Bacterial vaginosis (BV), a disorder of the female reproductive tract (FRT) in which a healthy Lactobacillus-dominant microflora is replaced by BV-associated bacteria (BVAB), can significantly increase the incidence of human immunodeficiency virus (HIV) acquisition. Discerning the effect of BV on the mucosal epithelium of the FRT may yield novel preventatives and therapeutics for HIV infection. Here, we investigated barrier dysfunction of the endocervix by host-derived factors, secreted in response to BV, as a potential cause of HIV infection.
KEYWORDS: bacterial vaginosis, female reproductive tract, human immunodeficiency virus, matrix metalloproteinases, mucosal immunity
ABSTRACT
Bacterial vaginosis (BV), a disorder of the female reproductive tract (FRT) in which a healthy Lactobacillus-dominant microflora is replaced by BV-associated bacteria (BVAB), can significantly increase the incidence of human immunodeficiency virus (HIV) acquisition. Discerning the effect of BV on the mucosal epithelium of the FRT may yield novel preventatives and therapeutics for HIV infection. Here, we investigated barrier dysfunction of the endocervix by host-derived factors, secreted in response to BV, as a potential cause of HIV infection. Using a polarized endocervical cell culture system, we determined that conditioned media (CM) from endocervical cells cocultured with BVAB (endocervical+BVAB CM), as well as cervicovaginal fluid (CVF) from women with BV, disrupted epithelial polarization. We assessed host matrix metalloproteinases (MMPs) as the BV-associated secreted factors which disrupt the endocervical epithelium. MMPs were overexpressed in endocervical+BVAB CM and CVF from women with BV and were capable of disrupting endocervical epithelial polarization. When we cocultured polarized endocervical cells with HIV-1-infected lymphocyte-derived cells, we discovered endocervical+BVAB CM and MMPs significantly increased the transmigration of virus through the epithelium, and treatment with an MMP inhibitor decreased these effects. When we examined the effect of CVF on HIV-1 transmigration through endocervical epithelium, we demonstrated that CVF samples with greater concentrations of BV-associated MMPs increased viral transmigration. Our results suggest MMPs increase HIV-1 infection by disrupting the endocervical epithelium, permitting transmigration of virus through the epithelium to infect underlying target cells.
INTRODUCTION
Bacterial vaginosis (BV) is a microbial disorder characterized by a shift in female reproductive tract (FRT) microflora from one dominated by Lactobacillus species to a diverse microflora with an increased proportion of anaerobes. BV is one of the most commonly diagnosed gynecological disorders among reproductive-aged women (1). Beyond excessive vaginal discharge, BV is asymptomatic in ∼50% of women (2). BV increases the risk of preterm birth, pelvic inflammatory disease, and sexually transmitted infections. Of significance, BV increases the risk of male-to-female transmission of human immunodeficiency virus (HIV) by approximately 60% (3). The cause of increased HIV infection due to BV is still incompletely understood. Elucidating these mechanisms may lead to novel treatments and preventatives for HIV infection in this vulnerable population.
Multiple BV-associated processes are hypothesized to increase HIV infection. BV is associated with increased inflammatory cytokines and chemokines, including tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and IL-8 (4, 5). This low-grade inflammation may attract CD4+ lymphocytes, the primary target cells of HIV, to the mucosa where they can be infected. In addition, BV is associated with decreased innate immune factors, such as defensins and other cationic antimicrobial (poly)peptides, which may increase the amount of viable HIV-infected cells or intact virions able to penetrate the FRT mucosa (6). However, the HIV target cells in the FRT mucosa, mostly CD4+ T cells, and dendritic and Langerhans cells usually reside below the epithelium of the FRT, in the stroma, or are imbedded in the tissue, and so virus must pass through the protective epithelium for infection. A healthy intact FRT mucosa blocks most transmissions, with infection rates in a healthy FRT as low as 0.01% per exposure (7).
Expectedly, physical damage to the epithelial barrier of the FRT is also associated with increased risks of HIV infection. This can include ulcers caused by other sexually transmitted infections (STIs), microabrasions from sexual intercourse, or damage due to vaginal medications (8, 9). For instance, nonoxynol, a topical antimicrobial with in vitro activity against HIV, increased rates of HIV infection due to toxicity in the FRT mucosa (10).
Susceptibility to BV and HIV infection in the FRT can also differ based on tissue location. The lower FRT includes the vaginal wall and ectocervix, which has a thick squamous epithelium, while the upper FRT consists of the endocervix and endometrium, which have a simple columnar epithelium. Our group has previously reported endocervical cells as most responsive to bacterial vaginosis-associated bacteria (BVAB) compared to other epithelial cell types in the FRT (11). This reactivity to BVAB combined with the monolayer epithelium of the endocervix makes it a particularly vulnerable area for HIV transmission (8, 12, 13).
The columnar epithelium of the endocervix is composed of tight junctions (TJs) consisting of occludin, claudin-1, and cadherin, which are assembled into the actin network of the cell (14). In contrast, the lower FRT has intermittent adherens junctions (AJs) in the basal layers of cells and relies on the thick cell layer of stratified apical cells to block migration of pathogens in the upper cell layers (14). The regulation of mucosal epithelial TJs can be altered by a host of factors, including hormones, inflammatory mediators, and pathogens (15, 16). Decreased polarization of the endocervical and ectocervical epithelium caused by an inflammatory response is implemented in preterm birth (17). Some pathogens also target mucosal barriers directly for their entry through the epithelium. For example, Neisseria gonorrhoeae disassembles apical AJs and induces endocervical cell shedding to infect the endocervix (18, 19). Exposure to HIV and HIV envelope proteins has been found to destabilize TJs in FRT epithelial cells in vitro (20).
Dysregulation of proteases has also been implicated in mucosal barrier dysfunction. These enzymes, usually involved in tissue repair and growth, have been known to cause epithelial damage when dysregulated in the gut and lung mucosa (21, 22). However, their influence in the FRT mucosa is less defined. In the FRT, increased expression of proteases is associated with the luteal phase of the FRT menstrual cycle, considered the most vulnerable phase for HIV infection, while protease expression is decreased and antiprotease expression increased during the follicular stage, the stage considered most protective (23). Altered gene expression of barrier function and protease genes has been reported in the cervicovaginal fluid (CVF) from women with BV-associated microbiomes compared to that from women with healthy microbiomes (24). Toward this, women frequently exposed to HIV who remain uninfected have increased concentrations of the antiproteases elafin, trappin-2, serpin, and cystatin (25, 26). Of particular interest, matrix metalloproteinases (MMPs), a class of nonspecific proteinases implicated in growth and wound healing, are overexpressed in inflammatory responses and cleave a wide range of extracellular substrates, including TJ proteins (27, 28). MMP7 cleaves the TJ protein occludin in response to estrogen in the female reproductive tract (29). Overexpression of MMP8 is associated with preterm birth and BV (30–32).
Understanding how BV affects the mucosal epithelium may elucidate potential therapeutics and preventatives for HIV. However, work in this area is hindered by the lack of an accepted animal model for BV (33, 34). In this paper, we examine how factors secreted in response to BVAB disrupt epithelial integrity by using a polarized endocervical epithelial cell culture system (35). We explore the potential of MMPs as key secreted proteases which disturb the mucosa, allowing for increased transmission of HIV. We found that MMP concentrations increased both in conditioned media (CM) from endocervical cells cocultured with BVAB (endocervical+BVAB CM) and in human CVF obtained from women with BV. We also determined that CVF from women with BV, endocervical+BVAB CM, and MMPs alone damage the integrity of polarized endocervical cells. When cocultured with HIV-1-infected lymphocytes, endocervical+BVAB CM and MMPs increased transmigration of virus across the endocervical epithelium. Our work suggests that proteases secreted in response to BV increase HIV infection by disrupting TJs of the endocervical monolayer.
RESULTS
Factors secreted in response to BV disrupt polarization of endocervical epithelium.
In this study, we hypothesized that barrier dysfunction of the FRT mucosa increases HIV-1 infection in women with BV. We focused on the endocervix, which has a cell monolayer anatomy particularly susceptible to physical damage. To investigate the endocervical mucosa, we used the immortalized endocervix-derived cell line A2EN, which forms a polarized cell layer, complete with TJs, when grown at the air-liquid interface (ALI) on collagen-coated transwell inserts (35). To produce endocervical+BVAB conditioned media (CM) for treatment of polarized A2ENs, we cultured an immortalized, immunologically responsive endocervical cell line, End1 (36), with the BVAB Atopobium vaginae at the physiologically relevant multiplicity of infection (MOI) of 3 (End1+A. vaginae CM) (37–39). A. vaginae is a bacterium generally only found in women with BV, and we have independently observed that this bacterium induced a robust immune response from FRT cells (11, 40, 41). End1+A. vaginae CM and End1+mock (no bacteria) CM were concentrated with a vacuum centrifuge and desalted using a 3-kDa centrifugal filter. It should be noted that in preliminary experiments, CM derived from bacterium-exposed End1 and A2EN cells exhibited similar fold inductions of inflammatory biomarkers compared to the baseline. End1-derived CM was utilized for the presented experiments because it contained higher concentrations of most proteins that were measured and higher MMP activity (presented below in Fig. 4A).
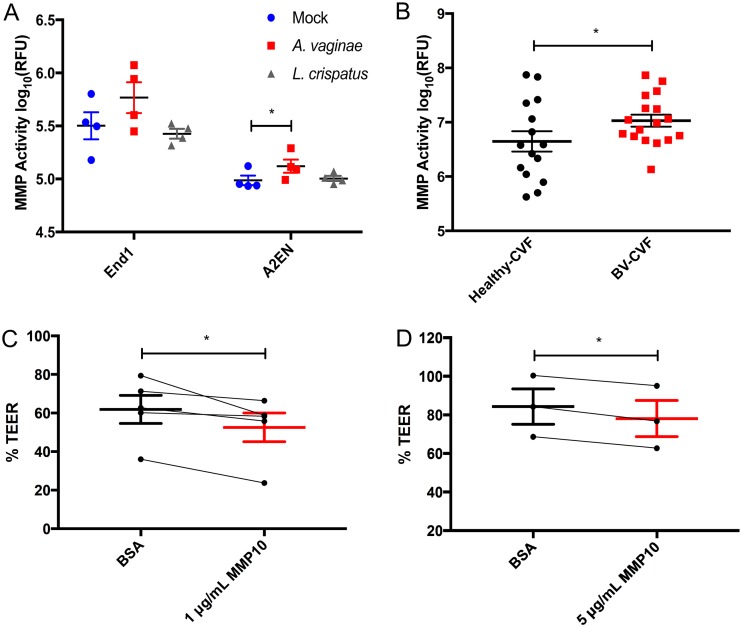
FIG 4.
BV-induced MMPs are enzymatically active and damage endocervical mucosa. MMP enzymatic activity, presented as log10 relative fluorescence units (RFU), of four separate batches of CM collected from mock- or bacterium-treated End1 or A2EN cells (A) or healthy CVF samples versus BV CVF samples (B). Polarized A2EN cells were treated topically with 1 μg/ml (C) or 5 μg/ml (D) MMP10 or a matched concentration of BSA (control) for 72 h. Data are presented as %TEER (TEER72 h/TEER0 h) for 4 independent experiments (C) and 3 independent experiments (D), all performed in duplicates. Connecting lines depict experimental pairs. *, P < 0.05 for two-tailed paired Student’s t tests (A, C, and D) and one-tailed unpaired t test (B). Error bars represent SEMs.
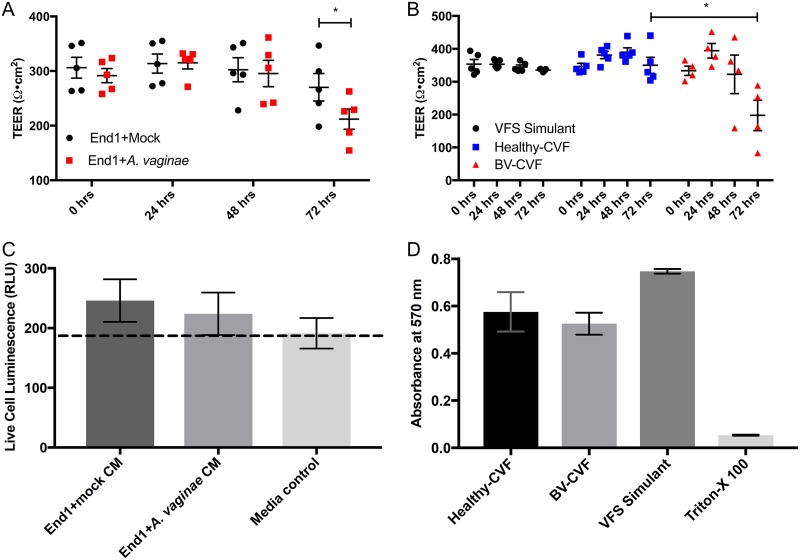
A2EN cells were treated apically with 4× concentrated End1+A. vaginae CM or End1+mock CM to determine if the cellular response to BV decreases epithelial resistance. The 4× concentration of CM was determined previously as the lowest concentration which was both active and noncytotoxic (42). Transepithelial electrical resistance (TEER) was measured using a calibrated ohmmeter before treatment and every 24 h for 72 h. We determined that soluble factors secreted by endocervical cells in response to BVAB were able to disrupt the endocervical epithelium. At 72 h, TEER of End1+A. vaginae CM-treated A2EN epithelium was significantly lower than under the End1+mock CM condition (P < 0.05) (Fig. 1A). The A. vaginae (no End1 cells) CM control caused no disturbance in TEER (see Fig. S1A in the supplemental material), further suggesting that the secreted factors from the endocervical cells, not factors from the bacteria, caused the disruption.
FIG 1.
Factors secreted in response to BV bacteria disrupt polarization of endocervical epithelium. A2EN cells grown at the air-liquid interface on collagen-coated transwells develop a transepithelial electrical resistance (TEER) of ∼300 Ω·cm2 when polarized. TEER was measured before treatment and then every 24 h for 3 days. (A) Polarized A2EN cells were treated with conditioned media (CM) from the endocervical End1 cells that were mock treated (End1+mock) or cocultured with A. vaginae (End1+A. vaginae). n = 5 experiments, each performed in triplicates; *, P < 0.05 by paired two-tailed Student's t test. (B) Polarized A2EN cells were treated for 72 h with a vaginal fluid simulant (VFS) (control) or clarified CVF from women diagnosed with BV (BV-CVF) or without (healthy-CVF). Each data point represents an individual CVF sample; *, P < 0.05. (C) To test whether CM-mediated reduction in TEER was due to cytotoxic effects, A2EN monolayers were treated with CM or medium control. After 24 h, a luminescence-based CytoTox-Glo assay was performed, and data are represented as live cell luminescence in relative luciferase units (RLU). (D) To confirm CVF was not cytotoxic to A2EN, polarized A2EN cells were treated with healthy CVF, BV CVF, VFS, or VFS containing 0.05% Triton X-100 as a positive control for cell death. After 72 h, an MTT metabolic toxicity assay was performed. Error bars are the standard errors of the means (SEMs). For panels C and D, n = 3, each experiment performed in duplicates.
CVF from women with BV disrupts endocervical epithelium.
As End1+A. vaginae CM disrupted endocervical barrier integrity, we next extended our studies to compare CVF from women with or without BV, to more closely represent the physiological conditions encountered in the FRT mucosa. CVF samples were collected from women diagnosed with BV according to Amsel’s criteria (2) and were sonicated and sedimented by centrifugation to remove mucus and bacteria, as we have reported previously (43). CVF was diluted 1:5 with a vaginal fluid simulant (VFS), which mimics the salt, nutrient, and protein concentrations of CVF (44). A 1:5 dilution of CVF was determined previously to be the highest concentration of CVF which did not disrupt polarized A2ENs. VFS alone was also used as a control for healthy and BV CVF. Polarized A2EN cells were treated with healthy CVF, BV CVF, or VFS for 72 h, and TEER was measured every 24 h. At 48 h, TEER of BV CVF-treated endocervical cells began to decrease and was significantly decreased compared to that of healthy CVF-treated transwells and VFS at 72 h posttreatment (P < 0.05) (Fig. 1B), matching the response observed from cells treated with End1-A. vaginae CM.
BVAB conditioned medium and CVF are not cytotoxic to endocervical cells.
We next confirmed the decrease in TEER observed in response to BV conditions was not due to cytotoxicity of End1+A. vaginae CM or BV CVF. A2EN epithelial cells grown on 96-well tissue culture-treated plates were treated for 24 h with 4× CM collected from BVAB or mock-treated End1, and then a CytoTox-Glo luminescence-based cytotoxicity assay was performed. End1+A. vaginae and End1+mock CM were not cytotoxic compared to the medium control (Fig. 1C). To investigate potential cytotoxicity of human CVF, polarized A2EN cells were treated with healthy or BV CVF for 72 h, and then a 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay was performed to determine cellular metabolic activity. While both CVF groups imparted a modest reduction in cellular dehydrogenase activity compared to that with VFS, there was no significant difference detected between healthy and BV CVF (Fig. 1D).
Matrix metalloproteinases are overexpressed by endocervical epithelial cells in response to BVAB.
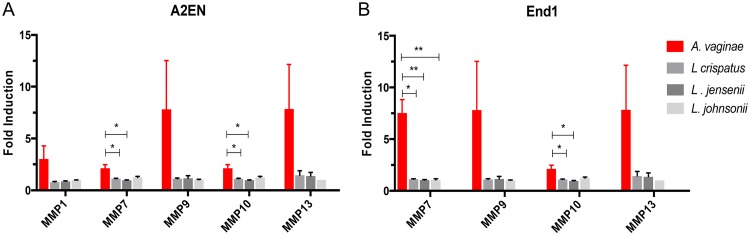
Our initial results suggested host-derived soluble factors secreted in response to BV disrupt the endocervical mucosa. We next hypothesized that MMPs are the potential secreted factor, based on their overexpression under inflammatory conditions and their ability to cleave TJ proteins. To determine if MMPs were expressed in response to BV, endocervical cell lines A2EN and End1 were cocultured with one of several FRT bacterial species at an MOI of 5 or with a medium change only. A. vaginae was used to represent BVAB, and the lactobacillus species L. crispatus, L. jensenii, and L. johnsonii were used to represent bacteria considered part of a healthy FRT microbiome. Supernatants were collected after 24 h and analyzed for expression of MMPs using a multiplex immunoassay for nine MMP isotypes. To compare differences in MMP expression induced by each FRT species, fold induction was calculated by comparison to mock-treated endocervical cells. MMP expression by endocervical epithelium was increased in response to A. vaginae compared to that in Lactobacillus species-treated cells in both cell lines (Fig. 2). Coculture with Lactobacillus spp. caused no significant change in expression of MMPs. MMP10 and MMP7 were significantly induced in A. vaginae-treated endocervical cells compared to that in cells cocultured with Lactobacillus spp. (P < 0.05). Of the eight MMP isotypes assayed, MMP7, -9, -10, and -13 were expressed by both A2EN and End1. MMP1, however, was expressed only in A2EN (Fig. 2). MMP7 and -10 were expressed at the highest concentrations by End1, while MMP10 and MMP13 were the isotypes expressed by A2EN at the highest concentrations.
FIG 2.
Endocervical epithelium overexpresses matrix metalloproteinases in response to BVAB. Endocervical cell lines A2EN (A) and End1 (B) were cocultured with A. vaginae (red bars) or one of three indicated Lactobacillus species (gray bars) representing healthy vaginal flora bacteria at an MOI of 5. After 24 h, conditioned media were collected, and concentrations of MMP isotypes were determined using a multiplex immunoassay (Bio-Rad’s Pro Human MMP panel). Fold induction was determined by comparison to MMP expression in mock-treated cells. Bars and error bars represent the means and SEMs, respectively. n = 4 or 5 for each bacterium tested. *, P < 0.05; **, P < 0.01 by paired two-tailed Student’s t tests.
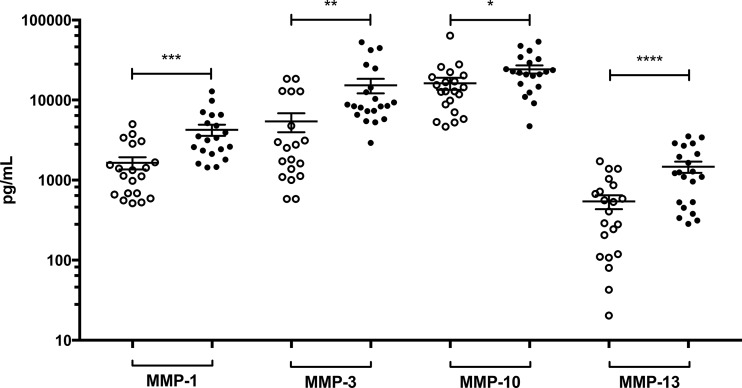
Concentrations of MMPs were increased in CVF from women with BV.
We next analyzed CVF to determine if MMPs were also increased in response to BV. We performed a multiplex immunoassay for MMPs on 21 CVF samples from healthy women and 20 CVF samples from women diagnosed with BV. When each individual MMP isotype was compared separately, the average concentrations of each isotype, MMP1, -2, -3, -7, -9, -10, -12, and -13, were increased in women with BV. The increase in the BV CVF group of isotypes MMP1, -3, -10, and -13 was statistically significant (P < 0.05) (Fig. 3), similar to the expression profile of the endocervical epithelia in Fig. 2. MMP10 and MMP13 were highly BV-inducible isotypes in both CVF- and BVAB-treated endocervical epithelia.
FIG 3.
Matrix metalloproteinases are overexpressed in vaginal fluid of women with BV. Concentrations of different MMP isotypes were evaluated in CVF from women with BV (●) and without BV (○). Each circle represents an individual CVF, and error bars show the SEMs. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 by one-tailed unpaired Student’s t tests.
MMP-specific protease activity was increased in endocervical+BVAB CM and CVF from women with BV.
After confirming an increase in concentration of MMPs under BV conditions, we next investigated if the increase in MMP concentration translated to increased protease activity. MMPs are expressed as an enzymatically inactive pro-MMP and must be processed further to reach the enzymatically active form, and MMP detection by antibody-based methods does not necessarily discriminate between the active and inactive forms. We therefore used an MMP activity assay, which detects MMP activity by fluorescence produced by cleavage of a nonspecific substrate for MMPs.
We first compared MMP activity of CM from mock-, A. vaginae-, or L. crispatus-treated A2EN and End1 cells. Both endocervical cell types displayed the same trend, in which the endocervical+A. vaginae CM exhibited the highest MMP activity compared to those of both endocervical+mock and endocervical+L. crispatus CM. A2EN+A. vaginae CM was significantly more active than A2EN+mock CM (P < 0.05) (Fig. 4A). We next compared MMP activity between healthy and BV CVF using the same technique (Fig. 4B). We measured MMP activity of 17 BV and 15 healthy CVF samples, using recombinant MMP10 activity as a reference (Fig. S1B). BV CVF had significantly higher MMP enzymatic activity than healthy CVF (P < 0.05). Interestingly, the range of activity in the healthy CVF group was quite large compared to that in BV CVF, with a >100-fold difference between the highest and lowest activity samples.
MMP10 disrupts TEER of endocervical epithelia.
MMPs have many diverse functions and substrates (45), and so it is possible they may not act on the endocervical epithelia during the BV state. To determine if MMPs could directly disrupt the endocervical epithelia, we treated polarized endocervical cells with recombinant MMP10 and MMP13. MMP10 was selected due to its high concentration in both endocervical culture and CVF. MMP13 was also selected, as its expression in endocervical culture was highly induced by BVAB and was significantly increased in BV CVF. Polarized A2EN cells were treated with 1 μg/ml or 5 μg/ml activated MMP10, MMP13, or a bovine serum albumin (BSA) protein equivalent for 72 h. Disruption of endocervical epithelium was measured by comparing the TEER at 72 h to the initial TEER before treatment. MMP10 significantly decreased TEER at both concentrations (P < 0.05) (Fig. 4C and D). Interestingly, MMP13 had no effect on TEER (not shown), suggesting potential differences between MMP isotypes with regard to substrate affinities and disruption of TJs.
End1 BVAB CM increased transepithelial migration of cell-associated HIV-1.
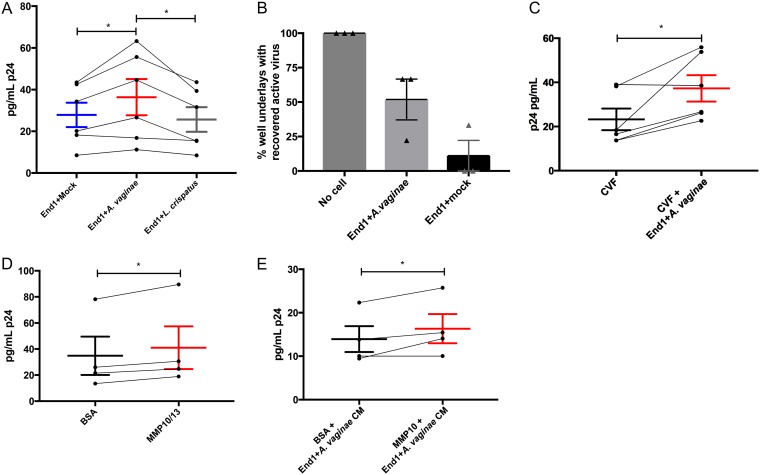
As both End1+A. vaginae CM and BV CVF disrupted the polarization of endocervical cells, we next tested whether decreased epithelial polarization could increase transmigration of HIV. The transmission of HIV from male to female usually occurs within the first few hours of exposure (46, 47), and so to model these early events in transmission experimentally, polarized A2EN cells, after a 72-h treatment with End1+A. vaginae, +L. crispatus, or +mock CM, were cocultured with HIV-1-infected PM1 cells at the apical surface for 3 h. Cell-associated HIV-1 was used because we determined that cell-free virus cannot pass through polarized A2EN cell layers under any treatment condition (not shown). This has also been reported in studies of Chlamydia trachomatis using the same A2EN cell system (48). After 3 h, the basolateral medium was collected, and passage of HIV-1 through the endocervical cell layers was measured by enzyme-linked immunosorbent assay (ELISA) of the viral capsid protein, p24. Decreased TEER at 72 h was associated with increased p24 in the basolateral medium. End1+A. vaginae CM increased the transmigration of virus to the basal side of the transwell compared to that for both End1+mock and End1+L. crispatus CM (P < 0.05) (Fig. 5A).
FIG 5.
BV and MMPs increase transmigration of cell-associated HIV across endocervical epithelium. Polarized A2EN cells were treated as indicated (x axes in A and C to E) for 72 h. Then, HIV-infected PM1 cells were added to the apical surface for an additional 3 h, and HIV-1 gag p24 protein recovered in the basolateral medium was measured by ELISA. (A) Treatment with End1+A. vaginae CM increased the recovery of basolateral p24 compared to that with End1+mock CM or End1+L. crispatus CM. (B) Transmigration of infectious HIV-1 across polarized A2EN cells was confirmed by incubating basolateral medium with uninfected PM1 cells to propagate recovered virions and applying PM1 supernatants to TZM-bl (HIV-1 infection reporter) cells as described in Materials and Methods. A cell-free transwell (“No cell”) was used as a positive control for transepithelial HIV migration. Data are presented as the percentage of transwell underlays containing infectious HIV (mean ± SEM, n = 3 separate CM preparations). (C) End1+A. vaginae CM, mixed with CVF, increased HIV transmigration compared to that with CVF alone. (D) Polarized A2EN cells treated with 0.5 μg/ml each of MMP10 and -13 resulted in increased basolateral HIV p24 recovery compared to that with BSA protein control. (E) MMP10 (1 μg/ml) mixed with End1+A. vaginae CM increased recovery of basolateral HIV p24 compared to that with BSA protein control. (A and C to E) Error bars represent SEMs. Each data point represents a separate CM batch, and connecting lines between groups indicate experimental pairs. *, P < 0.05 for paired Student’s t tests.
As antibody-based methods cannot distinguish between infectious and inactive virions of HIV-1, we next investigated if infectious virions migrated through the endocervical epithelium by collecting basolateral medium from each transwell at 3 h, incubating with fresh (uninfected) PM1 cells in the presence of Polybrene for 1.5 h, and then culturing for 6 days to amplify infection. After 6 days, PM1 supernatant was collected and assayed for HIV-1 using a luciferase-based infection assay, in which TZM-bl cells are treated with the collected supernatant and produce luminescence if productively infected with HIV-1. Infectious virus migrated through the epithelium, and End1+A. vaginae-treated CM tended to increase the number of transwells with recoverable virus (Fig. 5B).
We next considered if soluble factors secreted by the endocervical epithelium in response to BVAB could also disrupt the HIV-1 protective effect of healthy CVF (43). Polarized A2EN cells were treated with a 1:5 dilution of pooled CVF from healthy donors alone or in combination with 4× End1+A. vaginae CM for 72 h (Fig. 5C), and then basolateral medium was collected and p24 ELISA was used to measure the transmigration of virus. Importantly, End1+A. vaginae CM was capable of increasing transmigration of HIV-1, even in the presence of CVF (P < 0.05).
MMP10 and -13 increase transmigration of HIV-1 through endocervical epithelia.
We next assessed whether MMPs could directly increase transmigration of cell-associated HIV-1. First, we treated polarized endocervical cells with 1 or 5 μg/ml of MMP10 or MMP13 for 72 h, as in previous experiments, and at 72 h, endocervical cells were cocultured with HIV-1-infected PM1 cells for 3 h; then, cell underlays were analyzed for the presence of p24 by ELISA. MMP10 and MMP13 were not capable of increasing transmigration of virus alone (not shown). However, when this experiment was repeated with MMP10 and -13 combined, 0.5 μg/ml each, this MMP isotype mixture significantly increased transmigration of cell-associated HIV-1 across endocervical epithelia (P < 0.05) (Fig. 5D).
We then examined how recombinant MMPs would affect HIV-1 transmigration in the presence of End1+A. vaginae CM. The above-mentioned experiment was repeated with 1 μg/ml MMP10 in the presence of 4× End1+A. vaginae CM and compared to that with CM with a BSA protein control. MMP10 was chosen, as it disrupted TEER at 72 h and increased transmigration in the presence of MMP13. At 72 h, MMP10 in combination with End1+A. vaginae CM increased the transmigration of HIV-1 significantly more than End1+A. vaginae CM alone (P < 0.05) (Fig. 5E).
The MMP inhibitor actinonin decreases BVAB-associated HIV-1 transmigration.
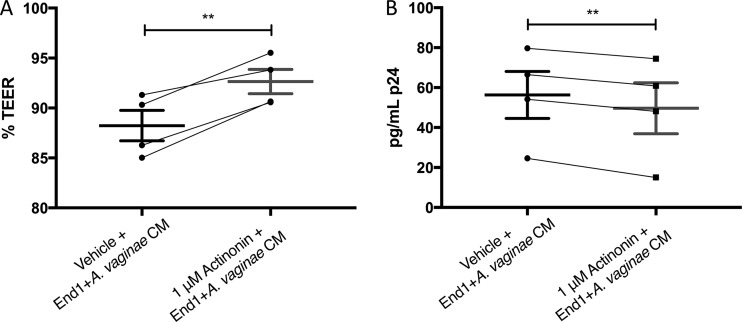
As MMPs were increased in response to BV, disrupted TEER, and increased HIV-1 transmigration, we next explored if MMP inhibitors could protect the endocervical epithelium from these BV-associated outcomes. We chose the MMP inhibitor actinonin, as it has broad-spectrum activity against most MMP isotypes. Polarized endocervical epithelial cells were treated with End1+A. vaginae CM with 1 μM actinonin or equivalent vehicle for 72 h, and then TEER was measured and cell layers were cocultured with HIV-1-infected PM1s, as in previous experiments. At 72 h, the endocervical cells treated with actinonin demonstrated significantly increased TEER (Fig. 6A) and decreased HIV-1 transmigration through the cell layers (Fig. 6B) compared to those in cells treated with CM and vehicle alone (P < 0.01).
FIG 6.
The MMP inhibitor actinonin decreases endocervical+BVAB CM-associated disruption of TEER and HIV-1 transmigration. Polarized A2EN cells were treated with End1+A. vaginae CM in combination with 1 μM actinonin or equivalent vehicle for 72 h, and effects on TEER (A) and HIV-1 transmigration (B) are shown. (A) %TEER = TEER72 h/TEER0 h for each of 4 experiments. (B) At 72 h posttreatment, HIV-infected PM1 cells were placed atop each A2EN transwell for 3 h. Then, the basal medium was collected, and concentration of p24 was determined by ELISA. Bars represent the means and error bars are the SEMs. Connecting lines between groups indicate experimental pairs. **, P < 0.01 by paired two-tailed Student’s t test.
BV-induced transepithelial migration of HIV-1 is associated with increased MMP activity.
As End1+A. vaginae CM increased HIV-1 transmigration through endocervical cell layers, we thereafter investigated if BV CVF would have the same effect. We treated polarized A2EN cells with 10 healthy CVF samples, 12 BV CVF samples, or VFS for 72 h, followed by 3-h coculture with HIV-infected PM1s, as in previous experiments. As CVF donor samples were tested in four separate experiments, to control for difference in overall p24 concentrations caused by PM1 cell variability, HIV-1 transmigration (Fig. 7) was calculated as the percent change in p24 in the basal chamber of the experimental well compared to that in the control well. Intriguingly, increased viral transmigration was not solely related to BV diagnosis by Amsel’s criteria. CVF samples which greatly increased transmigration, or which were highly protective, compared to that with VFS were found in both the healthy and BV groups. The healthy CVF group contained one sample which increased the passage of virus nearly 10-fold compared to that with VFS. Some BV CVF samples were also highly protective to virus, though detection of some protective BV CVF samples was not entirely unexpected, as CVF is a highly bioactive fluid with many other potentially antiviral components (49).
FIG 7.
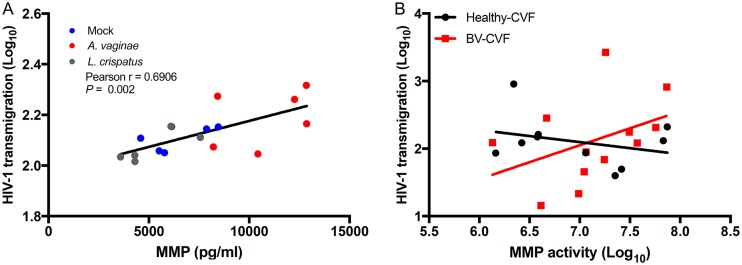
HIV-1 transmigration is associated with increased MMP activity. Polarized A2EN cells were treated with CM from mock-, A. vaginae-, or L. crispatus-treated End1 cells or with healthy or BV CVF for 72 h, and then HIV-1 transepithelial migration was measured as described in Materials and Methods. HIV-1 transmigration was calculated as the concentration of p24 in the basal medium of the experimental treatment compared to the p24 present in the control treatment (% change in p24). (A) Positive correlation between MMP concentration of each CM (x axis) and log-transformed HIV-1 transmigration (y axis). (B) MMP activity of each CVF sample (x axis) plotted versus HIV-1 transmigration. Each data point represents one preparation of CM (A) or 1 donor sample (B). For reference, a linear regression line was fit to all CM samples (A) and separately in the BV CVF and healthy CVF groups (B).
To better understand these results, we explored the characteristics of each CVF sample to find similarities between the highly protective and disruptive CVF specimens. We also performed these same analyses on endocervical CM, for comparison to a less complex and more homogenous fluid. First, we compared the change in HIV-1 transmigration to the concentration of epithelial derived MMPs present. In endocervical CM, the total MMP concentration correlated positively with HIV-1 transmigration (Fig. 7A), and End1+A. vaginae CM caused the greatest increase in HIV-1 transmigration and had the highest concentration of MMPs compared to that with End1+L. crispatus or End1+mock (no bacteria) CM. However, in CVF, there was no correlation between the concentration of MMPs in CVF and HIV-1 transmigration. Several healthy CVF samples had high concentrations of MMPs but did not increase transmigration, while several BV CVF samples had lower concentrations of MMPs but were still able to increase transmigration. However, when MMP enzymatic activity of each CVF sample was determined, there was a trend between increased MMP enzymatic activity and increased HIV-1 transmigration in the BV CVF group, though it did not reach statistical significance (Fig. 7B).
Next, we investigated how individual MMP isotypes present in CVF were associated with HIV-1 transmigration. When we compared the concentrations of 8 MMP isotypes in each CVF sample to the HIV-1 transmigration caused by each sample, we determined that isotypes MMP1 and MMP3 correlated positively with increased HIV-1 transmigration (P < 0.01) (Table 1). MMP13 levels were variable in CVF (range, 7 to 1,714 pg/ml, n = 20), but this isotype also appeared to associate with HIV-1 transepithelial migration (P = 0.05). Interestingly, these isotypes were all found to be significantly increased in BV CVF (Fig. 3). Overall, these results suggested that presence of these BV-associated MMP isotypes was the most predictive of CVF which increased HIV-1 transmigration.
TABLE 1.
CVF expression of MMP1, -3, and -13 associates with transepithelial migration of HIV-1
| MMPa type | HIV migration vs MMP |
||
|---|---|---|---|
| Pearson’s r | 95% CIb | P value | |
| MMP1 | 0.555 | 0.150 to 0.801 | 0.005 |
| MMP2 | −0.161 | −0.563 to 0.302 | 0.248 |
| MMP3 | 0.520 | 0.101 to 0.782 | 0.003 |
| MMP7 | −0.020 | −0.470 to 0.437 | 0.466 |
| MMP9 | −0.062 | −0.492 to 0.389 | 0.394 |
| MMP10 | 0.190 | −0.275 to 0.583 | 0.210 |
| MMP12 | 0.033 | −0.414 to 0.469 | 0.443 |
| MMP13 | 0.377 | −0.078 to 0.702 | 0.050 |
MMP, matrix metalloproteinase.
CI, confidence interval; n = 20.
DISCUSSION
Our study implicates barrier dysfunction of the endocervix by host-derived factors as an important mechanism of HIV-1 infection in the BV-afflicted mucosa. We determined MMPs as BV-associated secreted factors which can damage the endocervix, as they were overexpressed in response to BV, and importantly, enzymatically active, and capable of damaging endocervical epithelium.
MMP10 and MMP13 appeared to be the MMP isotypes most important to BV pathogenesis. Expression of these isotypes was greatly increased by BVAB. MMP10 was capable of disrupting polarization of endocervical epithelial cells, and MMP10 and MMP13 in combination increased HIV-1 transmigration. The function of these isotypes in the FRT mucosa is not well studied, though one group has reported that MMP10 is upregulated in women with pelvic organ prolapse (50). Additionally, analysis of MMP10 substrate affinity implicates it in the processing of other MMPs to their active form as well as degradation of type I collagen, the collagen type most prevalent in the stroma of the cervix (51, 52). MMP13 is usually expressed in tissues during wound healing, and its overexpression is associated with cancers and chronic inflammatory conditions such as osteoporosis and inflammatory bowel disease (53, 54). The overexpression of MMP10 and -13 together has been associated with chronic intestinal ulcers (55).
When we simulated early events in heterosexual HIV transmission using a polarized endocervical cell coculture system, we demonstrated that the transmigration of cell-associated HIV-1 through endocervical epithelium was increased in response to endocervical+BVAB CM compared to that with endocervical+lactobacillus CM (Fig. 5A). MMPs also disrupted the integrity of the endocervical epithelia. MMP10 increased the viral enhancing properties of endocervical+BVAB CM, and a combination of MMP10 and MMP13 increased viral transmigration (Fig. 5D and E). These results suggest increased MMP activity, and its ability to disrupt mucosa in response to BV may be a novel target for HIV preventatives. Importantly, we demonstrated the ability of an MMP inhibitor to decrease BV-associated HIV-1 transmigration and disruption of endocervical barrier dysfunction (Fig. 6). As the current recommended treatment for BV, the antibiotic metronidazole, is not completely effective, with many women relapsing to a BV state after just a few months (56–58), alternative treatments for BV are crucial. Given the polymicrobial nature of BV, treatments targeting an increase in MMP activity, a symptom of BV which increases HIV infection, may be more beneficial to HIV prevention than antibiotics alone.
When we compared the ability of healthy CVF and BV CVF to block viral transmigration through endocervical cell layers, we found a “BV-negative” diagnosis was not always HIV-1 protective. One CVF specimen considered healthy according to Amsel’s criteria was highly disruptive to endocervical polarization and increased viral transmigration, while several BV CVF specimens were protective against viral transmigration and disruption of endocervical polarization. However, CVF specimens which increased transmigration of HIV-1 generally had increased MMP enzymatic activity or increased presence of BV-associated MMP isotypes, regardless of BV diagnosis. It is possible that diagnosis according to Amsel’s criteria, in which 3 of 4 characteristics of BV must be met by vaginal fluid (2), may not be a fully effective predictor of BV-associated HIV infections. Many groups have suggested other methods of BV diagnosis, including the presence of the Gram-positive BVAB A. vaginae and Gardnerella vaginalis, BVAB biofilms, and increased concentrations of inflammatory cytokines (31, 59, 60). As the microbiomes of these samples were not analyzed in this study, it is also possible that similar bacterial communities existed in CVF samples which increased HIV-1 transmigration, warranting future investigation. Alternatively, different complications of the FRT besides BV, such as infection or chronic inflammation, may also increase MMP activity in CVF, subsequently weakening the epithelia. For example, Chlamydia trachomatis infection has also been associated with increased expression of the MMP10 gene (61). Here, we suggest increased MMP activity as a potential predictor of FRT barrier dysfunction and subsequent HIV-1 transmission in women and recommend future studies of MMP inhibition in the FRT as a potential HIV preventative.
MATERIALS AND METHODS
Cell lines, bacteria, and virus.
The human immortalized endocervical cell line End1 (CRL-265) was purchased from American Type Culture Collection (ATCC, Manassas, VA). End1 cells were maintained according to ATCC instructions in keratinocyte serum-free media (KSFM) (Thermo Fisher, Waltham, MA) supplemented with calcium chloride, bovine pituitary extract, recombinant epidermal growth factor, and the antibiotic Primocin (InvivoGen, San Diego, CA). A2EN, another endocervix-derived cell line (Alison J. Quayle, Louisiana State University), was maintained in EpiLife growth medium (InvivoGen, San Diego, CA) supplemented with calcium chloride, glutamine, a proprietary EpiLife defined growth medium, and Primocin. The lymphocyte-derived immortalized cell line, PM1 (Marvin Reitz), and the HeLa cell-derived line, TZM-bl (John C. Kappes, Xiaoyun Wu, and Tranzyme), were obtained from the AIDS Research and Reference Reagent Program (Division of AIDS, NIAID, NIH). PM1 cells were maintained in RPMI 1640 with 10 mM HEPES buffer, streptomycin, penicillin, and 20% (vol/vol) fetal bovine serum (FBS), referred to as R20. TZM-bl cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) with 10% (vol/vol) FBS, streptomycin, and penicillin.
The following bacteria were purchased from ATCC: Lactobacillus crispatus (33197), Lactobacillus jensenii (25258), Lactobacillus johnsonii (11506), and Atopobium vaginae (BAA-55). Lactobacilli were grown in MRS broth (or MRS agar plates) at 37°C with 5% CO2. A. vaginae was grown in tryptic soy broth (TSB) with 5% defibrinated rabbit blood (all media from Becton, Dickinson and Company, Franklin Lakes, NJ, USA) or on Colombia agar plates with 5% sheep’s blood in anaerobic chambers using GasPaks (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) at 37°C with 5% CO2. For experimental consistency, cultures of each bacteria were aliquoted and snap-frozen by submerging in liquid nitrogen for 2 h and then transferred to −80°C until use. Multiplicity of infection (MOI) was calculated as the number of bacterial CFU divided by the number of epithelial cells in each experimental condition. Bacterial CFU of each species was determined by serial dilution of the bacterial aliquots and plating on the appropriate medium.
The laboratory-adapted strain of HIV-1, BaL, was acquired from the AIDS Research and Reference Reagent Program. BaL was propagated in PM1 cells for 6 days, and then supernatant was collected, centrifuged, and filtered of residual cell debris, as described in our previous work (62). Aliquots were frozen at −80°C for future infections. Viral infectious titer was determined by infection of TZM-bl and with serial dilutions of virus-containing supernatants. Effective dilution of viral supernatant was determined as the dilution resulting in infection of 50% of culture cell wells. Detection of virus in supernatant and in experiments was determined by enzyme-linked immunosorbent assay (ELISA) for the HIV-1 capsid protein, p24, according to the manufacturer’s instructions (Perkin Elmer, Waltham, MA).
Polarization of A2EN on transwell inserts.
A2EN cells were seeded at 30,000 cells per transwell in complete EpiLife medium onto 24-well 0.4-μm-pore-size corning transwell inserts (Corning Inc., Kennebunk, ME) that were precoated with PureCol type I bovine collagen (Advanced BioMatrix, Carlsbad, CA) diluted 1:30 in growth medium. After 24 h, medium was replaced with complete EpiLife supplemented with additional calcium chloride to a concentration of 0.4 mM. At day 4 after seeding, the apical medium was removed, and cells were grown at the air-liquid interface. Cells were polarized and used by 10 to 12 days postseeding. Polarization was determined by measuring transepithelial electrical resistance (TEER) using an EVOM2 ohmmeter calibrated in EpiLife base medium that was used for all TEER measurements described below. Cells which did not leak at ALI and measured >280 Ω·cm2 were considered polarized (35).
Preparation of conditioned media.
To prepare large conditioned medium (CM) batches, End1 or A2EN cells were grown in tissue culture-treated 100-mm plates to ∼80% to 90% confluence, and then each plate was treated with 8 ml of Ham’s F12 medium containing A. vaginae or L. crispatus at an MOI of 3 or F12 alone for 24 h at 37°C with 5% CO2. MOI was determined as CFU divided by the number of cells per plate. CM was then collected and centrifuged at 1,000 × g for 10 min to pellet cell and bacterial debris, and then supernatants were vacuum concentrated with a Thermo SpeedVac and desalted using Amicon Ultra 3-kDa 15-ml centrifugal filters (MilliporeSigma, Burlington, MA). Aliquots of CM (typically 20- to 30-fold concentrated compared to the initial CM volume) were stored at −80°C until use. CM for MMP concentration analysis was prepared as follows. End1 or A2EN cells were plated in tissue culture-treated 12-well dishes at 50,000 cells/well. At ∼80% to 90% confluence, cells were treated in duplicates with growth medium alone or containing A. vaginae, L. crispatus, L. johnsonii, or L. jensenii at an MOI of 5 for 24 h, and then collected, clarified by centrifugation at 10,000 × g for 10 min, and stored at −80°C.
Collection and processing of cervicovaginal fluid.
CVF was collected from premenopausal postmenarchal women with consent according to the guidelines of the institutional review board of the University of Central Florida. Diagnosis of BV was determined by the clinical staff at UCF Women’s Care, UCF Student Health Center, according to Amsel’s criteria. An Instead SoftCup (Ultrafem, New York, NY) was used to collect vaginal fluid by insertion into the vagina for 30 min, per manufacturer’s instructions, and then removed, and the sample was centrifuged at 1,000 × g for 10 min in a sterile 50-ml conical tube and stored at −20°C. For experimental use, CVF was sonicated via ten 2- to 3-s pulses using a microtip ultrasound probe to break up mucus and then centrifuged at 10,000 × g for 5 min to clarify CVF of bacteria and mucus.
Treatment of polarized A2EN transwells.
For CM experiments, polarized A2EN cells were treated at the apical surface with 100 μl End1 CM at 4× concentration, diluted in EpiLife base medium (no growth supplements). For CVF experiments, A2EN cells were treated topically with 80 μl clarified CVF, diluted 1:5 with a vaginal fluid simulant, which resembles the nutrient, salt, and protein concentrations of vaginal fluid (44), and pH was adjusted to neutral. A2EN cells treated topically with CVF and End1+A. vaginae CM were treated with 100 μl of 1:5 CVF with 4× CM, diluted in VFS. Treatments with recombinant MMP10 (rMMP10) and -13 were performed as follows. MMP10 or MMP13 (R&D Systems, Minneapolis, MN) was activated by incubation with 1 mM 4-aminophenylmercuric acetate (APMA) at 37°C for 2 h or 30 min, respectively, according to company instructions. A2EN cells were treated topically with 100 μl 0.1 or 0.5 μg/ml MMP10 or -13 or a combination of 0.05 μg/ml of both MMP10 and MMP13 in EpiLife base medium without growth supplements. Bovine serum albumin (BSA) of an equivalent concentration was used as a protein and APMA activation control. For actinonin experiments, A2EN cells were treated topically with 100 μl of 4× End1+A. vaginae CM in combination with 1 μM actinonin resuspended in dimethyl sulfoxide (DMSO; Santa Cruz Biologics, Santa Cruz, CA) or with the equivalent DMSO vehicle. For each experiment, treatments were replaced every 24 h. A2EN cells treated with CVF were washed twice with 100 μl phosphate-buffered saline (PBS) with penicillin and streptomycin before the medium for treatment was replaced.
Measurement of TEER.
A2EN cell TEER was measured using an EVOM2 epithelial voltohmmeter and Endohm 6 tissue resistance chamber for 6.5-mm transwell culture cups (World Precision Instruments, Sarasota, FL). Each day, the system was calibrated in EpiLife base medium using an empty transwell insert (baseline resistance) versus a company-provided “CALICELL” transwell cup representative of polarized epithelial tissue. TEER measurements of A2EN transwells were made before treatment and then every 24 h for 72 h.
HIV transmigration assays.
At 72 h posttreatment, treatments were removed, and A2EN cells were cocultured with HIV-1 BaL-infected PM1s at the apical side of the transwell for 3 h. HIV-infected PM1 cells were resuspended at 1 million live cells per/ml in EpiLife without growth supplements. After 3 h, basal medium was removed and stored at −20°C until detection of p24 using ELISA. To detect intact active virus in the basolateral medium after the 3-h transmigration assay, 150 μl of each basolateral well medium was incubated at 37°C and 5% CO2 with 150 μl PM1 cells, resuspended at 3 million cells/ml in R2 (RPMI plus 2% FBS) for 90 min with 2 μg/ml Polybrene. Cells were then resuspended in fresh R2, centrifuged (200 × g, 5 min), and resuspended at 1 million cells/ml in R20 in 12-well plates. Cells were cultured for 6 days in R20 to allow for propagation of virus. On day 3, PM1 cells were collected, spun, and resuspended at 1 million cells/ml in fresh R20. On day 6, cell supernatant was collected and used for a luciferase infection assay to detect active virus propagated from each transwell basolateral chamber.
Luciferase infection assay.
TZM-bl cells were plated at 7,000 cells/well in black 96-well tissue culture-treated plates. When 70% confluent, growth medium was replaced with day 6 supernatants from basolateral medium-incubated PM1s. TZM-bl cells have been transfected with the luciferase gene, controlled by the HIV Tat promoter, and so productive infection can be measured by luminescence. After 24 h, supernatant was removed, and the assay substrate, BrightGlo, was added according to the manufacturer’s instructions to detect infected TZM-bl (Bright Glo luciferase system, Promega, Madison, WI).
Bioplex immunoassay.
To measure MMP concentrations in CM and CVF, the BioPlex Pro Human MMP multiplex immunoassay for 9 different MMP isotypes (MMP-1, -2, -3, -7, -8, -9, -10, -12, and -13) was used according to step-by-step company instructions for the generation of standard curves, sample preparation, and instrument settings (catalog number 171AM001M; Bio-Rad Laboratories, Hercules, CA). CM and CVF were not diluted unless there was less than 50 μl remaining after functional assays. Analysis of the neutrophil-derived MMP8 was not included, as concentrations were too high for standard curve fitting. MMP concentrations (picograms per milliliter) in endocervical cell-bacterium CM are presented as fold induction versus that in mock bacterium control (Fig. 2, 3, and 7 and Table 1).
MMP activity assay.
For detection of MMP enzymatic activity in CM and CVF, we employed a fluorometric MMP activity detecting assay, SensoLyte 520 Generic MMP assay (AnaSpec, Inc., Fremont, CA). This assay has a proprietary fluorescent substrate which shifts in fluorescence spectrum from 490 to 520 nm when cleaved by an active MMP. Concentrated CM collected from treated End1 and A2EN cells was diluted in the provided assay buffer, and fluorescence was measured after 60 min at room temperature. CVF was diluted 1:40 using assay buffer, and fluorescence was measured after 30 min at room temperature.
Cytotoxicity assays.
Cytotoxicity of CM was tested using the Promega CytoTox-Glo (Promega, Madison, WI). A2EN cells were plated at 5,000 cells/well into black 96-well plates and grown to ∼90% confluence. Cells were then treated with 4× End1 CM diluted in complete EpiLife, complete EpiLife alone, or complete EpiLife with 0.05% Triton X-100, as a positive control for total cell death. After 24 h, the company-provided proprietary assay substrate was added, which is cleaved by proteases released from dead cells, producing luminescence. Dead cell luminescence was measured, and then cells were lysed and total cell luminescence was measured, per assay instructions. Results were reported as live cell luminescence (total cell luminescence minus dead cell luminescence). To test CVF for cytotoxicity, healthy CVF or BV CVF was pooled from 3 to 5 donor samples, diluted 1:5 with VFS, and pH adjusted to neutral. Polarized A2EN cells were treated with CVF, VFS alone, or VFS with 0.05% Triton X-100, according to the protocol described in “Measurement of TEER,” above. At 72 h, CVF treatments were removed, and cells were treated with MTT reagent diluted in EpiLife base medium for 2 h at 37°C with 5% CO2, per the manufacturer’s instructions (TACS MTT cell proliferation assay; Trevigen, Gaithersburg, MD). After 2 h, cells were removed from transwells with 0.25% trypsin, sonicated, and resuspended in 50 μl DMSO in clear 96-well plates, and then absorbance was read at 570 nm.
Statistical analyses.
All statistical analyses were performed using GraphPad Prism 7 software (GraphPad Software, La Jolla, CA). Student’s t tests were used to determine statistical significance.
Supplementary Material
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Kenyon C, Colebunders R, Crucitti T. 2013. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol 209:505–523. doi: 10.1016/j.ajog.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. 1983. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74:14–22. doi: 10.1016/0002-9343(83)91112-9. [DOI] [PubMed] [Google Scholar]
- 3.Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya-Achola JO, Bwayo J, Kreiss J. 1999. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 180:1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 4.Sturm-Ramirez K, Gaye-Diallo A, Eisen G, Mboup S, Kanki PJ. 2000. High levels of tumor necrosis factor-α and interleukin-1β in bacterial vaginosis may increase susceptibility to human immunodeficiency virus. J Infect Dis 182:467–473. doi: 10.1086/315713. [DOI] [PubMed] [Google Scholar]
- 5.Cauci S, Guaschino S, de Aloysio D, Driussi S, De Santo D, Penacchioni P, Quadrifoglio F. 2003. Interrelationships of interleukin‐8 with interleukin‐1β and neutrophils in vaginal fluid of healthy and bacterial vaginosis positive women. Mol Hum Reprod 9:53–58. doi: 10.1093/molehr/gag003. [DOI] [PubMed] [Google Scholar]
- 6.Valore EV, Wiley DJ, Ganz T. 2006. Reversible deficiency of antimicrobial polypeptides in bacterial vaginosis. Infect Immun 74:5693–5702. doi: 10.1128/IAI.00524-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boily M-C, Baggaley RF, Wang L, Masse B, White RG, Hayes RJ, Alary M. 2009. Heterosexual risk of HIV-1 infection per sexual act: systematic review and meta-analysis of observational studies. Lancet Infect Dis 9:118–129. doi: 10.1016/S1473-3099(09)70021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shattock RJ, Moore JP. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol 1:25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 9.Norvell MK, Benrubi GI, Thompson RJ. 1984. Investigation of microtrauma after sexual intercourse. J Reprod Med 29:269–271. [PubMed] [Google Scholar]
- 10.Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Tshibaka LM, Ettiègne-Traoré V, Uaheowitchai C, Karim SSA, Mâsse B, Perriëns J, Laga M, COL-1492 Study Group. 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360:971–977. doi: 10.1016/S0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 11.Eade CR, Diaz C, Wood MP, Anastos K, Patterson BK, Gupta P, Cole AL, Cole AM. 2012. Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PLoS One 7:e50106. doi: 10.1371/journal.pone.0050106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carias AM, McCoombe S, McRaven M, Anderson M, Galloway N, Vandergrift N, Fought AJ, Lurain J, Duplantis M, Veazey RS, Hope TJ. 2013. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J Virol 87:11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blaskewicz CD, Pudney J, Anderson DJ. 2011. Structure and function of intercellular junctions in human cervical and vaginal mucosal epithelia. Biol Reprod 85:97–104. doi: 10.1095/biolreprod.110.090423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Someya M, Kojima T, Ogawa M, Ninomiya T, Nomura K, Takasawa A, Murata M, Tanaka S, Saito T, Sawada N. 2013. Regulation of tight junctions by sex hormones in normal human endometrial epithelial cells and uterus cancer cell line Sawano. Cell Tissue Res 354:481–494. doi: 10.1007/s00441-013-1676-9. [DOI] [PubMed] [Google Scholar]
- 16.Ye D, Ma I, Ma TY. 2006. Molecular mechanism of tumor necrosis factor-alpha modulation of intestinal epithelial tight junction barrier. Am J Physiol Gastrointest Liver Physiol 290:G496–G504. doi: 10.1152/ajpgi.00318.2005. [DOI] [PubMed] [Google Scholar]
- 17.Nold C, Anton L, Brown A, Elovitz M. 2012. Inflammation promotes a cytokine response and disrupts the cervical epithelial barrier: a possible mechanism of premature cervical remodeling and preterm birth. Am J Obstet Gynecol 206:208.e1–208.e7. doi: 10.1016/j.ajog.2011.12.036. [DOI] [PubMed] [Google Scholar]
- 18.Wang L-C, Yu Q, Edwards V, Lin B, Qiu J, Turner JR, Stein DC, Song W. 2017. Neisseria gonorrhoeae infects the human endocervix by activating non-muscle myosin II-mediated epithelial exfoliation. PLoS Pathog 13:e1006269. doi: 10.1371/journal.ppat.1006269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodríguez-Tirado C, Maisey K, Rodríguez FE, Reyes-Cerpa S, Reyes-López FE, Imarai M. 2012. Neisseria gonorrhoeae induced disruption of cell junction complexes in epithelial cells of the human genital tract. Microbes Infect 14:290–300. doi: 10.1016/j.micinf.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Nazli A, Chan O, Dobson-Belaire WN, Ouellet M, Tremblay MJ, Gray-Owen SD, Arsenault AL, Kaushic C. 2010. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog 6:e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antalis TM, Shea-Donohue T, Vogel SN, Sears C, Fasano A. 2007. Mechanisms of disease: protease functions in intestinal mucosal pathobiology. Nat Clin Pract Gastroenterol Hepatol 4:393–402. doi: 10.1038/ncpgasthep0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed CE. 2007. Inflammatory effect of environmental proteases on airway mucosa. Curr Allergy Asthma Rep 7:368–374. doi: 10.1007/s11882-007-0056-5. [DOI] [PubMed] [Google Scholar]
- 23.Birse K, Arnold KB, Novak RM, McCorrister S, Shaw S, Westmacott GR, Ball TB, Lauffenburger DA, Burgener A. 2015. Molecular signatures of immune activation and epithelial barrier remodeling are enhanced during the luteal phase of the menstrual cycle: implications for HIV susceptibility. J Virol 89:8793–8805. doi: 10.1128/JVI.00756-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley F, Birse K, Hasselrot K, Noël-Romas L, Introini A, Wefer H, Seifert M, Engstrand L, Tjernlund A, Broliden K, Burgener AD. 2018. The vaginal microbiome amplifies sex hormone‐associated cyclic changes in cervicovaginal inflammation and epithelial barrier disruption. Am J Reprod Immunol 80:e12863. doi: 10.1111/aji.12863. [DOI] [PubMed] [Google Scholar]
- 25.Iqbal SM, Ball TB, Levinson P, Maranan L, Jaoko W, Wachihi C, Pak BJ, Podust VN, Broliden K, Hirbod T, Kaul R, Plummer FA. 2009. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS 23:1669–1677. doi: 10.1097/QAD.0b013e32832ea643. [DOI] [PubMed] [Google Scholar]
- 26.Burgener A, Rahman S, Ahmad R, Lajoie J, Ramdahin S, Mesa C, Brunet S, Wachihi C, Kimani J, Fowke K, Carr S, Plummer F, Ball TB. 2011. Comprehensive proteomic study identifies serpin and cystatin antiproteases as novel correlates of HIV-1 resistance in the cervicovaginal mucosa of female sex workers. J Proteome Res 10:5139–5149. doi: 10.1021/pr200596r. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JS, Elrod JW. 2002. Extracellular matrix, junctional integrity and matrix metalloproteinase interactions in endothelial permeability regulation. J Anat 200:561–574. doi: 10.1046/j.1469-7580.2002.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ichikawa Y, Ishikawa T, Momiyama N, Kamiyama M, Sakurada H, Matsuyama R, Hasegawa S, Chishima T, Hamaguchi Y, Fujii S, Saito S, Kubota K, Hasegawa S, Ike H, Oki S, Shimada H. 2006. Matrilysin (MMP-7) degrades VE-cadherin and accelerates accumulation of beta-catenin in the nucleus of human umbilical vein endothelial cells. Oncol Rep 15:311–315. [PubMed] [Google Scholar]
- 29.Gorodeski GI. 2007. Estrogen decrease in tight junctional resistance involves matrix-metalloproteinase-7-mediated remodeling of occludin. Endocrinology 148:218–231. doi: 10.1210/en.2006-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon BH, Oh S-Y, Romero R, Shim S-S, Han S-Y, Park JS, Jun JK. 2001. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 185:1162–1167. doi: 10.1067/mob.2001.117678. [DOI] [PubMed] [Google Scholar]
- 31.Arnold KB, Burgener A, Birse K, Romas L, Dunphy LJ, Shahabi K, Abou M, Westmacott GR, McCorrister S, Kwatampora J, Nyanga B, Kimani J, Masson L, Liebenberg LJ, Abdool Karim SS, Passmore J-A, Lauffenburger DA, Kaul R, McKinnon LR. 2016. Increased levels of inflammatory cytokines in the female reproductive tract are associated with altered expression of proteases, mucosal barrier proteins, and an influx of HIV-susceptible target cells. Mucosal Immunol 9:194–205. doi: 10.1038/mi.2015.51. [DOI] [PubMed] [Google Scholar]
- 32.Diaz-Cueto L, Cuica-Flores A, Ziga-Cordero F, Ayala-Mendez JA, Tena-Alavez G, Dominguez-Lopez P, Cuevas-Antonio R, Arechavaleta-Velasco F. 2006. Vaginal matrix metalloproteinase levels in pregnant women with bacterial vaginosis. J Soc Gynecol Invest 13:430–434. doi: 10.1016/j.jsgi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Rivera AJ, Frank JA, Stumpf R, Salyers AA, Wilson BA, Olsen GJ, Leigh S. 2011. Differences between the normal vaginal bacterial community of baboons and that of humans. Am J Primatol 73:119–126. doi: 10.1002/ajp.20851. [DOI] [PubMed] [Google Scholar]
- 34.Yildirim S, Yeoman CJ, Janga SC, Thomas SM, Ho M, Leigh SR, Consortium PM, White BA, Wilson BA, Stumpf RM. 2014. Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. ISME J 8:2431. doi: 10.1038/ismej.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buckner LR, Schust DJ, Ding J, Nagamatsu T, Beatty W, Chang TL, Greene SJ, Lewis ME, Ruiz B, Holman SL, Spagnuolo RA, Pyles RB, Quayle AJ. 2011. Innate immune mediator profiles and their regulation in a novel polarized immortalized epithelial cell model derived from human endocervix. J Reprod Immunol 92:8–20. doi: 10.1016/j.jri.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fichorova RN, Anderson DJ. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol Reprod 60:508–514. doi: 10.1095/biolreprod60.2.508. [DOI] [PubMed] [Google Scholar]
- 37.Onderdonk AB, Zamarchi GR, Rodriguez ML, Hirsch ML, Munoz A, Kass EH. 1987. Qualitative assessment of vaginal microflora during use of tampons of various compositions. Appl Environ Microbiol 53:2779–2784. doi: 10.1128/AEM.53.12.2779-2784.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fichorova RN, Yamamoto HS, Delaney ML, Onderdonk AB, Doncel GF. 2011. Novel vaginal microflora colonization model providing new insight into microbicide mechanism of action. mBio 2:e00168-11. doi: 10.1128/mBio.00168-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Onderdonk AB, Lee ML, Lieberman E, Delaney ML, Tuomala RE. 2003. Quantitative microbiologic models for preterm delivery. J Clin Microbiol 41:1073–1079. doi: 10.1128/jcm.41.3.1073-1079.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bradshaw CS, Tabrizi SN, Fairley CK, Morton AN, Rudland E, Garland SM. 2006. The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J Infect Dis 194:828–836. doi: 10.1086/506621. [DOI] [PubMed] [Google Scholar]
- 41.Ferris MJ, Masztal A, Aldridge KE, Fortenberry JD, Fidel PL, Martin DH. 2004. Association of Atopobium vaginae, a recently described metronidazole resistant anaerobe, with bacterial vaginosis. BMC Infect Dis 4:5. doi: 10.1186/1471-2334-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eade CR, Cole AL, Diaz C, Rohan LC, Parniak MA, Marx P, Tarwater PM, Gupta P, Cole AM. 2013. The anti-HIV microbicide candidate RC-101 inhibits pathogenic vaginal bacteria without harming endogenous flora or mucosa. Am J Reprod Immunol 69:150–158. doi: 10.1111/aji.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Venkataraman N, Cole AL, Svoboda P, Pohl J, Cole AM. 2005. Cationic polypeptides are required for anti-HIV-1 activity of human vaginal fluid. J Immunol 175:7560–7567. doi: 10.4049/jimmunol.175.11.7560. [DOI] [PubMed] [Google Scholar]
- 44.Owen DH, Katz DF. 1999. A vaginal fluid simulant. Contraception 59:91–95. doi: 10.1016/s0010-7824(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 45.Manicone AM, McGuire JK. 2008. Matrix metalloproteinases as modulators of inflammation. Semin Cell Dev Biol 19:34–41. doi: 10.1016/j.semcdb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen R, Richter HE, Smith PD. 2011. Early HIV-1 target cells in human vaginal and ectocervical mucosa. Am J Reprod Immunol 65:261–267. doi: 10.1111/j.1600-0897.2010.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hu J, Gardner MB, Miller CJ. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol 74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Buckner LR, Amedee AM, Albritton HL, Kozlowski PA, Lacour N, McGowin CL, Schust DJ, Quayle AJ. 2016. Chlamydia trachomatis infection of endocervical epithelial cells enhances early HIV transmission events. PLoS One 11:e0146663. doi: 10.1371/journal.pone.0146663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole AM. 2006. Innate host defense of human vaginal and cervical mucosae. Curr Top Microbiol Immunol 306:199–230. [PubMed] [Google Scholar]
- 50.Wang H, Zhang Z-Q, Wang S-Z, Lu J-L, Wang X-L, Zhang Z-Y. 2015. Association of matrix metalloproteinase-10 polymorphisms with susceptibility to pelvic organ prolapse. J Obstet Gynaecol Res 41:1972–1981. doi: 10.1111/jog.12809. [DOI] [PubMed] [Google Scholar]
- 51.Schlage P, Egli FE, Nanni P, Wang LW, Kizhakkedathu JN, Apte SS, Auf Dem Keller U. 2014. Time-resolved analysis of the matrix metalloproteinase 10 substrate degradome. Mol Cell Proteomics 13:580–593. doi: 10.1074/mcp.M113.035139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kleissl HP, Van der Rest M, Naftolin F, Glorieux FH, de Leon A. 1978. Collagen changes in the human uterine cervix at parturition. Am J Obstet Gynecol 130:748–753. doi: 10.1016/0002-9378(78)90003-0. [DOI] [PubMed] [Google Scholar]
- 53.Tardif G, Reboul P, Pelletier JP, Martel-Pelletier J. 2004. Ten years in the life of an enzyme: the story of the human MMP-13 (collagenase-3). Mod Rheumatol 14:197–204. doi: 10.1007/s10165-004-0292-7. [DOI] [PubMed] [Google Scholar]
- 54.Vizoso FJ, Gonzalez LO, Corte MD, Corte MG, Bongera M, Martinez A, Martin A, Andicoechea A, Gava RR. 2006. Collagenase-3 (MMP-13) expression by inflamed mucosa in inflammatory bowel disease. Scand J Gastroenterol 41:1050–1055. doi: 10.1080/00365520600554667. [DOI] [PubMed] [Google Scholar]
- 55.Vaalamo M, Karjalainen-Lindsberg ML, Puolakkainen P, Kere J, Saarialho-Kere U. 1998. Distinct expression profiles of stromelysin-2 (MMP-10), collagenase-3 (MMP-13), macrophage metalloelastase (MMP-12), and tissue inhibitor of metalloproteinases-3 (TIMP-3) in intestinal ulcerations. Am J Pathol 152:1005–1014. [PMC free article] [PubMed] [Google Scholar]
- 56.Löfmark S, Edlund C, Nord CE. 2010. Metronidazole is still the drug of choice for treatment of anaerobic infections. Clin Infect Dis 50:S16–S23. doi: 10.1086/647939. [DOI] [PubMed] [Google Scholar]
- 57.Larsson PG, Forsum U. 2005. Bacterial vaginosis–a disturbed bacterial flora and treatment enigma. APMIS 113:305–316. doi: 10.1111/j.1600-0463.2005.apm_113501.x. [DOI] [PubMed] [Google Scholar]
- 58.Mitchell C, Balkus J, Agnew K, Lawler R, Hitti J. 2009. Changes in the vaginal microenvironment with metronidazole treatment for bacterial vaginosis in early pregnancy. J Womens Health (Larchmt) 18:1817–1824. doi: 10.1089/jwh.2009.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menard JP, Fenollar F, Henry M, Bretelle F, Raoult D. 2008. Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin Infect Dis 47:33–43. doi: 10.1086/588661. [DOI] [PubMed] [Google Scholar]
- 60.Swidsinski A, Mendling W, Loening-Baucke V, Ladhoff A, Swidsinski S, Hale LP, Lochs H. 2005. Adherent biofilms in bacterial vaginosis. Obstet Gynecol 106:1013–1023. doi: 10.1097/01.AOG.0000183594.45524.d2. [DOI] [PubMed] [Google Scholar]
- 61.Vicetti Miguel RD, Harvey SAK, LaFramboise WA, Reighard SD, Matthews DB, Cherpes TL. 2013. Human female genital tract infection by the obligate intracellular bacterium Chlamydia trachomatis elicits robust type 2 immunity. PLoS One 8:e58565. doi: 10.1371/journal.pone.0058565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole AL, Yang OO, Warren AD, Waring AJ, Lehrer RI, Cole AM. 2006. HIV-1 adapts to a retrocyclin with cationic amino acid substitutions that reduce fusion efficiency of gp41. J Immunol 176:6900–6905. doi: 10.4049/jimmunol.176.11.6900. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.