Abstract
Although multidrug therapy (MDT) has been widely used for the treatment of leprosy for nearly 40 y, the disease remains a public health concern in some areas. The early detection of leprosy cases is vital to interrupt Mycobacterium leprae transmission, but currently diagnosis is typically achieved during the recognition of clinical symptoms by professional staff performing physical examinations in conjunction with microbiological assessment of slit skin smears (SSSs) and histopathology. In the last 10 y, serum antibody detection tests have emerged to aid leprosy diagnosis. Here we evaluated the ability of antigens NDO-BSA and LID-1 (ML0405 and ML2331) and the conjugate of these, NDO-LID, to detect antibodies in the sera of 113 leprosy patients and 166 control individuals in Yunnan province in southwest China. We found that each antigen was readily detected by sera from multibacillary (MB) patients, with sensitivities of 97.3%, 97.3% and 98.6% for NDO-BSA, LID-1 and NDO-LID, respectively. Even among paucibacillary (PB) patients the antigens detected antibodies in 74.4%, 56.4% and 69.2% of serum samples, respectively. Receiver operating characteristics (ROC) curve analysis indicated that, irrespective of the leprosy case classification as MB or PB, the detection efficiency obtained with NDO-LID was better than that obtained with the other two antigens (with LID-1 being a slightly better than NDO-BSA). Our results indicate the utility of NDO-LID in assisting in the diagnosis of PB and MB leprosy patients and that these antibody detection assays represent powerful diagnostic tools. We suggest that could be implemented into the procedures of local health centres in leprosy-endemic regions to assist in earlier diagnosis.
Keywords: Leprosy, Antibody, Diagnosis, Antigen
Introduction
Leprosy, also known as Hansen’s disease, is one of the most ancient human diseases. It is a chronic granulomatous disease caused by infection with Mycobacterium leprae that manifests in the skin and peripheral nerve system.1 Determined by the host immune response against M. leprae and characterized by histopathology examination, leprosy can be divided into five distinct escalating presentations, ranging from true tuberculoid leprosy (TT) through borderline tuberculoid (BT), mid-borderline (BB), borderline lepromatous (BL) and lepromatous leprosy (LL).2 To simplify diagnosis and align treatment guidelines, the World Health Organization (WHO) recommends operational classification according to the number of skin lesions and involved nerves.3 Leprosy cases are thus defined as either paucibacillary (PB; including TT and BT) or multibacillary (MB; including BB, BL and LL). PB patients have a cellular immune response that contains the bacteria within host macrophages such that slit skin smears (SSSs) are negative and patients are characterized by having less than five asymmetrical skin lesions and one involved nerve. Conversely, MB patients have more than five skin lesions and one or more involved nerves and exhibit weak cellular responses but abundant circulating antibodies that do not limit M. leprae multiplication, such that they present with higher bacteria indices (BIs) than PB patients.
In 1981, researchers4,5 extracted phenolic glycolipid (PGL)-I, a membrane component specific to M. leprae, and developed it into one of the most significant tools to date for assisting in the diagnosis of leprosy.6 MB patients typically have high levels of circulating anti-PGL-I antibodies, mainly of the immunoglobulin M (IgM) subclass.7 Natural disaccharide-octyl (NDO) is a synthetic mimetic of PGL-I that, when conjugated with either bovine or human serum albumin (BSA and HSA, respectively), is amenable to conventional diagnostic methods such as enzyme-linked immunosorbent assay (ELISA).8,9 Although performance in PB and pure neural leprosy cases is limited, the examination of serum anti-PGL-I antibody responses is a relatively reliable and simple method with which to confirm diagnosis of MB presentations.10–12
More recently sequencing of the M. leprae genome provided the opportunity for informed screening and led to the generation of protein diagnostic candidates.13 Numerous studies have now indicated the utility of various antigens in supporting the diagnosis of leprosy, in particular the MB presentations, although the individual antigen that provides the greatest sensitivity can vary between studies.14,15 The single fusion protein Leprosy IDRI Diagnostic (LID)-1, generated from a continuous linkage and expression of the ml0405 and ml2331 genes, has emerged as a diagnostic alternative.14 Furthermore, LID-1 can be used as a carrier protein for NDO to yield NDO-LID.16–19
Multidrug therapy (MDT) has been freely provided for the treatment of leprosy for nearly 40 y, and the number of newly detected leprosy cases sharply declined from 5.2 million reported cases in 1985 to 214 783 in 2016.20,21 However, newly detected leprosy case rates have become stable at around 200 000 per year over the last decade. While India and Brazil account for the largest proportion of cases, new cases of leprosy are also reported in China. Since 1981, the focus of the national control program has been on bringing prevalence rates down to <1 case per 100 000 at the county level,22 and the incidence has decreased in recent years.23
Although the perceived interruptions in the M. leprae transmission chain as well as reductions in the grade 2 disability rate are great achievements of the national control program, it should be noted that, as with most countries, the current leprosy control strategy in China remains founded on the recognition of clinical features. This is occasionally assisted by microbiology and histopathology techniques to detect, diagnose and treat cases as they are reported.24,25 This is particularly tenuous in some provinces, where diagnosis depends on relatively few professional leprologists, alongside an increasing number of clinicians that have, at best, limited experience with leprosy patients, thus delaying diagnosis and increasing the disabilities related to advanced leprosy.25 Leprosy remains a public health problem in provinces of southwest Chinese such as Yunnan and Guizhou, where multiple genetic variants of M. leprae have been identified.26 Thus a validated tool for early diagnosis would be very useful. The Beijing Tropical Medicine Research Institute accordingly launched a combined strategy to provide control and surveillance of leprosy in these provinces, including serological evaluations. The aim of this study was to evaluate the relative abilities of NDO-BSA, LID-1 and NDO-LID to support the diagnoses of suspected and clinically confirmed leprosy cases.
Materials and methods
Subjects
This study was reviewed and approved by the Medical Ethics Committee of Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China. All participants (n=279) signed an informed consent form before participating, with recruits <14 y of age having their informed consent form signed by either a parent or legal guardian. Leprosy cases were clinically diagnosed in 2012–2014 in Honghe prefecture, a leprosy-endemic region of Yunnan province that had a 2012 new case detection rate of 0.65/100 000 population (Figure 1).
Figure 1.
Location of Honghe prefecture, Yunnan province in southwest China.
Each leprosy patient was characterized operationally as MB or PB for treatment by WHO criteria and microbiologically by acid-fast staining of SSSs and histopathology to be classified into one of the five types characterized by the Ridley–Jopling criteria (TT, BT, BB, BL and LL).2 In total 74 MB patients, comprised of 10 LL, 61 BL and 3 BB, and 39 PB leprosy patients, comprised of 29 BT and 10 TT, were identified and recruited along with 166 control individuals. All patients were newly diagnosed at the time of serum collection and none were presenting with reactional episodes. The epidemiological and demographic characteristics recorded for each patient included age, gender, ethnicity and origin, estimated date of initial onset of symptoms and time elapsed due to misdiagnosis, disability grade, pathological type and bacterial index. The controls consisted of 108 household contacts (HHCs) of a leprosy patient,27 27 tuberculosis (TB) cases (each of whom were confirmed as Mycobacterium tuberculosis sputum positive at diagnosis) and 31 healthy endemic controls (ECs) from the same endemic region. Blood was collected before the initiation of MDT and sera were stored at −20°C until being thawed for assay.
Antigen-specific antibody ELISA
Circulating antibodies against NDO-BSA, LID-1 and NDO-LID were quantified by ELISA. In brief, 96-well plates (Corning, Corning, NY, USA) were blocked with 1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS)/Tween-20 and then coated with saturating amounts of either NDO-BSA (5 ng/well), LID-1 (50 ng/well) or NDO-LID (5 ng/well). Serum samples were diluted 1:200 in 0.1% BSA then added in duplicate and incubated for 2 h at room temperature. Wells were then washed before incubating with a mixture of anti-human IgG and IgM–horse radish peroxidase conjugate diluted in 0.1% BSA (both from Rockland Immunochemicals, Gilbertsville, PA, USA). After washing, peroxidase-specific substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) was added and the reaction was allowed to develop before quenching by the addition of 1 N H2SO4. The optical density (OD) of each well was confirmed and read at 450 nm using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA, USA). Results from each individual serum sample were determined as the mean OD of the duplicate wells.
Statistical analyses
Data were input into SPSS 17.0 software (SPSS, Chicago, IL, USA) for analysis of variance with the post hoc Tukey’s test to evaluate mean differences between groups, assuming that OD values were normally distributed. The cut-offs used for sensitivity and specificity calculations were calculated using optimized OD thresholds generated by receiver operating characteristics (ROC) curve analysis, assuming the following different scenarios for use: diagnosis of MB leprosy within the general population of the endemic region, only MB patients and only PB patients. The diagnostic performance of the NDO-BSA, LID-1 and NDO-LID ELISAs was evaluated by comparing each area under the curve (AUC) with DeLong’s test. All statistical analyses were performed in R (R Foundation for Statistical Computing, Vienna, Austria), assuming a statistical significance was obtained when p-values were <0.05.
Results
Antigen-specific antibody responses among leprosy patients
We determined the relative levels of antibodies against NDO-BSA, LID-1 and NDO-LID within each serum sample by ELISA. As expected, and based on operational classifications, MB leprosy cases presented with higher levels of circulating antigen-specific antibodies when compared against all other groups (Figure 2). Positive responses were detected in 97.3%, 97.3% and 98.6% of the MB patients (comprised of 10 LL, 61 BL and 3 BB) for antibodies against NDO-BSA, LID- 1 and NDO-LID, respectively. These rates decreased for PB patients (comprised of 29 BT and 10 TT), for whom 74.4%, 56.4% and 69.2% had antibodies against NDO-BSA, LID-1 and NDO-LID, respectively (Table 1). Accordingly, when further characterized by Ridley–Jopling classification, the seropositivity rates for antibodies against each of the antigens were significantly different between the polar extreme TT and LL groups (Figure 2). In general, both the proportion of responders and the magnitude of response declined as the presentation moved toward the TT end of the spectrum.
Figure 2.
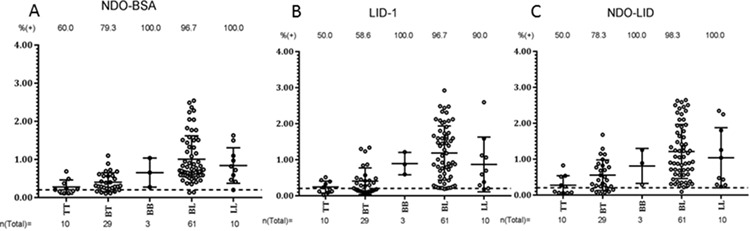
Antigen-specific responses of leprosy patients, stratified by antigen. Sera from fully characterized leprosy patients were analysed for antibodies against (A) LID-1 (IgG), (B) NDO-LID (IgG and IgM) and (C) NDO-BSA (IgM). The MB group comprised 10 LL, 61 BL and 3 BB and the PB group comprised 29 BT and 10 TT patients. Each point represents the OD of an individual serum sample. The mean OD and 95% CI are represented by the horizontal bar while the traced horizontal line depicts the threshold for determining a positive result (OD=0.2). The number above each data set is the percentage of positive responders and the number below represents the total number of participants in each group.
Table 1.
Sensitivity of antigen-specific antibodies among various groups
| Groups | n | BI, mean (range) | Male:female | NDO-BSA positive, n (%) | LID-1 positive, n (%) | NDO-LID positive, n (%) | |
|---|---|---|---|---|---|---|---|
| Patients | MB | 74 | 3.01 (1.5–5.5) | 56:18 | 72 (97.3) | 72 (97.3) | 73 (98.6) |
| PB | 39 | 0.22 (0–1.8) | 26:13 | 29 (74.4) | 22 (56.4) | 27 (69.23) | |
| Controls | HHC-MB | 93 | – | 31:62 | 52 (57.4) | 21 (22.5) | 16 (14.8) |
| HHC-PB | 15 | – | 8:7 | 6 (40.0) | 4 (26.7) | 0 (0) | |
| TB | 27 | – | 20:7 | 6 (22.2) | 2 (7.4) | 1 (3.7) | |
| EC | 31 | – | 18:13 | 5 (16.1) | 4 (12.9) | 5 (16.1) | |
HHC-MB: household contact of an MB patient; HHC-PB: household contact of a PB patient.
Antibody responses among control populations
It is well recognized that HHCs are at higher risk of M. leprae infection and subsequent development of leprosy than the general population. Responses of HHCs and the general population of the leprosy-endemic region, either TB patients or healthy EC, were also assessed. As expected, a small percentage of HHCs had antibodies against of NDO-BSA, LID-1 and NDO-LID in their serum, with the proportion being slightly greater among contacts of MB patients than among contacts of PB patients. A smaller proportion of positive responses was also detected among TB patients and healthy ECs (Figure 3). Together, these data suggest a proportion of the general population in the leprosy-endemic region may be harbouring M. leprae without any clinical symptoms of disease.
Figure 3.
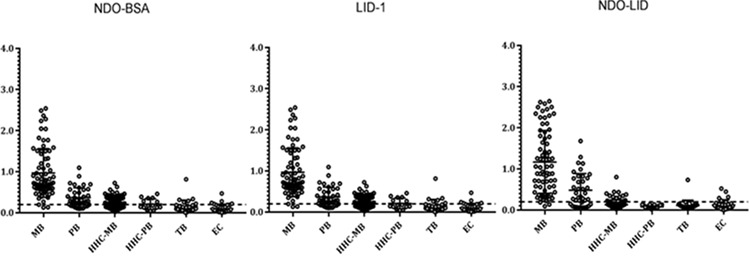
Antigen-specific responses among the broader population. Sera from multiple groups were analysed for antibodies against LID-1 (IgG), NDO-LID (IgG and IgM) and NDO-BSA (IgM). The leprosy patient group contained 113 total samples, the HHC-MB group contained 93, the HHC-PB group contained 15, TB patients contained 27 and ECs contained 31. Each point represents the OD of an individual serum sample, with the mean OD and 95% CI represented by the horizontal bar. The traced horizontal line depicts the threshold for determining a positive result (OD=0.2).
Diagnostic performance of each antigen
To directly compare the capacity of each antigen to discriminate all leprosy patients, MB patients or PB patients from control individuals, we constructed ROC curves (Figure 4). For the combined grouping of all (i.e. MB and PB) patients, the largest observed AUC of 0.923 was seen with NDO-LID (Table 2). Similarly, when only MB patients were considered, NDO-LID provided the greatest AUC (0.978), slightly larger than that derived from using either NDO-BSA (0.961) or LID-1 (0.963). Again, when only PB patients were considered, the AUC for NDO-LID (0.819) was greatest among the antigens evaluated. Together, ROC curve analyses with a focus on these three patient groupings indicated that the efficiency of the combined conjugate of NDO-LID was better than that delivered independently by the other two antigens, LID-1 and NDO-BSA. Taken together, our data indicate the benefit of using the NDO-LID conjugate to support the diagnosis of leprosy and surveillance for M. leprae infection.
Figure 4.
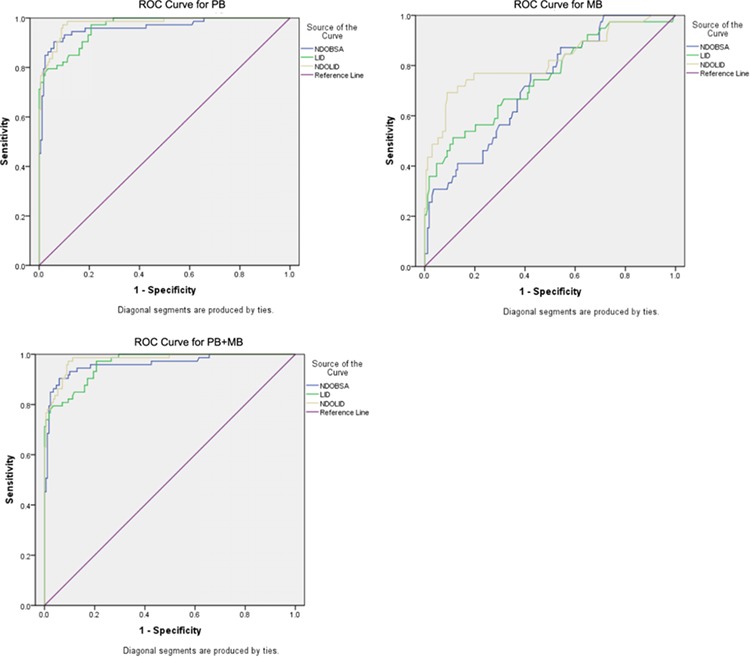
Capacity of each antigen to confirm the diagnosis of leprosy. ROC curves were generated with data from LID-1, NDO-LID-1 and NDO-BSA ELISAs and were configured for diagnosis of either PB leprosy, MB leprosy or combined (PB+MB) leprosy.
Table 2.
Diagnostic performance of each antigen. ROC curves were generated and AUC for various conditions calculated
| Variable | AUC | SE | Significance | 95% CI | |
|---|---|---|---|---|---|
| MB+PB | NDO-BSA | .877 | .021 | .000 | .835 to .919 |
| LID-1 | .890 | .021 | .000 | .850 to .930 | |
| NDO-LID | .923 | .018 | .000 | .887 to .959 | |
| MB | NDO-BSA | .961 | .014 | .000 | .933 to .990 |
| LID-1 | .963 | .011 | .000 | .942 to .983 | |
| NDO-LID | .978 | .008 | .000 | .962 to .995 | |
| PB | NDOBSA | .723 | .043 | .000 | .640 to .807 |
| LID-1 | .749 | .046 | .000 | .659 to .840 | |
| NDO-LID | .819 | .044 | .000 | .732 to .905 |
SE: standard error.
Discussion
Serological analyses have the potential to facilitate leprosy diagnosis and could be an extremely useful tool in countries where decreasing numbers of new case reports have led to reduced expertise among clinicians. In this study we evaluated the diagnostic performance of LID-1 (a fusion protein), NDO-BSA (the mimetic of M. leprae–specific PGL-I) and their conjugate, NDO-LID, to assess their ability to support the clinical diagnosis of leprosy in China. We evaluated the efficiency with which these antigens could distinguish leprosy cases in Yunnan province from control individuals (HHC, TB and EC). Our data indicate that the NDO-LID conjugate yields an incremental increase in the rate of confirmation of MB presentations over that obtained with either LID-1 or NDO-BSA alone.
Numerous studies have now indicated the utility of various antigens in supporting the diagnosis of leprosy, in particular the MB presentations, although the individual antigen that provides the greatest sensitivity varies between studies.14,15 In agreement with our findings, theoretical analyses generally indicate that the LID-1 and NDO-BSA antigens could complement each other to yield even greater sensitivities.16 Analyses of sera from 781 MB patients reported in Yunnan, Guizhou, Sichuan and Hunan provinces indicated that LL patients had the highest seropositivity rates of 78.7% and 71.7% against NDO-BSA and LID-1, respectively, while the combined results for NDO-BSA and LID-1 indicated a rate of 86.3% for MB leprosy patients.28 In a study of 396 patients in Colombia, serum IgM antibodies against the NDO-LID conjugate were detected in 78% of all patients, a level greater than the 64% sensitivity attained by detecting anti-PGL-I antibodies alone. The inclusion of the LID-1 protein, and the use of protein A to simultaneously detect both antigen-specific IgG and IgM isotypes, yielded the highest overall sensitivity of 86.3%.29 Responses among 98 leprosy patients from Rio Grande do Norte, Brazil revealed a diagnostic sensitivity for MB cases of 89% for anti-LID-1 antibodies that increased to 95% for NDO-LID.30 Given that NDO-LID generated the largest AUC following ROC curve analyses, our data are consistent with these recent reports and support the use of NDO-LID for confirming the diagnosis of leprosy.
Serum antibody detection tests could also potentially simplify and standardize the screening of individuals to facilitate detection of new MB cases. Epidemiological surveys using serology have routinely revealed that anti-PGL-I positive rates are higher among HHCs than the general population and represent a risk for the development of leprosy,31–34 but analysis of anti-PGL-I antibodies in samples collected in Bangladesh demonstrated no association between anti-PGL-I antibody levels and onset of disease.35 This contrasts with analyses of sera collected in the Philippines.14,36 Among the Filipino cohorts, antibodies against LID-1 were found to be elevated 6–8 months before clinical diagnosis in 7 of 11 HHCs that developed MB leprosy.14 These data were the first indication that the detection of responses against LID-1 could assist in recognizing early stage leprosy as well as potentially identify individuals most in need of follow-up clinic examination. The LID-1 protein has subsequently been used to replace the carrier BSA or HSA proteins within conjugates with the PGL-I mimetic NDO to simplify the fabrication of ELISAs.16,37 Our data further reveal that the NDO-LID conjugate enhances sensitivity to assist in the rapid and consistent detection of MB leprosy and suggest this as a useful tool for diagnostic screening in China.
Evidence for the early detection of emergent leprosy cases has been generated independently with protein and glycolipid antigens.36,38,39 NDO-LID has been integrated into a rapid protein-based serological assay that can be used in field situations40,41 and the use of such rapid diagnostic tests could facilitate screening to rapidly detect early signs of leprosy, especially in endemic areas. It should be noted, however, that a screening study in Bangladesh reported that responses to NDO-HSA required supplementation with other biomarkers in order to discriminate M. leprae–infected from uninfected individuals.42 In agreement with other studies, our results indicate that the simultaneous detection of antibody responses to M. leprae protein (LID-1) and glycolipid (PGL-I) antigens provides a benefit in monitoring not only leprosy cases, but also those HHCs and ECs most at risk of developing leprosy.7,14,43,44 Consistent with increased transmission of M. leprae from heavily infected patients, among MB HHCs we observed circulating IgG and IgM antibodies against NDO-LID at a rate much higher than that observed among PB HHCs. The relatively higher seropositive rate among recruited MB HHCs indicates asymptomatic M. leprae infection and therefore potential emergence as a leprosy case.27 Given that leprosy is a chronic disease that typically develops slowly after infection, there appears to be a need to monitor both serological responses and clinical features over an extended timeframe. Tests detecting serum antibodies can provide an objective and quantifiable assessment to inform or trigger clinical examinations. Alternatively, or in addition, evaluating individuals who have weak positive responses with other emerging detection methods such as M. leprae–specific DNA fragment polymerase chain reaction or more rigorous nerve assessments could lead to early diagnosis and intervention.
Our study suggests that NDO-LID ELISA represents a potentially powerful tool that can assist in the diagnosis of leprosy. Extending the duration and expanding the sample size are likely required to validate our results in a real-time setting. Conducting such a study will likely require coordination of multiple centres within different leprosy-affected provinces. Such a program appears warranted to ensure sustained interruption of M. leprae transmission and long-term leprosy control.
Authors’ contributions:
LJ was responsible for data acquisition and methodology. SX was responsible for the data analysis. LJ, YY, XY and YL were responsible for the investigation. WY and LJ were responsible for project administration. LJ and MSD wrote the draft manuscript.
Acknowledgements:
The authors are extremely grateful to the study volunteers for their cooperation and would like to thank the field and laboratory staff for their assistance in making this work possible.
Funding:
This work was supported by the Li Huan-ying Medical Foundation (funding number 20160725) with proteins produced by a grant from the American Leprosy Missions.
Competing interests:
MSD has provided the antigens described herein to commercial diagnostic companies for the generation of rapid diagnostic tests. All other authors have declared no conflicts of interest.
Ethical approval:
The study was reviewed and approved by the Medical Ethics Committee of the Beijing Friendship Hospital, Capital Medical University, Beijing, China. The participants, or legal guardians when the participant was <14 y old), provided written informed consent.
References
- 1. Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19(2):338–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ridley DS, Jopling WH. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34(3):255–273. [PubMed] [Google Scholar]
- 3. Norman G, Joseph G, Richard J. Validity of the WHO operational classification and value of other clinical signs in the classification of leprosy. Int J Lepr Other Mycobact Dis. 2004;72(3):278–283. [DOI] [PubMed] [Google Scholar]
- 4. Brennan PJ, Barrow WW. Evidence for species-specific lipid antigens in Mycobacterium leprae. Int J Lepr Other Mycobact Dis. 1980;48(4):382–387. [PubMed] [Google Scholar]
- 5. Hunter SW, Brennan PJ. A novel phenolic glycolipid from Mycobacterium leprae possibly involved in immunogenicity and pathogenicity. J Bacteriol. 1981;147(3):728–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Young DB, Buchanan TM. A serological test for leprosy with a glycolipid specific for Mycobacterium leprae. Science. 1983;221(4615):1057–1059. [DOI] [PubMed] [Google Scholar]
- 7. Spencer JS, Kim HJ, Wheat WH et al. Analysis of antibody responses to Mycobacterium leprae phenolic glycolipid I, lipoarabinomannan, and recombinant proteins to define disease subtype-specific antigenic profiles in leprosy. Clin Vaccine Immunol. 2011;18(2):260–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujiwara T, Hunter SW, Cho SN, Aspinall GO, Brennan PJ. Chemical synthesis and serology of disaccharides and trisaccharides of phenolic glycolipid antigens from the leprosy bacillus and preparation of a disaccharide protein conjugate for serodiagnosis of leprosy. Infect Immun. 1984;43(1):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cho SN, Fujiwara T, Hunter SW, Rea TH, Geiber RH, Brennan PJ. Use of an artificial antigen containing the 3,6-di-O-methyl-beta-D-glucopyranosyl epitope for the serodiagnosis of leprosy. J Infect Dis. 1984;150(3):311–322. [DOI] [PubMed] [Google Scholar]
- 10. Oskam L, Slim E, Bührer-Sékula S. Serology: recent developments, strengths, limitations and prospects: a state of the art overview. Lepr Rev. 2003;74(3):196–205. [PubMed] [Google Scholar]
- 11. Bührer-Sékula S, Visschedijk J, Grossi MAF et al. The ML flow test as a point of care test for leprosy control programmes: potential effects on classification of leprosy patients. Lepr Rev. 2007;78(1):70–79. [PubMed] [Google Scholar]
- 12. Bührer-Sékula S, Illarramendi X, Teles RB et al. The additional benefit of the ML flow test to classify leprosy patients. Acta Trop. 2009;111(2):172–176. [DOI] [PubMed] [Google Scholar]
- 13. Cole ST, Eiglmeier K, Parkhill J et al. Massive gene decay in the leprosy bacillus. Nature. 2001;409(6823):1007–1011. [DOI] [PubMed] [Google Scholar]
- 14. Duthie MS, Goto W, Ireton GC et al. Use of protein antigens for early serological diagnosis of leprosy. Clin Vaccine Immunol 2007;14(11):1400–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marcal PHF, Fraga LAO, De Mattos AMM et al. Utility of immunoglobulin isotypes against LID-1 and NDO-LID for, particularly IgG1, confirming the diagnosis of multibacillary leprosy. Mem Inst Oswaldo Cruz. 2018;113(5):e170467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duthie MS, Raychaudhuri R, Tutterrow YL et al. A rapid ELISA for the diagnosis of MB leprosy based on complementary detection of antibodies against a novel protein-glycolipid conjugate. Diagn Microbiol Infect Dis. 2014;79(2):233–239. [DOI] [PubMed] [Google Scholar]
- 17. Serrano-Coll H, Muñoz M, Beltrán JC, Duthie MS, Cardona-Castro N. Anti-natural octyl disaccharide-leprosy IDRI diagnostic (NDO-LID) antibodies as indicators of leprosy reactions and neuritis. Trans R Soc Trop Med Hyg. 2017;111(3):125–131. [DOI] [PubMed] [Google Scholar]
- 18. Devides AC, Rosa PS, Belone AFF et al. Can anti-PGL-1 and anti-NDO-LID-1 antibody titers be used to predict the risk of reactions in leprosy patients? Diagn Microbiol Infect Dis. 2018;91(3):260–265. [DOI] [PubMed] [Google Scholar]
- 19. Hungria EM, Bührer-Sékula S, Oliveira RM et al. Mycobacterium leprae-specific antibodies in multibacillary leprosy patients decrease during and after treatment with either the regular 12 doses multidrug therapy (MDT) or the uniform 6 doses MDT. Front Immunol. 2018;9:915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization Global leprosy update, 2014: need for early case detection. Wkly Epidemiol Rec. 2015;90(36):461–474. [PubMed] [Google Scholar]
- 21. World Health Organization Global leprosy update, 2017: reducing the disease burden due to leprosy. Wkly Epidemiol Rec. 2018;93(35):445–456. [Google Scholar]
- 22. Chen XS, Li WZ, Jiang C, Ye GY. Leprosy in China: epidemiological trends between 1949 and 1998. Bull World Health Org. 2001;79(4):306–312. [PMC free article] [PubMed] [Google Scholar]
- 23. Lun ZR, Zhu XQ, Yang TB. Leprosy in China. Lancet Infect Dis. 2012;12(1):11. [DOI] [PubMed] [Google Scholar]
- 24. Li J, Yang L, Wang Y, Liu H, Liu J, Cross H. How to improve early case detection in low endemic areas with pockets of leprosy: a study of newly detected leprosy patients in Guizhou Province, People’s Republic of China. Lepr Rev. 2016;87(1):23–31. [PubMed] [Google Scholar]
- 25. Jian L, Lianchao Y, Shiguo Z, Wang S, Junchao G. Delayed diagnosis of leprosy cases that persist in China. Lepr Rev. 2017;88(3):354–363. [Google Scholar]
- 26. Wang N, Wang Z, Wang C et al. Prediction of leprosy in the Chinese population based on a weighted genetic risk score. PLoS Negl Trop Dis. 2018;12(9): e0006789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moura ML, Dupnik KM, Sampaio GAA et al. Active surveillance of Hansen’s disease (leprosy): importance for case finding among extra-domiciliary contacts. PLoS Negl Trop Dis. 2013;7(3): e2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Le W, Haiqin J, Danfeng H et al. Monitoring and detection of leprosy patients in southwest China: a retrospective study, 2010–2014. Sci Rep. 2018;8(1):11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Munoz M, Beltrán-Alzate JC, Duthie MS et al. Comparison of enzyme-linked immunosorbent assay using either natural octyl disaccharide-leprosy IDRI diagnostic or phenolic glycolipid-I antigens for the detection of leprosy patients in Colombia. Am J Trop Med Hyg. 2018;98(1):274–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Amorim FM, Nobre ML, Ferreira LC et al. Identifying leprosy and those at risk of developing leprosy by detection of antibodies against LID-1 and LID-NDO. PLoS Negl Trop Dis. 2016;10(9): e0004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bührer-Sékula S, Smits HL, Gussenhoven GC et al. Simple and fast lateral flow test for classification of leprosy patients and identification of contacts with high risk of developing leprosy. J Clin Microbiol. 2003;41(5):1991–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Araújo S, Lobato J, EdM R et al. Unveiling healthy carriers and subclinical infections among household contacts of leprosy patients who play potential roles in the disease chain of transmission. Mem Inst Oswaldo Cruz. 2012;107(Suppl 1):55–59. [DOI] [PubMed] [Google Scholar]
- 33. Bazan-Furini R, Motto ACF, Simão JCL et al. Early detection of leprosy by examination of household contacts, determination of serum anti-PGL-1 antibodies and consanguinity. Mem Inst Oswaldo Cruz. 2011;106(5):536–540. [DOI] [PubMed] [Google Scholar]
- 34. Düppre NC, Camacho LAB, Sales AM et al. Impact of PGL-I seropositivity on the protective effect of BCG vaccination among leprosy contacts: a cohort study. PLoS Negl Trop Dis. 2012;6(6): e1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Richardus RA, Zwet K, Hooij A et al. Longitudinal assessment of anti-PGL-I serology in contacts of leprosy patients in Bangladesh. PLoS Negl Trop Dis. 2017;11(12): e0006083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Douglas JT, Callona RV, Fajardo TT Jr, Abalos RM, Balagon MVF, Klatser PR. Prospective study of serological conversion as a risk factor for development of leprosy among household contacts. Clin Diagn Lab Immunol. 2004;11(5):897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fabri ACOC, Carvalho APM, Vieira NF et al. Integrative literature review of the reported uses of serological tests in leprosy management. Rev Soc Bras Med Trop. 2016;49(2):158–164. [DOI] [PubMed] [Google Scholar]
- 38. Qiong-Hua P, Zhong-Yi Z, Jun Y et al. Early revelation of leprosy in China by sequential antibody analyses with LID-1 and PGL-I. J Trop Med. 2013;2013: 352689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Souza MM, Netto EM, Nakatani M, Duthie MS. Utility of recombinant proteins LID-1 and PADL in screening for Mycobacterium leprae infection and leprosy. Trans R Soc Trop Med Hyg. 2014;108(8):495–501. [DOI] [PubMed] [Google Scholar]
- 40. Cardoso LPV, Dias RF, Freitas AA et al. Development of a quantitative rapid diagnostic test for multibacillary leprosy using smart phone technology. BMC Infect Dis. 2013;13(1):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Duthie MS, Balagon MF, Maghanoy A et al. Rapid quantitative serological test for detection of infection with Mycobacterium leprae, the causative agent of leprosy. J Clin Microbiol. 2014;52(2):613–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hooij A, Fat EMTK, Richardus R et al. Quantitative lateral flow strip assays as user-friendly tools to detect biomarker profiles for leprosy. Sci Rep. 2016;6: 34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duthie MS, Hay MN, Rada EM et al. Specific IgG antibody responses may be used to monitor leprosy treatment efficacy and as recurrence prognostic markers. Eur J Clin Microbiol Infect Dis. 2011;30(10):1257–1265. [DOI] [PubMed] [Google Scholar]
- 44. Spencer JS, Duthie MS, Geluk A et al. Identification of serological biomarkers of infection, disease progression and treatment efficacy for leprosy. Mem Inst Oswaldo Cruz. 2012;107(Suppl 1):79–89. [DOI] [PubMed] [Google Scholar]


