were transfected with NC siRNA or ACE2 siRNA (10 nM each), grown for 72 h, and then incubated with control IgG-DT3C conjugate or H8R64-DT3C conjugate for another 72 h. Results are presented as means ± SD from triplicate cultures. *P < 0.05.
3.2. HCC827 parental cells barely express ACE2
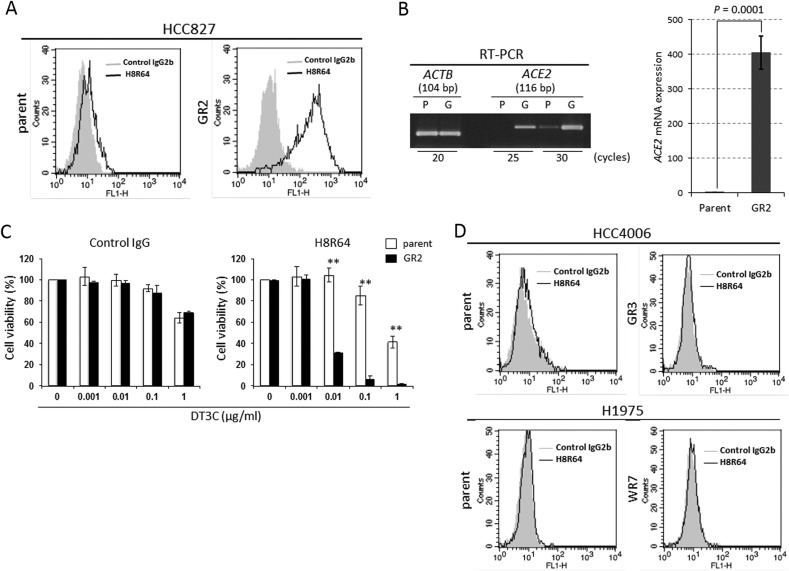
HCC827 GR2 cells clearly expressed ACE2 whereas its parental cells barely expressed the molecule both at the protein and mRNA levels (Fig. 2 A + B). In addition, the parental cells were significantly more resistant to H8R64-DT3C conjugates than GR2 cells (Fig. 2C), supporting the concept that ACE2 expression in HCC827 parental cells was limited. These findings led us to examine whether and to what extent TKI-resistant, mesenchymal EGFR-mutant HCC4006 and H1975 cells or their parental cells expressed ACE2. However, it was not expressed in the TKI-resistant or parental cells (Fig. 2D).
Fig. 2.
HCC827 parental cells barely express ACE2. (A) Flow cytometry results show that ACE2 is clearly expressed at the protein level in HCC827 GR2 cells while it is barely expressed in the parental cells. (B) Conventional and quantitative RT-PCR for the expression of ACE2 mRNA in HCC827 parental and GR2 cells. bp, base pair; P, parent; G, GR2. (C) Viability of HCC827 parental cells and GR2 cells incubated with control IgG-DT3C conjugate or H8R64-DT3C conjugate for 72 h. Results are presented as means ± SD from triplicate cultures. **P < 0.01. (D) Flow cytometry results reveal that ACE2 is hardly expressed in parental cells or TKI-resistant cells derived from HCC4006 and H1975 cells.
3.3. Primary EGFR-mutant lung adenocarcinomas contain cancer cells that express higher levels of ACE2 compared with normal lung epithelial cells
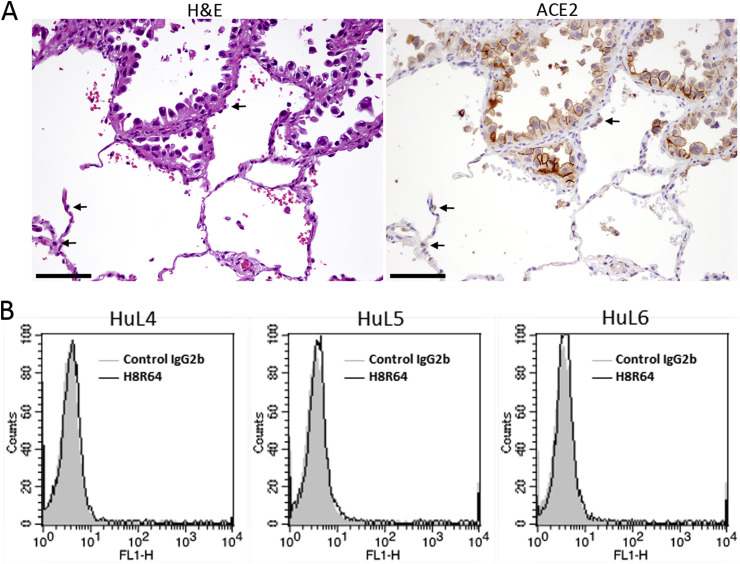
Most of the TKI-naïve EGFR-mutant lung adenocarcinomas examined contained carcinoma cells that showed membranous expression of ACE2 (Table 1; Fig. 3 A). On the other hand, normal bronchial or alveolar epithelial cells stained negative for or barely expressed ACE2 (Fig. 3A), although these cells were previously reported to express the molecule [10]. Moreover, the HuL4–6 cells, normal human alveolar epithelial cells [18], barely expressed ACE2 by flow cytometer (Fig. 3B). Although not all carcinoma cells expressed ACE2 even in ACE2-positive tumors, the expression level of ACE2 in EGFR-mutant carcinoma cells seemed much higher than that in normal lung epithelial cells.
Fig. 3.
ACE2 is expressed in EGFR-mutant lung adenocarcinoma tissues. (A) Representative images of H&E staining and ACE2 immunohistochemistry of a primary EGFR-mutant lung adenocarcinoma tissue (patient 5). The arrows indicate normal alveolar epithelial cells that weakly express ACE2. Scale bars, 100 μm. (B) Flow cytometry results showing that ACE2 is barely detected in HuL4–6 cells.
4. Discussion
We have developed a new anti-ACE2 mouse mAb H8R64 that is internalized by ACE2-expressing cells. We found that ACE2 was expressed to a greater degree in TKI-resistant, EGFR-mutant HCC827 GR2 cells with an EMT phenotype than in their parental cells. If an ADC consisting of a humanized mAb based on H8R64 and a potent anticancer drug were produced, the new ADC could induce cell death efficiently only in cancer cells expressing ACE2. If HCC4006 GR3 cells and H1975 WR7 cells, mesenchymal EGFR-mutant lung adenocarcinoma sublines, expressed ACE2, we could see it as a promising therapeutic target to fully eradicate EGFR-mutant cancer cells. Unfortunately, neither of these two sublines expressed ACE2. However, we have also demonstrated in this study that TKI-naïve EGFR-mutant lung adenocarcinoma tissues mostly express ACE2 at least partially, and that the expression levels of ACE2 in carcinoma cells appears to be higher than those in normal lung bronchial or alveolar epithelial cells. These findings suggest that ADCs incorporating anti-ACE2 mAb could induce cell death in EGFR-mutated cancer cells more efficiently than in normal lung epithelia. It remains to be established whether ACE2-positive EGFR-mutant lung cancer cells resist EGFR-TKIs and survive TKI treatment.
As mentioned earlier, ACE2 is a lung-protective molecule against SARS-CoV or influenza virus-induced acute lung injury [11], [15]. It thus seems dangerous to suppress the function(s) of ACE2 in the lung. However, we have shown here that TKI-naïve EGFR-mutant lung adenocarcinomas mostly contain cancer cells expressing ACE2 to a greater extent than normal lung epithelia, and that mesenchymal, TKI-resistant HCC827 GR2 cells strongly express the molecule, suggesting that ADCs incorporating anti-ACE2 mAb could be another therapeutic option for EGFR-mutant lung cancers and might be able to suppress their growth.
Conflict of interest statement
None of the authors of the present study have a conflict of interest to declare.
Acknowledgments
This work was supported in part by Grants-in-Aid for Scientific Research from JSPS (15K06876 for MY; 15K08364 for YS).
References
- 1.Pao W., Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nat. Rev. Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong C.R., Jänne P.A. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat. Med. 2013;19:1389–1400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sequist L.V., Waltman B.A., Dias-Santagata D D. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3002003. 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakuma Y., Matsukuma S., Nakamura Y. Enhanced autophagy is required for survival in EGFR-independent EGFR-mutant lung adenocarcinoma cells. Lab. Invest. 2013;93:1137–1146. doi: 10.1038/labinvest.2013.102. [DOI] [PubMed] [Google Scholar]
- 5.Sakuma Y., Nishikiori H., Hirai S. Prolyl isomerase Pin1 promotes survival in EGFR-mutant lung adenocarcinoma cells with an epithelial-mesenchymal transition phenotype. Lab. Invest. 2016;96:391–398. doi: 10.1038/labinvest.2015.155. [DOI] [PubMed] [Google Scholar]
- 6.Panowski S., Bhakta S., Raab H. Site-specific antibody drug conjugates for cancer therapy. MAbs. 2014;6:34–45. doi: 10.4161/mabs.27022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamaguchi M., Nishii Y., Nakamura K. Development of a sensitive screening method for selecting monoclonal antibodies to be internalized by cells. Biochem. Biophys. Res. Commun. 2014;454:600–603. doi: 10.1016/j.bbrc.2014.10.133. [DOI] [PubMed] [Google Scholar]
- 8.Nishii Y., Yamaguchi M., Kimura Y. A newly developed anti-Mucin 13 monoclonal antibody targets pancreatic ductal adenocarcinoma cells. Int. J. Oncol. 2015;46:1781–1787. doi: 10.3892/ijo.2015.2880. [DOI] [PubMed] [Google Scholar]
- 9.Jiang F., Yang J., Zhang Y. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat. Rev. Cardiol. 2014;11:413–426. doi: 10.1038/nrcardio.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hamming I., Timens W., Bulthuis M.L. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuba K., Imai Y., Rao S. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jia H.P., Look D.C., Tan P. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z., Huang X.R., Chen H.Y. Loss of angiotensin-converting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy. Lab. Invest. 2012;92:650–661. doi: 10.1038/labinvest.2012.2. [DOI] [PubMed] [Google Scholar]
- 14.Meng Y., Yu C.H., Li W. Angiotensin-converting enzyme 2/angiotensin-(1-7)/Mas axis protects against lung fibrosis by inhibiting the MAPK/NF-κB pathway. Am. J. Respir. Cell Mol. Biol. 2014;50:723–736. doi: 10.1165/rcmb.2012-0451OC. [DOI] [PubMed] [Google Scholar]
- 15.Zou Z., Yan Y., Shu Y. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat. Commun. 2014;5:3594. doi: 10.1038/ncomms4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tao L., Qiu Y., Fu X. Angiotensin-converting enzyme 2 activator diminazene aceturate prevents lipopolysaccharide-induced inflammation by inhibiting MAPK and NF-κB pathways in human retinal pigment epithelium. J. Neuroinflammation. 2016;13:35. doi: 10.1186/s12974-016-0489-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlén M., Fagerberg L., Hallström B.M. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi M., Hirai S., Tanaka Y. Fibroblastic foci, covered with alveolar epithelia exhibiting epithelial-mesenchymal transition, destroy alveolar septa by disrupting blood flow in idiopathic pulmonary fibrosis. Lab. Invest. 2017;97:232–242. doi: 10.1038/labinvest.2016.135. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki K., Nakamura K., Kato K. Exploration of target molecules for prostate cancer gene therapy. Prostate. 2007;67:1163–1173. doi: 10.1002/pros.20613. [DOI] [PubMed] [Google Scholar]
- 20.Allred D.C., Harvey J.M., Berardo M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998;11:155–168. [PubMed] [Google Scholar]