Research highlights
► The red algal lectin KAA-2 specifically binds to high mannose type N-glycans. ► KAA-2 shows a broad spectrum of anti-influenza activity strain-independently. ► The antiviral activity of KAA-2 is very strong with EC50s of low nanomolar range. ► KAA-2 effectively inactivates recent emerged swine origin H1N1 influenza virus. ► KAA-2 inhibits virus entry into the cells by binding to viral envelope glycoproteins.
Abbreviations: CV-N, cyanovirin-N; ESA-2, Eucheuma serra agglutinin 2; HA, hemagglutinin; HM, high mannose; KAA-2, Kappaphycus alvarezii agglutinin; MVL, Microcystis viridis lectin; MVN, microvirin; NR, neutral red; OAA, Oscillatoria agardhii agglutinin; PA, pyridylaminated; SP-D, surfactant protein D; SVN, scytovirin
Keywords: Lectin, Red algae, High mannose type N-glycan, Anti-influenza activity
Abstract
The carbohydrate binding profile of the red algal lectin KAA-2 from Kappaphycus alvarezii was evaluated by a centrifugal ultrafiltration–HPLC method using pyridylaminated oligosaccharides. KAA-2 bound exclusively to high mannose type N-glycans, but not to other glycans such as complex type, hybrid type, or the pentasaccharide core of N-glycans. This lectin exhibited a preference for an exposed α1–3 Man on a D2 arm in a similar manner to Eucheuma serra agglutinin (ESA-2), which shows various biological activities, such as anti-HIV and anti-carcinogenic activity. We tested the anti-influenza virus activity of KAA-2 against various strains including the recent pandemic H1N1-2009 influenza virus. KAA-2 inhibited infection of various influenza strains with EC50s of low nanomolar levels. Immunofluorescence microscopy using an anti-influenza antibody demonstrated that the antiviral activity of KAA-2 was exerted by interference with virus entry into host cells. This mechanism was further confirmed by the evidence of direct binding of KAA-2 to a viral envelope protein, hemagglutinin (HA), using an ELISA assay. These results indicate that this lectin would be useful as a novel antiviral reagent for the prevention of infection.
1. Introduction
High mannose (HM)-binding algal lectins from blue-green algae (cyanobacteria) and marine macro-algae are promising compounds as potential drugs for the prevention of transmission of various enveloped viruses, such as human immunodeficiency virus (HIV), influenza virus, hepatitis C virus (HCV), Ebola virus, and severe acute respiratory syndrome coronavirus (SARS-CoV) [1]. Suppression of virus infection by algal lectins is based on the specific interaction of lectins with target glycans on virus surfaces [2]. For example, cyanobacterial lectins such as cyanovirin-N (CV-N) from Nostoc ellipsosporum [3], [4], scytovirin (SVN) from Scytonema varium [5], MVL from Microcystis viridis [6], microvirin (MVN) from Microcystis aeruginosa [7] and OAA from Oscillatoria agardhii [8], as well as red algal lectins such as griffithsin from Griffithsia sp. [9] and ESA-2 from Eucheuma serra [10], display strong activity with EC50s of pico- or nanomolar levels against HIV infection through specific recognition of HM glycans on the HIV envelope glycoprotein. These anti-HIV lectins are structurally dissimilar and their modes of carbohydrate binding are significantly different except for OAA and ESA-2, both of which have been recently classified into a new lectin family [8]. However, these anti-HIV lectins have several common characteristics, such as amino acid sequence redundancy and exclusive HM oligosaccharide recognition [1].
N-linked glycans on viral envelopes have a pivotal role in tertiary structure folding, in viral invasion into susceptible cells, and in evasion from the host immune response [2]. In some enveloped viruses, HM type N-glycans are predominantly present on the glycoproteins that are involved in interaction with host cells. Gp120 on the HIV surface, which mediates virus infection through direct binding to the CD4 receptor, is heavily glycosylated with HM glycans [11]. HCV expresses a high level of HM oligosaccharides on its envelope glycoproteins [12]. HM glycans are a major component of the N-glycans of the SARS-CoV spike glycoprotein [13]. Together with the relative abundance of HM glycans on viral surfaces, HM glycans located in close proximity to receptor-binding sites are promising and attractive targets for antiviral HM-binding lectins. In fact, HM-binding algal lectins such as CV-N show a broad range of antiviral activity against various viruses [14], [15], [16], [17], by efficiently inhibiting the initial steps of virus entry into the cells and preventing the further establishment of infection.
Currently, there is a growing public health concern due to the emergence of new influenza viruses, which can potentially cause pandemics. The recently emerged swine-origin H1N1 influenza viruses were very rapidly transmitted from human to human and caused a global pandemic [18]. Antiviral reagents and novel therapeutic concepts are still in high demand, to control these outbreaks, because sufficient vaccine supply is not always available. Highly pathogenic virus strains such as avian H5N1 have been a global threat in recent years and mutations of these viruses, which can result in easier human-to-human transmission, has become a reality due to reassortment in host animals. In fact, pandemic H1N1-2009 and a H5N1 virus are reported to have a high genetic compatibility and reassortment between these two viruses can occur, which could create pandemic H5N1 viruses [19]. The emergence of recent pandemic swine-origin influenza virus as well as the ongoing threat of the outbreak of highly pathogenic H5N1 influenza viruses led us to explore new antiviral agents. During the course of this survey, we found that the lectin KAA-2 from the marine red alga Kappaphycus alvarezii has strong anti-influenza activity. In the present study, we have clarified and defined the oligosaccharide binding specificity of KAA-2 and evaluated its inhibitory potency against influenza virus infection.
2. Materials and methods
2.1. Materials
K. alvarezii agglutinin (KAA-2, previously called ECA-2 of Eucheuma cottonii) was prepared as described previously [20] and stored at −20 °C until used. Influenza virus vaccines against hemagglutinin (HA) of A/California/7/09 (H1N1), A/Victoria/210/09 (H3N2), and B/Brisbane/60/08 were purchased from Denka-Seiken (Niigata, Japan).
2.2. Centrifugal ultrafiltration–HPLC method
The oligosaccharide-binding activity of KAA-2 was determined using a centrifugal ultrafiltration–HPLC method as described previously [10]. Briefly, 90 μl of 500 nM KAA-2 in 50 mM Tris–HCl, pH 7.0, and 10 μl of 300 nM pyridylaminated (PA)-oligosaccharide were mixed and kept at room temperature for 60 min. Subsequently, unreacted PA-oligosaccharides were collected by centrifugation (10,000g, 30 s) using Nanospin Plus (Gelman Sciences, Pensacola, FL). An aliquot of the filtrate was applied to a TSKgel ODS 80TM column (4.6 × 150 mm) and unbound PA-oligosaccharide was quantified. The amount of bound PA-oligosaccharide was obtained by subtracting the amount of unbound oligosaccharide from the amount of added oligosaccharide, which was determined from the filtrate of the reaction solution without a lectin. The binding activity (%) was calculated as the ratio of the amount of bound oligosaccharide to that of added oligosaccharide.
2.3. Cells and viruses
The following strains of influenza virus were kindly provided by Dr. T. Sakaguchi (Hiroshima University, Japan): A/WSN/33 (H1N1), A/PR8/34 (H1N1), A/FM/1/47 (H1N1), A/Kyoto/1/81 (H1N1), A/Bangkok/10/83 (H1N1), A/Beijing/262/95 (H1N1), A/Aichi/2/68 (H3N2), A/Udorn/72 (H3N2), A/Philippines/2/82 (H3N2), B/Ibaraki/2/85. A clinical isolate of pandemic H1N1 virus 2009, A/Oita/OU1 P3-3/09, was generously provided by Dr. A. Nishizono (Oita University, Japan). The influenza viruses were grown in the chorioallantonic fluid of 10-day-old chicken eggs. Madin-Darby canine kidney (MDCK) cells were grown in Dulbecco’s modified Eagle medium (DMEM, Wako, Osaka, Japan) supplemented with 10% fetal bovine serum and penicillin–streptomycin.
2.4. Anti-influenza virus activity
Evaluation of anti-influenza activity was determined using the neutral red (NR) dye uptake assay. Various concentrations of KAA-2 were prepared in DMEM containing 10 μg/ml trypsin and added to MDCK cells cultured in 96-well microplates. The cells were infected with influenza virus at a multiplicity of infection of approximately 0.001 infectious particles per cell. After incubation at 37 °C for 48 h, 100 μl of NR dye (150 μg/ml in DMEM) was added and further incubated for 2 h. The NR dye incorporated into the cells was extracted from the cells by adding 100 μl of 1% acetic acid/50% ethanol. The color intensity of the dye absorbed by, and subsequently eluted from, the cells was measured using a microplate reader (1420 multilabel counter, Perkin–Elmer, MA) at 540 nm and indicates survival following virus infection.
2.5. Immunofluorescence microscopy
Immunofluorescence staining was performed to visualize and evaluate KAA-2 inhibition of virus infection. Briefly, MDCK cells were grown on cover glass and subsequently infected with A/Oita/OU1 P3-3/09 at a multiplicity of infection of approximately 0.001 infectious particles per cell, in the presence or absence of test compounds (200 nM KAA-2 or 1 μM Amantadine (1-aminoadamantane) in DMEM containing 10 μg/ml trypsin). After infection (24 h later), the infected cells were fixed with 80% acetone for 5 min. Following washing with phosphate buffered saline, pH 7.4 (PBS), the cells were incubated with mouse monoclonal anti-HA antibody (HyTest, Turku, Finland) at 37 °C for 1 h. After washing with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG antibody (Anticorps Secondaires, Compiègne, France) at 37 °C for 1 h. After further PBS washing, the cells were mounted using Vectashield with 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA) and were observed under a fluorescence-microscope (OLYMPUS BX51, Olympus, Japan).
2.6. ELISA assay
Binding of KAA-2 to viral HA was assayed using an enzyme-linked immunosorbent assay (ELISA) as follows. One hundred microliters of KAA-2 (5 μg/ml) in carbonate buffer (pH 9.6) was coated on ELISA plates (BD Biosciences, Bedford, MA) and left overnight at 4 °C. After washing three times with PBS containing 0.1% Tween20 (PBST), the wells were blocked with 3% skim milk for 1 h at 37 °C and then washed with PBST as above. Various concentrations of an influenza vaccine preparation (Astellas, Tokyo, Japan) enriched for virus HA were added to each well and incubated for 1 h at 37 °C. After washing with PBST as above, the wells were incubated with mouse anti-HA monoclonal antibody (HyTest) for 1 h at 37 °C followed by incubation with horse-radish peroxidase (HRP)-conjugated goat anti-mouse IgG antibody (GE Healthcare, UK) for 1 h at 37 °C. Each well was washed with PBST, and subsequently 3,3′,5,5′-tetramethylbenzidine (TMB) substrate (Sigma–Aldrich, Saint-Louis, MI) was added. The reaction was stopped using TMB stop reagent (Sigma–Aldrich) and absorbance at 450 nm was measured using a microplate reader (1420 multilabel counter, Perkin–Elmer). Inhibition of the interaction between KAA-2 and HA by yeast mannan was determined by the same ELISA assay, except that various concentrations of yeast mannan were added to the plate coated with KAA-2 prior to incubation with HA (3 μg/ml).
3. Results
3.1. Oligosaccharide binding specificity of KAA-2
The oligosaccharide binding specificity of the algal lectin KAA-2 from K. alvarezii was determined using a conventional carbohydrate binding assay with fluorescence-labeled oligosaccharide. The oligosaccharide structures used in this study are shown in Fig. 1 A. Of the 38 kinds of PA-oligosaccharides tested, KAA-2 exclusively bound to HM type N-glycans (Nos. 13–22) (Fig. 1B) and did not recognize other N-glycans including complex type (Nos. 1–12), hybrid type (Nos. 23–25) and the pentasaccharide core of N-glycans (No. 26) (Fig. 1B). Furthermore, oligosaccharides from glycolipids (Nos. 27–33) exhibited no interaction with this lectin. KAA-2 exhibited a binding preference for an exposed α1–3 Man on the D2 arm of a HM type oligosaccharide in a similar manner to ESA-2 and OAA, which constitute a novel anti-HIV lectin family [8]. KAA-2 bound with high binding activity to oligosaccharides with α1–3 Man that was highly exposed at the D2 position (Nos. 13, 14, 16, 18 and 21). In contrast, masking of this α1–3 Man with α1–2 Man dramatically impaired lectin–carbohydrate interaction as observed for oligosaccharides Nos. 15, 17, 19, 20 and 22 (Fig. 1B). These results clearly indicate that KAA-2 specifically recognizes branched oligomannosides of HM type N-glycans and accurately distinguishes subtle differences in the branched portion of the sugar. However, KAA-2 did not interact with any branched oligomannose alone, such as Man(α1–2)Man (No. 34), Man(α1–3)Man (No. 35), Man(α1–6)Man (No. 36), Man(α1–6)[Man(α1–3)]Man (No. 37), or Man(α1–6)[Man(α1–3)]Man(α1–6)[Man(α1–3)]Man (No. 38). This result indicates that a portion of the core N-acetyl-d-glucosamine (GlcNAc) residue(s) of HM type N-glycans is also essential for interaction with KAA-2. The importance of reducing terminal GlcNAc residue(s) was most obvious when the binding activities of Man5GlcNAc2 (No. 13, 95.8%) and Man5 (No. 38, 1.5%) were compared.
Fig. 1.
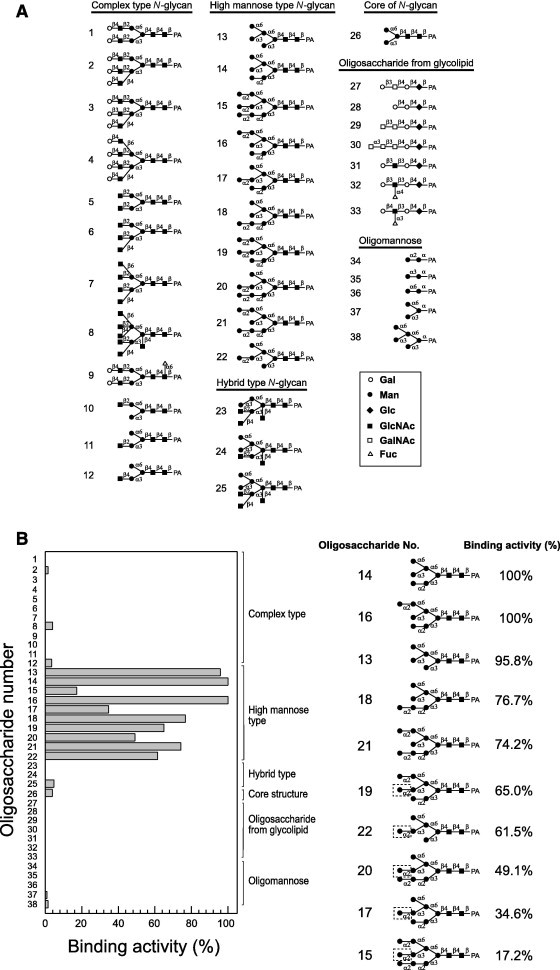
Oligosaccharide-binding specificity of KAA-2. (A) The structures of the pyridylaminated (PA)-oligosaccharides used in this study. Open circles, galactose; closed circles, mannose; closed diamonds, glucose; closed squares, N-acetylglucosamine; open squares, N-acetylgalactosamine; open triangles, fucose; open pentagons, xylose. (B) Binding activity of KAA-2 to PA-oligosaccharides. Binding activity was determined using the centrifugal ultrafiltration–HPLC method, and is expressed as the ratio (%) of the amount of bound oligosaccharide to that of the added oligosaccharide. The structures of the oligosaccharides that are selectively recognized by KAA-2 are shown. The non-reducing terminal α1–2 Man in the D2 arm (surrounded by a dotted line in the figure) negatively participates in binding to KAA-2. The assay was performed in duplicate for each PA-oligosaccharide and binding activity is expressed as the average value of duplicate assays.
3.2. Anti-influenza virus activity of KAA-2
The anti-influenza virus activity of KAA-2 was evaluated by assay of its inhibition of infection in MDCK cells using the dye uptake assay. Ten influenza A virus strains and one influenza B virus strain were used to examine the effect of KAA-2 on infection. As shown in Fig. 2 , KAA-2 strongly inhibited infection by all of the influenza virus strains except for that by an earlier laboratory-adapted strain, A/PR8/34 (H1N1). KAA-2 inhibited influenza virus infection in a strain-independent but dose-dependent manner with EC50s of low nanomolar levels, mostly ranging from 1 to 10 nM (Table 1 ). Cytotoxicity was not observed up to 1000 nM, the highest dose used in this experiment. Among the influenza viruses tested, influenza B (Ibaraki/2/85) was the most sensitive to KAA-2, exhibiting an EC50 of 1.7 nM. Infection of clinical isolates of recent pandemic swine-origin influenza virus, A/Oita/OU1 P3-3/09 (H1N1) was also efficiently inhibited by KAA-2 with an EC50 of 12.3 nM.
Fig. 2.
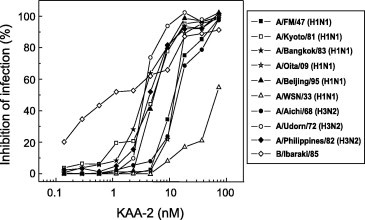
Anti-influenza activity of KAA-2 in MDCK cells infected with various influenza virus strains. Cell viability following incubation for 48 h with various influenza viruses in the presence of varying concentrations of KAA-2 was assayed using the NR dye uptake assay. Percent inhibition of infection is expressed as the average value of duplicate assays. Closed squares, A/FM/1/47; open squares, A/Kyoto/1/81; closed stars, A/Bangkok/10/83; open stars, A/Oita/OU1 P3-3/09; closed triangles, A/Beijing/262/95; open triangles, A/WSN/33; closed circles, A/Aichi/2/68; open circles, A/Udorn/72; closed diamonds, A/Philippines/2/82; open diamonds, B/Ibaraki/2/85.
Table 1.
In vitro activity of KAA-2 against various influenza strains.
| Virus | Strain | EC50 (nM) | Antiviral indexa |
|---|---|---|---|
| Influenza A | PR8/34 (H1N1) | −b | − |
| FM/1/47 (H1N1) | 11.52 ± 1.05 | >87.0 | |
| Kyoto/1/81 (H1N1) | 5.36 ± 0.38 | >186.6 | |
| Bangkok/10/83 (H1N1) | 3.59 ± 0.27 | >278.6 | |
| Beijing/262/95 (H1N1) | 5.42 ± 0.46 | >184.5 | |
| Oita/OU1 P3-3/09 (H1N1) | 12.30 ± 0.24 | >81.3 | |
| WSN/33 (H1N1) | 68.56 ± 4.17 | >14.6 | |
| Aichi/2/68 (H3N2) | 13.59 ± 1.04 | >73.6 | |
| Udorn/72 (H3N2) | 3.86 ± 0.09 | >259.1 | |
| Philippines/2/82 (H3N2) | 4.40 ± 0.27 | >227.3 | |
| Influenza B | Ibaraki/2/85 | 1.71 ± 0.97 | >584.6 |
The IC50 value of KAA-2, determined using MDCK cells, was >1000 (nM).
The antiviral index represents the ratio of IC50 to EC50.
PR8/34 was insensitive to KAA-2 up to 75 nM, a highest dose in this experiment.
3.3. Immunofluorescence microscopy
To investigate whether KAA-2 acts as an inhibitor of virus entry into cells, the presence of viral antigen in the infected cells was determined in the presence or absence of KAA-2 using immunofluorescence microscopy. After 24-h infection with A/Oita/OU1 P3-3/09 (H1N1), viral antigens were detected with the anti-HA monoclonal antibody followed by FITC-conjugated second antibody. In the absence of KAA-2, the virus invaded and proliferated in the host cells as shown in Fig. 3 . In contrast, in the presence of 200 nM KAA-2, no viral antigens were detected in the MDCK cells, indicating KAA-2 clearly inhibited virus entry into the cells. Treatment with 1 μM Amantadine, an anti-influenza drug which is known to be a specific inhibitor of the viral ion channel (M2 protein) and not an entry inhibitor, did not block virus invasion into the cells (Fig. 3).
Fig. 3.
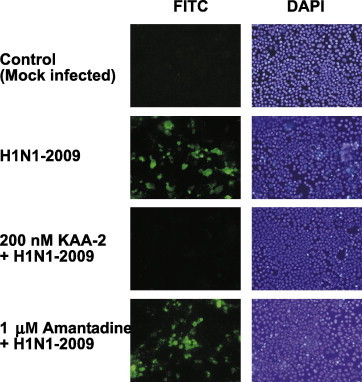
Inhibition of influenza virus entry into MDCK cells by KAA-2. MDCK cells grown on glass coverslips were infected with A/Oita/OU1 P3-3/09 (H1N1) in the presence or absence of KAA-2 or Amantadine. After 24-h infection, the cells were fixed with 80% acetone for 5 min. Viral antigens in the infected cells were observed under a fluorescence microscope after treatment with an anti-HA mouse monoclonal antibody followed by FITC-conjugated goat anti-mouse IgG. Nuclei within the cells were stained with DAPI (200× magnification).
3.4. Direct interaction of KAA-2 with influenza viral hemagglutinin (HA)
To assess the possibility that KAA-2 directly binds to the oligosaccharide on enveloped glycoprotein (HA), and that this oligosaccharide is the KAA-2 target for virus inactivation, we performed ELISA assays using an influenza vaccine preparation. This commercially available vaccine contains HA of A/California/7/09 (H1N1), A/Victoria/210/09 (H3N2), and B/Brisbane/60/08 as a major component. As shown in Fig. 4 A, direct interaction between KAA-2 and these HA glycoproteins was clearly demonstrated. HA bound to immobilized KAA-2 in a dose-dependent manner, but did not bind to yeast mannan, which was used as a reference. The interaction between HA and KAA-2 was inhibited when immobilized KAA-2 was incubated with yeast mannan bearing HM glycans prior to incubation with HA (Fig. 4B). These results indicate that KAA-2 binds to the HA of influenza virus through HM glycans.
Fig. 4.
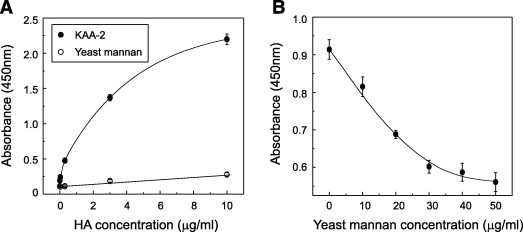
Interaction between KAA-2 and influenza envelope glycoprotein HA. (A) Direct binding of KAA-2 to HA was analyzed using an ELISA assay. KAA-2 or yeast mannan was immobilized onto the ELISA plate and incubated with an influenza vaccine which contains HA from a mixture of influenza viruses: A/California/7/09 (H1N1); A/Victoria/210/09 (H3N2), and B/Brisbane/60/08. The wells were incubated with mouse anti-HA monoclonal antibody for 1 h at 37 °C followed by with HRP-conjugated goat anti-mouse IgG antibody for 1 h at 37 °C. Subsequently, the HRP substrate, TMB, was added to each well and the absorbance at 450 nm was measured. Absorbance is expressed as the average value of duplicate assays. (B) The plate coated with KAA-2 was incubated with varying concentrations of yeast mannan at the room temperature for 1 h. Subsequently, the plate was incubated with the influenza vaccine (3 μg/ml) and assayed in the same way as above.
4. Discussion
Small carbohydrate-binding agents that target glycans on a virus surface are promising potential viral-inactivating agents that could be used for the prevention and control of virus infections [2]. In this study, we demonstrated that the HM-binding lectin KAA-2 from the red alga K. alvarezii showed strong anti-influenza activity against a broad range of influenza virus strains, including the recently emerged swine-origin H1N1 influenza viruses. This lectin efficiently inhibited influenza virus infection with EC50s of low nanomolar levels. Furthermore, KAA-2 showed intense activity against various influenza strains, regardless of the virus subtype and strain. Intriguingly, it was reported that recent pandemic H1N1 viruses were resistant to the antiviral activities of HM-binding innate immune proteins such as surfactant protein D (SP-D) and mannose-binding lectin (MBL), but the seasonal H1N1 strains are susceptible to these proteins [21]. In contrast, KAA-2 strongly inhibited infection of pandemic H1N1 virus as well as seasonal strains, although, A/PR8/34 (H1N1), which is devoid of glycosylation site on HA [21], was the only resistant strain to KAA-2. In terms of practical application for medicinal use, this strain-independent inhibition by KAA-2 might be more advantageous than antibody-based medicines that are susceptible to antigenic drift or antigenic shift.
As demonstrated in this study, inhibition of virus infection by KAA-2 was caused by prevention of viral entry into the host cells by directly binding to the surface envelope glycoprotein HA. The interaction between KAA-2 and HA was competitively inhibited by yeast mannan bearing HM glycans, indicating that KAA-2 binds to HA via HM glycans.
As revealed in this study, the HM type oligosaccharide recognition by KAA-2 is highly specific, and no binding to other glycans such as complex type, hybrid type or the pentasaccharide core of N-glycans was detected. It has been reported that site 65 of hemagglutinin 1 (HA1) subunit is exclusively occupied by HM N-glycans [22]. Although it is still unknown what determines the site specificity of attached glycans, the target HM glycan(s) for KAA-2 binding is invariably present on the HA of the virus surface. In this context, the number of sequons (potential N-glycosylation sites of HA) has increased during influenza epidemics over the past decades [23]. The antiviral activity of some lectins such as SP-D has been shown to increase in proportion to the number of attached glycans near the receptor binding site [24]. More recently-isolated H3N2 strains are needed to examine such an effect of KAA-2 on H3N2 subtypes.
The oligosaccharide binding profile of KAA-2 was almost identical to that of ESA-2 from E. serra (red alga) and OAA from O. agardhii (cyanobacterium), both of which were recently classified as members of a new lectin family found in lower organisms [8]. These lectins prefer the α1–3 Man branched from the α1–6 Man of the pentasaccharide core. Both ESA-2 and OAA strongly inhibited HIV infection by direct binding with the HIV envelope glycoprotein gp120 [8]. Strong antiviral activity of these lectins is presumed due to the high binding affinity (association constant of 108 M−1 level) for HM oligosaccharide [8], [10]. The close similarity in carbohydrate-binding profiles between KAA-2 and these anti-HIV proteins suggests that KAA-2 might be classified into the same lectin family and probably has anti-HIV activity.
KAA-2 was originally isolated from the red alga K. alvarezii (previously called E. cottonii) [20]. Red algae belonging to the genus Eucheuma (or Kappaphycus) are economically important species and have been widely cultivated as edible seaweeds or as the sources of carrageenans in subtropical coastal areas. These algae contain abundant and various isolectins. The yield of ESA-2, for example, corresponds to about 0.9% of the alga on a dry weight basis. Besides its high content of lectins, the genus Eucheuma has attracted attention because algae-derived lectins such as ESA-2 show various biological activities such as mitogenic activity for mouse and human lymphocytes and in vitro growth inhibition of tumor cells [25] in addition to anti-HIV activity. Therefore, these algae are promising organisms to furnish novel biochemically active compounds such as antiviral lectins, which have potential importance for the pharmaceutical industry and public health.
In conclusion, this study provides the first evidence that the red algal lectin KAA-2 from K. alvarezii strongly inhibits cell entry of various influenza viruses regardless of the virus strain. KAA-2 inhibits influenza virus propagation by directly binding to HM glycans on the envelope glycoprotein HA. The results of this paper indicate that KAA-2 would be useful as a novel antiviral agent.
Acknowledgments
We are grateful to Dr. Takemasa Sakaguchi and Dr. Akira Nishizono for their generous gifts of influenza viruses.
References
- 1.Ziόłkowska N.E., Wlodawer A. Structural studies of algal lectins with anti-HIV activity. Acta Biochim. Pol. 2006;53:617–626. [PubMed] [Google Scholar]
- 2.Balzarini J. Targeting the glycans of glycoproteins: a novel paradigm for antiviral therapy. Nat. Rev. Microbiol. 2007;5:583–597. doi: 10.1038/nrmicro1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd M.R., Gustafson K.R., Mcmahon J.B., Shoemaker R.H., O’Keefe B.R., Mori T., Gulakowski R.J., Wu L., Rivera M.I., Laurencot C.M., Currens M.J., Cardellina J.H., II, Buckheit R.W., Jr., Nara P.L., Pannell L.K., Sowder R.C., II, Henderson L.E. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential application to microbicide development. Antimicrob. Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bewley C.A., Gustafson K.R., Boyd M.R., Covell D.G., Bax A., Clore G.M., Gronenborn A.M. Solution structure of cyanovirin-N, a potent HIV-inactivating protein. Nat. Struct. Biol. 1998;5:571–578. doi: 10.1038/828. [DOI] [PubMed] [Google Scholar]
- 5.Bokesch H.R., O’Keefe B.R., McKee T.C., Pannell L.K., Patterson M.L., Gardella R.S., Sowder R.C., II, Turpin J., Watson K., Buckheit R.W., Jr., Boyd M.R. A potent novel anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry. 2003;42:2578–2584. doi: 10.1021/bi0205698. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi M., Ogawa T., Muramoto K., Kamio Y., Jimbo M., Kamiya H. Isolation and characterization of a mannan-binding lectin from the freshwater cyanobacterium (blue-green algae) Microcystis viridis. Biochem. Biophys. Res. Commun. 1999;265:703–708. doi: 10.1006/bbrc.1999.1749. [DOI] [PubMed] [Google Scholar]
- 7.Huskens D., Férir G., Vermeire K., Kehr J.C., Balzarini J., Dittmann E., Schols D. Microvirin, a novel alpha(1,2)-mannose specific lectin isolated from Microcystis aeruginosa, has anti-HIV-1 activity comparable with that of cyanovirin-N but a much higher safety profile. J. Biol. Chem. 2010;285:24845–24854. doi: 10.1074/jbc.M110.128546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sato Y., Okuyama S., Hori K. Primary structure and carbohydrate-binding specificity of a potent anti-HIV lectin isolated from the filamentous cyanobacterium, Oscillatoria agardhii. J. Biol. Chem. 2007;282:11021–11029. doi: 10.1074/jbc.M701252200. [DOI] [PubMed] [Google Scholar]
- 9.Mori T., O’Keefe B.R., Sowder R.C., II, Bringans S., Gardella R.S., Berg S., Cochran P., Turpin J.A., Buckheit R.W., Jr., McMahon J.B., Boyd M.R. Isolation and characterization of Griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 10.Hori K., Sato Y., Fujiwara Y., Ito K., Iwamoto Y., Makino H., Kawakubo A. Strict specificity for high mannose-type N-glycans and primary structure of a red alga Eucheuma serra lectin. Glycobiology. 2007;17:479–491. doi: 10.1093/glycob/cwm007. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Cleveland B., Klots I., Travis B., Richardson B.A., Anderson D., Montefiori D., Polacino P., Hu S. Removal of a single N-linked glycan in human immunodeficiency virus type 1 gp120 results in an enhanced ability to induce neutralizing antibody responses. J. Virol. 2008;82:638–651. doi: 10.1128/JVI.01691-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duvet S., Cocquerel L., Pillez A., Cacan R., Verbert A., Moradpour D., Wychowski C., Dubuisson J. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 1998;273:32088–32095. doi: 10.1074/jbc.273.48.32088. [DOI] [PubMed] [Google Scholar]
- 13.Ritchie G., Harvey D.J., Feldmann F., Stroeher U., Feldmann H., Royle L., Dwek R.A., Rudd P.M. Identification of N-linked carbohydrates from severe acute respiratory syndrome (SARS) spike glycoprotein. Virology. 2010;399:257–269. doi: 10.1016/j.virol.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Helle F., Wychowski C., Vu-Dac N., Gustafson K.R., Voisset C., Dubisson J. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 2006;281:25177–25183. doi: 10.1074/jbc.M602431200. [DOI] [PubMed] [Google Scholar]
- 15.O’Keefe B.R., Smee D.F., Turpin J.A., Saucedo C.J., Gustafson K.R., Mori T., Blakeslee D., Buckheit R., Boyd M.R. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barrientos L.G., O’Keefe B.R., Bray M., Sanchez A., Gronenborn A.M., Boyd M.R. Cyanovirin-N binds to the viral surface glycoprotein, gp1, 2 and inhibits infectivity of Ebola virus. Antiviral Res. 2003;58:47–56. doi: 10.1016/s0166-3542(02)00183-3. [DOI] [PubMed] [Google Scholar]
- 17.O’Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B., Jr. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neumann G., Noda T., Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Octaviani C.P., Ozawa M., Yamada S., Goto H., Kawaoka Y. High level of genetic compatibility between swine-origin H1N1 and highly pathogenic avian H5N1 influenza viruses. J. Virol. 2010;84:10918–10922. doi: 10.1128/JVI.01140-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawakubo A., Makino H., Ohnishi J., Hirohara H., Hori K. Occurrence of highly yielded lectins homologous within the genus Eucheuma. J. Appl. Phycol. 1999;11:149–156. [Google Scholar]
- 21.Job E.R., Deng Y., Tate M.D., Bottazzi B., Crouch E.C., Dean M.M., Mantovani A., Brooks A.G., Reading P.C. Pandemic H1N1 influenza A viruses are resistant to the antiviral activities of innate immune proteins of the collectin and pentraxin superfamilies. J. Immunol. 2010;185:4284–4291. doi: 10.4049/jimmunol.1001613. [DOI] [PubMed] [Google Scholar]
- 22.Mir-Shekari S.Y., Ashford D.A., Harvey D.J., Dwek R.A., Schulze I.T. The glycosylation of the influenza A virus hemagglutinin by mammalian cells. J. Biol. Chem. 1997;272:4027–4036. doi: 10.1074/jbc.272.7.4027. [DOI] [PubMed] [Google Scholar]
- 23.Zhang M., Gaschen B., Blay W., Foley B., Haigwood N., Kuiken C., Korber B. Tracking global patterns of N-linked glycosylation site variation in highly variable viral glycoproteins: HIV, SIV, and HCV envelopes and influenza hemagglutinin. Glycobiology. 2004;14:1229–1246. doi: 10.1093/glycob/cwh106. [DOI] [PubMed] [Google Scholar]
- 24.Hartshorn K.L., Webby R., White M.R., Tecle T., Pan C., Boucher S., Moreland R.J., Crouch E.C., Scheule R.K. Role of viral hemagglutinin glycosylation in anti-influenza activities of recombinant surfactant protein D. Respir. Res. 2008;9:1–12. doi: 10.1186/1465-9921-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukuda Y., Sugahara T., Ueno M., Fukuta Y., Ochi Y., Akiyama K., Miyazaki T., Masuda S., Kawakubo A., Kato K. The anti-tumor effect of Eucheuma serra agglutinin on colon cancer cells in vitro and in vivo. Anticancer Drugs. 2006;17:943–947. doi: 10.1097/01.cad.0000224458.13651.b4. [DOI] [PubMed] [Google Scholar]


