Abstract
Workers in swine operations are exposed to dust, bacteria, and virus, and are at increased risk of respiratory problems. Toll-like receptors (TLR) play an important role in human immune responses to respiratory hazards. Worker gender and age may significantly modify the involvement of TLR in the etiology of these respiratory outcomes. The aim of this study was to investigate whether modification effects of worker gender and age altered associations between polymorphisms in the TLR genes and lung function. This study included 374 full-time workers from large swine operations from Saskatchewan. Information on demography, lifestyle, pulmonary function, and blood samples were obtained. Multiple linear regression and decision tree model were used in the analysis. Among females aged <45.8 years, workers with polymorphisms of rs4696480 in the TLR2 gene exhibited markedly better lung function than workers with wild-type. These associations were not observed among female workers aged ≥45.8 years and males. Among males, workers with polymorphisms of rs187084 in the TLR9 gene displayed significantly lower lung function than those with wild-type. This male-specific association was not dependent on worker age. This is the first study to report gender-specific correlations between lung function and polymorphisms of TLR genes, and modification effects of worker age on these associations, suggesting the importance of considering gender and age in genetic association studies of airway diseases due to exposure of high concentration of respiratory hazards.
Keywords: airway
Introduction
Gender differences in incidence, susceptibility, and severity of many lung diseases have been long recognized. Females are at increased risk of development of asthma, airway hype-responsiveness, and severe exacerbation (Becklake and Kauffmann 1999; Leynaert et al. 1997; Manfreda et al. 2004; Townsend, Miller, and Prakash 2012). Males are more susceptible to bacteria and virus infections, infectious complications after surgery, severe sepsis and septic shock (Merkel et al. 2001; Offner, Moore, and Biffl 1999; Traub et al. 2012; Wichmann et al. 2000; Yamamoto et al. 1991). Although the biological mechanisms underlying gender differences in pulmonary responsiveness are not fully understood, recent evidence suggests the involvement of sex-related hormones (Lange et al. 2001; Redline and Gold 1994; Troisi et al. 1995). Epidemiological studies demonstrated significant differences in many lung diseases prior to and after puberty, menopause, and andropause, when sex hormones undergo marked changes. Before the onset of puberty, when sex hormones are generally present at low concentration levels, boys are at higher risk of developing asthma than girls (Redline and Gold 1994). However, after puberty, which is related to an increase in sex hormones levels, females are more likely to be diagnosed with asthma (Redline and Gold 1994). In women, menopause initiates complex biological changes which include alterations in sex hormone levels. The onset of menopause is associated with a decrease in sex hormone levels and thus reported to provide protection against asthma, while the use of postmenopausal hormones may reverse the protective effects of menopause (Lange et al. 2001; Troisi et al. 1995). Significant lung function changes during the menstrual cycle phases and enhanced asthma exacerbations during pregnancy were reported in many studies (Driver et al. 2005; Murphy, Clifton, and Gibson 2006; Stanford et al. 2006). Taken together results from these investigations suggested sex hormones play a major role in many chronic lung diseases.
Toll-like receptors (TLRs), a family of pattern recognition receptors (PRR), play an important role in both innate and adaptive immune responses (Iwasaki and Medzhitov 2004; Lee and Lawrence 2018; Tipping 2006). Several investigators demonstrated gender differences in immunological responses after stimulation of TLR ligand (Berghofer et al. 2006; Clifford et al. 2012; Griesbeck et al. 2015; Iyer and Cheng 2012; Torcia et al. 2012; Traub et al. 2012). Further ovariectomy and estradiol replacement therapy significantly modified these gender-specific immune responses (Marriott, Bost, and Huet-Hudson 2006; Soucy et al. 2005). However, fewer studies examined gender differences in these associations in a human population. The aim of this study was to (1) examine the gender-specific associations between lung function and polymorphisms of TLR genes and (2) determine the possible modification effects of age on these associations in a human population.
Methods and materials
Ethics approval
The study was approved by the Biomedical Research Ethics Board of the University of Saskatchewan. Informed written consent was obtained from all subjects.
Recruitment and data collection
Detailed information on the recruitment and data collection of this cross-sectional study are described elsewhere (Gao et al. 2013, 2014; Senthilselvan et al. 2009, 2007, 1997, 2008). Briefly, 374 full-time workers were recruited from large swine production companies in Saskatchewan. Only those workers who were 17 years or older and worked for a minimum of 4 days per week with a total work-duration of 20 hr or more per week were included in this study. The participation rate was approximately 70%.
A previously validated questionnaire was used to collect anthropomorphic data, respiratory symptoms, smoking history, past illnesses, and occupational history (Gao et al. 2013, 2014, 2009, 2007; Senthilselvan et al. 1997, 2008). Each participant also had measurements taken of height (cm), weight (kg), pulmonary function, and systolic and diastolic blood pressure (mmHg).
Pulmonary function measurements including forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced expiratory flow between 25% and 75% of FVC (FEF25%−75%) were obtained by trained technicians using a volume displacement spirometer (model 1022; Sensor-Medics, Yorba Linda, California) who followed the American Thoracic Society recommendations (1987, 136:1285–1298). All interviews and pulmonary function tests were conducted in local communities near the swine production sites.
Skin prick tests (SPT) for 5 allergens (Alternaria sp, swine, mixed grass allergens, house dust mite, and cat dander) were performed on the same occasion as the pulmonary function tests as described in previously (Gao et al. 2013, 2014; Senthilselvan et al. 2008). Solutions for the 5 allergens were obtained from Western Allergy Services Ltd, Burnaby, BC, Canada. Histamine and saline were also included as the positive and negative control (Gao et al. 2013, 2014; Senthilselvan et al. 2008). The tests were performed on normal skin of each worker’s forearms with a distance of 2 cm between two SPT to avoid cross-contamination. A positive test was defined as one or more SPT had a raised wheal of 3 mm or larger than the saline control (Gao et al. 2013, 2014; Senthilselvan et al. 2008). The technicians conducting the SPT were not aware of the genotypes of each worker.
Smoking status was defined from the questionnaire as following: a current smoker (a person currently smoking cigarettes); an ex-smoker (a person who has smoked more than 400 cigarettes (or equivalent amount of tobacco) in his/her lifetime but was not currently smoking); and a non-smoker (a person who had not smoked more than 400 cigarettes (or equivalent amount of tobacco in his/her lifetime).
DNA isolation and genotyping
Detailed information on blood sample collection and genotyping methods was previously described (Pahwa et al. 2009). Briefly, Qiagen PAXgene tubes with blood cards spotted for each sample (S&S, catalog #10538414) were used to collect blood samples. DNA was isolated by a Gentra Autopure robot (Qiagen Corporation, Hilden, Germany), and DNA for genotyping was quantified by PicoGreen assays. Human TLR4 Asp299Gly and Thr399Ile polymorphisms were genotyped using TaqMan assays and standard protocols on the ABI 7900 Sequence Detection System. Plasmids carrying mutant and wild-type sequences were included as controls. All other TLR single nucleotide polymorphisms (SNP) were genotyped as a part of a 96-plex GoldenGate genotyping assay on Veracode beads. Assays were performed according to manufacturer’s protocols and scanned on a BeadXpress reader (Illumina, Inc., San Diego, CA, USA). The BeadStudio software (Illumina® GenomeStudio) was employed to cluster and clean raw genotyping data.
Selection of TLR SNP
The selection of single nucleotide polymorphism (SNP) in the TLR genes was based upon the following criteria as described previously (Gao et al. 2013, 2014; Senthilselvan et al. 2009): (i) SNP that were associated with one or more disease phenotypes in multiple cohorts or shown to alter gene function in biological assays; and (ii) tagging SNP (tSNP) of TLR genes identified using high-density SNP maps generated by the HapMap project. The tSNP of each TLR gene were selected using the SNPSelector software which prioritizes SNP on their tagging for linkage disequilibrium, SNP allele frequencies and source, function, regulatory potential, and repeat status (Xu et al. 2005). A total of 11 SNP from all TLR genes except TLR8 were selected in our study.
Statistical analysis
Descriptive statistics, mean (STD) and N (%) were provided for continuous and categorical variables, respectively. Significant differences in characteristics and genotypes between male and female workers were examined by chi-square test for categorical variables and Student’s t-test for continuous variables. Fisher’s exact test was used if the expected count for any cell was less than 5. Only those SNP which followed HWE (Hardy–Weinberg Equilibrium) and are common variances, Minor Allele Frequency (MAF) ≥5%, were included in this study. HWE was examined among females for the SNP of the TLR7 and TLR8 genes on the X chromosome. For the SNP in high Linkage disequilibrium (LD), D’>0.95, the SNP with the highest polymorphism information content (PIC) value was selected. Both dominant and recessive inheritance models were considered. However, only the inheritance model with the smallest AIC value (the best-fitted model) was selected.
To investigate the gender-specific association between lung function and polymorphisms of TLR genes, gender-stratified multivariable analysis was carried out after controlling for potential confounders including age, height, weight, and smoking status.
To examine the modification effects of age on the gender-specific associations between lung function and a SNP, a decision tree analysis was utilized by including age, gender, and a SNP (dominant and recessive inheritance) as independent variables in the model (SAS 2015). This method is based upon recursively splitting a dataset by independent variables into non-overlapping segments in such a way that all individuals within each segment are as similar as possible, which is measured by Gini index, entropy, and residual sum of squares (SAS 2015). In this study, age was included as a continuous variable. In order to reduce the number of computations for searching candidate cut-points of the age variable, the INTERVALBINS = 5 was defined. A minimum of 5 observations was also defined in child segments of a split. Based upon the results from a decision model, age group-stratified multivariable analysis was carried to further examine these gender-specific associations between a SNP and lung function in each age group after controlling for potential confounders including height, weight and smoking habit. The Bonferroni adjustment was applied in this study. The criterion for significance was set at p < 0.05.
Sensitivity analysis
To confirm the modification effects of age on the gender-specific associations between lung function and a SNP, in addition to age, gender, and a SNP variable, smoke, height, and weight were also included in the decision tree models. As the cut-points of age variable given by a decision tree analysis were based upon maximizing the homogeneity in all segments, a series of sensitivity analyses were conducted by using different cut-points of the age variable at 40, 45, and 50 years, respectively.
Results
As indicated in Table 1, male workers were significantly heavier and taller on average than females accompanied by significantly higher mean values of lung function parameters except for FEV1/FVC ratio, which was significantly lower in males. The genotype distributions of most SNP were similar between female and male workers, except for TLR5-rs5744168 and TLR10-rs4129009 polymorphisms. The two polymorphisms in the TLR4 gene (rs4986790 and rs4986791) were in high linkage disequilibrium (D’ = 0.97) and TLR4-rs4986790 polymorphisms were selected for further analysis because of its higher polymorphism information content (PIC) value. The TLR2-rs 5743708 and the TLR5-rs5744168 polymorphisms were excluded due to their minor allele frequency (MAF) less than 5%. This yielded a total of 7 SNP.
Table 1.
Comparison of characteristics between male and female workers*.
| Characteristic | Workers in swine operations | |
|---|---|---|
| Males (N = 240) | Females (N = 134) | |
| Age, year | 36.2 ±11.9 | 34.9 ± 10.7 |
| Weight, kg | 88.1 ± 16.6 | 75.5 ± 15.0# |
| Height, cm | 170.8 ± 6.1 | 164.4 ± 5.8# |
| Smoking Status, N (%) | ||
| Current smoker | 73 (30.4) | 44 (32.8) |
| Former smoker | 68 (28.3) | 33 (24.6) |
| Non smoker | 99 (41.3) | 57 (42.5) |
| Atopy, N (%) | ||
| Yes | 86 (38.7) | 37 (29.4) |
| No | 136 (61.3) | 89 (70.6) |
| Lung function | ||
| Observed | ||
| FEV1, L | 4.1 ± 0.7 | 3.1 ± 0.6# |
| FVC, L | 5.3 ± 0.8 | 3.9 ± 0.6# |
| FEV1/FVC, % | 77.8 ± 6.6 | 78.0 ± 6.2# |
| FEF25%−75%, L | 3.8 ± 1.2 | 3.2 ± 1.0# |
| TLR1, N (%) | ||
| RS5743551 | ||
| AA | 78 (44.6) | 58 (56.3) |
| AG | 78 (44.6) | 38 (36.9) |
| GG | 19 (10.9) | 7 (6.8) |
| MAF(G) | 116 (33.1) | 52 (25.2) |
| TLR2, N (%) | ||
| RS4696480 | ||
| AA | 40 (22.9) | 28 (27.2) |
| AT | 89 (50.9) | 55 (53.4) |
| TT | 46 (26.3) | 20 (19.4) |
| MAF(A) | 181 (51.7) | 95 (46.1) |
| RS5743708 | ||
| AG | 9 (5.2) | 9 (8.8) |
| GG | 164 (94.8) | 93 (91.2) |
| MAF(A) | 9 (2.6) | 9 (4.4) |
| LD (D′) | 0.5523 | |
| TLR3, N (%) | ||
| RS3775291 | ||
| AA | 27 (15.3) | 9 (8.8) |
| AG | 72 (40.9) | 45 (44.1) |
| GG | 77 (43.8) | 48 (47.1) |
| MAF(A) | 126 (35.8) | 63 (30.9) |
| TLR4, N (%) | ||
| RS4986790 | ||
| AA | 150 (86.7) | 88 (88.0) |
| AG | 23 (13.3) | 12 (12.0) |
| MAF(G) | 23 (6.7) | 12 (6.0) |
| RS4986791 | ||
| CT | 20 (11.6) | 13 (13.0) |
| TT | 153 (88.4) | 87 (87.0) |
| MAF(C) | 20 (5.8) | 13 (6.5) |
| LD (D′) | 0.9652 | |
| TLR5, N (%) | ||
| RS5744168 | ||
| AG | 9 (5.1) | 16 (15.8)# |
| GG | 169 (94.9) | 85 (84.2) |
| MAF(A) | 9 (2.5) | 16 (7.9) |
| TLR6, N (%) | ||
| RS5743810 | ||
| AA | 21 (12.0) | 17 (16.7) |
| AG | 80 (45.7) | 43 (42.2) |
| GG | 74 (42.3) | 42 (41.2) |
| MAF(A) | 122 (34.9) | 77 (37.8) |
| TLR9, N (%) | ||
| RS187084 | ||
| AA | 66 (37.7) | 27 (26.5) |
| AG | 76 (43.4) | 54 (52.9) |
| GG | 33 (18.9) | 21 (20.6) |
| MAF(G) | 142 (40.6) | 96 (47.1) |
| TLR10, N (%) | ||
| RS4129009 | ||
| AA | 97 (55.4) | 74 (72.6)# |
| AG | 64 (36.6) | 23 (22.6) |
| GG | 14 (8.0) | 5 (4.9) |
| MAF(G) | 92 (26.3) | 33 (16.2) |
Data are presented as mean ±SD unless otherwise indicated.
The polymorphisms of rs2302267 in the TLR7 gene were excluded from our study due to the violation of the HWE in female workers.
The polymorphism of rs5743708 in the TLR2 gene and the polymorphisms of rs5744168 in the TLR5 gene were excluded due to their minor allele frequency (MAF) less than 5%.
Significant from males p < 0.05
The gender-specific associations from multivariate analysis after controlling for potential confounders (age, height, weight, and smoking habit) are shown in Table 2. Among males, workers with the TLR3-rs 3775291 polymorphisms exhibited significantly higher mean values of FEV1 and FVC than those with wild-type, and individuals with the TLR6-rs 5743810 polymorphisms displayed significantly higher mean values of FEV1/FVC and FEF25%−75% than those with wild-type. Workers with the TLR9-rs187084 polymorphisms on average exhibited significantly higher FEV1/FVC, but significantly lower FVC than those with wild-type. After adjusting for multiple comparisons using Bonferroni’s correction at α = 0.007 (0.05/7) significance level, only the association between the TLR9-rs187084 polymorphisms and FVC remained significant among males. Among females with the TLR2-rs4696480 polymorphisms they on average displayed significantly higher lung function in FEV1, FVC and FEF25%−75% than those with wild-type. After adjusting for multiple comparisons, at α = 0.007 (0.05/7) significance level, associations with FEV1 and FVC remained significant among females.
Table 2.
Gender-specific effects of polymorphisms of the TLR genes on lung function in the workers in multivariate analysis*.
| Gene | Lung function | Male workers | Female workers | ||||
|---|---|---|---|---|---|---|---|
| Polymorphism mean (SE) | Wild-type mean (SE) | Difference (SE) | Polymorphism mean (SE) | Wild-type mean (SE) | Difference (SE) | ||
| TLR1 | |||||||
| RS5743551 | FEV1, L | 3.84 (0.06) | 3.86 (0.06) | −0.03 (0.07)d | 3.22 (0.07) | 3.22 (0.07) | −0.01 (0.07)d |
| FVC, L | 4.92 (0.07) | 4.98 (0.09) | −0.06 (0.09)d | 4.17 (0.09) | 4.24 (0.08) | −0.07 (0.09)d | |
| FEV1/FVC, % | 78.35 (1.55) | 77.67 (0.66) | 0.68 (1.55)r | 76.82 (0.99) | 75.76 (0.94) | 1.06(0.98)d | |
| FEF25%–75%, L | 3.70 (0.27) | 3.62 (0.11) | 0.09 (0.27)r | 2.84 (0.28) | 2.94 (0.13) | −0.10 (0.29)r | |
| TLR2 | |||||||
| RS4696480 | FEV1, L | 3.93 (0.08) | 3.81 (0.05) | 0.12 (0.09)r | 3.39 (0.08) | 3.14 (0.06) | 0.25 (0.08)r‡ |
| FVC, L | 5.01 (0.09) | 4.92 (0.06) | 0.08 (0.10)r | 4.38 (0.09) | 4.11 (0.08) | 0.27 (0.09)r‡ | |
| FEV1/FVC, % | 78.41 (1.06) | 77.25 (0.72) | 1.16 (1.19)r | 76.82 (1.12) | 75.92 (0.91) | 0.90 (1.13)r | |
| FEF25%–75%, L | 3.88 (0.18) | 3.48 (0.12) | 0.40 (0.21)r | 3.16 (0.16) | 2.82 (0.13) | 0.34 (0.16)r‡ | |
| TLR3 | |||||||
| RS3775291 | FEV1, L | 4.02 (0.09) | 3.80 (0.05) | 0.21 (0.10)r† | 3.15 (0.13) | 3.26 (0.06) | −0.11 (0.12)r |
| FVC, L | 5.16 (0.12) | 4.89 (0.06) | 0.27 (0.12)r† | 4.20 (0.15) | 4.23 (0.08) | −0.04 (0.15)r | |
| FEV1/FVC, % | 77.31 (0.75) | 78.20 (0.87) | −0.89 (0.99)d | 74.91 (1.75) | 76.64 (0.87) | −1.73 (1.71)r | |
| FEF25%–75%, L | 3.84 (0.22) | 3.56 (0.12) | 0.28 (0.24)r | 2.66 (0.25) | 3.02 (0.13) | −0.36 (0.25)r | |
| TLR4 | |||||||
| RS4986790 | FEV1, L | 3.91 (0.11) | 3.87 (0.05) | 0.04 (0.12)d | 3.17 (0.11) | 3.29 (0.07) | −0.12 (0.12)d |
| FVC, L | 5.13 (0.14) | 4.97 (0.06) | 0.16 (0.14)d | 4.05 (0.12) | 4.23 (0.08) | −0.18 (0.13)d | |
| FEV1/FVC, % | 76.31 (1.44) | 77.82 (0.66) | −1.50 (1.45)d | 77.65 (1.39) | 77.59 (0.92) | 0.06 (1.51)d | |
| FEF25%–75%, L | 3.58 (0.25) | 3.65 (0.11) | −0.06 (0.25)d | 3.01 (0.22) | 3.19 (0.14) | −0.18 (0.23)d | |
| TLR6 | |||||||
| RS5743810 | FEV1, L | 4.03 (0.11) | 3.82 (0.05) | 0.21 (0.11)r | 3.23 (0.07) | 3.21 (0.08) | 0.02 (0.07)d |
| FVC, L | 5.02 (0.14) | 4.93 (0.06) | 0.09 (0.14)r | 4.23 (0.08) | 4.18 (0.09) | 0.05 (0.09)d | |
| FEV1/FVC, % | 80.40 (1.49) | 77.34 (0.66) | 3.06 (1.50)r† | 77.34 (1.37) | 76.01 (0.86) | 1.33 (1.29)r | |
| FEF25%–75%, L | 4.14 (0.26) | 3.55 (0.11) | 0.59 (0.26)r† | 3.07 (0.20) | 2.91 (0.13) | 0.16 (0.19)r | |
| TLR9 | |||||||
| RS187084 | FEV1, L | 3.74 (0.09) | 3.88 (0.05) | −0.14 (0.09)r | 3.25 (0.09) | 3.21 (0.07) | 0.03 (0.09)r |
| FVC, L | 4.68 (0.10) | 5.02 (0.06) | −0.34 (0.11)r‡ | 4.30 (0.11) | 4.17 (0.08) | 0.13 (0.10)r | |
| FEV1/FVC, % | 79.59 (1.16) | 77.09 (0.69) | 2.51 (1.24)r† | 75.01 (1.20) | 76.67 (0.89) | −1.66 (1.19)r | |
| FEF25%–75%, L | 3.79 (0.20) | 3.56 (0.12) | 0.24 (0.22)r | 2.98 (0.13) | 2.82 (0.17) | 0.16 (0.16)d | |
| TLR10 | |||||||
| RS4129009 | FEV1, L | 3.94 (0.13) | 3.84 (0.05) | 0.11 (0.13)r | 3.18 (0.17) | 3.23 (0.06) | −0.04 (0.17)r |
| FVC, L | 5.04 (0.16) | 4.94 (0.06) | 0.10 (0.16)r | 4.13 (0.20) | 4.21 (0.08) | −0.08 (0.20)r | |
| FEV1/FVC, % | 77.83 (0.83) | 77.51 (0.79) | 0.32 (0.98)d | 76.55 (1.17) | 76.11 (0.89) | 0.45 (1.09)d | |
| FEF25%–75%, L | 3.57 (0.14) | 3.65 (0.14) | −0.09 (0.17)d | 2.76 (0.34) | 2.95 (0.13) | −0.19 (0.34)r | |
Only those SNPs which follow HWE and are common variances (MAF>5.0%) were selected into the multivariate models adjusting for age, height, weight, and smoking habit.
Significant at α = 0.05 level.
Significant after adjusting for multiple comparison at α = 0.007 (0.05/7).
(dominant inheritance has the best-fitted model).
(recessive inheritance has the best-fitted model).
The modification effects of work’s age on female-specific associations between TLR2-rs4696480 polymorphisms and lung function are presented in Table 3. The age cut-off from the decision tree models was at 45.8 years in females and 36.2 years in males (see the outputs from decision tree models in Figures 1 and 2). The Figures 1 and 2 illustrate the TLR2-rs4696480 polymorphisms selected by the decision tree among females aged <45.8 years in the model of FEV1 and FVC, and were not selected among females aged ≥45.8 years and males. Among females who were younger than 45.8 years, individuals with the TLR2-rs4696480 polymorphisms exhibited significantly higher average lung function in FEV1 and FVC than those with wild-type after adjusting for height, weight and smoking habit. These associations remained significant after adjusting for multiple comparisons at α = 0.007 (0.05/7) significance level. No marked associations were observed among female workers who were older than 45.8 years, and among males.
Table 3.
Modification effects of age on the female-specific associations between the TLR2-rs4696480 polymorphisms and lung function (N = 103)*.
| TLR2-RS4696480 polymorphisms | |||
|---|---|---|---|
| Polymorphism mean (STE) | Wild-type mean (STE) | Comparison† difference (STE) | |
| Female workers aged <45.8 years | |||
| (N = 83, Mean = 31, range:18–45) | |||
| Lung function | |||
| FEV1, L | 3.68 (0.09) | 3.39 (0.08) | 0.30 (0.09)‡ |
| FVC, L | 4.59 (0.09) | 4.28 (0.08) | 0.30 (0.10)‡ |
| Female workers aged ≥45.8 years | |||
| (N = 20, Mean = 50 Range:46–57) | |||
| FEV1, L | 2.66 (0.25) | 2.56 (0.15) | 0.11 (0.27) |
| FVC, L | 3.76 (0.34) | 3.79 (0.21) | −0.03 (0.38) |
The cut-point of the age variable at 45.8 years was provided by the decision tree models.
p values were from multivariate models adjusting for height, weight and smoking habit.
Significant after adjusting for multiple comparison at α = 0.007 (0.05/7).
Figure 1.
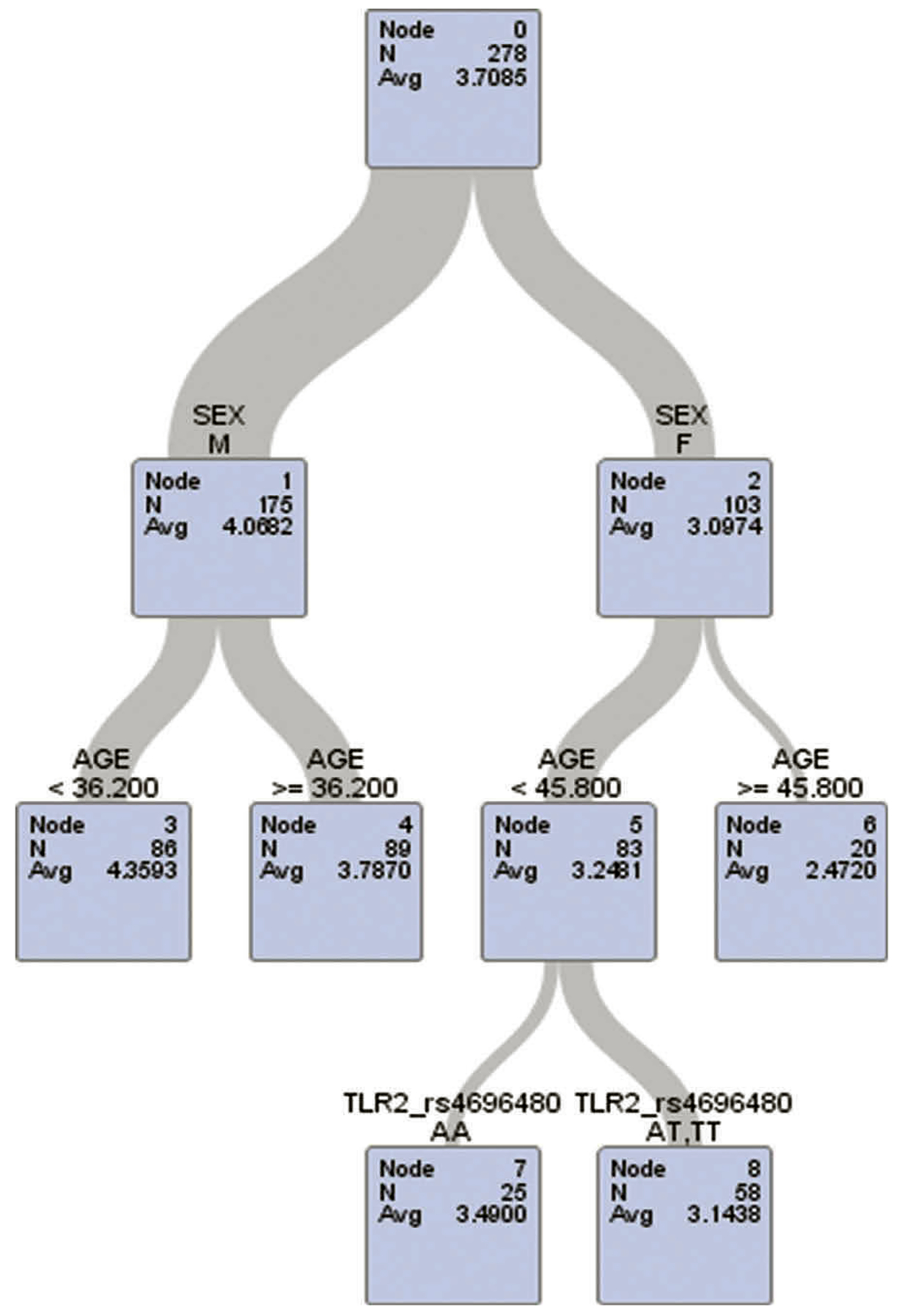
Decision tree analysis of FEV1 with age, sex, and TLR2-rs4696480 in the model.
Figure 2.
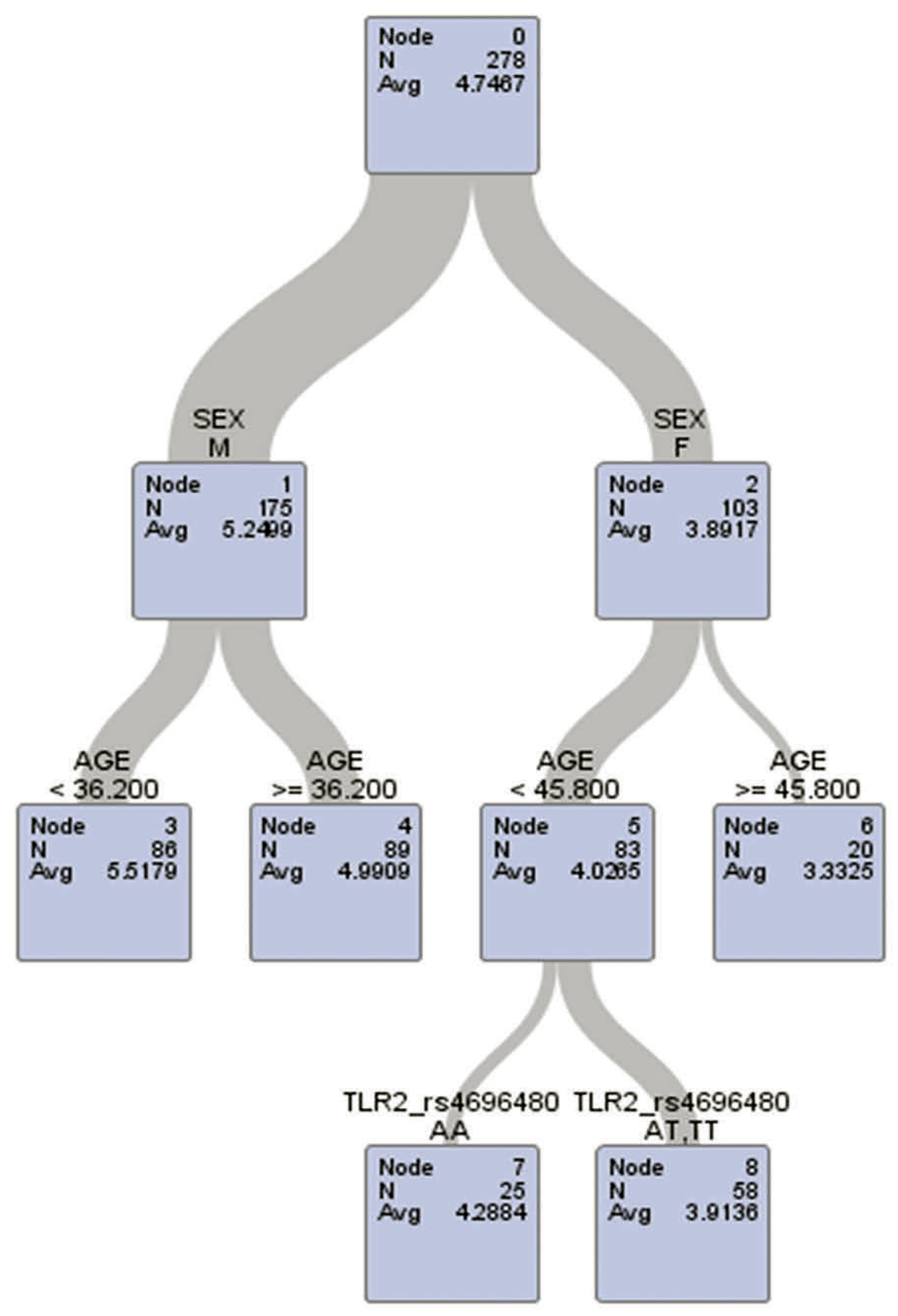
Decision tree analysis of FVC with age, sex, and TLR2-rs4696480 in the model.
Sensitivity analysis confirmed the female-specific associations between TLR2-rs4696480 polymorphisms and lung function (FEV1 and FVC) were significant among the females only in the younger age group, but not in the older age group using different cut-offs of age at 40, 45 or 50 years (Table 4). Further, decision tree analysis was conducted by including age, gender, TLR2-rs4696480, weight, height, and smoke status. The results from the decision tree analysis also confirmed modification effects of age on these female-specific associations only in the younger age group since TLR2-rs4696480 polymorphism was not selected by the decision tree among female workers aged ≥45 years (see the outputs from decision tree models in Figures 3 and 4).
Table 4.
Female-specific associations between the TLR2-rs 696480 polymorphisms and lung function in different age groups using age cut-offs at 40, 45 and 50 years (N = 103).
| Lung Function | Comparison in female workers* (Polymorphisms - Wild-type) | |
|---|---|---|
| Difference (STE) | Difference (STE) | |
| Age cut-off at 40 years | ||
| <40 years | ≥40 years | |
| (N = 67) | (N = 36) | |
| FEV1, L | 3.71 (0.08)† | −0.31 (0.26) |
| FVC, L | 0.30 (0.10)† | 0.40 (0.28) |
| Age cut-off at 45 years | ||
| <45 years | ≥45 years | |
| (N = 82) | (N = 21) | |
| FEV1, L | 0.30 (0.09)† | 0.10 (0.28) |
| FVC, L | 0.30 (0.10)† | −0.04 (0.38) |
| Age cut-off at 50 years | ||
| <50 years | ≥50 years | |
| (N = 94) | (N = 9) | |
| FEV1, L | 0.30 (0.10)† | N/A |
| FVC, L | 0.30 (0.10)† | N/A |
p values were from multivariate models adjusting for height, weight and smoking habit.
Significant after adjusting for multiple comparison at α = 0.007 (0.05/7).
Figure 3.
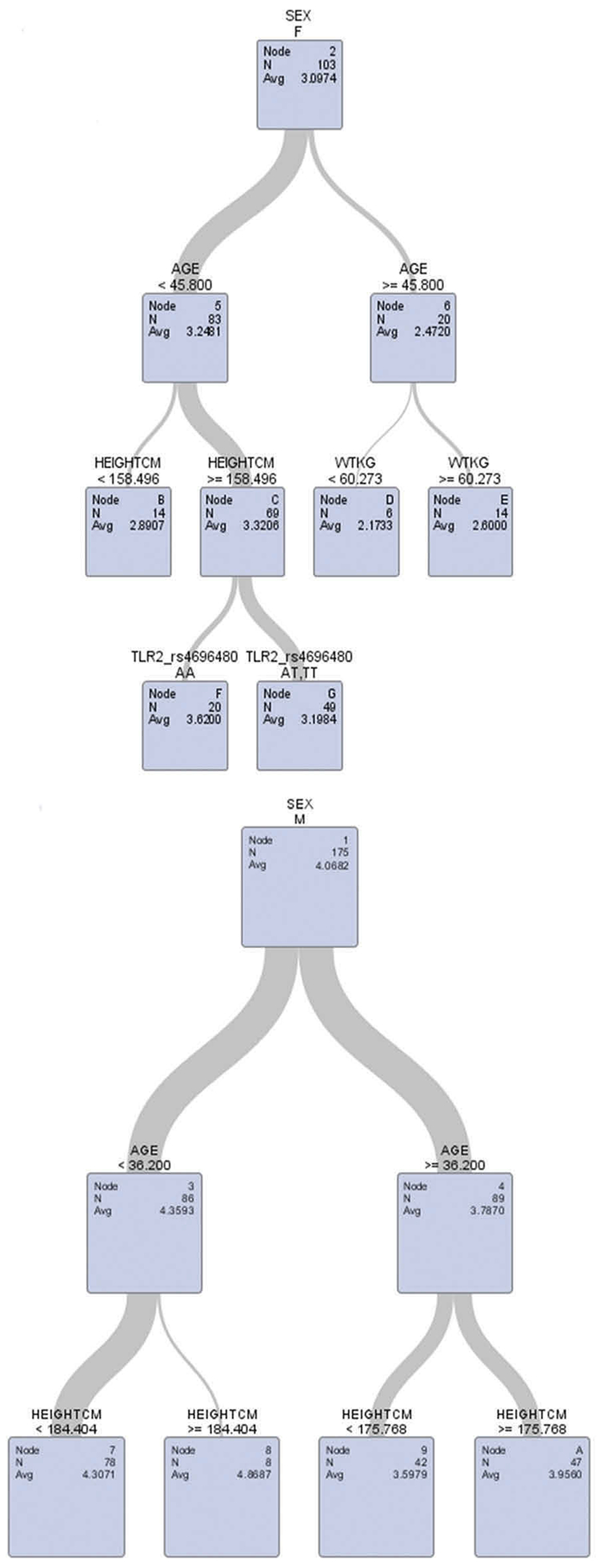
Decision tree analysis of FEV1 with age, sex, TLR2-rs 4696480, smoke, weight, and height in the model.
Figure 4.
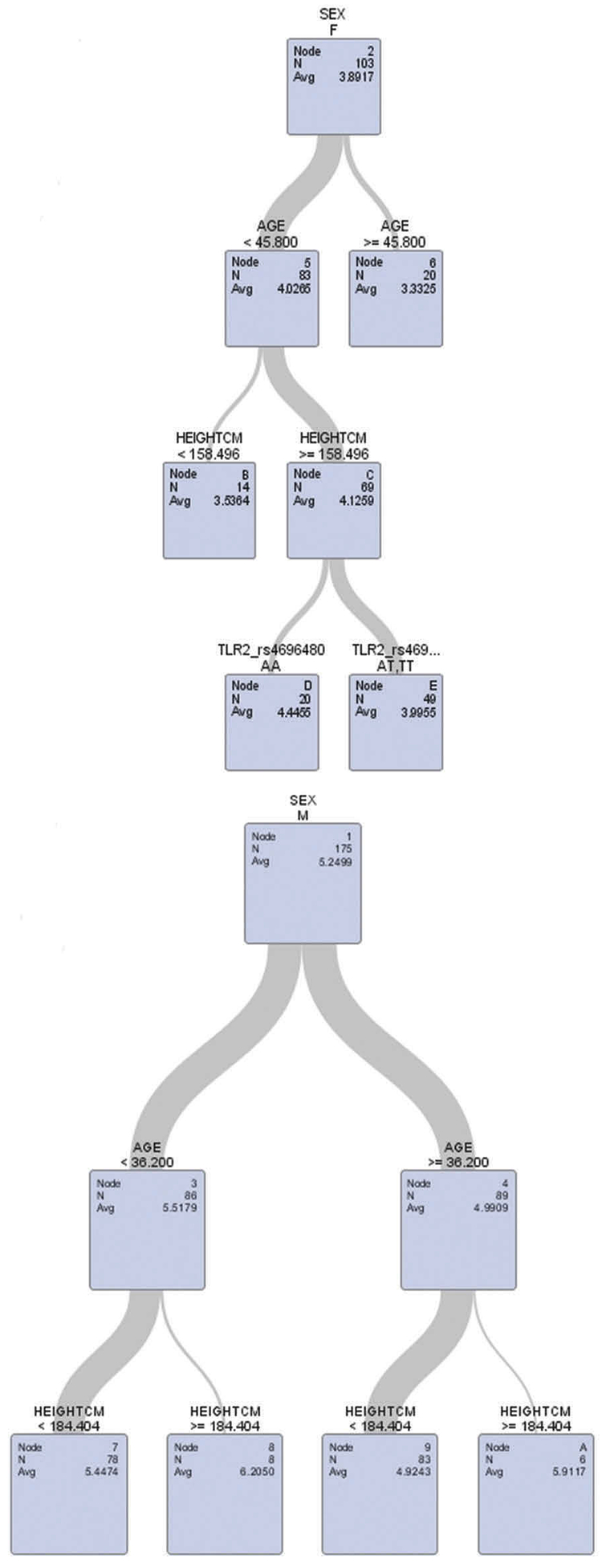
Decision analysis of FVC with age, sex, TLR2-rs 4696480, smoke, weight, and height in the model.
Discussion
Data demonstrated that female workers with TLR2 -rs 4696480 polymorphisms displayed significantly higher mean values of FEV1 and FVC than females with wild-type, but this relationship was not observed in males. Further, these female-specific associations were dependent upon age and only noted in the younger but not older age group. Male-specific association was also found where male workers with TLR9-rs187084 polymorphisms were significantly correlated with lower mean FVC values than males with wild-type. However, this male-specific association was not dependent upon age.
Workers in animal confinement facilities are at higher risk of lung dysfunction due to exposure to a large variety of hazards including bacteria (gram-positive and gram-negative), viruses, fungi, dust particles, and gases (Dosman et al. 2004; Eduard, Pearce, and Douwes 2009; Harting et al. 2012; May, Romberger, and Poole 2012, 2007; Senthilselvan et al. 1997; Viegas et al. 2013). Several investigators showed that the involvement of TLR2 in the airway diseases among workers in swine operation and the important role of TLR9 in lung inflammation following exposure to chicken barn air (Gao et al. 2013; Harting et al. 2012; Just, Duchaine, and Singh 2009; May, Romberger, and Poole 2012; Schneberger et al. 2016). TLR2 mainly responds to cell wall structure components such as peptidoglycan from gram-positive bacteria, and the ligand of TLR9 is un-methylated DNA which is found in bacterial and viral DNA (Hemmi et al. 2000; Iwasaki and Medzhitov 2004). The activation of TLR induces a series of intracellular signaling cascades to produce both pro- and anti-inflammatory cytokines and chemokines, which all work together to respond to invading pathogens (Hemmi et al. 2000; Iwasaki and Medzhitov 2004; Tipping 2006).
TLR2-16934 (rs4696480) is a promoter variant of TLR2 gene. An experimental study of cord blood mononuclear cells showed the carriers of AA genotype of this SNP exhibited significantly increased gene expression of several Treg (regulatory T cells) marker genes including FOXP3 (forkhead box protein p3), GITR (glucocorticoid-induced tumor necrosis factor receptor) and LAG3 (lymphocyte activation gene 3) as well as Th2 (T helper cell type 2) cytokines and TNF-α (tumor necrosis factor alpha) secretion in the presence of maternal atopy and postulated modification effects of maternal atopy status on this association may be partially explained by epigenetic regulation (Liu et al. 2011). Eder et al. (2004) reported that farm children from rural areas in Austria and Germany with a T allele of TLR2-16934 (rs4696480) were less susceptible to the diagnosis of asthma and atop, but not among children who did not reside on a farm. Kerkhof et al (2010) noted that this SNP significantly modified the influence of PM2.5 exposure on doctor-diagnosed asthma in children in the Netherlands.
Animal studies showed the TLR2 gene is responsive to sex hormones (Li and Bai 2014; Poole et al. 2011; Scotland et al. 2011; Soucy et al. 2005; Yang et al. 2009). Soucy et al. (2005) demonstrated that a single injection of LPS to mice increased transcriptional activation of TLR2 gene in the brain, which was completely inhibited by ovariectomy. Further estradiol replacement therapy was able to reverse the inhibitory effects of ovariectomy by stimulation of estrogen receptor (ER) α. Scotland et al. (2011) noted an elevated number of leukocytes in naïve peritoneal and pleural cavities of females compared to males, and resident peritoneal macrophages from females exhibited significantly higher expression of TLRs including TLR2, TLR3, and TLR4 than males. It is of interest that ovariectomy significantly reduced mRNA expression levels of these TLR, as well as protein expression of TLR2 and TLR4 in female resident macrophages. Li and Bai (2014) using human THP-1 cells confirmed not only the involvement of 17β-estradiol (E2) in promoting transcription activity of TLR2 mRNA, but also identified an ER response element in the 5′-flanking region, 251 bases upstream of the TLR2 promoter region, which regulates E2-mediated upregulation of transcription activity and protein expression of the TLR2 gene. Our study extended current knowledge from experimental studies using cells and animal models to a human population by providing direct evidence showing female-specific associations between polymorphisms of TLR2 gene and lung function.
In humans, levels of sex hormones not only vary between males and female, but change according to age. Loss of sex hormones begins around the age of 35–40 in males and 40–50 in females, and these natural alterations in sex hormones provide a unique opportunity to study sex-hormone sensitive genes (Horstman et al. 2012). Population studies demonstrated the median age at natural menopause in Caucasian women is approximately 50 years, and perimenopause starts at 47.5 years, when female hormone levels start to markedly decrease (Horstman et al. 2012; McKinlay 1996). As a result of female hormone levels falling due to aging and menopause, female hormone sensitive genes may function differently, especially after menopause. In our study, female-specific associations between TLR2 -rs 4696480 polymorphisms and lung functions were only found among the younger age group, when female hormones are at higher levels.
Our study showed a male-specific association between FVC and the TLR9-rs187084 polymorphisms. Sex differences in susceptibility to infection were reported in various experimental and population studies. In comparison with female mice, males are more susceptible to infection and endotoxin shock induced by LPS (Merkel et al. 2001; Yamamoto et al. 1991). Human population investigations also confirmed males are more susceptible to bacterial infection (Offner, Moore, and Biffl 1999; Wichmann et al. 2000). A large prospective cohort of surgical intensive care patients from Germany demonstrated female patients displayed a lower chance to be referred to the ICU, and were less likely to develop severe sepsis/septic shock than male patients (Wichmann et al. 2000). Another prospective cohort study of 545 trauma patients from the USA also noted that males were 1.6-fold more likely to develop major infectious complications after surgery (Offner et al. Biffl 1999).
The involvement of the TLR9 gene in the regulation of inflammation was reported in animal model by Schneberger et al. (2016). Expression of TLR9 and production of cytokines following TLR9 stimulation varied significantly between males and females (Torcia et al. 2012; Traub et al. 2012). The TLR9 mRNA expression was 5-fold higher in male than female mice following infection of mouse cytomegalovirus (MCMV) (Traub et al. 2012). Torcia et al. (2012) examined IL10 production from the peripheral blood mononuclear cell (PBMC) and reported that following TLR9 stimulation or infection with Herpes simplex virus (HSV-1), IL10 production was significantly higher in males and females in post-menopausal age than females in reproductive age. A large case-control study from Taiwan also showed the male-specific association between polymorphisms in the TLR9 gene and Graves’ disease (Liao et al. 2010). The mechanisms underlying sex hormones modification of the TLR9 gene are still unknown. However, several studies demonstrated that testosterone may upregulate production of IL10, which plays a key role in limiting host immune response to pathogens by activating anti-inflammatory immune pathways, reducing the production of inflammatory cytokines and enhancing activation of regulatory T cells, whereas estrogens downregulate the TLR9 expression in ER + breast cancer cells (Iyer and Cheng 2012; Jukkola-Vuorinen et al. 2009; Liva and Voskuhl 2001; Torcia et al. 2012).
Sex hormones significantly modulate cell signaling pathways to control gene regulation and expression. Activation of the NF-κB family of transcription factors is a major result of TLR ligation with pathogen-associated molecular patterns and plays an important role in inflammatory responses. Estrogen receptors (ERs) might inhibit NF-κB activity in multiple ways in cytoplasm and in nucleus (Kalaitzidis and Gilmore 2005) by the following mechanisms (as illustrated in Supplementary Figure 1): (a) ERs directly interacting with NF-κB to block its binding to DNA, (b) ERs inhibiting IκB kinase complex (IKK) activity, (c) ERs inhibiting IκB degradation, (d) ERs inhibiting NF-κB activity by competing for NF-κB coactivators, and (e) ERs interfering with binding to NF-κB coactivators.
Decision tree analysis has gained attention among computational, biomedical, and medical researchers. The advantages and disadvantages of this method were comprehensively reviewed by Song and Zhang (2014) and Song and Lu (2015). Recently, due to rapid advances in microarray technology, investigators are facing the challenge of how to effectively analyze large numbers of genetic markers from limited numbers of samples in microarray data. Machine learning has been widely used for microarray analysis to identify genetic markers to improve diagnosis and prediction of prognosis or responses in patients receiving a particular treatment of many diseases such as systemic lupus erythematosus, primary antiphospholipid syndrome (Armananzas et al. 2009) and cancers (Deist et al. 2018; Wang 2014). Simplicity and easy interpretation makes this method popular. However, this method has the disadvantage of being unstable, i.e. the optimal decision tree based upon a small dataset is generally unstable, and suffers from overfitting, which limits its generalizability and robustness.
There were some limitations to our study. This investigation did not have an objective measure of peptidoglycan exposure levels associated with gram-positive bacteria. Lack of sex hormone measurement or self-report of menopause information also limited our ability to examine gender-specific associations and compare the female-specific associations prior to and post-menopause. The age cut-off at 45.8 years from decision tree is a rough measure of loss of female hormones generally beginning at the age of 40–50 years in females (Horstman et al. 2012). However, consistent results from our sensitivity analyses using three different cut-offs of age at 40, 45 and 50 years confirmed these female-specific associations in the younger age group. Small sample size of this study (n = 374) also limits further stratification of our statistical analysis and results from this study need to be confirmed in a large cohort.
Conclusions
This is the first human population study, to our knowledge, to report gender-specific associations between polymorphisms of TLR genes and lung function among workers in swine operations, and modification effects of age on these gender-specific associations. These findings emphasize the importance of considering gender and age in all genetic association studies of respiratory diseases since the underlying causes of these diseases might vary not only between males and females, but also between different age groups. Our study provides useful information for the prevention and treatment of lung diseases among workers exposed to high levels of respiratory hazards.
Supplementary Material
Funding
This work was supported by the Canadian Institutes of Health Research [MOP-57907].
Footnotes
Declarations of interest
None.
Color versions of one or more of the figures in the article can be found online at www.tandfonline.com/uteh.
Supplemental data for this article can be accessed here.
References
- Standardization of spirometry–1987 update. Statement of the American thoracic society. Am. Rev. Respir. Dis 136:1285–98. doi: 10.1164/ajrccm/136.5.1285. [DOI] [PubMed] [Google Scholar]
- Armananzas R, Calvo B, Inza I, Lopez-Hoyos M, Martinez-Taboada V, Ucar E, Bernales I, Fullaondo A, Larranaga P, and Zubiaga AM. 2009. Microarray analysis of autoimmune diseases by machine learning procedures. IEEE Trans. Inf. Technol. Biomed 13:341–50. doi: 10.1109/TITB.2008.2011984. [DOI] [PubMed] [Google Scholar]
- Becklake MR, and Kauffmann F. 1999. Gender differences in airway behaviour over the human life span. Thorax 54:1119–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghofer B, Frommer T, Haley G, Fink L, Bein G, and Hackstein H. 2006. TLR7 ligands induce higher IFN-alpha production in females. J. Immunol 177:2088–96. [DOI] [PubMed] [Google Scholar]
- Clifford HD, Yerkovich ST, Khoo SK, Zhang G, Upham J, Le Souef PN, Richmond P, and Hayden CM. 2012. Toll-like receptor 7 and 8 polymorphisms: Associations with functional effects and cellular and antibody responses to measles virus and vaccine. Immunogenetics 64:219–28. doi: 10.1007/s00251-011-0574-0. [DOI] [PubMed] [Google Scholar]
- Deist TM, Dankers FJWM, Valdes G, Wijsman R, Hsu IC, Oberije C, Lustberg T, van SJ, Hoebers F, Jochems A, El NI, Wee L, Morin O, Raleigh DR, Bots W, Kaanders JH, Belderbos J, Kwint M, Solberg T, Monshouwer R, Bussink J, Dekker A, and Lambin P. 2018. Machine learning algorithms for outcome prediction in (chemo)radiotherapy: An empirical comparison of classifiers. Med. Phys 45:3449–59. doi: 10.1002/mp.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosman JA, Lawson JA, Kirychuk SP, Cormier Y, Biem J, and Koehncke N. 2004. Occupational asthma in newly employed workers in intensive swine confinement facilities. Eur. Respir. J 24:698–702. doi: 10.1183/09031936.04.00112102. [DOI] [PubMed] [Google Scholar]
- Driver HS, McLean H, Kumar DV, Farr N, Day AG, and Fitzpatrick MF. 2005. The influence of the menstrual cycle on upper airway resistance and breathing during sleep. Sleep 28:449–56. [DOI] [PubMed] [Google Scholar]
- Eder W, Klimecki W, Yu L, von Mutius E, Riedler J, Braun-Fahrlander C, Nowak D, and Martinez FD, Alex Study Team. 2004. Toll-like receptor 2 as a major gene for asthma in choldren of European farmers. J. Allergy Clin. Immunol 113:482–88. doi: 10.1016/j.jaci.2003.12.374. [DOI] [PubMed] [Google Scholar]
- Eduard W, Pearce N, and Douwes J. 2009. Chronic bronchitis, COPD, and lung function in farmers: The role of biological agents. Chest 136:716–25. doi: 10.1378/chest.08-2192. [DOI] [PubMed] [Google Scholar]
- Gao Z, Dosman JA, Rennie DC, Schwartz DA, Yang IV, Beach J, and Senthilselvan A. 2013. Association of Toll-like receptor 2 gene polymorphisms with lung function in workers in swine operations. Ann. Allergy Asthma Immunol 110:44–50. doi: 10.1016/j.anai.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Gao Z, Dosman JA, Rennie DC, Schwartz DA, Yang IV, Beach J, and Senthilselvan A. 2014. NOS3 polymorphism, lung function, and exposure in swine operations: Results of 2 studies. J. Allergy Clin. Immunol 134:485–88. doi: 10.1016/j.jaci.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Griesbeck M, Ziegler S, Laffont S, Smith N, Chauveau L, Tomezsko P, Sharei A, Kourjian G, Porichis F, Hart M, Palmer CD, Sirignano M, Beisel C, Hildebrandt H, Cenac C, Villani AC, Diefenbach TJ, Le GS, Schwartz O, Herbeuval JP, Autran B, Guery JC, Chang JJ, and Altfeld M. 2015. Sex differences in plasmacytoid dendritic cell levels of IRF5 drive higher IFN-alpha production in women. J. Immunol 195:5327–36. doi: 10.4049/jimmunol.1501684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JR, Gleason A, Romberger DJ, Von Essen SG, Qiu F, Alexis N, and Poole JA. 2012. Chronic obstructive pulmonary disease patients have greater systemic responsiveness to ex vivo stimulation with swine dust extract and its components versus healthy volunteers. J. Toxicol. Environ. Health Part A 75:1456–1470. doi: 10.1080/15287394.2012.722186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, Matsumoto M, Hoshino K, Wagner H, Takeda K, and Akira S. 2000. A toll-like receptor recognizes bacterial DNA. Nature 408:740–45. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- Horstman AM, Dillon EL, Urban RJ, and Sheffield-Moore M. 2012. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A Biol. Sci. Med. Sci 67:1140–52. doi: 10.1093/gerona/gls068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A, and Medzhitov R. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol 5:987–95. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Iyer SS, and Cheng G. 2012. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol 32:23–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukkola-Vuorinen A, Rahko E, Vuopala KS, Desmond R, Lehenkari PP, Harris KW, and Selander KS. 2009. Toll-like receptor-9 expression is inversely correlated with estrogen receptor status in breast cancer. J. Innate. Immun 1:59–68. doi: 10.1159/000151602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just N, Duchaine C, and Singh B. 2009. An aerobiological perspective of dust in cage-housed and floor-housed poultry operations. J. Occup. Med. Toxicol 4:13. doi: 10.1186/1745-6673-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaitzidis D, and Gilmore TD. 2005. Transcription factor cross-talk: The estrogen receptor and NF-kappaB. Trends Endocrinol. Metab 16:46–52. doi: 10.1016/j.tem.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Kerkhof M, Postma DS, Brunekreef B, Reijmerink NE, Wijga AH, de Jongste JC, Gehring U, and Koppelman GH. 2010. Toll-like receptor 2 and 4 genes influence susceptibility to adverse effects of traffic-related air pollution on childhood asthma. Thorax 65:690–97. doi: 10.1136/thx.2009.119636. [DOI] [PubMed] [Google Scholar]
- Lange P, Parner J, Prescott E, Ulrik CS, and Vestbo J. 2001. Exogenous female sex steroid hormones and risk of asthma and asthma-like symptoms: A cross sectional study of the general population. Thorax 56:613–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F, and Lawrence DA. 2018. From infections to anthropogenic inflicted pathologies: Involvement of immune balance. J. Toxicol. Environ. Health B 21:24–46. doi: 10.1080/10937404.2017.1412212. [DOI] [PubMed] [Google Scholar]
- Leynaert B, Bousquet J, Henry C, Liard R, and Neukirch F. 1997. Is bronchial hyperresponsiveness more frequent in women than in men? A population-based study. Am. J. Respir. Crit Care Med 156:1413–20. doi: 10.1164/ajrccm.156.5.9701060. [DOI] [PubMed] [Google Scholar]
- Li X, Li M, and Bai X. 2014. Upregulation of TLR2 expression is induced by estrogen via an estrogen-response element (ERE). Arch. Biochem. Biophys 549:26–31. doi: 10.1016/j.abb.2014.01.028. [DOI] [PubMed] [Google Scholar]
- Liao WL, Chen RH, Lin HJ, Liu YH, Chen WC, Tsai Y, Wan L, and Tsai FJ. 2010. Toll-like receptor gene polymorphisms are associated with susceptibility to Graves’ ophthalmopathy in Taiwan males. BMC. Med. Genet 11:154. doi: 10.1186/1471-2350-11-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Rädler D, Illi S, Klucker E, Turan E, von Mutius E, Kabesch M, and Schaub B. 2011. TLR2 polymorphisms influence neonatal regulatory T cells depending on maternal atopy. Allergy 66:1020–29. doi: 10.1111/j.1398-9995.2011.02683.x. [DOI] [PubMed] [Google Scholar]
- Liva SM, and Voskuhl RR. 2001. Testosterone acts directly on CD4+ T lymphocytes to increase IL-10 production. J. Immunol 167:2060–67. [DOI] [PubMed] [Google Scholar]
- Manfreda J, Sears MR, Becklake MR, Chan-Yeung M, Dimich-Ward H, Siersted HC, Ernst P, Sweet L, Van TL, Bowie DM, and Anthonisen NR. 2004. Geographic and gender variability in the prevalence of bronchial responsiveness in Canada. Chest 125:1657–64. [DOI] [PubMed] [Google Scholar]
- Marriott I, Bost KL, and Huet-Hudson YM. 2006. Sexual dimorphism in expression of receptors for bacterial lipopolysaccharides in murine macrophages: A possible mechanism for gender-based differences in endotoxic shock susceptibility. J. Reprod. Immunol 71:12–27. doi: 10.1016/j.jri.2006.01.004. [DOI] [PubMed] [Google Scholar]
- May S, Romberger DJ, and Poole JA. 2012. Respiratory health effects of large animal farming environments. J. Toxicol. Environ. Health B 15:524–41. doi: 10.1080/10937404.2012.744288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinlay SM 1996. The normal menopause transition: An overview. Maturitas 23:137–45. [DOI] [PubMed] [Google Scholar]
- Merkel SM, Alexander S, Zufall E, Oliver JD, and Huet-Hudson YM. 2001. Essential role for estrogen in protection against Vibrio vulnificus-induced endotoxic shock. Infect. Immun 69:6119–22. doi: 10.1128/IAI.69.10.6119-6122.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy VE, Clifton VL, and Gibson PG. 2006. Asthma exacerbations during pregnancy: Incidence and association with adverse pregnancy outcomes. Thorax 61:169–76. doi: 10.1136/thx.2005.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offner PJ, Moore EE, and Biffl WL. 1999. Male gender is a risk factor for major infections after surgery. Arch. Surg 134:935–38. [DOI] [PubMed] [Google Scholar]
- Pahwa P, Karunanayake CP, Rennie DC, Chen Y, Schwartz DA, and Dosman JA. 2009. Association of the TLR4 Asp299Gly polymorphism with lung function in relation to body mass index. BMC. Pulm. Med 9:46. doi: 10.1186/1471-2466-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Wyatt TA, Kielian T, Oldenburg P, Gleason AM, Bauer A, Golden G, West WW, Sisson JH, and Romberger DJ. 2011. Toll-like receptor 2 regulates organic dust-induced airway inflammation. Am. J. Respir. Cell Mol. Biol 45:711–19. doi: 10.1165/rcmb.2010-0427OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline S, and Gold D. 1994. Challenges in interpreting gender differences in asthma. Am. J. Respir. Crit Care Med 150:1219–21. doi: 10.1164/ajrccm.150.5.7952543. [DOI] [PubMed] [Google Scholar]
- SAS. 2015. The HPSPLIT Procedure.
- Schneberger D, Aulakh G, Channabasappa S, and Singh B. 2016. Toll-like receptor 9 partially regulates lung inflammation induced following exposure to chicken barn air. J. Occup. Med. Toxicol 11:31. doi: 10.1186/s12995-016-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Stables MJ, Madalli S, Watson P, and Gilroy DW. 2011. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118:5918–27. doi: 10.1182/blood-2011-03-340281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthilselvan A, Chenard L, Kirychuk S, Predicala B, Schwartz DA, Burch LH, Rennie DC, Willson PJ, and Dosman JA. 2009. Gender-related tumor necrosis factor-alpha responses in naive volunteers with toll-like receptor 4 polymorphisms exposed in a swine confinement facility. J. Interferon Cytokine Res 29:781–90. doi: 10.1089/jir.2009.0002. [DOI] [PubMed] [Google Scholar]
- Senthilselvan A, Chenard L, Ulmer K, Gibson-Burlinguette N, Leuschen C, and Dosman JA. 2007. Excess respiratory symptoms in full-time male and female workers in large-scale swine operations. Chest 131:1197–204. doi: 10.1378/chest.06-2323. [DOI] [PubMed] [Google Scholar]
- Senthilselvan A, Dosman JA, Kirychuk SP, Barber EM, Rhodes CS, Zhang Y, and Hurst TS. 1997. Accelerated lung function decline in swine confinement workers. Chest 111:1733–41. [DOI] [PubMed] [Google Scholar]
- Senthilselvan A, Rennie D, Chenard L, Burch LH, Babiuk L, Schwartz DA, and Dosman JA. 2008. Association of polymorphisms of toll-like receptor 4 with a reduced prevalence of hay fever and atopy. Ann. Allergy Asthma Immunol 100:463–68. doi: 10.1016/S1081-1206(10)60472-3. [DOI] [PubMed] [Google Scholar]
- Song C, and Zhang H. 2014. Comments on fifty years of classification and regression trees. Int. Stat Rev 82:359–61. doi: 10.1111/insr.12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song YY, and Lu Y. 2015. Decision tree methods: Applications for classification and prediction. Shanghai Arch. Psychiat 27:130–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soucy G, Boivin G, Labrie F, and Rivest S. 2005. Estradiol is required for a proper immune response to bacterial and viral pathogens in the female brain. J. Immunol 174:6391–98. [DOI] [PubMed] [Google Scholar]
- Stanford KI, Mickleborough TD, Ray S, Lindley MR, Koceja DM, and Stager JM. 2006. Influence of menstrual cycle phase on pulmonary function in asthmatic athletes. Eur. J. Appl. Physiol 96:703–10. doi: 10.1007/s00421-005-0067-7. [DOI] [PubMed] [Google Scholar]
- Tipping PG 2006. Toll-like receptors: The interface between innate and adaptive immunity. J. Am. Soc. Nephrol 17:1769–71. doi: 10.1681/ASN.2006050489. [DOI] [PubMed] [Google Scholar]
- Torcia MG, Nencioni L, Clemente AM, Civitelli L, Celestino I, Limongi D, Fadigati G, Perissi E, Cozzolino F, Garaci E, and Palamara AT. 2012. Sex differences in the response to viral infections: TLR8 and TLR9 ligand stimulation induce higher IL10 production in males. PLoS. One 7: e39853. doi: 10.1371/journal.pone.0039853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, and Prakash YS. 2012. Sex differences and sex steroids in lung health and disease. Endocr. Rev 33:1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub S, Demaria O, Chasson L, Serra F, Desnues B, and Alexopoulou L. 2012. Sex bias in susceptibility to MCMV infection: Implication of TLR9. PLoS. One 7:e45171. doi: 10.1371/journal.pone.0045171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi RJ, Speizer FE, Willett WC, Trichopoulos D, and Rosner B. 1995. Menopause, postmenopausal estrogen preparations, and the risk of adult-onset asthma. A prospective cohort study. Am. J. Respir. Crit Care Med 152:1183–88. doi: 10.1164/ajrccm.152.4.7551368. [DOI] [PubMed] [Google Scholar]
- Viegas S, Mateus V, Almeida-Silva M, Carolino E, and Viegas C. 2013. Occupational exposure to particulate matter and respiratory symptoms in Portuguese swine barn workers. J. Toxicol. Environ. Health Part A 76:1007–14. doi: 10.1080/15287394.2013.831720. [DOI] [PubMed] [Google Scholar]
- Wang X 2014. Identification of marker genes for cancer based on microarrays using a computational biology approach. Curr. Bioinform 9:140–46. doi: 10.2174/1574893608999140109115649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann MW, Inthorn D, Andress HJ, and Schildberg FW. 2000. Incidence and mortality of severe sepsis in surgical intensive care patients: The influence of patient gender on disease process and outcome. Intensive Care Med. 26:167–72. [DOI] [PubMed] [Google Scholar]
- Xu H, Gregory SG, Hauser ER, Stenger JE, Pericak-Vance MA, Vance JM, Zuchner S, and Hauser MA. 2005. SNPselector: A web tool for selecting SNPs for genetic association studies. Bioinformatics 21:4181–86. doi: 10.1093/bioinformatics/bti682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Saito H, Setogawa T, and Tomioka H. 1991. Sex differences in host resistance to Mycobacterium marinum infection in mice. Infect. Immun 59:4089–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HZ, Cui B, Liu HZ, Chen ZR, Yan HM, Hua F, and Hu ZW. 2009. Targeting TLR2 attenuates pulmonary inflammation and fibrosis by reversion of suppressive immune microenvironment. J. Immunol 182:692–702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


