Sexually transmitted Chlamydia, which can cause fibrotic pathology in women’s genital tracts, is also frequently detected in the gastrointestinal tract. However, the medical significance of the gastrointestinal Chlamydia remains unclear. A murine Chlamydia readily spreads from the mouse genital tract to the gastrointestinal tract while inducing oviduct fibrotic blockage or hydrosalpinx. We previously proposed a two-hit model in which the mouse gastrointestinal Chlamydia might induce the second hit to promote genital tract pathology, and we are now providing experimental evidence for testing the hypothesis.
KEYWORDS: gut chlamydia, hydrosalpinx, genital pathology, Chlamydia, gastrointestinal colonization, pathogenesis, tubal fibrosis
ABSTRACT
Sexually transmitted Chlamydia, which can cause fibrotic pathology in women’s genital tracts, is also frequently detected in the gastrointestinal tract. However, the medical significance of the gastrointestinal Chlamydia remains unclear. A murine Chlamydia readily spreads from the mouse genital tract to the gastrointestinal tract while inducing oviduct fibrotic blockage or hydrosalpinx. We previously proposed a two-hit model in which the mouse gastrointestinal Chlamydia might induce the second hit to promote genital tract pathology, and we are now providing experimental evidence for testing the hypothesis. First, chlamydial mutants that are attenuated in inducing hydrosalpinx in the genital tract also reduce their colonization in the gastrointestinal tract, leading to a better correlation of chlamydial induction of hydrosalpinx with chlamydial colonization in the gastrointestinal tract than in the genital tract. Second, intragastric coinoculation with a wild-type Chlamydia rescued an attenuated Chlamydia mutant to induce hydrosalpinx, while the chlamydial mutant infection in the genital tract alone was unable to induce any significant hydrosalpinx. Finally, the coinoculated gastrointestinal Chlamydia failed to directly spread to the genital tract lumen, suggesting that gastrointestinal Chlamydia may promote genital pathology via an indirect mechanism. Thus, we have demonstrated a significant role of gastrointestinal Chlamydia in promoting pathology in the genital tract possibly via an indirect mechanism. This study provides a novel direction/dimension for further investigating chlamydial pathogenic mechanisms.
INTRODUCTION
Chlamydia trachomatis is the leading infectious cause of infertility due to its ability to induce tubal inflammation/adhesion/fibrosis/hydrosalpinx in women (1–4). Chlamydia muridarum has been used for investigating C. trachomatis pathogenesis because of its induction of long-lasting tubal fibrosis/hydrosalpinx in mice (5–9). The vaginal C. muridarum ascends to the oviduct to induce tubal inflammation (10) that may both clear infection and damage tubal epithelia, triggering transient fibrosis. For reasons not yet known, mouse tubal fibrosis continues in many cases, resulting in oviduct lumen occlusion, hydrosalpinx, and/or infertility (5, 7, 9). Both chlamydial plasmid genes (11–13) such as pgp3 (14) and chromosomal genes such as tc0237/0668 (15, 16) have been shown to impact chlamydial ascension and/or tubal inflammation/fibrosis. Host pathways may also regulate chlamydial pathogenesis (9, 17–20). CD8+ T cells promote (21, 22) while CD4+ T cells prevent (23) chlamydial induction of hydrosalpinx. However, the accumulated knowledge is still insufficient to fully explain how the long-lasting tubal fibrosis is maintained after the oviduct infection is cleared.
The human genital tract pathogen Chlamydia is routinely detected in gastrointestinal (GI) tracts (24–28). When we tracked a murine Chlamydia ascension in the female mouse genital tract (9, 29), we accidentally found that genital Chlamydia spread to and colonized the GI tract for long periods of time (30), which is consistent with an earlier observation that orally introduced Chlamydia persisted in the gut (31). The spreading might occur via blood circulation (30, 32) since any mucosal lumenal inoculation with Chlamydia always led to a transient systemic spreading in mice (33). The disseminated organisms were cleared within 2 to 3 weeks, and only those that reached the GI tract lumen could persist for long periods of time (32, 34). Systemic distribution of human Chlamydia has also been reported in women (35, 36). However, Chlamydia that traveled to mouse genital tissue via blood circulation failed to reach the genital tract lumen and could not induce hydrosalpinx (30, 32, 34, 37). It has been hypothesized that GI Chlamydia may serve as a reservoir for autoinoculating the genital tract lumen to promote chlamydial pathogenicity (31, 38). However, mice intragastrically inoculated with Chlamydia failed to autoinoculate their genital lumen (34) and did not develop any significant pathology (37, 39).
The present study used the murine Chlamydia model to determine whether GI Chlamydia can affect chlamydial pathogenicity in the upper genital tract. Murine Chlamydia infection in the mouse genital tract is self-limited, but the induced hydrosalpinx is long lasting (9, 10), correlating with chlamydial long-lasting colonization in the GI tract (30). Attenuated Chlamydia mutants were defective in both inducing hydrosalpinx and colonizing GI tract (39–41). Nevertheless, all mutants were still able to ascend to the oviduct with some at a reduced level (11, 14, 42), while others maintained a robust ascension as wild-type (Wt) Chlamydia (15). We have proposed a two-hit model to explain chlamydial pathogenicity in the upper genital tract (43). Genital wild-type Chlamydia may be able to both ascend to the oviduct to produce a first hit and spread to the GI tract to induce a second hit. Both hits may be required for chlamydial induction of long-lasting hydrosalpinx. To test the two-hit model, we used intravaginal inoculation with plasmid-free (Pf) mutant Chlamydia to induce the first hit in the oviduct since Pf Chlamydia can still ascend to the oviduct (11), but with significantly reduced spreading to the GI tract (40) and without inducing hydrosalpinx in the genital tract (11, 41). We then intragastrically coinoculated the same mice with a wild-type Chlamydia to produce the second hit. We found that coinfection significantly rescued Pf Chlamydia to induce hydrosalpinx. This rescue was mediated via an indirect mechanism since GI Chlamydia did not directly infect the genital lumen (34). Thus, we demonstrate that chlamydial spreading to the GI tract is able to promote chlamydial pathogenicity in the upper genital tract.
RESULTS
Chlamydial pathogenicity in the upper genital tract correlates with chlamydial colonization in the GI tract.
Although chlamydial infection in the mouse genital tract is self-limited, the genital organisms are able to both induce long-lasting hydrosalpinx in the upper genital tract and spread to the GI tract to establish a long-lasting colonization there, suggesting a temporal correlation between the upper genital pathogenicity and colonization in the GI tract (43). We have obtained various attenuated chlamydial organisms that are significantly reduced in inducing hydrosalpinx but still maintaining robust colonization in the mouse genital tract (11, 14–16, 42). All attenuated organisms were still able to ascend to the oviduct, with some at a reduced level (11, 14, 42), while others maintained ascension as efficiently as wild-type C. muridarum (15). However, all of them were consistently reduced in colonizing the GI tract (39–41). We have now systematically compared chlamydial mutants with mutations in either plasmid-borne genes or chromosomal genes for their colonization in the genital tract and spreading to the GI tract (Fig. 1). Intravaginal inoculation with Wt chlamydial organisms either transformed with a plasmid that expresses mCherry gene (designated CMpmCherry) or with the endogenous plasmid (clone 13.32.1) both established robust colonization in the genital tract and spread to the GI tract for long-lasting colonization. However, chlamydial organisms deficient in either the plasmid-encoded pGP3 (designated CMpGP3s) or the entire plasmid (Pf, designated CMUT3) or with loss-of-function mutations in chromosomal genes were inefficient in spreading to the GI tract, although they all established robust colonization in the genital tract. We further compared their ability to induce hydrosalpinx on day 56 after intravaginal inoculation (Fig. 2). Although both Wt organisms induced 80% or more mice to develop hydrosalpinx, the mutants were highly attenuated in inducing hydrosalpinx. When we used Spearman’s rank correlation to analyze the relationship of chlamydial induction of hydrosalpinx with chlamydial colonization in the genital tract versus the GI tract, we found that the mouse hydrosalpinx scores correlated better with chlamydial colonization in the GI tract with a coefficient of rho of 0.7252, while the correlation coefficient between hydrosalpinx scores and chlamydial burdens in the genital tract was 0.5838. Pearson correlation analysis revealed a similar pattern: R2 = 0.4294 in the GI tract versus R2 = 0.2565 in the genital tract (Fig. 3).
FIG 1.
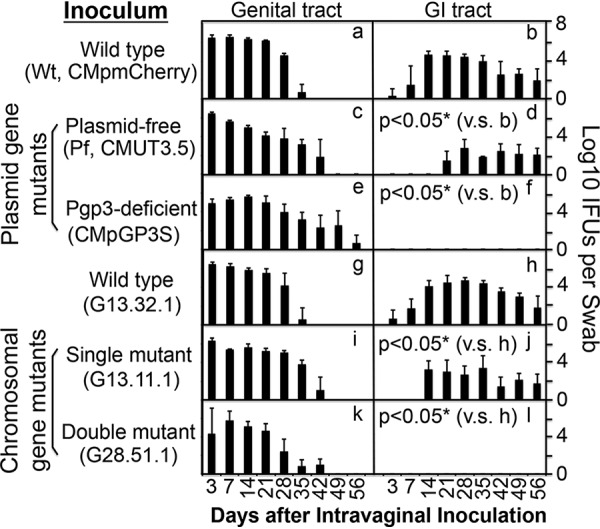
Monitoring live organism shedding from mouse genital and GI tracts after intravaginal inoculation with chlamydial organisms with or without mutations. Groups of CBA/J mice (n = 5) intravaginally inoculated with plasmid-free (Pf) C. muridarum transformed without (CMUT3.5, panels c and d) or with pCM plasmid coding for mCherry (CMpmCherry, panels a and b, as wild-type [Wt]) or pCM with a premature stop codon installed in the pgp3 gene (CMpGP3S, panels e and f) or C. muridarum clone G13.32.1 (panels g and h) or its isogenic clones with a single chromosomal gene mutation (clone G13.11.1, panels i and j) or double gene mutation (clone 28.51.1, panels k and l) were monitored for live organism shedding from both genital (panels a, c, e, g, i, and k) and gastrointestinal (GI; panels b, d, f, h, j, and l) tracts in log10 IFU (y axis) on days 3 and 7 and weekly thereafter (x axis). *, P < 0.05 (area under the curve comparison between panels b versus [v.s] panel d or f or between panel h versus panel j or i using the Wilcoxon rank sum test).
FIG 2.
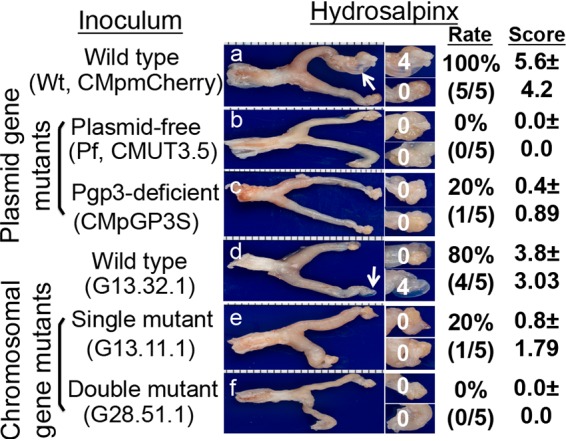
Detecting gross pathology from mouse genital tract following intravaginal inoculation with chlamydial organisms with or without mutations. Groups of CBA/J mice (n = 5) intravaginally inoculated with different C. muridarum organisms as described in the Fig. 1 legend (panel a for mice infected with CMpmCherry, panel b for Pf, CMUT3.5, panel c for CMpGP3S, panel d for C. muridarum wild-type clone G13.32.1, panel e for mutant clone G13.11.1, and panel f for mutant clone 28.51.1). On day 56 after infection, all mice were sacrificed for observing hydrosalpinx pathology, as described in Materials and Methods. One representative genital tract image from each group is shown with the vagina on the left and oviduct/ovary on the right. Oviducts with hydrosalpinges are indicated by white arrows. The oviduct/ovary from each side is magnified in the panels on the right, with the corresponding hydrosalpinx scores marked. The hydrosalpinx was counted and semiquantitatively graded to calculate the incidence rate and severity score in a given group.
FIG 3.
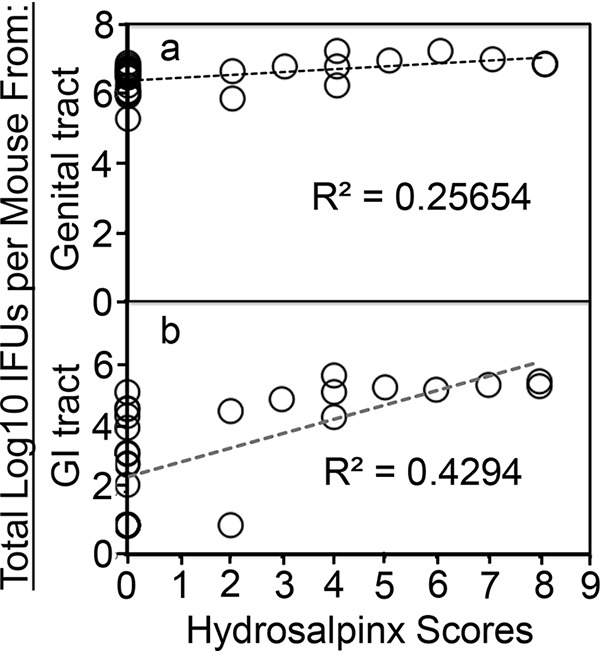
Correlating mouse gross pathology hydrosalpinx with live organism recoveries from mouse genital or GI tracts. For Pearson correlation analyses, the hydrosalpinx score from each CBA1/J mouse (as displayed along the x axis) was plotted against live organism shedding in log10 total numbers of IFU from genital (a) or GI (b) tract swab specimens collected from a mouse over time (y axis). Each circle represents a mouse. The total number of IFU from each mouse was obtained by adding the numbers of IFU from all time points observed. The correlation coefficient r between the hydrosalpinx scores and the number of genital or vaginal IFU was 0.5065, while that between the hydrosalpinx scores and the number of GI tract or rectal IFU was 0.6553, given that r is the square root of coefficient of determination R2 in linear regression.
GI Chlamydia is able to restore pathogenicity of attenuated Chlamydia in the upper genital tract.
To test whether genital Chlamydia spreading to gastrointestinal tract can promote chlamydial pathogenicity in the upper genital tract, mice intravaginally infected with Pf mutant Chlamydia were coinoculated intragastrically with wild-type Chlamydia that expresses an mCherry gene so that the mutant and wild-type organisms can be differentiated (Fig. 4). As shown above, although intravaginal inoculation with either Wt or Pf organisms led to robust colonization of both organisms in the genital tract with a time course of up to 42 days, Pf organisms displayed significantly delayed and reduced spreading to the GI tract. In a parallel group of mice that were intravaginally inoculated with Pf Chlamydia for 7 days, an additional intragastric inoculation with Wt Chlamydia still led to robust colonization of the Wt organisms in the GI tract, while low levels of the original Pf organisms were also detectable in the same GI samples. This coinfection scheme did not significantly alter the original Pf organism colonization in either the genital or GI tracts. Similarly, under this coinfection scheme, the preexisting Pf organisms did not significantly alter the gastrointestinal colonization of the coinoculated Wt organisms. Thus, we used this coinfection mouse model to further determine whether the coinoculated Wt organisms in the GI tract could restore the Pf Chlamydia-infected mice to develop pathology in the upper genital tract by monitoring genital tract pathology on day 56 after the initial infection (Fig. 5). We found that although 90% of mice inoculated intravaginally with Wt organisms developed significant hydrosalpinx, only 1 of 14 mice inoculated intravaginally with Pf organisms developed hydrosalpinx. However, intragastric coinfection with Wt organisms restored the Pf-infected mice to develop hydrosalpinx in 9 of the 14 coinfected mice. The hydrosalpinges were confirmed microscopically with semiquantitation of oviduct dilation (Fig. 6). The coinfected mice displayed oviduct dilation significantly higher than that of mice with Pf organisms alone. Thus, Pf organisms alone are insufficient for inducing hydrosalpinx, although Pf organisms are known to ascend to the upper genital tract (11). Importantly, intragastric coinoculation with Wt Chlamydia may provide a second hit for driving the development of long-lasting hydrosalpinx in the genital tract.
FIG 4.
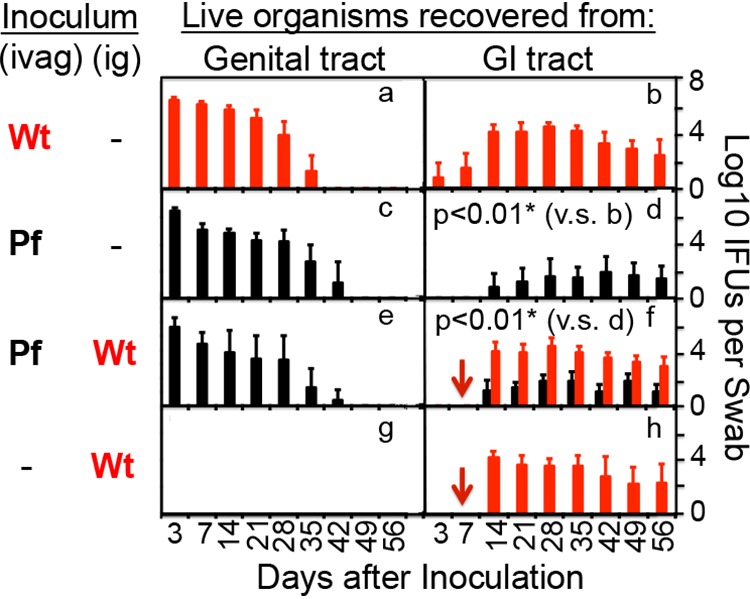
Monitoring live organism shedding from mouse genital and GI tracts after coinfection. As indicated on the left of the figure, groups of female CBA/1J mice were intravaginally (ivag) infected with a mCherry-expressing wild-type (clone CMpmCHerry, red bar [panel a, n = 10]) or plasmid-free (clone CMUT3.5, black bar [panel c, n = 14; panel e, n = 14]) chlamydial organisms. Seven days after the intravaginal infection, mice were intragastrically (ig) infected with the same Wt chlamydial organisms, as indicated by red arrows (panel f, n = 14; panel h, n = 11). All mice were monitored for live chlamydial organism shedding from both vaginal (panels a, c, e, and g) and rectal (panels b, d, f, and h) swabs, and the titers of Wt (red) and Pf (black) organisms were quantitated separately and are expressed as the log10 IFU per swab (y axis) on different days after intravaginal infection (x axis). *, P < 0.01 (area-under-the-curve comparison using a Wilcoxon rank sum test).
FIG 5.
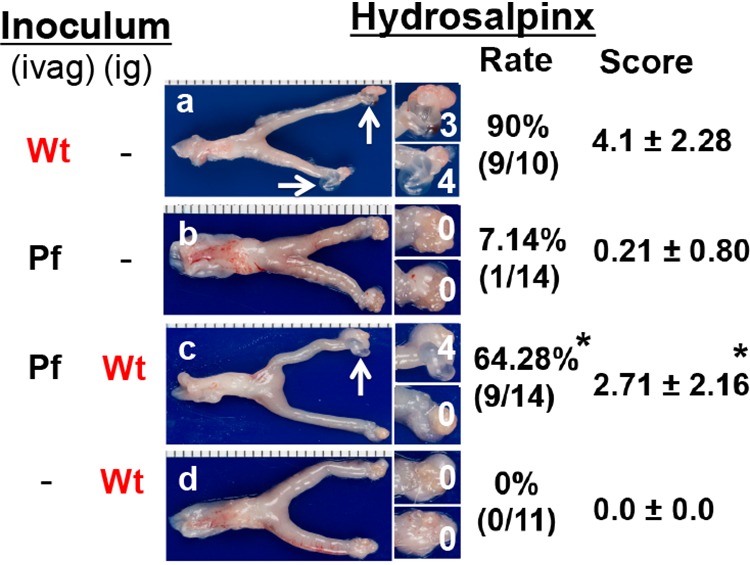
Detecting gross pathology from mouse genital tract after coinfection. Groups of CBA/J mice (n = 10 to 14 per group) with or without coinfection as described in the Fig. 4 legend (panel a for mice infected intravaginally [ivag] with Wt CMpmCherry alone, panel b for ivag Pf alone, panel c for both ivag Pf and intragastric infection [ig] with Wt, and panel d for ig Wt alone). On day 56, all mice were sacrificed to determine the genital pathology macroscopically for hydrosalpinx. Only one genital tract gross image is presented as representative for the corresponding group, with the vagina on the left and the oviduct/ovary on the right (panels a to d). The hydrosalpinx (dilated oviduct accumulated with serous fluid) is marked by a white arrow. The oviduct portion is magnified for viewing the hydrosalpinx. Hydrosalpinx scores are indicated in white. Both hydrosalpinx incidence and severity score are listed on the right of the corresponding group images. *, P < 0.05 (the Wilcoxon test was used for comparing hydrosalpinx scores, while the Fisher exact test was used for comparing incidence). The data were acquired from three independent experiments.
FIG 6.
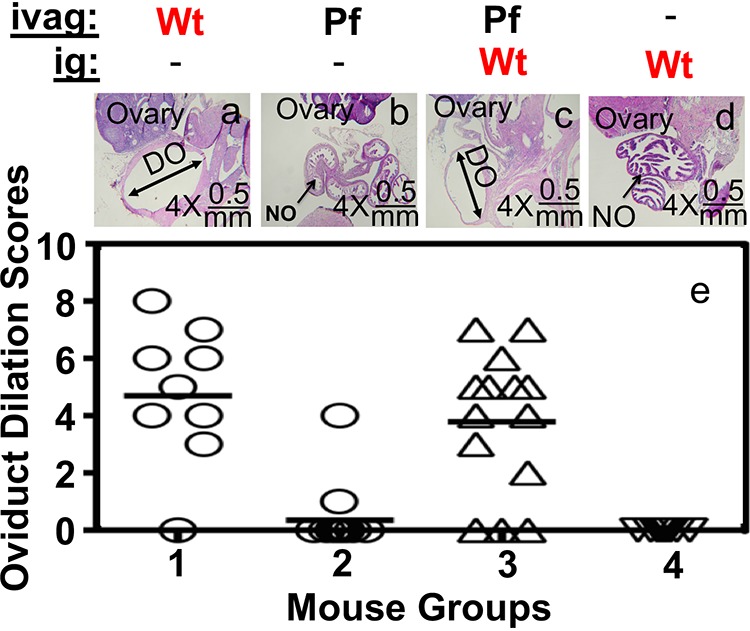
Detecting oviduct dilation under microscopy after coinfection. Groups of CBA/J mice (n = 10 to 14 per group) with or without coinfection as described in the Fig. 4 legend (panel a or group 1 for mice infected ivag with Wt CMpmCherry alone, panel b or group 2 for ivag Pf alone, panel c or group 3 for both ivag Pf and ig infection with Wt, and panel d or group 4 for ig Wt alone). On day 56, all mice were sacrificed for observing microscopically for oviduct dilation. Only one microscopic image (taken under a 4× lens objective) of the genital tract tissue is presented as a representative for a given group (panels a to d). Dilated oviducts (DO) are marked by double-headed arrows, while normal oviduct (NO) are marked by single-headed arrows. The ovary is also indicated. The oviduct dilation scores from all 4 groups are displayed in panel e. Open circles are used for mouse dilation scores in groups 1 and 2, while open upright triangles are used for group 3 and upside down triangles are used for group 4.
GI Chlamydia cannot directly reach the genital tract lumen to promote pathogenicity.
To determine whether intragastrically coinoculated Wt Chlamydia promoted pathology in the upper genital tract by directly infecting the genital tract lumen or via an indirect mechanism, we obtained the following data. First, no live organisms were detected in vaginal swabs of mice intragastrically inoculated wild-type Chlamydia throughout the experiment (see the intragastric-inoculation-alone group in Fig. 4 above; see also references 34 and 37). This observation suggests that GI Chlamydia is not able to infect the genital tract lumen since mice with chlamydial infection in the oviduct lumen consistently shed live organisms in the vaginal swabs (30, 44, 45). Second, intragastrically infected mice failed to develop pathology in the genital tract, which is consistent with a lack of oviduct lumenal infection. Third, when wild-type Chlamydia was coinoculated into the GI tracts of mice with prior intravaginal infection with Pf Chlamydia, GI Chlamydia failed to spread to the genital tract lumen throughout the infection course (see the coinfected group from Fig. 4 above). Although both the intravaginally inoculated Pf chlamydial organisms and intragastrically coinoculated Wt chlamydial organisms were detected in the rectal swabs, only Pf organisms were detected in the vaginal swabs. Finally, we further monitored tissue distribution of chlamydial organisms in the coinfected mice (Fig. 7). The intragastrically coinoculated wild-type Chlamydia was not detected in genital tissue. In order to increase detection sensitivity, we monitored chlamydial genomes using qPCR and found no Wt Chlamydia genome in the genital tissue (Fig. 8). These observations together demonstrate that GI Chlamydia may promote hydrosalpinx via an indirect mechanism.
FIG 7.
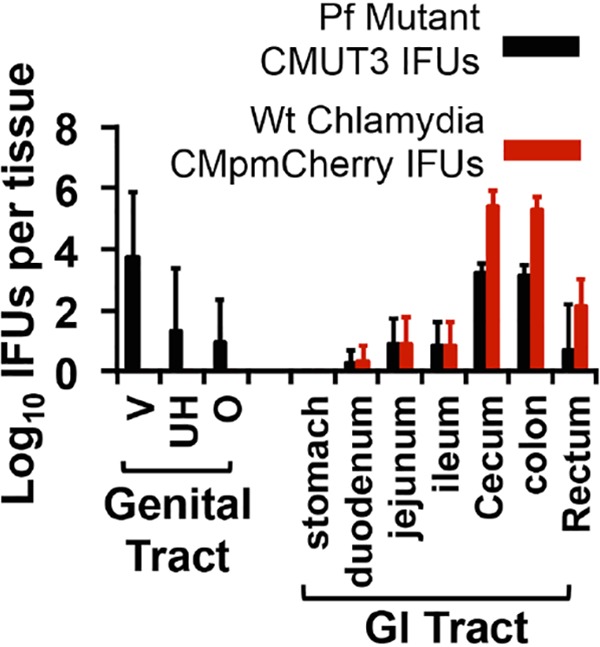
Monitoring live organism shedding from mice coinoculated with both plasmid-free and wild-type chlamydial organisms. CBA/J (n = 5) intravaginally inoculated with Pf C. muridarum (clone CMUT3.5) were intragastrically coinoculated with Wt Chlamydia (CMpmCherry) 7 days after intravaginal inoculation (see the Fig. 4 legend above). On day 28 after intravaginal inoculation, mice were sacrificed for recovering live organisms (black bar for Pf and red bar for Wt log10 IFU along the y axis) from both genital (vagina/cervix [V], uterine horn [UH], and oviduct and ovary [O]) and GI (stomach, duodenum, jejunum, ileum, cecum, colon, and rectum) tissues (x axis).
FIG 8.
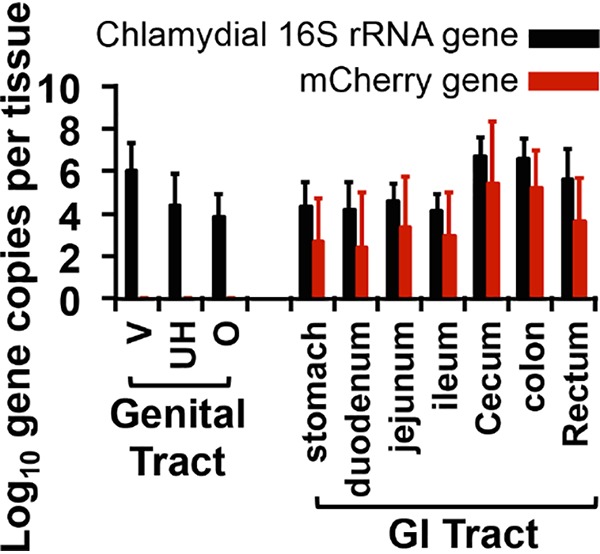
Monitoring chlamydial genomes from mice coinoculated with both plasmid-free and wild-type chlamydial organisms. Tissue samples from the same CBA/J mice (n = 5) intravaginally inoculated with Pf C. muridarum and intragastrically coinoculated with wild-type (red) Chlamydia 7 days after intravaginal inoculation as described in the Fig. 7 legend were also used for detecting chlamydial DNA using qPCR. The results are expressed as the log10 chlamydial 16S rRNA (black bars) or mCherry (red bars) gene copy numbers, as shown along the y axis. Genital tissues include vagina/cervix (V), uterine horn (UH), and oviduct and ovary (O), while GI tissues include stomach, duodenum, jejunum, ileum, cecum, colon, and rectum tissues, as listed along the x axis.
DISCUSSION
Although Chlamydia is known to induce long-lasting tubal pathology and infertility in women and female mice (2–5, 7, 9), the precise mechanisms remain unclear. In the present study, we have presented experimental evidence for supporting a role of gastrointestinal Chlamydia in promoting chlamydial pathogenicity in the upper genital tract. First, in addition to the temporal correlation between chlamydial induction of long-lasting tubal fibrosis/hydrosalpinx and stable colonization in the GI tract, we confirmed a positive correlation of chlamydial induction of hydrosalpinx with chlamydial burden in the GI tract but not in the genital tract. All Chlamydia mutants were consistently attenuated in both inducing hydrosalpinx and colonizing the GI tract. However, these mutants still maintained a robust colonization in the genital tract. Importantly, all mutants were still able to ascend to the oviduct, with some at a reduced level (11, 14), while others maintained ascension as efficiently as wild-type C. muridarum (15, 16, 39). It is clear that factors (such as GI tract colonization) beyond tubal infection and inflammation may play important roles in chlamydial pathogenicity in the female genital tract. Second, intragastric coinoculation with wild-type Chlamydia rescued attenuated C. muridarum to induce hydrosalpinx, providing the first-ever direct evidence for GI Chlamydia to promote chlamydial pathogenicity in the upper genital tract. Third, GI Chlamydia failed to directly infect the genital tract lumen, and GI Chlamydia alone failed to induce hydrosalpinx, suggesting that GI Chlamydia must use an indirect mechanism to promote chlamydial pathogenicity in the upper genital tract. Efforts are under way to investigate the mechanisms by which the gastrointestinal chlamydial organisms promote chlamydial pathogenicity in the genital tract.
In the coinfection experiment, the GI coinoculation was delayed by 7 days. This is because the timing of coinfection makes a significant difference. In our pilot experiments, we tested three different coinfection schedules: (i) GI coinoculation with Wt Chlamydia 7 days before genital inoculation with Pf Chlamydia, (ii) GI and genital inoculations at the same time, or (iii) GI coinoculation 7 days after the genital inoculation. We found that the first two schemes did not result in any enhanced pathology in the genital tract. Furthermore, these coinoculation conditions shortened the genital infection course by Pf Chlamydia. It is likely that the protective immunity induced by Wt Chlamydia in the GI tract reduced Pf Chlamydia infection in the genital tract. In contrast, when the GI coinoculation was carried out 7 days after the genital inoculation, the GI coinoculation significantly increased hydrosalpinx but no longer shortened the Pf chlamydial infection course in the genital tract. The 7-day delay might be sufficient for avoiding the interference with the genital Pf Chlamydia infection by the immune responses induced in the GI tract. The 7-day delay GI coinoculation schedule is also consistent with the kinetics of the natural spreading of genital chlamydial organisms to the GI tract. Thus, we used the 7-day delay coinfection protocol for testing the two-hit model in the present study.
We have previously proposed a two-hit model for explaining C. muridarum induction of long-lasting hydrosalpinx in mice (43). The first hit may consist of the initial epithelial infection/damage, antigen processing and presentation, and a wound-healing response such as transient fibrosis. C. muridarum ascends to the mouse oviduct to induce pyosalpinx followed by clearance of C. muridarum and a reparative response (10, 46). Chlamydial antigen processing and presentation must occur in the infected oviducts since Chlamydia-specific CD4+ Th1 cells are recruited to clear the tubal infection (23, 47). Similarly, major histocompatibility complex class I-restricted epitopes may also be produced in the oviduct. The question is whether antigen-presenting cells that present chlamydial epitopes can persist in the upper genital tract for a long period of time after the tubal infection is cleared. The second hit emphasizes the recruitment of pathogenic CD8+ T cells to oviduct for promoting tubal fibrosis and hydrosalpinx. This is because antigen-specific CD8+ T cells have been previously shown by the Murthy group to be required for hydrosalpinx induction in mice genitally infected with Wt C. muridarum (21, 22, 48). We now know that that intravaginally inoculated Wt C. muridarum organisms are able to both ascend to the upper genital tract and spread to the GI tract (30). Thus, it is reasonable to hypothesize that CD8+ T cells induced or educated by Chlamydia in the GI tract may act as a second hit to promote chlamydial pathogenicity in the upper genital tract. This hypothesis is consistent with these observations: mice deficient in either TNFR1 (49) or interleukin-13 (IL-13) (50) significantly reduced hydrosalpinx induction by Chlamydia. IL-13+ CD8+ T cells have been isolated from Chlamydia-infected mice (51). IL-13+ CD8+ T cells have been shown to promote pathogenic fibrosis in other systems (52–54). GI tract infections are known to induce fibrosis-promoting lymphocytes (55–57). Regardless of how gastrointestinal chlamydial organisms induce pathogenic CD8+ T cells and how these pathogenic cells work, the present study has established an experimental system for testing this hypothesis.
It is worth pointing out the limitation of the coinfection experiment, in which the intravaginal infection with Pf Chlamydia is meant to provide the first hit, while the intragastric coinoculation with Wt Chlamydia provides the second hit. First, the Pf Chlamydia still spreads to the GI tract, although at a significantly delayed and reduced level (40), which may make the first hit not from the genital tract only. Thus, alternative approaches are needed to produce a clean first hit. Second, although once Wt Chlamydia establishes colonization in the GI tract it is strictly restricted to the GI tract (34, 37), there is always a transient bacteremia phase following any mucosal inoculation with C. muridarum, as initially revealed by Perry and Hughes (33). However, this transient bacteremia is rapidly cleared within 2 to 3 weeks (32). The chlamydial organisms that travel to the genital tract tissues via blood circulation cannot infect the genital tract lumen and fail to cause any significant pathology (32, 34, 37). The hematogenous chlamydial organisms can only enter the lumen of the GI tract and establish long-lasting colonization there (32), which may mediate the spreading of genital Chlamydia into the GI tract (30). However, it is not clear whether the transient bacteremia can induce immune responses that may function as the second hit. Finally, although the coinfection experiment provided evidence that the GI tract-derived second hit is sufficient for promoting chlamydial pathogenicity in the genital tract, it fails to address whether the GI tract-derived second hit is necessary. Efforts are under way to determine whether blocking genital chlamydial spreading to the GI tract can attenuate chlamydial pathogenicity in the genital tract.
Caution should also be taken when applying the mouse model-based information to human C. trachomatis pathogenesis. Although murine bacterium C. muridarum infection in the mouse genital tract can induce tubal fibrosis/hydrosalpinx similar to that observed in C. trachomatis-infected women (2–4), C. muridarum may be naturally transmitted among mice via oral-fecal route instead of sexual transmission. Various C. muridarum virulence factors have been shown to be more important for C. muridarum to colonize the GI tract than to infect the genital tract (39, 40), suggesting that these factors may be selected for adapting to the GI tract mucosal barriers. In addition, C. muridarum genome still maintains three copies of a full-length cytotoxin gene with significant homology to large clostridial cytotoxins (58). C. muridarum may have acquired the cytotoxin gene from other enteric bacteria and maintaining the full-length cytotoxins may be necessary for C. muridarum to compete against other enteric bacteria in the GI tract. However, C. trachomatis may have been transmitted sexually among humans, forcing C. trachomatis to adapt to the human genital mucosa. Since the genital microbiota are less robust than those in the GI tract, C. trachomatis may not need to maintain the full-length cytotoxins. Indeed, the cytotoxin genes are lost or significantly shortened in C. trachomatis (58). Nevertheless, C. trachomatis has been frequently detected in the GI tracts of humans but without any significant association with GI pathologies (24–28). Thus, C. trachomatis may have experienced selection pressures from both the genital and the GI tracts during spreading among humans. It will be worth testing whether GI C. trachomatis can impact C. trachomatis pathogenicity in the genital tracts in women, which is doable using well-controlled large-scale clinic studies. Information obtained from mouse models may be useful in designing human studies.
MATERIALS AND METHODS
Chlamydial organism growth.
All Chlamydia muridarum clones used in the present study were derived from strain Nigg3 (GenBank accession no. CP009760.1). These include various passaged clones (15): G13.32.1 (retaining the wild-type Nigg3 genome sequence), G13.11.1 (a frameshift mutation in tc0668 resulting in a premature stop codon at the 216th codon, also called single mutant), and G28.51.1 (with mutations in both tc0668 and tc0237, a so-called double mutant). The overall genome sequences of these three clones are isogenic to each other. We also used a plasmid-free (Pf) C. muridarum (clone CMUT3 or CMUT3.G5, GenBank accession no. CP006974.1) derived from Nigg3 (11) in the present study. The CMUT3 clone was further used for transformation with pmCherry:CM to create CMpmCherry or pCM installed with a premature termination codon in pgp3 gene to create Pgp3-deficient CM (designated CMpGP3S), as described previously (14, 59). CMUT3 is referred to as plasmid-free (Pf) mutant, while CMpmCherry as wild-type (Wt) chlamydial organisms. All clones were propagated in HeLa cells (human cervical carcinoma epithelial cells, ATCC catalog no. CCL2.1) and purified as elementary bodies (EBs) as reported previously (60). Aliquots of purified EBs were stored at −80°C until use. The storage buffer was SPG (sucrose, phosphate, and glutamate) consisting of 220 mM sucrose, 12.5 mM phosphate, and 4 mM l-glutamic acid (pH 7.5).
Mouse infection and coinfection.
Mouse experiments were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Committee on the Ethics of Laboratory Animal Experiments of the University of Texas Health Science Center at San Antonio.
C. muridarum EBs were used to inoculate 6- to 7-week-old female CBA1/J mice (stock 000656; Jackson Laboratories, Inc., Bar Harbor, ME) intravaginally and/or intragastrically as described previously (30, 39). For intravaginal inoculation, stock EBs diluted in 10 μl of SPG that contains 2 × 105 inclusion forming units (IFU) or the desired IFU as indicated in individual experiments were delivered to ectocervix area using a 20-μl micropipette tip. Five days prior to inoculation, each mouse was injected subcutaneously with 2.5 mg of Depo-Provera (Pharmacia Upjohn, Kalamazoo, MI) suspended in sterile phosphate-buffered saline. For intragastric inoculation, EBs diluted in 100 μl of SPG that contains 2 × 105 IFU or the desired number of IFU, as indicated in individual experiments, were delivered to the stomach using a straight-balled end needle designed for mouse oral gavage (N-PK 020; Braintree Scientific, Inc., Braintree, MA). In some experiments, the same mice were coinoculated intragastrically with Wt C. muridarum (CMpmCHerry) 7 days after the intravaginal inoculation with Pf C. muridarum (CMUT3.G5). The 7-day delay of coinoculation protocol was established based on temporal titration in pilot experiments to achieve maximal exacerbation of hydrosalpinx without affecting the infectivity of genital C. muridarum. This was because an advanced or simultaneous coinoculation with Chlamydia in the GI tract could induce protective immunity against subsequent chlamydial infection in the genital tract, as described previously (37). After the initial inoculation, both vaginal and rectal swabs were taken periodically or organs/tissues were harvested (after mice were sacrificed) for titrating viable organisms as described previously (30, 32). On day 56 after intravaginal inoculation, all mice were sacrificed to determine genital tract pathology, as described below.
Titrating live chlamydial organisms recovered from swabs and tissue homogenates.
To quantitate live chlamydial organisms in vaginal or rectal swabs, each swab was soaked in 0.5 ml of SPG and vortexed with glass beads, and the chlamydial organisms released into the supernatants were titrated on HeLa cell monolayers in duplicate. The infected cultures were processed for immunofluorescence assay as described previously (45) and below. Inclusions were counted in five random fields per coverslip under a fluorescence microscope. For coverslips with <1 IFU per field, entire coverslips were counted. Coverslips showing obvious cytotoxicity of HeLa cells were excluded. The total number of IFU per swab was calculated based on the mean IFU per view, the ratio of the view area to that of the well, dilution factor, and inoculation volumes. Where possible, a mean IFU/swab was derived from the serially diluted and duplicate samples for any given swab. The total number of IFU/swab was converted into log10, which was used to calculate the means and standard deviations across mice from the same group at each time point.
For quantitating live organisms from mouse organs and tissue segments, each organ or tissue segment was transferred to a tube containing 0.5 to 5 ml of SPG depending on the sizes of the organs. Each GI tract was cut into seven segments/portions, including the stomach, duodenum, jejunum, ileum, cecum, colon, and anorectum (rectum), while each genital tract was divided into three segments, including the vagina/cervix (abbreviated as V), uterine horn (UH), and oviduct and ovary (O). The organs and tissue segments were homogenized in cold SPG using a 2-ml tissue grinder (K885300-0002; Fisher Scientific, Pittsburg, PA) or an automatic homogenizer (Omni Tissue Homogenizer, TH115; Omni International, Kennesaw, GA). The homogenates were briefly sonicated and spun at 3,000 rpm for 5 min to pellet the remaining large debris. The supernatants were titrated for live C. muridarum organisms on HeLa cells as described above. The results were expressed as log10 IFU per organ or tissue segment.
Immunofluorescence assay.
An immunofluorescence assay used for titration of live organisms was carried out as described previously (61). A rabbit antibody (designated R1604, raised with purified C. muridarum EBs) was used as a primary antibody to label all C. muridarum in HeLa cells and was visualized with a goat anti-rabbit IgG conjugated with Cy2 (green, catalog no. 111-225-144; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). The DNA dye Hoechst 3328 (blue; Sigma-Aldrich, St. Louis, MO) was used to visualize nuclei. The doubly labeled samples were used for counting for C. muridarum under a fluorescence microscope (AX70; Olympus) equipped with a charge-coupled device camera (Hamamatsu). For samples containing two different types of C. muridarum, such as Pf C. muridarum and Wt C. muridarum that expresses mCherry, we used a rat anti-mCherry monoclonal antibody (M11217; Thermo Fisher Scientific), together with R1604, as the primary antibodies to differentiate them. The mCherry-expressing wild-type C. muridarum was visualized with goat anti-rat IgG conjugated with Cy3 (red; Jackson Immuno Research Laboratories). Wild-type C. muridarum was labeled with both green and red, while plasmid-free C. muridarum was labeled with green only. The distinct inclusions were counted separately using AX70 for calculating IFU recoveries.
qPCR for quantitating C. muridarum in terms of the mCherry gene and the chlamydial 16S RNA gene.
To quantitate C. muridarum with or without mCherry gene, a portion of each tissue homogenate was transferred to the lysis buffer provided with a Quick-gDNA miniPrep kit (11-317C; Genesee Scientific, San Diego, CA) and subjected to DNA extraction according to the manufacturer’s instructions. Each DNA preparation was eluted in 100 μl of elution buffer, and 2 μl was used for quantitative PCR (qPCR). The primers used for quantitating Chlamydia 16S rRNA gene were as follows: forward primer (5′-CGCCTGAGGAGTACACTCGC-3AGGA), reverse primer (5′-CCAACACCTCACGGCACGAG-3′), and double-quenched probe (5′-CACAAGCAGTGGAGCATGTGGTTTAA-3′) (Integrated DNA Technologies, Coralville, IA). The primers used for quantitating mCherry gene were as follows: forward primer (5′-AAGGCTGAAGCTGAAGGAC-3′), reverse primer (5′-GATGGTGT AGTCCTCGTTGTG-3′), and double-quenched probe (5′-CCAACTTGA/ZEN/TGTTGACGTTGTAGGCG-3′) (Integrated DNA Technologies). PCR was carried out in a total volume of 10 μl in a CFX96 Touch Deep Well real-time PCR detection system with iQ Supermix real-time PCR reagent (Bio-Rad, Hercules, CA). The qPCR conditions included an initial denaturation step at 95°C for 3 min, followed by 40 cycles of amplification at 95°C for 15 s and 60°C for 1 min. Gene copy numbers for a given sample in triplicate were calculated based on a standard plasmid DNA preparation in the corresponding samples and are expressed as log10 copies per sample.
Evaluating genital tract pathology macroscopically and microscopically.
On day 56 after intravaginal infection, mice were euthanized for evaluating genital tract pathology. The focus was on the upper genital tract hydrosalpinx. Before the tissues were removed, an in situ gross examination was performed for evidence of oviduct hydrosalpinx or any other related abnormalities of oviducts. The severity of oviduct hydrosalpinx was scored based on the following criteria: no hydrosalpinx (score 0), hydrosalpinx detectable only under microscopic examination (score 1), hydrosalpinx clearly visible with naked eyes but the size was smaller than the ovary on the same side (score 2), equal to the ovary on the same side (score 3), or larger than the ovary on the same side (score 4). The excised tissues, after photographing, were fixed in 10% neutral formalin, embedded in paraffin and serially sectioned longitudinally (with 5 μm/each section). Efforts were made to include the cervix, both uterine horns, and the oviducts, as well as lumenal structures of each tissue in each section. The sections were stained with hematoxylin and eosin (H&E) as described elsewhere (45). The H&E-stained sections were assessed by a pathologist blinded to mouse treatment and scored for severity of inflammation and pathologies based on modified schemes established previously (15). The oviducts were scored for both lumenal dilation (0, no significant dilatation; 1, mild dilation of a single cross section; 2, one to three dilated cross sections; 3, more than three dilated cross sections; and 4, confluent pronounced dilation) and inflammatory cell infiltration (0, no significant infiltration; 1, infiltration at a single focus; 2, infiltration at two to four foci; 3, infiltration at more than four foci; and 4, confluent infiltration). Scores assigned to individual mice were calculated as means ± the standard errors for each group of animals.
Statistical analyses.
All data, including the time courses of live organism shedding (IFU), genome copies, and pathology scores, were compared using the area under the curve between two groups using Wilcoxon rank sum test (an in-house Excel sheet), while category data, including the numbers of of mice positive for live organism shedding or hydrosalpinx, were analyzed by using the Fisher exact test (http://vassarstats.net/tab2x2.html). Correlations of chlamydial pathogenicity in the upper genital tract with chlamydial colonization in different tissues were analyzed by calculating Spearman’s rank correlation coefficients and Pearson correlation coefficients.
ACKNOWLEDGMENTS
This study was supported in part by U.S. National Institutes of Health grants R01AI047997 and R01AI121989 to G.Z.
REFERENCES
- 1.Centers for Disease Control and Prevention. 2017. Sexually transmitted disease surveillance, 2016. Centers for Disease Control and Prevention, Atlanta, GA: https://www.cdc.gov/std/stats16/default.htm. [Google Scholar]
- 2.Budrys NM, Gong S, Rodgers AK, Wang J, Louden C, Shain R, Schenken RS, Zhong G. 2012. Chlamydia trachomatis antigens recognized in women with tubal factor infertility, normal fertility, and acute infection. Obstet Gynecol 119:1009–1016. doi: 10.1097/AOG.0b013e3182519326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodgers AK, Budrys NM, Gong S, Wang J, Holden A, Schenken RS, Zhong G. 2011. Genome-wide identification of Chlamydia trachomatis antigens associated with tubal factor infertility. Fertil Steril 96:715–721. doi: 10.1016/j.fertnstert.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodgers AK, Wang J, Zhang Y, Holden A, Berryhill B, Budrys NM, Schenken RS, Zhong G. 2010. Association of tubal factor infertility with elevated antibodies to Chlamydia trachomatis caseinolytic protease P Am J Obstet Gynecol 203:494.e7–494.e14. doi: 10.1016/j.ajog.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de la Maza LM, Pal S, Khamesipour A, Peterson EM. 1994. Intravaginal inoculation of mice with the Chlamydia trachomatis mouse pneumonitis biovar results in infertility. Infect Immun 62:2094–2097. doi: 10.1128/IAI.62.5.2094-2097.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison RP, Feilzer K, Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 63:4661–4668. doi: 10.1128/IAI.63.12.4661-4668.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shah AA, Schripsema JH, Imtiaz MT, Sigar IM, Kasimos J, Matos PG, Inouye S, Ramsey KH. 2005. Histopathologic changes related to fibrotic oviduct occlusion after genital tract infection of mice with Chlamydia muridarum. Sex Transm Dis 32:49–56. doi: 10.1097/01.olq.0000148299.14513.11. [DOI] [PubMed] [Google Scholar]
- 8.Murthy AK, Li W, Guentzel MN, Zhong G, Arulanandam BP. 2011. Vaccination with the defined chlamydial secreted protein CPAF induces robust protection against female infertility following repeated genital chlamydial challenge. Vaccine 29:2519–2522. doi: 10.1016/j.vaccine.2011.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Zhang H, Zhou Z, Yang Z, Ding Y, Zhou Z, Zhong E, Arulanandam B, Baseman J, Zhong G. 2014. Chlamydial induction of hydrosalpinx in 11 strains of mice reveals multiple host mechanisms for preventing upper genital tract pathology. PLoS One 9:e95076. doi: 10.1371/journal.pone.0095076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Zhou Z, Chen J, Wu G, Yang Z, Zhou Z, Baseman J, Zhang J, Reddick RL, Zhong G. 2014. Lack of long lasting hydrosalpinx in A/J mice correlates with rapid but transient chlamydial ascension and neutrophil recruitment in the oviduct following intravaginal inoculation with Chlamydia muridarum. Infect Immun 82:2688–2696. doi: 10.1128/IAI.00055-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei L, Chen J, Hou S, Ding Y, Yang Z, Zeng H, Baseman J, Zhong G. 2014. Reduced live organism recovery and lack of hydrosalpinx in mice infected with plasmid-free Chlamydia muridarum. Infect Immun 82:983–992. doi: 10.1128/IAI.01543-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connell CM, Ingalls RR, Andrews CW, Scurlock AM, Darville T. 2007. Plasmid-deficient Chlamydia muridarum fail to induce immune pathology and protect against oviduct disease. J Immunol 179:4027–4034. doi: 10.4049/jimmunol.179.6.4027. [DOI] [PubMed] [Google Scholar]
- 13.Zhong G. 2017. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol 25:141–152. doi: 10.1016/j.tim.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Huang Y, Yang Z, Sun Y, Gong S, Hou S, Chen C, Li Z, Liu Q, Wu Y, Baseman J, Zhong G. 2014. Plasmid-encoded Pgp3 is a major virulence factor for Chlamydia muridarum to induce hydrosalpinx in mice. Infect Immun 82:5327–5335. doi: 10.1128/IAI.02576-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Conrad TA, Gong S, Yang Z, Matulich P, Keck J, Beltrami N, Chen C, Zhou Z, Dai J, Zhong G. 2016. The chromosome-encoded hypothetical protein TC0668 is an upper genital tract pathogenicity factor of Chlamydia muridarum. Infect Immun 84:467–479. doi: 10.1128/IAI.01171-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Zhou Z, Conrad T, Yang Z, Dai J, Li Z, Wu Y, Zhong G. 2015. In vitro passage selects for Chlamydia muridarum with enhanced infectivity in cultured cells but attenuated pathogenicity in mouse upper genital tract. Infect Immun 83:1881–1892. doi: 10.1128/IAI.03158-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang Z, Conrad T, Zhou Z, Chen J, Dutow P, Klos A, Zhong G. 2014. Complement factor C5 but not C3 contributes significantly to hydrosalpinx development in mice infected with Chlamydia muridarum. Infect Immun 82:3154–3163. doi: 10.1128/IAI.01833-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Tian Q, Wang L, Xue M, Zhong G. 2017. IL-6-mediated signaling pathways limit Chlamydia muridarum infection and exacerbate its pathogenicity in the mouse genital tract. Microbes Infect 19:536–545. doi: 10.1016/j.micinf.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cheng W, Shivshankar P, Li Z, Chen L, Yeh IT, Zhong G. 2008. Caspase-1 contributes to Chlamydia trachomatis-induced upper urogenital tract inflammatory pathologies without affecting the course of infection. Infect Immun 76:515–522. doi: 10.1128/IAI.01064-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Igietseme JU, Omosun Y, Partin J, Goldstein J, He Q, Joseph K, Ellerson D, Ansari U, Eko FO, Bandea C, Zhong G, Black CM. 2013. Prevention of Chlamydia-induced infertility by inhibition of local caspase activity. J Infect Dis 207:1095–1104. doi: 10.1093/infdis/jit009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy AK, Li W, Chaganty BK, Kamalakaran S, Guentzel MN, Seshu J, Forsthuber TG, Zhong G, Arulanandam BP. 2011. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect Immun 79:2928–2935. doi: 10.1128/IAI.05022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlcek KR, Li W, Manam S, Zanotti B, Nicholson BJ, Ramsey KH, Murthy AK. 2016. The contribution of Chlamydia-specific CD8+ T cells to upper genital tract pathology. Immunol Cell Biol 94:208–212. doi: 10.1038/icb.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morrison RP, Caldwell HD. 2002. Immunity to murine chlamydial genital infection. Infect Immun 70:2741–2751. doi: 10.1128/iai.70.6.2741-2751.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig AP, Kong FY, Yeruva L, Hocking JS, Rank RG, Wilson DP, Donovan B. 2015. Is it time to switch to doxycycline from azithromycin for treating genital chlamydial infections in women? Modeling the impact of autoinoculation from the gastrointestinal tract to the genital tract. BMC Infect Dis 15:200. doi: 10.1186/s12879-015-0939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peters RPH, Dubbink JH, van der Eem L, Verweij SP, Bos MLA, Ouburg S, Lewis DA, Struthers H, McIntyre JA, Morré SA. 2014. Cross-sectional study of genital, rectal, and pharyngeal chlamydia and gonorrhea in women in rural South Africa. Sex Transm Dis 41:564–569. doi: 10.1097/OLQ.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 26.Gratrix J, Singh AE, Bergman J, Egan C, Plitt SS, McGinnis J, Bell CA, Drews SJ, Read R. 2015. Evidence for increased Chlamydia case finding after the introduction of rectal screening among women attending 2 Canadian sexually transmitted infection clinics. Clin Infect Dis 60:398–404. doi: 10.1093/cid/ciu831. [DOI] [PubMed] [Google Scholar]
- 27.Musil K, Currie M, Sherley M, Martin S. 2016. Rectal chlamydia infection in women at high risk of chlamydia attending Canberra Sexual Health Centre. Int J STD AIDS 27:526–530. doi: 10.1177/0956462415586317. [DOI] [PubMed] [Google Scholar]
- 28.Gratrix J, Singh AE, Bergman J, Egan C, McGinnis J, Drews SJ, Read R. 2014. Prevalence and characteristics of rectal chlamydia and gonorrhea cases among men who have sex with men after the introduction of nucleic acid amplification test screening at 2 Canadian sexually transmitted infection clinics. Sex Transm Dis 41:589–591. doi: 10.1097/OLQ.0000000000000176. [DOI] [PubMed] [Google Scholar]
- 29.Campbell J, Huang Y, Liu Y, Schenken R, Arulanandam B, Zhong G. 2014. Bioluminescence imaging of Chlamydia muridarum ascending infection in mice. PLoS One 9:e101634. doi: 10.1371/journal.pone.0101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeruva L, Spencer N, Bowlin AK, Wang Y, Rank RG. 2013. Chlamydial infection of the gastrointestinal tract: a reservoir for persistent infection. Pathog Dis 68:88–95. doi: 10.1111/2049-632X.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai J, Zhang T, Wang L, Shao L, Zhu C, Zhang Y, Failor C, Schenken R, Baseman J, He C, Zhong G. 2016. Intravenous inoculation with Chlamydia muridarum leads to a long-lasting infection restricted to the gastrointestinal tract. Infect Immun 84:2382–2388. doi: 10.1128/IAI.00432-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perry LL, Hughes S. 1999. Chlamydial colonization of multiple mucosae following infection by any mucosal route. Infect Immun 67:3686–3689. doi: 10.1128/IAI.67.7.3686-3689.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, Zhang Q, Zhang T, Zhang Y, Zhu C, Sun X, Zhang N, Xue M, Zhong G. 2016. The Chlamydia muridarum organisms fail to autoinoculate the mouse genital tract after colonization in the gastrointestinal tract for 70 days. PLoS One 11:e0155880. doi: 10.1371/journal.pone.0155880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dan M, Tyrrell LD, Goldsand G. 1987. Isolation of Chlamydia trachomatis from the liver of a patient with prolonged fever. Gut 28:1514–1516. doi: 10.1136/gut.28.11.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carter JD, Hudson AP. 2017. Recent advances and future directions in understanding and treating Chlamydia-induced reactive arthritis. Expert Rev Clin Immunol 13:197–206. doi: 10.1080/1744666X.2017.1233816. [DOI] [PubMed] [Google Scholar]
- 37.Wang L, Zhu C, Zhang T, Tian Q, Zhang N, Morrison S, Morrison R, Xue M, Zhong G. 2018. Nonpathogenic colonization with chlamydia in the gastrointestinal tract as oral vaccination for inducing transmucosal protection. Infect Immun 86:e00630-17. doi: 10.1128/IAI.00630-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rank RG, Yeruva L. 2014. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 82:1362–1371. doi: 10.1128/IAI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shao L, Zhang T, Liu Q, Wang J, Zhong G. 2017. Chlamydia muridarum with mutations in chromosomal genes tc0237 and/or tc0668 is deficient in colonizing the mouse gastrointestinal tract. Infect Immun 85:e00321-17. doi: 10.1128/IAI.00321-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao L, Melero J, Zhang N, Arulanandam B, Baseman J, Liu Q, Zhong G. 2017. The cryptic plasmid is more important for Chlamydia muridarum to colonize the mouse gastrointestinal tract than to infect the genital tract. PLoS One 12:e0177691. doi: 10.1371/journal.pone.0177691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shao L, Zhang T, Melero J, Huang Y, Liu Y, Liu Q, He C, Nelson DE, Zhong G. 2018. The genital tract virulence factor pGP3 is essential for Chlamydia muridarum colonization in the gastrointestinal tract. Infect Immun 86:e00429-17. doi: 10.1128/IAI.00429-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y, Zhang Q, Yang Z, Conrad T, Liu Y, Zhong G. 2015. Plasmid-encoded Pgp5 is a significant contributor to Chlamydia muridarum induction of hydrosalpinx. PLoS One 10:e0124840. doi: 10.1371/journal.pone.0124840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong G. 2018. Chlamydia spreading from the genital tract to the gastrointestinal tract: a two-hit hypothesis. Trends Microbiol 26:611–623. doi: 10.1016/j.tim.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang L, Yang Z, Zhang H, Zhou Z, Arulanandam B, Baseman J, Zhong G. 2014. Induction of protective immunity against Chlamydia muridarum intracervical infection in DBA/1j mice. Vaccine 32:1407–1413. doi: 10.1016/j.vaccine.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tang L, Zhang H, Lei L, Gong S, Zhou Z, Baseman J, Zhong G. 2013. Oviduct infection and hydrosalpinx in DBA1/j mice is induced by intracervical but not intravaginal inoculation with Chlamydia muridarum. PLoS One 8:e71649. doi: 10.1371/journal.pone.0071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueha S, Shand FH, Matsushima K. 2012. Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol 3:71. doi: 10.3389/fimmu.2012.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. 2015. Vaccines: a mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manam S, Nicholson BJ, Murthy AK. 2013. OT-1 mice display minimal upper genital tract pathology following primary intravaginal Chlamydia muridarum infection. Pathog Dis 67:221–224. doi: 10.1111/2049-632X.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong X, Liu Y, Chang X, Lei L, Zhong G. 2014. Signaling via tumor necrosis factor receptor 1 but not Toll-like receptor 2 contributes significantly to hydrosalpinx development following Chlamydia muridarum infection. Infect Immun 82:1833–1839. doi: 10.1128/IAI.01668-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Asquith KL, Horvat JC, Kaiko GE, Carey AJ, Beagley KW, Hansbro PM, Foster PS. 2011. Interleukin-13 promotes susceptibility to chlamydial infection of the respiratory and genital tracts. PLoS Pathog 7:e1001339. doi: 10.1371/journal.ppat.1001339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson RM, Kerr MS, Slaven JE. 2014. An atypical CD8 T-cell response to Chlamydia muridarum genital tract infections includes T cells that produce interleukin-13. Immunology 142:248–257. doi: 10.1111/imm.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brodeur TY, Robidoux TE, Weinstein JS, Craft J, Swain SL, Marshak-Rothstein A. 2015. IL-21 promotes pulmonary fibrosis through the induction of profibrotic CD8+ T cells. J Immunol 195:5251–5260. doi: 10.4049/jimmunol.1500777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long X, Chen Q, Zhao J, Rafaels N, Mathias P, Liang H, Potee J, Campbell M, Zhang B, Gao L, Georas SN, Vercelli D, Beaty TH, Ruczinski I, Mathias R, Barnes KC, Chen X. 2015. An IL-13 promoter polymorphism associated with liver fibrosis in patients with Schistosoma japonicum. PLoS One 10:e0135360. doi: 10.1371/journal.pone.0135360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fuschiotti P, Larregina AT, Ho J, Feghali-Bostwick C, Medsger TA Jr. 2013. Interleukin-13-producing CD8+ T cells mediate dermal fibrosis in patients with systemic sclerosis. Arthritis Rheum 65:236–246. doi: 10.1002/art.37706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Metwali A, Setiawan T, Blum AM, Urban J, Elliott DE, Hang L, Weinstock JV. 2006. Induction of CD8+ regulatory T cells in the intestine by Heligmosomoides polygyrus infection. Am J Physiol Gastrointest Liver Physiol 291:G253–G259. doi: 10.1152/ajpgi.00409.2005. [DOI] [PubMed] [Google Scholar]
- 56.Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, Miller HR, Cua DJ, Goldschmidt M, Hunter CA, Kastelein RA, Artis D. 2006. Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 203:843–849. doi: 10.1084/jem.20051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koch KN, Muller A. 2015. Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory T-cells, establish persistent infection and promote tolerance to allergens. Gut Microbes 6:382–387. doi: 10.1080/19490976.2015.1105427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belland RJ, Scidmore MA, Crane DD, Hogan DM, Whitmire W, McClarty G, Caldwell HD. 2001. Chlamydia trachomatis cytotoxicity associated with complete and partial cytotoxin genes. Proc Natl Acad Sci U S A 98:13984–13989. doi: 10.1073/pnas.241377698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y, Chen C, Gong S, Hou S, Qi M, Liu Q, Baseman J, Zhong G. 2014. Transformation of Chlamydia muridarum reveals a role for Pgp5 in suppression of plasmid-dependent gene expression. J Bacteriol 196:989–998. doi: 10.1128/JB.01161-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mukhopadhyay S, Clark AP, Sullivan ED, Miller RD, Summersgill JT. 2004. Detailed protocol for purification of Chlamydia pneumoniae elementary bodies. J Clin Microbiol 42:3288–3290. doi: 10.1128/JCM.42.7.3288-3290.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fan T, Lu H, Hu H, Shi L, McClarty GA, Nance DM, Greenberg AH, Zhong G. 1998. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med 187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]


