Abstract
Myopia and glaucoma are both increasing in prevalence and are linked by an unknown mechanism as many epidemiologic studies have identified moderate to high myopia as an independent risk factor for glaucoma. Myopia and glaucoma are both chronic conditions that lead to connective tissue remodeling within the sclera and optic nerve head. The mechanobiology underlying connective tissue remodeling differs substantially between both diseases, with different homeostatic control mechanisms. In this article, we discuss similarities and differences between connective tissue remodeling in myopia and glaucoma; selected multi-scale mechanisms that are thought to underlie connective tissue remodeling in both conditions; how asymmetric remodeling of the optic nerve head may predispose a myopic eye for pathological remodeling and glaucoma; and how neural tissue deformations may accumulate throughout both pathologies and increase the risk for mechanical insult of retinal ganglion cell axons.
Keywords: myopia, glaucoma, ocular biomechanics, remodeling, optic nerve head, mechanobiology
1. Introduction
The link between myopia (nearsightedness) and glaucoma has been recognized, at least, since the early 20th century [1]. High myopia is a major risk factor for glaucoma in various ethnic groups, and even low levels of myopia have a significant effect on glaucoma risk [2]. The prevalence of myopia has been increasing worldwide over the past 60 years, with epidemic levels higher than 80% in some East Asian populations [3], increasing the vision-related health care burden and the risk for associated blinding diseases such as glaucoma and retinal detachment. Globally, glaucoma is the second leading cause of blindness with an estimated 65 million people suffering from primary open angle glaucoma, one of the most common forms of the disease [4, 5]. The growing prevalence of myopia and glaucoma makes these ocular diseases a significant global health concern; therefore, it is critical to gain an understanding of the interrelated pathophysiologic mechanisms of both conditions.
Connective tissue remodeling is thought to play a key role in both conditions [6, 7]. However, the mechanobiology of remodeling differs significantly between myopia and glaucoma. Since elevated intraocular pressure (IOP) is the primary risk factor of glaucoma, connective tissue remodeling in glaucoma is thought to be load-driven and to involve connective tissue turnover to maintain mechanical homeostasis [7]. In this sense, glaucoma- related remodeling has similarities to other load-driven remodeling processes, such as those associated with vascular hypertension [8]. Significant progress has been made over the last couple of decades in deciphering the fundamental mechanisms underlying load-driven remodeling. The remodeling mechanisms involved in eye development and myopia, however, are to some extend unique to the eye and not common across other organs, as they are not driven by load but by visual cues [6]. In the following section, we discuss why, despite the differences, connective tissue remodeling is a plausible link between both pathologies, and how connective tissue remodeling that leads to myopia early in life may increase the risk for pathologic connective tissue remodeling and vision loss in glaucoma later in life.
2. Connective Tissue Remodeling in Myopia
A myopic eye is too long for its focal length, which is typically caused by an elongated posterior scleral shell [6]. During normal eye development, a vision-guided feedback mechanism actively modulates scleral remodeling to match the eye’s axial length to its focal length at the organ-level. By adjusting the eye length, this homeostatic control mechanism aims to achieve and maintain clear vision (emmetropia) using visual cues that are locally detected by the retina [9, 10]. The retina is thought to send biochemical signals to the fibroblasts of the sclera, altering its composition, biomechanical properties and remodeling rate, and ultimately the eye’s axial length [6]. Figure 1 illustrates the multi-scale mechanisms that are involved in scleral remodeling in myopia.
Figure 1:
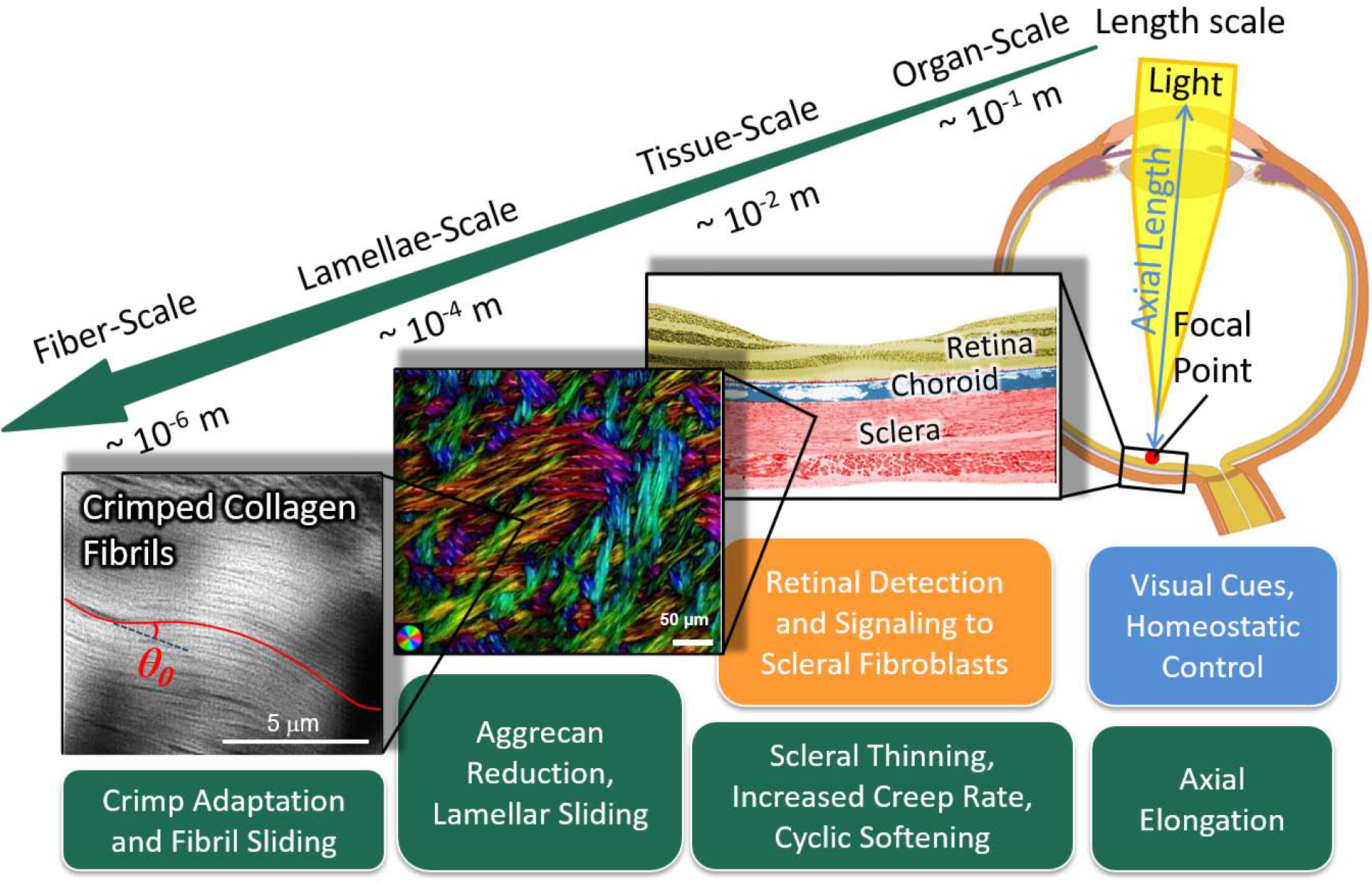
Multi-scale mechanisms of scleral remodeling in myopia. The green boxes represent structural and material alterations due to remodeling; the blue box represents the primary stimulus; and the orange box represents stimulus detection and signaling pathways. Scleral remodeling during eye development is driven by visual cues and a homeostatic control mechanism that matches the eye’s axial length to its focal length at the organ-scale. Visual cues are detected by the retina, which sends signals through the choroid to the sclera to alter the scleral remodeling rate. Scleral remodeling in myopia leads to axial elongation at the organ-scale, scleral thinning, increased creep rate and cyclic softening at the tissue-scale. We propose that scleral remodeling involves lamellar sliding at the lamellae-scale, and fibril sliding and adaptation of the collagen fibril crimp at the fiber-scale. The colors at the lamellae-scale indicate the local fiber orientation, whereas the intensity is proportional to the collagen fiber density.
There is a fascinating multi-scale dilemma inherent in the emmetropization process. Visual cues such as defocus, light intensity, contrast, and light chromaticity alter axial eye elongation [11, 6]. Some visual cues lead to signals that accelerate scleral remodeling, whereas others slow it down. These competing visual cues can vary across the retina (tissue-level) and over time. Based on the local visual cues, a photoreceptor cell, or a small collection of cells have to determine if the organ has to elongate faster or slower. This difficult decoding and decision-making task is even more astonishing since it functions without a connection to the brain and is, therefore, thought to solely occur at the cellular-level of the retina [9, 10]. However, the homeostatic control mechanism aims to match the organ-level size of the eye with its optics. This discrepancy between length scales presents a potential explanation for continued scleral remodeling and myopia progression in children that receive common lens correction (single-vision lenses). Single-vision lens are designed to correct defocus along the central axis but not in the periphery. Typically, myopic eyes have a hyperopic shift in the periphery, which remains after single-vision correction. Consequently, a peripheral photoreceptor cell will continue to receive local visual cues that suggest the eye is too short accelerating scleral remodeling while the eye is already too long.
Abundant evidence suggests that axial elongation in myopia involves accelerated connective tissue remodeling and not accelerated tissue growth of the posterior sclera [12, 13, 14, 6]. Although some remodeling mechanisms are thought to be universal across soft tissues and organs, scleral remodeling during eye development and myopia is unique in at least two ways: (i) remodeling is driven by visual cues, as described previously; and (ii) may be modulated by aggrecan. Aggrecan is a major structural proteoglycan (PG) that is found in cartilage but not in soft collagenous tissues except the sclera. Multiple studies have concluded that PGs and glycosaminoglycans (GAGs) play no significant role in the elastic and viscoelastic response of soft tissues [15, 16]. This may be different in the sclera due to its aggrecan content. Indeed, Murienne et al. [17] have shown in porcine sclera that enzymatic digestion of GAGs leads to a similar shift in the stress-strain response that was seen tree shrews when comparing control eyes to those developing myopia [13]. The composition of the sclera has been shown to change during experimental myopia with a reduction in aggrecan and sulfated GAG levels [18, 19]. During myopia development, several changes in scleral material properties and micro-structure have been reported, including changes in the elastic, viscoelastic, creep, cyclic softening, and collagen fiber crimping response [20, 13, 21]. Interestingly, these material property changes seem to prevail as long as scleral remodeling is accelerated and myopia progresses but return to normal levels once remodeling stabilizes (Figure 2).
Figure 2:
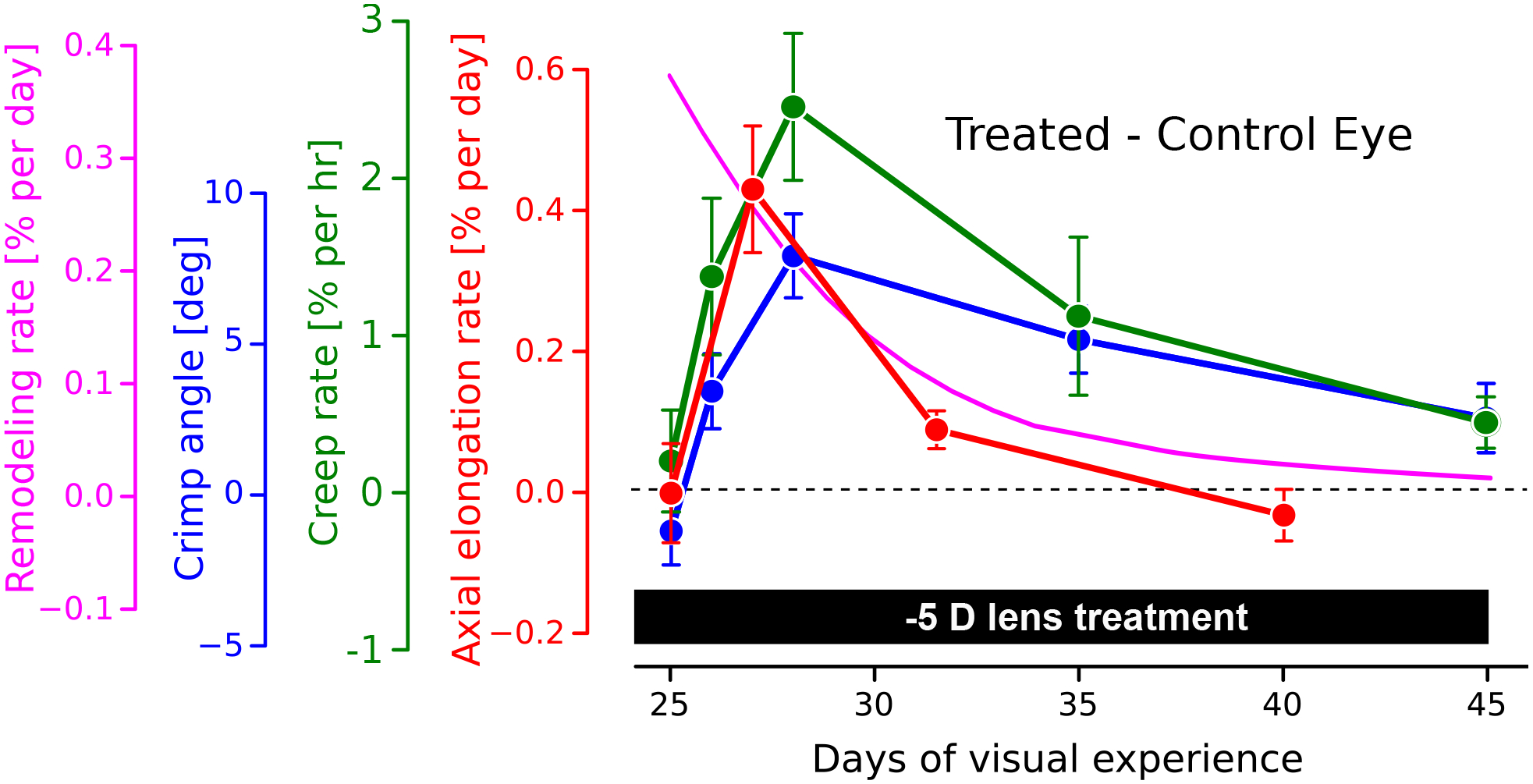
Time-dependent changes in scleral biomechanics during experimentally induced myopia using a −5D lens in tree shrews. Plotted are the differences between lens treated and control eyes, showing a similar time-dependent trend for all of the study variables. The axial elongation rate (red curve, [20]) increases rapidly after lens placement, followed by a decline to normal levels as the eye adapted its axial length to the new focal length. The creep rate (green curve:, [20]) and the collagen fiber crimp angle (blue curve, [13]) show a similar time-dependent trend compared to the axial elongation rate. Based on the model assumptions, the computationally predicted remodeling rate (magenta curve, [22]) predicts an immediate increase in the remodeling rate after lens placement, followed by a gradual decrease that is consistent with the time-dependent biomechanical changes seen in the other curves.
Based on the aforementioned evidence, it seems plausible that connective tissue remodeling in myopia is a creep-like deformation response. Scleral fibroblasts receive biochemical signals from the retina to accelerate or slow scleral remodeling by altering the composition and biomechanical properties of the sclera. Initial mechanistic theories assumed that the scleral collagen architecture consisted of lamellae and collagen fibers with distinct endings. In this case, connective tissue remodeling can be imagined as collagen sliding or relative deformations between neighboring lamellae and/or fibers within each lamella [23]. This theory of scleral remodeling is based on a highly simplified view of the hierarchical structure of the sclera and does not consider that lamellae are highly interwoven (see lamellae-scale in Figure 1). Neither scleral lamellae nor their collagen fibers show clear endings. In contrast, scleral lamellae are tightly interwoven with each other, where lamellae split and merge, and collagen fibers can be connected to multiple lamellae. Although the highly interwoven lamellar structure of the sclera may limit relative micro-deformations, the collagen sliding theory is still plausible but likely more complicated than originally thought. If collagen fibers have no endings, collagen fiber elongation could lead to a sliding-like mechanism at higher length scales. Indeed, experimental results in tree shrews indicate that the collagen fiber diameter decreases in myopia [12]. Lower scale mechanisms may allow for a continuous and permanent elongation of collagen fibers by adding new monomers to existing fibrils as they are being stretched [24]. Aggrecan is reduced during accelerated sclera remodeling, suggesting that its removal may promote collagen sliding and/or collagen fibril stretching. This perspective counters observations in tendon, where the removal of PGs and GAGs is thought to inhibit collagen fibril sliding [25]. However, the architecture of tendons is fundamentally different from the sclera, with no interweaving of collagen fibers and without aggrecan.
3. Connective Tissue Remodeling in Glaucoma
The optic nerve head (ONH) is of particular interest in glaucoma as it is thought to be the primary site of retinal ganglion cell (RGC) axonal injury in glaucoma [26]. From the biomechanical perspective, the ONH is generally thought to be a weak spot within the otherwise relatively strong collagenous corneo-scleral shell [27]. Progressive remodeling of the ONH connective tissues is the defining feature of glaucoma as compared to other optic neuropathies [28]. Figure 3 illustrates the multi-scale mechanisms hypothesized to be involved in ONH remodeling in glaucoma. The connective tissues of the ONH include the peripapillary sclera (PPS) and lamina cribrosa (LC), which are mainly composed of Type I collagen. The LC is thought to mechanically protect RGC axons from organ-scale pressure loads as the axons pass from a relative higher pressure environment within the eye (IOP) to a lower pressure region in the retrobulbar cerebrospinal space [29]. The LC consists of a complex, load-bearing, three-dimensional network of collagenous beams, nourished by a capillary bed primarily arising from the short posterior ciliary arteries penetrating the PPS. The anatomy of the LC and sclera raises several considerations regarding the etiology of pathologic remodeling and bowing of the LC in glaucoma and implies that the classic mechanical and vascular mechanisms of axonal insult are inseparably intertwined [30].
Figure 3:
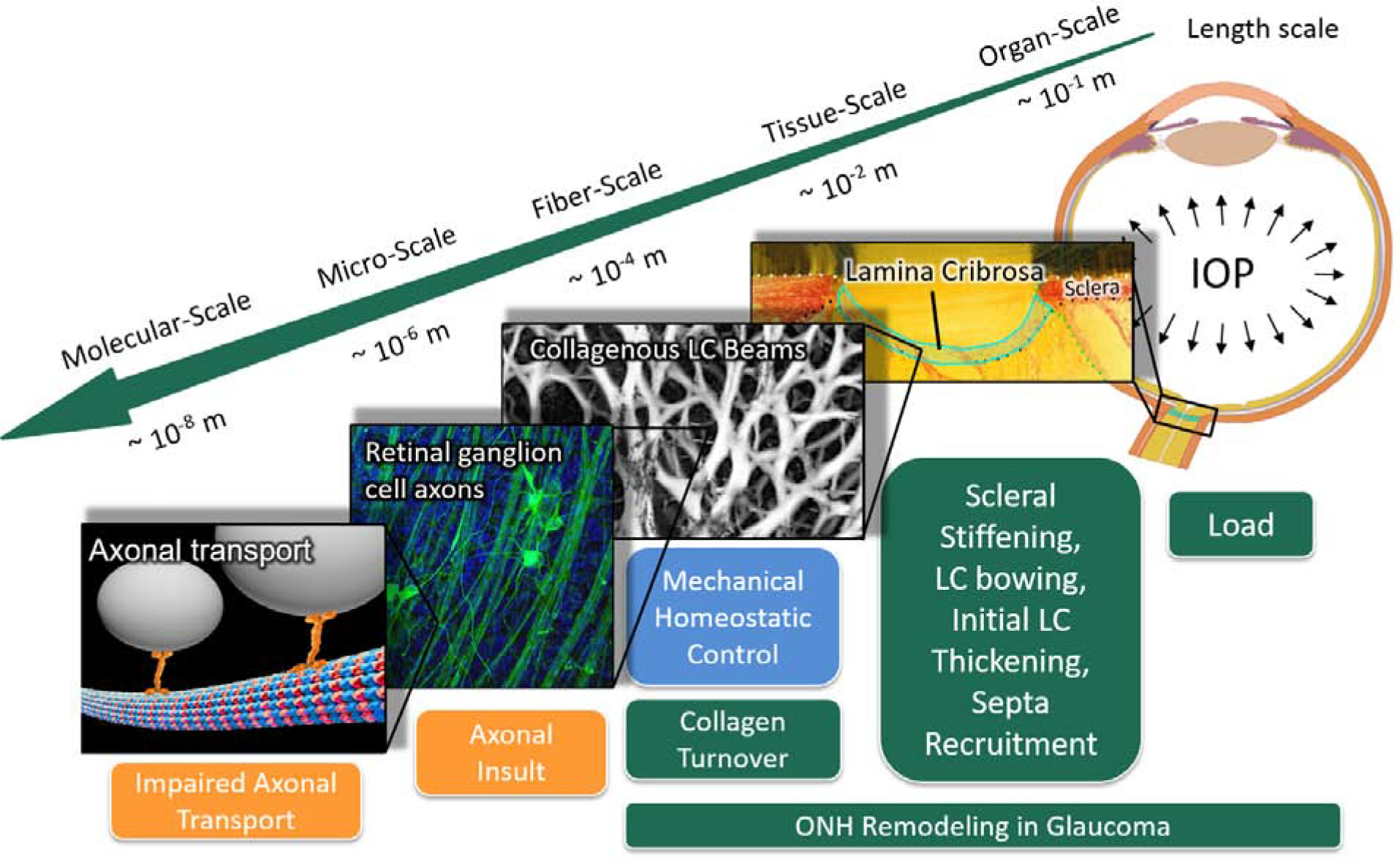
Multi-scale mechanisms of ONH remodeling in glaucoma. The green boxes represent structural and material alterations due to ONH remodeling; the blue box represents the primary stimulus; and orange boxes represent mechanisms related to RGC axonal injury. The eye is subjected to IOP load at the organ-scale and chronic IOP elevation is the main risk factor for glaucoma. Pathologic ONH remodeling in glaucoma includes changes in scleral stiffness, LC bowing, initial LC thickening, and septa recruitment at the tissue-scale [31, 32, 33, 34, 35, 36]; and the synthesis of new LC beams at the fiber-scale [37]. We propose that these remodeling mechanisms are driven by a mechanical homeostatic control mechanism in an effort to maintain a homeostatic strain condition at the fiber-scale by altering collagen turnover in the ONH connective tissue. Pathologic ONH remodeling is thought to impair axonal transport by a direct or indirect mechanical insult to the RGC axons that pass through the LC porous microstructure. Fiber scale image is modified from a study by Brazile et al. [38]. ONH, optic nerve head; LC, lamina cribrosa; RGC, retinal ganglion cell; IOP, intraocular pressure.
At the tissue-scale, early experimental glaucoma in non-human primates shows a thicker LC [31, 37] that is bowed posterior [31,39] than contralateral control eyes. Structural changes at the ONH occur before any detectable loss of retinal nerve fiber layer thickness, suggesting that pathologic ONH remodeling precedes axonal insult [40]. Computational and experimental studies based on experimental glaucoma in monkeys suggest that these morphologic changes result from connective tissue remodeling due to increased collagen synthesis in the retrolaminar septa [37, 41]. Furthermore, initial bowing of LC may be caused by elastic deformations, but these deformations are thought to become permanent and progress due to connective tissue turnover [7]. Recent findings by Fazio et al. [36] suggest that scleral remodeling in glaucoma follows a connective tissue wound healing response, where the sclera becomes more compliant initially, followed by a scarring period in which the sclera progressively stiffens. A similar process has been proposed for the LC, where after an initial thickening, the LC becomes thinner with associated scar formation at the advanced stage of glaucoma [7]. Although the scaring response remains poorly understood, the primary remodeling response is thought to be load-driven, involving a mechanical homeostatic control mechanism that modulates collagen turnover.
Several findings suggest that connective tissue remodeling in glaucoma is driven by a homeostatic control mechanism at the fiber-scale. Fiber-scale strain was identified as a critical stimulus in mechanotransduction pathways of LC cells and scleral fibroblasts [42]. Camp et al. [43] have shown that the fiber-scale strain protects fibrillar collagen against enzymatic degradation. Computational models suggest that the LC thickens in early experimental glaucoma to maintain a homeostatic strain level at the fiber-scale [41]. Brazile et al. [38] found that collagen fibers are less wavy in thin LC beams than in their thicker counterparts. This difference may allow thin beams to support similar amounts of IOP-induced force as thicker beams at physiologic IOP, despite the differences in beam width (Figure 4). A remodeling mechanism that alters the collagen fiber crimp can maintain a homeostatic strain condition without changing the LC beam diameter. In addition, studies of the distribution of collagen crimp characteristics throughout the globe show well-defined regional patterns consistent across eyes and between sheep [44] and human [45], suggesting that the mechanisms regulating these parameters might be shared across species and act at multiple scales.
Figure 4:
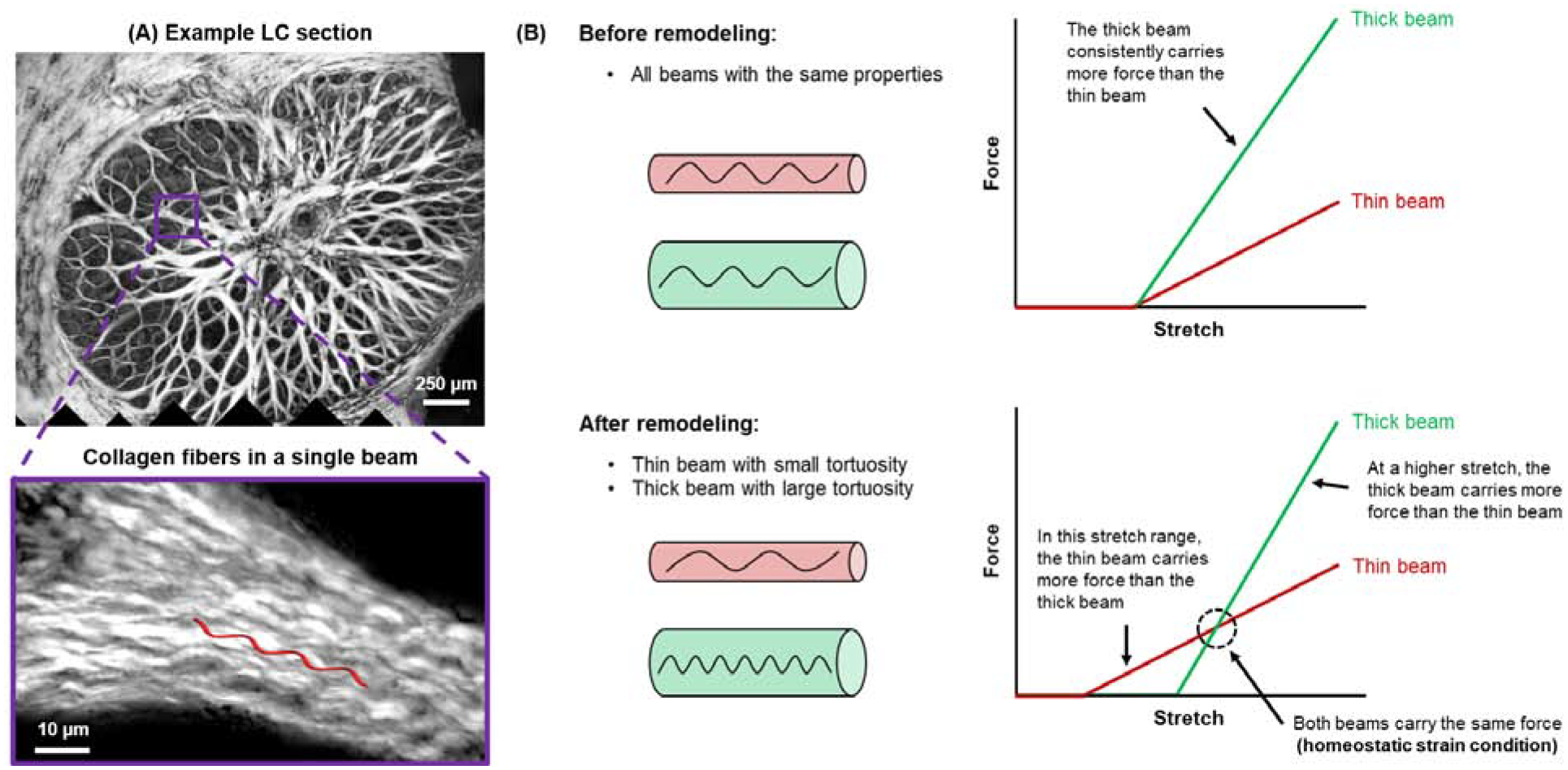
Schematic illustration of mechanical implications of remodeling of LC beam collagen. (A) Example LC section of a sheep eye fixed at 5 mmHg and the close-up showing the crimped collagen fibers in a single beam. (B) Without remodeling, all LC beams have the same mechanical properties, that is, thin and thick beams have the same tortuosity (top). Thin and thick beams stiffen at the same level of stretch, with the thick beam consistently carrying more force than the thin beam. After remodeling, the thin beam has a lower tortuosity than the thick beam (bottom). The thin beam stiffens at a lower level of stretch. Hence, for this beam, there is a range of stretches within the thin beam carries more force than the thick beam. This also results in the existence of a crossover point at which both beams carry the same force (i.e., the homeostatic strain condition). At a higher level of stretch, the thick beam carries more force than the thin beam. LC, lamina cribrosa.
4. Connective Tissue Remodeling Linking Myopia and Glaucoma
Connective tissue remodeling in myopia leads to profound morphological changes of the posterior pole and the ONH, which alters the biomechanical environment of the RGC axons permanently. Recent advancements in optical coherence tomography (OCT) allow in vivo assessment of ONH remodeling. Here, we present characteristic morphological changes that occur at the ONH in myopia and how these changes might increase the risk for glaucoma development. Figure 5 presents a summary of the interacting mechanisms.
Figure 5:
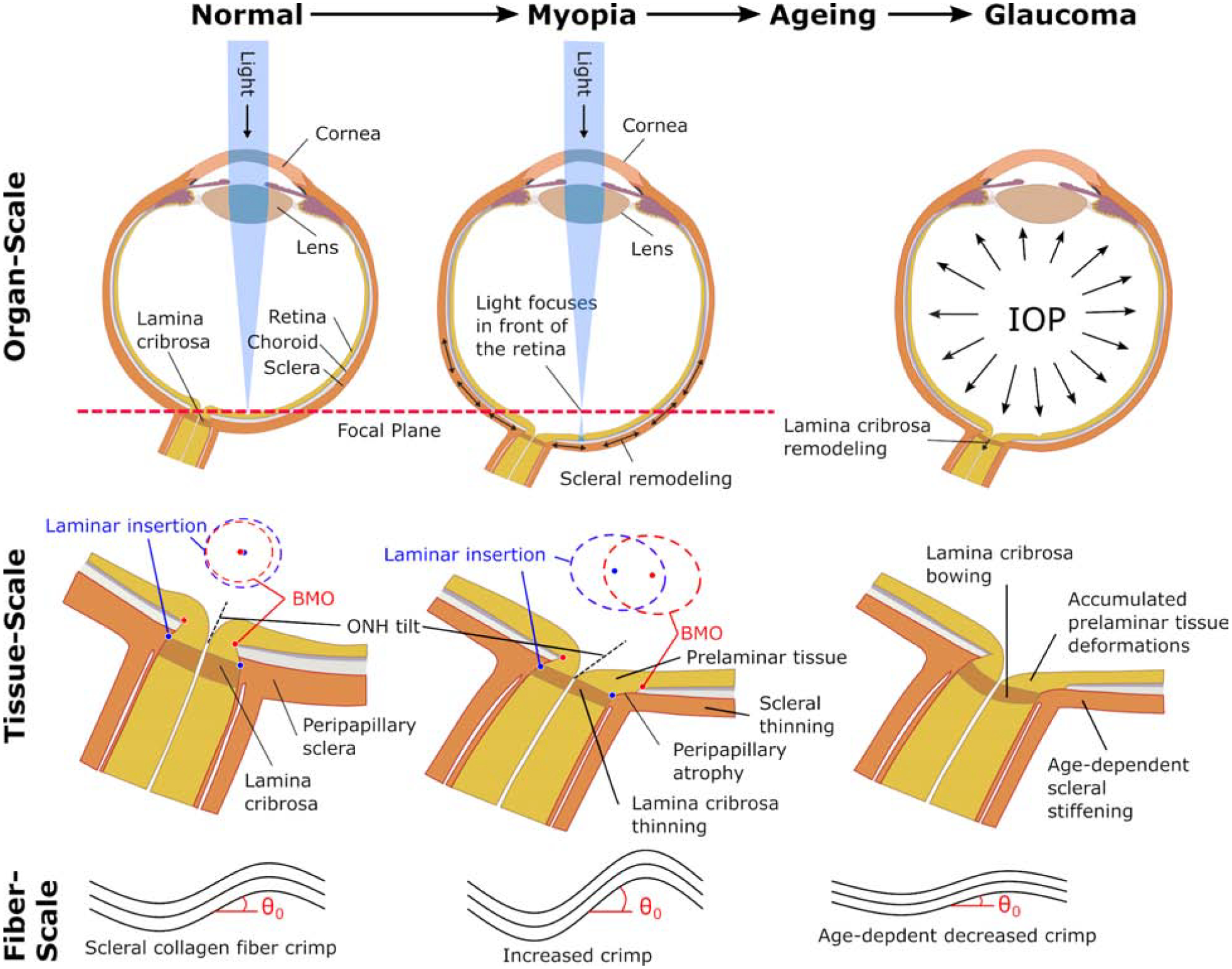
Possible interactions at multiple scales between scleral and ONH remodeling in myopia, aging, and glaucoma. At the organ-scale, visual cues drive scleral remodeling that leads to axial elongation and myopia whereas IOP is thought to be the primary load that drives glaucomatous ONH remodeling in adults. At the tissue-scale, the vision-guided remodeling leads to asymmetric morphological changes of the ONH due to differential remodeling in the nasal/temporal sides. The LC and sclera thin as the LC shifts nasally with respect to the Bruch’s membrane opening (BMO). The blue and red ellipses represent en face views of the anterior laminar insertion and BMO, respectively, illustrating the increase in canal area, development of an elliptical canal shape, and relative deformations between the LC and BMO. These relative deformations lead to the tilted and rotated appearance of the myopic ONH, and potentially contribute to the development of peripapillary atrophy. Both, the sclera and the LC stiffen with age [46, 47]. In glaucoma, pathological ONH remodeling involves an initial thickening of the LC and posterior bowing of LC and sclera. ONH remodeling in myopia, age-dependent scleral stiffening, and pathologic remodeling in glaucoma, are all thought to promote glaucomatous LC bowing and tissue deformations in the prelaminar tissues. These prelaminar tissue deformations may accumulate as the eye develops myopia during childhood, ages, and develops glaucoma later in life. At the fiber-scale, the scleral collagen fiber crimp is temporarily increased during myopia development followed by a decrease with age. The age-dependent decrease in crimp angle θ0, is partially responsible for the increased scleral stiffness with age [45]. Experimental evidence supports the notion that mechanical homeostatic conditions are defined at the fiber-scale. ONH, optic nerve head; IOP, intraocular pressure; LC, lamina cribrosa.
During development of myopia, the ONH shape becomes more elliptical at multiple depths, including the Bruch’s membrane opening (BMO) and the scleral canal opening [48]. The increased BMO and scleral canal opening areas in myopia suggest that myopic remodeling leads to an overall expansion of the ONH [48]. In monkeys this expansion process involves thinning of the PPS and LC [49]. Tissue thinning supports the notion that connective tissue remodeling in myopia involve micro-deformations (e.g. collagen sliding) that are mostly volume-preserving at the tissue-level. Age-dependent tissue stiffening may play a critical role in deciphering the interaction between connective tissue remodeling in myopia and glaucoma. The human sclera was shown to stiffen with age due to increased shear stiffness and a reduction collagen fiber crimp [46, 45]. Using computational modeling, Sigal [50] predicted that a stiffer sclera and increased scleral canal opening (i.e. LC diameter) lead to an increase in peak LC strain, which can result in load-driven remodeling. When the sclera is compliant (as it is in children), LC radius had no influence on the peak LC strain [50]. This finding supports the idea that scleral stiffening due to aging may be an important aspect of the increased susceptibility to glaucoma of highly myopic eyes that typically have a larger scleral canal opening. Furthermore, a thinner LC was found to be more sensitive to IOP-induced posterior displacement of the LC (LC bowing) and this effect increased with scleral canal size [51]. Consequently, both the thinning of the LC and the expansion of the scleral canal in myopia may promote pathologic ONH remodeling that leads to LC bowing and mechanical insult to the RGC axons passing through the LC, even at normal IOP.
In contrast to a normal eye, the myopic ONH is characterized by an asymmetric morphology [52]. Besides the already mentioned elliptical shape of the ONH, this asymmetric changes involve a vertical tilting and tissue-level deformations that make the ONH appear as if it was rotated (also called ONH torsion) [53]. This asymmetric morphology suggests that scleral remodeling in myopia is heterogeneous and/or anisotropic. If scleral remodeling involves collagen fiber sliding, the collagen fiber architecture will impact the tissue-level deformations that result from such a remodeling mechanism. Consequently, the complex collagen fiber architecture of the sclera, with radial, circumferential, interweaving and tangential fibers [54, 55] may explain the asymmetric morphological changes of the ONH observed in myopia.
As connective tissue remodeling in myopia is driven by visual cues and not by mechanical stimuli, an asymmetric ONH remodeling will likely cause local and/or regional deviation from the mechanical homeostatic state. These deviations can lead to over- and under-loaded connective tissues and may change the principal stress/strain directions, which may lead to secondary load-driven remodeling. Changes in principal stress directions are thought to alter the anisotropic collagen architecture in the LC and the sclera [56]. Indeed, Markov et al. [57] have found notable alterations in the collagen architecture of the PPS in highly myopic eyes. We have two hypotheses to explain these micro-structural changes in the PPS. First, it is possible that micro-structural changes are the result of load-driven remodeling secondary to the asymmetric vision-guided remodeling of the ONH in myopia. Alternatively, the microstructural changes may be a direct consequence of vision-driven remodeling of collagen fibers in the posterior sclera. Fibers and fiber bundles in the sclera are several orders of magnitude longer than their diameter. Thus, they may interact with cells and other fibers at small scales, for example by interweaving, but can also transmit forces across long distances. In this perspective, vision-guided remodeling that increases the length of scleral fibers far away from the ONH may still impact the biomechanics and micro-structure of the ONH. The exact implications of anisotropic and heterogenous vision-guided and load-driven remodeling interactions on glaucoma risk are unclear at this point.
Anatomically, the retina is thought to be fully developed between 15–45 months of age [58, 59], which is also the age rage at which scleral growth ceases [60]. Typically, connective tissue remodeling that leads to high myopia occurs at young age, but still long after the retina has fully developed. Consequently, the retina is stretched as it deforms with the remodeling sclera during the development of myopia. At the ONH, the interaction between neural and connective tissues is more complex as the ONH remodels into an asymmetric shape with relative deformations between the connective and neural tissues (see tissue-scale in Figure 5). Lee et al. [61] provided longitudinal evidence that the LC is shifted or “dragged” nasally relative to the prelaminar tissue and BMO during axial elongation in myopic children. These relative deformations are likely involved in the tilted and rotated appearance of the ONH in high myopia and in the development of peripapillary atrophy often seen at the temporal side of the myopic optic disc [61]. The myopia-associated deformations of the prelaminar tissues will likely remain or worsen with age. As mentioned previously, pathologic ONH remodeling in glaucoma typically involves posterior deformations or bowing of the LC, which pulls the prelaminar tissues posteriorly. In addition, patients with glaucoma also have peripapillary changes that can be seen in clinic [62, 63]. Beta-zone atrophy associated with glaucoma corresponds to loss of retinal pigment epithelium cells and photoreceptors, as well as changes in the choriocapillaris. Consequently, stretching and shearing of the prelaminar tissues may accumulate with age due to vision-guided ONH remodeling during childhood myopia and load-driven remodeling in glaucoma later in life. This age-dependent accumulation of prelaminar deformation in myopic eyes may lead to mechanical axonal insult at lower levels of pathological ONH remodeling compared to non-myopic eyes. If this is true, more severe levels of ONH remodeling due to myopia should lead to more profound vision loss in glaucoma with otherwise comparable conditions (e.g. IOP). Sawada et al. [64] investigated this idea using a clever study design based on paired eyes of patients with open-angle glaucoma whose IOP was similar. The study revealed that myopic papillary and parapapillary deformations (more elliptical ONH, larger peripapillary atrophy area without Bruch’s membrane), rather than the refractive error, were related to the visual field loss in open-angle glaucoma, which supports our speculation.
5. Discussion
We have presented an opinion about connective tissue remodeling in myopia and glaucoma. We suggest that the primary remodeling mechanisms are substantially different between these two comorbidities. Differences exist in the stimuli and in the primary mechanisms that restructure the connective tissues. In myopia, visual cues drive remodeling by altering the tissue composition that can accelerate or slow collagen fiber sliding. In glaucoma, mechanical homeostasis seems to be defined at the collagen fiber level and deviation from this state can alter connective tissue turnover by increased and decreased collagen synthesis. We propose that connective tissue remodeling in myopia can increase the risk for glaucoma in two ways: (i) Asymmetric ONH remodeling in childhood myopia predisposes the eye to pathologic ONH remodeling and glaucoma even at normal IOP later in life. Aging is thought to play an important role in the increased risk of myopic eyes to pathologic ONH remodeling. Age-dependent stiffening of the connective tissues and reduced collagen fiber crimping in the PPS is thought to promote progressive LC bowing in high myopic eyes. (ii) The risk for mechanical insult of retinal ganglion cell axons increases due to accumulation of neural tissue deformations that result from connective tissue remodeling at the ONH in myopia and glaucoma.
Others have proposed biomechanical mechanisms to explain the link between myopia and glaucoma [65]. It is worth discussing here one of the most popular explanations, which, unfortunately, has some important flaws. The explanations generally originate from modeling the eye in accordance with Laplace’s law σ = pr/(2t), where σ is the wall stress, p is IOP, and t is scleral thickness. With this construction, it can be argued that a myopic eye would have a larger radius and/or a thinner sclera, resulting in larger stresses. Presupposing these stresses are then transmitted to the neural tissues, others infer this could lead to neural tissue damage and glaucoma. Whilst attractive, largely because it is simple, there are several flaws with this interpretation. Although we have discussed these at length elsewhere [66], the most salient ones are that the myopic eyes do not have a larger radius at the posterior pole nor are stresses directly transmitted to the neural tissues. In fact, myopic eyes are longer but not wider, which reduces the radius of curvature at the posterior pole [66]. In our opinion, morphological changes due to connective tissue remodeling of the ONH link myopia and glaucoma risk, and not the increased axial length of the eye.
Our opinions are based on the data and models available to date. To the best of our knowledge, no longitudinal data set exists that encompasses the development of both conditions, which is critical for clarifying the interactions between myopia and glaucoma. Acquiring these data presents a significant challenge as it would require longitudinal data collection from childhood until late adulthood to realistically capture the development of childhood myopia and glaucoma later in life. Another challenge is rooted in the limitations of current imaging techniques that are insufficient to characterize all aspects of connective tissue remodeling in response to IOP changes, with age and disease in humans or typical animal models. Specialized animal models, such as the tree shrew (tupaia belangeri), may provide unique advantageous for this purpose. Tree shrews have been used as a model of myopia for decades [67, 68], but more recently a glaucoma model has been established in these animals [69]. The tree shrew eye has inherent magnification and appears to have little corneal or lenticular aberration as spectral domain optical coherence tomography (SD-OCT) images are astonishingly clear. SD-OCT imaging of the ONH reveals exceptional detail of the collagenous LC, including microstructural beam definition. Furthermore, tree shrew eye ages rapidly, where 20 weeks of eye development correlate to 20 years in humans [70]. This animal model may provide a special opportunity to identify and follow collagen remodeling throughout both conditions in vivo.
Connective tissue remodeling is a promising and underutilized treatment target for both, myopia and glaucoma. In myopia, collagen crosslinking has been proposed as a potential treatment modality for slowing scleral remodeling by inhibiting collagen sliding and biomechanically strengthening the sclera [21]. Scleral crosslinking may also inhibit asymmetric remodeling of the ONH in myopia. Animal studies confirmed that scleral crosslinking can inhibit myopia progression but with some controversial results [71, 72]. As ONH remodeling in glaucoma is thought to be driven by load, surgical or pharmaceutical interventions that alter the biomechanical state of the ONH could not only reduce the mechanical insult to the RGC axons but also guide and potentially reverse pathologic ONH remodeling. This may be of particular interest in patients with glaucoma with myopia and asymmetric ONH morphology. Personalized alterations in the stress-strain environment of the myopic ONH may be needed to effectively prevent or reverse pathologic load-driven ONH remodeling. The best approaches for these treatments will depend on whether the changes in the ONH and PPS associated with myopia are primary or secondary. Although IOP-lowering drugs can slow glaucoma progression, the load reduction occurs globally, which may be insufficient in high myopic eyes. Scleral crosslinking was also proposed for glaucoma treatment [73, 74]. However, crosslinking the entire sclera will increase the scleral stiffness overall (similar to aging), which may explain the increased glaucomatous damage in mouse experiments conducted by Kimball et al. [73]. Coudrillier et al. [74] demonstrated ex vivo that localized crosslinking of the PPS reduces LC strains, supporting our opinion that localized biomechanical treatment could be used to guide load-driven remodeling. The asymmetric shape of the ONH in high myopic eyes may require a personalized treatment strategy to effectively control load-driven remodeling at the ONH. Predictive computational simulations could be used to guide biomechanical interventions at the ONH. Simulation-based diagnostic and treatment planning is being tested for anterior segment interventions [75] but has not been applied to the posterior segment.
In our opinion, significant progress toward the effective treatment of myopia and glaucoma could be made as follows: (i) by gaining a deeper understanding of the interacting remodeling mechanisms through longitudinal animal experiments where both conditions can be induced; (ii) development of treatment strategies that can locally alter ONH biomechanics and guide load-driven remodeling; and (iii) development of eye-specific computational models that can predict the progression of connective tissue remodeling and be used to personalize ONH biomechanics interventions.
Acknowledgments
This work was supported in part by the National Institutes of Health Grants R01-EY027759, R01-EY026588, R01-EY028662 and R01-EY023966 (Bethesda, Maryland); Eye Sight Foundation of Alabama (Birmingham, Alabama); and Research to Prevent Blindness (New York, New York).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Knapp A, Glaucoma in myopic eyes., Transactions of the American Ophthalmological Society 23 (1925) 61–70. [PMC free article] [PubMed] [Google Scholar]
- [2].Shen L, Melles RB, Metlapally R, Barcellos L, Schaefer C, Risch N, Herrinton LJ, Wildsoet C, Jorgenson E, The association of refractive error with glaucoma in a multiethnic population., Ophthalmology 123 (2016) 92–101. doi: 10.1016/j.ophtha.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, Cook DG, Owen CG, Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention., The British journal of ophthalmology 100 (2016) 882–890. doi: 10.1136/bjophthalmol-2015-307724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kapetanakis VV, Chan MPY, Foster PJ, Cook DG, Owen CG, Rudnicka AR, Global variations and time trends in the prevalence of primary open angle glaucoma (poag): a systematic review and meta-analysis., The British journal of ophthalmology 100 (2016) 86–93. doi: 10.1136/bjophthalmol-2015-307223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y, Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis., Ophthalmology 121 (2014) 2081–2090. URL: 10.1016/j.ophtha.2014.05.013. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- [6].Grytz R, Scleral Remodeling in Myopia, Kugler Publications, Amsterdam, 2018, pp. 383–403. [Google Scholar]
- [7].Grytz R, Girkin C, Libertiaux V, Downs J, Perspectives on biomechanical growth and remodeling mechanisms in glaucoma, Mechanics Research Communications 42 (2012) 92–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cyron CJ, Humphrey JD, Growth and remodeling of load-bearing biological soft tissues, Meccanica 52 (2017) 645–664. URL: 10.1007/s11012-016-0472-5. doi: 10.1007/s11012-016-0472-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Troilo D, Gottlieb MD, Wallman J, Visual deprivation causes myopia in chicks with optic nerve section., Current eye research 6 (1987) 993–999. doi: 10.3109/02713688709034870. [DOI] [PubMed] [Google Scholar]
- [10].McBrien NA, Moghaddam HO, Cottriall CL, Leech EM, Cornell LM, The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks., Vision Res 35 (1995) 1141–1152. [DOI] [PubMed] [Google Scholar]
- [11].Gawne TJ, Siegwart JT, Ward AH, Norton TT, The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews., Experimental eye research 155 (2017) 75–84. doi: 10.1016/j.exer.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].McBrien NA, Cornell LM, Gentle A, Structural and ultrastructural changes to the sclera in a mammalian model of high myopia., Invest Ophthalmol Vis Sci 42 (2001) 2179–2187. [PubMed] [Google Scholar]
- [13].Grytz R, Siegwart JT, Changing material properties of the tree shrew sclera during minus lens compensation and recovery., Invest Ophthalmol Vis Sci 56 (2015) 2065–2078. URL: 10.1167/iovs.14-15352. doi: 10.1167/iovs.14-15352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harper AR, Summers JA, The dynamic sclera: Extracellular matrix remodeling in normal ocular growth and myopia development., Exp Eye Res 133 (2015) 100–111. URL: 10.1016/j.exer.2014.07.015. doi: 10.1016/j.exer.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lujan TJ, Underwood CJ, Henninger HB, Thompson BM, Weiss JA, Effect of dermatan sulfate glycosaminoglycans on the quasi-static material properties of the human medial collateral ligament., J Orthop Res 25 (2007) 894–903. URL: 10.1002/jor.20351. doi: 10.1002/jor.20351. [DOI] [PubMed] [Google Scholar]
- [16].Lujan TJ, Underwood CJ, Jacobs NT, Weiss JA, Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament., J Appl Physiol 106 (2009) 423–431. URL: 10.1152/japplphysiol.90748.2008. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murienne BJ, Jefferys JL, Quigley HA, Nguyen TD, The effects of glycosaminoglycan degradation on the mechanical behavior of the posterior porcine sclera, Acta Biomaterialia 12 (2015) 195–206. URL: 10.1016/j.actbio.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Moring AG, Baker JR, Norton TT, Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery., Invest Ophthalmol Vis Sci 48 (2007) 2947–2956. URL: 10.1167/iovs.06-0906. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Siegwart JT, Strang CE, Selective modulation of scleral proteoglycan mrna levels during minus lens compensation and recovery., Mol Vis 13 (2007) 1878–1886. [PubMed] [Google Scholar]
- [20].Siegwart JT, Norton TT, Regulation of the mechanical properties of tree shrew sclera by the visual environment., Vision Res 39 (1999) 387–407. URL: 10.1016/s0042-6989(98)00150-3 [DOI] [PubMed] [Google Scholar]
- [21].Levy AM, Fazio MA, Grytz R, Experimental myopia increases and scleral crosslinking using genipin inhibits cyclic softening in the tree shrew sclera., Ophthalmic & Physiological Optics 38 (2018) 246–256. doi: 10.1111/opo.12454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grytz R, El Hamdaoui M, Multi-scale modeling of vision-guided remodeling and age-dependent growth of the tree shrew sclera during eye development and lens-induced myopia, Journal of Elasticity 129 (2017) 171–195. URL: 10.1007/s10659-016-9603-4. doi: 10.1007/s10659-016-9603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Baldivia S, Levy A, Hegde S, Aper SJA, Merkx M, Grytz R, A novel organ culture model to quantify collagen remodeling in tree shrew sclera, PLoS ONE 11 (2016) e0166644 URL: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0166644. doi: 10.1371/journal.pone.0166644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chang S-W, Flynn BP, Ruberti JW, Buehler MJ, Molecular mechanism of force induced stabilization of collagen against enzymatic breakdown., Biomaterials 33 (2012) 3852–3859. doi: 10.1016/j.biomaterials.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rigozzi S, Müller R, Stemmer A, Snedeker JG, Tendon glycosaminoglycan proteoglycan sidechains promote collagen fibril sliding-afm observations at the nanoscale., J Biomech 46 (2013) 813–818. URL: 10.1016/j.jbiomech.2012.11.017. doi: 10.1016/j.jbiomech.2012.11.017. [DOI] [PubMed] [Google Scholar]
- [26].Balaratnasingam C, Morgan WH, Bass L, Matich G, Cringle SJ, Yu D-Y, Axonal transport and cytoskeletal changes in the laminar regions after elevated intraocular pressure., Invest Ophthalmol Vis Sci 48 (2007) 3632–3644. doi: 10.1167/iovs.06-1002. [DOI] [PubMed] [Google Scholar]
- [27].Burgoyne CF, A biomechanical paradigm for axonal insult within the optic nerve head in aging and glaucoma, Exp. Eye. Res 93 (2011) 120–132. URL: 10.1016/j.exer.2010.09.005. doi: 10.1016/j.exer.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yang H, Reynaud J, Lockwood H, Williams G, Hardin C, Reyes L, Stowell C, Gardiner SK, Burgoyne CF, The connective tissue phenotype of glaucomatous cupping in the monkey eye - clinical and research implications, Progress in Retinal and Eye Research 59 (2017) 1–52. doi: 10.1016/j.preteyeres.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Downs JC, Roberts MD, Burgoyne CF, Mechanical environment of the optic nerve head in glaucoma, Optometry & Vision Science 85 (2008) 425–435. URL: 10.1097/OPX.0b013e31817841cb. doi: 10.1097/OPX.0b013e31817841cb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brazile B, Yang B, Voorhees A, Sigal IA, Simultaneous in-situ visualization and quantification of lamina cribrosa collagen beams and capillaries at normal and elevated iops, Investigative Ophthalmology & Visual Science 59 (2018) 1220. [Google Scholar]
- [31].Yang H, Downs JC, Girkin C, Sakata L, Bellezza A, Thompson H, Burgoyne CF, 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: lamina cribrosa and peripapillary scleral position and thickness, Invest. Ophthalmol. Vis. Sci 48 (2007) 4597–4607. URL: 10.1167/iovs.07-0349. doi: 10.1167/iovs.07-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang H, Downs JC, Bellezza A, Thompson H, Burgoyne CF, 3-D histomorphometry of the normal and early glaucomatous monkey optic nerve head: prelaminar neural tissues and cupping, Invest. Ophthalmol. Vis. Sci 48 (2007) 5068–5084. URL: 10.1167/iovs.07-0790. doi: 10.1167/iovs.07-0790. [DOI] [PubMed] [Google Scholar]
- [33].Yang H, Williams G, Downs JC, Sigal IA, Roberts MD, Thompson H, Burgoyne CF, Posterior (outward) migration of the lamina cribrosa and early cupping in monkey experimental glaucoma, Invest. Ophthalmol. Vis. Sci 52 (2011) 7109–7121. URL: 10.1167/iovs.11-7448. doi: 10.1167/iovs.11-7448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yang H, Ren R, Lockwood H, Williams G, Libertiaux V, Downs C, Gardiner SK, Burgoyne CF, The connective tissue components of optic nerve head cupping in monkey experimental glaucoma part 1: Global change., Invest Ophthalmol Vis Sci 56 (2015) 7661–7678. URL: 10.1167/iovs.15-17624. doi: 10.1167/iovs.15-17624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Girard MJA, Suh J-KF, Bottlang M, Burgoyne CF, Downs JC, Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations, Invest. Ophthalmol. Vis. Sci 52 (2011) 5656–5669. doi: 10.1167/iovs.10-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Fazio MA, Girard MJA, Lee W, Morris JS, Burgoyne CF, Downs JC, The relationship between scleral strain change and differential cumulative intraocular pressure exposure in the nonhuman primate chronic ocular hypertension model., Investigative ophthalmology & visual science 60 (2019) 4141–4150. doi: 10.1167/iovs.19-27060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Roberts MD, Grau V, Grimm J, Reynaud J, Bellezza AJ, Burgoyne CF, Downs JC, Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma, Invest. Ophthalmol. Vis. Sci 50 (2009) 681–690. doi: 10.1167/iovs.08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Brazile BL, Hua Y, Jan N-J, Wallace J, Gogola A, Sigal IA, Thin lamina cribrosa beams have different collagen microstructure than thick beams, Investigative Ophthalmology & Visual Science 59 (2018) 4653–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]; * In this article it was shown that collagen fibers are less wavy in thin LC beams than in their thicker counterparts. This difference may allow thin beams to support similar amounts of IOP-induced force as thicker beams at physiologic IOP.
- [39].Downs JC, Roberts MD, Sigal IA, Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism., Exp. Eye Res 93 (2011) 133–140. URL: 10.1016/j.exer.2010.08.004. doi: 10.1016/j.exer.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fortune B, Reynaud J, Wang L, Burgoyne CF, Does optic nerve head surface topography change prior to loss of retinal nerve fiber layer thickness: a test of the site of injury hypothesis in experimental glaucoma., PLoS One 8 (2013) e77831 URL: 10.1371/journal.pone.0077831. doi: 10.1371/journal.pone.0077831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Grytz R, Sigal IA, Ruberti JW, Meschke G, Downs JC, Lamina cribrosa thickening in early glaucoma predicted by a microstructure motivated growth and remodeling approach, Mech. Mat 44 (2011) 99–109. doi: 10.1016/j.mechmat.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Qu J, Chen H, Zhu L, Ambalavanan N, Girkin CA, Murphy-Ullrich JE, Downs JC, Zhou Y, High-magnitude and/or high-frequency mechanical strain promotes peripapillary scleral myofibroblast differentiation., Invest Ophthalmol Vis Sci 56 (2015) 7821–7830. URL: 10.1167/iovs.15-17848. doi: 10.1167/iovs.15-17848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Camp RJ, Liles M, Beale J, Saeidi N, Flynn BP, Moore E, Murthy SK, Ruberti JW, Molecular mechanochemistry: low force switch slows enzymatic cleavage of human type I collagen monomer., J. Am. Chem. Soc 133 (2011) 4073–4078. URL: 10.1021/ja110098b. doi: 10.1021/ja110098b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jan N-J, Brazile BL, Hu D, Grube G, Wallace J, Gogola A, Sigal IA, Crimp around the globe; patterns of collagen crimp across the corneoscleral shell, Experimental Eye Research 172 (2018) 159–170. doi: 10.1016/j.exer.2018.04.003, [DOI] [PMC free article] [PubMed] [Google Scholar]; * In this article the distribution of collagen crimp was characterized throughout the globe showing well-defined regional patterns that are consistent across sheep eyes.
- [45].Gogola A, Jan N-J, Brazile B, Lam P, Lathrop KL, Chan KC, Sigal IA, Spatial patterns and age-related changes of the collagen crimp in the human cornea and sclera, Investigative Ophthalmology /& Visual Science 59 (2018) 2987–2998. doi: 10.1167/iovs.17-23474, [DOI] [PMC free article] [PubMed] [Google Scholar]; * In this article the distribution of collagen crimp was characterized throughout the globe showing well-defined regional patterns that are consistent across human eyes.
- [46].Grytz R, Fazio MA, Libertiaux V, Bruno L, Gardiner SK, Girkin CA, Downs JC, Age- and race-related differences in human scleral material properties., Invest Ophthalmol Vis Sci 55 (2014) 8163–8172. doi: 10.1167/iovs.14-14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Albon J, Purslow PP, Karwatowski WS, Easty DL, Age related compliance of the lamina cribrosa in human eyes, Br. J. Ophthalmol 84 (2000) 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jeoung JW, Yang H, Gardiner S, Wang Y, Hong SW, Girard MJ, Hardin C, Wei P, Vianna JR, Chauhan BC, Burgoyne CF, Optical coherence tomography (OCT) optic nerve head (ONH) neural canal direction, obliqueness and minimum cross-sectional area in high myopic versus age-matched healthy eyes, in: Invest Ophthalmol Vis Sci, volume 59 of ARVO E-Abstract 5552, 2019. [Google Scholar]
- [49].Jonas JB, Kutscher JN, Panda-Jonas S, Hayreh SS, Lamina cribrosa thickness correlated with posterior scleral thickness and axial length in monkeys., Acta ophthalmologica 94 (2016) e693–e696. doi: 10.1111/aos.13070. [DOI] [PubMed] [Google Scholar]
- [50].Sigal IA, Interactions between geometry and mechanical properties on the optic nerve head., Invest. Ophthalmol. Vis. Sci 50 (2009) 2785–2795. URL: 10.1167/iovs.08-3095. doi: 10.1167/iovs.08-3095. [DOI] [PubMed] [Google Scholar]
- [51].Sigal IA, Yang H, Roberts MD, Burgoyne CF, Downs JC, IOP-induced lamina cribrosa displacement and scleral canal expansion: an analysis of factor interactions using parameterized eye-specific models, Invest. Ophthalmol. Vis. Sci 52 (2011) 1896–1907. URL: 10.1167/iovs.10-5500. doi: 10.1167/iovs.10-5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Sawada Y, Araie M, Shibata H, Ishikawa M, Iwata T, Yoshitomi T, Optic disc margin anatomic features in myopic eyes with glaucoma with spectral-domain oct., Ophthalmology 125 (2018) 1886–1897. doi: 10.1016/j.ophtha.2018.07.004, [DOI] [PubMed] [Google Scholar]; * This article illustrates anatomic features of the optic disc in myopic eyes with glaucoma.
- [53].Tan NYQ, Sng CCA, Ang M, Myopic optic disc changes and its role in glaucoma., Current opinion in ophthalmology 30 (2019) 89–96. doi: 10.1097/ICU.0000000000000548, [DOI] [PubMed] [Google Scholar]; * This article summarizes optic disc changes in myopia and provides evidence that these changes are secondary to changes of the posterior globe.
- [54].Gogola A, Jan N-J, Lathrop KL, Sigal IA, Radial and circumferential collagen fibers are a feature of the peripapillary sclera of human, monkey, pig, cow, goat, and sheep, Investigative Ophthalmology & Visual Science 59 (2018) 4763–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This article provides insight into the complex collagen fiber architecture of the sclera, with radial, circumferential, interweaving and tangential fibers, which may play a critical role in the asymmetric morphological changes of the ONH observed in myopia.
- [55].Voorhees AP, Jan N-J, Hua Y, Yang B, Sigal IA, Peripapillary sclera architecture revisited: A tangential fiber model and its biomechanical implications., Acta biomaterialia (2018). doi: 10.1016/j.actbio.2018.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Grytz R, Meschke G, Jonas JB, The collagen fibril architecture in the lamina cribrosa and peripapillary sclera predicted by a computational remodeling approach, Biomech. Model. Mechanobiol 10 (2011) 371–382. doi: 10.1007/s10237-010-0240-8. [DOI] [PubMed] [Google Scholar]
- [57].Markov PP, Eliasy A, Pijanka JK, Htoon HM, Paterson NG, Sorensen T, Elsheikh A, Girard MJ, Boote C, Bulk changes in posterior scleral collagen microstructure in human high myopia, Molecular Vision 24 (2019) 818–833. [PMC free article] [PubMed] [Google Scholar]
- [58].Hendrickson AE, Yuodelis C, The morphological development of the human fovea., Ophthalmology 91 (1984) 603–612. doi: 10.1016/s0161-6420(84)34247-6. [DOI] [PubMed] [Google Scholar]
- [59].Yuodelis C, Hendrickson A, A qualitative and quantitative analysis of the human fovea during development., Vision research 26 (1986) 847–855. doi: 10.1016/0042-6989(86)90143-4. [DOI] [PubMed] [Google Scholar]
- [60].Jonas JB, Holbach L, Panda-Jonas S, Scleral cross section area and volume and axial length., PLoS One 9 (2014) e93551 URL: 10.1371/journal.pone.0093551. doi: 10.1371/journal.pone.0093551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lee KM, Choung H-K, Kim M, Oh S, Kim SH, Positional change of optic nerve head vasculature during axial elongation as evidence of lamina cribrosa shifting: Boramae myopia cohort study report 2, Ophthalmology 125 (2018) 1224–1233. [DOI] [PubMed] [Google Scholar]; ** This article provides longitudinal evidence that the lamina cribrosa is shifted or “dragged” nasally relative to the prelaminar tissue and BMO during axial elongation in myopic children. These relative deformations are likely involved in the tilted and rotated appearance of the ONH in high myopia, and in the development of peripapillary atrophy often seen at the temporal side of the myopic optic disc.
- [62].Jonas JB, Clinical implications of peripapillary atrophy in glaucoma, Curr. Opin. Ophthalmol 16 (2005) 84–88. [DOI] [PubMed] [Google Scholar]
- [63].Dai Y, Jonas JB, Huang H, Wang M, Sun X, Microstructure of parapapillary atrophy: beta zone and gamma zone., Investigative ophthalmology & visual science 54 (2013) 2013–2018. doi: 10.1167/iovs.12-11255. [DOI] [PubMed] [Google Scholar]
- [64].Sawada Y, Hangai M, Ishikawa M, Yoshitomi T, Association of myopic optic disc deformation with visual field defects in paired eyes with open-angle glaucoma: A cross-sectional study., PloS one 11 (2016) e0161961. doi: 10.1371/journal.pone.0161961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Burgoyne CF, Downs JC, Bellezza AJ, Suh J-KF, Hart RT, The optic nerve head as a biomechanical structure: a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage., Prog. Retina Eye. Res 24 (2005) 39–73. URL: 10.1016/j.preteyeres.2004.06.001. doi: 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- [66].Chung CW, Girard MJA, Jan N-J, Sigal IA, Use and misuse of Laplace’s law in ophthalmology, Invest. Ophthalmol. Vis. Sci 56 (2016) 236 URL: 10.1167/iovs.15-18053. doi: 10.1167/iovs.15-18053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sherman SM, Norton TT, Casagrande VA, Myopia in the lid-sutured tree shrew (Tupaia glis)., Brain Res. 124 (1977) 154–157. [DOI] [PubMed] [Google Scholar]
- [68].Norton TT, Experimental myopia in tree shrews., Ciba Found Symp 155 (1990) 178–94; discussion 194–9. [PubMed] [Google Scholar]
- [69].Samuels BC, Siegwart JT, Zhan W, Hethcox L, Chimento M, Whitley R, Downs JC, Girkin CA, A novel tree shrew (tupaia belangeri) model of glaucoma., Investigative ophthalmology & visual science 59 (2018) 3136–3143. doi: 10.1167/iovs.18-24261, [DOI] [PMC free article] [PubMed] [Google Scholar]; * This article provides insight into the special oportunity of using tree shrews as glaucoma model.
- [70].Siegwart J, Jr TT Norton, The susceptible period for deprivation-induced myopia in tree shrew., Vision Res 38 (1998) 3505–3515. [DOI] [PubMed] [Google Scholar]
- [71].Lin X, Naidu RK, Dai J, Zhou X, Qu X, Zhou H, Scleral cross-linking using glyceraldehyde for the prevention of axial elongation in the rabbit: Blocked axial elongation and altered scleral microstructure, Current Eye Research 44 (2019) 162–171. doi: 10.1080/02713683.2018.1522647. [DOI] [PubMed] [Google Scholar]
- [72].Grytz R, El Hamdaoui M, Levy AM, Girkin C, Samuels B: Scleral crosslinking using genipin has a dose-dependent effect on form-deprivation myopia in tree shrews. Invest Ophthalmol Vis Sci 2018, 58 of ARVO E-Abstract 708. [Google Scholar]
- [73].Kimball EC, Nguyen C, Steinhart MR, Nguyen TD, Pease ME, Oglesby EN, Oveson BC, Quigley HA, Experimental scleral cross-linking increases glaucoma damage in a mouse model., Exp Eye Res (2014). URL: 10.1016/j.exer.2014.08.016. doi: 10.1016/j.exer.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Coudrillier B, Campbell IC, Read AT, Geraldes DM, Vo NT, Feola A, Mulvihill J, Albon J, Abel RL, Ethier CR, Effects of peripapillary scleral stiffening on the deformation of the lamina cribrosa., Invest Ophthalmol Vis Sci 57 (2016) 2666–2677. URL: 10.1167/iovs.15-18193. doi: 10.1167/iovs.15-18193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dupps WJ, Seven I, A large-scale computational analysis of corneal structural response and ectasia risk in myopic laser refractive surgery., Transactions of the American Ophthalmological Society 114 (2016) T1. [PMC free article] [PubMed] [Google Scholar]


