Abstract
Objective
Despite the high prevalence of non-motor impairments reported in patients with amyotrophic lateral sclerosis (ALS), little is known about the functional neural markers underlying such dysfunctions. In this study, a new dual-task multimodal framework relying on simultaneous electroencephalogram (EEG) and functional near-infrared spectroscopy (fNIRS) recordings was developed to characterize integrative non-motor neural functions in people with ALS.
Approach
Simultaneous EEG-fNIRS data were recorded from six subjects with ALS and twelve healthy controls. Through a proposed visuo-mental paradigm, subjects performed a set of visuo-mental arithmetic operations. The data recorded were analyzed with respect to event-related changes both in the time and frequency domains for EEG and de/oxygen-hemoglobin level (HbR/HbO) changes for fNIRS. The correlation of EEG spectral features with fNIRS HbO/HbR features were then evaluated to assess the mechanisms of ALS on the electrical (EEG)-vascular (fNIRS) interrelationships.
Main results
We observed overall smaller increases in EEG delta and theta power, decreases in beta power, reductions in HbO responses, and distortions both in early and later EEG event-related potentials in ALS subjects compared to healthy controls. While significant correlations between EEG features and HbO responses were observed in healthy controls, these patterns were absent in ALS patients. Distortions in both electrical and hemodynamic responses are speculated to be associated with cognitive deficits in ALS that center primarily on attentional and working memory processing.
Significance
Our results highlight the important role of ALS non-motor dysfunctions in electrical and hemodynamic neural dynamics as well as their interrelationships. The insights obtained through this study can enhance our understanding of the underlying non-motor neural processes in ALS and enrich future diagnostic and prognostic techniques.
Keywords: amyotrophic lateral sclerosis (ALS), non-motor dysfunction, electroencephalogram (EEG), functional near-infrared spectroscopy (fNIRS), multimodal frameworks
1. Introduction
Amyotrophic lateral sclerosis (ALS) is known widely as a progressive motor neurodegenerative disease. However, within the past several years, ALS has been recognized as a multi-system disorder in which, not only the motor system degenerates but the non-motor system (i.e. behavior and cognition) is affected as well [1–4]. Up to 50% of patients with ALS develop cognitive impairments, while estimates of frontotemporal dementia (FTD) in these patients range from 15% to 41% [2, 3]. In a study by Montuschi et al [5] involving 207 ALS patients, they found that 13% of the patients showed symptoms of dementia, while 37% demonstrated other undemented executive impairments. Generally, frontal impairments in ALS are largely associated with executive dysfunctions [1, 5–7]. Several studies have shown that ALS patients have considerable executive dysfunction, including poor working memory (WM), poor sustained attention, poor response inhibition, and loss of visual attention [8–10]. Additionally, there is evidence of non-motor dysfunction beyond the behavioral and executive domains, including language [11] and social cognition [1, 11] which are extremely heterogeneous in these patient cohorts [12]. Notably, it has been reported that ALS patients’ cognitive impairments are correlated negatively with their survival [5].
Despite the high prevalence of non-motor dysfunctions in ALS, little is known about their underlying functional neural variations [6] and their relation to disease duration or the severity of motor symptoms [11, 13, 14]. Burke et al [15] suggested that distinct cognitive-behavioral phenotypes may relate to differential disruption of extramotor cortical networks, but the connections between neural and cognitive findings require further investigation, specifically in ALS. Non-motor dysfunctions in ALS may also be explained by structural changes in the non-motor cortical regions associated with cognition and behavior [12, 16]. For example, a structural study by Consonni et al [12] indicated cortical thinning of the right inferior temporal gyrus and sulcus in ALS patients with cognitive impairment as compared to both cognitively normal patients and neurotypical controls [12]. While the main body of ALS studies have discussed non-motor issues largely through behavioral and observational evaluations [1–4, 17], cognitive and behavioral screens such as the ALS cognitive behavioral screen (ALS-CBS™) typically do not assess associated neural markers [17], and little research has explored the neural associations of cognitive dysfunctions in these patients. Thus, there is an enormous need for more research to elucidate neural markers of non-motor dysfunction in ALS. Exploration of the neural correlates of non-motor dysfunctions in ALS can advance our understanding of neural abnormalities in the disease. This may provide complementary objective signatures of disease progression and enrich current diagnostic and prognostic techniques.
To investigate the characteristics of neural signatures in ALS, most studies have relied on electroencephalography (EEG) because of its high temporal resolution, cost-effectiveness, and portability [18]. These studies either used resting-state or activation paradigms in which subjects were asked to perform an active task. In the former case, power spectral bands [19] or functional connectivity [20] are features reported commonly to address differences between participants with ALS and controls. In activation paradigms that can reflect cognitive dysfunctions directly, event-related potentials (ERPs) are the commonly evaluated temporal responses (responses in the time domain). For example, abnormalities in the P300 response, together with early components, such as P100 or N200, elicited during an oddball paradigm, are the temporal features most frequently reported to be impaired in ALS patients [6, 7, 18]. P300 tasks include cognitive substrates of oddball paradigms, associated largely with attentional demand and working memory, which are referred to normally as mental workload [21] and have been associated with ALS markers [6, 7, 18]. The reported distortions in evoked ERP features make them probable candidates to possess information about cognitive deficits in ALS patients, although more work is required to quantify robust signatures. In contrast to early components, later ERPs related to higher cognitive processes in patients with ALS have received comparatively less attention. Although studies have reported the absence of higher cognitive neural responses in people with similar symptoms such as consciousness disorders [22, 23] they have not reported quantified measures for ALS patients. Thus, to achieve reliable event-related markers in ALS, further quantification is required with suitable paradigms that evoke later ERP components.
However, because of EEG’s low spatial resolution and signal-to-noise ratio (SNR), ERP analysis depends on the number of trials (for averaging), and thus, ERP-based paradigms are limited and inconclusive for ALS patients. This is an issue in typical ERP-based visual paradigms, particularly, in the later stages of ALS, when ocular problems develop and lack of fine eye-gaze control can affect visual paradigms’ efficiency [24]. Hence, there is a need for compensatory methods to overcome these shortcomings [6]. Two complementary paths may address these issues. The first is to look for other distinctive EEG features (rather than temporal features), such as power spectral activities in new types of activation paradigms. Few studies on ALS patients have characterized EEG spectral power responses [25, 26], in which the slow cortical potential (SCP) is most commonly used in the learned self-regulation process or motor-related paradigms, rather than cognitive activation designs. However, given that executive dysfunction is a major impairment reported in ALS patients, more studies are needed to explore neural oscillatory characteristics of executive dysfunction through cognitive paradigms in ALS patients. The second path is to incorporate other neuroimaging modalities with EEG simultaneously to capture complementary neural dynamical features, such as hemodynamic activities in addition to EEG electrical responses. To do so, functional near-infrared spectroscopy (fNIRS) is the only neuroimaging technique that can be used plausibly for ALS patients, considering their disease’s progression and their immobility [7], as fNIRS measures the brain’s hemodynamic activities, is portable and can be used longitudinally at patients’ bedsides [27–29].
In this study, we introduced a novel compensatory method to explore non-motor neural degeneration in ALS patients. We developed an innovative visuo-mental dual-task that combined a visuo-spatial oddball paradigm with a set of arithmetic operations to induce and record both hemodynamic and electrical responses simultaneously using EEG-fNIRS. Studies have shown that while fNIRS signals are effective in mirroring cerebral oxygenation changes in response to various cognitive functions [27–31], including mental arithmetic tasks, they also can reflect distinctive patterns between patients with ALS and healthy controls [32, 33]. The proposed paradigm allows us to obtain the following advantages over the methods used previously. First, our paradigm’s dual-task nature is speculated to be particularly effective in capturing cognitive dysfunctions in ALS that single-task paradigms do not fully reflect [21, 34]. Second, the hemodynamic information gained through fNIRS recording can compensate for the EEG-based systems’ shortcomings, such as the patient’s inability to perform fine visual tasks particularly in the later stages of their disease—this complementary information can offer unique opportunities for future neuroimaging studies of ALS. Third, the associations between EEG and fNIRS can be further explored to investigate how ALS affects the interrelations between electrical (EEG) and vascular (fNIRS) dynamics. While previous studies have largely used a non-dual-task design [31, 33, 35], such as a P300 speller for EEG or mental arithmetic task for fNIRS, or used a single recording modality (e.g. EEG or fNIRS) [36, 37], our novel experimental protocol integrated two simultaneous tasks (visual and mental) and two simultaneous recording modalities (EEG and fNIRS) to explore multi-aspect non-motor cortical dysfunctions in ALS. The insights obtained through this study can advance our understanding of integrative non-motor markers in ALS, aid in identifying relations between electrical and vascular responses in ALS and can ultimately introduce novel neural markers of ALS that can be exploited as diagnostic and prognostic predictors.
2. Methods
2.1. Subjects
A total of 18 subjects were recruited and assigned to two groups: six individuals with ALS (five males) and 12 age-matched healthy controls (three males). The study protocol was approved by the Institutional Review Board (IRB) of the University of Rhode Island (URI) and written informed consents were provided directly by each subject or patient’s caregiver. The average age of the patient group was 57.0 ± 15.7 years old and the average age of the control group was 56.4 ± 15.4 years old. Specifically, in our patient group we have one young (No. 1) subject, whom we matched with two young healthy controls. Excluding the young participants, the average of the elder patients’ age was 62.6 ± 8.4 years old compared to 62.7 ± 4.8 years old for the elder healthy participants. Average ALS revised functional rating scale (ALSFRS-R) scores were 11.6 ± 9.5 on a 48-point scale [38]. Half of the patients required mechanical ventilation, the youngest one of whom, was in a completely locked-in state (CLIS). Age-matched control subjects had no reported history of visual, mental, or substance-related issues. All participants in both groups had at least some level of post-secondary education. Two healthy controls were excluded from fNIRS data analysis because of their poor signal quality in fNIRS calibration settings. All subjects provided informed consent (or assent) for the study and were reimbursed financially. Participants with ALS underwent recording at their homes or care centers. Both groups’ participants took the ALS-CBS™ [17], a cognitive-behavioral screen used commonly in ALS patient care, to assess their cognitive competence. Because several patients had difficulty speaking or writing, the information and retrieval (fluency) section of the ALS-CBS test could not be used effectively, and consequently, only the attention, concentration, and tracking portions of the ALS-CBS test were performed. Because of communication and/or ocular impairments in three of our ALS patients, two used eye-tracking systems (Tobii EyeX) and the patient in a CLIS used the P300 speller to answer CBS questions. Table 1 shows the ALS subjects’ demographics, including age, gender, disease duration, ALSFRS-R, and ALS-CBS scores. For more information about healthy controls’ demographic information see supplementary table 1 (stacks.iop.org/JNE/16/066036/mmedia).
Table 1.
ALS subjects demographic information.
| Participant no. | Age | Gender | Disease duration (years) | ALSFRS-R (max 48) | Average ALS-CBS score (%) | Educational level |
|---|---|---|---|---|---|---|
| 1 | 29 | M | 4 | 0 | 100.0 | College degree |
| 2 | 55 | M | 11 | 4 | 93.8 | Graduate degree |
| 3 | 70 | M | 8 | 14 | 93.3 | Some postsecondary |
| 4 | 67 | M | 2 | 7 | 86.6 | College degree |
| 5 | 69 | F | 11 | 23 | 80.0 | College degree |
| 6 | 52 | M | 3 | 22 | 89.1 | Some postsecondary |
| Mean ± SD | 57.0 ± 15.7 | — | 6.5 ± 4.0 | 11.6 ± 9.5 | 90.5 ± 6.9 | — |
2.2. Experimental protocol
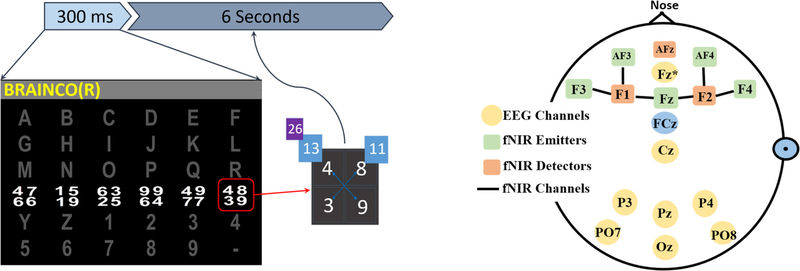
Subjects participated in several sessions (4.7 ± 3.8), with two runs per session. To familiarize the subjects with our BCI set up, including the recording protocol and the task, they all participated in training sessions before the main experimental recordings. In addition, in each session, there were a few quick test runs to make sure that the subjects understood the task, which were evaluated by discussing their results. We continued performing test runs until we were sure the subjects were comfortable with the task. Following the conventional oddball P300 paradigm, a 2 × 2 matrix of digits was displayed over the intensified letter in our visuo-mental dual-task paradigm (figure 1, left). Each subject was instructed to focus on a target character (14 targets per run), while each row and column was intensified once per trial to cause two target intensifications per character. Upon each target intensification, subjects were instructed to perform predefined mental arithmetic tasks, i.e. add pairs of numbers in the matrix either diagonally (first target flash) or vertically (second target flash), and then double the larger result from their addition. The stimulation intensification time was set to 300 ms, followed by a 6 s inter-stimulus interval (ISI). The relatively long ISI adopted compared to conventional EEG-based oddball tasks allowed the fNIRS recordings to reflect evoked hemodynamic activities. Although fNIRS paradigms use longer stimulus and resting periods, normally ranging from 8 to 12 s [28], these times were reduced in our paradigm because of the potential to extend our work in future communication studies of ALS. Generally, longer pauses increase the amount of time required to select each character in communication systems—this can make the communication system slow and impractical in real-life scenarios. The inherent dual nature of our visuo-mental paradigm is hypothesized to provoke both electrical and hemodynamic responses associated with visual oddball stimulations and mental arithmetic operations.
Figure 1.
The oddball-based visuo-mental dual-task paradigm and an example arithmetic operation the participants performed (left). A schematic montage of the EEG sensors-f NIRS optodes (right).
2.3. Data acquisition
Both signals were recorded simultaneously using a single cap mounted both with EEG electrodes and fNIRS optodes. fNIRS data were recorded using NIRScout (NIRx Inc.) with two NIR lights (760 nm and 850 nm wavelengths) and digitized at 7.81 Hz. EEG data were recorded simultaneously using the g.USBamp amplifier (g.tec Medical Tech.) and digitized at 256 Hz. Figure 1 (figure 1, right) shows a schematic head model of the fNIRS-EEG sensors’ placement. As depicted in this figure, five emitters and two detectors acquired seven fNIRS channels covering the frontal areas responsible for higher cognitive functions associated with mathematical operations paradigms [34]. Following the modified combinatorial nomenclature (MCN) montage, emitters were placed at Fz, F3, F4, AF3, and AF4, and detectors at F1 and F2. EEG was recorded from eight channels: Fz*, Cz, P3, Pz, P4, PO7, PO8, and Oz covering all frontal, central, parietal, and occipital areas used commonly in conventional P300 paradigms (FAF2, denoted, Fz* was the nearest electrode placement to fNIRS occupied Fz according to 128-channel montage) [39]. This montage was intended to capture one aspect of the dual-task, i.e. the visuospatial task, with conventional EEG montages [39], and the arithmetic operations with frontal fNIRS and EEG channels reported previously [40]. This montage follows standards closely and is convenient to mount, making it an appropriate candidate for future applications. All experimental protocols, data acquisition, and stimulus presentation labels were controlled using BCI2000 and NIRStar software [41, 42].
2.4. EEG data analysis
EEG data were bandpass filtered at 0.5–30 Hz and detrended. Then, the data were checked for extreme values and outliers which led to the removal of a total of three runs from two ALS participants. ERP segments were extracted from 0–800 ms after the onset of each target stimulus and averaged across all runs for each subject. ERP features were then extracted as follows: Peaks and latencies of the P200, P300, and P600 components were defined as the maximum peaks between 100–250, 250–400, and 650–800 ms post-stimulus, respectively, while N200 and N400 components were defined as the minimum peaks between 150–280 and 360–560 ms post-stimulus, respectively. For time-frequency analysis, EEG data were normalized and segmented into 9 s epochs, beginning from 4 s pre-stimulus to 5 s post-stimulus. A set of 30 complex Morlet wavelets ranging from 1 to 30 Hz and 3–10 cycles was used for time-frequency decomposition. The baseline-corrected spectrograms were obtained by dividing each frequency bin and time point by the baseline −2 to −1 s average and log-transforming. The spectrograms from the first 5 s post-stimulus were then averaged across four traditional frequency bands: delta (1–3 Hz), theta (4–7 Hz), alpha (8–12 Hz), and beta (13–30 Hz).
2.5. fNIRS data analysis
fNIRS data were band-pass filtered at 0.01–0.2 Hz to mitigate physiological noises such as respiratory (0.2–0.3 Hz) [43] and cardiac signals (0.8–1.3 Hz) [44]. Oxy-hemoglobin (HbO) and deoxy-hemoglobin (HbR) concentration changes were extracted from raw optical intensity data using the modified Beer–Lambert Law [45]. To synchronize the fNIRS data with the EEG data and stimulus triggers, HbO data were up-sampled to the 256 Hz EEG sampling rate. Six-second post-stimulus epochs were extracted with the −2 to −1 s pre-stimulus window as the baseline for further normalization. To normalize the relative hemodynamic changes during arithmetic operations, the average baseline was subtracted from the following post-stimulus signal for each epoch. First, for fNIRS feature extraction with respect to the stimulus onset, post-stimulus epochs with three different time window lengths (0–2, 2–4, 4–6 s) were extracted. Then, for each epoch and time segment, the peaks of both HbO and HbR were calculated, resulting in a total of six features for each target segment in each channel.
2.6. Statistical analysis
To statistically compare the EEG results (both time-frequency maps and ERP analysis) between groups, a nonparametric bootstrapping procedure was used. This method is useful for small or unequal sample sizes [46] and has greater statistical power and makes fewer assumptions about the data’s distribution compared to canonical methods [47]. For the time-frequency analysis, all of each subject’s runs were averaged, and then the delta, theta, alpha, and beta power features were extracted to create time-frequency maps. In each frequency band, features were the averages of band power over sliding 500 ms windows from 0–5 s post-stimulus with 50% overlap. Then, through the bootstrapping procedure, resamples were generated for each feature. The same analysis was done for ERP features (i.e. peaks and latencies of the P200, N200, P300, N400, and P600). Each resample was obtained by randomly selecting six healthy control subjects (equal to the number of ALS subjects), averaging each feature across all six randomly selected subjects, and finally, subtracting the group means to generate the histogram (probability distribution) of group differences. This procedure was iterated 1000 times to create a distribution. The proportion of resamples less than zero (or greater than zero depending on which tail of the histogram hits the zero point) to all resamples yielded p-values [46]. The difference in the means was determined to be statistically significant if the p-value was less than 0.05.
As in the EEG analysis, in the fNIRS analysis, the bootstrap method was used to perform a between-group statistical comparison of ALS and controls’ fNIRS responses. To do so, HbO/HbR values were first averaged over subjects in each group, and then the peak of averaged HbO/HbR was calculated for each windowed epoch (i.e. 0–2, 2–4, 4–6 s). For each feature, we generated the resamples iteratively and subtracted the group means to construct the histogram and extract the corresponding p-values.
To account for multiple comparisons in both the fNIRS and EEG statistical analyses, the false discovery rate (FDR) method was used to compute adjusted p-values (p < 0.05) [48].
2.7. Correlation analysis
Spearman correlation analysis was conducted to explore the relations between significant EEG and fNIRS features over time. To do so, correlations were calculated between a set of windowed EEG spectral features (i.e. delta, theta, and beta power) and windowed peak HbO/HbR values for the frontal EEG-fNIRS channels. EEG features used 1 s sliding windows with a 0.5 s overlap in the time range of 0–4 s post-stimulus, while fNIRS used 2 s windows with a 1 s overlap in the time range of 0–6 s post-stimulus. Spearman correlation analysis was also used to investigate interrelations between EEG/fNIRS features and ALS clinical scores, including disease duration, ALSFRS-R, and ALS-CBS.
3. Results
3.1. EEG results
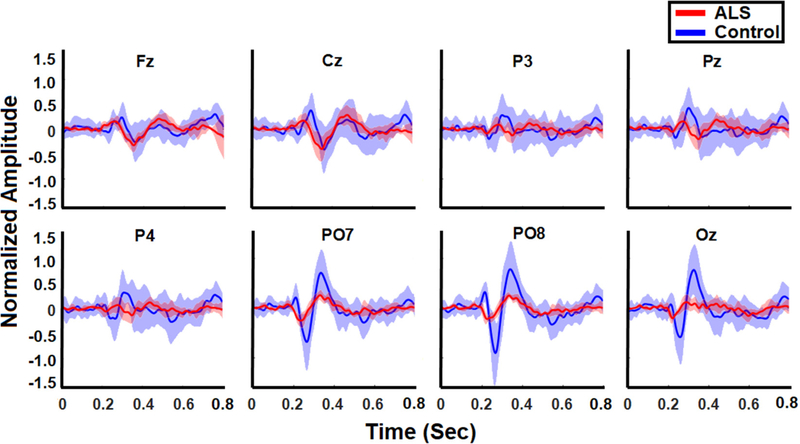
The averaged normalized target ERPs for healthy controls and ALS patients are illustrated in figure 2 for all channels. We observed attenuated ERP features overall in ALS patients compared to healthy controls. In particular, frontal (Fz), parietal (P3, Pz, P4), parieto-occipital (PO7, PO8), and occipital (Oz) P200 amplitudes were attenuated significantly in ALS patients (p < 0.05), and averaged 0.19 ± 0.06 in the frontal channel (Fz) compared to healthy controls, averaging 0.31 ± 0.16 in the same channel. P300 amplitudes were attenuated significantly in all channels in patients (p < 0.001), and averaged 0.21 ± 0.07 in the frontal channel compared to healthy controls, with an average of 0.38 ± 0.14 in the same channel (figure 3, top left). P600 amplitudes also were attenuated significantly in all channels (p < 0.001), and averaged 0.11 ± 0.04 for patients in the frontal channel compared to healthy controls, with an average of 0.43 ± 0.19 (figure 3, top right).
Figure 2.
Normalized target ERPs averaged across all ALS participants (red) and all healthy controls (blue) in the 800 ms following stimulus onset. The shaded bands represent the standard deviation within each group of controls and patients.
Figure 3.
Boxplots showing changes (mean ± SD) in average P300 amplitude (top left), P600 (top right), N200 (bottom left), and N400 amplitude (bottom right) for ALS participants (red) and healthy controls (blue). For each plot, the maximum p-value among channels is shown at the top.
N200 amplitudes were attenuated significantly in all channels (p < 0.001), and averaged −0.0.10 ± 0.0.05 in the frontal channel for patients compared to healthy controls, who averaged −0.25 ± 0.16 (figure 3, bottom left). Similarly, N400 amplitudes were attenuated significantly in all channels (p < 0.03), and averaged −0.30 ± 0.11 in the frontal channel for patients compared to healthy controls, who averaged −0.41 ± 0.13 (figure 3, bottom right).
The P300 and N200 features were observed to have significantly shorter latencies in patients compared to the healthy controls. Patients’ P300 latencies, which averaged 263 ± 12, 267 ± 13, and 275 ± 9 ms, preceded those of healthy controls significantly at 291 ± 25, 307 ± 22, and 303 ± 15 ms in the frontal (Fz), central (Cz), and parietal (Pz) channels, respectively (p < 0.001). Parieto-occipital (PO8) and occipital (Oz) N200 latencies were significantly shorter in patients (p < 0.05) and averaged 238 ± 19 and 222 ± 5 ms compared to healthy controls, whose latencies averaged 269 ± 14 and 258 ± 16 ms in channels PO8 and Oz, respectively.
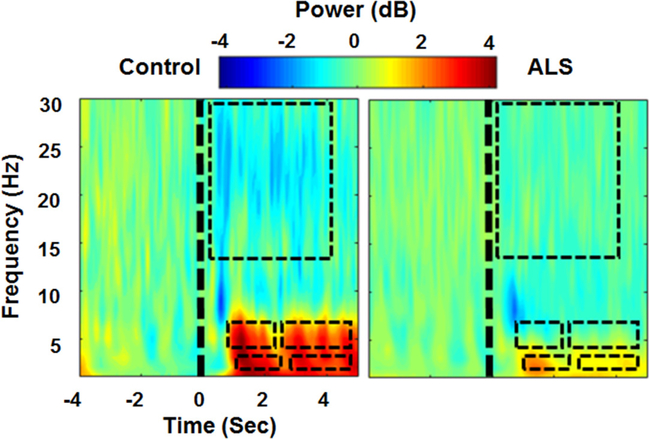
Figure 4 illustrates the average time-frequency decomposition across all healthy controls (left) and all ALS participants (right) for the frontal EEG channel (Fz). We observed that delta power was significantly lower in participants with ALS than in healthy controls within the 1–2.5 s (ALS: 1.7 ± 1.9 dB, Control: 3.8 ± 2.1 dB) and 2.75–4.75 s post-stimulus windows (ALS: 1.1 ± 1.5 dB, Control: 3.0 ± 2.3 dB) (p < 0.01). Similarly, in the theta band, we observed that power in participants with ALS was significantly lower than in healthy controls within the 0.75–2.25 s (ALS: 1.1 ± 3.4 dB, Control: 3.6 ± 2.2 dB) and 2.5–4.75 s post-stimulus windows (ALS: 0.5 ± 1.6 dB, Control: 3.5 ± 4.3 dB) (p < 0.001). In the beta band, we observed a significantly lower power in healthy controls than in participants with ALS within the 0.5–4 s post-stimulus window (ALS: –0.3 ± 1.4 dB, Control: –1.2 ± 1.9 dB) (p < 0.02). No significant changes between the two groups were observed in the alpha band.
Figure 4.
Average time-frequency decomposition across all healthy controls (left) and ALS participants (right) for channel Fz. The significant differences between each group (healthy versus ALS) for each frequency band are illustrated by a black dashed rectangle.
3.2. fNIRS results
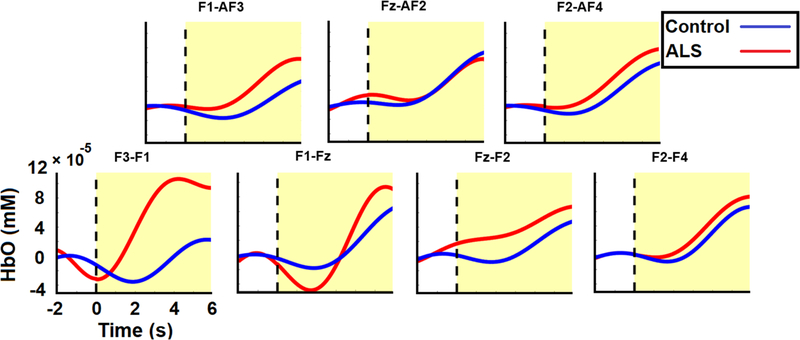
Event-related HbO and HbR activities were calculated, epoched, and grand-averaged across all runs and subjects in both groups. Figure 5 illustrates the grand-average HbO responses from 2 s target pre-stimulus to 6 s target post-stimulus for both groups. We observed that our designed task-evoked hemodynamic responses in the ISI following target stimulus presentation in both the patient and control groups. Most channels showed an initial dip in HbO responses in both groups, followed by a rise. Between-group differences were particularly clear in the channels located on the left frontal and prefrontal lobes (primarily F3-F1 and F1-Fz). In channel F3-F1, the peak value of the average HbO occurred 4.23 s post-stimulus with (1.1 ± 1.3) × 10−4 mM for the ALS group compared to 5.71 s post-stimulus with (2.3 ± 13.2) × 10−5 mM for controls. The average initial dip in this channel reached its minimum of (−3.2 ± 5.1) × 10−5 mM 0.06 s post-stimulus in the ALS group compared with the minimum of (−3.5 ± 4.8) × 10−5 mM at 1.89 s post-stimulus for the healthy group. In channel F1-Fz, the peak value of average HbO occurred 5.5 s post-stimulus with (9.4 ± 13.2) × 10−5 mM for the ALS group compared to 6.0 s post-stimulus with (6.5±13.4) × 10−5 mM for the controls. This channel’s initial dip was greater in the ALS group with a minimum of (−4.85 ± 14.5) × 10−5 mM at 1.72 s post-stimulus compared to (−1.8 ± 3.9) × 10−5 mM at 1.88 s post-stimulus for the healthy controls.
Figure 5.
Average target HbO responses evolving from 2 s pre-stimulus to 6 s post-stimulus for both ALS (red) and control (blue) groups. The vertical dashed line and the shaded yellow area denote the target stimulus onset and post-stimulus respectively.
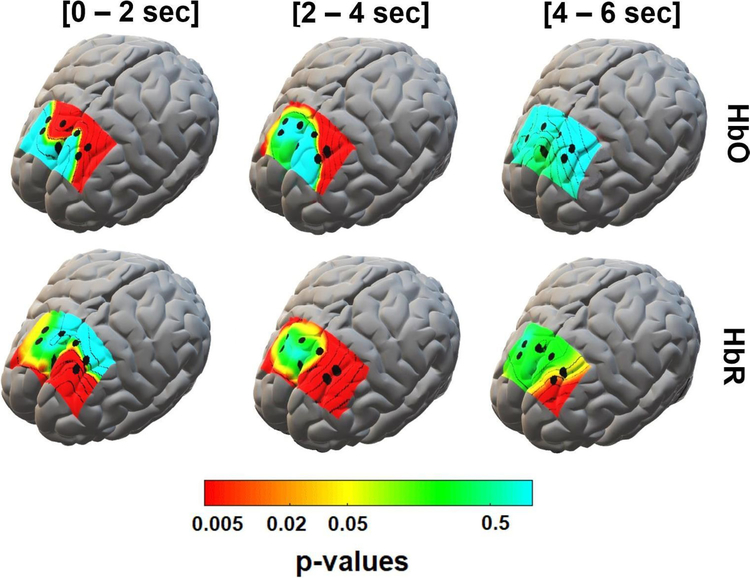
Figure 6 illustrates the between-group (ALS versus healthy controls) p-value maps corresponding to the statistical difference between the peak value of the grand-averaged HbO and HbR concentrations in the post-stimulus segments. These maps show significant HbO and HbR differences in three non-overlapping windows of 0–2, 2–4, and 4–6 s post-stimulus. For the HbO features, channel F3-F1, on the left prefrontal area, showed the greatest between-group difference (p < 0.03) in nearly all time windows. However, the most significant differences (p < 0.001) for this channel (i.e. F3-F1) occurred in the 0–2 and 2–4 s post-stimulus windows with average peak HbO concentration of (4.8 ± 10.5) × 10−5 and (11.8 ± 16.3) × 10−5 mM respectively in the ALS group compared to (−0.9 ± 2.8) × 10−5 and (1.16 ± 7.5) × 10−5 mM in the control group, respectively. Also, for the 0–2 s time window, channel F3-AF3 (p < 0.03) and Fz-F2 (p < 0.001) showed a significant difference in the HbO peak between two groups with an average (0.6 ± 2.3) × 10−5 and (3.3 ± 4.7) × 10−5 mM, respectively, in the ALS group in comparison with (−0.4 ± 1.3) × 10−5 and (0.6 ± 0.2) × 10−5 mM, respectively, in the control group.
Figure 6.
p-value maps derived from statistical comparison of peak values of HbO (top) and HbR (bottom) concentrations in different time windows (0–2, 2–4, and 4–6 s post-stimulus). The maps are superimposed on a standard 3D brain model for illustration purposes only. Dots show the corresponding fNIRS channel in the frontal cortex. The color bar is in a logarithmic scale to magnify significant p-values.
For the HbR peak, channel F3-AF3 showed a significant difference (p < 0.03) between the two groups in all three time windows with average (−0.5 ± 1.1) × 10−6, (−0.5 ± 1.1) × 10−6, and (−0.2 ± 0.5) × 10−6 mM, respectively, in the ALS group compared to (−7.5 ± 7.9) × 10−6, (12.0 ± 11.9) × 10−6, and (14.1 ± 18.8) × 10−6 mM, respectively, in the control group. Also, channel F3-F1 showed significant differences between groups in 2–4 s (p < 0.001) and 4–6 s (p < 0.04) for the HbR peaks with average (0.9 ± 2.3) × 10−6 and (0.59 ± 1.1) × 10−6 mM, in the ALS group, respectively, compared to (−3.3 ± 3.6) × 10−6 and (−2.9 ± 4.8) × 10−6 mM respectively in the control group.
3.3. Correlation results
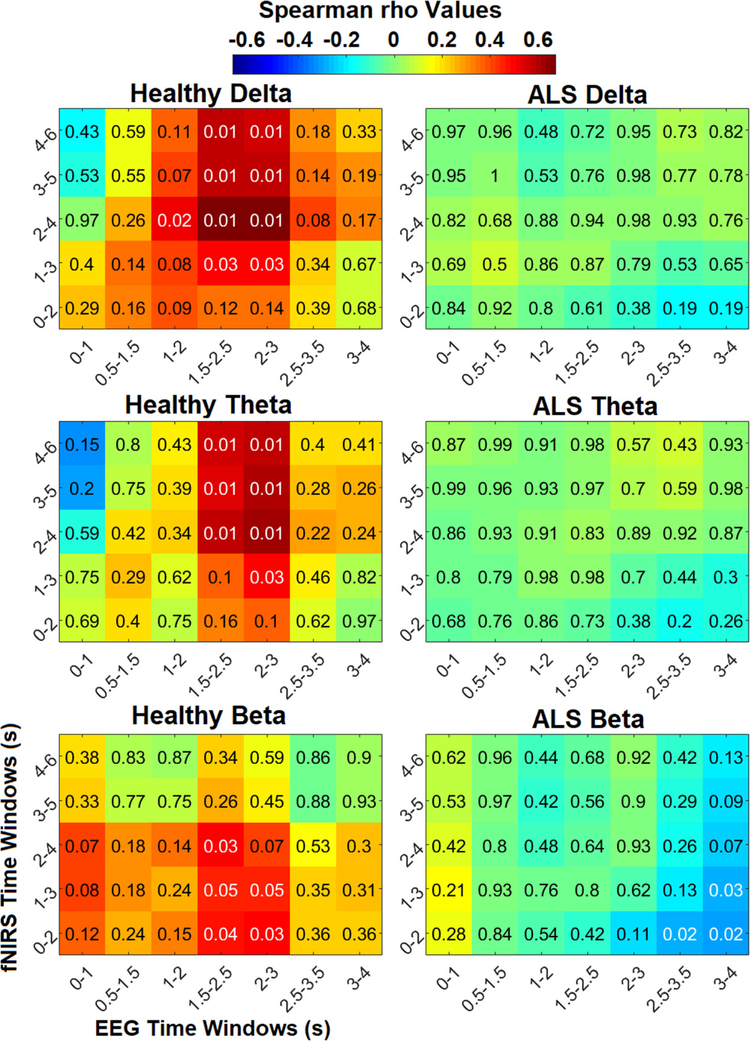
As shown in figure 7, generally significant (p ⩽ 0.02) positive correlation was observed between windowed peak HbO 2–6 s post-stimulus and windowed EEG-delta features 1.5–3 s post-stimulus in healthy subjects. Additionally, delta band features in the 1–3 s windows were significantly (p ⩽ 0.02) correlated with peak HbO in the 2–4 s windows in healthy controls. Significant (p ⩽ 0.03) positive correlation was also generally observed between EEG-theta features in the 1.5–3 s windows and peak HbO in the 1–6 s windows in healthy controls. Significance in correlation remains between both delta and theta band features in the 1.5–3 s windows and peak HbO in the 2–6 s windows after FDR correction in healthy subjects. However, no significant correlation was observed between patient group peak HbO and delta or theta band features in any of the time windows. In the control group, significant positive correlation (p < 0.05) was observed between beta band features in the 1.5–3 s windows and peak HbO features in the 0–3 s windows, as well as between the 1.5–2.5 s beta band window and 2–4 s post-stimulus peak HbO window (p ⩽ 0.03). In contrast, significant (p ⩽ 0.04) negative correlation was observed between later beta band features and earlier peak HbO windows in the patient group. However, the significance in the beta band in both healthy and patient groups was not present after FDR-correction. No significant correlation between peak HbR features and EEG features in ay frequency band or time window was observed. Some sporadic significant (p < 0.05) negative correlation was observed between peak HbR and EEG features in the delta and theta bands for the patient group, but they did not remain significant after FDR correction.
Figure 7.
Spearman correlation (rho) between obtained significant EEG features in the delta, theta, and beta frequency bands and windowed fNIRS peak HbO in the healthy control (left) and patient (right) groups. Significant corrected p-values (p < 0.05 before rounding to the nearest 100th) are shown in white, and all other values are displayed in black. Only correlation maps with significant p-values and their counterparts in the patient group are displayed.
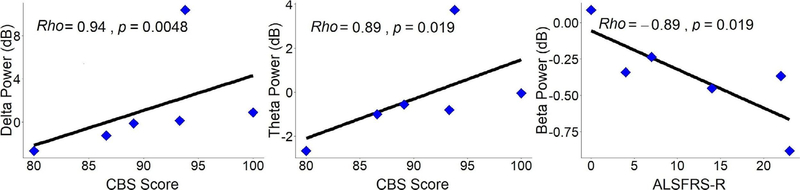
Figure 8 illustrates the significant results derived from correlation analysis of fNIRS/EEG features and clinical scores of ALS patients in the same time windows shown in figure 7. As the figure shows, delta band power was significantly positively correlated (rho = 0.94; p < 0.005) with ALS-CBS scores in the 0.5–1.5 s post-stimulus window (top left). Theta band power showed a significant positive correlation (rho = 0.89; p < 0.02) with cognitive screens obtained from ALS-CBS scores in the 0–1 s post-stimulus window (bottom left). Beta band power was significantly negatively correlated (rho = −0.89; p < 0.02) with ALSFRS-R scores in the 1.5–2.5 s post-stimulus window (top right). No significant correlations were found between other EEG/fNIRS features and other clinical scores. For more information about nonsignificant correlation values see supplementary figures 1–3.
Figure 8.
Spearman correlations between delta power 0.5–1.5 s post-stimulus and CBS scores (left), theta power 0–1 s post-stimulus and CBS scores (middle), and beta power 1–2 s post-stimulus and ALSFRS-R scores (right) for patients with ALS. Each correlation’s individual rho and p-values are at the top left of their respective plots.
4. Discussion
In this study, a new multimodal framework was developed to understand the multi-aspect neural correlates of non-motor mechanisms in both normal and ALS conditions. As the multimodal paradigm developed captures both electrophysiological and hemodynamic responses during the dual visuo-mental task, such a task can then elucidate cognitive deficits, such as executive dysfunctions in ALS, that single-task paradigms do not reveal [34]. In general, dual-task paradigms have been claimed to be effective in capturing cognitive dysfunctions in ALS that single-task paradigms do not reflect properly [21, 34]. For example, Pettit et al [34] designed a dual-task that combined visual inspection with digit recall that reflected executive dysfunctions in ALS patients associated with middle frontal gyrus impairments. Our visuo-mental dual-task paradigm engaged several cognitive components simultaneously, including arousal (at target stimulation onset), numerical representation (digit matrix presentation), mental arithmetic (adding and multiplying digits), logical thinking (picking the greater result after addition), and decision-making (deciding on the greater result and whether to add diagonally or vertically). In general, the task developed in this study can be divided into two major and cognitively different segments that represent our paradigm’s dual nature. Segment-1 (SG1), 0–300 ms after stimulus onset, is associated with general early attentional components involving arousal or visuospatial selective attention, followed by segment-2 (SG2), >300 ms after stimulus onset, which reveals task-specific processes, including mathematical operations as well as working memory.
Complementary to previous studies, we observed new characteristics of early (sensory) ERP components, as well as later (higher cognitive) markers reported occasionally in ALS patients. In the SG1 period of our dual-task paradigm, we observed generally attenuated averaged target ERPs in ALS subjects compared to healthy controls. In particular, we observed weaker P200 and N200 in participants with ALS. Although these early components have been acknowledged to be present in ALS patients [49] and individuals with consciousness disorders [50], to the best of our knowledge, little work has been performed to characterize the neural dynamics of these components in clinical populations. Our findings highlight the importance of quantifying these markers in ALS patients rather than merely contending with their existence. The decrease of P300 observed in our ERP analysis of ALS is consistent with the results of studies that have reported that people with ALS may have reduced P300 amplitudes [7, 51, 52]. Generally, the P300 is associated with selective attention [53], the ability to filter out visuospatial distractions attentively, and working memory [51]. Attentional scores have also been previously found to correlate with P300 feature characteristics [14]. Therefore, the decreased P300 amplitude observed in ALS patients can be interpreted as a reflection of an attentional problem. Participants with ALS also demonstrated shorter P300 latencies than did controls, which is unusual among conventional oddball paradigms [51], but not unique, as shorter P300 latencies in ALS in response to unattended targets have been reported previously [53]. This shortened latency in response to unattended targets also is interpreted as a sign of impaired selective attention in these patients. This supports previous studies that reported distortions in P300 latency and amplitude when attention is divided between dual tasks [54, 55].
As expected, we could elicit later ERP components associated with the SG2 period of our dual-task paradigm both in controls and ALS patients, although these responses were weaker in the patients. In general, we observed a lower elicitation of N400 in patients, which are speculated to indicate deficits in patients’ semantic memory. As in previous studies, we observed a late component in evoked responses (~650 ms post-stimulus) associated with typical mental arithmetic tasks in the healthy group (figure 3) [54]. However, this pattern was significantly weaker in the ALS group, indicating a possible deficit in these patients’ computational processing [56]. These later (higher cognitive) processing components, which normally are reported to have failed [49, 50] in ALS patients, can potentially introduce new candidates for ALS disease signatures.
While most prior studies of ALS have analyzed EEG spectral power features in either the resting-state [57] or single modal activation paradigms (e.g. motor-tasks) [58], we explored EEG neural dynamics here through a multimodal activation paradigm.
Overall, we observed a significantly greater increase in frontal delta power in healthy controls than in patients. Delta power increases in mental calculation tasks are interpreted typically as relevant to ‘internal concentration’ blocking interference during task performance [59]. As in our proposed visuo-mental dual-task paradigm, subjects attempt to concentrate on performing a set of internal arithmetic tasks independent of external visual stimuli, thus, we expected to observe increased delta during math operations. Interestingly, the increase in the delta was observed largely after ~1 s delay (figure 4) in both groups, showing that the delta deviations refer to later cognitive/calculation components (SG2). The suppressed delta increase in the ALS group is speculated to be related to a degraded internal concentration during calculations [59]. Interestingly, this signature of delta in patients was associated with lower cognitive scores in patients (figure 8, left).
In the theta frequency band, healthy participants showed a profound power increase, while this pattern was attenuated significantly in the patient group. Notably, the smaller increase in theta power was also associated with lower cognitive scores in patients (figure 8, middle). Generally, theta oscillations are associated with several cognitive components, including attention to stimuli and working memory processes [60–62]. Therefore, it is plausible to associate increased theta power in our mental task with an inherent increased workload and the task’s attentional demand. Thus, the mental dimension (i.e. the arithmetic task) we added to the conventional visual oddball paradigm would engage more working memory and attentional components.
While we did not observe any significant difference in the alpha frequency band between participants with ALS and controls, a significant decrease in the beta was seen in the healthy group compared to the patients. Frontal beta activity is associated with top-down control processes [63, 64], particularly in general task-related processing. Beta power is reported to be enhanced during the intended maintenance of mental status, or status quo [65]. Thus, the initial beta suppression observed after target presentation (in the SG1 period) might be attributable to the changing mental state after stimulus onset. Later beta suppression (in the SG2 period) can be attributed to content-specific frontal beta modulation parallel with working memory processing and decision making which confirms the results of recent studies [66, 67]. Therefore, ALS patients’ lack of beta power modulation while performing the mental task can be interpreted as dysfunctions in the top-down control process related to general workload processing.
The healthy group’s hemodynamic responses confirmed that our relatively short ISI can evoke sufficient hemodynamic activity to generate a reliable comparative framework between the groups. This can be explained by the small ratio of targets/non-targets (2/10), so in a random presentation routine, non-target flashes are likely to provide sufficient resting time after target intensifications. In addition, a few fNIRS studies have reported plausible classification results with shorter ISI because of inherent initial dips in hemodynamic responses [68, 69]. As expected, the general observation of an evoked hemodynamic response during the arithmetic tasks supports previous findings of an elevated hemodynamic response during mental arithmetic operations [27, 40, 70]. Interestingly, in a similar study using simultaneous functional magnetic resonance imaging (fMRI)-EEG recordings, Sammer et al [70] identified increased hemodynamic activity evoked by mental arithmetic workload accompanied by enhanced EEG-theta power. In this study, this pattern appears to be associated with working memory processes required for mental arithmetic calculations, such as keeping numbers in mind, searching memory, strategic manipulation, and using numbers in computations. Therefore, in our dual-task, we might conclude that while hemodynamic elevation in response to the arithmetic workload paralleled increases in theta power in the healthy group [70], this interactive pattern was absent in participants with ALS. This can explain the overall weaker correlation observed between frontal fNIRS and frontal EEG-theta in ALS group compared to controls depicted in figure 7. In particular, the significant hemodynamic contrast was observed primarily in channels F3-F1 and F3-AF3 which correspond to the dorsolateral prefrontal cortex (DLPFC), a region critical to working memory rocessing [71]. This strengthens our speculation that participants with ALS may have executive dysfunctions that affect workload processing reflected in the DLPFC area. In addition to the weaker correlations observed between hemodynamic levels and EEG-theta in ALS, no significant correlation between HbO levels and delta powers was observed in these patients. However, we observed a significant positive correlation between EEG-delta and HbO level in healthy controls. Notably, while EEG-fNIRS correlation maps largely were positive for healthy controls, they were mainly negative in ALS and revealed a desynchronization pattern of electrical (EEG)-vascular (fNIRS) responses during mental tasks. Interestingly, as figure 7 illustrates, later time windows in fNIRS showed a more significant correlation between frontal HbO elevation and EEG-spectral features in healthy controls confirming the intrinsic slowness of hemodynamic responses relative to electrical activities [72]. Overall, the lack of hemodynamic signatures in ALS patients combined with the absence of correlative patterns with EEG-features can potentially introduce new spatial candidates for disease-specific cognitive markers.
The significant correlations between CBS scores and EEG-delta and theta power, in both SG1 and early SG2 segments, corroborates the cognitive interpretations made here, confirming ALS patients’ weaker concentration and deficits in attentional processing. On the other hand, the significant correlation between beta power and ALSFRS-R scores suggests beta oscillations’ potential to predict disease progression or staging. Together with our correlation analysis between the CBS test and the spectral neural markers obtained in ALS patients, our results indicate these markers’ important complementary role alongside cognitive-behavioral screens. In summary, the significant correlations between clinical scores (ALSFRS-R and CBS) and EEG-spectral features may provide information about cognitive deficits and disease staging. Furthermore, the lack of significant correlations with ERP features aligns with prior results in which ERP features do not depend on disease duration or the severity of motor symptoms [11, 13, 14].
Generally, our exploratory multimodal framework allows us to integratively capture potential non-motor markers that previous single-modality/single-task studies have reported only sporadically. The obtained integrative signatures through the developed visuo-mental dual-task make two simultaneous responses of EEG and fNIRS potentially suitable candidates to quantify multi-aspect cognitive dysfunction in patients with ALS. Our general finding of a deficit in hemodynamic responses, together with spatial information derived from fNIRS data, as well as EEG markers of the mental workload in ALS suggest potential attentional, working memory, and decision-making dysfunctions, either in general task or task-specific demands in these patients. Although deficits in attention (e.g. impaired arousal or selective attention) have also been reported in ALS patients [53, 73], our paradigm’s dual nature provides potential complementary neural markers that reveal new aspects of non-motor dysfunctions in ALS patients.
Overall, this study’s findings demonstrated the integrative characteristics of non-motor neural signatures in patients with severe motor deficits that are reflected in both electrical and hemodynamic neural features and suggest future exploitation of these signatures as potential diagnostic and prognostic markers. The results could improve our integrative understanding of mental workload in healthy brain functions while elucidating the potential mechanisms of ALS’s effect on non-motor functions.
4.1. Limitations and future works
The statistical power of our study was limited by the small number of ALS patients recruited because of the relative difficulty of recruiting and/or recording from this population. For future work, bolstering our results with larger sample sizes is necessary to further explore the generalizability of the obtained neuro-markers to the neurogenesis of ALS. In addition, we did not analyze differences in gender and age, which might affect the neuro-markers’ measures. Future research with larger samples of ALS patients should be conducted to further consider demographic information in smaller sub-groups.
In general, this study was exploratory in nature and primarily focused on characterization rather than application. However, the outcomes of this study may also be extended for application purposes. For example, the identified markers can be further extended as potential integrative control signals for brain-computer interface (BCI) systems applicable for patients with advanced ALS, particularly those who are unable to use typical BCIs that rely on fine ocular control. In general, in the later stages of the disease when the patient’s vision and normal EEG-based communicative BCIs fail, the fNIRS-EEG dual-task proposed may help compensate for these limitations. Thus, future studies can expand this modality further as a potential BCI design through which ALS patients can communicate more efficiently. In addition, the integrative cognitive markers in ALS obtained here can be used to develop BCIs embedded with predictive phases to address intra-individual variations in ALS patients’ daily BCI use we reported previously [52].
More specific clinical information such as emotional impairments which appear to affect neural features such as ERP characteristics [74] is another potential direction to further expand our study. For example, apathy is a common issue in ALS [75], and motivation is known to affect BCI performance [76], which can be critical for ALS patients as one of the main BCI end-users. Our cognitive testing did not address these specific clinical changes, which could potentially affect the results. Future studies to further expand the cognitive and behavioral tests which consider more specific clinical information particular to ALS will no doubt provide valuable insights into existing ALS research.
Furthermore, although our study indicated that despite a strong relation between fNIRS and EEG in healthy controls during mental tasks, these patterns were absent in ALS patients, no causal investigation was conducted. Further analyses must be performed to explore the causal relations in neurovascular coupling and their potential associations with cognitive dysfunction in ALS.
Supplementary Material
Acknowledgments
The authors would like to thank the participants without whom this study would not have been possible. We would also like to thank the ALS Association Rhode Island Chapter and the National Center for Adaptive Neurotechnologies for their continuous support. This study was supported by the Institutional Development Award (IDeA) Network for Biomedical Research Excellence from the NIGMS of the NIH (P20GM103430) and National Center for Adaptive Neurotechnologies (EB018783).
Footnotes
Supplementary material for this article is available online
References
- [1].Beeldman E, Raaphorst J, Klein Twennaar M, de Visser M, Schmand B A and de Haan R J 2016. The cognitive profile of ALS: a systematic review and meta-analysis update J. Neurol. Neurosurg. Psychiatry 87 611–9 [DOI] [PubMed] [Google Scholar]
- [2].Lomen-Hoerth C, Murphy J, Langmore S, Kramer JH, Olney RK and Miller B 2003. Are amyotrophic lateral sclerosis patients cognitively normal? Neurology 60 1094–7 [DOI] [PubMed] [Google Scholar]
- [3].Murphy JM, Henry RG, Langmore S, Kramer JH, Miller BL and Lomen-Hoerth C 2007. Continuum of frontal lobe impairment in amyotrophic lateral sclerosis Arch. Neurol 64 530–4 [DOI] [PubMed] [Google Scholar]
- [4].Turner MR and Swash M 2015. The expanding syndrome of amyotrophic lateral sclerosis: a clinical and molecular odyssey J. Neurol. Neurosurg. Psychiatry 86 667–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Montuschi A, Iazzolino B, Calvo A, Moglia C, Lopiano L, Restagno G, Brunetti M, Ossola I, Presti AL and Cammarosano S 2015. Cognitive correlates in amyotrophic lateral sclerosis: a population-based study in Italy J. Neurol. Neurosurg. Psychiatry 86 168–73 [DOI] [PubMed] [Google Scholar]
- [6].Kellmeyer P, Grosse-Wentrup M, Schulze-Bonhage A, Ziemann U and Ball T 2018. Electrophysiological correlates of neurodegeneration in motor and non-motor brain regions in amyotrophic lateral sclerosis-implications for brain-computer interfacing J. Neural Eng 15 041003. [DOI] [PubMed] [Google Scholar]
- [7].Raggi A, Iannaccone S and Cappa SF 2010. Event-related brain potentials in amyotrophic lateral sclerosis: a review of the international literature Amyotroph. Lateral Scler 11 16–26 [DOI] [PubMed] [Google Scholar]
- [8].Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM and Schulz P E 2005. Prevalence and patterns of cognitive impairment in sporadic ALS Neurology 65 586–90 [DOI] [PubMed] [Google Scholar]
- [9].Silvoni S, Volpato C, Cavinato M, Marchetti M, Priftis K, Merico A, Tonin P, Koutsikos K, Beverina F and Piccione F 2009. P300-based brain-computer interface communication: evaluation and follow-up in amyotrophic lateral sclerosis Frontiers Neurosci. 3 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zaehle T, Becke A, Naue N, Machts J, Abdulla S, Petri S, Kollewe K, Dengler R, Heinze H and Vielhaber S 2013. Working memory in ALS patients: preserved performance but marked changes in underlying neuronal networks PloS One 8 e71973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Consonni M, Catricalà E, Dalla Bella E, Gessa VC, Lauria G and Cappa S F 2016. Beyond the consensus criteria: multiple cognitive profiles in amyotrophic lateral sclerosis? Cortex 81 162–7 [DOI] [PubMed] [Google Scholar]
- [12].Consonni M, Contarino VE, Catricalà E, Dalla Bella E, Pensato V, Gellera C, Lauria G and Cappa SF 2018. Cortical markers of cognitive syndromes in amyotrophic lateral sclerosis NeuroImage 19 675–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McCane LM, Heckman SM, McFarland DJ, Townsend G, Mak JN, Sellers EW, Zeitlin D, Tenteromano LM, Wolpaw JR and Vaughan TM 2015. P300-based brain-computer interface (BCI) event-related potentials (ERPs): people with amyotrophic lateral sclerosis (ALS) versus age-matched controls Clin. Neurophysiol 126 2124–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Geronimo A, Simmons Z and Schiff S J 2016. Performance predictors of brain–computer interfaces in patients with amyotrophic lateral sclerosis J. Neural Eng 13 026002. [DOI] [PubMed] [Google Scholar]
- [15].Burke T, Pinto-Grau M, Lonergan K, Bede P, O’Sullivan M, Heverin M, Vajda A, McLaughlin R L, Pender N and Hardiman O 2017. A cross-sectional population-based investigation into behavioral change in amyotrophic lateral sclerosis: subphenotypes, staging, cognitive predictors, and survival Ann. Clin. Transl. Neurol 4 305–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kasper E, Schuster C, Machts J, Kaufmann J, Bittner D, Vielhaber S, Benecke R, Teipel S and Prudlo J 2014. Microstructural white matter changes underlying cognitive and behavioural impairment in ALS–an in vivo study using DTI PloS One 9 e114543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Woolley S C, York M K, Moore D H, Strutt A M, Murphy J, Schulz P E and Katz J S 2010. Detecting frontotemporal dysfunction in ALS: utility of the ALS cognitive behavioral screen (ALS-CBS) Amyotroph. Lateral Scler 11 303–11 [DOI] [PubMed] [Google Scholar]
- [18].Abiri R, Borhani S, Sellers E W, Jiang Y and Zhao X 2018. A comprehensive review of EEG-based brain-computer interface paradigms J. Neural Eng 16 011001. [DOI] [PubMed] [Google Scholar]
- [19].Santhosh J, Bhatia M, Sahu S and Anand S 2005. Decreased electroencephalogram alpha band [8–13 Hz] power in amyotrophic lateral sclerosis patients: a study of alpha activity in an awake relaxed state Neurol. India 53 99. [DOI] [PubMed] [Google Scholar]
- [20].Iyer P M, Egan C, Pinto-Grau M, Burke T, Elamin M, Nasseroleslami B, Pender N, Lalor E C and Hardiman O 2015. Functional connectivity changes in resting-state EEG as potential biomarker for amyotrophic lateral sclerosis PloS One 10 e0128682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Putze F, Hesslinger S, Tse C, Huang Y, Herff C, Guan C and Schultz T 2014. Hybrid fNIRS-EEG based classification of auditory and visual perception processes Frontiers Neurosci. 8 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Koenig M A and Kaplan P W 2013. Clinical neurophysiology in acute coma and disorders of consciousness Seminars in Neurology pp 121–32 [DOI] [PubMed] [Google Scholar]
- [23].De Massari D, Ruf CA, Furdea A, Matuz T, Van Der Heiden L, Halder S, Silvoni S and Birbaumer N 2013 Brain communication in the locked-in state Brain 136 1989–2000 [DOI] [PubMed] [Google Scholar]
- [24].Murguialday AR, Hill J, Bensch M, Martens S, Halder S, Nijboer F, Schoelkopf B, Birbaumer N and Gharabaghi A 2011. Transition from the locked in to the completely locked-in state: a physiological analysis Clin. Neurophysiol 122 925–33 [DOI] [PubMed] [Google Scholar]
- [25].Kübler A, Neumann N, Kaiser J, Kotchoubey B, Hinterberger T and Birbaumer NP 2001. Brain-computer communication: self-regulation of slow cortical potentials for verbal communication Arch. Phys. Med. Rehabil 82 1533–9 [DOI] [PubMed] [Google Scholar]
- [26].Kübler A, Kotchoubey B, Hinterberger T, Ghanayim N, Perelmouter J, Schauer M, Fritsch C, Taub E and Birbaumer N 1999. The thought translation device: a neurophysiological approach to communication in total motor paralysis Exp. Brain Res 124 223–32 [DOI] [PubMed] [Google Scholar]
- [27].Schudlo LC and Chau T 2014. Dynamic topographical pattern classification of multichannel prefrontal NIRS signals: II. Online differentiation of mental arithmetic and rest J. Neural Eng 11 016003. [DOI] [PubMed] [Google Scholar]
- [28].Naseer N and Hong K 2015. fNIRS-based brain-computer interfaces: a review Frontiers Hum. Neurosci 9 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lloyd-Fox S, Papademetriou M, Darboe MK, Everdell NL, Wegmuller R, Prentice AM, Moore SE and Elwell C E 2014. Functional near infrared spectroscopy (fNIRS) to assess cognitive function in infants in rural Africa Sci. Rep 4 4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shin J, Müller K and Hwang H 2016. Near-infrared spectroscopy (NIRS)-based eyes-closed brain-computer interface (BCI) using prefrontal cortex activation due to mental arithmetic Sci. Rep 6 36203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shin J, Müller K and Hwang H 2017. Hybrid EEG-NIRS brain-computer interface under eyes-closed condition Asia-Pacific Signal and Information Processing Association Annual Summit and Conf. (APSIPA ASC) pp 721–3 [Google Scholar]
- [32].Kuruvilla MS, Green JR, Ayaz H and Murman DL 2013. Neural correlates of cognitive decline in ALS: an fNIRS study of the prefrontal cortex Cogn. Neurosci 4 115–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chaudhary U, Xia B, Silvoni S, Cohen LG and Birbaumer N 2017. Brain–computer interface-based communication in the completely locked-in state PLoS Biol. 15 e1002593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [34].Pettit LD, Bastin ME, Smith C, Bak TH, Gillingwater TH and Abrahams S 2013. Executive deficits, not processing speed relates to abnormalities in distinct prefrontal tracts in amyotrophic lateral sclerosis Brain 136 3290–304 [DOI] [PubMed] [Google Scholar]
- [35].Khan MJ, Hong K, Naseer N and Bhutta MR 2014 A hybrid EEG-fNIRS BCI: motor imagery for EEG and mental arithmetic for fNIRS 14th Int. Conf. on Control, Autom. Syst. (ICCAS 2014) pp 275–8 [Google Scholar]
- [36].Amiri S, Fazel-Rezai R and Asadpour V 2013 A review of hybrid brain-computer interface systems Adv. Hum.-Comput. Interact 2013. 1 [Google Scholar]
- [37].Müller-Putz G, Leeb R, Tangermann M, Höhne J, Kübler A, Cincotti F, Mattia D, Rupp R, Müller K and del R Millán J 2015. Towards noninvasive hybrid brain–computer interfaces: framework, practice, clinical application, and beyond Proc. IEEE 103 926–43 [Google Scholar]
- [38].Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B and Nakanishi A 1999. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J. Neurol. Sci 169 13–21 [DOI] [PubMed] [Google Scholar]
- [39].Krusienski DJ, Sellers EW, McFarland DJ, Vaughan TM and Wolpaw JR 2008. Toward enhanced P300 speller performance J. Neurosci. Methods 167 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Bauernfeind G, Scherer R, Pfurtscheller G and Neuper C 2011. Single-trial classification of antagonistic oxyhemoglobin responses during mental arithmetic Med. Biol. Eng. Comput 49 979. [DOI] [PubMed] [Google Scholar]
- [41].Schalk G and Mellinger J 2010. A Practical Guide to Brain–computer Interfacing with BCI2000: General-Purpose Software for Brain-Computer Interface Research, Data Acquisition, Stimulus Presentation, and Brain Monitoring (Berlin: Springer; ) ( 10.1007/978-1-84996-092-2) [DOI] [Google Scholar]
- [42].Simis M, Santos K, Sato J, Fregni F and Battistella L 2018. Using functional near infrared spectroscopy (fNIRS) to assess brain activity of spinal cord injury patient, during robot-assisted gait Clin. Neurophysiol 129 e4–e44 [Google Scholar]
- [43].Coyle S, Ward T, Markham C and McDarby G 2004. On the suitability of near-infrared (NIR) systems for next-generation brain-computer interfaces Physiol. Meas 25 815–22 [DOI] [PubMed] [Google Scholar]
- [44].Scarpa F, Cutini S, Scatturin P, Dell’Acqua R and Sparacino G 2010. Bayesian filtering of human brain hemodynamic activity elicited by visual short-term maintenance recorded through functional near-infrared spectroscopy (fNIRS) Opt. Express 18 26550–68 [DOI] [PubMed] [Google Scholar]
- [45].Kocsis L, Herman P and Eke A 2006. The modified Beer–Lambert law revisited Phys. Med. Biol 51 N91. [DOI] [PubMed] [Google Scholar]
- [46].Oruç I, Krigolson O, Dalrymple K, Nagamatsu LS, Handy TC and Barton JJ 2011. Bootstrap analysis of the single subject with event related potentials Cogn. Neuropsychol 28 322–37 [DOI] [PubMed] [Google Scholar]
- [47].Vizioli L, Foreman K, Rousselet GA and Caldara R 2009. Inverting faces elicits sensitivity to race on the N170 component: a cross-cultural study J. Vis 10 15. [DOI] [PubMed] [Google Scholar]
- [48].Hochberg Y and Benjamini Y 1990. More powerful procedures for multiple significance testing Stat. Med 9 811–8 [DOI] [PubMed] [Google Scholar]
- [49].Bensch M, Martens S, Halder S, Hill J, Nijboer F, Ramos A, Birbaumer N, Bogdan M, Kotchoubey B and Rosenstiel W 2014. Assessing attention and cognitive function in completely locked-in state with event-related brain potentials and epidural electrocorticography J. Neural Eng 11 026006. [DOI] [PubMed] [Google Scholar]
- [50].Laureys S, Owen AM and Schiff ND 2004. Brain function in coma, vegetative state, and related disorders Lancet Neurol. 3 537–46 [DOI] [PubMed] [Google Scholar]
- [51].Riccio A, Simione L, Schettini F, Pizzimenti A, Inghilleri M, Olivetti Belardinelli M, Mattia D and Cincotti F 2013. Attention and P300-based BCI performance in people with amyotrophic lateral sclerosis Frontiers Hum. Neurosci 7 732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Shahriari Y, Vaughan TM, McCane L, Allison BZ, Wolpaw JR and Krusienski DJ 2019. An exploration of BCI performance variations in people with amyotrophic lateral sclerosis using longitudinal EEG data J. Neural Eng 16 056031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Pinkhardt EH, Jürgens R, Becker W, Mölle M, Born J, Ludolph A C and Schreiber H 2008. Signs of impaired selective attention in patients with amyotrophic lateral sclerosis J. Neurol 255 532–8 [DOI] [PubMed] [Google Scholar]
- [54].Wang L, Wang S and Kuang G 2013. A study of brain-computer interface paradigm based on mental arithmetic J. Biomed. Eng 30 469–75 [PubMed] [Google Scholar]
- [55].Polich J 2007. Updating P300: an integrative theory of P3a and P3b Clin. Neurophysiol 118 2128–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Van Beek L, Ghesquière P, De Smedt B and Lagae L 2015. Arithmetic difficulties in children with mild traumatic brain injury at the subacute stage of recovery Dev. Med. Child Neurol 57 1042–8 [DOI] [PubMed] [Google Scholar]
- [57].Fraschini M, Demuru M, Hillebrand A, Cuccu L, Porcu S, Di Stefano F, Puligheddu M, Floris G, Borghero G and Marrosu F 2016. EEG functional network topology is associated with disability in patients with amyotrophic lateral sclerosis Sci. Rep 6 38653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Burget F, Fiederer LDJ, Kuhner D, Völker M, Aldinger J, Schirrmeister RT, Do C, Boedecker J, Nebel B and Ball T 2017. Acting thoughts: towards a mobile robotic service assistant for users with limited communication skills 2017 European Conf. on Mobile Robots (ECMR) pp 1–6 [Google Scholar]
- [59].Fernández T, Harmony T, Rodríguez M, Bernal J, Silva J, Reyes A and Marosi E 1995. EEG activation patterns during the performance of tasks involving different components of mental calculation Electroencephalogr. Clin. Neurophysiol 94 175–82 [DOI] [PubMed] [Google Scholar]
- [60].Deligani RJ, Hosni SI, Vaughan TM, McCane LM, Zeitlin DJ, McFarlan DJ, Krusienski DJ and Shahriari Y 2019. Neural alterations during use of a P300-based BCI by individuals with amyotrophic lateral sclerosis 9th Int. IEEE/MBS Conf. on Neural Eng. pp 899–902 [Google Scholar]
- [61].Borgheai SB, Deligani RJ, McLinden J, Abtahi M, Ostadabbas S, Mankodiya K and Shahriari Y 2019. Multimodal evaluation of mental workload using a hybrid EEG-fNIRS brain-computer interface 9th Int. IEEE/EMBS Conf. on Neural Eng. pp 973–6 [Google Scholar]
- [62].Deiber M, Missonnier P, Bertrand O, Gold G, Fazio-Costa L, Ibañez V and Giannakopoulos P 2007. Distinction between perceptual and attentional processing in working memory tasks: a study of phase-locked and induced oscillatory brain dynamics J. Cogn. Neurosci 19 158–72 [DOI] [PubMed] [Google Scholar]
- [63].Bastos AM, Vezoli J, Bosman CA, Schoffelen J, Oostenveld R, Dowdall JR, De Weerd P, Kennedy H and Fries P 2015. Visual areas exert feedforward and feedback influences through distinct frequency channels Neuron 85 390–401 [DOI] [PubMed] [Google Scholar]
- [64].Siegel M, Donner TH and Engel AK 2012. Spectral fingerprints of large-scale neuronal interactions Nat. Rev. Neurosci 13 121. [DOI] [PubMed] [Google Scholar]
- [65].Engel AK and Fries P 2010. Beta-band oscillations—signalling the status quo? Curr. Opin. Neurobiol 20 156–65 [DOI] [PubMed] [Google Scholar]
- [66].Spitzer B and Haegens S 2017. Beyond the status quo: a role for beta oscillations in endogenous content (re) activation Eneuro 4 e0170–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Wimmer K, Ramon M, Pasternak T and Compte A 2016. Transitions between multiband oscillatory patterns characterize memory-guided perceptual decisions in prefrontal circuits J. Neurosci 36 489–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zafar A and Hong K 2017. Detection and classification of three-class initial dips from prefrontal cortex Biomed. Opt. Express 8 367–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Borgheai SB, Abtahi M, Mankodiya K, McLinden J and Shahriari Y 2019. Towards a single trial fNIRS-based brain-computer interface for communication 2019 9th Int. IEEE/EMBS Conf. on Neural Eng. pp 1030–3 [Google Scholar]
- [70].Sammer G, Blecker C, Gebhardt H, Bischoff M, Stark R, Morgen K and Vaitl D 2007. Relationship between regional hemodynamic activity and simultaneously recorded EEG-theta associated with mental arithmetic-induced workload Hum. Brain Mapp. 28 793–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Blumenfeld RS and Ranganath C 2006. Dorsolateral prefrontal cortex promotes long-term memory formation through its role in working memory organization J. Neurosci 26 916–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Plichta MM, Heinzel S, Ehlis A, Pauli P and Fallgatter AJ 2007. Model-based analysis of rapid event-related functional near-infrared spectroscopy (NIRS) data: a parametric validation study NeuroImage 35 625–34 [DOI] [PubMed] [Google Scholar]
- [73].Volpato C, Prats Sedano MA, Silvoni S, Segato N, Cavinato M, Merico A, Piccione F, Palmieri A and Birbaumer N 2016. Selective attention impairment in amyotrophic lateral sclerosis Amyotroph. Lateral Scler. Frontotemp. Degeneration 17 236–44 [DOI] [PubMed] [Google Scholar]
- [74].Campanella S, Vanhoolandt ME and Philippot P 2004. Emotional deficit in subjects with psychopathic tendencies as assessed by the Minnesota Multiphasic Personality Inventory-2: an event-related potentials study Neurosci. Lett 373 26–31 [DOI] [PubMed] [Google Scholar]
- [75].Unglik J, Bungener C, Delgadillo D, Salachas F, Pradat PF, Bruneteau G, Lenglet T, Le Forestier N, Couratier P and Vacher Y 2018. Emotional feeling in patients suffering from amyotrophic lateral sclerosis Geriatrie Et Psychologie Neuropsychiatrie Du Vieillissement 16 414. [DOI] [PubMed] [Google Scholar]
- [76].Kleih SC, Nijboer F, Halder S and Kubler A 2010. Motivation modulates the P300 amplitude during brain-computer interface use Clin. Neurophysiol 121 1023–31 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.