Abstract
IFNβ innate immune plays an essential role in antiviral immune. Previous reports suggested that many important regulatory proteins in innate immune pathway may be modified by ubiquitin and that many de-ubiquitination (DUB) proteins may affect immunity. Monocyte chemotactic protein-inducing protein 1 (MCPIP1), one of the CCCH Zn finger-containing proteins, was reported to have DUB function, but its effect on IFNβ innate immune was not fully understood. In this study, we uncovered a novel mechanism that may explain how MCPIP1 efficiently inhibits IFNβ innate immune. It was found that MCPIP1 negatively regulates the IFNβ expression activated by RIG-I, STING, TBK1, IRF3. Furthermore, MCPIP1 inhibits the nuclear translocation of IRF3 upon stimulation with virus, which plays a key role in type I IFN expression. Additionally, MCPIP1 interacts with important modulators of IFNβ expression pathway including IPS1, TRAF3, TBK1 and IKKε. Meanwhile, the interaction between the components in TRAF3-TBK1-IKKε complex was disrupted by MCPIP1. These results collectively suggest MCPIP1 as an innate immune regulator encoded by the host and point to a new mechanism through which MCPIP1 negatively regulates IRF3 activation and type I IFNβ expression.
Keywords: MCPIP1, Antiviral innate immune, Interferon, IRF3, TRAF3-TBK1-IKKε complex
1. Introduction
The innate immune system stands the first line of the defense that protects the host from viral intrusion, depending on pattern recognition receptors (PRRs) and the corresponding pathways [[1], [2], [3], [4]]. The pathogen-associated molecular patterns (PAMPs) of the invading viruses may be recognized by PRRs. Then, the adaptor proteins (TRIF forTLR3, MyD88 for TLR7/8/9, MAVS/IPS-1 for RIG-I) would be recruited, and the infecting signals would be transmitted to the downstream kinase complexes, followed by the activation of transcription factors, such as interferon regulatoryfactor-3 (IRF-3), nuclear factor kB (NF-kB) and ATF-2/c-jun [2,[5], [6], [7]]. Upon activated, the transcription factors may regulate the expression of type I Interferons which induce the expression of IFN-stimulated genes (ISGs) and ultimately establish the antiviral function of the host [4,[8], [9], [10]].
Many important regulatory proteins in innate immune pathways may be modified by ubiquitin [[11], [12], [13]]. On the other hand, Many de-ubiquitination (DUB) proteins may affect immunity. We have found that the papain-like proteases (PLPs) encoded by coronavirus (CoV) reduce the ubiquitinated modification of essential regulatory molecules of IFN innate immune pathway, such as RIG-1, MAVS, STING, TRAF3, TBK1 and IRF3. Additionally, CoV PLPs negatively regulate IFN expression of the host, acting as both deubiquitinases and IFN antagonists [[14], [15], [16]].
The C-terminal phosphorylation and activation of IRF3 requires noncanonical IκB kinases, TBK1 or IKK, which form signaling complexes with TRAF family members that transmit upstream signals to downstream effectors resulting in the expression of type I IFN. Previous studies suggest that TRAF family members are involved in the regulation of antiviral immune responses [[17], [18], [19], [20]]. We have reported that SARS-CoV PLP blocks the ubiquitination of STING-TRAF3-TBK1 complex and disrupts STING-TRAF3-TBK1 complex [21].
MCPIP1 (monocyte chemotactic protein-induced protein 1), a negative regulator of macrophage activation, was also found to negatively regulate JNK and NF-κB activity by removing ubiquitin moieties from proteins including TRAFs [22]. In this study, we observed that MCPIP1 inhibits the IFNβ expression activated by RIG-I, STING, TBK1, IRF3. Additionally, MCPIP1 inhibits the nuclear migration of IRF3. Furthermore, MCPIP1 interacts with TRAF3 and disrupts TRAF3-TBK1-IKKε complex which is essential for the activation of IFNβ production pathway. This report suggests that MCPIP1 may act as an IFN antagonist antiviral protein encoded by the host and uncovers the mechanism undergoes by MCPIP1 to inhibit IFNβ innate immune pathway.
2. Materials and methods
2.1. Cell and plasmids
HEK293T and HeLa cells were cultured in Dulbecco's modified Eagle's medium containing 10% (v/v) FCS supplemented with penicillin (100 U/ml) and streptomycin (100 mg/ml).
The plasmid expressing V5-MCPIP1 was cloned according to NCBI Reference SequenceNM_025079.2. The reporter plasmids IFNβ-Luc, PRD (III-I)4-Luc, NF-κB-Luc, and plasmids NL63-PLP2-TM, Myc-IRF3, Flag-IRF3, Flag-IRF7, Flag-IPS-1, Flag-STING, Flag-TBK1, Flag-IKKε, Flag-RIG-IN, A20, HA-TRAF3 were described previously [14,21,23,24].
2.2. SiRNA preparation
MCPIP1-target siRNA sequence (5′-CCAGCGUGUAUACUAAGCUTT-3′) were designed and chemically synthesized by Genescript Co. A siRNA with the sequence of 5′-UUCUCCGAACGUGUCACGU-3′ was selected as the negative control siRNA (NC-siRNA) as described previously [25,26].
2.3. Luciferase reporter gene assay
HEK293T cells were transfected with the indicated stimulator plasmid DNA (Flag-IPS-1, Flag-STING, Flag-TBK1, Flag-IKKε, Flag-RIG-IN), reporter plasmid DNA (pRL-TK, IFNβ-Luc, or PRD (III-I)4-Luc) and either V5-MCPIP1 or NL63PLP2-TM/A20 using Lipofectamine 2000 (Invitrogene) according to the manufacturer's protocol and incubated for 24 h. Then, firefly luciferase and renilla luciferase activities were assayed using the Dual Luciferase Reporter Assay Kit (Promega). Data were shown as mean relative luciferase (firefly luciferase activity divided by Renilla luciferase activity) with standard deviation from a representative experiment carried out in triplicate. The luciferase assay was performed as described previously [14,15].
2.4. Immunofluorescence assay
HEK293T or Hela cells were grown to confluence in a six-well plate. Plasmid DNA expressing V5-MCPIP1 and Flag-IRF3 (1.2 μg per well) were transfected. Twenty-four hours later, the cells were infected or mock-infected by SeV (100 HAU) and incubated for 18 h. Fluorescence was examined by using a confocal microscope. Immunoflurescence assay was performed as described previously [15].
2.5. Co-immuno-precipitation (Co-IP) analysis
HEK293T cells were seeded in 100-mm dishes at a density of1 × 106 cells/dish. Twelve hours later, cells were transiently transfected with a total of 10 μg of empty vector or indicated expression plasmids using Lipofectamine 2000 (Invitrogen). At 48 h post transfection, cells were lysed in a buffer containing 0.5% TritonX-100, 150 mmol/L NaCl, 12.5 mmol/L β-glycerolphosphate, 1.5 mmol/L MgCl2, 2 mmol/L EDTA, 10 mmol/L NaF, 1 mmol/LNa3VO4, 2 mmol/L DTT and protease inhibitor cocktail (Sigma). Cell extracts were clarified by centrifugation at5000 × g at 4 °C for 10 min, and protein concentration of lysatedetermined using BCA Protein Assay kit (Bio-med). The protein concentrations in cell lysates were adjusted to 1 μg/μL, and 500 μL of each lysate wasused for co-IP. Lysates were pre-cleared by adding 20 μL of proteinA + G Agarose (Beyotime) and 1 μg of normal IgGand incubating for 2 h at 4 °C, followed by spinning down the agarosebeads. The pre-cleared supernatant was then incubated with theindicated primary antibody [anti-V5 (MBL) or anti-HA (MBL)/anti-Myc (MBL)/anti-Flag (MBL)] with rocking overnight at 4 °C. Thereafter, the beads-antibody-antigen complex was pelleted and washed 3 times with 1 mL of lysis buffer. The protein complexes were then eluted from the beads in 30 μL of2 × SDS-PAGE sample buffer by boiling for 10 min. Samples were separated on SDS-PAGE and transferred to PVDF membranes for Western-blotting. Co-IP assay was performed as described previously [14,27].
3. Results
3.1. MCPIP1 is an IFNβ antagonist
According to the previous reports, MCPIP1 showed DUB activity targeting to TRAFs [22]. Our previous work demonstrated that several proteins encoded by viral, such as NL63 PLP2, PEDV PLP2, SARS PLpro, and MERS PLpro, showed DUB activity and negatively regulate IFN immune-response [[14], [15], [16],21].
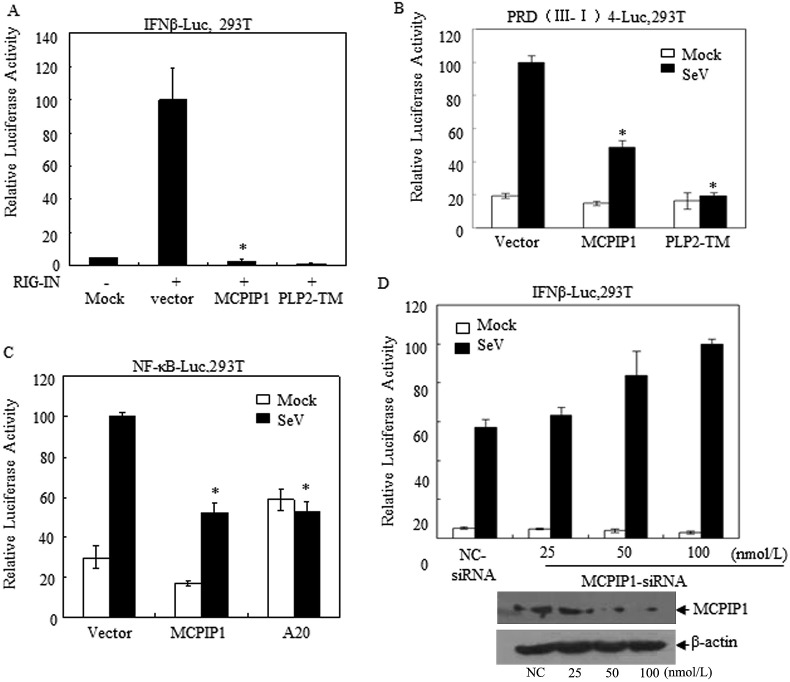
In order to further investigate the effect of MCPIP1 on IFNβ expression, HEK 293T cells were co-transfected with IFNβ-Luc, pRL-TK reporter plasmids and MCPIP1 expression conduct. Twenty four hours later, cell lysates were prepared and IFNβ promoter-driven luciferase activity was assessed. As shown in Fig. 1 , it was observed that MCPIP1 inhibits RIG-I activated IFNβ expression (Fig. 1A). Moreover, the SeV stimulated IFNβ expression pathway depending on IRF3 and NF-κB was also negatively-regulated by MCPIP1 (Fig. 1B and C). Meaning while, this negative effect of MCPIP1 could no longer be detected when its expression was silenced by MCPIP1 targeting siRNA in our experiments (Fig. 1D). These results suggest that MCPIP1 may be an IFNβ antagonist.
Fig. 1.
MCPIP1 inhibits the expression of IFNβ in cells. a HEK293T cells were co-transfected with the plasmids which expressing IFNβ-Luc and either MCPIP1 or NL63 PLP2-TM (positive control). Twenty-four hours later, cells were harvested and subjected to a Dual-luciferase assay. b HEK293T cells were transfected with PRD (III-I) 4-Luc and either MCPIP1 or NL63 PLP2-TM expressing plasmids (positive control). Twenty-four hours later, cells were harvested and subjected to a Dual-luciferase assay. c HEK293T cells were transfected with the plasmids which expressing NF-κB -Luc and either MCPIP1 or A20 (positive control). Twenty-four hours later, cells were harvested and subjected to a Dual-luciferase assay. d HEK293T cells were respectively co-transfected with the plasmids which expressing IFNβ-Luc and MCPIP1-siRNA (25, 50, 100 nmol/L). Nc-siRNA (sequence described in the text) was used as control. Twenty-four hours later, cells were harvested and subjected to Dual-luciferase assay. The expression of MCMIP1 was also detected by Western-blotting assay. The results were expressed as mean relative luciferase (firefly luciferase activity divided by Renilla luciferase activity) with standard deviation from a representative experiment carried out in triplicate. Data were presented as mean ± SEM, n = 3. *p < 0.05.
3.2. MCPIP1 antagonizes RIG-I/STING/TBK1/IRF3/IRF7 mediated IFNβ expression
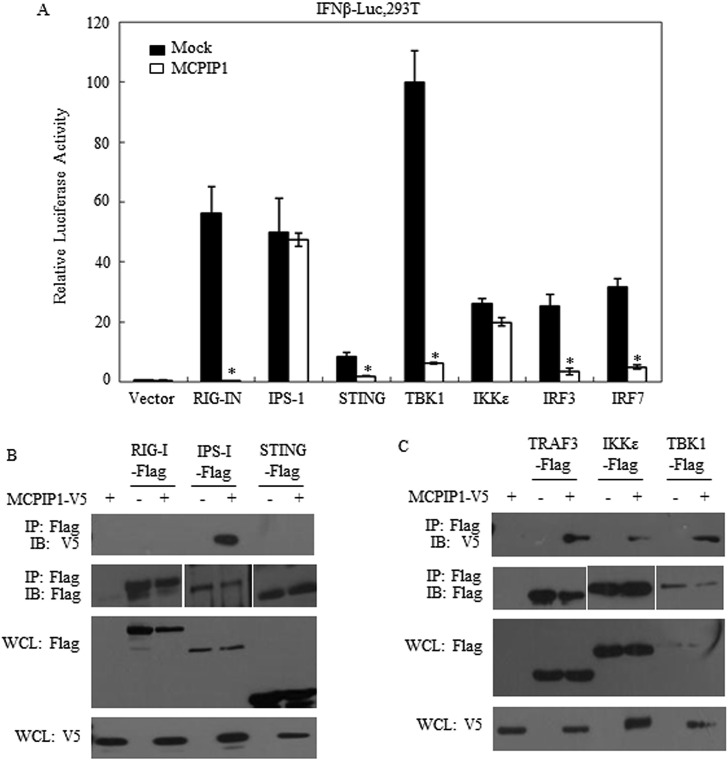
We have previously discovered that human coronavirus (NL63 and SARS) encoding anti-viral protein PLPs, which negatively regulate innate antiviral immune response by disrupting STING-mediated IFN induction [14,21]. Based on these findings, we hypothesized that MCPIP1 inhibits the IFN expression pathway through a similar mechanism. To test this hypothesis, we assessed IFNβ promoter activity level stimulated by several adaptor proteins, i.e. RIG-I, IPS-1, STING, TBK1, IKKε, IRF3 and IRF7, in the presence of MCPIP1. The results in Fig. 2 showed that RIG-I, STING, TBK1, IRF3 and IRF7mediated IFNβ expression were significantly antagonized by MCPIP1 IFNβ expression (Fig. 2A). Moreover, Co-IP assay showed that MCPIP1 may interact with IPS-1, TFAF3, TBK1 and IKKε (Fig. 2B). These findings suggested the probable negatively regulating sites of MCPIP1 on IFNβ expression pathway.
Fig. 2.
MCPIP1 negatively regulates the IFNβ expression activated by RIG-I, STING, TBK1, IRF3. a HEK293T cells were co-transfected with the plasmids which expressing MCPIP1 and IFNβ-Luc. RIG-IN, STING, TBK1, IRF3, IFN7, IPS-1 or IKKε was co-transfected to activate IFN expression pathway. Twenty-four hours later, the cells were harvested and subjected to a Dual-luciferase assay. Data were presented as mean ± SEM, n = 3. *p < 0.05. b HEK293T cells were respectively co-transfected with the plasmids which expressing MCPIP1 and RIG-I, STING, IPS-1, TRAF3, TBK1 or IKKε. Twenty-four hours later, the cells were harvested and Co-IP detection was assayed.
3.3. MCPIP1 inhibits the nuclear translocation of IRF3
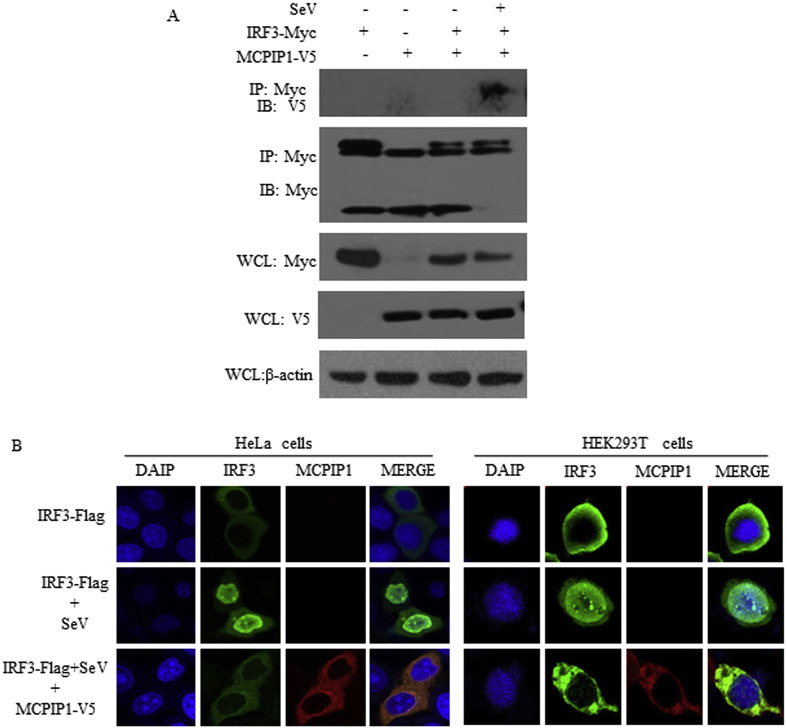
IFNβ transcription requires the activation of transcription factors NF-kB and IRF3 resulting in their subsequent binding to the IFNβ promoter [8,28,29]. To investigate the mechanisms used by MCPIP1 to inhibit antiviral IFNβ expression, the transcriptional activity of NF-kB and IRF3 was analyzed using the luciferase reporter gene detection and Co-IP assay. Luciferase reporter gene detection showed that IRF3-dependent IFNβ activation was significantly inhibited by MCPIP1 (Fig. 1B). It was uncovered by Co-IP detection that cellular MCPIP1 interacts with IRF3 upon SeV infection (Fig. 3 A). Furthermore, to determine whether MCPIP1 prevents IRF3 migration from the cytoplasm to the nucleus, HEK293T cells and Hela cells infected by SeV were transiently transfected with MCPIP1 and IRF3 expression constructs and the subcellular localization of the protein was analyzed using confocal microscopy. As shown in Fig. 3B, we observed that IRF3 was activated by SeV infection and migrated from the cytoplasm to the nucleus. Moreover, when MCPIP1 was co-transfected, the nuclear translocation of IRF3 was prevented.
Fig. 3.
MCPIP1 inhibits the nuclear translocation of IRF3. a HEK293T cells were co-transfected with the plasmids which expressing MCPIP1 and IRF3. Twenty-four hours later, cells were infected with SeV (100 HAU) for another 18 h. Then cells were harvested and detected by Co-IP assay. b HEK293T cells and Hela cells were co-transfected with the plasmids which expressing MCPIP1 and IRF3. Twenty-four hours later, cells were infected with SeV (100 HAU) for another 18 h. Then cells were harvested and detected by immunofluorescence assay.
3.4. MCPIP1 disrupts TRAF3-TBK1-IKKε complex
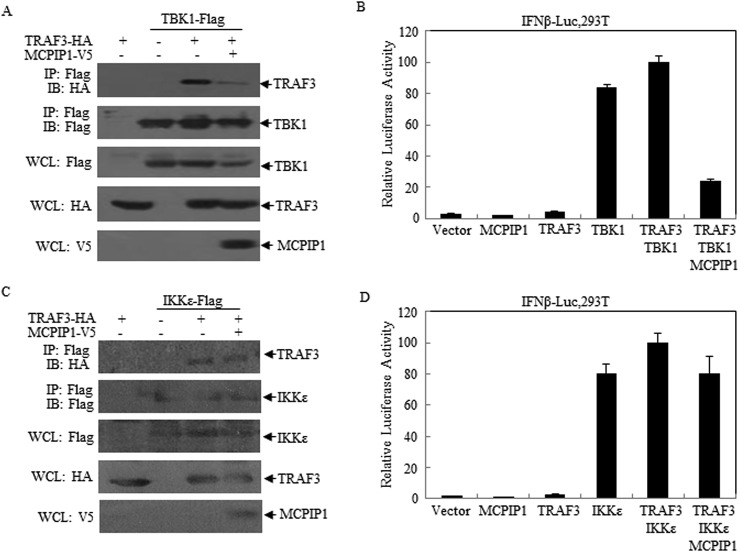
We have previously found that virus-encoded DUB proteins, such as SARS-CoV PLpro-TM, may disrupt STING-TRAF3-TBK1 complex and inhibit IFNβ expression [27]. In this research, we detected the effect of MCPIP1 on TRAF3 complex. It was observed that interaction of TRAF3 with TBK1 was disrupted in the presence of MCPIP1 (Fig. 4 A, lane 4). In agreement with this, luciferase activity assay showed that the IFNβ expression activated by TRAF3 and TBK1 were inhibited by MCPIP1 (Fig. 4B). Meaning while, the interaction of TRAF3 with IKKε, as well as the subsequent IFNβ expression, was not disrupted by MCPIP1 (Fig. 4C and D). These results suggest that MCPIP1 may negatively regulating IFNβ expression through disruption of TRAF complex.
Fig. 4.
MCPIP1 disrupts TRAF3-TBK1-IKKε interaction. a, b HEK293T cells were respectively co-transfected with the plasmids which expressing MCPIP1, IFNβ-Luc, TRAF3 and TBK1. Twenty-four hours later, the cells were harvested and subjected to Co-IP (a) or Dual-luciferase (b) assay. c, d HEK293T cells were respectively co-transfected with the plasmids which expressing MCPIP1, IFNβ-Luc, TRAF3 and IKKε. Twenty-four hours later, the cells were harvested and subjected to Co-IP (c) or Dual-luciferase (d) assay. Data were presented as mean ± SEM, n = 3. *p < 0.05.
4. Discussions
MCPIP1 was identified as a regulator of immunity and negatively regulated JNK and NF-κB activity by removing ubiquitin moieties from TRAFs [22]. However, the mechanisms underwent by MCPIP1 to control immunity have not been deeply understood. In this study, we uncovered a novel mechanism operated by MCPIP1 to negatively regulate IFNβinnate immune. We showed that (1) MCPIP1 inhibits NF-κB activity and IFNβ expression pathway activated by RIG-I, STING, TBK1 and IRF3 in a dose-dependent manner. (2) MCPIP1 interacts with IRF3 and inhibits the nuclear translocation of IRF3 upon stimulation with virus. (3) MCPIP1 interacts with the key modulators of IFNβ expression pathway including IPS1, TRAF3, TBK1 and IKKε. (4) MCPIP1 disrupts the interaction between the components of TRAF3-TBK1-IKKε complex and inhibits the corresponding IFNβ expression. These results collectively suggest a potent mechanism through which MCPIP1 negatively regulates IRF3 activation and IFNβ expression.
MCPIP1, also known as ZC3H12A, was identified in human peripheral blood monocytes treated with MCP-1 [30,31]. Several research groups have found that MCPIP1 is an immune response modifier, however, the novel mechanism remain not fully understood [[32], [33], [34], [35], [36]]. MCPIP1 protein curtains a CCCH Zn finger domain, which is thought as one of the characteristic structure of antiviral protein [37]. Matsushita et al. reported that MCPIP1 has RNase activity and may control immune response through regulating inflammatory mRNA decay [38].
There is a functional ubiquitin association (UBA) domain at the N terminus of MCPIP1. Liang et al. have discovered that MCPIP1 may be a deubiquitinase and defined a novel DUB domain of MCPIP1 [22]. Ubiquitin and deubiquitin modification emerge as the key mechanisms that regulate the virus-induced type I IFN signaling pathways [[39], [40], [41], [42]]. Several cellular DUB proteins play important roles in negative regulation of host innate immunity. We previously found that several CoV encoded proteins also negatively regulate IFNβ expression of the host through DUB activity [15,16,21]. Based on these, we hypothesis that MCPIP1 may also inhibits IFNβ innate immune in cells. The results in this study that MCPIP1 inhibits NF-κB activity and IFNβ expression pathway activated by RIG-I, STING, TBK1 and IRF3 in a dose-dependent manner yielded evidence for our hypothesis.
Innate immune defense mechanism characterized by production of type I interferons was launched by the host upon infection. This innate antiviral response is initiated when viral PAMPs were detected by the host via a number of cellular PRRs, such as the membrane bound Toll-like receptors (TLRs), retinoic acid inducible gene I (RIG-I), or melanoma differentiation-associated gene 5 (MDA5) [[1], [2], [3]]. These PRRs would recruit different adaptor molecules upon engagement of their respective ligands, submitting signals to downstream kinases that activate IRF3, NF-κB and other transcription factors that coordinately regulate IFNβ transcription [2,7]. IRF3 is a constitutively expressed, latent transcription factor that plays a pivotal role in type I IFN responses. The activation of IRF3 requires specific C-terminal phosphorylation. IRF3 may be activated upon phosphorylation mediated by TBK1 and IKKε, which leads to its homodimerization, nuclear translocation, and collaboration with activated NF-κB to induce IFNβ synthesis [[43], [44], [45]].
IFNβ innate antiviral response may be regulated by the host, as well as the viruses. We have previously reported that PLP and 3CLpro encoded by CoVs negatively regulate IFNβ expression of the host [14,16]. In this study, we observed that MCPIP1 inhibited NF-κB activity and IFNβ expression pathway activated by RIG-I, STING, TBK1 and IRF3 in the host cells. Furthermore, the formation of TRAF3-TBK1-IKKε complex is an essential step in the activation of IRF3 [46]. In this study, we found that when MCPIP1 was co-expressed in the cell, the interaction between TRAF3 and TBK1 was significantly disrupted, suggesting the TRAF3-TBK1-IKKε complex and the submitting signals to downstream kinases were blocked. Upon stimulation with virus, IRF3 migrated towards the nuclear. However, the nuclear translocation of IRF3 induced by viral was inhibited when co-expressed with MCPIP1. Taking our previously foundation together with the report by Liang et al. [21,22], we think that the DUB activation of MCPIP1 plays important role in its negatively regulation of IFN expression pathway, which is similar to A20 [12,[47], [48], [49]].
In summary, the results in this study uncovered a novel mechanism used by MCPIP1 to negatively regulated type I IFN antiviral defense. These findings may provide plentiful evidences which may be helpful to deeply understand the working mechanism employed by the antiviral proteins to control innate immune regulation.
Conflicts of interest
The authors have declared that no competing interest exists.
Acknowledgments
This work was supported by grants the National Natural Science Foundation of China (No. 81571982 to Z.C. and No. 81471947 to Y.X.).
References
- 1.Yoneyama M., Kikuchi M., Natsukawa T., Shinobu N., Imaizumi T., Miyagishi M., Taira K., Akira S., Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. PMID: 15208624. [DOI] [PubMed] [Google Scholar]
- 2.Seth R.B., Sun L., Chen Z.J. Antiviral innate immunity pathways. Cell Res. 2006;16:141–147. doi: 10.1038/sj.cr.7310019. PMID: 16474426. [DOI] [PubMed] [Google Scholar]
- 3.Meylan E., Tschopp J. Toll-like receptors and RNA helicases: two parallel ways to trigger antiviral responses. Mol. Cell. 2006;22:561–569. doi: 10.1016/j.molcel.2006.05.012. PMID: 16762830. [DOI] [PubMed] [Google Scholar]
- 4.Kingsolver M.B., Huang Z., Hardy R.W. Insect antiviral innate immunity: pathways, effectors, and connections. J. Mol. Biol. 2013;425:4921–4936. doi: 10.1016/j.jmb.2013.10.006. PMID: 24120681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nilsen N.J., Vladimer G.I., Stenvik J., Orning M.P., Zeid-Kilani M.V., Bugge M., Bergstroem B., Conlon J., Husebye H., Hise A.G., Fitzgerald K.A., Espevik T., Lien E. A role for the adaptor proteins TRAM and TRIF in toll-like receptor 2 signaling. J. Biol. Chem. 2015;290:3209–3222. doi: 10.1074/jbc.M114.593426. PMID: 25505250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S., Cai X., Wu J., Cong Q., Chen X., Li T., Du F., Ren J., Wu Y.T., Grishin N.V., Chen Z.J. Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science. 2015;347 doi: 10.1126/science.aaa2630. aaa2630. PMID: 25636800. [DOI] [PubMed] [Google Scholar]
- 7.Sen G.C. Viruses and interferons. Annu. Rev. Microbiol. 2001;55:255–281. doi: 10.1146/annurev.micro.55.1.255. PMID: 11544356. [DOI] [PubMed] [Google Scholar]
- 8.Ivashkiv L.B., Donlin L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014;14:36–49. doi: 10.1038/nri3581. PMID: 24362405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia-Sastre A., Biron C.A. Type 1 interferons and the virus-host relationship: a lesson in detente. Science. 2006;312:879–882. doi: 10.1126/science.1125676. PMID: 16690858. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T., Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. PMID: 16424890. [DOI] [PubMed] [Google Scholar]
- 11.Cohen P., Strickson S. The role of hybrid ubiquitin chains in the MyD88 and other innate immune signalling pathways. Cell Death Differ. 2017;24:1153–1159. doi: 10.1038/cdd.2017.17. PMID: 28475177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aksentijevich I., Zhou Q. NF-kappab pathway in autoinflammatory diseases: dysregulation of protein modifications by ubiquitin defines a new category of autoinflammatory diseases. Front. Immunol. 2017;8:399. doi: 10.3389/fimmu.2017.00399. PMID: 28469620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimizu Y., Taraborrelli L., Walczak H. Linear ubiquitination in immunity. Immunol. Rev. 2015;266:190–207. doi: 10.1111/imr.12309. PMID: 26085216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun L., Xing Y., Chen X., Zheng Y., Yang Y., Nichols D.B., Clementz M.A., Banach B.S., Li K., Baker S.C., Chen Z. Coronavirus papain-like proteases negatively regulate antiviral innate immune response through disruption of STING-mediated signaling. PLoS One. 2012;7 doi: 10.1371/journal.pone.0030802. e30802. PMID: 22312431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xing Y., Chen J., Tu J., Zhang B., Chen X., Shi H., Baker S.C., Feng L., Chen Z. The papain-like protease of porcine epidemic diarrhea virus negatively regulates type I interferon pathway by acting as a viral deubiquitinase. J. Gen. Virol. 2013;94:1554–1567. doi: 10.1099/vir.0.051169-0. PMID: 23596270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Chen X., Bian G., Tu J., Xing Y., Wang Y., Chen Z. Proteolytic processing, deubiquitinase and interferon antagonist activities of Middle East respiratory syndrome coronavirus papain-like protease. J. Gen. Virol. 2014;95:614–626. doi: 10.1099/vir.0.059014-0. PMID: 24362959. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima T., Matsuzawa S., Kress C.L., Bruey J.M., Krajewska M., Lefebvre S., Zapata J.M., Ronai Z., Reed J.C. Ubiquitin-conjugating enzyme Ubc13 is a critical component of TNF receptor-associated factor (TRAF)-mediated inflammatory responses. Proc. Natl. Acad. Sci. U. S. A. 2007;104:6371–6376. doi: 10.1073/pnas.0700548104. PMID: 17404240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B.B., Coon T.A., Glasser J.R., McVerry B.J., Zhao J., Zhao Y., Zou C., Ellis B., Sciurba F.C., Zhang Y., Mallampalli R.K. A combinatorial F box protein directed pathway controls TRAF adaptor stability to regulate inflammation. Nat. Immunol. 2013;14:470–479. doi: 10.1038/ni. 2565. PMID: 23542741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minassian A., Zhang J., He S., Zhao J., Zandi E., Saito T., Liang C., Feng P. An internally translated MAVS variant exposes its amino-terminal TRAF-binding motifs to deregulate interferon induction. PLoS Pathog. 2015;11 doi: 10.1371/journal.ppat.1005060. e1005060. PMID: 26221961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coutermarsh-Ott S., Eden K., Allen I.C. Beyond the inflammasome: regulatory NOD-like receptor modulation of the host immune response following virus exposure. J. Gen. Virol. 2016;97:825–838. doi: 10.1099/jgv.0.000401. PMID: 26763980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X., Yang X., Zheng Y., Yang Y., Xing Y., Chen Z. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell. 2014;5:369–381. doi: 10.1007/s13238-014-0026-3. PMID: 24622840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang J., Saad Y., Lei T., Wang J., Qi D., Yang Q., Kolattukudy P.E., Fu M. MCP-induced protein 1 deubiquitinates TRAF proteins and negatively regulates JNK and NF-kappaB signaling. J. Exp. Med. 2010;207:2959–2973. doi: 10.1084/jem.20092641. PMID: 21115689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clementz M.A., Chen Z., Banach B.S., Wang Y., Sun L., Ratia K., Baez-Santos Y.M., Wang J., Takayama J., Ghosh A.K., Li K., Mesecar A.D., Baker S.C. Deubiquitinating and interferon antagonism activities of coronavirus papain-like proteases. J. Virol. 2010;84:4619–4629. doi: 10.1128/jvi.02406-09. PMID: 20181693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K., Chen X., Yan F., Xing Y., Yang X., Tu J., Chen Z. 5'-triphosphate-siRNA against survivin gene induces interferon production and inhibits proliferation of lung cancer cells in vitro. J. Immunother. 2013;36:294–304. doi: 10.1097/CJI.0b013e318294183b. PMID: 23719240. [DOI] [PubMed] [Google Scholar]
- 25.Diakos C., Zhong S., Xiao Y., Zhou M., Vasconcelos G.M., Krapf G., Yeh R.F., Zheng S., Kang M., Wiencke J.K., Pombo-de-Oliveira M.S., Panzer-Grumayer R., Wiemels J.L. TEL-AML1 regulation of survivin and apoptosis via miRNA-494 and miRNA-320a. Blood. 2010;116:4885–4893. doi: 10.1182/blood-2009-02-206706. PMID: 20807887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Z., Dong Y., Maeda U., Xia W., Tanzi R.E. RNA interference silencing of the adaptor molecules ShcC and Fe65 differentially affect amyloid precursor protein processing and Abeta generation. J. Biol. Chem. 2007;282:4318–4325. doi: 10.1074/jbc.M609293200. PMID: 17170108. [DOI] [PubMed] [Google Scholar]
- 27.Chen X., Wang K., Xing Y., Tu J., Yang X., Zhao Q., Li K., Chen Z. Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell. 2014;5:912–927. doi: 10.1007/s13238-014-0104-6. PMID: 25311841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bovolenta C., Lou J., Kanno Y., Park B.K., Thornton A.M., Coligan J.E., Schubert M., Ozato K. Vesicular stomatitis virus infection induces a nuclear DNA-binding factor specific for the interferon-stimulated response element. J. Virol. 1995;69:4173–4181. doi: 10.1128/jvi.69.7.4173-4181.1995. PMID: 7539506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thanos D., Maniatis T. Virus induction of human IFN beta gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. PMID: 8548797. [DOI] [PubMed] [Google Scholar]
- 30.Zhou L., Azfer A., Niu J., Graham S., Choudhury M., Adamski F.M., Younce C., Binkley P.F., Kolattukudy P.E. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ. Res. 2006;98:1177–1185. doi: 10.1161/01.res.0000220106.64661.71. PMID: 16574901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang J., Wang J., Azfer A., Song W., Tromp G., Kolattukudy P.E., Fu M. A novel CCCH-zinc finger protein family regulates proinflammatory activation of macrophages. J. Biol. Chem. 2008;283:6337–6346. doi: 10.1074/jbc.M707861200. PMID: 18178554. [DOI] [PubMed] [Google Scholar]
- 32.Jura J., Skalniak L., Koj A. Monocyte chemotactic protein-1-induced protein-1 (MCPIP1) is a novel multifunctional modulator of inflammatory reactions. Biochim. Biophys. Acta. 2012;1823:1905–1913. doi: 10.1016/j.bbamcr.2012.06.029. PMID: 22771441. [DOI] [PubMed] [Google Scholar]
- 33.Xu J., Fu S., Peng W., Rao Z. MCP-1-induced protein-1, an immune regulator. Protein Cell. 2012;3:903–910. doi: 10.1007/s13238-012-2075-9. PMID: 23132255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dobosz E., Wilamowski M., Lech M., Bugara B., Jura J., Potempa J., Koziel J. MCPIP-1, alias Regnase-1, controls epithelial inflammation by posttranscriptional regulation of IL-8 production. J Innate Immun. 2016;8:564–578. doi: 10.1159/000448038. PMID: 27513529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Happel C., Ramalingam D., Ziegelbauer J.M. Virus-mediated alterations in miRNA factors and degradation of viral miRNAs by MCPIP1. PLoS Biol. 2016;14 doi: 10.1371/journal.pbio.2000998. e2000998. PMID: 27893764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monin L., Gudjonsson J.E., Childs E.E., Amatya N., Xing X., Verma A.H., Coleman B.M., Garg A.V., Killeen M., Mathers A., Ward N.L., Gaffen S.L. MCPIP1/Regnase-1 restricts IL-17A- and il-17C-dependent skin inflammation. J. Immunol. 2017;198:767–775. doi: 10.4049/jimmunol.1601551. PMID: 27920272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G., Guo X., Lv F., Xu Y., Gao G. p72 DEAD box RNA helicase is required for optimal function of the zinc-finger antiviral protein. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4352–4357. doi: 10.1073/pnas.0712276105. PMID: 18334637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsushita K., Takeuchi O., Standley D.M., Kumagai Y., Kawagoe T., Miyake T., Satoh T., Kato H., Tsujimura T., Nakamura H., Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–1190. doi: 10.1038/nature07924. PMID: 19322177. [DOI] [PubMed] [Google Scholar]
- 39.Bibeau-Poirier A., Servant M.J. Roles of ubiquitination in pattern-recognition receptors and type I interferon receptor signaling. Cytokine. 2008;43:359–367. doi: 10.1016/j.cyto.2008.07.012. PMID: 18707898. [DOI] [PubMed] [Google Scholar]
- 40.Bhoj V.G., Chen Z.J. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. PMID: 19325622. [DOI] [PubMed] [Google Scholar]
- 41.Isaacson M.K., Ploegh H.L. Ubiquitination, ubiquitin-like modifiers, and deubiquitination in viral infection. Cell Host Microbe. 2009;5:559–570. doi: 10.1016/j.chom.2009.05.012. PMID: 19527883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhong B., Zhang Y., Tan B., Liu T.T., Wang Y.Y., Shu H.B. The E3 ubiquitin ligase RNF5 targets virus-induced signaling adaptor for ubiquitination and degradation. J. Immunol. 2010;184:6249–6255. doi: 10.4049/jimmunol.0903748. PMID: 20483786. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S., TenOever B.R., Grandvaux N., Zhou G.P., Lin R., Hiscott J. Triggering the interferon antiviral response through an IKK-related pathway. Science. 2003;300:1148–1151. doi: 10.1126/science.1081315. PMID: 12702806. [DOI] [PubMed] [Google Scholar]
- 44.Fitzgerald K.A., McWhirter S.M., Faia K.L., Rowe D.C., Latz E., Golenbock D.T., Coyle A.J., Liao S.M., Maniatis T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. PMID: 12692549. [DOI] [PubMed] [Google Scholar]
- 45.Yoneyama M., Suhara W., Fujita T. Control of IRF-3 activation by phosphorylation. J. Interferon Cytokine Res. 2002;22:73–76. doi: 10.1089/107999002753452674. PMID: 11846977. [DOI] [PubMed] [Google Scholar]
- 46.Siu K.L., Kok K.H., Ng M.H., Poon V.K., Yuen K.Y., Zheng B.J., Jin D.Y. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J. Biol. Chem. 2009;284:16202–16209. doi: 10.1074/jbc.M109.008227. PMID: 19380580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun S.C. Deubiquitylation and regulation of the immune response. Nat. Rev. Immunol. 2008;8:501–511. doi: 10.1038/nri2337. PMID: 18535581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pujari R., Hunte R., Khan W.N., Shembade N. A20-mediated negative regulation of canonical NF-kappaB signaling pathway. Immunol. Res. 2013;57:166–171. doi: 10.1007/s12026-013-8463-2. PMID: 24242761. [DOI] [PubMed] [Google Scholar]
- 49.Coornaert B., Carpentier I., Beyaert R. A20: central gatekeeper in inflammation and immunity. J. Biol. Chem. 2009;284:8217–8221. doi: 10.1074/jbc.R800032200. PMID: 19008218. [DOI] [PMC free article] [PubMed] [Google Scholar]