The development of vaccines for prevention of diseases caused by pathogenic species can encounter major obstacles if high sequence diversity is observed between individual strains. Therefore, development might be restricted either to conserved antigens, which are often rare, or to multivalent vaccines, which renders the production more costly and cumbersome. In light of this complexity, we applied a structure-based surface shaping approach for the development of a Lyme borreliosis (LB) vaccine suitable for the United States and Europe.
KEYWORDS: Borrelia, Lyme, OspA, vaccine
ABSTRACT
The development of vaccines for prevention of diseases caused by pathogenic species can encounter major obstacles if high sequence diversity is observed between individual strains. Therefore, development might be restricted either to conserved antigens, which are often rare, or to multivalent vaccines, which renders the production more costly and cumbersome. In light of this complexity, we applied a structure-based surface shaping approach for the development of a Lyme borreliosis (LB) vaccine suitable for the United States and Europe. The surface of the C-terminal fragment of outer surface protein A (OspA) was divided into distinct regions, based primarily on binding sites of monoclonal antibodies (MAbs). In order to target the six clinically most relevant OspA serotypes (ST) in a single protein, exposed amino acids of the individual regions were exchanged to corresponding amino acids of a chosen OspA serotype. Six chimeric proteins were constructed, and, based on their immunogenicity, four of these chimeras were tested in mouse challenge models. Significant protection could be demonstrated for all four proteins following challenge with infected ticks (OspA ST1, OspA ST2, and OspA ST4) or with in vitro-grown spirochetes (OspA ST1 and OspA ST5). Two of the chimeric proteins were linked to form a fusion protein, which provided significant protection against in vitro-grown spirochetes (OspA ST1) and infected ticks (OspA ST2). This article presents the proof-of-concept study for a multivalent OspA vaccine targeting a wide range of pathogenic LB Borrelia species with a single recombinant antigen for prevention of Lyme borreliosis.
INTRODUCTION
Lyme borreliosis (LB) is the most common arthropod-borne infectious disease present in the temperate regions of the Northern hemisphere. The spirochetes that cause LB are transmitted by hard-bodied ticks of Ixodes spp. in a complex enzootic cycle that primarily includes small mammals and birds (1). When humans contract LB, the consequences are a spectrum of distinct clinical manifestations that to some degree are associated with the Borrelia species causing the LB (2). The disease symptoms range from early localized infection of the skin (erythema migrans) to progressive disseminated infections of the nervous system (neuroborreliosis), skin (acrodermatitis chronica atrophicans), heart (Lyme carditis), and joints (Lyme arthritis) (3). In Europe, four Borrelia species presenting six different outer surface protein A (OspA) serotypes (ST) are responsible for the majority of human clinical cases: Borrelia burgdorferi (OspA ST1), Borrelia afzelii (OspA ST2), Borrelia garinii (OspA ST3, OspA ST5, and OspA ST6), and Borrelia bavariensis (OspA ST4) (4). The number of LB cases annually in western Europe has recently been estimated as approximately 230,000 (5) and in the United States as approximately 300,000 (6, 7), thereby affirming LB as a major health burden with substantial economic impact. Hence, the increasing incidence of cases argues in favor of the development of a multivalent vaccine that could reduce the debilitating impact on health and the economic burden of the disease worldwide.
OspA is a 29-kDa lipoprotein attached by a lipid moiety to the outer membrane that is expressed by the spirochetes when present in the midgut of unfed ticks. During tick feeding, the incoming blood from the host results in environmental changes in the tick’s gut that cue the spirochetes to downregulate OspA and migrate to the salivary glands and further to the vertebrate host (1, 8). The spirochetes differentially regulate protein expression as a consequence of the changing environmental pressure encountered in their vector-host life cycle (1, 8). Therefore, several strategies for LB vaccine development have been assessed, focusing on outer membrane proteins of the pathogen and salivary gland proteins of the vector (9). Nevertheless, until now, primarily vaccines based on OspA have been assessed in clinical studies (10). Two monovalent OspA ST1 vaccines (LYMErix from SmithKline Beecham [11] and ImuLyme from Pasteur Mérieux-Connaught [12]) have been evaluated in clinical efficacy trials, and the former was licensed for human use in the United States between 1998 and 2002 (13, 14). Additionally, a recombinant hexavalent vaccine comprising three chimeric OspA proteins covering six OspA serotypes was developed by Baxter Bioscience and tested in clinical trials (15, 16). The hexavalent LB vaccine candidate VLA15, developed by Valneva Austria GmbH, is based on the C-terminal fragment of six different OspA serotypes (ST1 to ST6) linked together in pairs to form three fusion proteins and has been extensively evaluated preclinically (17–19). VLA15 is now being assessed in two phase 2 clinical trials (ClinicalTrials registration numbers NCT03769194 and NCT03970733).
We have investigated a novel OspA-based vaccine approach with the rationale to design a single recombinant protein without compromising protection against the majority of Borrelia species and OspA serotypes associated with LB. Having a single-protein antigen is associated with considerably reduced costs and complexity in the vaccine production processes. Furthermore, taking into account newly emerging pathogenic Borrelia species, the number of different OspA serotypes to be included in a multivalent vaccine may increase in the future. The vaccine candidates were designed with a structure-based surface-shaping approach initially described by Scarselli and coworkers (20). They introduced multiple immunodominant antigenic surface features on one conserved protein scaffold of factor H binding protein (fHBP), a major Neisseria meningitidis serogroup B antigen. The sequence of one variant group was taken as a conserved backbone (to preserve protein folding), and surface epitopes of the other two variant groups were artificially introduced on this scaffold. We have applied a similar approach to the surface-exposed C-terminal fragment of OspA, which contains most of the epitopes associated with protection (21–23). On the surface of the C-terminal fragment, several adjacent regions (patches) were defined, mainly corresponding to the mapped binding sites of monoclonal antibodies (MAbs) described in the literature, as well as to additional binding regions reported for the C-terminal fragment of OspA ST1 (21, 23–35). The surface-exposed amino acids within these patches were exchanged to represent different OspA serotypes, which, in sum, accommodated multiple OspA serotypes on one protein. The protein backbones of all six chimeric variants were based on the sequence of the C-terminal fragment of B. afzelii OspA ST2, which represents the most prevalent OspA ST causing LB in Europe (36–39).
The immunogenicity in mice of all six variants was assessed with enzyme-linked immunosorbent assay (ELISA) for surface binding and with serum bactericidal assays (SBAs) for functional antibodies. Based on the serological readouts, four variants were selected for further evaluation in two different mouse challenge models (bacterial challenge via ticks and syringe). Finally, two of the four variants assessed in the animal models were linked to form a fusion protein that provided significant protection against B. burgdorferi OspA ST1 and B. afzelii OspA ST2, the most important Borrelia species pathogenic to humans in the United States and Europe, respectively (40). This study demonstrates that the novel multivalent OspA-based vaccine approach has the potential to achieve broad protection with a single recombinant antigen.
RESULTS
Rational design of multivalent variants of the OspA C-terminal fragment.
The sequences of the C-terminal fragments of the six OspA serotypes (B. burgdorferi [ST1], B. afzelii [ST2], B. bavariensis [ST4], and B. garinii OspA [ST3, ST5, and ST6]) used in this study for the development of a chimeric OspA vaccine shared a high percentage of pairwise sequence identity that ranged from 86% (OspA ST5 versus ST6) to 67% (OspA ST2 versus ST3). Therefore, we expected good structural fold conservation, which was a prerequisite for the design of multivalent OspA variants.
As a basis for our design, we used available crystal structures of B. burgdorferi B31 OspA (ST1) bound to Fab LA-2 (binds to a site located at the C-terminal half [PDB ID 1FJ1]) (28) and to Fab 184.1 (binds to the N-terminal half [PDB ID 1OSP]) (41). The latter complex offers a structure of the C-terminal half that is unaffected by direct antibody-OspA contacts and therefore served as structural template for homology modeling and subsequent fold conservation analysis. Based on the PDB ID 1OSP crystal structure and structural models of OspA (41, 42), we generated homology models of the C-terminal fragment for each of the six OspA serotypes, ST1 to ST6. Similarly to our previously described LB vaccine candidate (17–19), thermal instability of the C-terminal fragments was expected. Therefore, stabilization was introduced in form of a disulfide-bond either linking the C-terminal helix to the surrounding β-sheet (named “B”) or joining the linker at the N-terminal side of the helix to the last β-strand in the C-terminal sheet (named “C”) (17). High fold conservation was observed when the structure models of OspA fragments for ST1 to ST6, with the insertion of the disulfide-bond “B,” were relaxed in short molecular mechanics simulations (PyMol [43] and GROMACS [44–48] with OPLS-AA [49]), which provided freedom to choose any of the six OspA C-terminal fragments as the structural scaffold. However, we decided to use the C-terminal fragment of OspA ST2 (strain K78; GenBank accession number AJY72832.1), consisting of amino acids (aa) 126 to 273, which has been shown to be a potent immunogenic subunit and sufficient for protection (17–19), as the structural scaffold. By using the C-terminal fragment of OspA ST2 as the common structural scaffold, we avoided the sequence of a putative T-cell epitope in OspA ST1 (amino acids 165 to 173) (50–52). The structural scaffold consists of the protein backbone together with those amino acid residues whose side chains are buried within the folded protein. The serotype-specific surface residue side chains were present on this common scaffold.
As a next step, the surface of the OspA ST2 C-terminal fragment was partitioned into surface areas (“patches”) with the potential to harbor possible B-cell epitopes (Fig. 1). This was based mainly on OspA ST1 data, such as the binding sites of monoclonal antibodies at atomic resolution (Fab LA-2 crystal structure of complex; PDB ID 1FJ1), nuclear magnetic resonance (NMR) data (chemical shift perturbation maps), point mutation analyses and low resolution data, and binding analyses to subdomains/fragments or peptide scanning results (21, 23–35). For the definition of patches, which individually should be able to represent a diversity of serotypes, several assumptions were made, as follows. (i) The relevant antibody contact area is contained within the surface of a patch. (ii) The defined patches are, to a high degree, transferable across serotypes, i.e., epitopes are expected to be located in the same area if fold conservation is high. This allowed for a free choice of serotypes for a patch. (iii) It is sufficient to exchange the exposed surface residues to change binding specificity, i.e., we expected no complications by serotype-specific OspA fold changes upon antibody binding. However, the following restrictions might apply: (i) patches might not contain a protective epitope for the selected serotypes, and (ii) epitopes may be located off-center on a patch and might then only be partially represented on one patch; thus, they could overlap two or more patches, which might result in a restriction in the possible patch combinations or even in requiring a modified patch layout to accommodate certain combinations.
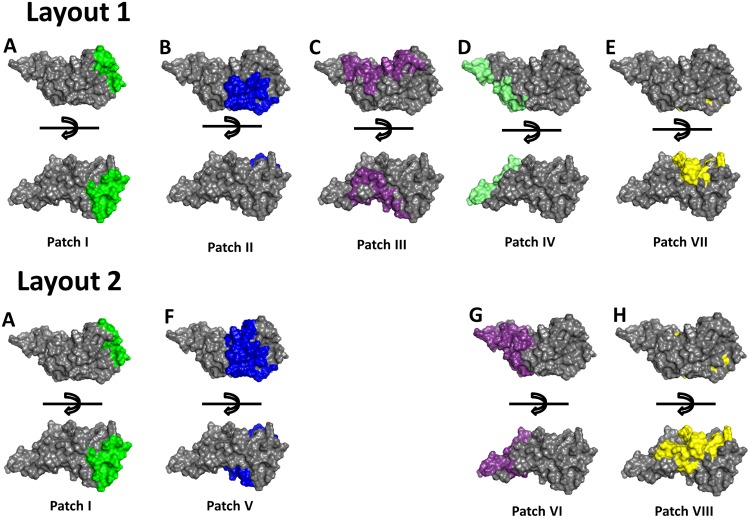
FIG 1.
Schematic representation of the surface partitions on the C-terminal fragment of OspA. The surface of the C-terminal fragment was partitioned into eight different areas (patch I through patch VIII) on a conserved protein scaffold of B. afzelii OspA ST2. Patches I, II, and III are based on the binding of monoclonal antibodies LA-2, 336, and 105.5, respectively, to B. burgdorferi OspA ST1 (A, B, and C). Patch IV represents parts of the binding region of MAb 4C10C2 (D). Patch V is an enlarged version of patch II and represents the binding region of MAb 336 and binding region of MAb 105.5 (F). Patch VI includes the binding sites of MAb 4C10C2 and MAb 105.5 (G). Patch VII includes the MAb CIII.78 binding site and the tick gut binding domain (TGBD) of OspA (E). Patch VIII is an extended version of patch VII (H).
The C-terminal fragment is relatively small and can only harbor a restricted number of conformational epitopes with an expected surface area of 900 to 2,000 Å2 (53–55). Therefore, the areas of the patches were designed as large as possible to offer a surface large enough for a complete antibody footprint and to allow for some uncertainty in case knowledge about the antibody binding position was less precise. The defined patches cover areas analogous to the described binding sites/regions of the different MAbs, LA-2 (28), 336 (27), 105.5 (31), 4C10C2 (29), CIII.78 (21), and to the tick gut binding domain (TGBD) (30). MAbs LA-2 and CIII.78 have been shown to provide protection in mice, which points to the importance of these epitopes (21, 28), whereas information regarding protection for the other MAbs was not publicly available. The crystal structure of OspA (ST1) in a complex with MAb LA-2 enabled a precise definition of the antibody footprint and was used for the definition of the main area of patch I (24, 28, 34, 35). MAb 336, which was generated with OspA from the B. afzelii strain PGau, is capable of binding to strains of other species, namely, B. burgdorferi and B. bavariensis (23). The binding site of MAb 105.5 on OspA ST1 is concave and covers a large surface area according to NMR analysis (31). Legros et al. showed that binding of MAb 4C10C2 was strain specific, since it binds to OspA ST1 of B. burgdorferi strain B31 but not to OspA ST1 of B. burgdorferi strain Sta3 (29).
Based on the assumptions listed above, the information about the sites/regions of different MAbs, and the expected surface area of an epitope, partition scheme layout 1 was designed with five different patches, patches I (LA-2), II (336), III (105.5), IV (4C10C2), and VII (CIII.78 and TGBD) (Fig. 1 and 2). A second patch scheme, layout 2, was defined to probe alternative positions and sizes of patches. Hence, layout 2 includes epitopes incompatible with layout 1 partitioning. Layout 2 entails four different patches, patches I (LA-2), V (336 and 105.5), VI (105.5 and 4C10C2), and VIII (CIII.78 and TGBD) (Fig. 1 and 2). The partitioning scheme for layout 2 does not contain the large patch III, which allowed us to increase the surface area of patch II and enabled us to include the C-terminal helix of OspA in its entirety within patch V (Fig. 1B and F). In addition, patch VIII in layout 2 is an enlargement of patch VII from layout 1 (Fig. 1E and H).
FIG 2.
Sequence alignment of the chimeric vaccine candidates compared to the B. afzelii (OspA ST2) C-terminal fragment. Sequence alignment comparing the multivalent OspA antigen variants to the sequence of a B. afzelii OspA C-terminal fragment (OspA ST2, aa 126 to 273, B. afzelii strain K78; GenBank accession number AJY72832.1) as a reference sequence (here also referred to as the “conserved backbone” or “structural scaffold”) showing the modified amino acid residues. The locations of the amino acids in the three-dimensional structure are shown in the line labeled “Surface exposed.” +, exposed amino acid; o, partially exposed amino acid; −, buried amino acid. The locations of the residues making up patches I through VIII are represented as numerals 1 to 8 in layouts 1 and 2.
The orientation of amino acids in the OspA C-terminal fragment was determined based on the structure models and crystal structures to distinguish amino acids having side chains, which are oriented to the core (buried), partially surface exposed, or surface exposed. The less accessible amino acid core residues were mostly hydrophobic, conserved, and tightly packed. They were kept for the OspA ST2 scaffold to ensure fold retention of the molecule. Exposed side chains and most partially exposed amino acids were exchanged for each patch to match the assigned OspA serotype.
When different OspA STs were assigned to adjacent patches, ideal combinations were chosen, with the least possible interference between the surface-exposed amino acid residues. In the best case, the assigned serotype-specific surface areas extended to the adjacent patch, providing a contiguous serotype-specific surface. Therefore, to optimize the serotype selection for the different patches, a procedure was implemented that sums up penalty scores (a combination of the interference between neighboring amino acids, surface exposure, and a switching function) for each serotype-specific residue in a patch to all non-serotype-matching residues in the adjacent patches. This was done for each patch to generate a score for every possible serotype combination. The scoring process selected residues which were highly (factor 1, represented as “+” in Fig. 2) or partially (factor 0.5, represented as “o” in Fig. 2) surface exposed and were located near the adjacent patch. The analysis of the interference between exposed and partially exposed amino acids used a cutoff of 0.5 nm with a 0.3-nm switching function by Cα-Cα distance. Buried residues (core, factor 0, represented as “−” in Fig. 2) were not included. Lists were generated and filtered to only show entries that matched the serotype of interest and showed the best possible combinations of serotype population on different patches. Several different patch assignments were designed with a minimum of interference between serotypes on neighboring patches to achieve broad protection with a final vaccine candidate.
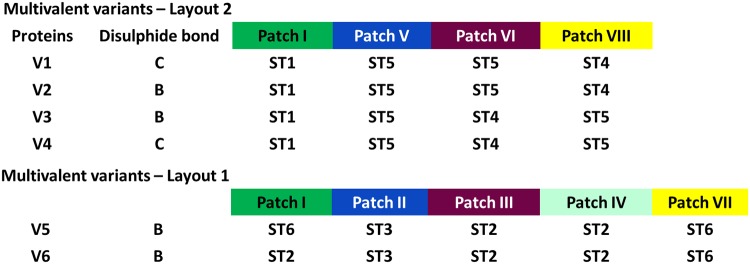
Finally, a set of six multivalent OspA variants, referred to as variants 1 to 6 (V1 to V6) (Fig. 2 and 3), was selected for evaluation of immunogenicity and protection. V1 to V4 (layout 2) had patch I populated with OspA ST1, together with OspA ST4 and ST5 on patches V, VI, and VIII. In V1 to V4, OspA ST5 and ST4 were put on patches VI and VIII, respectively, or the inverse, to probe serotype specificity in a simple patch exchange situation (Fig. 2 and 3). V1 to V4 have the same patch layouts, but different disulfide bond location C and B, respectively. The location of disulfide bond C, in contrast to that of B, avoids mutating exposed side chains placed in patch V in layout 2 or at the border of patch II (which is defined as a subarea of patch V) in layout 1 to cysteines. V5 and V6 (disulfide bond B) followed layout 1, in which patch I is populated with either OspA ST6 or ST2, respectively, and the other patches were populated to represent OspA ST2, ST3, and ST6. For a final vaccine candidate, at least 2 multivalent variants, which can be fused together to obtain a single antigen LB vaccine, would be required to target all six serotypes.
FIG 3.
Tabular representation of multivalent chimeric OspA vaccine candidates designed by a surface shaping approach. V1 through V4 are based on patches I, V, VI, and VIII. Patch I and patch V represent amino acid residues of OspA ST1 and ST5, respectively, on the surface of the chimeras. V1 and V2 represent OspA ST5 residues on patch VI and OspA ST4 residues on patch VIII, whereas the serotypes are reversed on these patches in V3 and V4. V1 and V4 are stabilized by disulfide bond C and V2 and V3 with disulfide bond B. V5 and V6 are designed based on patches I, II, III, IV, and VII and are stabilized by disulfide bond B. Patch I is populated with OspA ST6 and ST2 residues on the surface in V5 and V6, respectively. Patches II, III, IV, and VII are populated with OspA ST3, ST2, ST2, and ST6, respectively, in both variants.
Chimeric OspA fragments induced significant antibody titers against all major OspA serotypes.
OspA-based vaccines primarily protect via production of circulating antibodies that are ingested by ticks during their blood meal. In the tick’s midgut, these antibodies bind, neutralize, and promote elimination of the spirochetes, such that no pathogen can be transmitted and subsequently cause an infection in the vertebrate host (56). Since high levels of anti-OspA antibodies following immunization are of primary importance, we first studied the immune response generated by the multivalent variants against the most clinically relevant OspA serotypes (ST1 to ST6) by ELISA. A recent study has shown that immunogenicity was 10-fold higher in mice with an OspA-based vaccine formulated with 0.15% aluminum hydroxide than that with the vaccine formulated without adjuvant (17). Therefore, all immunizations in this study were performed using antigens formulated with 0.15% aluminum hydroxide. Mice were immunized 3 times at 2-week intervals with a dose of 5 μg of each of the individual multivalent variants (V1 to V6). Since all multivalent variants only contained the C-terminal half of OspA, the 96-well plates for ELISA were coated with stabilized C-terminal fragments of OspA, and the immune response was compared to the response induced by full-length OspA (FL-OspA).
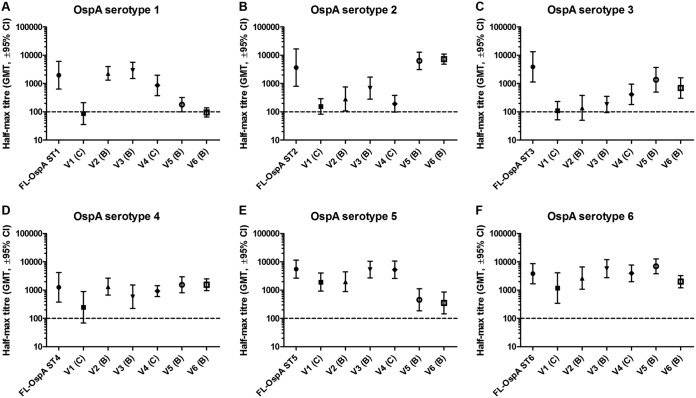
V1 and V2 have the same patch layout but different disulfide bonds, C and B, respectively, for stabilization (Fig. 3). Despite this similarity, V1 induced significantly lower anti-OspA ST1 antibody titers than V2 (Fig. 4A). However, the immunogenicity was comparable to that of other serotypes (Fig. 4B to F). V3 and V4 also had the same patch layout and different disulfide bonds, B and C, respectively, for stabilization (Fig. 3), but induced comparable immune responses with respect to all included serotypes (Fig. 4). Comparing the induced immune response to that induced by full-length OspA, V2 to V4 induced similar antibody titers to those of FL-OspA ST1, FL-OspA ST4, and FL-OspA ST5 (homologous serotypes present on their surface; Fig. 4A, D, and E) and cross-reactive antibodies comparable to those induced by FL-OspA ST6 (Fig. 4F). The immune response was lower than the response induced by FL-OspA ST2 and FL-OspA ST3 (Fig. 4B and C). Comparing V1 to V4, V1 induced the lowest level of anti-OspA ST1 antibodies, possibly because disulfide bond C slightly distorts the structure of patch I, where OspA ST1 sequences are located. However, a similarly reduced level of anti-OspA ST1 antibodies was not observed with V4, although it also had disulfide bond C, possibly because patches VI and VIII were populated with interchanged OspA ST4 and ST5, respectively. V5 and V6, both with disulfide bond B (Fig. 3), induced immune responses comparable to those induced by FL-OspA ST2, FL-OspA ST3, and FL-OspA ST6, the serotypes presented on their surfaces (Fig. 4B, C, and F), as well as cross-reactive antibodies comparable to those induced by FL-OspA ST4 (Fig. 4D). However, they induced weaker immune responses than FL-OspA ST1 and FL-OspA ST5, which were serotypes not present on these multivalent variants (Fig. 4A and E). When OspA ST6 was only populated on patch VII (V6), a weaker immune response than that for the constellation with OspA ST6 populated on both patches I and VII (V5) (Fig. 4F) was observed.
FIG 4.
Antibody response of chimeric vaccine candidates with C-terminal fragment of OspA (monomers) as coating antigens. The immunogenicity of the individual chimeric proteins when administered at a dose of 5 μg and formulated with 0.15% aluminum hydroxide was assessed in groups of 10 mice. Immune sera collected from mice 2 weeks after the final immunization were serially diluted and tested in duplicates. The plates were coated with C-terminal fragments of the respective OspA ST, and the immune response of the chimeric candidates was compared to the FL-OspA of the respective serotypes. The results are represented as half-maximal geometric mean titers (GMT) with a 95% confidence interval, and the dotted lines represent the detection limit.
These results showed that the immune responses to the multivalent variants varied according to the distribution of serotypes on different patches. The data also revealed that disulfide bond B performed better for stabilization of the multivalent variants than disulfide bond C. For this reason, V1 and V4 were excluded from further analyses. These data also indicated that two multivalent variants may have to be combined to allow generation of a strong immune response against all six clinically most relevant OspA serotypes.
Chimeric variants generated surface binding and functional antibodies against multiple OspA serotypes.
The multivalent V2, V3, V5, and V6 variants were evaluated for their ability to induce surface binding and functional antibodies against the clinically most relevant OspA serotypes (ST1 to ST6). The assessment was based on the ability of antibodies to bind to LB Borrelia species expressing OspA ST1 to ST6 on their surfaces. In addition, functional antibodies were also assessed in a serum bactericidal assay (SBA).
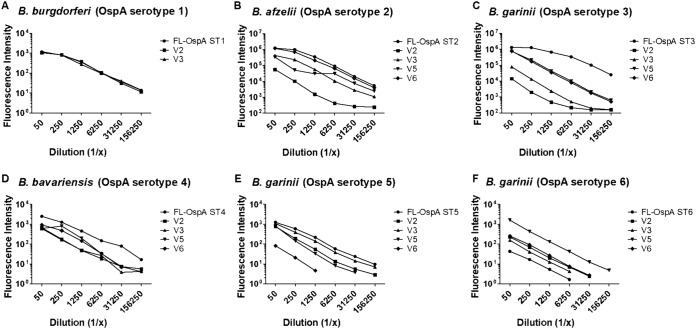
Heat-inactivated pooled serum samples from mice immunized with each multivalent variant were tested in 5-fold dilutions for their ability to bind to spirochetes expressing OspA ST1 to ST6. V2-induced sera showed equivalent surface binding to spirochetes expressing OspA ST1 as immune sera induced with FL-OspA ST1 (Fig. 5A) but lower surface binding to spirochetes expressing OspA ST2, ST4, and ST5 than to spirochetes expressing the homologous FL-OspA (Fig. 5B and D and E). V3-induced antibodies showed surface binding comparable to those induced with FL-OspA ST1 and FL-OspA ST5 (Fig. 5A and E) but lower surface binding than those induced with FL-OspA ST2 and FL-OspA ST4 (Fig. 5B and D). Both of these variants induced strong surface binding antibodies compared to FL-OspA ST6 (Fig. 5F).
FIG 5.
Surface binding of spirochetes to determine vaccine-induced antibodies. The antibodies generated by the chimeric vaccine candidates were tested by surface binding assay. The binding of vaccine-induced antibodies to OspA of the corresponding serotype was compared to the binding of antibodies generated by the respective FL-OspA. The surface binding assay was performed with B. burgdorferi OspA ST1 ZS7, B. afzelii OspA ST2 Pra10, B. garinii OspA ST3 PFr, B. bavariensis OspA ST4 PFin, B. garinii OspA ST5 PHei, and OspA ST6 KL11. The results are presented as fluorescence intensity.
V5- and V6-generated sera demonstrated surface binding equivalent to FL-OspA ST2 and FL-OspA ST4-generated sera (Fig. 5B and D). V5-induced antibodies showed significantly stronger surface binding than V6-induced antibodies, and both showed stronger binding than FL-OspA ST6 (Fig. 5F). Both of these variants induced lower levels of surface binding than FL-OspA ST5 (Fig. 5E) and did not induce any surface binding to B. burgdorferi ZS7 (OspA ST1).
V2- and V3-induced sera showed only weak binding to B. garinii strain PFr (OspA ST3); on the other hand, for V5- and V6-generated antibodies, significant surface binding almost as strong as that induced by FL-OspA ST3 (Fig. 5C) was observed.
In order to determine the titer of borreliacidal antibodies induced by the four variants, SBAs in the presence of guinea pig complement (ZS7 OspA ST1, LU171 OspA ST2, and PFr OspA ST3) or baby rabbit complement (DK6 OspA ST4, PHei OspA ST5, and KL11 OspA ST6) were performed. For V2 and V3, we observed bactericidal titers comparable to those with FL-OspA ST1, while low bactericidal activity was seen for both multivalent variants compared to FL-OspA ST4 and FL-OspA ST5 (Table 1). None of the V2 and V3 variants induced significant bactericidal activity against B. garinii OspA ST3 or B. afzelii OspA ST2 (Table 1). V5 and V6 induced higher bactericidal titers than FL-OspA ST6, similar titers as FL-OspA ST4, and no or low bactericidal activity compared to FL-OspA ST1, FL-OspA ST3, and FL-OspA ST5 (Table 1). V5 generated similar titers, whereas V6 generated lower levels of bactericidal antibodies than FL-OspA ST2 (Table 1). In conclusion, V5 generated higher bactericidal activity than V6.
TABLE 1.
Functional antibodies generated by the chimeric OspA variants, as determined in serum bactericidal assaysa
| Immune serum | SB titer forb
: |
|||||
|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | |
| FL-OspA | 160 | 2,500 | 2,500 | 160 | 12,500 | <20 |
| V2 | 160 | <20 | <20 | 80 | 2,500 | 500 |
| V3 | 160 | <20 | <20 | 20 | 500 | 500 |
| V5 | <20 | 2,500 | <20 | 160 | 20 | 62,500 |
| V6 | <20 | 500 | <20 | 320 | <20 | 500 |
Serum bactericidal assay was performed with B. burgdorferi ZS7 (ST1), B. afzelii LU171 (ST2), B. garinii PFr (ST3), B. bavariensis DK6 (ST4), B. garinii PHei (ST5), and KL11 (ST6).
Serum bactericidal (SB) titer is defined as the reciprocal of the lowest dilution showing ≥50% reduction in spirochetes compared to negative sera.
Chimeric variants provided significant protection against major LB Borrelia species pathogenic to humans.
V2, V3, V5, and V6 were next assessed in challenge experiments using Borrelia strains expressing four OspA serotypes (ST1, ST2, ST4, and ST5). Mice were immunized with the individual multivalent variants and were challenged with Ixodes ricinus ticks infected with B. burgdorferi OspA ST1, B. afzelii OspA ST2, or B. bavariensis OspA ST4, as described previously (19), or by subcutaneous injection of in vitro-grown spirochetes (B. burgdorferi OspA ST1 or B. garinii OspA ST5). Ticks were monitored daily until detachment, and only mice with at least one tick (B. afzelii OspA ST2) or two ticks (B. burgdorferi OspA ST1 and B. bavariensis OspA ST4) feeding for >48 h were included in the final infection readout.
V2 and V3 generated significant protection against Borrelia strains expressing OspA ST1, ST2, and ST4 in the tick challenge models (Table 2), and V3 protected better against OspA ST5 than V2 (Table 2). Thus, V2 and V3 provided cross-protection against B. afzelii OspA ST2. V5 and V6 provided no cross-protection against a challenge with ticks harboring B. burgdorferi OspA ST1 or against a subcutaneous injection of B. burgdorferi OspA ST1 or B. garinii OspA ST5, but both variants provided significant protection, as well as cross-protection, against challenge with ticks infected with B. afzelii OspA ST2 and B. bavariensis OspA ST4, respectively (Table 2).
TABLE 2.
Vaccine efficacy of chimeric OspA variants in tick and subcutaneous challenge experimentsa
| Immunogen | Serotypes | Dose (μg) | Tick challenge (no. infected/total no.) with: |
Subcutaneous challenge (no. infected/total no.) with: |
||||
|---|---|---|---|---|---|---|---|---|
| B. burgdorferi (ST1) | B. afzelii (ST2) | B. bavariensis (ST4) | B. burgdorferi (ST1) | B. garinii (ST5) | B. garinii (ST5) | |||
| FL-OspA | 5 | 3/9b | 1/7d | 0/6d | 0/10d | 0/10d | 0/10d | |
| V2 | 1, 4, 5 | 5 | 1/7c | 3/8b | 0/4c | 0/10d | 3/10e | 4/10c |
| V3 | 1, 4, 5 | 5 | 0/8d | 4/10b | 1/9d | 0/10d | 0/10d | 1/10c |
| V5 | 2, 3, 6 | 5 | 5/8e | 1/9d | 0/7d | 9/10e | 3/5e | 8/10e |
| V6 | 2, 3, 6 | 5 | 6/9e | 1/9d | 0/7d | 7/10e | 7/10e | 9/10e |
| Placebo | 12/14 | 13/14 | 9/10 | 10/10 | 8/10 | 10/10 | ||
aMice were challenged with laboratory-reared ticks infected with B. burgdorferi (ST1; strain Pra1), B. afzelii (OspA ST2; strain IS1), or B. bavariensis (OspA ST4; strain Marx1), or by subcutaneous injection with in vitro-grown spirochetes of B. burgdorferi (OspA ST1; strain ZS7) or B. garinii (OspA ST5; strain PHei [results from two independent experiments are shown in the last two columns]). For the tick challenge experiments, only mice with at least one tick (ST2) or two ticks (ST1 and ST4) feeding for >48 h were included in the readout. Statistical significance was calculated with Fisher’s exact test (two-tailed).
P < 0.05.
P < 0.01.
P < 0.001.
Not significant.
Fusion protein V3-L2-V5 generated potent immune responses against major LB Borrelia species pathogenic to humans.
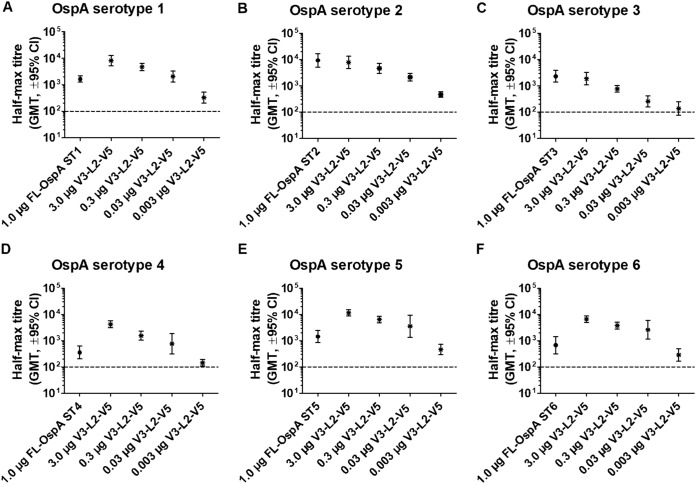
In order to generate a single antigen that can provide protection against the major LB Borrelia species, V3 and V5 were selected for further analysis based on the immunogenicity and protection data for the individual variants. These two variants were fused together with a 23-amino-acid linker sequence derived from an immunogenic loop of the surface-exposed protein P66 (57–59) from B. garinii strain PBr, and the fusion protein was referred to as V3-L2-V5. The immunogenicity of V3-L2-V5 was compared to that of FL-OspA from the six OspA serotypes. Mice were immunized with V3-L2-V5 (3.0, 0.3, 0.03, and 0.003 μg) and FL-OspA (1 μg) 3 times at 2-week intervals. Immune sera were collected 1 week after the final immunization for analyses of anti-OspA IgG antibodies by ELISA. The V3-L2-V5 vaccine generated higher antibody titers than FL-OspA ST1, FL-OspA ST4, FL-OspA ST5, and FL-OspA ST6, and similar titers with respect to FL-OspA ST2 and FL-OspA ST3 (Fig. 6).
FIG 6.
Antibody response of vaccine candidate V3-L2-V5 versus the corresponding FL-OspA serotypes. The immunogenicity of V3-L2-V5 was studied in a dose titration experiment and was compared to FL-OspA of the respective serotype (ST1-ST6). Immune sera were collected 2 weeks after the final immunization and tested in duplicates. The plates were coated with C-terminal OspA fragments of the respective serotype. The results are represented as half-maximal geometric mean titers (GMT) with a 95% confidence interval, and the dotted lines represent the detection limit.
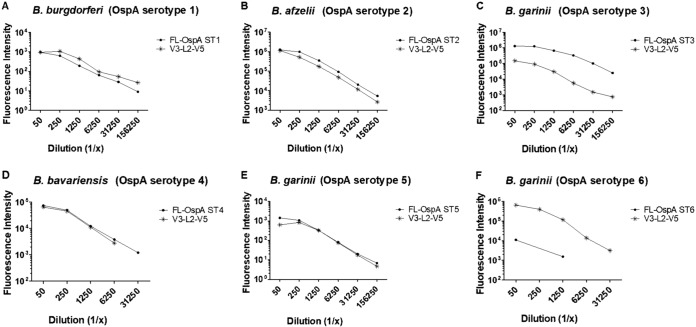
The ability of V3-L2-V5 to generate antibodies was assessed by surface binding and SBA. For these in vitro assays, only sera from mice immunized with a dose of 3.0 μg were used for comparison. V3-L2-V5 generated comparable surface binding to spirochetes expressing OspA ST1, ST2, ST4, and ST5 and to spirochetes expressing the respective FL-OspA STs (Fig. 7A, B, D, and E). The surface binding of V3-L2-V5-induced antibodies to spirochetes expressing OspA ST6 was higher than that to the corresponding FL-OspA ST6 (Fig. 7F), and the surface binding to spirochetes expressing OspA ST3 was lower than that generated by the corresponding FL-OspA ST3 (Fig. 7C). The functional antibody titers generated by V3-L2-V5 were comparable to or higher than those generated by the respective FL-OspA for spirochetes expressing OspA ST1, ST2, and ST6 and lower with respect to OspA ST3, ST4 and ST5 (Table 3).
FIG 7.
Antibodies generated by V3-L2-V5 versus the corresponding FL-OspA serotypes. The antibodies generated by V3-L2-V5 were tested by surface binding assay. The binding of vaccine-induced antibodies to OspA was compared to the binding of antibodies generated by FL-OspA of the corresponding serotypes (ST1 to ST6). The surface binding assay was carried out with B. burgdorferi OspA ST1 ZS7, B. afzelii OspA ST2 Pra10, B. garinii OspA ST3 PFr, B. bavariensis OspA ST4 PFin, B. garinii OspA ST5 PHei, and OspA ST6 KL11. The results are represented as fluorescence intensity.
TABLE 3.
Functional antibodies generated by the V3-L2-V5 fusion vaccine candidate as determined in serum bactericidal assaysa
| Immune serum | SB titerb
: |
|||||
|---|---|---|---|---|---|---|
| ST1 | ST2 | ST3 | ST4 | ST5 | ST6 | |
| FL-OspA | 160 | 500 | 2,500 | 160 | 12,500 | 20 |
| V3-L2-V5 | 160 | 500 | <20 | 40 | 500 | 2,500 |
Growth inhibition assay was analyzed with B. burgdorferi ZS7 (ST1), B. afzelii LU171 (ST2), B. garinii PFr (ST3), B. bavariensis DK6 (ST4), B. garinii PHei (ST5), and KL11 (ST6) strains.
Serum bactericidal (SB) titer is defined as the reciprocal of the lowest dilution with ≥50% reduction in spirochetes compared to negative sera.
Fusion protein V3-L2-V5 protected against two major human-pathogenic LB Borrelia species.
The protective capacity of the V3-L2-V5 vaccine was tested against the two most clinically relevant LB Borrelia species, i.e., B. burgdorferi (OspA ST1) and B. afzelii (OspA ST2). For this study, V3-L2-V5 (3.0, 0.3, 0.03, and 0.003 μg) was compared to the respective FL-OspA (1.0 μg) after three immunizations against a challenge with in vitro-grown B. burgdorferi (OspA ST1) and ticks infected with B. afzelii (OspA ST2). The V3-L2-V5 protein demonstrated highly significant protection against B. burgdorferi (OspA ST1) at the three highest immunization doses (3.0, 0.3, and 0.03 μg) (Table 4). Interestingly, V3-L2-V5 still demonstrated significant protection at even the lowest dose of 3 ng (Table 4). For the challenge with B. afzelii OspA ST2-infected ticks, the results of two experiments were combined (using the same batch of infected ticks). V3-L2-V5 demonstrated 100% protection against a challenge with B. afzelii OspA ST2-infected ticks at a dose as low as 0.03 μg, comparable to the protection obtained with 1.0 μg of FL-OspA ST2.
TABLE 4.
Efficacy of V3-L2-V5 fusion vaccine candidate in comparison to full-length OspA against B. burgdorferi OspA ST1 subcutaneous challenge and B. afzelii OspA ST2 tick challengea
| Immunogen | Dose (μg) | No. infected/total in: |
|
|---|---|---|---|
| Subcutaneous challenge with B. burgdorferi (ST1) | Tick challenge with B. afzelii (ST2) | ||
| FL-OspA ST1 | 1.0 | 6/10d | |
| FL-OspA ST2 | 1.0 | 0/18c | |
| V3-L2-V5 | 3.0 | 0/10c | 0/9c |
| V3-L2-V5 | 0.3 | 0/10c | 0/9c |
| V3-L2-V5 | 0.03 | 0/10c | 0/9c |
| V3-L2-V5 | 0.003 | 2/10b | 6/9d |
| Placebo | 8/10 | 15/16 | |
Mice were either challenged with ticks infected with B. afzelii (OspA ST2; strain IS1) or subcutaneously injected with in vitro-grown spirochetes of B. burgdorferi (OspA ST1; strain ZS7). For B. afzelii, only mice with at least one tick feeding for >48 h were included in the readout. Statistical significance was calculated with Fisher’s exact test (two-tailed).
P < 0.05.
P < 0.001.
Not significant.
DISCUSSION
A structure-based surface-shaping method was used to create multivalent OspA variants based on the OspA C-terminal fragment. The multivalent variants were designed with the goal of increasing the breadth of protection against diverse Borrelia species and OspA serotypes using as few antigens as possible. Thus, the ultimate approach was to design a single recombinant protein that could induce a protective immune response for all six major OspA serotypes and therefore reduce the cost and complexity of vaccine production. Scarselli et al. (20) described several criteria required to generate a multivalent vaccine that induces broad coverage in immunogenicity and, ideally, protection against a multitude of variants of the targeted pathogen. These include (i) detailed information of the three-dimensional structure of the antigen, (ii) knowledge of immunogenic epitopes on the antigen as well as on its variants, (iii) presence of surface areas that are large enough to hold a conformational epitope, and (iv) sequence and fold conservation between the variants of the antigen. Using this set of criteria, Scarselli and colleagues (20) modeled an fHBP-derived protein that was broadly immunogenic against a plethora of Neisseria meningitidis serogroup B strains.
Applying a similar approach to the design of multivalent variants of the OspA C-terminal fragment, we had to rely mainly on the crystal structure and information on antibody binding sites/regions that were available for B. burgdorferi OspA ST1. Structure models for each of the six OspA serotypes suggested high fold conservation, which provided us with the freedom to choose any of the six OspA C-terminal fragments as the structural scaffold. Since OspA ST2 is the most prevalent serotype in Europe, we chose OspA from B. afzelii (strain K78; GenBank accession number AJY72832.1) as the structural scaffold for the design of multivalent OspA variants. By using the sequence of OspA ST2 as the structural scaffold, a putative T-cell epitope present only in OspA ST1 was avoided (50–52). The surface was partitioned into patches that have the potential to harbor possible B-cell epitopes based on the described binding sites/regions of MAbs LA-2 (28), 336 (27), 105.5 (31), 4C10C2 (29), and CIII.78 (21) and of the tick gut binding domain (TGBD) (30) (Fig. 1). Several different patch assignments were designed in order to ensure minimum interference between amino acid residues of different serotypes on neighboring patches. Two layouts were designed with different patch assignments (Fig. 1) to achieve broad protection in a final vaccine design. Surface-exposed and most partially exposed amino acids were replaced for each patch to match the respective OspA ST. We constructed six variants (V1 to V6) based on the two layouts and assessed two different locations of stabilization by a disulfide bond (Fig. 3).
V1 to V4 have the same patch layout, composed of patches I, V, VI, and VIII, but V1 and V4 contained disulfide bond C and were difficult to produce because of low protein expression and low yields after purification. In addition, these proteins were equally or less immunogenic than V2 and V3, which have the same serotype assignment but stabilizing disulfide bond B (Fig. 4). These results underline the importance of the position of the disulfide bond for expression, purification yield, and immunogenicity. OspA ST5 and ST4 were interchanged between patches VI and VIII on V2 and V3 to study the effect on immunogenicity, as well as protection generated against these serotypes (Fig. 4; Tables 1 and 2). However, no significant difference was observed in immunogenicity (Fig. 4D and E, Fig. 5C and D; Table 1) or protection with respect to OspA ST4 and ST5 (Table 2). Likewise, V2 and V3 showed significant cross-protection against OspA ST2 (Table 2), although V3 showed slightly higher protection in two experiments using B. garinii OspA ST5 for the challenge (Table 2).
V5 and V6 were designed with OspA ST2 and ST6 interchanged on patch I (Fig. 3) to study the effect on immunogenicity as well as that on protection. With respect to immunogenicity, both variants generated similar immune responses against FL-OspA ST2, and the immune response is similar to the one generated by FL-OspA ST2 (Fig. 4 and 5; Table 1). Both variants also provided significant protection against a challenge with B. afzelii OspA ST2-infected ticks (Table 2). This indicated that OspA ST2 on patch I does not additionally contribute to protection when patches III and IV are also populated with OspA ST2 residues. In contrast, the presence of OspA ST6 residues on patch I had a significant contribution to OspA ST6-specific immunogenicity, since V5 demonstrated better immunogenicity than V6 against OspA ST6 (Fig. 4F and 5E; Table 1). In general, V5 and V6 display 98% overall sequence identity, which suggested that a serotype interchange on patch I might not have a significant effect on immunogenicity and protection generated by these candidates (Fig. 4 and 5; Tables 1 and 2).
Even though V5 and V6 were not designed with OspA ST4 residues on any of the patches, they induced a strong immune response and protection against challenge with B. bavariensis OspA ST4. Patch VII populated with OspA ST6 in both variants shared a sequence identity of 94% with OspA ST4. Therefore, it can be assumed that the cross-reactivity of V5 and V6 with OspA ST4 could likely be attributed to patch VII.
V5 and V6 also demonstrated a significantly lower immune response, as well as partial nonsignificant cross-protection against a challenge with B. burgdorferi OspA ST1 and B. garinii OspA ST5.
Based on all data obtained with the individual variants, V3 and V5 were linked with a short and flexible linker sequence (23 amino acids derived from P66) to produce a single fusion protein, V3-L2-V5. The linker sequence used in V3-L2-V5 was reported to be immunogenic in humans (60), but the specific contribution of the linker sequence to immunogenicity and protection was not assessed in this study. OspA-based vaccines protect via a transmission blocking mechanism of action and are therefore dependent on high level of circulating antibodies that neutralize the spirochetes in the midgut of the tick. In this respect, antibodies recognizing the P66 linker may increase the efficacy of the LB vaccine by targeting spirochetes in the tissue of the host that were not neutralized by anti-OspA antibodies in the tick. However, this hypothesis needs to be investigated. The high antibody titers in conjunction with the functionality of the antibodies generated by V3-L2-V5 indicate that the single antigen has the potential to generate a broad protective immune response against the major OspA serotypes (Fig. 5 and 6; Table 3). This was further substantiated by the demonstration that the fusion vaccine V3-L2-V5 provided 100% protection against challenge with in vitro-grown B. burgdorferi OspA ST1 and B. afzelii OspA ST2-infected ticks at the first three immunization doses (3.0, 0.3, and 0.03 μg). Interestingly, V3-L2-V5 was shown to be highly potent as a vaccine, since protection against challenge with in vitro-grown B. burgdorferi OspA ST1 was observed following immunization with only 3 ng. The results presented here provide the proof of principle of a new multivalent LB vaccine that may prospectively attain broad protection with a single protein.
MATERIALS AND METHODS
Ethics statement.
The animal experiments in the study were conducted according to Austrian law (Tierversuchsgesetz 2012, BGBl. I Nr. 114/2012) and approved by Magistratsabteilung 58. All experimental procedures were reviewed and approved by Valneva’s animal welfare committee and in accordance with the 3R principle (replacement, reduction, and refinement). The number of animals used in the study was kept as low as possible. Mice were housed in the animal facility at Valneva in Vienna at 20 to 24°C with a 12/12 h light/dark cycle in standard individually vented cages (IVCs; Euro standard type II long) in groups of five female mice in each cage. No mortalities occurred prior to the conclusion of the experiments. Mortalities were not expected, since mice are the natural reservoir of Borrelia in nature.
Design of chimeric constructs.
Crystal structures of B. burgdorferi OspA ST1 (PDB ID 1OSP [41] and PDB ID 1FJ1 [28]) and homology models reflecting the other serotypes (42) were analyzed for the design of the chimeric constructs. The conserved protein backbone of B. afzelii OspA ST2 (strain K78; GenBank accession number AJY72832.1) served as a structural scaffold for determination of sequence positions where adaptive mutations were required.
Residues with surface-exposed (and partially surface-exposed) side chains were distinguished from buried residues to adapt the outer surface accordingly. Patches were defined and optimized to optimally accommodate the binding of known antibodies or to match with results from, e.g., peptide library scanning experiments, mutagenesis, and fragment binding experiments. Surface residues in these regions were mutated to represent the serotype of choice, while buried residues were retained (ST2-based scaffold), making use of the fold conservation observed in the structural models. An overlap of anticipated antibody footprints was addressed by defining two different surface partitioning schemes. Also, the combinations of serotypes for individual patches were optimized in order to minimize the interference of mutations for antibody binding in one patch of a selected serotype with residues of adjacent patches. For this optimization, the interresidue distances (Cα-Cα) on the ST2 reference structure were scanned, summing up penalty scores for each interfering residue combination (determined from a sequence structure alignment with candidate OspA sequences of serotypes ST1 to ST6). The residues within a cutoff 0.8 nm (from 0.5 to 0.8 nm, faded off with a scaling function) were scaled by exposition factors of 0, 0.5, and 1, i.e., core, partially exposed, and surface accessible, respectively. This scoring was repeated for each possible patch attribution and serotype combination. Within the patches of a selected layout and according to the choice of serotype for them, the subset of surface-exposed amino acid residues was substituted by a set of amino acid residues of the respective serotypes, and the chosen type of disulfide bond was introduced into the sequence.
Cloning.
The serotype-specific OspA amino acid sequences were derived from B. burgdorferi ST1 (aa 126 to 273, strain B31; GenBank accession number NP_045688.1), B. afzelii ST2 (aa 126 to 273; strain K78, accession number AJY72832.1), B. garinii ST3 (aa 126 to 274, strain PBr; accession number WP_015939522.1), B. bavariensis ST4 (aa 126 to 273, strain PBi; accession number WP_011187157.1), B. garinii ST5 (aa 126 to 273, strain PHei; accession number CAA56544.1), and B. garinii ST6 (aa 126 to 274, strain DK29; accession number CAA45010.1). The sequence alignment of chimeric variants compared to the C-terminal fragment of B. afzelii OspA ST2 (providing the structural scaffold) is depicted, and identities are represented as dots, only showing the amino acids that have been substituted in each variant (Fig. 2). The nucleotide sequence of each of the hybrid constructs was codon optimized and synthesized (GeneArt Gene Synthesis; Thermo Fisher Scientific). The constructs were digested using HindIII and XhoI and were cloned into the expression vector pET28b+ (Merck Millipore, USA), with the inclusion of a 23-amino-acid signal sequence for lipidation (MKATKLVLGAVILGSTLLAGCSS) from Escherichia coli major outer membrane lipoprotein and a histidine tag (LEHHHHHH) at the C terminus. Two chimeric variants, V3 and V5, were fused together with a 23-amino-acid linker sequence, ANNQAGQKSSGSTQATTPNLTFE (L2), to form the fusion vaccine denoted V3-L2-V5. The linker sequence was derived from a loop region of P66 (a major Borrelia porin) from B. garinii strain PBr (GenBank accession number EED29356.1). For the final fusion protein, V3 was inserted into the pET28b+ vector immediately after the lipid signal sequence, using the HindIII and SpeI restriction sites, followed by the linker sequence using SpeI and ScaI and V5 using ScaI and XhoI.
Protein expression and purification.
Protein expression and purification up to the step that includes phase separation with Triton X-114 followed the procedure as described by Comstedt et al. (18). Briefly, induction of protein expression was performed at 25°C with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). A protease inhibitor cocktail II [PIC II: 2 ml Bestatin, 2 ml 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride (AEBSF), and 2 ml E-64] was added to the lysis buffer (50 mM Tris-HCl, 500 mM NaCl, and 5 mM EDTA [pH 8.0]), and cell lysis was carried out with a high-pressure homogenizer (Panda 2K). Triton X-114 was added to the crude lysate (0.06 times the volume of crude lysate), and the solution was incubated at 4°C with gentle stirring overnight and then centrifuged for 1 h at 4°C. The supernatant was incubated at 28°C for 30 min. The lipid phase was recovered by centrifugation at 7,000 × g for 40 min at 28°C.
The His-tagged chimeric proteins were purified by immobilized metal ion affinity chromatography (IMAC). Briefly, the lipid phase was diluted 1:20 in lipid phase dilution buffer (50 mM Tris-HCl [pH 8.0], 500 mM NaCl, 0.05% Tween 20, and 10% ethanol) and loaded onto a column with Ni+ Sepharose beads (Ni Sepharose 6 Fast Flow; GE Healthcare) equilibrated with the lipid-phase dilution buffer. The bound His-tagged proteins were eluted with an imidazole elution buffer (50 mM Tris-HCl, 500 mM NaCl, 0.05% Tween 20, and 100 mM imidazole) with increasing imidazole concentrations (100 mM, 250 mM, and 500 mM).
The PyroGene recombinant factor C kit (Lonza) was used to determine the concentration of endotoxin in the lipidated chimeric protein samples.
Immunization and challenge.
Immunization and challenge studies were performed as described by Comstedt et al. (17–19). The purified lipidated chimeric proteins were formulated with 0.15% aluminum hydroxide (Alhydrogel; Brenntag) as an adjuvant. Groups of 10 female C3H/HenRj mice (8 to 10 weeks old) per antigen were injected subcutaneously with 5.0 μg of individual chimeric proteins. Full-length OspA protein (5 μg) was used as the positive control, and adjuvant was used alone as the placebo. Three immunizations were administered at 2-week intervals, and immune sera were collected 1 (challenge experiments) or 2 (immunogenicity experiments) weeks after the final immunization. Two weeks following the final immunization, mice were challenged subcutaneously with in vitro-grown spirochetes expressing OspA from B. burgdorferi (ST1) or B. garinii (ST5) (17, 18). A dose of 5 × 104 spirochetes in 100 μl was used for all strains per mouse. OspA expression was confirmed by flow cytometry, and only cultures where at least 80% of spirochetes expressed OspA on their surface were used for challenge.
Ticks infected with B. afzelii (strain IS1), B. burgdorferi (strain Pra1), and B. bavariensis (strain Marx1) were used to challenge mice, as described previously (17, 19). For strains IS1 and Marx1, two ticks were applied to each mouse, and for Pra1, three ticks were applied. Mice with at least one (IS1) or two (Pra1 and Marx1) fully fed ticks were included in the subsequent readouts. Four weeks after challenge, mice were anesthetized with isoflurane prior to terminal bleeding and sacrificed by cervical dislocation. The infection status was determined by VlsE ELISA as described previously by Comstedt et al. (17).
OspA ELISA.
Immune sera derived from mice after the third immunization were analyzed for OspA-specific IgG titers. Indirect ELISA was performed using stabilized C-terminal fragments of OspA of the respective serotypes as a coating antigen. The ELISA was performed as previously described (17). Briefly, 96-well plates were coated with 0.05 μg protein in 50 μl phosphate-buffered saline (PBS) per well overnight at 4°C. The plates were blocked with 100 μl blocking buffer (PBS with 0.05% Tween 20 [PBST]) per well for 1 h at room temperature. Serum samples were serially diluted and tested in duplicates by incubating for 1 h at room temperature. A horseradish peroxidase (HRP)-conjugated polyclonal rabbit anti-mouse IgG (Dako) was used as a secondary antibody, ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)] was used as the substrate, and the reaction was stopped with 1% SDS. Absorbance was read at 405 nm (Synergy 2; BioTek), and antibody titers were presented as half-maximal titers, which is the reciprocal of the serum dilution corresponding to the mean absorbance between the highest and the lowest dilution.
Surface binding of Borrelia.
Spirochetes were stained to determine OspA expression as described previously (17). Briefly, spirochetes were fixed by the addition of an equal volume of 4% paraformaldehyde. The heat-inactivated serum pools were serially diluted (1:5) in washing buffer in a separate dilution plate. Diluted sera were added to the fixed spirochetes and incubated for 45 min at room temperature. Phycoerythrin (PE)-conjugated goat anti-mouse IgG (Beckman Coulter, USA) was used as the secondary antibody, and LDS 751 (Life Technologies, USA) was used to stain the DNA of the spirochetes. The stained spirochetes were analyzed with a flow cytometer (Cytomics FC 500; Beckman Coulter) by gating for positive LDS 751 events.
Serum bactericidal assay.
To determine the amount of functional antibodies, a serum bactericidal assay was used. The heat-inactivated immune serum pools were serially diluted and incubated with spirochetes (1.0 × 103 to 2.0 × 104/well). Different sources of complement were used, namely, guinea pig (B. burgdorferi ZS7 OspA ST1, B. afzelii LU171 OspA ST2, and B. garinii PFr OspA ST3) or baby rabbit (B. bavariensis DK6 OspA ST4, B. garinii PHei OspA ST5, and B. garinii KL11 OspA ST6) complement in 96-well plates for 3 to 5 days at 32°C with 5% CO2. The amount of spirochetes was assessed with BacTiter-Glo, and luminescence was measured in an ELISA reader (Synergy 2; BioTek). The values are represented as serum bactericidal titers, which is defined as the reciprocal of the serum dilution with at least 50% reduction in spirochetes compared to negative-control sera.
VlsE ELISA.
ELISA with the invariable region 6 (IR6) of the variable major protein-like sequence E protein (VlsE) was performed as described earlier (17). Briefly, 96-well streptavidin-precoated plates were coated with 25-amino-acid-long biotinylated IR6 peptide. Immune sera were diluted (1:2) and tested in duplicates, and an HRP-conjugated polyclonal rabbit anti-mouse IgG (Dako) was used as the secondary antibody. ABTS was added as a substrate, and the reaction was continued for 30 min; absorbance was read at 450 nm.
Experiment evaluation and statistics.
The infection status of mice was determined by VlsE ELISA. All groups were compared to the placebo group for assessment of infection/protection, and statistical significance was calculated with Fisher’s exact test (two-tailed); *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
ACKNOWLEDGMENTS
We thank Christina Satke, Sandra Jost, Ursula Bartuschka, Ana Isabel Kremers, and Julia Hable for their excellent technical support. The ticks used in the study were provided by Hans Dautel (Insect Services, Germany), and LB Borrelia strains were obtained from Volker Fingerle (National Reference Center for Borrelia, Bavarian Health and Food Safety Authority, Oberschleißheim, Germany).
This study was supported by the European Union Seventh Framework Programme (FP7) under grant agreement 316655 (VacTrain).
REFERENCES
- 1.Radolf JD, Caimano MJ, Stevenson B, Hu LT. 2012. Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol 10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franke J, Hildebrandt A, Dorn W. 2013. Exploring gaps in our knowledge on Lyme borreliosis spirochaetes–updates on complex heterogeneity, ecology, and pathogenicity. Ticks Tick Borne Dis 4:11–25. doi: 10.1016/j.ttbdis.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Stanek G, Wormser GP, Gray J, Strle F. 2012. Lyme borreliosis. Lancet 379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- 4.Rizzoli A, Hauffe H, Carpi G, Vourc HG, Neteler M, Rosa R. 2011. Lyme borreliosis in Europe. Euro Surveill 16:pii=19906. doi: 10.2807/ese.16.27.19906-en. [DOI] [PubMed] [Google Scholar]
- 5.Sykes RA, Makiello P. 2017. An estimate of Lyme borreliosis incidence in Western Europe. J Public Health (Oxf) 39:74–81. doi: 10.1093/pubmed/fdw017. [DOI] [PubMed] [Google Scholar]
- 6.Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS. 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59:676–681. doi: 10.1093/cid/ciu397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS. 2015. Incidence of clinician-diagnosed lyme disease, United States, 2005–2010. Emerg Infect Dis 21:1625–1631. doi: 10.3201/eid2109.150417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer R, Caimano MJ, Luthra A, Axline D Jr, Corona A, Iacobas DA, Radolf JD, Schwartz I. 2015. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol Microbiol 95:509–538. doi: 10.1111/mmi.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuijt TJ, Hovius JW, van der Poll T, van Dam AP, Fikrig E. 2011. Lyme borreliosis vaccination: the facts, the challenge, the future. Trends Parasitol 27:40–47. doi: 10.1016/j.pt.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Embers ME, Narasimhan S. 2013. Vaccination against Lyme disease: past, present, and future. Front Cell Infect Microbiol 3:6. doi: 10.3389/fcimb.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, Krause DS, Lyme Disease Vaccine Study Group . 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. N Engl J Med 339:209–215. doi: 10.1056/NEJM199807233390401. [DOI] [PubMed] [Google Scholar]
- 12.Sigal LH, Zahradnik JM, Lavin P, Patella SJ, Bryant G, Haselby R, Hilton E, Kunkel M, Adler-Klein D, Doherty T, Evans J, Molloy PJ, Seidner AL, Sabetta JR, Simon HJ, Klempner MS, Mays J, Marks D, Malawista SE, Recombinant Outer-Surface Protein A Lyme Disease Vaccine Study Consortium . 1998. A vaccine consisting of recombinant Borrelia burgdorferi outer-surface protein A to prevent Lyme disease. N Engl J Med 339:216–222. doi: 10.1056/NEJM199807233390402. [DOI] [PubMed] [Google Scholar]
- 13.Nigrovic LE, Thompson AD, Fine AM, Kimia A. 2008. Clinical predictors of Lyme disease among children with a peripheral facial palsy at an emergency department in a Lyme disease-endemic area. Pediatrics 122:e1080–e1085. doi: 10.1542/peds.2008-1273. [DOI] [PubMed] [Google Scholar]
- 14.Poland GA. 2011. Vaccines against Lyme disease: what happened and what lessons can we learn? Clin Infect Dis 52(Suppl 3):s253–s258. doi: 10.1093/cid/ciq116. [DOI] [PubMed] [Google Scholar]
- 15.Wressnigg N, Barrett PN, Pöllabauer E-M, O’Rourke M, Portsmouth D, Schwendinger MG, Crowe BA, Livey I, Dvorak T, Schmitt B, Zeitlinger M, Kollaritsch H, Esen M, Kremsner PG, Jelinek T, Aschoff R, Weisser R, Naudts IFK, Aichinger G. 2014. A novel multivalent OspA vaccine against Lyme borreliosis is safe and immunogenic in an adult population previously infected with Borrelia burgdorferi sensu lato. Clin Vaccine Immunol 21:1490–1499. doi: 10.1128/CVI.00406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wressnigg N, Pollabauer EM, Aichinger G, Portsmouth D, Low-Baselli A, Fritsch S, Livey I, Crowe BA, Schwendinger M, Bruhl P, Pilz A, Dvorak T, Singer J, Firth C, Luft B, Schmitt B, Zeitlinger M, Muller M, Kollaritsch H, Paulke-Korinek M, Esen M, Kremsner PG, Ehrlich HJ, Barrett PN. 2013. Safety and immunogenicity of a novel multivalent OspA vaccine against Lyme borreliosis in healthy adults: a double-blind, randomised, dose-escalation phase 1/2 trial. Lancet Infect Dis 13:680–689. doi: 10.1016/S1473-3099(13)70110-5. [DOI] [PubMed] [Google Scholar]
- 17.Comstedt P, Hanner M, Schüler W, Meinke A, Lundberg U. 2014. Design and development of a novel vaccine for protection against Lyme borreliosis. PLoS One 9:e113294. doi: 10.1371/journal.pone.0113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Comstedt P, Hanner M, Schüler W, Meinke A, Schlegl R, Lundberg U. 2015. Characterization and optimization of a novel vaccine for protection against Lyme borreliosis. Vaccine 33:5982–5988. doi: 10.1016/j.vaccine.2015.07.095. [DOI] [PubMed] [Google Scholar]
- 19.Comstedt P, Schüler W, Meinke A, Lundberg U. 2017. The novel Lyme borreliosis vaccine VLA15 shows broad protection against Borrelia species expressing six different OspA serotypes. PLoS One 12:e0184357. doi: 10.1371/journal.pone.0184357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarselli M, Arico B, Brunelli B, Savino S, Di Marcello F, Palumbo E, Veggi D, Ciucchi L, Cartocci E, Bottomley MJ, Malito E, Lo Surdo P, Comanducci M, Giuliani MM, Cantini F, Dragonetti S, Colaprico A, Doro F, Giannetti P, Pallaoro M, Brogioni B, Tontini M, Hilleringmann M, Nardi-Dei V, Banci L, Pizza M, Rappuoli R. 2011. Rational design of a meningococcal antigen inducing broad protective immunity. Sci Transl Med 3:91ra62. doi: 10.1126/scitranslmed.3002234. [DOI] [PubMed] [Google Scholar]
- 21.Sears JE, Fikrig E, Nakagawa TY, Deponte K, Marcantonio N, Kantor FS, Flavell RA. 1991. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol 147:1995–2000. [PubMed] [Google Scholar]
- 22.Wilske B, Luft B, Schubach WH, Zumstein G, Jauris S, Preac-Mursic V, Kramer MD. 1992. Molecular analysis of the outer surface protein A (OspA) of Borrelia burgdorferi for conserved and variable antibody binding domains. Med Microbiol Immunol 181:191–207. doi: 10.1007/bf00215765. [DOI] [PubMed] [Google Scholar]
- 23.Jiang W, Gorevic PD, Dattwyler RJ, Dunn JJ, Luft BJ. 1994. Purification of Borrelia burgdorferi outer surface protein A (OspA) and analysis of antibody binding domains. Clin Diagn Lab Immunol 1:406–412. doi: 10.1128/CDLI.1.4.406-412.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schaible UE, Kramer MD, Eichmann K, Modolell M, Museteanu C, Simon MM. 1990. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A 87:3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubach WH, Mudri S, Dattwyler RJ, Luft BJ. 1991. Mapping antibody-binding domains of the major outer surface membrane protein (OspA) of Borrelia burgdorferi. Infect Immun 59:1911–1915. doi: 10.1128/IAI.59.6.1911-1915.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong W, Wiesmüller KH, Kramer MD, Wallich R, Simon MM. 1996. Plasmid DNA and protein vaccination of mice to the outer surface protein A of Borrelia burgdorferi leads to induction of T helper cells with specificity for a major epitope and augmentation of protective IgG antibodies in vivo. Eur J Immunol 26:2749–2757. doi: 10.1002/eji.1830261130. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Yang X, Luft BJ, Koide S. 1998. NMR identification of epitopes of Lyme disease antigen OspA to monoclonal antibodies. J Mol Biol 281:61–67. doi: 10.1006/jmbi.1998.1930. [DOI] [PubMed] [Google Scholar]
- 28.Ding W, Huang X, Yang X, Dunn JJ, Luft BJ, Koide S, Lawson CL. 2000. Structural identification of a key protective B-cell epitope in Lyme disease antigen OspA. J Mol Biol 302:1153–1164. doi: 10.1006/jmbi.2000.4119. [DOI] [PubMed] [Google Scholar]
- 29.Legros V, Jolivet-Reynaud C, Battail-Poirot N, Saint-Pierre C, Forest E. 2000. Characterization of an anti-Borrelia burgdorferi OspA conformational epitope by limited proteolysis of monoclonal antibody-bound antigen and mass spectrometric peptide mapping. Protein Sci 9:1002–1010. doi: 10.1110/ps.9.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pal U, de Silva AM, Montgomery RR, Fish D, Anguita J, Anderson JF, Lobet Y, Fikrig E. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J Clin Invest 106:561–569. doi: 10.1172/JCI9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koide S, Yang X, Huang X, Dunn JJ, Luft BJ. 2005. Structure-based design of a second-generation Lyme disease vaccine based on a C-terminal fragment of Borrelia burgdorferi OspA. J Mol Biol 350:290–299. doi: 10.1016/j.jmb.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 32.Gipson CL, de Silva AM. 2005. Interactions of OspA monoclonal antibody C3.78 with Borrelia burgdorferi within ticks. Infect Immun 73:1644–1647. doi: 10.1128/IAI.73.3.1644-1647.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shandilya S, Kurt Yilmaz N, Sadowski A, Monir E, Schiller ZA, Thomas WD Jr, Klempner MS, Schiffer CA, Wang Y. 2017. Structural and molecular analysis of a protective epitope of Lyme disease antigen OspA and antibody interactions. J Mol Recognit 30:e2595. doi: 10.1002/jmr.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golde WT, Piesman J, Dolan MC, Kramer M, Hauser P, Lobet Y, Capiau C, Desmons P, Voet P, Dearwester D, Frantz JC. 1997. Reactivity with a specific epitope of outer surface protein A predicts protection from infection with the Lyme disease spirochete, Borrelia burgdorferi. Infect Immun 65:882–889. doi: 10.1128/IAI.65.3.882-889.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson BJ, Sviat SL, Happ CM, Dunn JJ, Frantz JC, Mayer LW, Piesman J. 1995. Incomplete protection of hamsters vaccinated with unlipidated OspA from Borrelia burgdorferi infection is associated with low levels of antibody to an epitope defined by MAb LA-2. Vaccine 13:1086–1094. doi: 10.1016/0264-410X(95)00035-Y. [DOI] [PubMed] [Google Scholar]
- 36.Ornstein K, Berglund J, Nilsson I, Norrby R, Bergström S. 2001. Characterization of Lyme borreliosis isolates from patients with erythema migrans and neuroborreliosis in southern Sweden. J Clin Microbiol 39:1294–1298. doi: 10.1128/JCM.39.4.1294-1298.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fingerle V, Schulte-Spechtel UC, Ruzic-Sabljic E, Leonhard S, Hofmann H, Weber K, Pfister K, Strle F, Wilske B. 2008. Epidemiological aspects and molecular characterization of Borrelia burgdorferi s.l. from southern Germany with special respect to the new species Borrelia spielmanii sp. nov. Int J Med Microbiol 298:279–290. doi: 10.1016/j.ijmm.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Grygorczuk S, Péter O, Kondrusik M, Moniuszko A, Zajkowska J, Dunaj J, Żukiewicz-Sobczak W, Pancewicz S. 2013. Assessment of the frequency of different Borrelia burgdorferi sensu lato species in patients with Lyme borreliosis from north-east Poland by studying preferential serologic response and DNA isolates. Ann Agric Environ Med 20:21–29. [PubMed] [Google Scholar]
- 39.Stupica D, Lusa L, Maraspin V, Bogovič P, Vidmar D, O’Rourke M, Traweger A, Livey I, Strle F. 2015. Correlation of culture positivity, PCR positivity, and burden of Borrelia burgdorferi sensu lato in skin samples of erythema migrans patients with clinical findings. PLoS One 10:e0136600. doi: 10.1371/journal.pone.0136600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piesman J, Gern L. 2004. Lyme borreliosis in Europe and North America. Parasitology 129(Suppl):S191–S220. doi: 10.1017/s0031182003004694. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Dunn JJ, Luft BJ, Lawson CL. 1997. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci U S A 94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T. 2009. The SWISS-MODEL repository and associated resources. Nucleic Acids Res 37:D387–D392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeLano WL. 2002. The PyMOL Molecular Graphics System. DeLano Scientific, San Carlos, CA: http://www.pymol.org. [Google Scholar]
- 44.Hess B, Kutzner C, van der Spoel D, Lindahl E. 2008. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 45.Berendsen HJC, van der Spoel D, van Drunen R. 1995. GROMACS: a message-passing parallel molecular dynamics implementation. Comp Phys Comm 91:43–56. doi: 10.1016/0010-4655(95)00042-E. [DOI] [Google Scholar]
- 46.Miyamoto S, Kollman PA. 1992. SETTLE: an analytical version of the SHAKE and RATTLE algorithms for rigid water models. J Comput Chem 13:952–962. doi: 10.1002/jcc.540130805. [DOI] [Google Scholar]
- 47.Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG. 1995. A smooth particle mesh Ewald method. J Chem Phys 103:8577–8592. doi: 10.1063/1.470117. [DOI] [Google Scholar]
- 48.Hess B. 2008. P-LINCS: a parallel linear constraint solver for molecular simulation. J Chem Theory Comput 4:116–122. doi: 10.1021/ct700200b. [DOI] [PubMed] [Google Scholar]
- 49.Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL. 2001. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B 105:6474–6487. doi: 10.1021/jp003919d. [DOI] [Google Scholar]
- 50.Drouin EE, Glickstein L, Kwok WW, Nepom GT, Steere AC. 2008. Searching for borrelial T cell epitopes associated with antibiotic-refractory Lyme arthritis. Mol Immunol 45:2323–2332. doi: 10.1016/j.molimm.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gross DM, Forsthuber T, Tary-Lehmann M, Etling C, Ito K, Nagy ZA, Field JA, Steere AC, Huber BT. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science 281:703–706. doi: 10.1126/science.281.5377.703. [DOI] [PubMed] [Google Scholar]
- 52.Drouin EE, Glickstein LJ, Steere AC. 2004. Molecular characterization of the OspA161–175 T cell epitope associated with treatment-resistant Lyme arthritis: differences among the three pathogenic species of Borrelia burgdorferi sensu lato. J Autoimmun 23:281–292. doi: 10.1016/j.jaut.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Davies DR, Cohen GH. 1996. Interactions of protein antigens with antibodies. Proc Natl Acad Sci U S A 93:7–12. doi: 10.1073/pnas.93.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lo Conte L, Chothia C, Janin J. 1999. The atomic structure of protein-protein recognition sites. J Mol Biol 285:2177–2198. doi: 10.1006/jmbi.1998.2439. [DOI] [PubMed] [Google Scholar]
- 55.Chakrabarti P, Janin J. 2002. Dissecting protein-protein recognition sites. Proteins 47:334–343. doi: 10.1002/prot.10085. [DOI] [PubMed] [Google Scholar]
- 56.de Silva AM, Telford SR III, Brunet LR, Barthold SW, Fikrig E. 1996. Borrelia burgdorferi OspA is an arthropod-specific transmission-blocking Lyme disease vaccine. J Exp Med 183:271–275. doi: 10.1084/jem.183.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bunikis J, Luke CJ, Bunikiene E, Bergström S, Barbour AG. 1998. A surface-exposed region of a novel outer membrane protein (P66) of Borrelia spp. is variable in size and sequence. J Bacteriol 180:1618–1623. doi: 10.1128/JB.180.7.1618-1623.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ornstein K, Östberg Y, Bunikis J, Noppa L, Berglund J, Norrby R, Bergström S. 2002. Differential immune response to the variable surface loop antigen of P66 of Borrelia burgdorferi sensu lato species in geographically diverse populations of lyme borreliosis patients. Clin Diagn Lab Immunol 9:1382–1384. doi: 10.1128/cdli.9.6.1382-1384.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bunikis J, Noppa L, Östberg Y, Barbour AG, Bergström S. 1996. Surface exposure and species specificity of an immunoreactive domain of a 66-kilodalton outer membrane protein (P66) of the Borrelia spp. that cause Lyme disease. Infect Immun 64:5111–5116. doi: 10.1128/IAI.64.12.5111-5116.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ntchobo H, Rothermel H, Chege W, Steere AC, Coburn J. 2001. Recognition of multiple antibody epitopes throughout Borrelia burgdorferi p66, a candidate adhesin, in patients with early or late manifestations of Lyme disease. Infect Immun 69:1953–1956. doi: 10.1128/IAI.69.3.1953-1956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]