Chagas disease, caused by Trypanosoma cruzi, is a major public health issue. Limitations in immune responses to natural T. cruzi infection usually result in parasite persistence with significant complications. A safe, effective, and reliable vaccine would reduce the threat of T. cruzi infections; however, no suitable vaccine is currently available due to a lack of understanding of the requirements for induction of fully protective immunity.
KEYWORDS: Trypanosoma cruzi, immunity, immunization
ABSTRACT
Chagas disease, caused by Trypanosoma cruzi, is a major public health issue. Limitations in immune responses to natural T. cruzi infection usually result in parasite persistence with significant complications. A safe, effective, and reliable vaccine would reduce the threat of T. cruzi infections; however, no suitable vaccine is currently available due to a lack of understanding of the requirements for induction of fully protective immunity. We established a T. cruzi strain expressing green fluorescent protein (GFP) under the control of dihydrofolate reductase degradation domain (DDD) with a hemagglutinin (HA) tag, GFP-DDDHA, which was induced by trimethoprim-lactate (TMP-lactate), which results in the death of intracellular parasites. This attenuated strain induces very strong protection against reinfection. Using this GFP-DDDHA strain, we investigated the mechanisms underlying the protective immune response in mice. Immunization with this strain led to a response that included high levels of gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α), as well as a rapid expansion of effector and memory T cells in the spleen. More CD8+ T cells differentiate to memory cells following GFP-DDDHA infection than after infection with a wild-type (WT) strain. The GFP-DDDHA strain also provides cross-protection against another T. cruzi isolate. IFN-γ is important in mediating the protection, as IFN-γ knockout (KO) mice failed to acquire protection when infected with the GFP-DDDHA strain. Immune cells demonstrated earlier and stronger protective responses in immunized mice after reinfection with T. cruzi than those in naive mice. Adoptive transfers with several types of immune cells or with serum revealed that several branches of the immune system mediated protection. A combination of serum and natural killer cells provided the most effective protection against infection in these transfer experiments.
INTRODUCTION
Chagas disease caused by Trypanosoma cruzi is a major public health issue, particularly in the Americas. There are approximately 10 million infected people and more than 10,000 deaths annually due to this parasite (1–3). T. cruzi infection usually results in parasite persistence in the infected host. The available therapeutic agents have limited efficacy in these chronic infections and have significant side effects that limit their usefulness (4, 5). A prospective, multicenter, randomized study examining trypanocidal therapy with benznidazole in patients with established Chagasic cardiomyopathy demonstrated that treatment resulted in a reduction in parasite detection in blood samples but did not significantly reduce cardiac clinical deterioration over a 5-year period of observation (6).
T. cruzi invades a wide variety of nucleated host cells. It has a complex network of antioxidant enzymes to protect itself from lysosomal reactive oxygen and nitrogen species (7–12). The blood form, trypomastigote, expresses calreticulin and GP160 proteins, which disrupt key components of the complement pathway (13–16). In addition, T. cruzi expresses large amounts of highly polymorphic immunogenic surface proteins, which delay production of effective antibodies and priming of CD8+ T cells (17–19). As a result, T. cruzi infection does not initiate a strong innate immune response (20–22), and the adaptive immune response is slow; thereby, the host fails to eliminate the infection.
A safe, effective, and reliable vaccine could play an important role in reducing the threat of T. cruzi infections and preventing the development of chronic infection, i.e., Chagas disease. However, no suitable vaccine is currently available for T. cruzi infection, in spite of considerable research in this area. Examination of the literature demonstrates that dead parasites, surface proteins, virus-based vaccines, and DNA vaccines only provide partial protection against this infection (23–27). A major hurdle in T. cruzi vaccine development has been a lack of understanding of the requirements for the induction of effective protective immunity. As the relationship between T. cruzi and the host immune system is intricate, it is difficult to unveil each factor. Although many of the mechanisms of immunity are understood, effective immunity induced by immunization to prevent T. cruzi infection requires further research.
In order to establish a model to study the protective immunity against T. cruzi infection, we developed an effective inducible system for T. cruzi employing a protein degradation domain based on Escherichia coli dihydrofolate reductase (ecDHFR) (the DHFR degradation domain [DDD]), which can be regulated by trimethoprim-lactate (TMP-lactate). This system can be used to express detrimental or toxic proteins, resulting in the rapid death of intracellular parasites. Subsequently, we constructed T. cruzi lines with alpha-toxin, cecropin A, and green fluorescent protein (GFP) under the control of DDD with a hemagglutinin (HA) tag (28). Interestingly, all of these DDDHA strains, including GFP-DDDHA, were attenuated in mouse experiments, producing no pathological changes and providing complete protection against a syngeneic wild-type (WT) T. cruzi infection at doses that were lethal in nonimmunized mice (28). These experiments established that stabilization of the DDDHA alone in T. cruzi was sufficient to attenuate this parasite (28).
In this report, we further characterize the effectiveness of protective immunity induced by a GFP-DDDHA transgenic parasite and report on our investigations of the mechanisms of this protective immune response. The GFP-DDDHA Tulahuen strain induces cross-protection against other T. cruzi strains. Infection with the GFP-DDDHA Tulahuen strain does not induce latent infection, and no reactivation of infection is seen with immune suppression. The immunological profile in mice immunized with the GFP-DDDHA strain demonstrates increased T-cell expansion and T-cell memory development, as well as a fast and robust protective immune response against reinfection. Adoptive transfers reveal that several branches of the immune system are important in protecting against reinfection. These studies demonstrate that a combination of serum and natural killer (NK) cells provides very effective protection in recipient mice, indicating that these rapid-acting components of the immune system are important criteria for T. cruzi vaccine design.
RESULTS
Immunization with the GFP-DDDHA T. cruzi strain provides cross-protection and does not cause persistent infection.
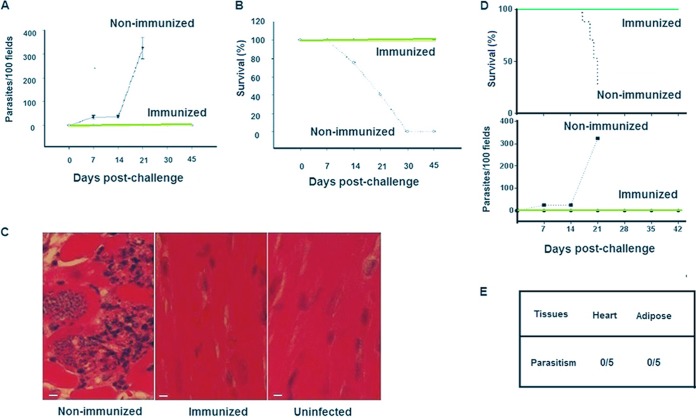
Previously, we reported that immunization with DDDHA transgenic T. cruzi strains induced strong protection against challenge with WT T. cruzi of the same background (28). Since T. cruzi strains have genetic variations, we conducted a cross-protection study to evaluate if inoculation of the GFP-DDDHA Tulahuen strain could protect mice against challenge with the Brazil strain. C3H mice were inoculated with 5,000 tissue trypomastigotes of the GFP-DDDHA Tulahuen strain per mouse, treated with TMP-lactate in drinking water after 7 days postinfection (dpi), and challenged with 5 × 105 WT Brazil strain tissue trypomastigotes per mouse after 42 dpi with the GFP-DDDHA Tulahuen strain. In nonimmunized mice, this challenge resulted in high parasitemia, and all of the infected mice died during the acute phase of the infection. However, all of the mice immunized with the GFP-DDDHA Tulahuen strain survived the challenge infection with the Brazil strain, and none of them developed parasitemia (Fig. 1A and B). Pathological examination of the heart (hematoxylin and eosin [H&E]-stained tissue sections) demonstrated that nonimmunized C3H mice infected with 5 × 105 Brazil strain trypomastigotes had extensive tissue infection, with many amastigotes being seen in the myocardium, along with extensive myocarditis. In contrast, mice immunized with the GFP-DDDHA Tulahuen strain did not exhibit myocarditis after challenge with the WT Brazil strain (Fig. 1C).
FIG 1.
The GFP-DDDHA strain provides cross-protection and does not cause persistent infection. (A) C3H mice were inoculated with the GFP-DDDHA Tulahuen strain and then challenged with the Brazil strain. Immunized mice did not develop parasitemia, while nonimmunized mice developed high parasitemia (n = 10). (B) Immunized mice survived the challenge, while nonimmunized mice all died during the acute infection phase (n = 10). (C) Histology (hematoxylin and eosin [H&E] staining) of heart tissues at day 14 postchallenge demonstrates that nonimmunized C3H mice infected with the Brazil strain had numerous amastigotes within myocardial tissue, associated with extensive myocarditis, while GFP-DDDHA-immunized mice infected with the Brazil strain had no amastigotes and did not exhibit myocarditis. ×20 magnification. Bar, 25 μm. (D) C57BL/6 mice immunized with the GFP-DDDHA Tulahuen strain and then challenged with half a million wild-type Tulahuen strain parasites. Similarly to C3H mice, immunized mice did not develop parasitemia and survived, while nonimmunized mice developed high parasitemia and all died in the acute phase (n = 10). (E) C57BL/6 mice immunized with the GFP-DDDHA Tulahuen strain did not have persistent infection, as real-time PCR did not detect T. cruzi DNA in heart and adipose tissues (n = 5). See Materials and Methods for details. Data presented are one representative example of two separate experiments. Both experiments produced similar results.
Because the majority of gene knockout (KO) mice that were required for further immune studies were on a C57BL/6 background, we also examined if immunization with the GFP-DDDHA strain in C57BL/6 mice provided the same protection as that demonstrated in C3H mice (28). C57BL/6 mice were inoculated with 5,000 GFP-DDDHA Tulahuen strain trypomastigotes, and, after 7 dpi, mice were treated with TMP-lactate for 35 days and then challenged with 5 × 105 WT T. cruzi parasites. A similar level of protection to that seen in C3H mice was found in C57BL/6 mice (Fig. 1D). In addition, immunized mice were still fully protected at 4, 6, 7, 9, 12, and 15 months after immunization and showed strong protection at each time point when challenged with the lethal wild-type strain (Fig. S1). Interestingly, as the GFP-DDDHA strain is attenuated, immunization with this strain, even without further treatment with TMP-lactate, also protected mice from challenge with wild-type T. cruzi. C3H and C57BL/6 mice that were immunized with the GFP-DDDHA strain without TMP-lactate treatment still showed strong protection and cross-protection against reinfection (Fig. S2). Interestingly, the heat-killed GFP-DDDHA strain did not induce any protection, indicating that infection was required to elicit the protection (data not shown).
To determine if inoculation with the GFP-DDDHA strain in C57BL/6 mice might cause persistent infection, immunized mice (5 × 103 GFP-DDDHA parasites) were immunosuppressed at 240 dpi with cyclophosphamide (200 mg/kg) by intraperitoneal (i.p.) injection at 3- to 4-day intervals, for a total of 3 doses. None of these mice developed detectable parasitemia during the 30-day postimmunosuppression follow-up period (data not shown). Furthermore, quantitative PCR (qPCR) using T. cruzi-specific primers (29) did not detect T. cruzi DNA in any tested tissue at the end of this 30-day period. These data indicate that immunization with the GFP-DDDHA Tulahuen strain followed by treatment with TMP-lactate in C57BL/6 mice does not result in a chronic latent infection and that there was no persistence of the GFP-DDDHA strain following infection (Fig. 1E).
Immunization with the GFP-DDDHA T. cruzi strain induces cytokine production.
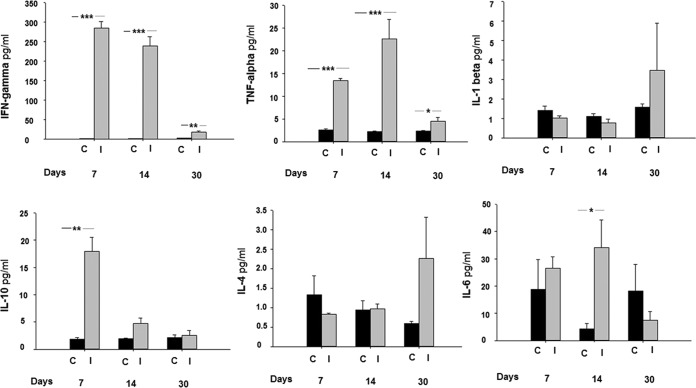
To examine the profile of cytokines induced by immunization with the GFP-DDDHA strain, serum samples from C57BL/6 mice immunized with the GFP-DDDHA strain from 7 to 30 dpi were analyzed using a multiplex assay. The most striking finding was a marked increase in serum gamma interferon (IFN-γ) in mice immunized with the GFP-DDDHA strain. Unimmunized mice did not have detectable IFN-γ, while immunized mice displayed a strong induction of serum IFN-γ at both 7 and 14 dpi that persisted at 30 dpi (Fig. 2). Similarly, tumor necrosis factor alpha (TNF-α) levels were markedly elevated in the sera of immunized mice at 7 and 14 dpi and remained significantly higher at 30 dpi than those in unimmunized mice (Fig. 2). There were also increases in the levels of interleukin 10 (IL-10) at 7 dpi and IL-6 at 14 dpi (Fig. 2). There were no significant changes in the levels of IL-1β or IL-4 from 7 to 30 dpi (Fig. 2).
FIG 2.
Mice immunized with the GFP-DDDHA strain exhibited high systemic IFN-γ and TNF-α levels. C57BL/6 mice immunized with the GFP-DDDHA Tulahuen strain from 7 to 30 dpi had serum cytokines measured using a multiplex assay. Note the elevated levels of IFN-γ and TNF-α in the GFP-DDDHA mice. C, control; I, inoculated with the GFP-DDDHA strain. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (unpaired two-tailed t test between immunized and unimmunized mice; n = 5). Data presented are one representative example of two separate experiments. Both experiments produced similar results.
Immunization with the GFP-DDDHA T. cruzi strain induces CD4+ and CD8+ T cell expansion and generates T-cell memory.
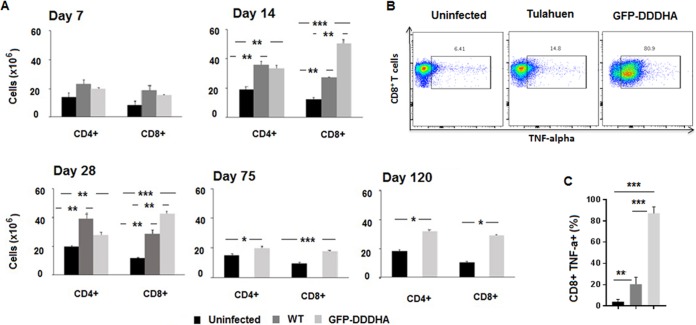
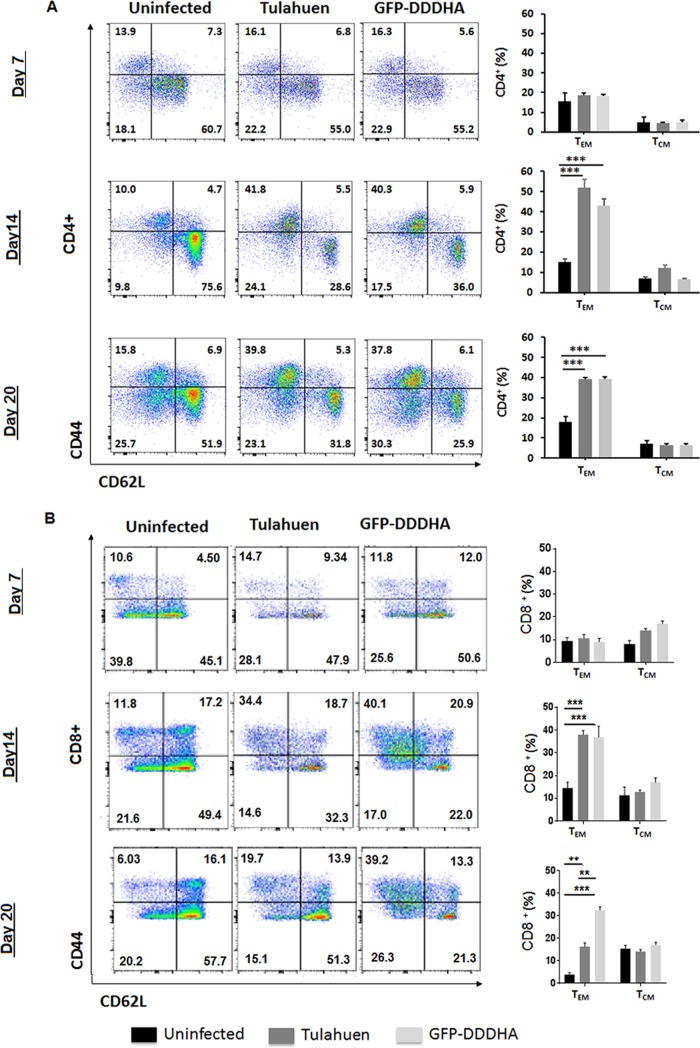
To investigate the effects of infection with the GFP-DDDHA strain on CD4+ and CD8+ T cells, we conducted a kinetic analysis of the expansion of the CD4+ and CD8+ T-cell compartment and compared the differences between uninfected mice and mice infected with the GFP-DDDHA or the WT Tulahuen strains. C57BL/6 mice were inoculated with the GFP-DDDHA strain or the WT Tulahuen strain (5,000 tissue-derived trypomastigotes per mouse for each strain) and sacrificed at different time points following infection. The numbers of CD4+ and CD8+ T cells in the spleen were measured by fluorescence-activated cell sorting (FACS) and compared to those populations in uninfected mice. Expansions in the CD4+ and CD8+ T cell populations were observed in mice infected with either strain starting at day 7 and continuing 4 weeks into the infection. These expansions were still evident at 75 and 120 dpi in the GFP-DDDHA-infected mice. Mice infected with the WT strain did not survive the infection, so we could not assess these cells in the WT strain at these late time points. While the magnitude of the increase in the numbers of CD4+ T cells was similar in mice infected with either strain, mice inoculated with the GFP-DDDHA strain showed a significantly larger increase in the numbers of CD8+ T cells present in the spleen (Fig. 3A). The GFP-DDDHA strain induced CD8+ T cells to express much higher TNF-α levels at 20 dpi than those of the WT strain (Fig. 3B and C). In addition, expansion of T cells in mice immunized with the GFP-DDDHA strain was associated with higher generation of CD8+ CD44+ CD62Llow effector/effector memory T cells than that in uninfected or WT-infected mice. However, in GFP-DDDHA strain-immunized mice, the expansion of the CD4+ CD44high CD62Llow effector/effector T cell population, although greater than that in uninfected mice, was similar to that in WT-infected mice (Fig. 4A and B).
FIG 3.
CD4+ and CD8+ T-cell expansion during immunization with the GFP-DDDHA Tulahuen strain. (A) C57BL/6 mice were inoculated with either the GFP-DDDHA Tulahuen strain or a WT strain and then sacrificed at the indicated time points following infection. The numbers of CD4+ and CD8+ T cells in the spleen were measured by fluorescence-activated cell sorting (FACS) and compared to those populations in uninfected mice. Mice infected with the WT strain died after 28 days postinoculation. The graph represents the average ± standard error of the mean (SEM) of data obtained from 3 mice per time point and condition. Data were analyzed by analysis of variance (ANOVA) with Tukey’s posttest (days 7 to 28) or unpaired two-tailed t test (days 75 to 120). *, P < 0.05; **, P < 0.01; ***, P < 0.001 (infected versus uninfected mice). Data presented are one representative example of three separate experiments. All three experiments produced similar results. (B) Flow cytometry analysis show that CD8+ T cells in splenocytes from C57BL/6 mice with the GFP-DDDHA Tulahuen strain expressed much higher TNF-α levels at 21 dpi than those of uninfected mice and mice infected with the WT strain. (C) Plots from flow cytometry analysis. Data were analyzed by ANOVA with Tukey’s posttest **, P < 0.01; ***, P < 0.001 (n = 5). Data presented are one representative example of three separate experiments. All three experiments produced similar results.
FIG 4.
The GFP-DDDHA strain favors development of T-cell memory. C57BL/6 mice were infected with wild-type (WT) T. cruzi or the GFP-DDDHA strain. Mice were then sacrificed at different time points following infection, and the distribution of naive (CD44− CD62Lhigh), effector memory (CD44+ CD62Llow), and central memory (CD44+ CD62Lhigh) CD4+ and CD8+ T cells in the spleen was measured by FACS and compared to the distribution of those populations in uninfected mice and WT strain-infected mice. (A) FACS analysis of the distribution of the populations of CD4+ T cells (left). Plots from the data (right, n = 3). (B) FACS analysis of the distribution of the populations of CD8+ T cells (left). Plots from the data (right, n = 3). Note that immunization with the GFP-DDDHA strain stimulates more CD8+ T-cell effector memory than that in WT strain infection. TEM, effector memory T cells; TCM, central memory T cells. Data were analyzed by ANOVA with Tukey’s posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data presented are one representative example of three separate experiments. All three experiments produced similar results.
IFN-γ is important in mediating GFP-DDDHA strain-induced protections.
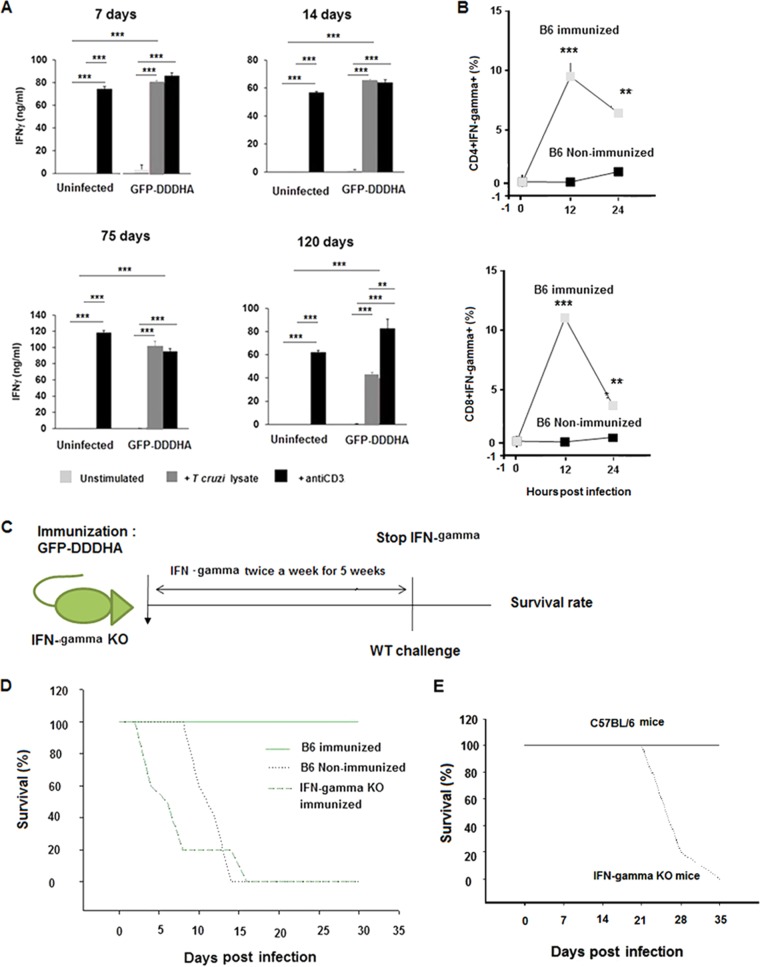
To determine if the expansion of the effector T cell populations was specific for T. cruzi, we analyzed the production of cytokines in response to parasite antigens. Total splenocytes from the GFP-DDDHA-infected and uninfected mice were activated with protein lysates from T. cruzi or with anti-CD3 antibody. While, as expected, splenocytes from uninfected mice failed to produce IFN-γ in response to T. cruzi lysates, those isolated from GFP-DDDHA-infected mice showed a robust response with high levels of IFN-γ secretion. The magnitude of IFN-γ production in cells activated with parasite lysates was similar to the response to anti-CD3 antibody, indicating that most of the T cells in the spleen of infected mice were responsive to T. cruzi (Fig. 5A). No significant levels of IL-4 or IL-17 production were detected in T. cruzi lysate-stimulated cells from the GFP-DDDHA-infected mice (data not shown). To determine if CD4+ and CD8+ T cells expressed IFN-γ during a recall response, GFP-DDDHA-immunized mice were challenged with half a million WT parasites, and the expression of IFN-γ in CD4+ and CD8+ T cells was analyzed by FACS. Consistent with the in vitro assays, expression of IFN-γ in CD4+ and CD8+ T cells from immunized mice was readily detected at 12 and 24 h postchallenge, whereas it was barely detectable in CD4+ and CD8+ T cells from naive mice (Fig. 5B).
FIG 5.
Immunization with the GFP-DDDHA strain enhances IFN-γ expression during antigen reencounter. (A) C57BL/6 mice were inoculated with the GFP-DDDHA Tulahuen strain and sacrificed at the indicated time points. Single-cell suspensions of splenocytes were then left unstimulated or were stimulated with T. cruzi lysates or anti-CD3 antibodies. IFN-γ production was measured by enzyme-linked immunosorbent assay (ELISA). Graphs represent the average ± SEM of data obtained from 3 mice per time point and condition. Data were analyzed by ANOVA with Tukey’s posttest (days 7 to 14) or unpaired two-tailed t test (days 75 to 120). In summary, all uninfected cells stimulated with antibodies were significantly different (P < 0.001) from resting cells or cells stimulated from lysates, whereas in GFP-DDDHA strain-infected mice no differences were found between cells stimulated with lysates or with antibodies (except at day 120; P < 0.01), and both of them are different from the resting condition (P < 0.001) at any time point. All GFP-DDDHA strain-infected cells stimulated with lysates were different from uninfected cells stimulated with lysates (P < 0.001). **, P < 0.01; ***, P < 0.001. Data presented are one representative example of three separate experiments. All three experiments produced similar results. (B) Immunized C57BL/6 mice at 42 days postimmunization were reinfected with half a million WT Tulahuen parasites at 12 and 24 h postinfection. Splenocytes were analyzed by FACS and gated on CD4+ and CD8+ T cells, and the percentage of cells expressing IFN-γ was determined. Note that CD4+ and CD8+ T cells from immunized mice expressed much higher IFN-γ levels than those of nonimmunized mice. Data were analyzed with an unpaired two-tailed t test. **, P < 0.01; ***, P < 0.001. Data presented are one representative example of three separate experiments. All three experiments produced similar results. (C) Schematic representation of IFN-γ knockout mouse immunization, treatment with recombinant IFN-γ, and then challenge with WT parasites. Eight-week-old IFN-γ knockout mice and age-matched C57BL/6 mice were immunized with the GFP-DDDHA strain and then challenged with a WT infection. To allow protection to establish and to specifically analyze the role of IFN-γ in the secondary response to lethal infection, IFN-γ KO mice were administered recombinant IFN-γ (1.2 μg [1,000 U] per /mouse) twice weekly i.p. for 5 weeks starting 1 day postadministration of the GFP-DDDHA strain. After 5 weeks, treatment with IFN-γ was stopped, and the next day, following IFN-γ removal, the immunized IFN-γ-treated IFN-γ KO mice and the immunized C57BL/6 mice, as well as a group of nonimmunized C57BL/6 mice, were infected with 5 × 105 wild-type Tulahuen strain parasites. (D) Survival curve of IFN-γ knockout mice and control mice. Nonimmunized C57BL/6 mice and the immunized treated IFN-γ KO mice died with this lethal infection by day 17, while the immunized C57BL/6 mice all survived. This suggests that IFN-γ is important in mediating protection after immunization. Data presented are one representative example of two separate experiments. Both experiments produced similar results. (E) IFN-γ knockout mice died during GFP-DDDHA strain infection. Eight-week-old IFN-γ knockout mice were infected with 5 × 103 GFP-DDDHA Tulahuen strain parasites and received TMP-lactate treatment on day 7 postinfection. All mice died within 35 days postinfection, indicating that IFN-γ is essential for the development of immunity against T. cruzi (n = 10).
To further examine the role of IFN-γ in mediating protection, 8-week-old B6.129S7-Ifngtm1Ts/J mice (IFN-γ knockout mice) and age-matched C57BL/6 mice were immunized with GFP-DDDHA T. cruzi, and then challenged with a lethal infection. IFN-γ−/− mice were not able to survive the same dosage of GFP-DDDHA strain used for wild-type mouse immunization, even with TMP-lactate treatment. This indicates a critical role for IFN-γ in the control of the primary infection (Fig. 5E). To specifically analyze the role that this cytokine might play in the generation of protection and to specifically evaluate the role of IFN-γ in the secondary response to lethal infection, IFN-γ KO mice were administered recombinant IFN-γ (1.2 μg [1,000 U] per mouse) twice weekly i.p. for 5 weeks starting 1 day postimmunization with the GFP-DDDHA strain, so they would survive the primary infection with this strain. After 5 weeks, treatment with IFN-γ was stopped, and then immunized IFN-γ-treated IFN-γ KO mice, immunized C57BL/6 mice (i.e., WT-immunized controls), and nonimmunized C57BL/6 mice were infected with 5 × 105 wild-type Tulahuen strain parasites. Nonimmunized C57BL/6 mice and immunized IFN-γ KO mice treated with IFN-γ during immunization died from this lethal infection by day 17, while all of the immunized C57BL/6 mice survived. These data suggest that IFN-γ is important in mediating protection against T. cruzi (30, 31), that IFN-γ mediates protection during the response to lethal reinfection after immunization in this system, and/or that IFN-γ is required to prevent reactivation of the vaccine strain (Fig. 5C and D).
Immunization with the GFP-DDDHA strain induces early and strong recall responses in immune cells.
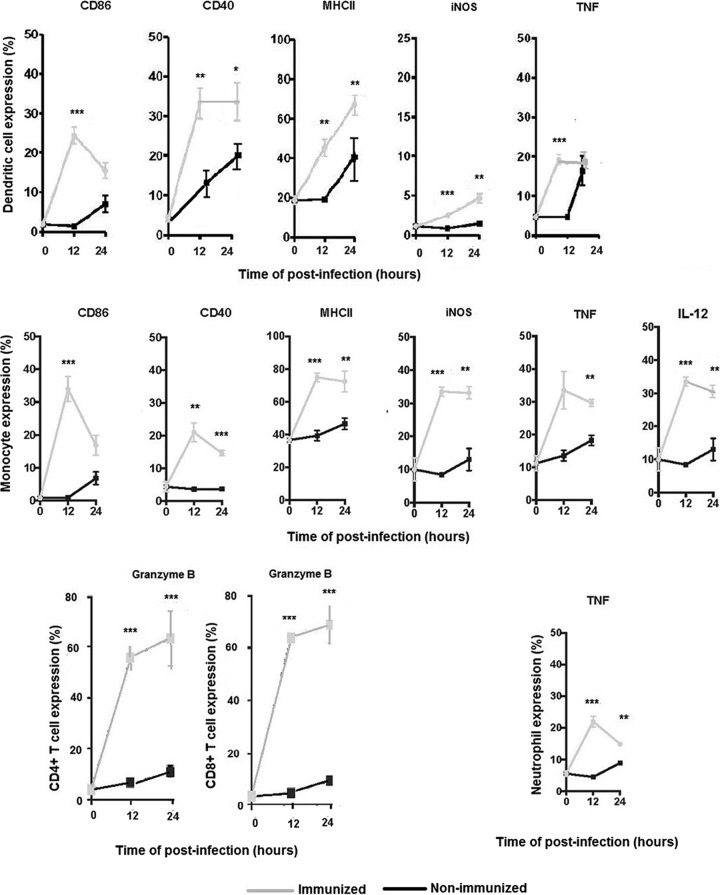
During immunization with the GFP-DDDHA strain, T. cruzi-specific T cells undergo priming, expand, and differentiate into memory cells that acquire enhanced functional features and quickly express high levels of effector cytokines. IFN-γ always appears as a key cytokine produced by all subsets of T cells and is often essential for effective protection. Cells of the innate immune system are also essential for early sensing and for protective inflammatory responses against infection (32). Therefore, we characterized the recall responses in GFP-DDDHA strain-immunized and nonimmunized mice. We evaluated CD4+ and CD8+ T cells, dendritic cells (DCs), blood-derived monocytes, and neutrophils. CD4+ and CD8+ T cells expressed high levels of IFN-γ and granzyme B in immunized mice early during the recall response (Fig. 5B and 6 and Fig. S5), which were not observed in nonimmunized mice, suggesting that memory T cells in immunized mice were rapidly recalled to become effector cells. Dendritic cells and monocytes showed increased expression of costimulatory proteins, as well as effector molecules such as MHCII, CD86, CD40, iNOS, and TNF-α, in immunized mice but not in naive mice. Monocytes also expressed IL-12 in immunized mice (Fig. 6 and Fig. S3 and S4). In addition, neutrophils quickly expressed TNF-α in immunized mice during recall response (Fig. 6 and Fig. S3). These data indicate that innate immune cells can quickly be mobilized and differentiate into robust effector cells important for the initial control of T. cruzi reinfection in mice immunized with the GFP-DDDHA strain.
FIG 6.
Immunization with the GFP-DDDHA strain induces early and strong recall responses in immune cells. Immunized C57BL/6 mice at 42 days postimmunization were reinfected with half a million WT Tulahuen parasites at 12 and 24 h postinfection. Splenocytes were analyzed by FACS and gated on dendritic cells, monocytes, neutrophils, and CD4+ and CD8+ T cells, and the percentage of cells expressing activation or effector molecules was determined. Note that dendritic cells and monocytes from immunized mice have early strong responses, by expressing CD86, CD40, MHCII, iNOS, TNF-α, and IL-12, compared to those of nonimmunized mice. A subset of effector memory T cells (CD44+ CD62L− CD4+ and CD44+ CD62L− CD8+) from immunized mice quickly and strongly expressed granzyme B compared to nonimmunized mice, and neutrophils expressed a higher level of TNF-α than that of nonimmunized mice. Data were analyzed with an unpaired two-tailed t test. *, P < 0.05; **, P < 0.01; ***, P < 0.001. Data presented are one representative example of three separate experiments. All three experiments produced similar results.
Immunization with the GFP-DDDHA strain induces both humoral and cellular protective responses.
The T. cruzi life cycle has a replicative phase within the host intracellular environment, and, for a few decades, the cytotoxic T lymphocyte response has been the predominant subject investigated as a protective response. CD8+ T cells are an established factor in controlling parasite load in infected hosts (18, 33–35). However, after immunization, which cells of the immune system may be responsible for providing strong protection and limiting T. cruzi initial infection remains unanswered. Thus, to investigate this subject, we performed adoptive transfers of serum or immune cells from GFP-DDDHA strain-immunized mice into naive mice and infected them with wild-type T. cruzi.
Although all mice receiving T cells still developed parasitemia at 7 dpi, reduced parasitemia was observed at 14 dpi, and at 21 dpi, no recipient mice had detectable parasitemia (Table 1). Furthermore, at 60 dpi, mice that received total T cells from immunized mice appeared healthy, with no mortality when using a sublethal dose for infection (Table 2), suggesting that while T cells alone do not act early enough to prevent infection, they play an important role in controlling T. cruzi growth at later phases of the infection, which is consistent with the literature (18, 33–35).
TABLE 1.
Adoptive transfer and parasitemiaa
| No. of days postinfection | Parasitemia (mean ± SEM) in micee
|
||
|---|---|---|---|
| Nontransfer | Naive control | Immunized | |
| T cell | |||
| 7 | 22 ± 5.8 | 16 ± 4.00 | 12 ± 3.74 |
| 14 | 16 ± 4.00 | 8 ± 3.74 | 2 ± 2.00b |
| 21 | 36 ± 10.77 | 25 ± 6.45 | 0 ± 0b ,d |
| NK cell | |||
| 7 | 14 ± 9.27 | 20 ± 9.27 | 12 ± 4.9 |
| 14 | 6 ± 2.45 | 20 ± 3.16 | 0 ± 0b ,c |
| 21 | 8 ± 2.00 | 15 ± 7.7 | 1.67 ± 1.67b ,c |
| Serum | |||
| 7 | 14 ± 9.27 | 10 ± 5.48 | 0 ± 0b ,d |
| 14 | 6 ± 2.45 | 12 ± 3.74 | 0 ± 0b ,c |
| 21 | 8 ± 2.00 | 10 ± 2.5 | 1.67 ± 1.67b ,c |
| Serum and NK cell | |||
| 7 | 14 ± 9.27 | 18 ± 3.47 | 0 ± 0b ,d |
| 14 | 6 ± 2.45 | 16 ± 5.1 | 0 ± 0b ,c |
| 21 | 8 ± 2.00 | 12 ± 3.5 | 0 ± 0b ,c |
| B cell | |||
| 7 | 22 ± 5.8 | 20 ± 9.57 | 8 ± 8.00 |
| 14 | 16 ± 4.00 | 25 ± 11 | 0 ± 0b ,c |
| 21 | 36 ± 10.77 | 30 ± 5.5 | 0 ± 0b ,d |
Several types of immune cells and serum from GFP-DDDHA strain immunized mice were given to naive C57BL/6 mice (aged between 10 and 14 weeks) by adoptive transfer. Mice without adoptive transfer or with naive control adoptive transfer were used for comparison (see Materials and Methods for details). After 24 h of postadoptive transfer, mice were infected with 5 × 103 WT Tulahuen trypomastigotes, which is sublethal for 10- to 14-week-old C57BL/6 mice. Two batches of mice were used for these experiments, one batch for T-cell and B-cell experiments and another batch for serum and NK cell experiments.
P < 0.05 compared to nontransfer mice.
P < 0.05 compared to mice that received the naive control.
P < 0.01 compared to mice that received the naive control.
Parasitemia (number of parasites/100 fields) was determined by light microscopy with 100 random fields at ×20 magnification. Parasitemia values were analyzed by unpaired two-tailed t test to determine the statistical significance between transferred mice, nontransfer mice, and mice with naive control. n = 5 to 10. Data presented are one representative example of four separate experiments. All four experiments produced similar results.
TABLE 2.
Adoptive transfer and survival ratea
| No. of days postinfection | Mouse survival rate (%)d
|
||
|---|---|---|---|
| Nontransfer | Naive control | Immunized | |
| T cell | |||
| 7 | 100 | 100 | 100 |
| 14 | 100 | 100 | 100 |
| 21 | 100 | 100 | 100 |
| 28 | 80 | 80 | 100 |
| 35 | 60 | 60 | 100 |
| 60 | 60 | 60 | 100b ,c |
| NK cell | |||
| 7 | 100 | 100 | 100 |
| 14 | 100 | 100 | 100 |
| 21 | 100 | 100 | 100 |
| 28 | 80 | 80 | 100 |
| 35 | 60 | 60 | 100 |
| 60 | 60 | 60 | 100b ,c |
| Serum | |||
| 7 | 100 | 100 | 100 |
| 14 | 100 | 100 | 100 |
| 21 | 100 | 100 | 100 |
| 28 | 80 | 80 | 100 |
| 35 | 60 | 60 | 100 |
| 60 | 60 | 60 | 100b ,c |
| Serum and NK cell | |||
| 7 | 100 | 100 | 100 |
| 14 | 100 | 100 | 100 |
| 21 | 100 | 100 | 100 |
| 28 | 80 | 80 | 100 |
| 35 | 60 | 60 | 100 |
| 60 | 60 | 60 | 100b ,c |
| B cell | |||
| 7 | 100 | 100 | 100 |
| 14 | 100 | 100 | 100 |
| 21 | 100 | 100 | 100 |
| 28 | 80 | 80 | 100 |
| 35 | 60 | 60 | 100 |
| 60 | 60 | 60 | 100b ,c |
Several types of immune cells and serum from GFP-DDDHA strain-immunized mice were given to naive C57BL/6 mice (aged between 10 and 14 weeks) by adoptive transfer. Mice without adoptive transfer or with naive control adoptive transfer were used for comparison (see Materials and Methods for details). After 24 h of postadoptive transfer, mice were infected with 5 × 103 WT Tulahuen trypomastigotes, which is sublethal for 10- to 14-week-old C57BL/6 mice. Survival rates were documented through the acute phase to the subacute phase.
P < 0.01 compared to nontransfer mice.
P < 0.01 compared to mice that received the naive control.
The survival rates of mice were analyzed by unpaired two-tailed t test to determine the statistical significance between transferred mice, nontransfer mice and mice with naive control. n = 5 to 10. Data presented are one representative example of four separate experiments. All four experiments produced similar results.
Recipient mice that received NK cells from immunized mice developed parasitemia at 7 dpi but did not show parasitemia at 14 dpi and had significant lower parasitemia at 21 dpi (Table 1). These mice did not die when infected with a sublethal dose (Table 2). Transfer of immunized sera significantly reduced parasitemia. Mice did not show parasitemia at 7 or 14 dpi and had significant lower parasitemia at 21 dpi, suggesting that antibodies may mediate early killing of T. cruzi (Table 1). Serum transfer also prevented mortality in a sublethal dose infection (Table 2). Mice receiving immunized total B cells developed parasitemia at 7 dpi but eliminated the parasite at 14 and 21 dpi (Table 1). In addition, none of these mice died in response to a sublethal dose infection (Table 2). Strikingly, when mice received both immunized serum and immunized NK cells, no mice developed parasitemia at any time point after infection (Table 1), and all mice were healthy with no mortality (Table 2), indicating that there is a synergetic effect of antibody and NK cells to develop a strong early protective response and that antibody-dependent cellular cytotoxicity may be an important mechanism for initial killing of T. cruzi.
DISCUSSION
It has been generally believed that an efficient protective immune response to T. cruzi requires the elicitation of Th1 cytokines, lytic antibodies, and the concerted activities of phagocytes, T-helper cells and cytotoxic T lymphocytes. In spite of innate and adapted immune responses, infection usually results in parasite persistence in the infected host. T. cruzi has the ability to evade the immune system (7–19), and initial T. cruzi invasion of mammalian cells does not induce strong innate immune responses (22). The development of adaptive immunity to T. cruzi infection is relatively slow, with delayed development of T. cruzi-specific CD8+ T effector cells (22), which is caused by various factors, including a weak innate immunity against this organism. Therefore, immunization should be the solution to induce effective immune responses.
One of the main reasons preventing the development of an efficient T. cruzi vaccine is our limited knowledge of how protective immunity against T. cruzi infection may develop. Among the most important questions we are asking are the following. What are the most effective immune responses mediating effective killing of an initial T. cruzi infection? How can we immunize a host so that its immune system can prevent T. cruzi from reaching sites where it persists, such as muscle and fat? A reliable model to investigate these questions is likely to yield some very valuable answers.
Previously, we reported the establishment of an inducible gene expression system for T. cruzi employing a degradation domain based on Escherichia coli dihydrofolate reductase (ecDHFR). The DHFR degradation domain (DDD) can be stabilized by trimethoprim-lactate and can be used to express detrimental or toxic proteins. T. cruzi lines with alpha-toxin, cecropin A, and GFP under the control of DDD with a hemagglutinin tag (HA) were developed (28). When the DDDHA degradation domain is stabilized by TMP-lactate, it induces amastigote death (28). Inoculation of any of the DDDHA strains we produced did not establish parasitemia in C3H and C57BL/6 mice and provided fully protective immunity against a lethal syngeneic WT T. cruzi infection (28).
In this study, we tested if immunization with the GFP-DDDHA strain can provide protection against T. cruzi parasites of a different genetic background. The diversity of the T. cruzi genome is well recognized (36–38). Currently, six discrete typing units (DTUs) are defined (37, 39). Mice immunized with the GFP-DDDHA Tulahuen strain (DTU Tc VI) became highly resistant to WT Brazil strain (DTU Tc I) challenge and did not develop parasitemia or cardiac inflammation, while unimmunized mice developed high parasitemia and parasitism in the heart with severe myocarditis, which caused all unimmunized mice to die during the acute phase of infection (Fig. 1). The cross-protection also suggests that conserved regions of the genomes of T. cruzi from different genetic backgrounds may generate certain common antigens that can stimulate the immune system against different types of T. cruzi. In addition, the cross-protection may be due to the enhancement of innate immune system responses by immunization.
We also examined if inoculation of the GFP-DDDHA strain would result in persistent infection in the inoculated mice. C57BL/6 mice inoculated with the GFP-DDDHA Tulahuen strain that were immunosuppressed on day 240 dpi did not develop parasitism in heart and adipose tissues, which are reservoirs for T. cruzi infection (29), even when assessed by qPCR, indicating that there is no persistent infection from the GFP-DDDHA T. cruzi strain. Immunized mice were still fully protected at 9, 12, and 15 months after immunization and showed strong protection at each time point when challenged with the lethal wild-type strain (Fig. S1). This provides evidence that protective immunity was induced without parasite persistence in tissue.
To understand the systemic immune responses to GFP-DDDHA T. cruzi, we examined the profile of cytokines in mice immunized with this strain. Two cytokines, IFN-γ and TNF-α, were significantly elevated in sera from immunized mice, while barely detectable in unimmunized mice. IFN-γ is critical in orchestrating Th1 responses, which have been thought to be more effective in eliminating T. cruzi and other intracellular pathogens (30). Both IFN-γ and TNF-α are strong inducers of inducible nitric oxide synthase, which produces nitric oxide and is largely responsible for macrophage-mediated killing of T. cruzi (31). Overall, the cytokines induced by the GFP-DDDHA strain likely skew the immune response toward a more effective Th1 response that should facilitate better control of WT T. cruzi infections (e.g., challenge infections).
We also investigated the expansion of CD4+ and CD8+ T cell populations in mice immunized with the GFP-DDDHA strain and compared these to those of mice infected with WT T. cruzi. The WT T. cruzi-infected mice died during the acute phase of the infection and showed an induced expansion of CD4+ T cells to levels similar to those in the GFP-DDDHA T. cruzi-infected mice. However, the expansion of CD8+ T cells and TNF-α-positive CD8+ T cells was significantly higher in GFP-DDDHA strain-inoculated mice. This increase may be due to the death of the GFP-DDDHA strain induced by TMP-lactate stabilization of the DDDHA domain, which could release a wider repertoire of antigenic signals or prevent any immunomodulatory effect of live T. cruzi on the generation of CD8+ T cell responses. The expansion of CD8+ T cells was still evident at 75 and 120 dpi with the GFP-DDDHA strain, supporting the generation of robust T-cell memory in these mice. Inoculation of the GFP-DDDHA strain induced significantly more differentiation of effector memory CD8+ T cells at 20 dpi than WT infection. Indeed, the profile of those cells supports the expansion of effector memory T cells, which could play a key role in the generation of protection against T. cruzi infection.
The expansion of T cells induced by the GFP-DDDHA strain was specific for T. cruzi. T. cruzi lysate was able to induce a robust secretion of IFN-γ that was similar to the response elicited by an anti-CD3 antibody, indicating that most of the expanded T cells in the spleen of GFP-DDDHA-infected mice were responsive to T. cruzi antigens. CD4+ and CD8+ T cells in GFP-DDDHA-immunized mice rapidly expressed IFN-γ when the mice were challenged with WT parasites. GFP-DDDHA-immunized IFN-γ KO mice failed to survive WT T. cruzi challenge, indicating that IFN-γ is important in mediating protection. Therefore, the induction of IFN-γ could be an important factor to determine the effectiveness of protection against T. cruzi infection (40–42), as IFN-γ plays a key role in the regulation of both innate and acquired antimicrobial immunity (32, 43).
Immune cells in GFP-DDDHA-immunized mice mounted an early and robust reaction when mice were challenged with WT T. cruzi, while there was only a weak and slow reaction in nonimmunized mice. CD4+ and CD8+ T cells expressed IFN-γ and granzyme B, indicating that memory T cells differentiate to effector cells and participate in controlling T. cruzi infection. Dendritic cells and monocytes expressed molecules involved in antigen presentation, costimulation, and effector functions in controlling T. cruzi infection. In addition, neutrophils, a first-line defender of T. cruzi infection, expressed TNF-α as a fast-activation molecule. The mechanisms for quick onset of innate response after immunization require further investigation. It is likely that innate cells receive early signals from memory T cells (32) or that innate cells detect antibody-bound T. cruzi antigens that trigger cell activation. Immunization has also been reported to increase innate cell responses due to “trained immunity” (44). Overall, immunization with the GFP-DDDHA strain in mice induces a fast and strong innate and adaptive immune response to T. cruzi infection, which is not seen in naive mice.
Adoptive transfer of T cells provided protection in reducing parasitemia at late times during infection but failed to prevent early parasitemia, indicating that T-cell killing of initial T. cruzi bacteria is not sufficient to prevent infection. T cells need to recognize cognate antigens in order to mount a response. It should be noted that tissue-resident memory T cells act fast locally; however, they normally cannot survive in recipient mice because they lose association with the support environments once they are isolated (45).
Recipients of B cells from immunized mice had low parasitemia at 7 dpi, which disappeared by day 14. All recipient mice survived the infection. B cells secrete antibodies and cytokines and participate in antigen presentation to control T. cruzi infection (46, 47). Recipients of NK cells from immunized mice still developed parasitemia at early time points but showed reduced parasitemia at later time points. Sera from immunized mice prevented parasitemia at early time points, suggesting that antibodies act in a rapid way to prevent establishment of T. cruzi infection.
Transfer of NK cells from immunized mice also improved the survival rate. NK cells play a central role in the innate immune response, especially in infections by intracellular pathogens. Cytotoxicity directed to the elimination of infected or damaged cells is one of their main effector mechanisms. Activated NK cells are also potent producers of IFN-γ, which activates macrophages and biases naive T-cell differentiation toward a TH1 profile. Memory NK cells have also been discovered in some settings of infection or contact hypersensitivity (48–55). Whether NK cells can differentiate to memory cells by GFP-DDDHA strain immunization will require further investigation.
The most striking observation is the protection conferred by the combination of NK cells and serum. The recipient mice never developed parasitemia, and no mice died, indicating that this combination is important in an early effective response to prevent T. cruzi infection. It is likely that antibodies and NK cells kill T. cruzi via antibody-dependent cellular cytotoxicity.
In summary, we have created a suicidal T. cruzi strain, based on the DDDHA domain, that results in fully protective immunity against T. cruzi challenge infections. Furthermore, this GFP-DDDHA T. cruzi strain induces cross-protective immunity against T. cruzi infection from other genetic linages. GFP-DDDHA T. cruzi does not persist and does not cause a chronic or latent infection in mice. The immune response to GFP-DDDHA T. cruzi suggests that this protective response is based on a Th1 response and that IFN-γ is important in controlling T. cruzi infection. Recall responses of immune cells in GFP-DDDHA T. cruzi-immunized mice are fast and robust. Interestingly, adoptive transfers reveal that several mechanisms of immune protection are induced by the immunization. The combination of sera and NK cells provides effective protection against T. cruzi initial infection. Therefore, both components should be major considerations for vaccine design against T. cruzi infection.
MATERIALS AND METHODS
Cell culture.
T. cruzi epimastigotes (Tulahuen and Brazil) were grown at 26°C in liver digest-neutralized tryptose medium (LDNT) supplemented with 10% fetal calf serum (FCS; Life Technologies, Gaithersburg, MD). Trypomastigotes were obtained by growth in human foreskin fibroblast (American Type Culture Collection, VA, USA) cultures. The transgenic strains of trypomastigotes were obtained from inoculation of metacyclic trypomastigotes in human foreskin fibroblast cultures. Human foreskin fibroblasts were cultured in Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Norwalk, CT) supplemented with 10% fetal bovine serum (Invitrogen, Norwalk, CT), 100 units each of penicillin and streptomycin per ml, and 2 mM l-glutamine (Invitrogen, Norwalk, CT). Cells were maintained in an incubator at 37°C and 5% CO2.
Transgenic parasites.
The inducible suicidal vector pTREX-GFP-DDDHA was introduced into epimastigotes (Tulahuen) by electroporation as previously described (28). Briefly, epimastigotes in the late logarithmic growth phase in LDNT broth were collected and washed. The parasite suspension (375 μl; 1.4 × 108 to 2.0 × 108 cells/ml) was incubated with 50 μg construct DNA and adjusted to 400 μl of the final volume. Electroporation was performed in a disposable cuvette using an Electro cell manipulator (BTX Genetronics, Inc., San Diego, CA), with one pulse delivered to the parasites at settings of 375 V, 25 Ω, and 50 μF. Subsequently, the transfected parasite suspension was diluted with 10 ml of LDNT medium, and G418 was included in the LDNT medium at a final concentration of 100 μg/ml. G418 was maintained for more than 4 weeks until parasites transfected with no DNA were no longer viable.
Fluorescent epimastigotes bearing pTREX-GFP-DDDHA induced by 60 nM TMP-lactate for 24 h were sorted with a MoFlo XDP cell sorter (Beckman Coulter, NJ, USA). The sorted parasites were then cultured for inoculation of mice. The sorted parasites were maintained in LDNT medium with G418 at a final concentration of 100 μg/ml.
Cross-protection test.
To test whether the transgenic T. cruzi strain could provide immune cross-protection in mice against a different background of T. cruzi, we used 7- to 8-week-old male C3H mice (Charles River Laboratories, Kingston, NY), which are highly susceptible to T. cruzi infection. Eight-week-old male C57BL/6 mice (Jackson Laboratory, Farmington, CT) were also used for cross-protection experiments. Tulahuen strain epimastigotes bearing pTREX-GFP-DDDHA (sorted fluorescent cells) were used to differentiate into tissue trypomastigotes in human foreskin fibroblasts. The C3H and C57BL/6 mice were inoculated with 5,000 tissue-derived trypomastigotes per mouse by intraperitoneal injection. Control mice also received the same amount of phosphate-buffered saline (PBS) by intraperitoneal injection without pTREX-GFP-DDDHA parasites. Starting 7 days postinfection (dpi), mice were administered TMP-lactate in drinking water (30 mg/100 ml per day) until day 42 dpi, while another group was not treated with TMP-lactate. At 42 dpi, all mice with preinoculation of the transgenic Tulahuen strain and control mice were challenged with 5 × 105 tissue-derived wild-type (WT) Brazil strain parasites. Parasitemia of the infected mice was observed by light microscope. Survival rates of each group were recorded daily. Parasitemia for blood and tissues was observed by light microscope. A separate set of mice were sacrificed at 14 dpi, and heart tissues were harvested, fixed, and paraffin embedded for subsequent hematoxylin and eosin staining.
Transgenic strain persistence and immune cell response tests.
To determine if inoculation of the GFP-DDDHA strain (Tulahuen) in mice results in persistent infection, C57BL/6 mice (male, 8 weeks old; Jackson Laboratory, Farmington, CT) were inoculated with 5,000 GFP-DDDHA Tulahuen strain parasites, and, after 7 dpi, the mice were treated with TMP-lactate for 35 days. At 240 dpi, these mice were immunosuppressed with cyclophosphamide (200 mg/kg) by i.p. injection at 3- to 4-day intervals for 3 doses. Parasitemia was examined using tail blood and light microscope. After 30 days of cyclophosphamide treatment, these mice were sacrificed and tissues were collected and stored at −80°C for DNA isolation and real-time PCR detection.
For real-time PCR detection of tissue parasitism, heart and white adipose tissues were used for DNA isolation using a Trizol DNA extraction protocol (Invitrogen, MA, USA) following the manufacturer’s instructions. A standard curve in the range of 0.01 pg to 1 ng for the quantification of T. cruzi DNA by real-time PCR was developed using the T. cruzi 195-bp repeat DNA-specific primers TCZ-F (5′-GCTCTTGCCCACAAGGGTGC-3′) and TCZ-R (5′-CCAAGCAGCGGATAGTTCAGG-3′) (47) and genomic DNA purified from T. cruzi epimastigotes. Real-time PCR was performed using a SYBR Green Ex Taq II kit (TaKaRa, Clontech, CA, USA) with 100 ng genomic DNA and 0.5 μM TCZ-F and TCZ-R primers, following the manufacturer’s protocol. The mouse microglobulin primers β2F2 (5′-TGGGAAGCCGAACATACTG-3′) and β2R2 (5′-GCAGGCGTATGTATCAGTCTCA-3′) were included as an internal control (29).
To examine the differences in immune cell expansion and memory development, we used 8-week-old C57BL/6 mice to compare the GFP-DDDHA strain and the WT Tulahuen strain (5,000 parasites/mouse for both types of parasites) for a time course and sacrificed them at different time points. For recall response, GFP-DDDHA-immunized C57BL/6 mice were challenged with 5 × 105 WT Tulahuen strain parasites, and splenocytes were prepared from these mice at 12 and 24 h of challenge.
IFN-γ knockout mouse model.
Eight-week-old B6.129S7-Ifngtm1Ts/J mice (IFN-γ knockout mice; Jackson Laboratory, Farmington, CT) age-matched C57BL/6 mice were immunized with the GFP-DDDHA strain (5,000 parasites/mouse). To allow protection to establish and specifically analyze the role of IFN-γ in the secondary response to lethal infection, IFN-γ KO mice were administered recombinant IFN-γ (1.2 μg [1,000 U]; R&D Systems, Inc., MN, USA) twice weekly i.p. for 5 weeks starting 1 day postadministration of the GFP-DDDHA strain. After 5 weeks, treatment with IFN-γ was stopped, the immunized IFN-γ-treated IFN-γ KO mice and the immunized C57BL/6 mice, as well as a group of nonimmunized C57BL/6 mice, were infected with 5 × 105 wild-type Tulahuen strain parasites, and the survival rate was documented.
Measurement of cytokines by multiplex assay.
Mouse serum samples were stored at −80°C and sent to the UT Southwestern Metabolic Phenotyping Core facility for cytokine measurements using a Millipore (Billerica, MA) mouse cytokine/chemokine magnetic bead panel immunology multiplex assay kit (MCYTOMAG-70k-06; TNF-α, IFN-γ, IL-1-β, IL-6, and IL-10) and a Magpix multiplex analyzer. Spline and 5-parameter logistic curve-fitting models were used to calculate the different cytokine levels from the samples.
Isolation and preparation of splenocytes.
Spleens were placed in 5 ml of complete DMEM (Fisher, MA, USA) supplemented with 10% FBS, penicillin-streptomycin, and l-glutamine. Tissue was dispersed with glass homogenizers and passed through a 40-μm wire mesh filter to create single-cell suspensions. Cells were then washed with 2 ml of ammonium-chloride-potassium (ACK) lysing buffer (Lonza, Inc., NJ, USA) to lyse red blood cells. Cells were subsequently washed with complete medium three times prior to total cell count. A total of 3 × 106 splenocytes were plated in a well of a 96-well U-bottom plate for staining with antibodies for flow cytometry analysis.
Flow cytometry assays.
Splenocytes harvested from infected and control mice were resuspended in a 96-well round-bottom plate and incubated with α-FcRII/III (CD16/CD32) antibody (clone 2.4G2; Tonbo Biosciences, CA, USA) to prevent nonspecific staining. Cells were then incubated with fluorescently labeled antibodies to identify specific cell subsets and their immunophenotype. For analyzing lymphocytes, the following antibodies (from BD Bioscience, MA, USA, unless otherwise indicated) were used along with a live/dead cell dye (UV Violet; Thermo Fisher Scientific, NJ, USA): α-CD3 (clone 17A2, allphycocyanin [APC] Cy7), α-CD4 (clone RM4-5, phycoerythrin [PE] CF594), α-CD8 (clone 53-6.7, fluorescein isothiocyanate [FITC]), α-CD44 (clone IM7, AF700), α-CD62L (clone MEL-14, APC), and IFN-γ (clone XMG1.2, AF700). For analyzing myeloid cells, splenocytes were stained with the following antibodies following incubation with live/dead cell dye (UV Violet): α-CD3 (clone 17A2, APC Cy7), α-CD11b (clone M1/10, AF700), α-CD11c (clone HL3, BV42), α-Ly6C (clone AL-21, PECF594), α-Ly6G (clone 1A8, FITC), α-CD40 (clone 3/23, BV 605), α-CD86 (clone GL1, PECy7), α-iNOS (clone CXNFT, APC), α-MHCII (M5/114.15.2, BV605), and α-TNF-α (MP6-XT22, PEPCCy5.5). Cells were acquired using an LSR II flow cytometer (BD Bioscience, MA, USA) and analyzed with FlowJo software (Tree Star, Inc., OR, USA).
Enzyme-linked immunosorbent assay.
For detection of IL-2, IL-4, IL-17A, and IFN-γ production, total splenocytes were cultured for 48 h in resting or stimulating conditions using either protein lysates from T. cruzi (10 μg/ml) or anti-CD3ε antibody (2 μg/ml) (145-2C11; BD Pharmingen, NJ, USA). After 48 h, supernatants for each sample were harvested. Cytokine concentration was measured using sandwich enzyme-linked immunosorbent assay (ELISA) using the following antibodies (anti-mouse IFN-γ is from BD Pharmingen, NJ, USA; all other antibodies are from eBioscience, MA, USA): anti-mouse IL-2 (JES6-1A12), anti-mouse IFN-γ (AN-18), anti-mouse IL-4 (11B-11), anti-mouse IL-17A (17CK15A5), biotinylated anti-mouse IL-2 (JES6-SH4), biotinylated anti-mouse IFN-γ (R4-6A2), biotinylated anti-mouse IL-4 (BVD6-24G2), and biotinylated anti-mouse IL-17A (17B7).
Adoptive transfer test.
To determine which branch of the immune system provides effective protection after GFP-DDDHA strain immunization, several types of immune cells and serum from GFP-DDDHA strain-immunized mice (male) were given to naive C57BL/6 mice (male, aged between 10 and 14 weeks) by adoptive transfer. Mice without adoptive transfer or with naive control adoptive transfer were used for comparison. T cells, B cells, or NK cells were purified using individual MagniSort cell isolation kits (Thermo Fisher, MA, USA). For immune cell adoptive transfer, 1 × 107 T cells, 6 × 106 NK cells, or 1 × 107 B cells were delivered to recipient mice by tail injection. For serum adoptive transfer, 300 μl of serum per mouse was administered to recipient mice by intraperitoneal injection once a week for 2 weeks before WT Tulahuen strain infection. For the combination of serum and NK cell adoptive transfer, mice received serum for the first week and serum and NK cells for the second week. After 24 h of postadoptive transfer, mice were infected with 5 × 103 WT Tulahuen trypomastigotes, and parasitemia was determined by light microscopy with 100 random fields at ×20 magnification. The survival rate was documented on a daily basis.
Statistical analysis.
For statistical analysis, we used the Prism program (analysis of variance [ANOVA] and t test) to determine significance based on the data. All experiments were performed at least twice (the number of replicated experiments for each data set, ranging from 2 to 4, is indicted in the respective figure legends) to confirm replication of observations and conclusions.
Ethics statement.
This study was performed in strict accordance with the provisions of the Guide for the Care and Use of Laboratory Animals (56) and the Public Health Service Policy on Humane Care and Use of Laboratory Animals (57). Mice that were genetically compatible were obtained from commercial sources and housed comfortably in the Albert Einstein College of Medicine AAALAC-accredited central animal facility. They were not deprived of water or food at any time, nor were they subjected to prolonged physical restraint. Any animals that became moribund were killed painlessly.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jun Shu for excellent technical assistance and Eva Mason and Emily Reynolds (UT Southwestern Metabolic Phenotyping Core) for assistance with cytokine measurements.
This work was supported by NIH grant AI1421110 (H. Huang).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.GBD 2013 Mortality and Causes of Death Collaborators. 2015. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotez PJ, Alvarado M, Basáñez M-G, Bolliger I, Bourne R, Boussinesq M, Brooker SJ, Brown AS, Buckle G, Budke CM, Carabin H, Coffeng LE, Fèvre EM, Fürst T, Halasa YA, Jasrasaria R, Johns NE, Keiser J, King CH, Lozano R, Murdoch ME, O’Hanlon S, Pion SDS, Pullan RL, Ramaiah KD, Roberts T, Shepard DS, Smith JL, Stolk WA, Undurraga EA, Utzinger J, Wang M, Murray CJL, Naghavi M. 2014. The global burden of disease study 2010: interpretation and implications for the neglected tropical diseases. PLoS Negl Trop Dis 8:e2865. doi: 10.1371/journal.pntd.0002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. 2015. Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Wkly Epidemiol Rec 90:33–43. [PubMed] [Google Scholar]
- 4.Bern C, Montgomery SP, Herwaldt BL, Rassi A Jr, Marin-Neto JA, Dantas RO, Maguire JH, Acquatella H, Morillo C, Kirchhoff LV, Gilman RH, Reyes PA, Salvatella R, Moore AC. 2007. Evaluation and treatment of Chagas disease in the United States: a systematic review. JAMA 298:2171–2181. doi: 10.1001/jama.298.18.2171. [DOI] [PubMed] [Google Scholar]
- 5.Garcia S, Ramos CO, Senra JF, Vilas-Boas F, Rodrigues MM, Campos-de-Carvalho AC, Ribeiro-Dos-Santos R, Soares MB. 2005. Treatment with benznidazole during the chronic phase of experimental Chagas’ disease decreases cardiac alterations. Antimicrob Agents Chemother 49:1521–1528. doi: 10.1128/AAC.49.4.1521-1528.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morillo CA, Marin-Neto JA, Avezum A, Sosa-Estani S, Rassi A, Rosas F, Villena E, Quiroz R, Bonilla R, Britto C, Guhl F, Velazquez E, Bonilla L, Meeks B, Rao-Melacini P, Pogue J, Mattos A, Lazdins J, Rassi A, Connolly SJ, Yusuf S, BENEFIT Investors. 2015. Randomized trial of benznidazole for chronic Chagas’ cardiomyopathy. N Engl J Med 373:1295–1306. doi: 10.1056/NEJMoa1507574. [DOI] [PubMed] [Google Scholar]
- 7.Marioni JC, Thorne NP, Valsesia A, Fitzgerald T, Redon R, Fiegler H, Andrews TD, Stranger BE, Lynch AG, Dermitzakis ET, Carter NP, Tavare S, Hurles ME. 2007. Breaking the waves: improved detection of copy number variation from microarray-based comparative genomic hybridization. Genome Biol 8:R228. doi: 10.1186/gb-2007-8-10-r228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epting CL, Coates BM, Engman DM. 2010. Molecular mechanisms of host cell invasion by Trypanosoma cruzi. Exp Parasitol 126:283–291. doi: 10.1016/j.exppara.2010.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrews NW. 1993. Living dangerously: how Trypanosoma cruzi uses lysosomes to get inside host cells, and then escapes into the cytoplasm. Biol Res 26:65–67. [PubMed] [Google Scholar]
- 10.De Pablos LM, Osuna A. 2012. Multigene families in Trypanosoma cruzi and their role in infectivity. Infect Immun 80:2258–2264. doi: 10.1128/IAI.06225-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bartholomeu DC, de Paiva RM, Mendes TA, DaRocha WD, Teixeira SM. 2014. Unveiling the intracellular survival gene kit of trypanosomatid parasites. PLoS Pathog 10:e1004399. doi: 10.1371/journal.ppat.1004399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piacenza L, Peluffo G, Alvarez MN, Kelly JM, Wilkinson SR, Radi R. 2008. Peroxiredoxins play a major role in protecting Trypanosoma cruzi against macrophage- and endogenously-derived peroxynitrite. Biochem J 410:359–368. doi: 10.1042/BJ20071138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norris KA, Bradt B, Cooper NR, So M. 1991. Characterization of a Trypanosoma cruzi C3 binding protein with functional and genetic similarities to the human complement regulatory protein, decay-accelerating factor. J Immunol 147:2240–2247. [PubMed] [Google Scholar]
- 14.Ferreira V, Valck C, Sánchez G, Gingras A, Tzima S, Molina MC, Sim R, Schwaeble W, Ferreira A. 2004. The classical activation pathway of the human complement system is specifically inhibited by calreticulin from Trypanosoma cruzi. J Immunol 172:3042–3050. doi: 10.4049/jimmunol.172.5.3042. [DOI] [PubMed] [Google Scholar]
- 15.Aguillón JC, Ferreira L, Pérez C, Colombo A, Molina MC, Wallace A, Solari A, Carvallo P, Galindo M, Galanti N, Orn A, Billetta R, Ferreira A. 2000. Tc45, a dimorphic Trypanosoma cruzi immunogen with variable chromosomal localization, is calreticulin. Am J Trop Med Hyg 63:306–312. doi: 10.4269/ajtmh.2000.63.306. [DOI] [PubMed] [Google Scholar]
- 16.Norris KA. 1998. Stable transfection of Trypanosoma cruzi epimastigotes with the trypomastigote-specific complement regulatory protein cDNA confers complement resistance. Infect Immun 66:2460–2465. doi: 10.1128/IAI.66.6.2460-2465.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bermejo DA, Amezcua Vesely MC, Khan M, Acosta Rodriguez EV, Montes CL, Merino MC, Merino MC, Toellner KM, Mohr E, Tayor D, Cunnigham AF, Gruppi A. 2011. Trypanosoma cruzi infection induces a massive extrafollicular and follicular splenic B-cell response which is a high source of non-parasite-specific antibodies. Immunology 132:123–133. doi: 10.1111/j.1365-2567.2010.03347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarleton RL. 2015. CD8+ T cells in Trypanosoma cruzi infection. Semin Immunopathol 37:233–238. doi: 10.1007/s00281-015-0481-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitcovsky TA, Buscaglia CA, Mucci J, Campetella O. 2002. A functional network of intramolecular cross-reacting epitopes delays the elicitation of neutralizing antibodies to Trypanosoma cruzi trans-sialidase. J Infect Dis 186:397–404. doi: 10.1086/341463. [DOI] [PubMed] [Google Scholar]
- 20.Vaena de Avalos S, Blader IJ, Fisher M, Boothroyd JC, Burleigh BA. 2002. Immediate/early response to Trypanosoma cruzi infection involves minimal modulation of host cell transcription. J Biol Chem 277:639–644. doi: 10.1074/jbc.M109037200. [DOI] [PubMed] [Google Scholar]
- 21.Tarleton RL. 2007. Immune system recognition of Trypanosoma cruzi. Curr Opin Immunol 19:430–434. doi: 10.1016/j.coi.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Kurup SP, Tarleton RL. 2013. Perpetual expression of PAMPs necessary for optimal immune control and clearance of a persistent pathogen. Nat Commun 4:2616. doi: 10.1038/ncomms3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhatia V, Garg NJ. 2005. Current status and future prospects for a vaccine against American trypanosomiasis. Expert Rev Vaccines 4:867–880. doi: 10.1586/14760584.4.6.867. [DOI] [PubMed] [Google Scholar]
- 24.Bhatia V, Garg NJ. 2008. Previously unrecognized vaccine candidates control Trypanosoma cruzi infection and immunopathology in mice. Clin Vaccine Immunol 15:1158–1164. doi: 10.1128/CVI.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatia V, Sinha M, Luxon B, Garg NJ. 2004. Utility of Trypanosoma cruzi sequence database for the identification of potential vaccine candidates: in silico and in vitro screening. Infect Immun 72:6245–6254. doi: 10.1128/IAI.72.11.6245-6254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatia V, Wen J-J, Zacks MA, Garg NJ. 2009. American trypanosomiasis and perspectives on vaccine development, p 1423–1450. In Stanberry LR, Barrett AD. (ed), Vaccines for biodefense and emerging and neglected diseases. Academic Press, New York, NY. [Google Scholar]
- 27.Gupta S, Garg NJ. 2010. Prophylactic efficacy of TcVac2 against Trypanosoma cruzi in mice. PLoS Negl Trop Dis 4:e797. doi: 10.1371/journal.pntd.0000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma YF, Weiss LM, Huang H. 2015. Inducible suicide vector systems for Trypanosoma cruzi. Microbes Infect 17:440–450. doi: 10.1016/j.micinf.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Combs TP, Nagajyothi, Mukherjee S, de Almeida CJ, Jelicks LA, Schubert W, Lin Y, Jayabalan DS, Zhao D, Braunstein VL, Landskroner-Eiger S, Cordero A, Factor SM, Weiss LM, Lisanti MP, Tanowitz HB, Scherer PE. 2005. The adipocyte as an important target cell for Trypanosoma cruzi infection. J Biol Chem 280:24085–24094. doi: 10.1074/jbc.M412802200. [DOI] [PubMed] [Google Scholar]
- 30.Kumar S, Tarleton RL. 2001. Antigen-specific Th1 but not Th2 cells provide protection from lethal Trypanosoma cruzi infection in mice. J Immunol 166:4596–4603. doi: 10.4049/jimmunol.166.7.4596. [DOI] [PubMed] [Google Scholar]
- 31.Cardoni RL, Rottenberg ME, Segura EL. 1990. Increased production of reactive oxygen species by cells from mice acutely infected with Trypanosoma cruzi. Cell Immunol 128:11–21. doi: 10.1016/0008-8749(90)90002-9. [DOI] [PubMed] [Google Scholar]
- 32.Soudja SM, Chandrabos C, Yakob E, Veenstra M, Palliser D, Lauvau G. 2014. Memory-T-cell-derived interferon-γ instructs potent innate cell activation for protective immunity. Immunity 40:974–988. doi: 10.1016/j.immuni.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Junqueira C, Caetano B, Bartholomeu DC, Melo MB, Ropert C, Rodrigues MM, Gazzinelli RT. 2010. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev Mol Med 12:e29. doi: 10.1017/S1462399410001560. [DOI] [PubMed] [Google Scholar]
- 34.Tarleton RL. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J Immunol 144:717–724. [PubMed] [Google Scholar]
- 35.Tarleton RL, Koller BH, Latour A, Postan M. 1992. Susceptibility of β2-microglobulin-deficient mice to Trypanosoma cruzi infection. Nature 356:338–340. doi: 10.1038/356338a0. [DOI] [PubMed] [Google Scholar]
- 36.Barnabé C, Brisse S, Tibayrenc M. 2000. Population structure and genetic typing of Trypanosoma cruzi, the agent of Chagas disease: a multilocus enzyme electrophoresis approach. Parasitology 120:513–526. doi: 10.1017/S0031182099005661. [DOI] [PubMed] [Google Scholar]
- 37.Brisse S, Dujardin JC, Tibayrenc M. 2000. Identification of six Trypanosoma cruzi lineages by sequence-characterised amplified region markers. Mol Biochem Parasitol 111:95–105. doi: 10.1016/s0166-6851(00)00302-9. [DOI] [PubMed] [Google Scholar]
- 38.Lewis MD, Llewellyn MS, Gaunt MW, Yeo M, Carrasco HJ, Miles MA. 2009. Flow cytometric analysis and microsatellite genotyping reveal extensive DNA content variation in Trypanosoma cruzi populations and expose contrasts between natural and experimental hybrids. Int J Parasitol 39:1305–1317. doi: 10.1016/j.ijpara.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zingales B, Andrade SG, Briones MRS, Campbell DA, Chiari E, Fernandes O, Guhl F, Lages-Silva E, Macedo AM, Machado CR, Miles MA, Romanha AJ, Sturm NR, Tibayrenc M, Schijman AG, Second Satellite Meeting. 2009. A new consensus for Trypanosoma cruzi intraspecific nomenclature: second revision meeting recommends TcI to TcVI. Mem Inst Oswaldo Cruz 104:1051–1054. doi: 10.1590/s0074-02762009000700021. [DOI] [PubMed] [Google Scholar]
- 40.Abrahamsohn IA, Coffman RL. 1996. Trypanosoma cruzi: IL-10, TNF, IFN-gamma, and IL-12 regulate innate and acquired immunity to infection. Exp Parasitol 84:231–244. doi: 10.1006/expr.1996.0109. [DOI] [PubMed] [Google Scholar]
- 41.Duthie MS, Kahn SJ. 2005. NK cell activation and protection occur independently of natural killer T cells during Trypanosoma cruzi infection. Int Immunol 17:607–613. doi: 10.1093/intimm/dxh239. [DOI] [PubMed] [Google Scholar]
- 42.Duthie MS, Kahn SJ. 2006. During acute Trypanosoma cruzi infection highly susceptible mice deficient in natural killer cells are protected by a single alpha-galactosylceramide treatment. Immunology 119:355–361. doi: 10.1111/j.1365-2567.2006.02439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue S, Niikura M, Mineo S, Kobayashi F. 2013. Roles of IFN-γ and γδ T cells in protective immunity against blood-stage malaria. Front Immunol 4:258. doi: 10.3389/fimmu.2013.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, Stunnenberg HG, O’Neill LAJ, Xavier RJ. 2016. Trained immunity: a program of innate immune memory in health and disease. Science 352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schenkel JM, Masopust D. 2014. Tissue-resident memory T cells. Immunity 41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bermejo DA, Jackson SW, Gorosito-Serran M, Acosta-Rodriguez EV, Amezcua-Vesely MC, Sather BD, Singh AK, Khim S, Mucci J, Liggitt D, Campetella O, Oukka M, Gruppi A, Rawlings DJ. 2013. Trypanosoma cruzi trans-sialidase initiates a program independent of the transcription factors RORγt and Ahr that leads to IL-17 production by activated B cells. Nat Immunol 14:514–522. doi: 10.1038/ni.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Popi AF, Longo-Maugéri IM, Mariano M. 2016. An overview of B-1 cells as antigen-presenting cells. Front Immunol 7:138. doi: 10.3389/fimmu.2016.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adams NM, O’Sullivan TE, Geary CD, Karo JM, Amezquita RA, Joshi NS, Kaech SM, Sun JC. 2016. NK cell responses redefine immunological memory. J Immunol 197:2963–2970. doi: 10.4049/jimmunol.1600973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeves RK, Li H, Jost S, Blass E, Li H, Schafer JL, Varner V, Manickam C, Eslamizar L, Altfeld M, von Andrian UH, Barouch DH. 2015. Antigen-specific NK cell memory in rhesus macaques. Nat Immunol 16:927–932. doi: 10.1038/ni.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Sullivan TE, Sun JC, Lanier LL. 2015. Natural killer cell memory. Immunity 43:634–645. doi: 10.1016/j.immuni.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. 2006. T cell and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 52.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. 2010. Critical role for the chemokine receptor CXCR6 in NK cell mediated antigen-specific memory of haptens and viruses. Nat Immunol 11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sun JC, Ugolini S, Vivier E. 2014. Immunological memory within the innate immune system. EMBO J 33:1295–1303. doi: 10.1002/embj.201387651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun JC, Beilke JN, Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. 2009. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A 106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC. [Google Scholar]
- 57.U.S. Department of Health and Human Services. 2015. Public Health Service policy on humane care and use of laboratory animals. U.S. Department of Health and Human Services, Washington, DC: https://olaw.nih.gov/sites/default/files/PHSPolicyLabAnimals.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.