Abstract
Definable habitats at the neighborhood level provide a wide range of favorable habitats with optimal conditions and environmental resources for mosquito survival. Problematic habitats for controlling mosquitoes in urban environments such as tire shops, bromeliad patches, and construction sites must be taken into consideration in the development of effective mosquito management and control in urban areas. Cemeteries are often located in highly urbanized areas serving as a haven for populations of vector mosquito species due to the availability of natural resources present in most cemeteries. Even though Miami-Dade County, Florida was the most affected area in the United States during the Zika virus outbreak in 2016 and is currently under a mosquito-borne illness alert after 14 confirmed locally transmitted dengue cases, the role of cemeteries in the proliferation of vector mosquitoes is unknown. Therefore, our objective was to use a cross-sectional experimental design to survey twelve cemeteries across Miami-Dade County to assess if vector mosquitoes in Miami can be found in these areas. Our results are indicating that vector mosquitoes are able to successfully exploit the resources available in the cemeteries. Culex quinquefasciatus was the most abundant species but it was neither as frequent nor present in its immature form as Aedes aegypti and Aedes albopictus. This study revealed that vector mosquitoes, such as Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus are successfully exploiting the resources available in these areas being able to thrive and reach high numbers. Mosquito control strategies should consider both long-term strategies, based on changing human behavior to reduce the availability of aquatic habitats for vector mosquitoes; as well as short-term strategies such as drilling holes or adding larvicide to the flower vases. Simple practices would greatly help improve the effectiveness of mosquito management and control in these problematic urban habitats.
Introduction
Vector mosquitoes such as Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus are expanding their range globally [1–3]. Invasive mosquito vector species often benefit from the decrease in the overall biodiversity of species due to an increase in urbanization [4]. Such biotic homogenization processes not only increase the contact between human hosts and mosquito vectors but also act as drivers that positively increase the range and abundance of populations of mosquito vector species [4–7].
The increase in range and abundance of vector mosquitoes can be deemed as one of the factors for the substantial increase in the incidence of vector-borne diseases (VBDs) [8,9]. In 2019 alone, 2,853,248 million cases of dengue have been reported in the Americas [10], and the current estimates for the global human dengue infections range around 390 million cases per year with no signs of slowing down [11]. Moreover, VBD outbreaks are not only being reported more frequently in endemic areas [10,12,13], but are also been reported in formerly non-endemic countries such as Croatia, France, and Italy [14–16].
There are only a few vaccines and treatments available to prevent arbovirus infections. However, they are not enough to control and prevent VBD outbreaks. Despite the availability of a safe and effective yellow fever vaccine, many logistic issues with its production and distribution as well as public adherence to vaccination campaigns have impaired its effectiveness [17–20]. Recent major outbreaks in South America caused hundreds of deaths [21]. The situation in Africa is even more troublesome, yellow fever virus outbreaks in Angola and in the Democratic Republic of the Congo have resulted in thousands of deaths and current death estimates in the entire continent range around 78,000 deaths per year [22,23].
Considering the above scenario, controlling vector mosquito populations is the most effective way to prevent the transmission of VBDs [24], and has been proven feasible under the Integrated Vector Management framework (IVM) [25]. Controlling vector mosquitoes is a complex and dynamic task that relies on many actions that logically add to each other [26]. Effective surveillance is an essential part of IVM, and the development of effective mosquito management and control relies on the behavior of the target species and where they are concentrated, abundant, and harder to reach.
Definable habitats at the neighborhood level provide a wide range of favorable habitats with optimal conditions and environmental resources for mosquito survival. Problematic habitats for controlling mosquitoes in urban environments such as tire shops [27], bromeliad patches [28], and construction sites [29] must be taken into consideration in the development of effective mosquito management and control in urban areas.
IVM is currently the gold standard for controlling vector mosquitoes [26]. It has achieved success in the past in eliminating vector mosquitoes from urban areas [30]. However, the implementation of mosquito management and control operations based on the IVM is complex and requires multiple approaches in an interdisciplinary framework that builds on each other in a systematic manner. Moreover, the lack of evidence of the effectiveness of new tools to control vector mosquitoes based on genetically modified mosquitoes added to their prohibitive cost put these approached far from being used in real-world conditions [31]. Therefore, understanding how vector mosquitoes are exploiting the resources available in urban habitats and how they are concentrated in urban areas is key for the identification of modifiable urban features to guide and improve the development of effective mosquito management and control strategies in urban areas.
In this context, the role of cemeteries in the proliferation of vector mosquitoes and in monitoring VBD transmission has been intensively studied over the years. [32–38]. Cemeteries are often located in highly urbanized areas serving as a haven for populations of vector mosquito species due to the availability of natural resources present in most cemeteries such as: (i) sugar sources, from both the natural vegetation and ornamental flowers; (ii) widely available vegetated areas for mosquitoes to rest; (iii) high availability of breeding habitats in the form of flower vases, ornamental bromeliads, and adornment items such as toys and fishbowls; and (iv) large presence of human hosts for blood-feeding.
Even though Miami-Dade County, Florida was the most affected area in the continental United States during the Zika virus outbreak in 2016 [39], 212 imported dengue cases were reported in 2019 [40] and Miami is currently under a mosquito-borne illnesses alert after 14 confirmed locally transmitted dengue cases [41]. The role of cemeteries in the proliferation of vector mosquitoes in Miami is unknown. Therefore, our objective was to use a cross-sectional experimental design to survey twelve cemeteries across Miami-Dade County to assess if vector mosquitoes can be found in these areas.
Methods
Study design
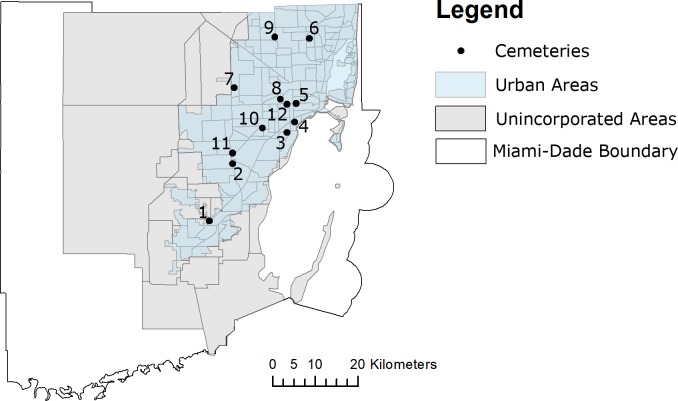
This study used a cross-sectional design to survey the presence of vector mosquitoes in 12 cemeteries located in urbanized areas of Miami-Dade County, Florida. Aiming to provide comprehensive coverage of the cemeteries in Miami-Dade County, we selected cemeteries considering their location in different urban areas as well as their types and layouts, such as cemetery with and without tombstones or plaques and apparent or buried coffin or sarcophagus. (Fig 1).
Fig 1. Map showing the location of the mosquito-surveyed cemeteries in Miami-Dade, Florida (latitude, 25.761681; longitude, -80.191788).
Fig 1 was produced using ArcGIS 10.2 (Esri, Redlands, CA) using freely available layers from the Miami-Dade County’s Open Data Hub— https://gis-mdc.opendata.arcgis.com/.
We included in this study cemeteries with different sizes and layouts. The perimeter of the cemeteries ranged from 0.38 to 2.88 Km and the area ranged from 0.01 Km2 to 0.5 km2. All the cemeteries surveyed during this study, but cemetery 3 and 4, had similar layouts with grassed areas comprising most of the cemetery with tombstones or plaques but no apparent coffin or sarcophagus. Flower vases were present in all cemeteries, but cemetery 3.
Cemetery 3 is not currently an active burial ground and the last known burial dates in the 1940s. This area has been serving as burial grounds since the 1850s and formally became a cemetery in the 1900s. This cemetery is completely covered by vegetation and has very few ornaments other than simple tombstones on top of the graves. Cemetery 4 has a similar history. It was first used as a graveyard in the late 1850s and the land was officially purchased in the 1910s by local families. This cemetery has a unique layout with all the burials done above ground with the coffins put inside a concrete sarcophagus. All the cemeteries surveyed in this study were well maintained and relatively clean.
Mosquito sampling and identification
Mosquitoes were collected in September and October 2019 in twelve cemeteries located in urban areas in Miami-Dade County, Florida with no on-going mosquito control. We used a standardized sampling effort for all collections. Each cemetery was surveyed once. Adult mosquitoes were collected with BG-Sentinel 2 traps (Biogents AG, Regensburg, Germany) baited with dry ice [42], for 24 hours. Two BG-Sentinel traps were used to collect adult mosquitoes at each cemetery. One trap was set near the main office and the second in a more secluded area. BG-Sentinel traps were placed in shaded areas protected from the elements to enhance the collection of mosquitoes.
Cemeteries were surveyed for immature mosquitoes for two hours or until all potential breeding sites were exhausted. Larvae and pupae were collected with manual plastic pumps (turkey basters) and stored in plastic containers (100 ml) for transport. The collected mosquitoes were transported to the Miami-Dade County Mosquito Control Laboratory. All specimens were morphologically identified to species using taxonomic keys [43]. Larvae were grown to L4 and then identified, pupae were allowed to emerge as adults and then identified, and all the adult mosquitoes collected in this study were kept at 4°C until identified.
Data analysis
The boxplot displaying the total number of mosquitoes collected in the twelve cemeteries and the diversity profiles were created using Past software (v.3.16) [44,45]. Diversity profiles were based on the Renyi index which depends upon a parameter α (alpha). Values at α = 0 represent the total number of species for each cemetery; values at α = 1 represents an index proportional to the Shannon index (i.e., a lesser amount of importance to the presence of rare species); and values at α = 2 represent an index proportional to the Simpson index (i.e., a higher amount of importance to the presence of frequent species rather than rare species) [46,47]. The Shannon and Simpson indices [48,49], have been widely used to asses diversity variation patterns in ecological communities [50]. The Shannon index considers species abundance and communities, in which lower values indicate less diversity. On the other hand, the Simpson index estimates species dominance, values close to 0 indicate the presence of dominant species whereas values close to 1 indicate high levels of diversity [51].
A bivariate linear regression using ordinary least squares was used to estimate the association between the perimeter, area and the Normalized Difference Vegetation Index (NDVI) of the cemeteries with the relative abundance of mosquitoes. We used the 30 m Landsat 8 OLI NDVI calculated from bands 5 (near-infrared) and 4 (red) [52], which were atmospherically corrected and obtained via the US Geological Survey website, Earth Explorer (https://earthexplorer.usgs.gov/). The Landsat image was acquired on 13 January 2018. As NDVI is directly proportional to photosynthetic activity and plant canopy greenness, it provides an indicator of surface moisture and plant cover, which can affect mosquito population densities [53]. Analyses were carried out with 10,000 randomizations without replacement and a 95% confidence interval using Past software (v.3.16) [44,45].
Since this study posed less than minimal risk to participants and did not involve endangered or protected species the Institutional Review Board at the University of Miami determined that the study was exempt from institutional review board assessment (IRB Protocol Number: 20161212). Collections of mosquitoes were conducted only upon authorization.
Results
A total of nine mosquito species were collected in the twelve cemeteries surveyed during this study. Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus were the most common and abundant species comprising 98% of all specimens collected.
Aedes aegypti was the most common species being found in all cemeteries, but cemetery 3, totaling 228 adults and 89 larvae and 83 pupae. Culex quinquefasciatus was the second most common species being found in all cemeteries but cemeteries 2 and 3, totaling 276 adults, 135 larvae and 3 pupae. Aedes albopictus was the third most common species being present in half of the surveyed cemeteries, totaling 51 adults, 26 larvae and 8 pupae (Table 1).
Table 1. Total number of mosquitoes collected at cemeteries in Miami-Dade County, Florida.
| Aedes aegypti | Aedes albopictus | Aedes triseriatus | Culex coronator | Culex quinquefasciatus | Culex interrogator | Culex nigripalpus | Wyeomyia mitchelli | Wyeomyia vanduzeei | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cemetery | A | I | A | I | A | I | A | I | A | I | A | I | A | I | A | I | A | I |
| 1 | 18 [20] | 1 | 1 | 11 | 15 [25] | 2 | 3 | |||||||||||
| 2 | 5 | |||||||||||||||||
| 3 | ||||||||||||||||||
| 4 | 13 [17] | 1 | 5 [6] | 7 | ||||||||||||||
| 5 | 7 [7] | 22 (17) | 17 [24] | |||||||||||||||
| 6 | 14 [17] | 1 | 25 [38] | |||||||||||||||
| 7 | 17 [5] | 15 (4) | 1 | 13 [31] | 1 (3) | 1 | ||||||||||||
| 8 | 1 [2] | 6 (9) | 37 [4] | 11 (8) | 1 | 16 [27] | 1 | 1 | ||||||||||
| 9 | 1 | (2) | 5 [1] | |||||||||||||||
| 10 | 6 [1] | 7 | 4 | 1 | 1 [5] | |||||||||||||
| 11 | 19 [12] | 40 (51) | 6 [3] | 134 | 2 | |||||||||||||
| 12 | 19 [32] | 6 [7] | ||||||||||||||||
| Total | 115 [113] | 89 (83) | 47 [4] | 26 (8) | 1 | 2 | 109 [167] | 135 (3) | 2 | 4 | 1 | 3 | 7 | |||||
A = adult mosquitoes, number of females and in brackets the number of males; I = immature mosquitoes; in parenthesis, the number of pupae.
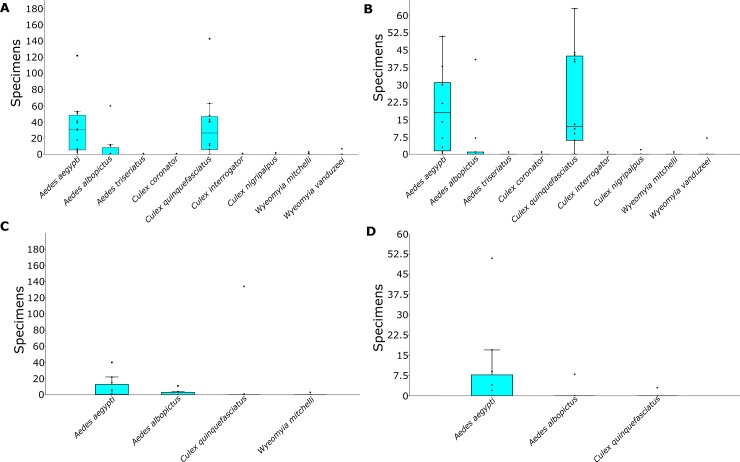
Culex quinquefasciatus and Ae. aegypti yielded the highest median values, 30.5 and 26.5 specimens respectively, among all specimens collected during this study (Fig 2A). A similar pattern was found for the adult mosquitoes, in which adult Ae. aegypti and Cx. quinquefasciatus mosquitoes were more abundant and yielded higher median values when compared to the other species found within cemeteries in Miami (Fig 2B). However, this pattern was not maintained in the immature mosquito population. Aedes aegypti was the most common species found in both the larval and pupal stage (Fig 2C and 2D).
Fig 2. Box plot graph displaying the total number of mosquitoes collected in the twelve cemeteries surveyed in Miami-Dade County, Florida.
(A) All collected mosquitoes; (B) adult mosquitoes; (C) larvae; and (D) pupae. Boxes represent the 25–75 percent quartiles; the horizontal line inside the box represents the median; the whiskers represent the largest data point less than 1.5 times the box height; and values further that limit are shown as outlier dots.
The Bivariate Linear Regression resulted in non-significant values considering the relative abundance of mosquitoes and the perimeter, area, and NDVI of the cemeteries surveyed in this study. These results are indicative that the population dynamics of the mosquito species found in cemeteries were being driven by factors other than the increased availability of green areas such as the availability of specific conditions and resources allowing mosquito development (Tables 2 and 3).
Table 2. Mosquito counts and area, perimeter, and NDVI of the 12 cemeteries surveyed in Miami-Dade County, Florida.
| Cemetery | Perimeter (Km) | Area (Km2) | Vases | NDVI | All Mosquitoes | Adults | Larvae | Pupae |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.54 | 0.15 | P | 0.604911 | 96 | 81 | 15 | 0 |
| 2 | 2.88 | 0.24 | P | 0.544393 | 5 | 0 | 5 | 0 |
| 3 | 0.66 | 0.02 | A | 0.813361 | 0 | 0 | 0 | 0 |
| 4 | 0.32 | 0.01 | P | 0.618182 | 49 | 49 | 0 | 0 |
| 5 | 2.46 | 0.28 | P | 0.323109 | 94 | 55 | 22 | 17 |
| 7 | 2.83 | 0.5 | P | 0.673231 | 95 | 95 | 0 | 0 |
| 8 | 2.25 | 0.27 | P | 0.492329 | 91 | 68 | 16 | 7 |
| 6 | 2.03 | 0.24 | P | 0.658885 | 124 | 90 | 17 | 17 |
| 9 | 1.96 | 0.23 | P | 0.641099 | 9 | 7 | 0 | 2 |
| 10 | 2.19 | 0.28 | P | 0.597605 | 25 | 21 | 4 | 0 |
| 11 | 2.25 | 0.24 | P | 0.44184 | 267 | 42 | 174 | 51 |
| 12 | 1 | 0.06 | P | 0.549094 | 64 | 64 | 0 | 0 |
NDVI = Normalized Difference Vegetation Index; P = Present, A = Absent.
Table 3. Bivariate Linear Regression for mosquitoes collected at cemeteries in Miami-Dade County, Florida.
| Perimeter | Area | NDVI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r | r2 | P | r | r2 | P | r | r2 | P | |
| All mosquitoes | 0.2393 | 0.0572 | 0.4537 | 0.2584 | 0.0667 | 0.4173 | -0.4704 | 0.2213 | 0.1227 |
| Adults | 0.1041 | 0.0108 | 0.7474 | 0.3292 | 0.1084 | 0.2959 | -0.1812 | 0.0328 | 0.5937 |
| Larvae | 0.2053 | 0.0421 | 0.5220 | 0.1063 | 0.0113 | 0.7421 | -0.4402 | 0.1938 | 0.1520 |
| Pupae | 0.2605 | 0.0678 | 0.4134 | 0.1640 | 0.0269 | 0.6104 | -0.5113 | 0.2615 | 0.0892 |
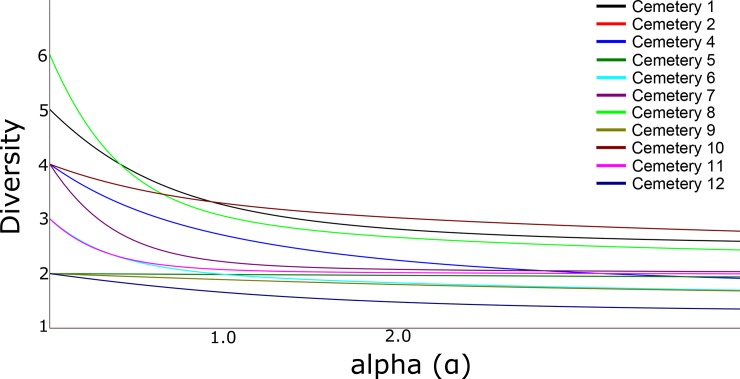
The Diversity profiles analysis shows that cemetery 8 had the highest mosquito richness among the cemeteries surveyed in this study comprising 6 different species followed by cemetery 1 with 5 species, and cemeteries 4, 7, and 10 with 4 species. However, even though the species richness was relatively high considering cemeteries only comprise small areas within the urban matrix, Ae. aegypti and Cx. quinquefasciatus were the most dominant species, being present in eleven and ten out of the twelve surveyed cemeteries, respectively (Fig 3). Therefore, the analysis suggests that despite the relatively high mosquito species richness found in the cemeteries the mosquito community yielded low evenness values due to the presence of the three most dominant species, Ae. aegypti, Cx. quinquefasciatus, and Ae. albopictus.
Fig 3. Diversity profiles considering the mosquito species collected in cemeteries in Miami-Dade County, Florida.
Values at α = 0 represent the total number of species for each cemetery; values at α = 1 represent an index proportional to the Shannon index (i.e., a lesser amount of importance to the presence of rare species); and values at α = 2 represent an index proportional to the Simpson index (i.e., a higher amount of importance to the presence of frequent species rather than rare species).
Discussion
Our results are indicating that vector mosquitoes are able to successfully exploit the resources available in the cemeteries allowing them to reach high densities. Culex quinquefasciatus was the most abundant species but it was neither as frequent nor present in its immature form as Ae. aegypti. Aedes aegypti was the most frequent and abundant species found in the pupal stage, which is a clear indication of mosquito production, revealing how Ae. aegypti is successfully exploiting the habitats available in the cemeteries. Aedes albopictus was also found in relatively high frequency and abundance in its immature form, while Cx. quinquefasciatus was found in large numbers in only one breeding site, a fishing bowl filled with rainwater placed in a shaded area.
Aedes albopictus is not commonly found in Miami. From August 2016 to November 2018, 150,588 adult Ae. aegypti were collected in Miami, while 11,405 Ae. albopictus were collected in the same period [6]. A similar result was found for their immature forms, from 2,488 inspections performed by Miami-Dade Mosquito Control inspectors, from April 2018 to June 2019, a total of 19,206 Ae. aegypti larvae and 2,997 pupae were collected, while only 325 Ae. albopictus larvae and 65 pupae were collected in the same period [54]. The fact that Ae. albopictus was the third most abundant species collected in the cemeteries reveals the importance of this environment to its maintenance in the urban areas of Miami-Dade County. The high epidemiological importance of both Ae. aegypti and Ae. albopictus highlights the importance of monitoring urban habitats that provide optimal conditions for mosquito development, such as in cemeteries.
Cemeteries and burial grounds are present in all urban settings around the globe. Cemeteries are complex environments and greatly vary in size, form, and shape. However, most cemeteries share similar features, such as vegetated areas with many sources of sugar and resting areas for vector mosquitoes, and widely available flower vases and ornamental plants serving as suitable aquatic habitats for vector mosquitoes. The lack of association between mosquito abundance and vegetation coverage, area, and perimeter of the cemeteries surveyed in this study shows the importance of considering local features, such as flower vases and other potential aquatic habitats for mosquitoes, for the development of effective control strategies.
Even though the role of cemeteries in the proliferation of vector mosquitoes has been studied for many years [32], not only the mosquito community composition varies from place to place but resource availability, local variation in climate and the increase in global temperatures due to global warming also have to be taken into consideration [54,55]. Constant surveillance of problematic areas for the proliferation of vector mosquitoes in urban areas is key to not only better understand how mosquito species may be exploiting these habitats but also to assess the spreading of invasive species in urban areas. Culex coronator has recently invaded the U.S. and was first detected in Miami-Dade County in 2008 [56]. The fact that it was found in two cemeteries highlights the importance of surveying these areas even though there is a solid body of literature on this subject.
Furthermore, as in other occupations such as in the construction workforce, cemetery workers that are responsible for landscaping and general maintenance spent a disproportional amount of time outdoors and are, therefore, subjected to vector mosquito bites being at a higher risk of being exposed to arboviruses [57–59].
In this context, cemeteries are problematic urban environments for the management and control of vector mosquitoes and should be considered key urban areas to be targeted and included in mosquito management and control strategies. The great availability of cryptic and difficult to reach aquatic habitats increases the difficulty in controlling vector mosquitoes in cemeteries. However, environmental management and removal of aquatic habitats proved to be an effective way to manage and control vector mosquitoes [30]. Furthermore, the fact that vector mosquitoes were collected all cemeteries but in cemetery 3, which had no available artificial aquatic habitat in its premises highlights the impact of anthropogenic alteration in the environment as a driver for the proliferation of vector mosquitoes in cemeteries and other problematic urban habitats.
The cross-sectional experimental design used in this study has limitations. It may have underestimated the species richness by failing to collect rare species and was unable to detect fluctuations in the mosquito community over time. However, it is appropriate to assess what are the most present and abundant species that should be targeted by mosquito control strategies.
Conclusion
The cross-sectional mosquito survey in the cemeteries of Miami-Dade County, Florida revealed that vector mosquitoes, such as Ae. aegypti, Ae. albopictus, and Cx. quinquefasciatus are successfully exploiting the resources available in these areas being able to thrive and reach high numbers. The better understanding of how vector mosquitoes are exploiting urban habitats is vital for the development of more effective and reliable long-term mosquito management and control strategies. Even though increasing community awareness aiming to change human behavior is the ultimate goal for the development of effective mosquito management and control strategies, it is an arduous task and rarely produces immediate results. There are, however, simple practices that could greatly impact the number of available aquatic breeding habitats and as a consequence the number of vector mosquitoes. Practices as simple as drilling holes or adding larvicide to the flower vases would have a substantial effect on the availability of suitable aquatic habitats for vector mosquitoes and would help decrease the number of mosquitoes in these areas.
Acknowledgments
We would like to thank the staff of the Miami-Dade County Mosquito Control Division for their help in the processing and identification of the mosquitoes.
Data Availability
All relevant data are within the manuscript
Funding Statement
JCB Miami-Dade Mosquito Control Division JCB CDC (https://www.cdc.gov/) grant 1U01CK000510-03: Southeastern Regional Center of Excellence in Vector-Borne Diseases: The Gateway Program.
References
- 1.Kraemer MUG, Sinka ME, Duda KA, Mylne A, Shearer FM, Brady OJ, et al. The global compendium of Aedes aegypti and Ae. albopictus occurrence. Sci Data. 2015;2: 150035 10.1038/sdata.2015.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samy AM, Elaagip AH, Kenawy MA, Ayres CFJ, Peterson AT, Soliman DE. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS One. 2016;11: e0163863 10.1371/journal.pone.0163863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benelli G, Wilke ABB, Beier JC. Aedes albopictus (Asian Tiger mosquito). Trends Parasitol. 2020. 10.1016/j.pt.2020.01.001 [DOI] [PubMed] [Google Scholar]
- 4.McKinney ML. Urbanization as a major cause of biotic homogenization. Biol Conserv. 2006;127: 247–260. [Google Scholar]
- 5.Johnson MTJ, Munshi-South J. Evolution of life in urban environments. Science. 2017;358: eaam8327 10.1126/science.aam8327 [DOI] [PubMed] [Google Scholar]
- 6.Wilke ABB, Vasquez C, Medina J, Carvajal A, Petrie W, Beier JC. Community composition and year-round abundance of vector species of mosquitoes make Miami-Dade County, Florida a receptive gateway for arbovirus entry to the United States. Sci Rep. 2019;9: 8732 10.1038/s41598-019-45337-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamal M, Kenawy MA, Rady MH, Khaled AS, Samy AM. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS One. 2018;13: e0210122 10.1371/journal.pone.0210122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, et al. Vital Signs: Trends in reported vectorborne disease cases—United States and Territories, 2004–2016. Morb Mortal Wkly Rep. 2018;67: 496–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Messina J, Brady O, Pigott D, Brownstein J, Hoen A, Hay S. A global compendium of human dengue virus occurrence. Sci Data. 2014;1: 140004 10.1038/sdata.2014.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PAHO/WHO. Reported Cases of Dengue Fever in The Americas, 2019. World Health Organization; Available at: http://www.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en/dengue-nacional-en/252-dengue-pais-ano-en.html [Google Scholar]
- 11.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paules CI, Fauci AS. Yellow fever—Once again on the radar screen in the Americas. N Engl J Med. 2017;376: 1397–1399. 10.1056/NEJMp1702172 [DOI] [PubMed] [Google Scholar]
- 13.Abdul-Ghani R, Mahdy MAK, Al-Eryani SMA, Fouque F, Lenhart AE, Alkwri A, et al. Impact of population displacement and forced movements on the transmission and outbreaks of Aedes-borne viral diseases: Dengue as a model. Acta Trop. 2019;197: 105066 10.1016/j.actatropica.2019.105066 [DOI] [PubMed] [Google Scholar]
- 14.Poletti P, Messeri G, Ajelli M, Vallorani R, Rizzo C, Merler S. Transmission potential of chikungunya virus and control measures: The case of italy. PLoS One. 2011;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gould EA, Gallian P, De Lamballerie X, Charrel RN. First cases of autochthonous dengue fever and chikungunya fever in France: From bad dream to reality! Clin Microbiol Infect. 2010;16: 1702–1704. 10.1111/j.1469-0691.2010.03386.x [DOI] [PubMed] [Google Scholar]
- 16.Gjenero-Margan I, Aleraj B, Krajcar D, Lesnikar V, Klobucar A, Pem-Novosel I, et al. Autochthonous dengue fever in Croatia, August-September 2010. Euro Surveill. 2011;16: 1–4. [PubMed] [Google Scholar]
- 17.Nathan N, Barry M, Van Herp M, Zeller H. Shortage of vaccines during a yellow fever outbreak in Guinea. Lancet. 2001;358: 2129–2130. 10.1016/S0140-6736(01)07185-9 [DOI] [PubMed] [Google Scholar]
- 18.Weitzel T, Vial P, Perret C, Aguilera X. Shortage of yellow fever vaccination: A travel medicine emergency for Chilean travellers. Travel Med Infect Dis. 2019;28: 1–2. 10.1016/j.tmaid.2019.01.016 [DOI] [PubMed] [Google Scholar]
- 19.Gershman MD, Angelo KM, Ritchey J, Greenberg DP, Muhammad RD, Brunette G, et al. Addressing a yellow fever vaccine shortage—United States, 2016–2017. Morb Mortal Wkly Rep. 2017;66: 457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrett ADT. Yellow Fever in Angola and Beyond—The problem of vaccine supply and demand. N Engl J Med. 2016;375: 301–303. 10.1056/NEJMp1606997 [DOI] [PubMed] [Google Scholar]
- 21.PAHO/WHO. Epidemiological Update: Yellow Fever, 2019. World Health Organization; Available at: https://reliefweb.int/sites/reliefweb.int/files/resources/2017-ago-2-phe-epi-update-yellow-fever.pdf [Google Scholar]
- 22.Hamlet A, Jean K, Perea W, Yactayo S, Biey J, Van Kerkhove M, et al. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Negl Trop Dis. 2018;12: e0006284 10.1371/journal.pntd.0006284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garske T, Van Kerkhove MD, Yactayo S, Ronveaux O, Lewis RF, Staples JE, et al. Yellow fever in Africa: Estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med. 2014;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roiz D, Wilson AL, Scott TW, Fonseca DM, Jourdain F, Müller P, et al. Integrated Aedes management for the control of Aedes-borne diseases. PLoS Negl Trop Dis. 2018;12: e0006845 10.1371/journal.pntd.0006845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trewin BJ, Darbro JM, Jansen CC, Schellhorn NA, Zalucki MP, Hurst TP, et al. The elimination of the dengue vector, Aedes aegypti, from Brisbane, Australia: The role of surveillance, larval habitat removal and policy. PLoS Negl Trop Dis. 2017;11: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lizzi KM, Qualls WA, Brown SC, Beier JC. Expanding Integrated Vector Management to promote healthy environments. Trends Parasitol. 2014;30: 394–400. 10.1016/j.pt.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilke ABB, Vasquez C, Petrie W, Beier JC. Tire shops in Miami-Dade County, Florida are important producers of vector mosquitoes. PLoS One. 2019;14: e0217177 10.1371/journal.pone.0217177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilke ABB, Vasquez C, Mauriello PJ, Beier JC. Ornamental bromeliads of Miami-Dade County, Florida are important breeding sites for Aedes aegypti (Diptera: Culicidae). Parasit Vectors. 2018;11: 283 10.1186/s13071-018-2866-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilke ABB, Vasquez C, Petrie W, Caban-Martinez AJ, Beier JC. Construction sites in Miami-Dade County, Florida are highly favorable environments for vector mosquitoes. PLoS One. 2018;13: e0209625 10.1371/journal.pone.0209625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Achee NL, Gould F, Perkins TA, Reiner RC, Morrison AC, Ritchie S a, et al. A critical assessment of vector control for dengue prevention. PLoS Negl Trop Dis. 2015;9: e0003655 10.1371/journal.pntd.0003655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilke ABB, Beier JC, Benelli G. Transgenic mosquitoes–Fact or fiction? Trends Parasitol. 2018;34: 456–465. 10.1016/j.pt.2018.02.003 [DOI] [PubMed] [Google Scholar]
- 32.Vezzani D. Review: Artificial container-breeding mosquitoes and cemeteries: A perfect match. Trop Med Int Heal. 2007;12: 299–313. [DOI] [PubMed] [Google Scholar]
- 33.O’Meara GF, Gettman AD, Evans LF, Scheel FD. Invasion of cemeteries in Florida by Aedes albopictus. J Am Mosq Control Assoc. 1992;8: 1–10. [PubMed] [Google Scholar]
- 34.Vezzani D, Velázquez SM, Soto S, Schweigmann NJ. Environmental characteristics of the cemeteries of Buenos Aires city (Argentina) and infestation levels of Aedes aegypti (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 2001;96: 467–471. 10.1590/s0074-02762001000400005 [DOI] [PubMed] [Google Scholar]
- 35.Walther D, Scheuch DE, Kampen H. The invasive Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in Germany: Local reproduction and overwintering. Acta Trop. 2017;166: 186–192. 10.1016/j.actatropica.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 36.Kampen H, Kuhlisch C, Fröhlich A, Scheuch DE, Walther D. Occurrence and spread of the invasive asian bush mosquito Aedes japonicus (diptera: Culicidae) in west and north Germany since detection in 2012 and 2013, respectively. PLoS One. 2016;11: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dávalos-Becerril E, Correa-Morales F, González-Acosta C, Santos-Luna R, Peralta-Rodríguez J, Pérez-Rentería C, et al. Urban and semi-urban mosquitoes of Mexico City: A risk for endemic mosquito-borne disease transmission. PLoS One. 2019;14: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fryxell RTT, Freyman K, Ulloa A, Hendricks B, Paulsen D, Odoi A, et al. Cemeteries are effective sites for monitoring la Crosse virus (LACv) and these environments may play a role in LACv infection. PLoS One. 2015;10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Likos A, Griffin I, Bingham AM, Stanek D, Fischer M, White S, et al. Local mosquito-borne transmission of Zika virus—Miami-Dade and Broward Counties, Florida, June–August 2016. Morb Mortal Wkly Rep. 2016;65: 1032–1038. [DOI] [PubMed] [Google Scholar]
- 40.Centers for Disease Control and Prevention. Imported Human disease cases Reported to CDC by county of residence. 2020. Available: https://wwwn.cdc.gov/arbonet/Maps/ADB_Diseases_Map/index.html
- 41.Florida Department of Health. Mosquito-Borne Illness Advisory. 2019. Available at: http://miamidade.floridahealth.gov/_newsroom/2019/_documents/2019-12-23-advisory.pdf
- 42.Wilke ABB, Carvajal A, Medina J, Anderson M, Nieves VJ, Ramirez M, et al. Assessment of the effectiveness of BG-Sentinel traps baited with CO2 and BG-Lure for the surveillance of vector mosquitoes in Miami-Dade County, Florida. PLoS One. 2019;14: e0212688 10.1371/journal.pone.0212688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Darsie RF Jr., Morris CD. Keys to the adult females and fourth instar larvae of the mosquitoes of Florida (Diptera, Culicidae). 1st ed. Vol. 1 Tech Bull Florida Mosq Cont Assoc (2000). [Google Scholar]
- 44.Morris EK, Caruso T, Buscot F, Fischer M, Hancock C, Maier TS, et al. Choosing and using diversity indices: Insights for ecological applications from the German Biodiversity Exploratories. Ecol Evol. 2014;4: 3514–3524. 10.1002/ece3.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammer Ø, Harper DATT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4: 9. [Google Scholar]
- 46.Tóthmérész B. Comparison of different methods for diversity ordering. J Veg Sci. 1995;6: 283–290. [Google Scholar]
- 47.Tóthmérész B. On the characterization of scale-dependent diversity. Abstr Bot. 1988;22: 149–156. [Google Scholar]
- 48.Simpson EH. Measurement of Diversity. Nature. 1949;163: 688–688. [Google Scholar]
- 49.Shannon CE. A mathematical theory of communication. Bell Syst Tech J. 1948;27: 379–423. [Google Scholar]
- 50.Biology E. Estimating terrestrial biodiversity through extrapolation. Philos Trans R Soc London Ser B Biol Sci. 1994;345: 101–118. [DOI] [PubMed] [Google Scholar]
- 51.Colwell RK. Biodiversity: Concepts, patterns, and measurement. Communities Ecosyst. 2009; 257–264. [Google Scholar]
- 52.Troyo A, Fuller DO, Calderón-Arguedas O, Solano ME, Beier JC. Urban structure and dengue incidence in Puntarenas, Costa Rica. Singap J Trop Geogr. 2009;30: 265–282. 10.1111/j.1467-9493.2009.00367.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jamison A, Tuttle E, Jensen R, Bierly G, Gonser R. Spatial ecology, landscapes, and the geography of vector-borne disease: A multi-disciplinary review. Appl Geogr. 2015;63: 418–426. [Google Scholar]
- 54.Wilke ABB, Chase C, Vasquez C, Carvajal A, Medina J, Petrie WD, et al. Urbanization creates diverse aquatic habitats for immature mosquitoes in urban areas. Sci Rep. 2019;9: 15335 10.1038/s41598-019-51787-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilke AB, Beier JC, Benelli G. Complexity of the relationship between global warming and urbanization–an obscure future for predicting increases in vector-borne infectious diseases. Curr Opin Insect Sci. 2019; [DOI] [PubMed] [Google Scholar]
- 56.Wilke ABB, Benelli G, Beier JC. Beyond frontiers: On invasive alien mosquito species in America and Europe. PLoS Negl Trop Dis. 2020;14: e0007864 10.1371/journal.pntd.0007864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilke ABB, Caban-Martinez AJ, Ajelli M, Vasquez C, Petrie W, Beier JC. Mosquito adaptation to the extreme habitats of urban construction sites. Trends Parasitol. 2019;35: 607–614. 10.1016/j.pt.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 58.Ajelli M, Moise IK, Hutchings TCSG, Brown SC, Kumar N, Johnson NF, et al. Host outdoor exposure variability affects the transmission and spread of Zika virus: Insights for epidemic control. PLoS Negl Trop Dis. 2017;11: e0005851 10.1371/journal.pntd.0005851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mutebi J-P, Hughes HR, Burkhalter KL, Kothera L, Vasquez C, Kenney JL. Zika virus MB16-23 in mosquitoes, Miami-Dade County, Florida, USA, 2016. Emerg Infect Dis. 2018;24: 808–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript