Abstract
Tumor associated fibroblasts (TAFs) are key stromal cells mediating the desmoplastic reaction and being partially responsible for the drug-resistance and immunosuppressive microenvironment formation in solid tumors. Delivery of genotoxic drugs off-targetedly to kill TAFs results in production of Wnt16 which renders the neighboring tumor cells drug resistant as shown in our previous study (PMC4623876). Our current approach looks for means to deactivate, rather than kill, TAFs. Reactive oxygen species (ROS) are the central hub of multiple profibrogenic pathways and indispensable for TAFs activation. Herein, puerarin was identified to effectively downregulate ROS production in the activated myofibroblast. In this study, a novel puerarin nanoemulsion (nanoPue) was developed to improve the solubility and bioavailability of puerarin. NanoPue significantly deactivated the stromal microenvironment (e.g., ~6-fold reduction of TAFs in nanoPue treated mice compared with the PBS control, p < 0.0001) and facilitated chemotherapy effect of nano-paclitaxel in the desmoplastic triple-negative breast cancer (TNBC) model. Moreover, the removal of the physical barrier increased intra-tumoral infiltration of cytotoxic T cell by 2-fold. This activated immune microenvironment allowed nanoPue to synergize PD-L1 blockade therapy in TNBC model.
Keywords: Reactive oxygen species, Puerarin, Tumor microenvironment, Paclitaxel, PD-L1 blockade therapy
1. Introduction
Most solid tumors contain reactive stromal cells including tumor-associated fibroblasts (TAFs) and immune cells, vasculature, and extracellular matrix (ECM). As the pivotal effector cells mediating desmoplasia, TAFs are indispensable for the tumor progression in these solid tumors. These highly proliferative TAFs can promote tumor growth through the production of a variety of growth factors [1]. They are also responsible for the recruitment of immunosuppressive cells, which protect tumor cells from immune surveillance [2]. Furthermore, the dense ECM produced by TAFs creates high interstitial fluid pressure, which serves as a physical barrier for both drug delivery and cytotoxic T cell penetration [3]. Past five years have witnessed a speeding progress in the immune checkpoint blockade therapy for a few types of solid tumors with high mutational burden. However, a recent study demonstrated a strong association of transforming growth factor-β (TGF-β) signaling, a hallmark of TAFs activation, with the compromised response to PD-L1 blockade even in the neoantigen-rich tumor [4]. For instance, PD-1/PD-L1 checkpoint blockers have durable response rate as high as 40% in melanoma, which nevertheless is a typical type of solid tumor rarely containing dense fibrous stroma [5,6]. In contrast, triple negative breast cancer (TNBC), which contains the highest mutational frequency of breast cancer subtypes and high PD-L1 expression but characteristic of geographical or central tumor fibrosis [7], only has up to 20% response to PD-L1 blockade [8]. This relatively ineffectiveness of PD-L1 blockade therapy might be attributed by the abundance of TAFs in TNBC. Therefore, desmoplasia depleting agents have a great potential to facilitate both chemo- and immunotherapy via tumor microenvironment (TME) remodulation.
Previous studies in our laboratory have shown that cisplatin, a chemotherapeutic drug, can cause damage to TAFs and inhibit the growth of tumors. However, it also increases the release of Wnt16 in TAFs, which is associated with drug-resistance in tumor cells and stroma reconstruction [9]. In this study, we aimed to deactivate TAFs rather than directly damage TAFs. Reactive oxygen species (ROS) is the key downstream mediator of multiple profibrogenic pathways such as TGF-β and platelet-derived growth factor (PDGF) initiated pathways. Increased ROS in the tumor nest further prolongs the fibrogenesis signaling and accelerates desmoplasia [10]. Moreover, chronic ROS stress activates apoptotic pathways of T cells and therefore is related to the impaired function of the effector T cells [11]. As such, we screened ten traditional Chinese medicines (TCMs) that were known to exhibit anti-fibrotic effect based on their capacity of ROS downregulation in the activated fibroblast. Compared with conventional screening methodology using cytotoxicity as the readout, the current screening strategy avoided the exclusion of drugs (e.g., TCMs) that show weak cytotoxic effect but can effectively deactivate TAFs.
TCMs have evolved over thousands of years with a unique system of theories for medicinal intervention. These natural active ingredients with low toxicity have been increasingly used as an adjuvant therapy to alleviate cancer symptoms in the last decades. Puerarin is an isoflavone derivative isolated from the kudzu root with the capacity of lowering blood pressure, reducing myocardial oxygen consumption, expanding coronary vessels, protecting liver, controlling blood sugar and inhibiting ischemia-reperfusion injury [12]. Particularly, puerarin shows a substantial anti-fibrosis effect in multiple organs including heart, lung, kidney, and liver [13,14]. Herein, we further confirmed puerarin showed a superior ROS reduction efficiency in the activated NIH3T3 murine fibroblasts. However, puerarin’s poor water solubility and bioavailability have limited its application as a pharmaceutical agent.
In the current study, a novel puerarin nanoemulsion (nanoPue) was engineered to improve its pharmacokinetic profile as well as tumor-specific accumulation. Based on the 4T1 murine TNBC tumor model, nanoPue was confirmed to successfully remodel the tumor stromal microenvironment and dwindle the physical barrier for particle and cell penetration (Fig. 1). Similar results were observed in a murine desmoplastic melanoma model. The combination of nanoPue and paclitaxel (PTX) polymer displayed a synergistic anti-tumor effect with negligible side effects. In addition, the activated immune microenvironment by nanoPue significantly improved the therapeutic efficacy of PD-L1 monoclonal antibody (α-PD-L1). These findings suggest nanoPue can be used as an adjuvant therapy to enhance chemo- and checkpoint blockade immunotherapy in highly desmoplastic solid tumors.
Fig. 1.
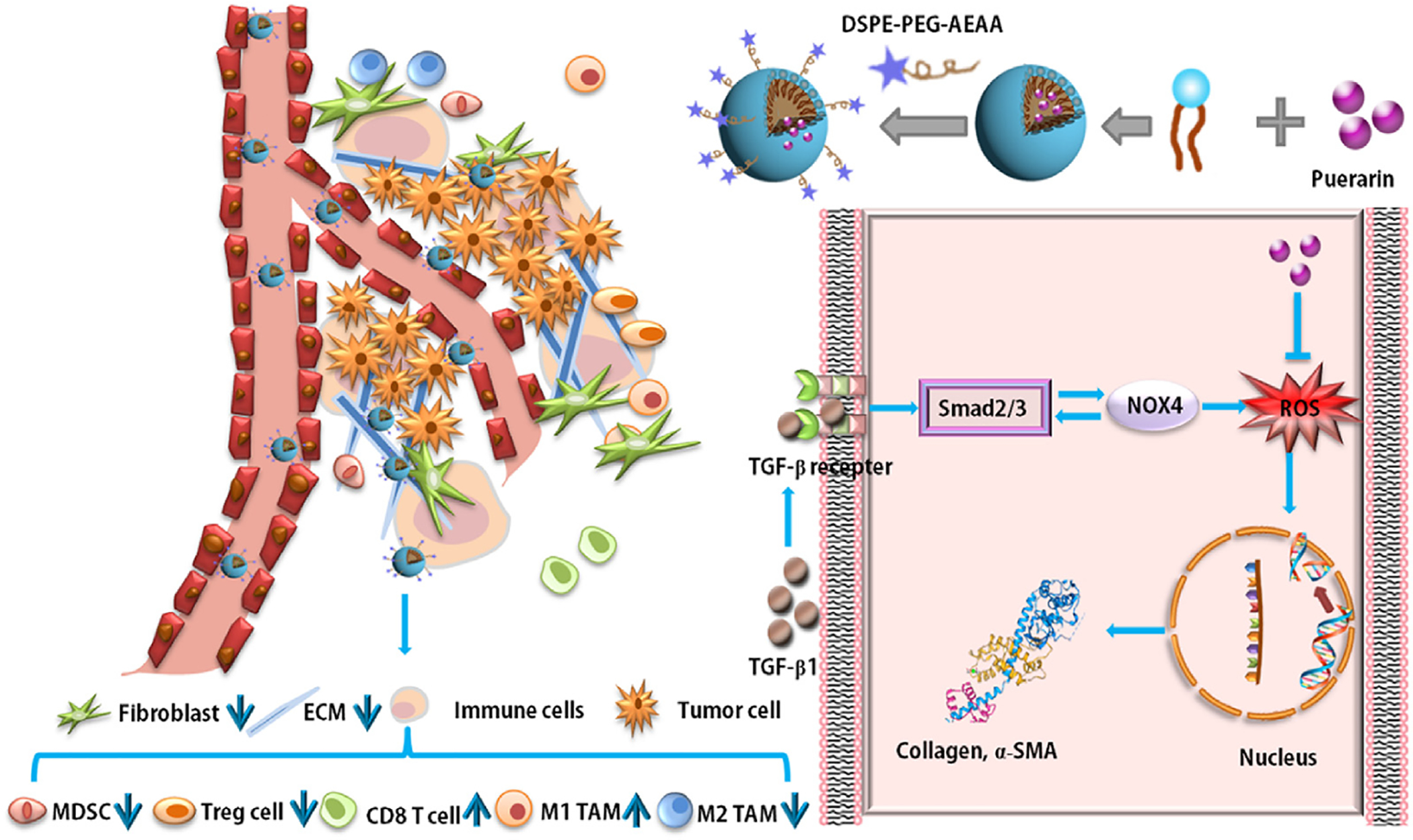
Illustration of TME remodulation by targeted puerarin delivery.
2. Results and discussion
2.1. Inhibitory effect of puerarin on ROS generation and construction of nanoPue
ROS plays an important role in the desmoplastic reaction. A small amount of ROS has an immune defense effect, however, excessive production of ROS can cause oxidative stress, activation of ERK1/2 pathway, differentiation of myofibroblasts and abnormal synthesis of ECM protein, which leads to fibrosis and tumor formation [15,16]. In this study, ROS assay was used to investigate and compare the inhibitory effects on ROS generation of ten natural compounds. As shown in Fig. 2A, compared with other natural compounds, puerarin significantly reduced the amount of ROS in TGF-β activated NIH3T3 cells. Thus, we decided to concentrate on this TCM for further studies.
Fig. 2.
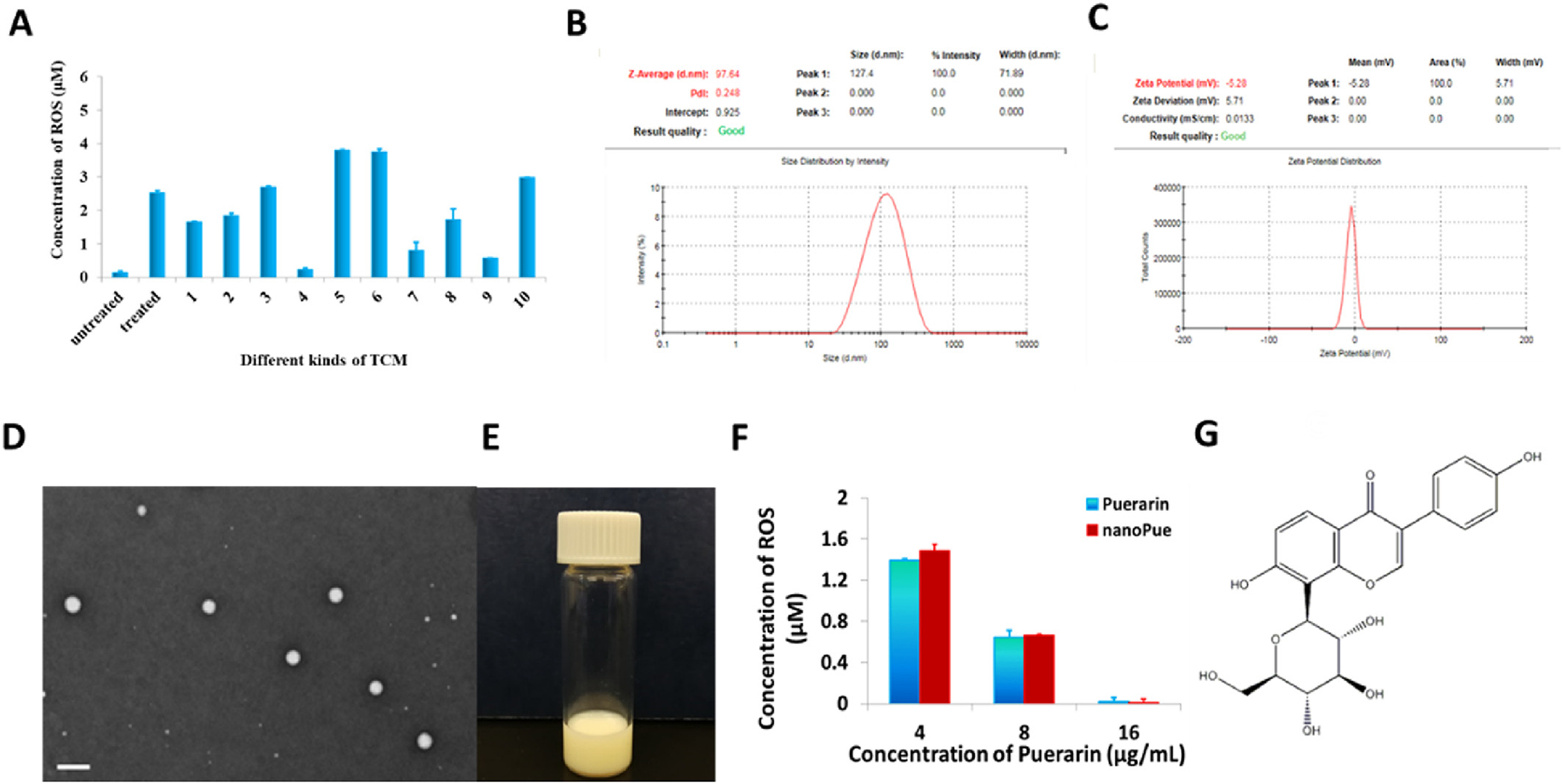
TCM screening based on ROS inhibition effect and characterization of nanoPue. (A) Effects of selected TCMs on ROS inhibition in TGF-β activated NIH3T3 cells. untreaeted: NIH3T3 cells were not treated with TGF-β; treated: NIH3T3 cells were treated with 10 ng/mL of TGF-β; (1) Astragalus total saponins (2) Matrine, (3) Panax notoginseng saponins R1, (4) Puerarin, (5) Jujuboside, (6) Quercetin, (7) Astragaloside IV, (8) Emodin, (9) Hydroxysafflor yellow A, (10) Tanshinone IIA (n = 6). Concentrations of all TCMs were 15 μg/mL. (B) Dynamic light scattering measurements of particle size and distribution of nanoPue. (C) Zeta potential of nanoPue. (D) TEM image of nanoPue. Scale bar represents 200 nm. (E) Appearance of nanoPue. (F) Concentration-dependent inhibition of ROS by puerarin and nanoPue in TGF-β activated NIH3T3 cells (n = 6). (G) The chemical structure of puerarin.
Puerarin is poorly soluble. Its low bioavailability and acute intravascular hemolysis further limit its pharmaceutical application [17–19]. Nanoemulsion is a colloidal particulate system allowing for the improvement of drug solubilization and therapeutic efficacy. In nanoemulsion-based delivery system, the combination of surfactants with oils offers a superior advantage over a cosolvent system or other nanocarriers in terms of safety profile and drug-loading capacity for hydrophobic compound. To avoid the toxicity of traditional small molecular surfactants, the biocompatible lecithin from soybean was chosen as the principle emulsifier for the preparation of nanoemulsion to carry puerarin (nanoPue). To achieve TAFs targeting ability, nanoPue was surface modified with the targeting ligand, aminoethyl anisamide (AEAA). AEAA is a potent ligand for the sigma receptor, which is overexpressed on most cancer cells and TAFs [20,21]. Recent studies have shown that the up-regulation of sigma receptor on TAFs, which is related to the increase of α-SMA [22]. Since most of the desmoplastic tumors have their vessels located in or near the stroma, which is enriched with TAFs, AEAA-modified nanoparticles accumulate mostly in TAFs rather than in tumor cells [23]. This phenomenon was observed in several different desmoplastic tumors. The phenomenon was called “binding site barrier” [23]. The encapsulation efficiency (EE) of nanoPue was 82.4 ± 3.2%. The average particle size and zeta potential of nanoPue was 112 ± 5 nm with a narrow size distribution (PDI = 0.242 ± 0.008) and −5.3 ± 0.6 mV, respectively (Fig. 2B and C), according to dynamic light scattering (DLS) analysis. The transmission electron microscopy (TEM) image confirmed the size of nanoPue and indicated the spherical shape and homogenous distribution (Fig. 2D). The nanoemulsion appeared milky white (Fig. 2E) and was remarkably stable under 4 °C with little free drug leakage and unchanged particle size within 40 days (Fig. S1A). Compared with puerarin suspension (with glycerol as the cosolvent), nanoPue exhibited improved release stability and had an accumulative drug release that only reached 58% within 24 h in PBS (pH 7.4) (Fig. S1B). The pharmacokinetics of nanoPue and puerarin suspension was further investigated in mice. NanoPue increased the half-life (t1/2α) and the area under the curve (AUC) of puerarin by 2-fold and 5-fold, respectively (Fig. S1C and S1D), which allows for the prolonged therapeutic effect of puerarin. Due to the poor solubility and pharmacokinetic profile of free puerarin, the following in vivo studies only focused on nanoPue.
The inhibitory effects of nanoPue and puerarin on ROS were compared. Both of them showed a concentration-dependent down-regulation pattern of ROS (Fig. 2F). Thus, the emulsification process in manufacturing nanoPue did not affect the pharmaceutical activity of the active ingredient. Different concentrations of puerarin and nanoPue has no cytotoxicity in NIH3T3 cells within 48 h (Fig. S2A), suggesting that the effect of puerarin on ROS production was not due to its cytotoxicity. Indeed, puerarin has been reported to significantly improve the activity of superoxide dismutase, block the automatic oxidation of lipids, and effectively scavenge oxygen free radicals. The potent anti-oxidant effect is associated with the presence of 3′-hydroxyl group of puerarin, which plays an important role in the clearance of ONOO- and total ROS (Fig. 2G) [24–26].
2.2. NanoPue is safe and attenuates desmoplastic reaction and remodels stromal TME
NanoPue was daily injected into Balb/C mice bearing the orthotopic 4T1 breast cancer for 6 days (Fig. 3A) to evaluate the safety profile of the formulation. No significant differences in alanine aminotransferase (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), and creatinine levels were observed after the administration of PBS, blank emulsion and nanoPue (Fig. 3B), which indicated repeated iv injection of nanoPue did not cause renal or hepatic dysfunctions. To evaluate the toxic effects of nanoPue on the major organs, H&E staining of the major organs were characterized in Fig. 3C. No inflammation or necrosis was found in the heart, liver, spleen and kidney of mice treated with blank emulsion and nanoPue. However, lung seems to be the major metastatic site in the PBS and blank emulsion group. The nanoPue seems to reduce lung metastasis to some extent (Fig. 3C). No significant weight loss occurred during the treatment for all mice (Fig. 3D). The results also showed that the maximum tolerable dose (MTD) of nanoPue exceeds the maximum dose of our injection and may be 500 mg/kg. The results of safety evaluation confirmed that the nanoPue did not cause toxicity or side effects in vivo. In addition, as shown in Fig. S2B, nanoPue at different concentrations showed a negligible hemolytic effect on the red blood cells (RBCs) (hemolyzed erythrocytes < 1%) compared with deionized water.
Fig. 3.
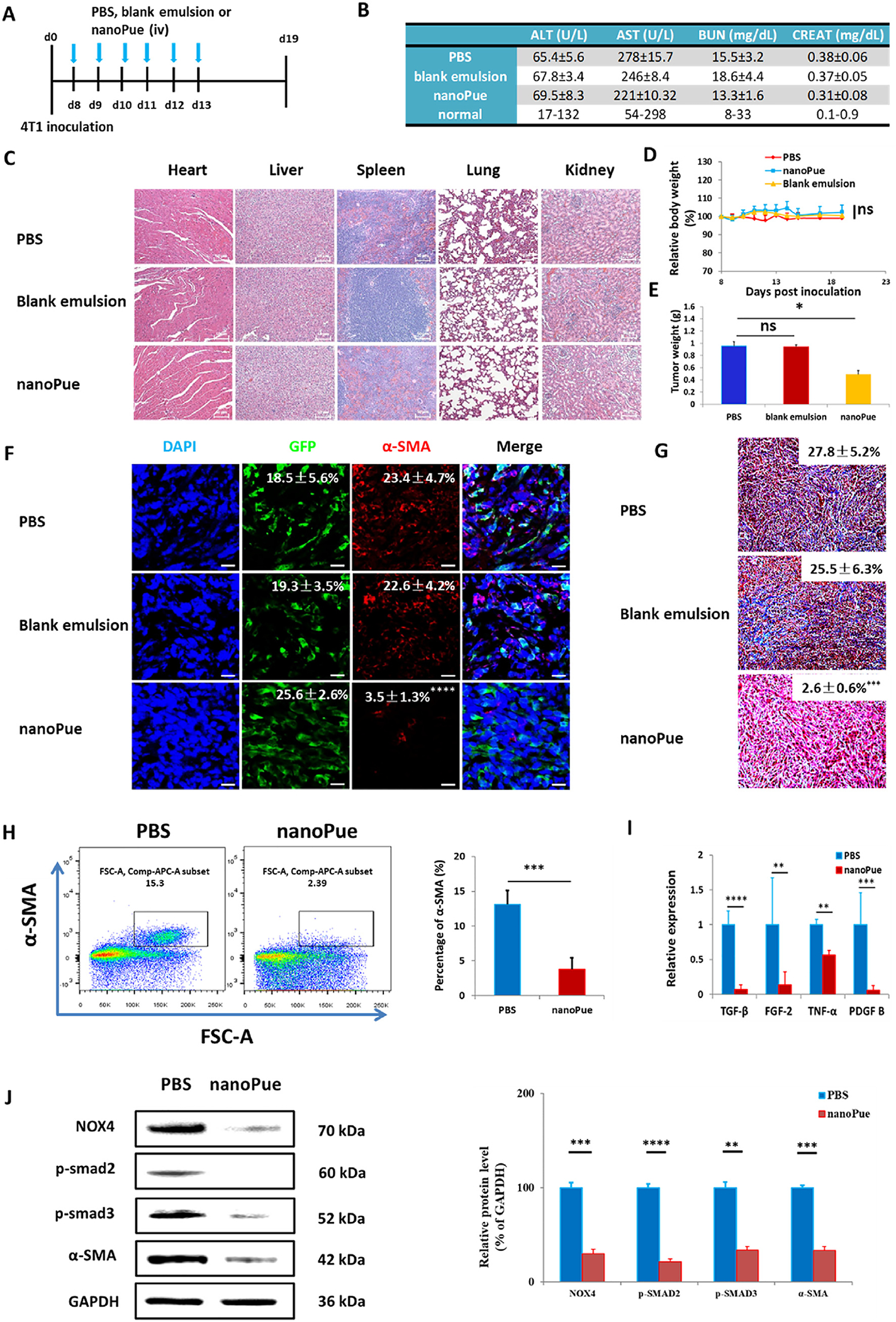
Toxicity evaluation of nanoPue (A–D) and attenuated 4T1 desmoplastic reaction by the nanoPue treatment (E–J). (A) Tumor inoculation and treatment scheme for PBS, blank emulsion and nanoPue therapy. (B) Serum ALP, ALT, AST, BUN, and creatinine levels (n = 4). (C) H&E staining of major drug accumulating organs after 6 injections of different treatments (n = 5). Scale bar represents 50 μm. (D) Changes of mice body weight after different treatments (n = 7). (E) Ex vivo tumor weight after 6 injections of different treatments (n = 7). (F) Confocal microscopy identifying α-SMA. The quantification results expressed as the percentage of total cell number (n = 5). Scale bar represents 20 μm. (G) Masson’s trichrome staining and quantification of collagen deposition expressed as the percentage of total cell number (n = 5). Scale bar represents 50 μm. (H) Depletion of α-SMA+ TAFs in the 4T1 tumors after the nanoPue treatment analyzed by flow cytometry (n = 4). (I) RT-PCR analysis of TGF-β, FGF-2, TNF-α and PDGF-B expression in the tumor tissue after different treatment (n = 6). (J) Western blot analysis of NOX4, p-SMAD2, p-SMAD3, α-SMA and GAPDH expression in the 4T1 tumor after different treatments. Protein expression levels were quantified by ImageJ, and normalized with GAPDH (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
After 6 consecutive daily injections of different formulations, the mice were sacrificed on day 19. The tumor was collected and weighed, followed by the characterization of the parameters of TME, which is orchestrated by various cells and cytokines. As shown in Fig. 3E, nanoPue significantly decreased the tumor weight compared with PBS or blank emulsion group (p < 0.05), although with only modest growth inhibition efficiency. Indeed, the major effect of nanoPue was TME remodeling. TAFs, the pivotal effector cells mediating the stromal TME, are activated myofibroblasts most reliably characterized by alpha smooth muscle actin (α-SMA) [27]. Figs. 3F, 2H and 2I demonstrated significant reduction of α-SMA positive TAFs in tumors of nanoPue treated animals compared with those treated with either PBS or blank emulsion. The immunofluorescence staining showed that diffuse intracytoplasmic α-SMA was abundant in PBS and blank emulsion group, while the expression of α-SMA in nanoPue group was very low (22.6–23.4% vs 3.5%, p < 0.0001) (Fig. 3F). Flow cytometry analysis further confirmed significantly lower expression of α-SMA in the nanoPue treated tumor vs the PBS group (Fig. 3H).
Collagen is the main ECM composition and serves as another important indicator of the severity of the desmoplastic reaction. Masson’s trichrome staining indicated abundant collagen deposition in PBS and blank emulsion groups, while that of the nanoPue group was significantly decreased (p < 0.001) (Fig. 3G). In conclusion, nanoPue could alleviate desmoplasia by deactivating TAFs and reducing collagen deposition. To be noted, repeated injections of blank emulsion did not show anti-tumor nor the stroma remodulation effect (Fig. 3E, F, and 3G). Therefore, in the follow-up study, PBS group was used as the only control group.
TGF-β is the most potent and ubiquitous profibrogenic cytokine promoting TAFs activation and ECM deposition [28,29]. RT-PCR results showed that TGF-β was more than 10-fold reduced by nanoPue treatment (p < 0.0001) (Fig. 3I). Other profibrogenic cytokines including fibroblast growth factor (FGF-2), platelet-derived growth factor B (PDGF-B), and tumor necrosis factor (TNF-α), are involved in promoting collagen synthesis and inhibiting extracellular matrix degradation [30], also displayed significantly decreased levels compared with those of PBS group (Fig. 3I).
The mechanism underlying the induction of fibrosis by TGF-β has been studied intensively in the past years. Increasing evidence indicates that ROS plays a central role in the profibrogenic activity of TGF-β. NADPH oxidases (NOX) enzymes are heme-containing proteins with a primary function of transporting electrons from NADPH to oxygen. Therefore, the NOX family have been confirmed to produce the majority of intracellular ROS in various cells. NOX4, in particular, is identified to be the most widely distributed in nonphagocytic myofibroblasts and could be upregulated in a SMAD2/3 dependent manner [31–33]. In turn, NOX4 activation and increased ROS level promote SMAD2/3 phosphorylation and facilitates the formation of a feed-forward loop in TGF-β/SMAD profibrogenic signaling [34]. Hypoxia-induced factor 1α (HIF-1α) is a heterodimeric transcription factor serving as the intracellular ROS sensor. Upon the activation via elevated ROS, upregulated HIF-1α enhances transcriptional activities linked to proliferation of myofibroblasts and inhibition of apoptosis. Meanwhile, the overexpressed HIF-1α could further promote intracellular ROS level and upregulate the expression and activity of NOXs [35,36]. Herein, the expression of NOX4, α-SMA, p-SMAD2 and p-SMAD3 in the tumor tissue were dramatically down-regulated by the nanoPue treatment according to the Western blot analysis (Fig. 3J), confirming that nanoPue could regulate the occurrence and development of desmoplasia.
2.3. Effect of nanoPue on biodistribution of second-wave injected nanoparticles
The stroma remodeling effect of the nanoPue treatment was further validated via the investigation of biodistribution of second-wave injected nanoparticles. The far-red fluorescence dye DiD was encapsulated in the same nanoemulsion formulation as in nanoPue. After 3 consecutive nanoPue or PBS treatments, 4T1 tumor-bearing mice were iv injected with DiD encapsulated testing nanoemulsion particles (nanoDiD) (Fig. 4A). In vivo and ex vivo imaging analyses demonstrated nearly 3-fold higher nanoDiD accumulation in the tumor of the animals pre-injected with nanoPue than with PBS (p < 0.01) (Fig. 4B). No significant difference in the fluorescence signals of heart, liver, spleen, lung and kidney was observed between PBS and nanoPue group (Fig. 4B). These results indicated that pre-injection of nanoPue had changed TME and render them more permeable for the subsequently injected nanoparticles.
Fig. 4.
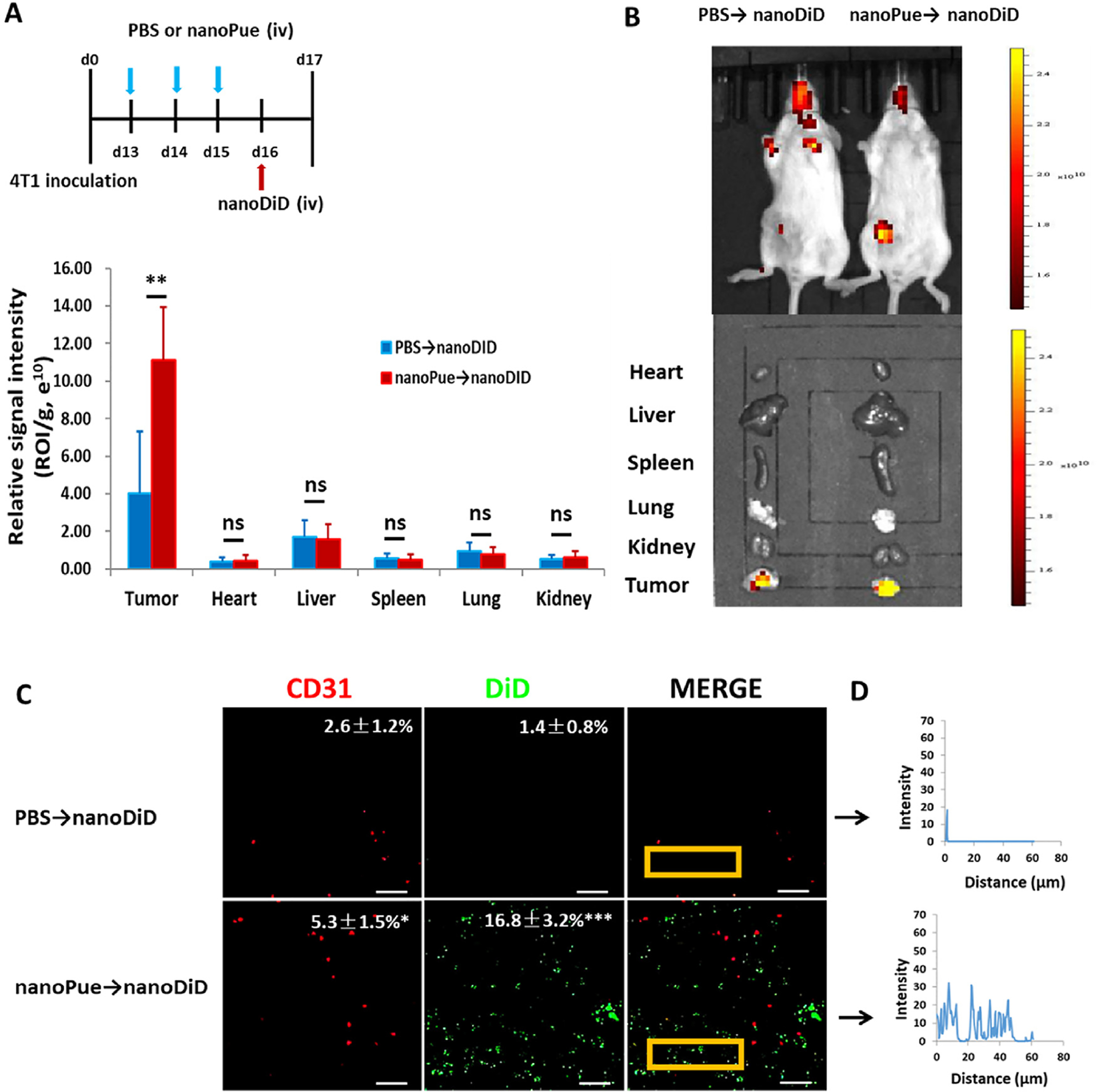
The concomitant effect of nanoPue treatment on the behaviors of nanoDiD within tumors. (A) nanoPue and nanoDiD treatment scheme. (B) Images and quantitative results of the nanoDiD in the mice and tumors at 24 h after test particle injection (n = 5). (C) The tumors were excised and sectioned into 10 μm thick slices and observed by laser scanning confocal microscope. The particles are shown in green (DiD) and the blood vessels are shown in red (CD31). Scale bar represents 50 μm. (D) The green fluorescence intensity profile as a function of distance from blood vessels (0–60 μm) in a representative region (indicated by the yellow rectangle) was plotted by using software ImageJ. *p < 0.05, **p < 0.01, ***p < 0.001, ns: not significant.
Frozen sections of tumors were stained for CD31 tumor vessel marker to observe the positional relationship between the nanoDiD and the blood vessels. In the mice pre-injected with PBS, not only there were few nanoDiD appeared in the tumor, their location was close to the vessels. Thus, these tumors in the PBS group showed limited nanoparticle extravasation. On the other hand, in the mice pre-injected with nanoPue, many more nanoDiD were found in the tumors (Fig. 4C). More importantly, these particles were located at positions far away from the vessels, as shown by ImageJ analysis of the data (Fig. 4D).
The results indicate that pre-injection of nanoPue improves the penetration of nanoparticles in the tumor, allowing more nanoDiD to accumulate in the tumor and diffuse deeper into the parenchyma of the tumor. Thus, nanoPue had significantly increased the enhanced permeability and retention (EPR) effect of the tumor [37,38]. The effect must be related to the fact that nanoPue could significantly reduce the fibrogenic status of the fibroblasts, increase vessel permeability and reduce the interstitial fluid pressure of the treated tumor. These profound changes of the tumor were resulted from the action of a single drug formulated in a simple emulsion without any noticeable toxicity. The treated tumors were no longer desmoplastic; there was a significant decrease in the α-SMA positive fibroblasts and increase of DiD (Fig. S3). It has also been reported that puerarin has a significant effect on vascular activity, which can directly affect vascular smooth muscle and lead to vasodilation [14]. In this study, we consider that nanoPue may have a good vasodilating effect on tumors.
The effect of nanoPue was examined in another desmoplastic tumor, i.e. BPD6 melanoma. The number of CD31-labeled blood vessels in nanoPue treated group was significantly higher than that in the PBS treated group (p < 0.0001). There was also a concomitant decrease in the α-SMA positive fibroblasts in the nanoPue treated group (p < 0.001) (Fig. S4A). These observations were mirrored in the 4T1 tumors which were studied as a comparison (Fig. S4B). These results indicate that nanoPue could be a general reagent to reduce the desmoplasia of solid tumors.
2.4. Combination of nanoPue and PTX polymer therapy on 4T1 tumor model
The chemotherapy treatment for TNBC is mainly based on PTX in the clinical applications since this cancer type is quite sensitive to PTX [39,40]. However, the overall prognosis after PTX therapy is still poor. Since nanoPue significantly increased the penetration of second-wave injected nanoparticles, we hypothesize that it can also facilitate the nano-formulated PTX chemotherapy in the highly desmoplastic TNBC. A PTX polymer nanoformulation (nanoPTX) (ZY Therapeutics Inc.) was utilized to investigate the synergistic effect of nanoPue and PTX. We gave the 4T1 tumor-bearing mice 3 injections of nanoPue and then three injections of nanoPTX according to the treatment scheme illustrated in Fig. 5A. In comparison with PBS→PBS group, the PBS→nanoPTX and nanoPue→PBS groups showed a partial effect on tumor inhibition. However, compared with the individual therapies, a combination therapy of nanoPue with nanoPTX significantly inhibited tumor growth (Fig. 5B). Fig. 5C shows that the average tumor weight at the endpoint in nanoPue→nanoPTX group was much lower than that in nanoPue→PBS group (p < 0.001) and PBS→nanoPTX group (p < 0.01). TUNEL assay demonstrated a large increase of apoptotic cells in nanoPue→nanoPTX combination treatment group compared with PBS→nanoPTX and nanoPue→PBS single treatment (Fig. 5D). Compared with the control and the single administration group, the expression of Ki67 protein (a marker for cell proliferation) in the tumor cells of the nanoPue→nanoPTX group was significantly down-regulated (P < 0.0001) (Fig. 5E). The results showed that nanoPue and nanoPTX had a synergistic inhibitory effect on 4T1 tumor growth. Pre-injection of nanoPue could render the subsequent injection of nanoPTX polymer nanoformulation more effective in inhibiting tumors. The result was consistent with the in vivo imaging (Fig. 4B). Thus, nanoPue’s activity in remodeling TME and alleviating tumor fibrosis was successfully translated to an improved efficacy of chemotherapy for the 4T1 tumor model.
Fig. 5.
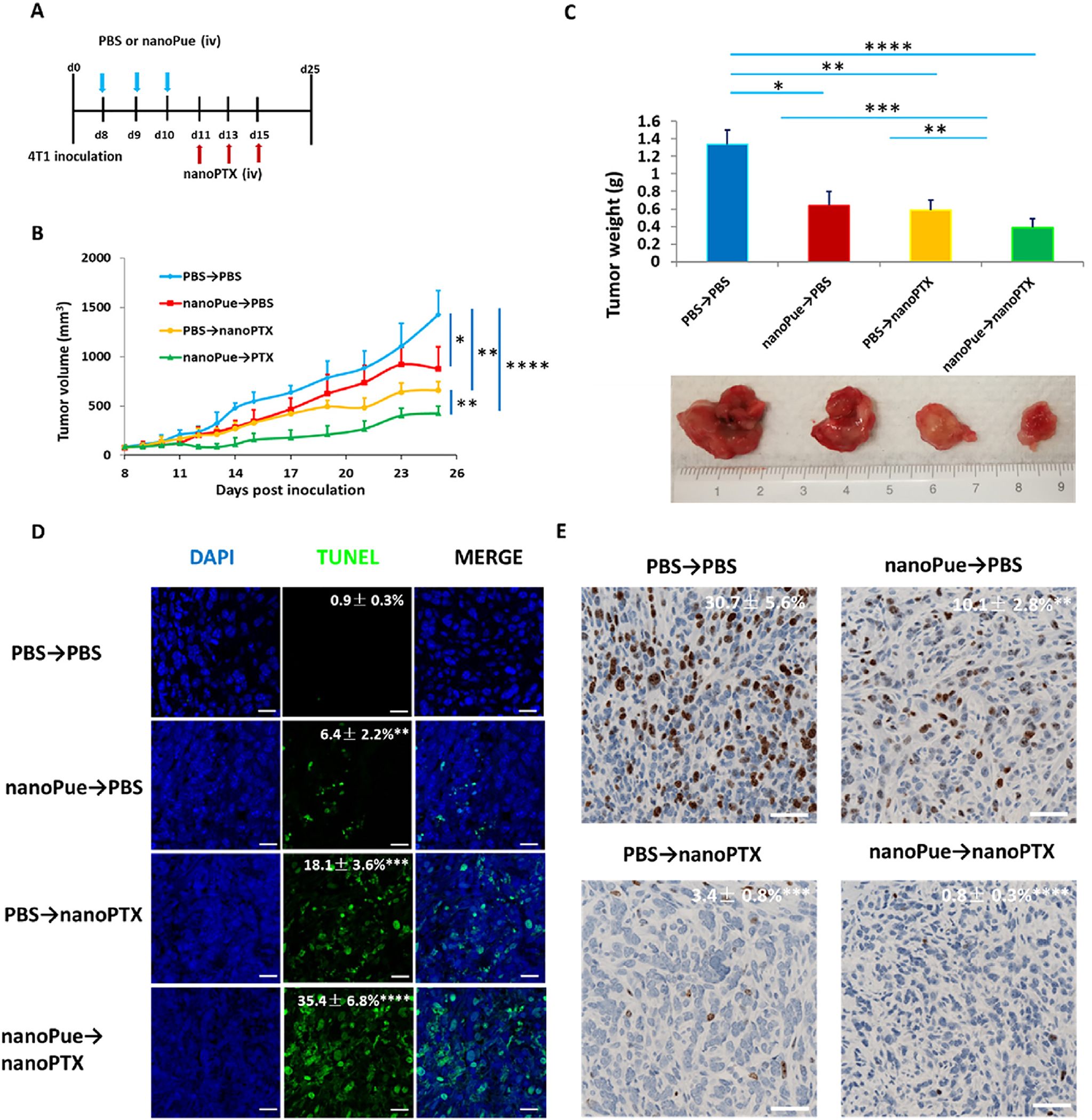
Combination of nanoPue and nanoPTX therapy on 4T1 tumor model (n = 5). (A) nanoPue and nanoPTX combination treatment scheme. (B) Tumor growth curves of 4T1 tumors in different treatment groups. (C) The tumor weight and the representative tumor image at the end of the experiment in different treatment groups. (D) TUNEL staining of differently treated 4T1 tumor tissues. TUNEL positive cells were quantified in 3 randomly selected fields per mouse (n = 5). (E) Comparison of Ki67 expression of 4T1 tumors in different treatment groups. Scale bar represents 20 μm *p < 0.05, **p < 0.01, ***p < 0.001 and ****p < 0.0001.
In order to investigate the toxic effects of combined drugs in mice, the body weight and serum chemistry of the treated mice were measured. The main organs of mice were examined by H&E staining method to evaluate any possible toxicity induced by the treatment. As shown in Fig. S5A, there were no significant differences in ALT, AST, BUN and creatinine between treatment group and PBS group, and all the parameters remained at normal levels. Interestingly, the weight of mice in PBS and nanoPue groups increased in varying degrees, while that of nanoPue→nanoPTX group remained unchanged and there was no significant difference among these three groups. However, the weight of mice in PBS→nanoPTX group decreased significantly compared with other groups (Fig. S5B). The results showed that the chemotherapeutic drugs have a significant effect on the body weight of mice. Pre-injection of nanoPue could significantly reduce the toxicity of chemotherapeutic drugs. In the survival analysis, nanoPue→nanoPTX also showed significantly prolonged median survival compared with the PBS→PBS, PBS→nanoPue, and PBS→nanoPTX group (Fig. S5C). The combination of nanoPue and nanoPTX not only shows a potent therapeutic effect but also achieves a long-lasting overall response. The results of H&E staining showed that no inflammation or necrosis in the heart, liver, spleen, kidney of nanoPue group compared with PBS group, indicating that the combined administration had no detectable toxicity in mice. However, lung seems to be the major metastatic site in the PBS and PBS→nanoPTX group. The combination of nanoPue and nanoPTX obviously suppressed metastasis in the lung (Fig. S5D).
2.5. NanoPue induced 4T1 tumor immune microenvironment changes
In the above experiments, we systematically investigated the effects of pre-injection of nanoPue on tumor stromal microenvironment (Fig. 3). We further explored whether nanoPue had a favorable regulatory effect on tumor immune microenvironment after repeated nanoPue or PBS treatments in 4T1 breast cancer (Fig. 6).
Fig. 6.
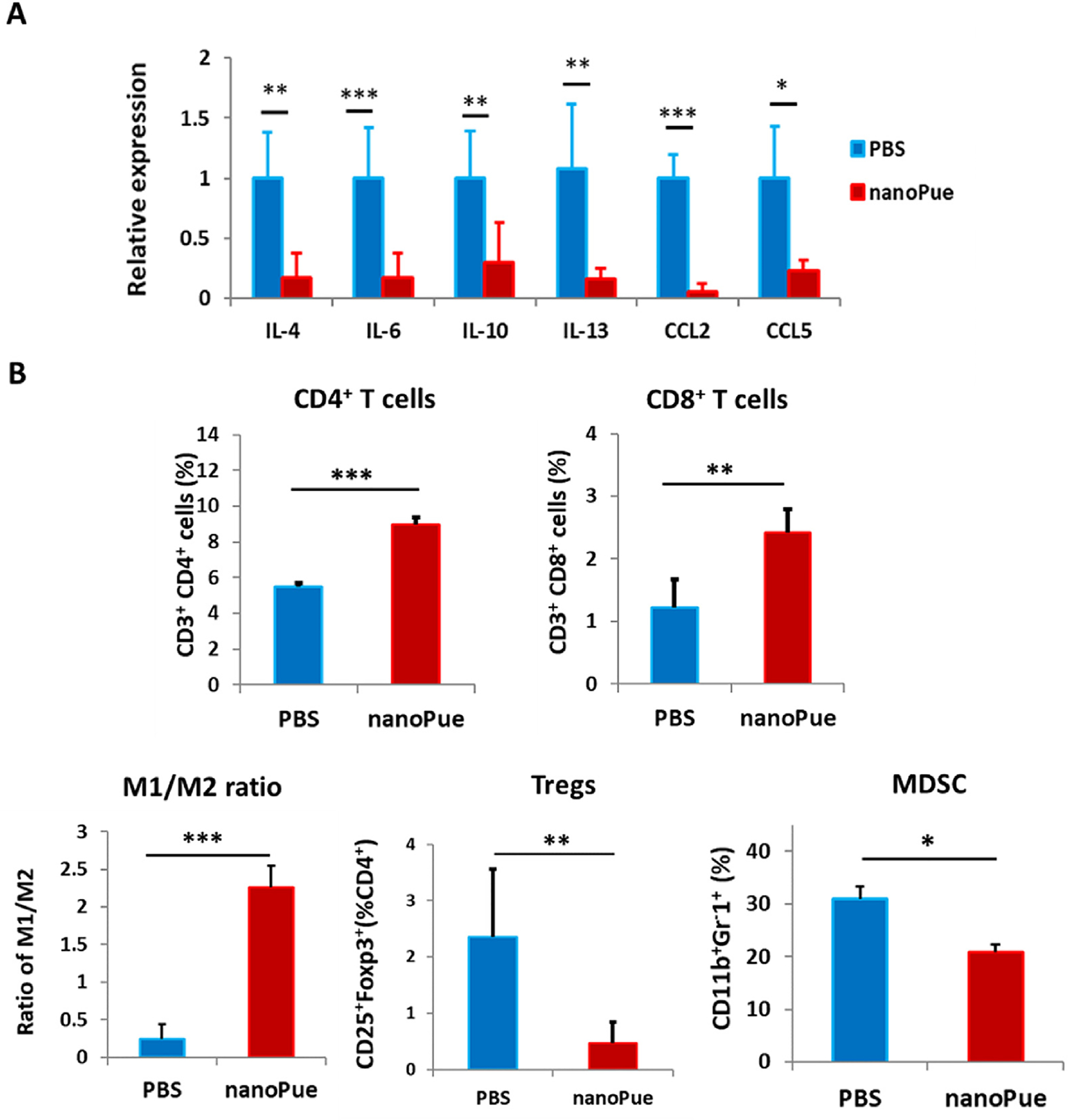
NanoPue induced 4T1 tumor immune microenvironment changes (A) RT-PCR analysis of IL-4, IL-6, IL-10, IL-13, CCL2 and CCL5 expression in the tumor tissue after different treatments (n = 6). (B) Analysis of CD4+, CD8+ T cells, MDSC, Tregs, M1/M2 ratios in the 4T1 tumors after various treatments by using flow cytometry (n = 4). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
T cell, especially the CD8+ cytotoxic T cell-mediated cellular immunity is the main form of anti-tumor immunity [41]. Unfortunately, activated TAFs not only serve as a physical barrier for T cell penetration, but have evolved multiple immunoregulatory mechanisms via the secretion of immunosuppressive cytokines (e.g., IL-4, IL-6, IL-10, IL-13 and TGF-β) and expression of immune inhibitory molecules (e.g., PDL1). Collectively, these immunosuppressive factors prevent T cell proliferation and differentiation, and trigger functional cytotoxic T cell death, thereby facilitating tumor cells to evade immune surveillance [42]. As expected, deactivation of TAFs by nanoPue significantly reduced intratumoral IL-4, IL-6, IL-10 and IL-13 (Fig. 6A), which led to increased infiltration of both CD8+ and CD4+ T cells into the tumor tissue (Fig. 6B and Fig. S6). Noticeably, CD8+ T cells were more intensively promoted compared with CD4+ T cells (2.0 vs 1.6-fold increase) after the nanoPue treatment. In addition to directly impairing T cell function, activated TAFs are also responsible for the recruitment of circulating myeloid cells and regulatory T cells (Tregs) mediated by C–C motif chemokine 2 (CCL2) and C–C motif chemokine 5 (CCL5). Upon the stimulation by the suppressive T helper cell 2 (Th2) cytokines (e.g., IL-4, IL-10, and IL-13), these infiltrated myeloid cells differentiate into myeloid-derived suppressor cells (MDSCs) and M2 macrophages [43]. These recruited Tregs, MDSCs, and M2 macrophages have been reported to negatively correlate with the number of intratumoral CD8+ T cells and hence are associated with adverse prognosis of breast cancer [44–48]. Therefore, the significant downregulation of CCL2 and CCL5 together with the reduced intratumoral Th2 cytokine levels (Fig. 6A) further improved the immune microenvironment through reducing Tregs and MDSCs infiltration as well as promoting M2 macrophage phenotype switch to pro-inflammatory M1 (Fig. 6B and Fig. S6).
2.6. Combination of nanoPue and α-PD-L1 in 4T1 tumor model
Although PD-1/L1 antibody has achieved great success in clinic, more than 80% of TNBC patients still fail to respond to the therapy [49]. Recent studies have demonstrated the correlation of better intratumoral T lymphocyte infiltration with higher patient response towards the PD-1/L1 antibody therapy [50,51]. Based on our findings that nanoPue can alleviate the immunosuppressive microenvironment of tumors, we hypothesized that nanoPue may enhance the activity of the checkpoint blockade immunotherapy. Accordingly, we attempted to combine nanoPue with α-PD-L1 in the treatment of 4T1 breast cancer (Fig. 7A) when examining the combination of nanoPue and nanoPTX. As shown in Fig. 7B, despite the moderate inhibition of tumor growth (p < 0.01) by the α-PD-L1 or nanoPue monotherapy, the combined administration of nanoPue and α-PD-L1 dramatically slowed down the tumor progression (p < 0.01) as also evidenced by the smallest tumor among all treatment groups 25 days after the tumor inoculation (Fig. 7C and Fig. S7A). TUNEL assay demonstrated significantly higher apoptosis by the nanoPue + α-PD-L1 combination therapy than the α-PD-L1 monotherapy (p < 0.01), which might be associated with better infiltration of cytotoxic T cells after nanoPue-mediated stroma depletion (Fig. 7D). Compared with α-PD-L1 group, the expression of Ki67 protein in the tumor cells of the nanoPue→α-PD-L1 group was significantly down-regulated (P < 0.05) (Fig. 7E).
Fig. 7.
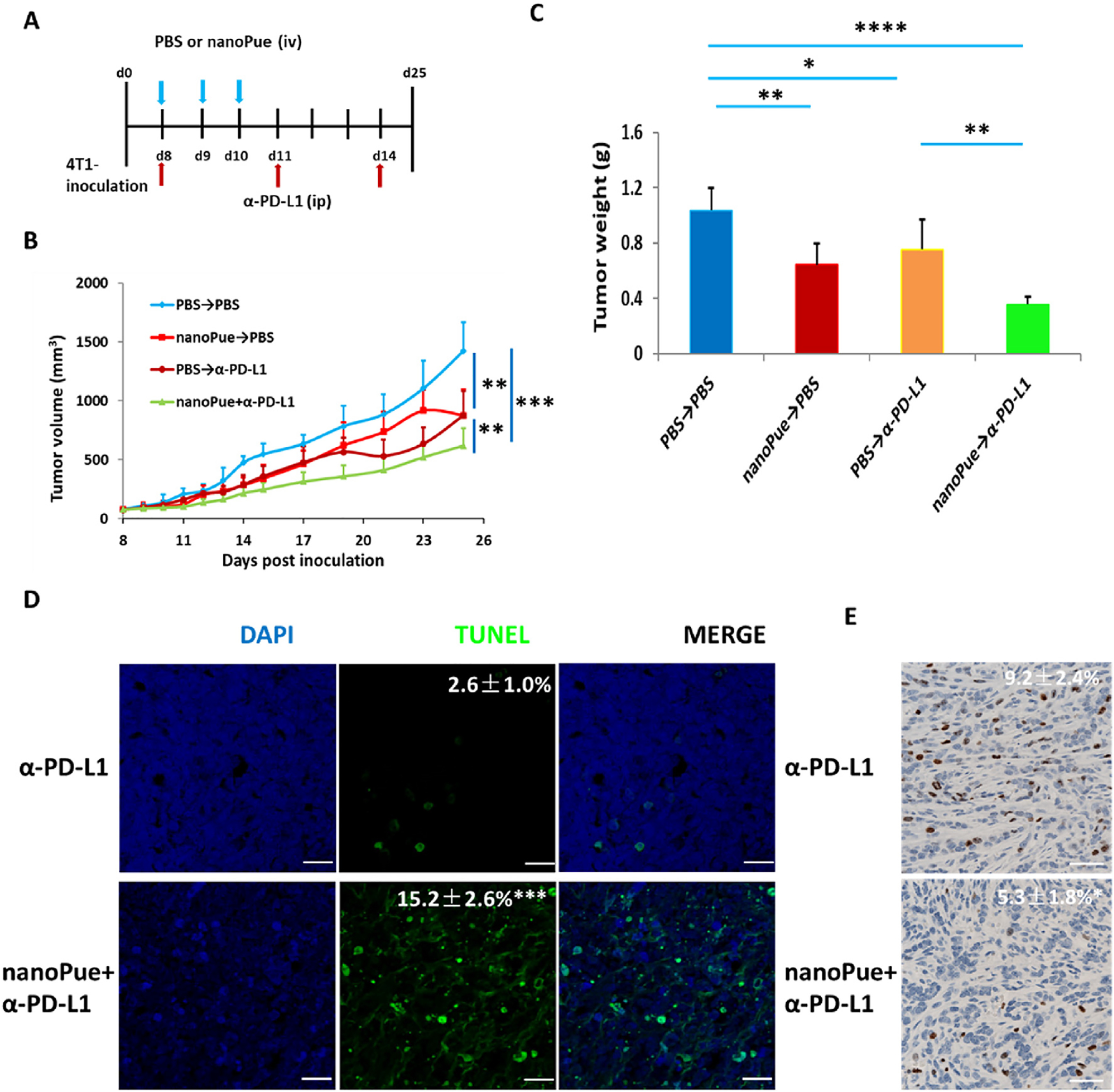
Combination of nanoPue and α-PD-L1 therapy on 4T1 tumor model (n = 5). (A) NanoPue and α-PD-L1 combination treatment scheme. (B) Tumor growth curves of 4T1 tumors in different treatment groups. (C) TUNEL staining of α-PD-L1 and nanoPue combined with α-PD-L1 treated 4T1 tumor tissues. TUNEL positive cells were quantified in 3 randomly selected fields per mouse (n = 5). (D) Comparison of Ki67 expression of 4T1 tumors in different treatment groups (n = 5). Scale bar represents 20 μm *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
This effect further validates the indispensable role of nanoPue in improving the immunogenicity in tumors and synergizing α-PD-L1 to activate the T cell immune response. No abnormal changes of serum ALT, AST, or BUN were observed 3 days after the last injection in each treatment group, suggesting high liver and kidney safety of the regimen (Fig. S7B). No significant weight loss occurred during various treatments (Fig. S7C). In the survival analysis, the nanoPue→α-PD-L1 group showed significantly prolonged median survival compared with the control and single treatment groups (Fig. S7D). No histological abnormity of heart, lung, spleen, and kidney was observed in any α-PD-L1 groups (Fig. S7E).
3. Conclusion
In the current study, an easy-to-scale-up nanoemulsion formulation was developed for the systemic delivery of puerarin with high EE and stability. This nanoPue formulation dramatically reduced the desmoplastic reaction in different types of solid tumors via downregulation of intratumoral ROS. The remodeled stromal microenvironment by the nanoPue treatment made better penetration of nanoparticles into the tumor parenchyma. Meanwhile, the nanoPue therapy significantly improved the tumor immune microenvironment and enhanced therapeutic efficiency of α-PD-L1 in a TNBC model. In summary, nanoPue, a robust TME modulator, could serve as an adjuvant therapy for both chemotherapeutic drugs and checkpoint blockade immunotherapies in highly desmoplastic tumors. Its relatively simple and scalable preparation also grants nanoPue a great potential for the clinical translation.
4. Materials and methods
Materials.
Medium-chain triglyceride (Kollisolv® MCT 70) was purchased from BASF (Ludwigshafen Germany). Polyethylene glycol (15)-hydroxystearate (Kolliphor® HS15), glycerol and Fluorometric Hydrogen Peroxide Assay Kit were obtained from Sigma-Aldrich (St. Louis, MO). Puerarin and lecithin from soybean was obtained from TCI (Tokyo Kasei Kogyo, Japan). nanoPTX (ZY-010) was prepared by utilizing a biodegradable polysaccharide, which was provided by ZY Therapeutics Inc. (Research Triangle Park, NC). ZY-010 was a lyophilized dosage form of PTX nano-formulation which contained 10% of PTX and 90% of Dextran-Folic acid conjugated polymer. The details of the synthesis of the polymer and the preparation of PTX entrapped nanoformulation can be found in US Patent PCT/US18/28,900 (Pharmaceutical Composition for in vivo Delivery, Method of Preparation of a Substantially Water-Insoluble Pharmacologically Active Agent for in vivo Delivery). After reconstituted in saline solution, the particle size assessed by Dynamic Light Scattering was about 100 nm with a Particle Dispersion Index (PDI) of less than 0.2. Zeta-potential of the nano-formulation in saline solution was evaluated by the Zetasizer Nano ZS instrument (Malvern) and measured to be in the range − 20 to − 25 mV. The details of the particle size and Zeta-potential was listed in Fig. S8. DSPE-PEG-AEAA was synthesized in our laboratory [23,24].
Cell lines, animals and antibodies.
Murine breast cancer 4T1 cells, mouse embryonic fibroblast cell line NIH3T3 and Murine BRAF mutant melanoma cell lines BPD6 were obtained from Tissue Culture Facility. 4T1 cells were stably transfected with the vector carrying the GFP, firefly luciferase, and the puromycin resistance gene. 4T1 cells and BPD6 cells were maintained in RPM-1640 media (Invitrogen) supplemented with FBS (10% v/v, Gibco), penicillin/streptomycin (1% v/v, Gibco), and puromycin (1 μg/mL, ThermoFisher) at 37 °C and 5% CO2 in a humidified atmosphere. NIH3T3 cells were cultured in Dulbecco’s Modified Eagle’s Media (DMEM) (Invitrogen, Carlsbad, CA), supplemented with FBS (10% v/v, Gibco) or 10% fetal bovine serum (Sigma, St. Louis MO), respectively, with penicillin (100 U/mL) (Invitrogen) and streptomycin (100 μg/mL) (Invitrogen).
Female Balb/C mice (8–10 weeks old) and female C57BL/6 mice (8–10 weeks old) were provided by Jackson Labs. All animal protocols were approved by the University of North Carolina at Chapel Hill’s Institutional Animal Care and Use Committee.
Antibodies and primers used in the study for Western blot, flow cytometry, and immunofluorescence staining are listed in Table S1 and S2 (Supporting Information).
Screening of TCMs for ROS inhibition.
Based on literatures and Chinese medicine pharmacology, 10 anti-fibrosis TCMs were selected to screen the ROS inhibition effects on TGF-β activated NIH3T3 cells. NIH3T3 cells were pre-stimulated with 10 ng/mL TGF-β for 24 h and 2 × 103 cells/well were seeded in a 96-well black plate. Then, the cells were treated with different TCMs. Cells were also treated with different concentration of puerarin and nanoPue as shown in Fig. 2A. After these drugs were added, the cells were further cultured for 48 h. Then the concentration of ROS was determined with the Fluorometric Hydrogen Peroxide Assay Kit according to the manufacturer’s instructions. The fluorescence intensity measured by using a fluorescence microplate reader (FLx800, Biotek Instrument Inc., Winooski, VT, USA) at excitation and emission wavelengths of 485 and 530 nm, respectively.
Cytotoxicity of puerarin.
The addition of puerarin and nanoPue to NIH3T3 cells was the same as the ROS assay. After 48 h of incubation, the culture medium was discarded and the cell layers were washed twice with PBS. Then 100 μL culture medium and 10 μL MTT solution (5 mg/mL) were added and incubated for 4 h at 37 °C. After the supernatant was removed, 150 μL DMSO was added and OD value was determined at 492 nm by using a multidetection microplate reader (Plate CHAMELEON™ V-Hidex). The cell viability rate of each concentration was calculated according to formula (1).
| (1) |
Preparation and characterization of nanoPue.
The aqueous phase was prepared by weighing an amount of glycerol (0.3 g) into 6 mL glucose solution (5%, w/w). DSPE-PEG-AEAA (1.5 mg) was added to the above mixture. The oil phase was prepared by dispersing 1.2 mg puerarin, 15% (w/w) of lecithin from soybean, 30% (w/w) Kolliphor® HS15 in MCT 70. The water phase was added into the oil phase quickly. The mixture was well mixed by a PC-351 hot plate-stirrer and incubated at 50 °C for 20 min. The crude emulsion was sonicated by using a fisher scientific sonic dismembrator model 100 at 600 w for 10 min. The emulsion was extruded through 0.22 μm polycarbonate membranes. NanoDiD was prepared by using the same method except that 2 mg of DiD was added to the oil phase. The particle size and zeta potential measurements were conducted with the Zetasizer (Nano ZS, Malvern Instruments Ltd., UK). The emulsion was negatively stained with 2% uranyl acetate and the emulsion morphology was observed by JEOL 100 CX II TEM (JEOL, Japan). The EE of nanoPue was measured by using the mini-column (Sephadex G-50) centrifugation method. The puerarin concentration was analyzed by a high-performance liquid chromatography (HPLC, Agilent LC1100) at the wavelength of 250 nm.
Stability of nanoPue In vitro.
To investigate the placement stability of nanoemulsion, nanoPue and puerarin suspension were incubated at 4 °C for different time. The change of particle size and EE% of samples were then measured by using the above methods separately.
The release of puerarin from emulsion was determined by using a dynamic dialysis method. A total of 1 mL nanoPue and puerarin suspension were loaded in a dialysis bag and incubated in 100 mL of PBS at pH 7.4 at 37 °C. At different times, 5 mL release medium was taken out and determined by using HPLC (Agilent LC1100) at the wavelength of 250 nm. The cumulative release of puerarin was calculated by the measured values of each time.
Pharmacokinetic study.
Mice were injected via the tail vein a single dose of nanoPue and suspension respectively at a dose of 35 mg/kg puerarin. Blood samples were collected via eye puncture at different time after administration. The blood samples were centrifuged at 5000×g for 10 min and stored in a freezer at −20 °C. Plasma samples (50 μL) were added 150 μL of methanol-acetonitrile (1:1, v/v). After vortex for 1 min, the mixture was centrifuged at 5000×g for 10 min and the supernatant was analyzed by HPLC. HPLC conditions were the same as the method mentioned in the determination of EE. The concentration-time data of puerarin was processed by the 3P97 pharmacokinetic calculation program to calculate the pharmacokinetic parameters.
Establishment of tumor model in mice.
4T1 and BPD6 tumor models were established in Balb/C and C57BL/6 female mice, respectively. 4T1 cells and BPD6 cells were harvested and washed in PBS (pH 7.4). For 4T1 tumor model, 1 × 106 cells suspension was injected into the mammary fat pads of the mice. For BPD6 tumor model, 1 × 106 BPD6 cells were injected into subcutaneous tissue in the lower flank area of Balb/C mice. The growth of 4T1 and BPD6 tumors were followed by directly measuring the tumor size by using a caliper.
Safety evaluation of nanoPue.
At the end of the endpoint, the mice were sacrificed and whole blood was obtained and centrifuged at 8000×g for 10 min to collect serum. ALT, AST, BUN, and creatinine, levels were determined as indicators of hepatic and renal damage. The body weights of mice were measured every other day from the beginning of treatment. The organs of mice, such as heart, liver, spleen, lung and kidney were fixed with 4% paraformaldehyde (PFA) and then were soaked in 70% ethanol overnight. H&E staining of organs was operated by UNC histology facility and the slides were observed by using fluorescence microscopy (Nikon, Tokyo, Japan) with 20 × objective.
NanoPue’s hemolytic effect in RBCs was investigated by using freshly collected blood from euthanized Wistar rats. 2% erythrocyte suspension was incubated with normal saline (negative control group, NG), distilled water (positive control group, PG) and different concentrations of nanoPue (experimental group, EG) at 37 °C for 1 h. The samples were centrifuged at 2000×g for 10 min, and the absorbance of the supernatants was measured at 540 nm by UV–Vis spectrophotometry (Shimadzu, Japan). The hemolysis rate (HR) was calculated according to the following equation:
| (2) |
Where AEG, ANG, and APG are the absorbance of EG, NG, and PG, respectively.
Another group of samples was incubated with different solutions (saline, distilled water, and nanoPue) at 37 °C for 1 h, and the degree of hemolysis was observed using the naked eye.
Effect of nanoPue on TME.
Mice bearing 4T1 tumors were randomized blindly into 3 treatment groups (n = 7): Untreated group (PBS), blank emulsion and nanoPue group (35 mg/kg). At the end of the endpoint, the mice were sacrificed and tumors were collected and weighed, then for H&E staining, Masson’s trichrome staining, immunofluorescence staining, Western Blot Analysis, flow cytometry analysis and RT-PCR assay.
Immunofluorescence staining and Masson’s Trichrome staining.
The tumor tissues were taken out from the mice and soaked in 4% PFA, 15% and 30% sucrose solutions for 24 h at 4 °C, respectively. The tumors were embedded in optimal cutting temperature embedding medium (Fisher Scientific) and cut into 10 μm sheets by Leica CM1850 cryostat (Germany). The slides were washed 3 times by 1 × PBS, permeabilized with 1% Triton and blocked by 5% goat serum. The primary antibodies with or without fluorescence were incubated with the slides 24 h at 4 °C. It is necessary that the slides were incubated with fluorescent secondary antibodies if the primary antibody doesn’t have fluorescence. Finally, the Nuclei were stained with DAPI. The slides were observed by using laser scanning confocal microscope (Zeiss, LSM 710).
The Masson’s trichrome assay was performed to investigate collagen among tumor tissues. Tumor tissues were fixed with 4% PFA and then were soaked in 70% ethanol overnight. The slides were stained by using a Masson’s trichrome Kit and Ki67 Cell Proliferation Kit by the UNC Tissue Procurement Core. The slides were observed by using fluorescence microscopy (Nikon, Tokyo, Japan). A minimum of 5 randomly selected microscopic fields were quantitatively analyzed by using ImageJ software.
Western blot analysis.
4T1 tumor bearing mice received six iv injection of nanoPue and PBS were sacrificed and the tumor tissues were collected. Fifty mg tumor sample was homogenized and lysed by using 500 μL radioimmunoprecipitation assay (RIPA) buffer containing 1% protease inhibitor (protease inhibitor cocktail and phosphatase inhibitor cocktail). The total protein concentration was determined by Pierce™ BCA Protein Assay Kit (Thermo Scientific, USA). Subsequently, 25 μg protein was loaded for Western Blot Analysis. The primary antibodies for α-SMA, p-SMAD2, p-SMAD3, NOX 4 were used for in vivo Western blot analysis and GAPDH was used as a control.
Flow cytometry.
The tumor tissues were placed on ice and incubated with collagenase A buffer and DNAase at 37 °C for 40 min. To obtain single cell suspension, FACS buffer (PBS containing 3% serum) was added and ground with filter. The mixture was centrifuged at 1200×g for 10 min. One mL ammonium-chloride-potassium buffer and 10 mL FACS were added to the sediment. Then the cell concentration was adjusted to 1–3 × 106 cells/mL. According to the protocol of manufacturer, cells are stained by antibodies on the surface or intracellular. The cells were fixed by adding 4% PFA and stored at 4 °C and analyzed via LSRFortessa (BD Biosciences).
Quantitative real-time PCR (RT-PCR) Assay.
20–30 mg tumor tissue was placed on ice and added 600 μL RNeasy lysis buffer, then broke by using the tissue tearor (Biospec products, Inc., USA). The total RNA was obtained from the tumor tissue homogenate followed the method of the RNeasy microarray tissue mini kit (Qiagen, Hilden, Germany). Subsequently RNA was reverse transcribed into cDNA by using the iScriptTM cDNA Synthesis Kit (BIO-RAD). The concentration of RNA and cDNA were both determined by using NanoDrop™ 2000 Spectrophotometers (ThermoFisher scientific, USA). The obtained cDNA was amplified by using the TaqManTM Gene Expression Master Mix. RT-PCR was performed by using the 7500 Real-Time PCR System and data were analyzed with the 7500 Software. The GAPDH RNA expression was used as normalized control.
Effect of repeated injection of nanoPue on the distribution of subsequently injected nanoDiD. After 3 injections of nanoPue (35 mg/kg, everyday), the mice were administered with nanoDiD at the DiD dose of 0.75 mg/kg. Twenty-four h later, the mice were imaged by using the IVIS® Kinetics Optical System (PerkinElmer, CA) at excitation and emission wavelengths of 640 and 670 nm, respectively. Then the mice were sacrificed and major organs and tumors were collected. The biodistribution of nanoDiD was quantitatively visualized with IVIS system as also.
Effect of nanoPue combined with chemotherapeutic drugs on tumor inhibition.
Mice bearing 4T1 tumors were randomized blindly into 4 treatment groups (n = 5): PBS→PBS group, nanoPue→PBS group, PBS→nanoPTX group and nanoPue→nanoPTX group and the treatment scheme is shown in Fig. 5A. The tumor volumes were measured by using a Vernier caliper and calculated through the following equations:
| (3) |
where a and b represent the long and short axis, respectively.
At the end of the endpoint, the mice were sacrificed and the tumors were collected and weighed, then H&E staining and TUNEL assay were performed.
Long-term survival was also monitored on 4T1-bearing mice with different treatments (n = 5, in each treatment group). Kaplan-Meier curves and median survival were quantified and calculated using GraphPad Prism 7.
TUNEL assay.
Apoptosis experiments were carried out by using a TUNEL assay kit (DeadEndTM Fluorometric TUNEL System, Promega) following the manufacturer’s protocol. Genomic fragmented cells were stained with green fluorescence of FITC and defined as TUNEL-positive nuclei. Then nuclei were stained with DAPI (ThermoFisher Scientific, USA). The images were taken by using laser scanning confocal microscope (Zeiss, LSM 710). A minimum of 5 randomly selected microscopic fields were quantitatively analyzed by using ImageJ software.
Statistical analysis.
Quantitative results were expressed as mean ± SD. The analysis of variance was completed using a one-way ANOVA or a two-tailed Student’s t-test. A P value less than 0.05 was considered statistically significant.
Supplementary Material
Acknowledgements
This work was financially supported by NIH grants CA198999, Natural Science Foundation of Liaoning Province (20170540575) and a grant from China Scholarship Council.
Footnotes
Declaration of competing interest
LH is a member of the Scientific Advisory Board of PDS Biotechnology, Inc. and Samyang Biopharmaceutical Co. All other authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.biomaterials.2020.119769.
References
- [1].Bremnes MR, Dønnem T, Al-Saad S, Al-Shibli K, Andersen S, Sirera R, Camps C, Marinez I, Busund LT, The role of tumor stroma in cancer progression and prognosis: emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer, J. Thorac. Oncol 6 (1) (2011) 209–217. [DOI] [PubMed] [Google Scholar]
- [2].Valkenburg KC, Groot AED, Pienta KJ, Targeting the tumour stroma to improve cancer therapy, Nat. Rev. Clin. Oncol 15 (6) (2018) 366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang B, Jiang T, Shen S, She XJ, Tuo YY, Hu Y, Pang ZQ, Jiang XG, Cyclopamine disrupts tumor extracellular matrix and improves the distribution and efficacy of nanotherapeutics in pancreatic cancer, Biomaterials 103 (2016) 12–21. [DOI] [PubMed] [Google Scholar]
- [4].Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y.l., Kadel EE, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang YG, Guan YG, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T, TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells, Nature 544 (7693) (2018) 544–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wiesner T, Kiuru M, Scott SN, Arcila M, Halpern AC, Hollmann T, Berger MF, Busam KJ, NF1 mutations are common in desmoplastic melanoma, Am. J. Surg. Pathol 39 (10) (2015) 1357–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhao XD, Subramanian S, Intrinsic resistance of solid tumors to immune checkpoint blockade therapy, Cancer Res. 77 (4) (2017) 817–822. [DOI] [PubMed] [Google Scholar]
- [7].Carey LA, Winer E, Viale G, Cameron D, Gianni L, Triple-negative breast cancer: disease entity or title of convenience? Nat. Rev. Clin. Oncol 7 (12) (2010) 683–692. [DOI] [PubMed] [Google Scholar]
- [8].Denkert C, Liedtke C, Tutt A, Minckwitz GV, Molecular alterations in triple-negative breast cancer-the road to new treatment strategies, The Lancet 389 (10087) (2017) 2430–2442. [DOI] [PubMed] [Google Scholar]
- [9].Miao L, Wang Y, Lin CM, Xiong Y, Chen N, Zhang L, Kim WY, Huang L, Nanoparticle modulation of the tumor microenvironment enhances therapeutic efficacy of cisplatin, J. Control. Release 217 (2015) 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arcucci A, Ruocco MR, Granato G, Sacco AM, Montagnani S, Cancer: an oxidative crosstalk between solid tumor cells and cancer associated fibroblasts, BioMed Res. Int 2016 (2016) 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yang YH, Bazhin AV, Werner J, Karakhanova S, Reactive oxygen species in the immune system, Int. Rev. Immunol 32 (3) (2013) 249–270. [DOI] [PubMed] [Google Scholar]
- [12].Bacanli M, Aydi S, Bsaran AA, Basaran N, A Phytoestrogen Puerarin and its Health Effects, Academic Press, 2018, pp. 425–431. [Google Scholar]
- [13].Wei SY, Chen Y, Xu XY, Progress on the pharmacological research of puerarin: a review, Chin. J. Nat. Med 12 (6) (2014) 407–414. [DOI] [PubMed] [Google Scholar]
- [14].Hou Y-X, Zhang H, Peng C, Puerarin: a review of pharmacological effects, Phytother Res 28 (7) (2014) 961–975. [DOI] [PubMed] [Google Scholar]
- [15].Meitzler JL, Antony S, Wu YZ, Juhasz A, Liu H, Jiang GJ, Lu JM, Roy K, Doroshow JH, NADPH oxidases: a perspective on reactive oxygen species production in tumor biology, Antioxidants Redox Signal 20 (17) (2014) 2873–2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Son B, Kwon T, Lee S, Han I, Kim W, Youn H, Youn B, CYP2E1 regulates the development of radiation-induced pulmonary fibrosis via ER stress-and ROS-dependent mechanisms, Am. J. Physiol-Lung C 313 (5) (2017) L916–L929. [DOI] [PubMed] [Google Scholar]
- [17].Wei D, Zhang X, Solubility of puerarin in the binary system of methanol and acetic acid solvent mixtures, Fluid Phase Equilib. 339 (2013) 67–71. [Google Scholar]
- [18].Quan DQ, Xu GX, Formulation optimization of self-emulsifying preparations of puerarin through self-emulsifying performances evaluation in vitro and pharmacokinetic studies in vivo, Acta Pharm. Sin 42 (8) (2007) 886–891. [PubMed] [Google Scholar]
- [19].Chung MJ, Sung NJ, Park C-S, Kweon D-K, Mantovani A-B, Moon T-W, Lee S-J, Park K-H, Antioxidative and hypocholesterolemic activities of water-soluble puerarin glycosides in HepG2 cells and in C57 BL/6J mice, Eur. J. Pharmacol 578 (2–3) (2018) 159–170. [DOI] [PubMed] [Google Scholar]
- [20].Banerjee R, Tyagi P, Li S, Huang L, Anisamide-targeted stealth liposomes: a potent carrier for targeting doxorubicin to human prostate cancer cells, Int. J. Cancer 112 (4) (2004) 693–700. [DOI] [PubMed] [Google Scholar]
- [21].Goodwin TJ, Huang L, On the article “Findings questioning the involvement of Sigma-1 receptor in the uptake of anisamide-decorated particles” [J. Control. Release 224 (2016) 229–238] Letter to the Editor 1 (September 14, 2016), J. Control. Release 243 (2016) 382–385. [DOI] [PubMed] [Google Scholar]
- [22].van Waarde A, Rybczynska AA, Ramakrishnan NK, Ishiwata K, Elsinga PH, Dierckx RA, Potential applications for sigma receptor ligands in cancer diagnosis and therapy, Biochim. Biophys. Acta 1848 (10) (2015) 2703–2714. [DOI] [PubMed] [Google Scholar]
- [23].Miao L, Newby JM, Lin CM, Zhang L, Xu F, Kim WY, Forest MG, Lai SK, Milowsky MI, Wobker SE, Huang L, The binding site barrier elicited by tumor-associated fibroblasts interferes disposition of nanoparticles in stroma-vessel type tumors, ACS Nano 10 (10) (2016) 9243–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Jin SE, Son YK, Min B-S, Jung HA, Choi JS, Anti-in-flammatory and anti-oxidant activities of constituents isolated fromPueraria lobata roots, Arch Pharm. Res. (Seoul) 35 (5) (2012) 823–837. [DOI] [PubMed] [Google Scholar]
- [25].Liu CM, Zheng GH, Ming QL, Sun JM, Cheng C, Protective effect of puerarin on lead-induced mouse cognitive impairment via altering activities of acetyl cholinesterase, monoamine oxidase and nitric oxide synthase, Environ. Toxicol. Pharmacol 35 (3) (2013) 502–510. [DOI] [PubMed] [Google Scholar]
- [26].Zhang SH, Guang J, Liu JW, Reversal of chemical-induced liver fibrosis in Wistar rats by puerarin, J. Nutr. Biochem 17 (7) (2006) 485–491. [DOI] [PubMed] [Google Scholar]
- [27].Clément S, Hinz B, Dugina V, Gabbiani G, Chaponnier C, The N-terminal Ac-EEED sequence plays a role in α-smooth muscle actin incorporation into stress fibers, J. Cell Sci 118 (7) (2005) 1395–1404. [DOI] [PubMed] [Google Scholar]
- [28].Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R, MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-β-induced collagen expression via inhibition of E-box repressors, Proc. Natl. Acad. Sci 104 (9) (2007) 3432–3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Souza, De Oliveira LS, De Araújo AA, Araujo Júnior RF, Barboza CAG, Borges BCD, Silva JSP, Low-level laser therapy (780 nm) combined with collagen sponge scaffold promotes repair of rat cranial critical-size defects and increases TGF-β, FGF-2, OPG/RANK and osteocalcin expression, Int. J. Exp. Pathol 98 (2) (2017) 75–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Selman M, Thannickal VJ, Pardo A, Zisman DA, Martinez FJ, Lynch Iii JP, Idiopathic pulmonary fibrosis: pathogenesis and therapeutic approaches, Drugs 64 (4) (2004) 405–431. [DOI] [PubMed] [Google Scholar]
- [31].Samarakoon R, Overstreet JM, Higgins PJ, TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities, Cell. Signal 25 (1) (2013) 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF, TGF-β regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells, Am. J. Physiol-Lung. C 300 (2) (2010) L295–L304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Veith C, Hristova M, Boots A, Van Schooten FJ, Van Der Vliet A, LSC-2017-Profibrotic signaling by TGF-β involves NADPH oxidase 4 dependent activation of tyrosine kinase Src and mitochondrial ROS, Eur. Respir. J 50 (2017) PA1021. [Google Scholar]
- [34].Chan EC, Peshavariya HM, Liu GS, Jiang F, Lim SY, Dusting GJ, Nox4 modulates collagen production stimulated by transforming growth factor β1 in vivo and in vitro, Biochem. Bioph. Res. Co 430 (3) (2013) 918–925. [DOI] [PubMed] [Google Scholar]
- [35].Feng HL, Wang J, Chen W, Shan BE, Guo Y, Xu JF, Wang L, Guo P, Zhang YZ, Hypoxia-induced autophagy as an additional mechanism in human osteosarcoma radioresistance, J. Bone. Oncol 5 (2) (2016) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Garrido-Urbani S, Jaquet V, Imhof BA, ROS and NADPH oxidase: key regulators of tumor vascularization, Med. Sci 30 (4) (2014) 415–421. [DOI] [PubMed] [Google Scholar]
- [37].Fang J, Nakamura H, Maeda H, The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect, Adv. Drug Deliv. Rev 63 (3) (2010) 136–151. [DOI] [PubMed] [Google Scholar]
- [38].Maeda H, Wu J, Sawa T, Matsumura Y, Hori K, Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review, J. Control. Release 65 (1) (2000) 271–284. [DOI] [PubMed] [Google Scholar]
- [39].Jean-Marc F, Anne-Claire HB, Olivier C, Alain L, Bruno S, Philippe F, Robert H, Mathilde D, Jérôme D, Mustapha A, Rémy L, Weekly paclitaxel, capecitabine, and bevacizumab with maintenance capecitabine and bevacizumab as first-line therapy for triple-negative, metastatic, or locally advanced breast cancer: results from the GINECO A-TaXel phase 2 study, Cancer 122 (2016) 3119–3126. [DOI] [PubMed] [Google Scholar]
- [40].Hu XC, Zhang J, Xu BH, Cai L, Ragaz J, Wang ZH, Wang BY, Teng YE, Tong ZS, Pan YY, Yin YM, Wu CP, Jiang ZF, Wang XJ, Lou GY, Liu, Feng JF, Luo JF, Sun K, Gu YJ, Wu J, Shao ZM, Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomized, openlabel, multicenter, phase 3 trial, Lancet Oncol 16 (4) (2015) 436–446. [DOI] [PubMed] [Google Scholar]
- [41].Han CY, Byoung SK, Chimeric antigen receptor T-cell therapy for cancer: a basic research-oriented perspective, Immunotherapy 10 (3) (2018) 221–234. [DOI] [PubMed] [Google Scholar]
- [42].Jiang H, Hegde S, DeNardo DG, Tumor-associated fibrosis as a regulator of tumor immunity and response to immunotherapy, Cancer Immunol. Immun 66 (8) (2017) 1037–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Qian BZ, Pollard JW, Macrophage diversity enhances tumor progression and metastasis, Cell 141 (1) (2010) 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Denardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM, CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages, Cancer Cell 16 (2) (2009) 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM, Macrophages in tumor microenvironments and the progression of tumors, Clin. Dev. Immunol 11 (2012) 1–11 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tan MCB, Goedegebuure PS, Belt BA, Flaherty B, Sankpal N, Gillanders WE, Eberlein TJ, Hsieh CS, Linehan DC, Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer, J. Immunol 182 (2009) 1746–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ, Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy, Cancer Immunol. Immunother 58 (1) (2009) 49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sinha P, Okoro C, Foell D, Freeze HH, Ostand-Rosenberg S, Srikrishna G, Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells, J. Immunol 81 (2018) 4666–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hugo W, Zaretsky JM, Sun L, Johnson DB, Ribas A, Lo RS, Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma, Cell 165 (1) (2016) 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Champiat S, Dercle L, Ammari S, Massard C, Hollebeaque A, Postel-Vinay S, Chaput N, Eggermont A, Marabelle A, Soria JC, Ferte C, Hyperprogressive disease (HPD) is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1, Clin. Cancer Res 23 (8) (2016) 1920–1928. [DOI] [PubMed] [Google Scholar]
- [51].Xiong Y, Wang Y, Karthik T, Tumor immune microenvironment and nano-immunotherapeutics in colorectal cancer, Nanomedicine: NBM (NMR Biomed.) 21 (2019) 102034, 10.1016/j.nano.2019.102034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


