Highlights
► Cellular membrane and viral cholesterol are important for the PRRSV infection on MARC-145 cells. ► Cholesterol depletion affects PRRSV entry. ► Cholesterol depletion did not change expression levels of CD163 in MARC-145 cells. ► Cholesterol depletion disturbs lipid-raft-dependent endocytosis.
Keywords: PRRSV, MARC-145, Lipid rafts, Cholesterol, Infection
Abstract
Lipid rafts play an important role in the life cycle of many viruses. Cholesterol is a critical structural component of lipid rafts. Although the porcine reproductive and respiratory syndrome virus (PRRSV) has restricted cell tropism for cells of the monocyte/macrophage lineage, a non-macrophage cell MARC-145 was susceptible to PRRSV because of the expression of virus receptor CD163 on the cell surface, therefore MARC-145 cells is used as model cell for PRRSV studies. In order to determine if cholesterol is involved in PRRSV infection in MARC-145 cells, we used three pharmacological agents: methyl-β cyclodextrin (MβCD), mevinolin, and filipin complex to deplete cholesterol in MARC-145. Although these agents act by different mechanisms, they all significantly inhibited PRRSV infection. The inhibition could be prevented by addition of exogenous cholesterol. Cell membrane cholesterol depletion after virus infection had no effect on PRRSV production and cholesterol depletion pre-infection did not reduce the virus attachment, suggesting cholesterol is involved in virus entry. Further results showed that cholesterol depletion did not change expression levels of the PRRSV receptor CD163 in MARC-145, had no effect on clathrin-mediated endocytosis, but disturbed lipid-raft-dependent endocytosis. Collectively, these studies suggest that cholesterol is critical for PRRSV entry, which is likely to be mediated by a lipid-raft-dependent pathway.
1. Introduction
Lipid rafts are lipid microdomains enriched in cholesterol and sphingolipids. Cholesterol is a critical structural component of lipid rafts. Cholesterol depletion leads to disorganization of lipid raft microdomains and dissociation of bound proteins [1], [2]. Lipid rafts play important roles in many cellular processes such as signal transduction, kinase activity modulation, cell migration and axonal guidance [3], [4], [5], [6]. They are also involved in multiple stages of the virus life cycle, including viral entry and fusion, viral protein transport and targeting, and viral assembly and budding [7], [8], [9], [10], [11].
The porcine reproductive and respiratory syndrome virus (PRRSV), an enveloped, positive-stranded RNA virus, belongs to the family Arteriviridae, order Nidovirales [12]. PRRSV has restricted cell tropism for cells of the monocyte/macrophage lineage [13]. Among non-macrophage cells, only the MA-104 cell line derived from African green monkey kidney and its replication derivative, MARC-145, were susceptible to PRRSV [14]. Therefore MARC-145 cell is the most used cell in PRRSV studies. The virus enters the target cells via receptor-mediated endocytosis, and the sialoadhesin and scavenger receptor CD163 are known as PRRSV entry mediators. However, MARC-145 cells do not express sialoadhesin [15].
In order to study if cholesterol is involved in PRRSV infection in MARC-145 cells, we used three pharmacological agents, MβCD, mevinolin, and filipin complex to deplete cellular cholesterol. We found that intact cell membrane cholesterol was indispensable for PRRSV entry into MARC-145 cells. Further results showed that cholesterol depletion did not change the expression level of CD163 on the surface of MARC-145 cells. Our data also showed that the uptake of transferrin, a marker for clathrin-mediated endocytosis, was not affected by cholesterol depletion, while cholera toxin subunit B, a marker for the presence of lipid rafts, was no longer efficiently internalized in the presence of MβCD. These results suggested that lipid-raft-dependent endocytosis is required for PRRSV infection.
2. Materials and methods
2.1. Cell culture and virus strains
MARC-145 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, USA), supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml). Porcine reproductive and respiratory syndrome virus (PRRSV-VR2332, North America strain) was propagated in MARC-145, and the cells were grown at 37 °C in a humidified incubator containing 5% CO2. The 50% tissue culture infectious dose (TCID50) was determined by serial titration of virus in MARC-145 cells.
2.2. Cholesterol depletion and replenishment on virus infection
To study the effects of cholesterol depletion on PRRSV infection, MARC-145 cells were washed three times with phosphate-buffered saline (PBS), and then were treated with 0, 1, 2 and 3 mM MβCD for 1 h before viral adsorption or throughout infection period (−1 to 24 h). The culture supernatant was collected and viral titers were measured by TCID50 and the cells infected with PRRSV were assayed using indirect immunofluorescent assay 24 hpi.
In order to pinpoint the stage at which cell membrane cholesterol was required for PRRSV infection, we used three different pharmacological agents to deplete cholesterol. MARC-145 cells were washed three times with PBS, and left in the absence or the presence of either serum-free DMEM containing varying concentrations of MβCD (Sigma) or filipin complex (Sigma) for 1 h, or serum-free DMEM containing varying concentrations of mevinolin (Sigma) for 48 h at 37 °C. As mevinolin and filipin complex were dissolved in DMSO (MβCD was dissolved in water), the control cells were also incubated in medium with the same amount of DMSO as used for mevinolin and filipin complex. After treatment, the cells were washed three times with PBS before flow cytometry analysis or PRRSV infection.
For cholesterol replenishment, MARC-145 cells were first pretreated with 0 or 10 mM MβCD for 1 h at 37 °C, and then supplemented with or without exogenous cholesterol (400 μg/ml) in DMEM and incubated for 1 h at 37 °C. After extensive washing with PBS, the cells were subjected to PRRSV infection.
2.3. Cell viability assays and cholesterol quantification
Cell viability was assessed by the MTT assay. Briefly, MARC-145 cells cultured in 96-well plates were first treated with varying concentrations of cholesterol depletion agents for different times at 37 °C (0, 1, 2 and 3 mM MβCD for 24 h; 0, 5, 10 and 20 mM MβCD or 0, 0.5, 1, 2 μg/ml filipin for 1 h; 0, 1, 2 and 4 μM mevinolin in serum-free DMEM for 48 h), and then 200 μl DMEM containing 20 μl MTT (5 mg/ml) was added to each well and incubated for 4 h at 37 °C. The culture medium was then removed and 100 μl DMSO was added to each well, and the plate was agitated on orbital shaker for 15 min. The light absorbance to each sample was measured at 570 nm with an enzyme-linked immunosorbent assay reader (East China Vacuum Tube Manufacturer, Nanjing, China).
To quantify cellular cholesterol levels, cholesterol depletion agents treated or control cells were washed once in PBS. The cells were then resuspended in the Amplex Red reaction buffer and vortexed. The amount of cholesterol was assayed with the Amplex Red cholesterol assay kit (Molecular Probes) according to the manufacturer’s instructions.
2.4. Virus titration
MARC-145 cells were seeded into 96-well plates 24 h before infection. Supernatants were diluted 10-fold serially. MARC-145 cells were infected by 100 μl/well of serial diluted supernatant in quintuplicate. Five days post-infection, the 50% tissue culture infected dose (TCID50) was determined by the Reed–Muench method [16].
2.5. Quantitative real-time PCR
Total mRNA was extracted from MARC-145 cells using Trizol RNA extract reagent (Takara Bio Inc., Japan) according to the manufacturer’s instructions. Reverse transcription was carried out by using Reverse Transcriptase M-MLV (Takara Bio Inc., Japan). Quantitative real-time PCR was performed and products were detected using an ABI PRISM 7300 sequence detection system (Applied Biosystems) according to Premix Ex Taq™’s instruction (Takara Bio Inc., Japan). The data were analyzed with ABI PRISM 7300SDS software. Each sample was run in triplicates. The relative amount of mRNA for target gene was normalized to β-Actin mRNA in the same sample. The primer sequences used are shown in Table 1 .
Table 1.
The primer sequences used for real-time PCR.
| Gene | Primer sequence (5′ to 3′) | |
|---|---|---|
| ORF7 | Forward | TTGTGTCTGTCGTCGATCCAG |
| Reverse | AAACTCCACAGTGTAACTTATCCTC | |
| Probe | (FAM) CGCTGGAACTTGTGCCCTGTCA (Eclipse) | |
| β-Actin | Forward | TGACTGACTACCTCATGAAGATCC |
| Reverse | TCTCCTTAATGTCACGCACGATT | |
| Probe | (FAM) CGGCTACAGCTTCACCACCACGGC (Eclipse) | |
2.6. Indirect immunofluorescence assay (IFA)
After 24 h infection, MARC-145 cells were fixed in cold ethanol for 30 min, washed with PBS (137 mM NaCl, 7 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl; pH 7.4), and then incubated for 1 h with 100 μl monoclonal antibody (McAb) against the N protein of PRRSV (1:100) at 37 °C. Unbound antibody was washed away with PBS containing 0.1% Tween-20. An optimum dilution (1:100) of Fluorescein (FITC)-conjugated AffiniPure Goat Anti-Mouse IgG (H + L) (Boster Bio-Tech Co. Ltd., Wuhan, China) was added and incubated for 45 min at 37 °C. After extensive washing with PBS in the dark, the cells were observed under a fluorescence microscope (Zeiss Axiovert 200).
2.7. Flow cytometry
Detached MARC-145 cells were washed once with PBS, fixed with 4% paraformaldehyde for 15 min at room temperature, and stained with mouse anti-human CD163 protein (Abcam, USA) or mouse anti-N protein of PRRSV for 1 h at 4 °C. The cells were washed with PBS and incubated with Fluorescein (FITC)-conjugated AffiniPure Goat Anti-Mouse IgG (H + L) (Boster Bio-Tech Co. Ltd., Wuhan, China) for 30 min. Unbound secondary antibody was washed away with PBS. The cells were resuspended in 200 μl PBS, and analyzed on a FACSCalibur cytometer (Becton Dickinson Immunocytometry Systems). At least 10,000 cells were analyzed for each sample.
2.8. Immunofluorescence confocal microscopy
MARC-145 cells were pretreated with 0 or 10 mM MβCD for 1 h, and then incubated in 10 μg/ml Fluorescein (FITC)-conjugated cholera toxin subunit B or 50 μg/ml transferrin in the presence of 0 or 10 mM MβCD for 30 min. After extensive washing with PBS, cells were fixed with 4% paraformaldehyde for 30 min at 37 °C followed by incubating with 4′,6′-diamidino-2-phenylindole (DAPI) for 10 min at room temperature to stain the cell nuclei. Cells were processed for immunofluorescence analysis using a confocal microscope.
2.9. Statistical analysis
All statistical analyses were performed by one-way ANOVA using a SPSS 16.0 software package (version 16.0, SPSS Inc., Chicago, IL, USA). Data were expressed as the mean ± standard deviation (SD). A value of P < 0.05 was considered as statistically significant.
3. Results
3.1. Cholesterol depletion reduces PRRSV infection
In order to study whether cholesterol plays a role in PRRSV infection in MARC-145 cells, we first used MβCD, which is widely used to sequester cholesterol from the plasma membrane. MARC-145 cells were treated with 0, 1, 2 and 3 mM MβCD for 1 h before viral adsorption or throughout infection (−1 to 24 h). After a 24 h infection period, the culture supernatant was collected and viral titers were measured by TCID50, and the infected cells were assayed using IFA with monoclonal antibody against N protein of PRRSV. The viral titers (Fig. 1 A) and numbers of fluorescein-stained cells (Fig. 1B) decreased in a dose-dependent manner on the cells pretreated with increasing concentrations of MβCD. The cell toxicity of MβCD on MARC-145 cells was measured by using MTT assays. The results showed that there was no significant difference in cell viability between the untreated cells and the cells treated with MβCD at concentrations of up to 3 mM for 24 h (Fig. 1C). The cholesterol level in MβCD-treated MARC-145 cells was determined using the Amplex Red cholesterol quantification assay. As shown in Fig. 1D, the cholesterol level in the cells decreased about 26%, 44% and 76% after treated with 1, 2 and 3 mM MβCD for 24 h, respectively, when compared to mock-treated cells.
Fig. 1.
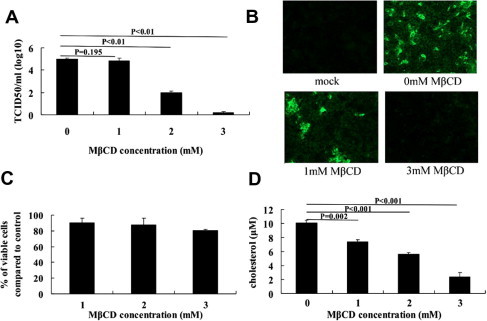
Depletion of cholesterol by MβCD inhibits PRRSV infection in MARC-145 cells. MARC-145 cells were pretreated with 0, 1, 2 and 3 mM MβCD for 1 h, followed by PRRSV infection in the presence of various concentrations of MβCD. After a 24 h infection period, the culture supernatants were harvested for viral titration using the TCID50 assay (A), and the PRRSV infection cells were assayed by IFA using monoclonal antibody against N protein (green) (B). Cell viability was determined using MTT assay (C) and cholesterol levels were measured with Amplex Red cholesterol assay kit (D). The averages from three independent experiments with standard deviation error bars are shown. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
3.2. Cholesterol depletion of MARC-145 cells inhibits PRRSV entry but has no effect on PRRSV binding
To pinpoint the stage at which cell membrane cholesterol is required for PRRSV infection, we first examined if cholesterol depletion affects PRRSV binding to the cells. MARC-145 cells were first treated with 0, 5, 10 and 20 mM MβCD for 1 h. After extensive washing with PBS, the cells were incubated on ice with PRRSV at MOI of 1.0 for 90 min. Unbound virus was washed away, and virus binding was assayed by flow cytometry and real-time PCR. As shown in Fig. 2 A and 2B, the amount of bound virions and virus specific genome copy number did not decrease, which inferred cholesterol depletion has no effects on virus attachment to the cells. We observed no major cytotoxicity in MβCD-treated cells (Fig. S1A) and the cholesterol level decreased about 20%, 50.5% and 65.1% after treatment with 5, 10 and 20 mM MβCD, respectively, compared to mock-treated cells (Fig. S1B).
Fig. 2.
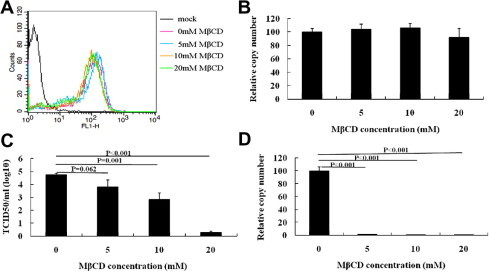
Depletion of cholesterol by MβCD affects PRRSV entry, but has no effect on binding in MARC-145 cells. For binding assays, MARC-145 cells were pretreated with 0, 5, 10 and 20 mM MβCD for 1 h, and then were inoculated with PRRSV at an MOI of 1.0 at 4 °C. After 90 min, the infection was either analyzed by FACS analysis using anti-N antibodies (A) or real-time PCR analysis of the PRRSV genome (B). For entry studies, the cells were shifted to 37 °C followed by washing with PBS. After 24 h, virus titer and virus reverse transcription products were detected using TCID50 (C) and real-time PCR (D), respectively. Data represent mean ± SD of results obtained with three independent experiments.
To examine whether cholesterol depletion affects PRRSV entry into MARC-145 cells, the cells were first pretreated with 0, 5, 10 and 20 mM MβCD for 1 h, and then incubated on ice with PRRSV at MOI of 1.0 for 90 min. The cells were shifted to 37 °C after unbound virus was washed away. After 24 h, virus in the supernatant was determined by TCID50 (Fig. 2C) and the genome copy number of PRRSV in MARC-145 cells was detected using real-time PCR (Fig. 2D). The data showed both viral production and genome copy numbers of PRRSV reduced in a dose-dependent manner in MβCD-treated cells. We observed a 99.8% reduction of PRRSV RNA in 20 mM MβCD-treated cells compared to that in untreated cells. The virus titers displayed significant difference in 0, 10, 20 mM MβCD-treated cells. These results suggested that cholesterol depletion inhibits PRRSV entry into MARC-145 cells.
3.3. Replenishment of envelope cholesterol restores PRRSV infection
Cholesterol replenishment experiments were performed to confirm the influence of cholesterol depletion on PRRSV infection. Briefly, MARC-145 cells were pretreated with 10 mM MβCD for 1 h, followed by addition of cholesterol to a final concentration of 400 μg/ml. The virus yield was investigated with the TCID50 assay (Fig. 3 A) and real-time PCR (Fig. 3B). As expected, PRRSV infection was partially restored by cholesterol supplementation, with the increase in both infectious viral production and viral protein expression.
Fig. 3.
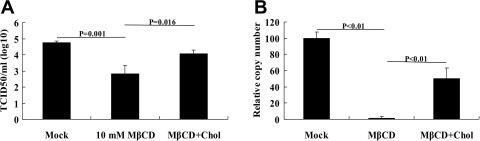
Exogenous cholesterol can partially restore virus infection. MARC-145 cells pretreated with 10 mM MβCD were supplemented with 400 μg/ml cholesterol before PRRSV infection. After infection for 24 h, viral titers were measured by TCID50 using the Reed–Muench method (A) and the copy number of PRRSV genome was analyzed using real-time PCR (B). Mock treated and MβCD treated without cholesterol supplemented cells were used as controls. Experiments were repeated three times, error bars represent deviation of the median from three experiments.
3.4. Cholesterol depletion at the post-entry stage has no effect on PRRSV production
In order to determine if cholesterol depletion affects the post-entry process in the PRRSV life cycle, MARC-145 cells were first infected with PRRSV for 6 h, and then treated with various concentrations of MβCD at 37 °C for 1 h. The genome copy numbers of PRRSV and viral titers were determined by real-time PCR (Fig. S1C) and TCID50 (Fig. S1D), respectively, at 18 h post-MβCD treatment (24 hpi). As shown in Fig. S1, a similar copy number (Fig. S1C) and TCID50 (Fig. S1D) for PRRSV were observed, likely supporting the notion that MβCD treatment at the post-entry stage has no inhibitory effect on virus production relative to that of untreated cells.
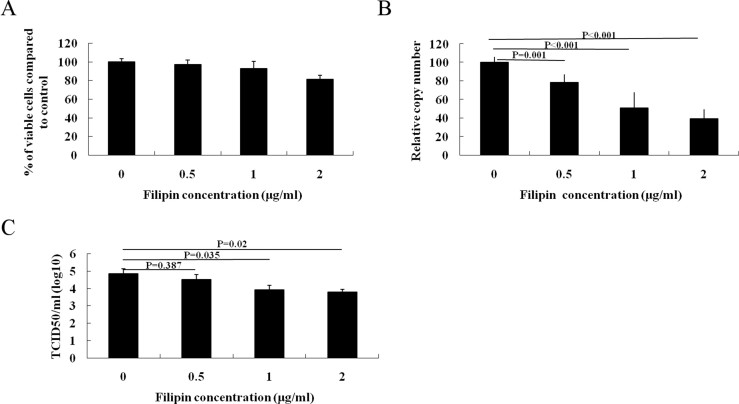
3.5. Chelating cholesterol with filipin complex inhibits PRRSV entry
To exclude any possible drug-specific effects of MβCD, we used another compound, filipin complex, to examine the role of cholesterol in PRRSV infection. Filipin complex is a polyene macrolide antibiotic that can insert into membranes and sequester cholesterol into complexes. At concentrations of 2 μg/ml, filipin complex did not compromise cell viability (Fig. S2A). PRRSV genome copies in infected cells after treatment with 2 μg/ml of filipin complex significantly reduced by 61% (Fig. S2B), and decreased titer of PRRSV was also observed (Fig. S2C). Cholesterol depletion by filipin complex did not affect PRRSV binding and its post-entry process (data not shown).
3.6. Inhibition of cholesterol synthesis inhibits PRRSV entry
Mevinolin is another lipid rafts depletion drug and it inhibits the 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase enzyme responsible for the production of mevalonate, a precursor in cholesterol biosynthesis. We first treated MARC-145 with mevinolin for 48 h in serum-free medium, and the cell viability and cholesterol level were determined. Up to 4 μM, cell viability was unaffected (Fig. S3A) and the cholesterol level reduced to 49.5% compared to mock-treated cells (Fig. S3B). Virus titers (Fig. S3C) and genome copies of PRRSV on mevinolin-treated cells (Fig. S3D) were reduced by 34-fold and 90%. However, no significant changes on virus attachment and post-entry stage were detected (data not shown).
3.7. Cholesterol depletion does not alter PRRSV receptor expression in MARC-145 cells
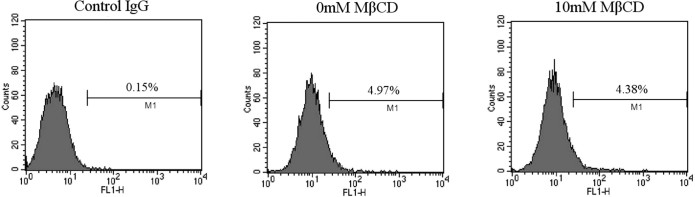
We next studied whether cholesterol depletion affects the expression level of CD163, we measured CD163 expression level by flow cytometry in MARC-145 cells pretreated or untreated with MβCD. As shown in Fig. S4, CD163 expression in MARC-145 cells pretreated with 10 mM MβCD was similar to that of on mock treated cells.
3.8. Cholesterol depletion affects lipid-raft-dependent pathway in MARC-145 cells
In order to determine if cholesterol depletion resulted in the inhibition of lipid-raft-dependent endocytosis in MARC-145 cells, we assessed the effect of cholesterol depletion on cholera toxin B (CTB) uptake. CTB is internalized in a lipid-raft-dependent manner after binding to its receptor GM1 ganglioside [17]. Our results showed that CTB internalization was blocked when MARC-145 cells were treated with MβCD, indicating lipid-raft-dependent endocytosis was compromised (Fig. 4 ). Furthermore, uptake of transferrin was assessed to determine if cholesterol depletion affects clathrin-mediated endocytosis [18]. MβCD treated cells were first incubated with fluorescent-labeled transferrin, and observed by confocal microscopy 20 min later. The results showed that transferrin uptake was not affected (data not shown). These data suggested that PRRSV infection is likely to be mediated by a lipid-raft-dependent pathway.
Fig. 4.
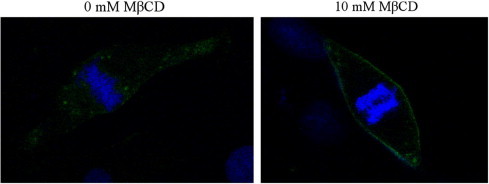
Cholesterol depletion affects cholera toxin B (CTB) uptake in MARC-145 cells. MARC-145 cells were treated with 0 or 10 mM MβCD for 1 h, prior to the internalization of FITC-conjugated CTB (shown in green) for 1 h. After washing with PBS, cells were fixed in 4% paraformaldehyde, and the nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole) (blue). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this paper.)
4. Discussion
Increasing evidences suggest that cholesterol, a major component of lipid rafts, is involved in virus infectivity and replication [19], [20], [21], [22]. As viruses belong to diverse families, the involvement of cholesterol in virus infection may represent an important feature in their life cycle.
The present work shows the importance of cholesterol on cellular membrane in establishing PRRSV infection in MARC-145 cells. We used three pharmacological agents to deplete the cholesterol in the cell membrane. The results showed that cholesterol depletion decreased the infection in a dose-dependent manner, and replenishment of cholesterol could partially restore viral infection. When a marked effect of cholesterol depletion on PRRSV entry was observed, no influence was seen on either virus binding or at the post-entry stage. These results are similar to the findings observed on HCoV-229E, MHV and SARS-CoV, which are also members of the order Nidovirales [23], [24], [25], [26].
CD163, a member of the scavenger receptor cysteine-rich family (SRCR), is exclusively expressed on cells of the monocyte lineage. Its expression in monocytes/macrophages is regulated by proinflammatory and antiinflammatory mediators [27]. CD163 has been shown to confer PRRSV-permissivity upon PRRSV non-susceptible cell lines, and is identified as a key molecule in PRRSV entry in MARC-145 cells [28], [29]. PRRSV entry occurs by binding to the outer cell membrane receptors followed by uptake into the cell via clathrin-dependent endocytosis [30]. Our results showed cholesterol depletion had no effect on the CD163 expression level.
Our studies on cellular uptake of transferrin, a marker for clathrin-mediated endocytosis, showed the localization of the fluorescently labeled transferrin did not significantly change in MARC-145 cells. To determine if cholesterol depletion inhibited lipid-raft-dependent endocytosis, uptake of FITC-conjugated cholera toxin subunit B was analyzed. In contrast to transferrin uptake, the localization of cholera toxin subunit B shifted from a mainly punctate cytoplasmic form to a predominantly plasma membrane location after treatment of cells with MβCD or filipin compared to untreated cells. These results suggested that cholesterol depletion disturbs lipid-raft-dependent endocytosis, but has no effect on clathrin-mediated endocytosis. Thus, we suspect that cholesterol depletion influences PRRSV entry by disturbing lipid-raft-dependent pathway in MARC-145 cells.
In conclusion, our data demonstrate the essential role of cholesterol in PRRSV entry in MARC-145 cells. However, due to aberrant and unexpected expression of CD163 in the non-macrophage MARC-145 cell line, the detailed mechanisms of CD163-mediated viral infection are yet to be fully explored. Future work has to address how cholesterol facilitates PRRSV entry, which will lead to the development of effective antiviral drug therapies that interfere with CD163 localization in lipid rafts and raft-mediated entry.
Acknowledgments
This project was funded by the priority academic program development of Jiangsu Higher Education Institutions. We thank Dr. Kevin D. Ridge, Dr. Eva Ramon and Dr. Xi Mo for revising the language of this manuscript and critical discussion of the experiments. We also thank Guoqing Huang for the assistance on flow cytometry experiments.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bbrc.2011.09.109.
Appendix A. Supplementary data
Supplementary Fig. 1.

MARC-145 cells were treated with 0, 5, 10, 20 mM MβCD for 24 h. Cell viability was determined using MTT assay (A) and cholesterol levels were measured with Amplex Red cholesterol assay kit (B). For PRRSV infection, MARC-145 cells infected with PRRSV for 6 h, and then treated with 0, 5, 10, 20 mM MβCD for 1 h. The virus genome was determined by real-time PCR(C) and the supernatants were harvested for viral titration using TCID50 assay after culturing another 18 h (D). Data represent mean ± SD of results obtained with three independent experiments.
Supplementary Fig. 2.
Filipin inhibit PRRSV entry into MARC-145 cells. (A) Cell viability was determined in the mock-infected MARC-145 cells treated with filipin (0, 0.5, 1, 2 μg/ml) for 1 h. (B and C) MARC-145 cells were pretreated with 0, 0.5, 1, 2 μg/ml filipin for 1 h, and then were inoculated with PRRSV at MOI of 1 at 4 °C. Ninety minutes later, the cells were shifted to 37 °C followed by washing with PBS. After incubation in 37 °C for 24 h, virus reverse transcription products and virus titer were detected using real-time PCR (B) TCID50 (C), respectively. Data represent mean ± SD of results obtained with three independent experiments.
Supplementary Fig. 3.
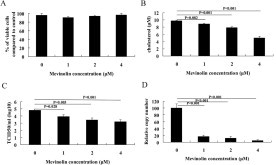
Mevinolin inhibit PRRSV entry into MARC-145 cells. (A) Cell viability and the effect of cholesterol level (B) were determined in the mock-infected MARC-145 cells treated with mevinolin (0, 1, 2, 4 μM) for 48 h. (C and D) MARC-145 cells were pretreated with 0, 1, 2, 4 μM mevinolin for 48 h, and then were inoculated with PRRSV at MOI of 1 at 4 °C. Ninety minutes later, the cells were shifted to 37 °C followed by washing with PBS. After incubation in 37 °C for 24 h, virus titer and virus reverse transcription products were detected using TCID50 (C) and real-time PCR (D), respectively. Data represent mean ± SD of results obtained with three independent experiments.
Supplementary Fig. 4.
Cholesterol depletion does not alter CD163 expression in MARC-145 cells. MARC-145 cells were treated with 0 or 10 mM MβCD, and then CD163 expression in MARC-145 cells was detected by FACS analysis.
References
- 1.Hannan L.A., Edidin M. Traffic, polarity, and detergent solubility of a glycosylphosphatidylinositol-anchored protein after LDL-deprivation of MDCK cells. J. Cell Biol. 1996;133:1265–1276. doi: 10.1083/jcb.133.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J., Pekosz A., Lamb R.A. Influenza virus assembly and lipid raft microdomains: a role for the cytoplasmic tails of the spike glycol-proteins. J. Virol. 2000;74:4634–4644. doi: 10.1128/jvi.74.10.4634-4644.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palazzo A.F., Eng C.H., Schlaepfer D.D., Marcantonio E.E., Gundersen G.G. Localized stabilization of microtubules by integrin- and FAK-facilitated Rho signaling. Science. 2004;303:836–839. doi: 10.1126/science.1091325. [DOI] [PubMed] [Google Scholar]
- 4.Young R.M., Holowka D., Baird B. A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J. Biol. Chem. 2003;278:20746–20752. doi: 10.1074/jbc.M211402200. [DOI] [PubMed] [Google Scholar]
- 5.Manes S., Mira E., Gomez-Mouton C., Zhao Z.J., Lacalle R.A., Martinez A.C. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol. Cell. Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierini L.M., Eddy R.J., Fuortes M., Seveau S., Casulo C., Maxfield F.R. Membrane lipid organization is critical for human neutrophil polarization. J. Biol. Chem. 2003;278:10831–10841. doi: 10.1074/jbc.M212386200. [DOI] [PubMed] [Google Scholar]
- 7.Nayak D.P., Barman S. Role of lipid rafts in virus assembly and budding. Adv. Virus Res. 2002;77:1977–1983. doi: 10.1016/s0065-3527(02)58001-5. [DOI] [PubMed] [Google Scholar]
- 8.Ono A., Freed E.O. Role of lipid rafts in virus replication. Adv. Virus Res. 2005;64:311–358. doi: 10.1016/S0065-3527(05)64010-9. [DOI] [PubMed] [Google Scholar]
- 9.Schmitt A.P., Lamb R.A. Influenza virus assembly and budding at the viral budozone. Adv. Virus Res. 2005;64:383–416. doi: 10.1016/S0065-3527(05)64012-2. [DOI] [PubMed] [Google Scholar]
- 10.Bender F.C., Whitbeck J.C., Ponce de Leon M., Lou H., Eisenberg R.J., Cohen G.H. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 2003;77:9542–9552. doi: 10.1128/JVI.77.17.9542-9552.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medigeshi G.R., Hirsch A.J., Streblow D.N., Nikolich-Zugich J., Nelson J.A. West Nile virus entry requires cholesterol-rich membrane microdomains and is independent of alphavbeta3 integrin. J. Virol. 2008;82:5212–5219. doi: 10.1128/JVI.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conzelmann K.K., Visser N., Van Woensel P., Thiel H.J. Molecular characterization of porcine reproductive and respiratory syndrome virus, a member of the arterivirus group. Virology. 1993;193:329–339. doi: 10.1006/viro.1993.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vanderheijden N., Delputte P.L., Favoreel H.W., Vandekerckhove J., Van Damme J., van Woensel P.A., Nauwynck H.J. Involvement of sialoadhesin in entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macro-phages. J. Virol. 2003;77:8207–8215. doi: 10.1128/JVI.77.15.8207-8215.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H.S., wang J.K., Yoon I.J., Joo H.S., Frey M.L. Enhanced replication of porcine reproductive and respiratory syndrome (PRRS) virus in a homogenous subpopulation of MA-104 cell line. Arch. Virol. 1993;133:477–483. doi: 10.1007/BF01313785. [DOI] [PubMed] [Google Scholar]
- 15.Delputte P.L., Nauwynck H.J. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J. Virol. 2004;78:8094–8101. doi: 10.1128/JVI.78.15.8094-8101.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reed L.J., Muench H. A simple method of estimating 50% endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 17.Fujinaga Y., Wolf A.A., Rodighiero C., Wheeler H., Tsai B., Allen L., Jobling M.G., Rapoport T., Holmes R.K., Lencer W.I. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulum. Mol. Biol. Cell. 2003;14:4783–4793. doi: 10.1091/mbc.E03-06-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanover J.A., Willingham M.C., Pastan I. Kinetics of transit of transferrin and epidermal growth factor through clathrin-coated membranes. Cell. 1984;39:283–293. doi: 10.1016/0092-8674(84)90006-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee C.J., Lin H.R., Liao C.L., Lin Y.L. Cholesterol effectively blocks entry of flavivirus. J. Virol. 2008;82:6470–6480. doi: 10.1128/JVI.00117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie J.M., Khromykh A.A., Parton R.G. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe. 2007;2:229–239. doi: 10.1016/j.chom.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Reyes-Del Valle J., Chavez-Salinas S., Medina F., Del Angel R.M. Heat shock protein 90 and heat shock protein 70 are components of dengue virus receptor complex in human cells. J. Virol. 2005;79:4557–4567. doi: 10.1128/JVI.79.8.4557-4567.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu Y.E., Cassese T., Kielian M. The cholesterol requirement for Sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J. Virol. 1999;73:4272–4278. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thorp E.B., Gallagher T.M. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 2004;78:2682–2692. doi: 10.1128/JVI.78.6.2682-2692.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi K.S., Aizaki H., Lai M.M. Murine coronavirus requires lipid rafts for virus entry and cell–cell fusion but not for virus release. J. Virol. 2005;79:9862–9871. doi: 10.1128/JVI.79.15.9862-9871.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li G.M., Li Y.G., Yamate M., Li S.M., Ikuta K. Lipid rafts play an important role in the early stage of severe acute respiratory syndrome-coronavirus life cycle. Microbes Infect. 2007;9:96–102. doi: 10.1016/j.micinf.2006.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nomura R., Kiyota A., Suzaki E., Kataoka K., Ohe Y., Miyamoto K., Senda T., Fujimoto T. Human coronavirus 229E binds to CD13 in rafts and enters the cell through caveolae. J. Virol. 2004;78:8701–8708. doi: 10.1128/JVI.78.16.8701-8708.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buechler C., Ritter M., Orso E., Langmann T., Klucken J., Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J. Leukoc. Biol. 2000;67:97–103. [PubMed] [Google Scholar]
- 28.Delrue I., Van Gorp H., Van Doorsselaere J., Delputte P.L., Nauwynck H.J. Research article susceptible cell lines for the production of porcine reproductive and respiratory syndrome virus by stable transfection of sialoadhesin and CD163. BMC Biotechnol. 2010;10:48. doi: 10.1186/1472-6750-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calvert J.G., Slade D.E., Shields S.L., Jolie R., Mannan R.M., Ankenbauer R.G., Welch S.K. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. J. Virol. 2007;81:7371–7379. doi: 10.1128/JVI.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nauwynck H.J., Duan X., Favoreel H.W., Van Oostveldt P., Pensaert M.B. Entry of porcine reproductive and respiratory syndrome virus into porcine alveolar macrophages via receptor-mediated endocytosis. J. Gen. Viral. 1999;80:297–305. doi: 10.1099/0022-1317-80-2-297. [DOI] [PubMed] [Google Scholar]