Abstract
Understanding the function of genes within staple crops will accelerate crop improvement by allowing targeted breeding approaches. Despite their importance, a lack of genomic information and resources has hindered the functional characterisation of genes in major crops. The recent release of high-quality reference sequences for these crops underpins a suite of genetic and genomic resources that support basic research and breeding. For wheat, these include gene model annotations, expression atlases and gene networks that provide information about putative function. Sequenced mutant populations, improved transformation protocols and structured natural populations provide rapid methods to study gene function directly. We highlight a case study exemplifying how to integrate these resources. This review provides a helpful guide for plant scientists, especially those expanding into crop research, to capitalise on the discoveries made in Arabidopsis and other plants. This will accelerate the improvement of crops of vital importance for food and nutrition security.
Introduction
Research in Arabidopsis and other model species has uncovered mechanisms regulating important biological processes in plants. However, as research in these model species does not always translate directly into crop species such as wheat, understanding gene function in crop species themselves is critical for crop improvement. With the advent of functional genomics resources in wheat and other crops, discoveries from model species can rapidly be tested and functional genetic studies can now be performed for agronomically-important traits directly in the crops themselves (Borrill, 2020).
The most common forms of domesticated wheat are tetraploid durum wheat (Triticum turgidum ssp. durum L.) and hexaploid bread wheat (Triticum aestivum L.). Polyploid wheat is derived from hybridisation events between different ancestral progenitor species reviewed in Matsuoka (2011), and thus each gene typically exists as two (tetraploid durum wheat) or three (hexaploid bread wheat) copies. These closely related copies, known as homoeologous genes, are on average >95% similar across their coding regions (Figure 1) and usually have a highly conserved gene structure. Tetraploid and hexaploid wheat have large genomes, 12 and 16 Gbp respectively, which consist mostly (>85%) of repetitive elements. The combination of these factors has, for a long time, hampered the development of genomics tools in wheat and other crops with large genomes, such as sugarcane (Garsmeur et al., 2018). Recent advances in sequencing technologies and bioinformatics tools has helped overcome these difficulties, and there are now a wide range of resources available for genomic analysis in wheat. The speed of wheat research has also been limited by its relatively long generation time, which ranges from four to six months depending on the requirement of cold periods (vernalisation) to induce flowering. Again, recent advances in the use of controlled growth conditions have radically changed these timeframes (Watson et al., 2018). Wheat has now become a tractable system for translational, comparative and functional genomics (Borrill et al., 2019).
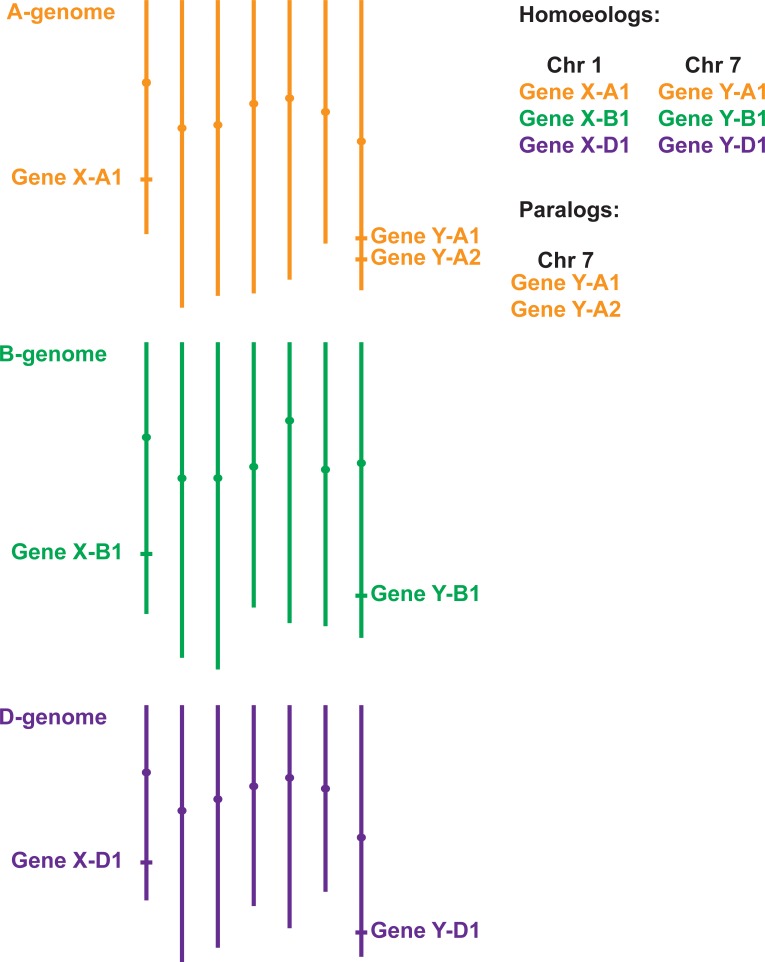
Figure 1. Gene homology within polyploid wheat.
Due to two separate hybridisation events, genes in polyploid wheat will be present in multiple copies called homoeologs, which usually have similar chromosome locations. In the example of hexaploid bread wheat illustrated here, Gene X has homoeologs on chromosomes 1A, 1B and 1D. Duplicated genes, called paralogs (e.g. two copies of Gene Y on chromosome 7A), have evolved either within wheat or in one of its ancestral species. Most paralogs arise from intra-chromosomal duplications, although inter-chromosomal duplications can also occur.
Here we describe some of the recent developments in wheat genomics, focussing on published and publicly available resources and tools, and lay out a roadmap for their use (Figure 2). We present available wheat genome assemblies and annotations and discuss a series of approaches to functionally characterise genes. We also outline strategies for growing, crossing and genotyping wheat using the latest available tools and techniques. Finally, we present a case study that encapsulates the above steps and highlights potential pitfalls. We focus mainly on the Ensembl Plants database, as it integrates many of the publicly available data on wheat. However, other databases such as URGI (https://wheat-urgi.versailles.inra.fr/; Alaux et al., 2018), the Wheat Information System (WheatIS; http://www.wheatis.org/), and GrainGenes (https://wheat.pw.usda.gov/GG3/; Blake et al., 2019) also host and integrate similar, but complementary, genetic, genomic and phenomic data for wheat. We expect this review will be a helpful guide for plant scientists who already work on wheat or who are considering expanding their research into crops with large genomes such as wheat.
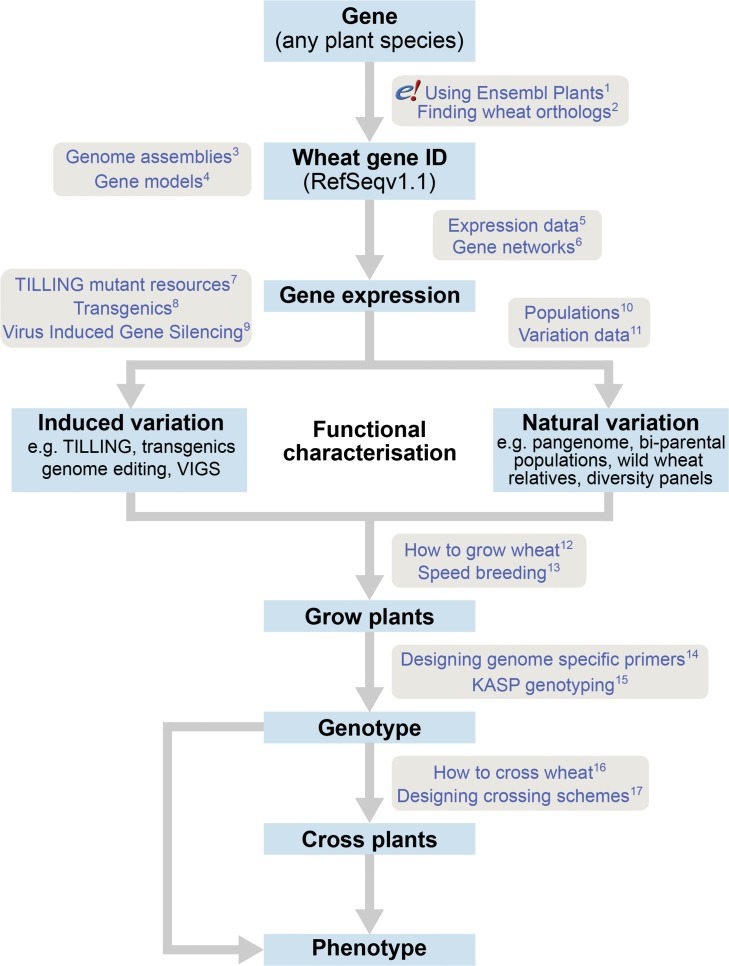
Figure 2. The roadmap for gene characterisation in wheat.
Overview of a proposed strategy to take a gene from any plant species, identify the correct wheat ortholog(s) using Ensembl Plants (https://plants.ensembl.org) and determine gene expression using expression browsers and gene networks. Suggestions for functional characterisation are provided including induced variation such as mutants, transgenics or Virus-Induced Gene Silencing (VIGs). In addition, publicly available populations incorporating natural variation are available. Finally steps for growing, genotyping and crossing plants are outlined. Links to detailed tutorials and further information are provided and can be found on www.wheat-training.com. (1) www.wheat-training.com/wp-content/uploads/Genomic_resources/pdfs/EnsemblPlants-primer.pdf (2) www.wheat-training.com/wp-content/uploads/Genomic_resources/pdfs/Finding-wheat-orthologs.pdf (3) www.wheat-training.com/wp-content/uploads/Genomic_resources/pdfs/Genome_assemblies.pdf (4) www.wheat-training.com/wp-content/uploads/Genomic_resources/pdfs/Gene-models.pdf (5) www.wheat-training.com/wp-content/uploads/Genomic_resources/pdfs/Expression-browsers.pdf (6) www.wheat-training.com/wp-content/uploads/Genomic_resources/pdfs/Gene-networks.pdf (7) www.wheat-training.com/wp-content/uploads/Functional_studies/PDFs/Selecting-TILLING-mutants.pdf (8) www.wheat-training.com/wp-content/uploads/Functional_studies/PDFs/Transgenics.pdf (9) www.wheat-training.com/wp-content/uploads/Functional_studies/PDFs/Virus_Induced_Gene_Silencing.pdf (10) www.wheat-training.com/wp-content/uploads/Functional_studies/PDFs/Populations.pdf (11) www.wheat-training.com/wp-content/uploads/Genomic_resources/Variation-data.pdf (2) www.wheat-training.com/wp-content/uploads/Wheat_growth/pdfs/Growing_Wheat_final.pdf (13) www.wheat-training.com/wp-content/uploads/Wheat_growth/pdfs/Speed_breeding.pdf (14) www.wheat-training.com/wp-content/uploads/Functional_studies/PDFs/Designing-genome-specific-primers.pdf (15) https://www.biosearchtech.com/support/education/kasp-genotyping-reagents/running-kasp-genotyping-reactions (16) http://www.wheat-training.com/wp-content/uploads/Wheat_growth/pdfs/How-to-cross-wheat-pdf.pdf (17) www.wheat-training.com/wp-content/uploads/Functional_studies/PDFs/Designing-crossing-schemes.pdf.
Wheat genome assemblies
A high-quality genome reference sequence is an essential resource for functional genetics and genomics in any species. Several hexaploid wheat genome assemblies have been released over the past eight years (Brenchley et al., 2012; Mayer et al., 2014; Chapman et al., 2015; Clavijo et al., 2017; Zimin et al., 2017). The most comprehensive assembly, called RefSeqv1.0, is a chromosome-level genome assembly annotated with high and low confidence gene models (The International Wheat Genome Sequencing Consortium (IWGSC) et al., 2018). Two tetraploid wheat genomes have also been sequenced, assembled, and annotated to the same standard as RefSeqv1.0 — the wild tetraploid progenitor of wheat, wild emmer (Avni et al., 2017), and a modern durum wheat variety (Maccaferri et al., 2019). Diploid ancestral progenitor species have also been assembled to varying levels of completeness (Luo et al., 2017; Zhao et al., 2017; Ling et al., 2018; Miki et al., 2019). We summarise the annotated assemblies for polyploid wheat in Table 1; in this review we will focus mainly on the RefSeqv1.0 assembly.
Table 1. Comparison of annotated genome assemblies in hexaploid and tetraploid wheat.
| CSS | TGACv1 | RefSeqv1.0 | Durum wheat | Wild emmer wheat | |
|---|---|---|---|---|---|
| Publication | Mayer et al., 2014 | Clavijo et al., 2017 | The International Wheat Genome Sequencing Consortium (IWGSC) et al., 2018 | Maccaferri et al., 2019 | Avni et al., 2017 |
| Contigs/Chromosomes | >1 million | 735,943 | 21 chromosomes + ChrU | 14 chromosomes + ChrU | 14 chromosomes + ChrU |
| Mean scaffold size | 7.7 kbp | 88.7 kbp | Chromosomes | Chromosomes | Chromosomes |
| Assembly Size | 10.2 Gbp | 13.4 Gbp | 14.6 Gbp | 10.5 Gbp | 10.5 Gbp |
| Order | Synteny/genetic order† | Large Bins | Physical order | Physical order | Physical order |
| Coding genes* | 133,090 HC 88,998 LC |
104,091 HC 103,660 LC |
107,891 HC 161,537 LC |
66,559 HC 303,404 LC |
67,182 HC 271,179 LC |
| Assembly-related resources | Archive Ensembl Plants | Archive Ensembl Plants | Ensembl Plants GrainGenes, URGI |
Ensembl Plants GrainGenes |
Ensembl Plants GrainGenes |
| TILLING mutants | TILLING mutants | ||||
| expVIP, wheatExp | expVIP | expVIP, eFP | |||
| Accession | Chinese Spring | Chinese Spring | Chinese Spring | Svevo | Zavitan |
*Number of high confidence (HC) and low confidence (LC) genes which are defined based on multiple criteria outlined in the published papers. Care must be taken when interpreting their nomenclature (see Figure 3).
†Chromosome arm assignment was derived from chromosome flow-sorting, while approximate intra-chromosomal ordering was established using synteny derived from grasses (GenomeZipper) and genetic mapping (POPSEQ) (Mascher et al., 2013; Mayer et al., 2014).
RefSeqv1.0 is the most widely used assembly and annotation of hexaploid wheat (available on Ensembl Plants https://plants.ensembl.org/wheat). The information from previous assemblies and annotations (Chromosome Survey Sequence (CSS) and TGACv1) are also available in the Ensembl Plants archive (https://oct2017-plants.ensembl.org) or as tracks in the Ensembl Plants genome browser interface. Ensembl Plants enables access to additional information such as SNP variation, gene trees, homoeolog assignments, and TILLING (Targeting Induced Local Lesions in Genomes) mutant information. Through this interface users can also combine knowledge from the bread, durum and wild emmer wheat genomes.
Like most of the previous hexaploid assemblies, RefSeqv1.0 is derived from the wheat landrace ‘Chinese Spring’. A combination of multiple Illumina and mate pair libraries were sequenced and assembled into scaffolds. Using a method of chromosome conformation capture called Hi-C, these scaffolds were further connected into pseudomolecules representing the 21 nuclear chromosomes of wheat, plus one additional ‘pseudo-chromosome’ (ChrU) containing all unassigned sequences (The International Wheat Genome Sequencing Consortium (IWGSC) et al., 2018).
The gene models for the RefSeqv1.0 assembly were annotated using two prediction pipelines, which were then consolidated into a single set of gene models (RefSeqv1.0 models). A subset of these (~2,000 gene models) were later re-annotated manually, resulting in the RefSeqv1.1 gene model set (Figure 3). Over half of high confidence protein coding genes are present as exactly three homoeologous copies (1:1:1 triads), while several other combinations exist (e.g. 2:1:1 whereby there are two paralogs on the A genome, and a single homoeolog each on the B and D genomes, e.g. Gene Y in Figure 1).
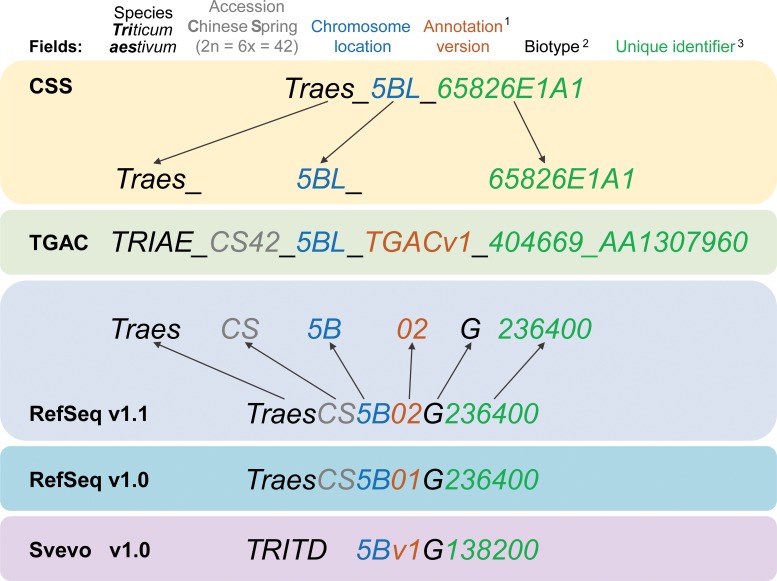
Figure 3. Gene model ID nomenclature description from the five available gene annotations for domesticated polyploid wheat.
Here, one gene is used as an example to highlight the differences in gene ID nomenclature. Fields represented in the nomenclature are shown at the top with matching colours for the corresponding features in the gene names. Yellow background shows the CSS gene names with dark grey arrows pointing towards the corresponding field in the TGAC gene annotation (TGACv1, green background). Blue backgrounds show the gene nomenclatures for RefSeqv1.0 and v1.1 annotations (as used in Ensembl Plants), while the lilac background shows the nomenclature for Svevo v1.0 (modern durum wheat). (1) Two annotation versions are available for the RefSeqv1.0 genome assembly: RefSeqv1.0 (release annotation) and RefSeqv1.1 (improved annotation). These are differentiated by the annotation version number; ‘01’ for RefSeqv1.0 and ‘02’ for RefSeqv1.1. Otherwise, the annotations follow the same rules. (2) In the RefSeq and Svevo annotations, the biotype is represented by an additional identifier, where G = gene. (3) In the RefSeqv1.0 and v1.1 annotation, identifiers are progressive numbers in steps of 100 reflecting the relative position between gene models. For example, gene TraesCS5B02G236400 would be adjacent to gene TraesCS5B02G236500. However, it is important to note that the relative positions of genes may change in future genome releases as the assembly is improved, for example, if scaffolds are rearranged. In these cases, the gene order would no longer be retained. In the gene annotation for the tetraploid durum wheat cv. Svevo, the species name is TRITD (TRITicum Durum) and gene identifiers increase in steps of 10, rather than by steps of 100 as in the RefSeq hexaploid wheat annotation. Note that RefSeqv1.0 and v1.1 comprises High Confidence (HC) and Low Confidence (LC) gene models. Low Confidence gene models are flagged by the ‘LC’ at the end (not shown). HC and LC genes which otherwise display the same unique identifier are not the same locus and are not in sequential order. Hence, TraesCS5B02G236400 and TraesCS5B02G236400LC are both located on chromosome 5B, but are not the same gene nor are they physically adjacent. Similarly, genes from homoeologous chromosomes with the same subsequent numeric identifier are not necessarily homoeologous genes. For example, TraesCS5A02G236400, TraesCS5B02G236400 and TraesCS5D02G236400 are not homoeologous genes.
The RefSeqv1.0 assembly and the RefSeqv1.1 gene models, as well as the durum and wild emmer assemblies and gene models, have been integrated into the publicly available Ensembl Plants genome browser (https://plants.ensembl.org) (Bolser et al., 2015; Howe et al., 2020). Existing variation data, both natural and induced, has been mapped to the RefSeqv1.0 hexaploid assembly and deposited in Ensembl Plants databases for visualisation via the genome browser. Integrating resources into a common reference facilitates their use and in the following sections we will discuss how to best access and utilise these resources.
Finding wheat orthologs
Although DNA sequence homology does not equate to functional homology, it represents a good starting point for translational and/or comparative genomics. Correctly identifying orthologous genes in another plant species can be a difficult task however, especially between distantly related species like Arabidopsis and wheat. These two species are separated by ~200 million years of evolution and as a result both nucleotide and protein similarities are relatively low compared to more closely related species, for example, wheat and rice (Oryza sativa L.).
Conveniently, all the data and tools necessary for identifying putative gene orthologs from different plant species are available through the Ensembl Plants website (https://plants.ensembl.org) (Bolser et al., 2015; Howe et al., 2020). The Plant Compara pipeline has been integrated into Ensembl Plants to create ‘gene trees’ that identify and clearly display the likely orthologs of any given gene for all of the species available on its website (Vilella et al., 2009; Herrero et al., 2016). This includes the RefSeqv1.1, Arabidopsis TAIR10 and rice IGRSP-1.0 gene models, amongst others. This represents a quick and reliable way to identify putative wheat orthologs of a given gene (Figure 2). Tutorials for using Ensembl Plants interactively or programmatically can be found on their website or at www.wheat-training.com.
When performing a search for putative wheat orthologs via the Ensembl Plants pipeline, we would expect to find three orthologs in hexaploid wheat for most gene queries. These orthologs would normally be located on homoeologous chromosome groups, for example chromosomes 1A, 1B and 1D (Figure 1). A well-documented exception to this rule is the long arm of chromosome 4A (4AL), which has undergone translocation events with chromosome arms 5AL and 7BS (Devos et al., 1995; Ma et al., 2013). Therefore, orthologs within these translocated regions will be physically located on different chromosome groups, for example three homoeologous genes could be on chromosome arms 4AL, 5BL and 5DL. Furthermore, gene structure of wheat orthologs is often conserved with respect to rice and other closely related monocot species; this comparison can usually be done within Ensembl Plants. If this is not possible, wheat RNA-Seq data can be used to determine the gene structure. As an alternative to the Ensembl Plants Gene Trees, one can perform reciprocal protein BLAST searches to identify putative wheat orthologs. We exemplify the above-mentioned approaches, along with potential pitfalls, in more detail in the ‘Case Study’ section.
Expression data
Determining if, when, where, and to what level a gene is expressed often constitutes one of the first steps towards its functional characterisation. Gene expression information can also be used to prioritise candidate genes underlying a quantitative trait locus (QTL) or to predict those members of a large gene family most relevant to a trait of interest. Numerous RNA-Seq datasets for wheat and many other crops have been generated and published. Although the raw data are often publicly available (e.g. via the NCBI sequence read archive, https://www.ncbi.nlm.nih.gov/sra), they are not sufficiently curated for rapid access and their use in direct comparisons is complicated due to the diversity of tissues, treatments, and origins of the samples. Expression browsers aim to centralise these public datasets and analyse them together, ideally allowing retrieval of expression information for a list of genes under different conditions. For wheat, four expression browsers are currently available: expVIP (http://www.wheat-expression.com; Borrill et al., 2016), wheat eFP browser (http://bar.utoronto.ca/efp_wheat/cgi-bin/efpWeb.cgi; Ramírez-González et al., 2018), EBI Gene Expression Atlas (https://www.ebi.ac.uk/gxa/experiments?species=triticum+aestivum), and WheatExp (https://wheat.pw.usda.gov/WheatExp; Pearce et al., 2015). Here we will focus on the first two given that they include a larger and more diverse set of samples and use the RefSeqv1.0 and v1.1 gene models described in Table 1.
Currently, expVIP includes expression data from 36 studies (1,016 RNA-Seq samples) across a diverse range of wheat tissues, developmental stages, cultivars, and environmental conditions including various abiotic and biotic stress treatments. It can display expression data for up to 250 genes at once, which can be particularly useful when working with a gene family, genes within a QTL interval, or genes involved in the same regulatory process. The expression values for each gene's homoeologs, based on the same homoeolog assignments as in Ensembl Plants, can also be displayed. The ‘homoeolog expression patterns’ of triads (genes that are present as exactly three homoeologous copies) can also be displayed as ternary plots and compared across tissues (Ramírez-González et al., 2018).
To allow comparisons across studies, the 1,016 RNA-Seq samples in expVIP were classified according to four high-level categories based on variety, tissue, developmental stage, and stress. These high-level categories are themselves divided into more detailed subcategories. These categories can be used to customise visualisation displays and allow users to select data relevant to their experimental comparisons. Data can be displayed both as transcripts per million (TPM) or as raw counts and can be directly downloaded to carry out differential gene expression analyses. Although the default gene model reference is RefSeqv1.1, users can also choose the CSS, TGACv1 and RefSeqv1.0 transcriptome references for legacy reasons. Tutorials describing expVIP are available on https://github.com/Uauy-Lab/expvip-web/wiki and www.wheat-training.com. Recently, expVIP was implemented for berry fruit species (Thole et al., 2019).
An additional resource is the electronic Fluorescent Pictograph (eFP) browser, which provides a simple visual assessment of expression data using pictures coloured according to a gene’s relative expression level. The eFP expression browser is available for several crops (e.g. potato, soybean, barley) and most recently wheat (http://bar.utoronto.ca/efp_wheat/cgi-bin/efpWeb.cgi). The wheat interface includes 209 RNA-Seq samples (also in expVIP) representing 22 tissue types from grain, root, leaf, and spike samples across multiple time points from a single hexaploid spring wheat cultivar (‘Azhurnaya’).
Gene networks
The available RNA-Seq data provides the opportunity to identify networks of co-expressed genes. Ramírez-González et al. (2018) constructed tissue and stress-specific co-expression networks in wheat to determine whether genes from the same triad showed variable spatiotemporal expression. In addition, a GENIE3 network was developed to predict transcription factor targets across the multiple RNA-Seq samples (Huynh-Thu et al., 2010; Ramírez-González et al., 2018). Together, these networks provide a powerful set of tools for hypothesis generation using wheat-specific datasets. We have recently validated the GENIE3 network using independent RNA-Seq data from tetraploid wheat (Harrington et al., 2019). Both co-expression and GENIE3 networks are incorporated into KnetMiner (https://knetminer.org/Triticum_aestivum/).
KnetMiner is a web-application for searching and visualising genome-scale knowledge networks of, for example, Arabidopsis, wheat, and human diseases (Hassani-Pak et al., 2016). It aims to provide research leads for scientists who are investigating the molecular basis of complex traits. KnetMiner accepts keywords in combination with a gene list and/or genomic regions as input and searches the underlying knowledge network to identify links between these user-provided genes and keywords. A network-based visualisation, named Network View, allows users to examine complex relationships between gene networks and traits. The networks contain nodes that represent different entities such as genes, single nucleotide polymorphisms (SNPs), publications, and traits (e.g. heat or drought tolerance) that are linked via different relation types (e.g. co-expression, GENIE3-targets, protein-protein-interaction, published-in). Together, KnetMiner and the integrated gene networks provide a powerful resource for gene discovery and hypothesis generation in wheat (see Case Study below).
Epigenomics
With the availability of the wheat genome, increasing interest has turned towards the wheat epigenome, that is heritable modifications to the genome that do not affect the DNA sequence itself, such as histone and DNA methylation. The global DNA methylome of polyploid wheat has been explored in multiple studies (Gardiner et al., 2015; Gardiner et al., 2018; Li et al., 2019). The methylome of the reference cultivar Chinese Spring was initially captured at the seedling stage (Gardiner et al., 2015), with more recent work focussing on the variation present in the seedling methylome of the 104 landraces from the Watkins core collection (Table 2; Gardiner et al., 2018). Researchers have also examined the changes in DNA methylation status as a result of biotic stress in wheat seedlings (Geng et al., 2019). The raw bisulfite sequencing data from these experiments is available through public archives, however it is not immediately accessible on genome browsers. More recently, new epigenomic data from Chinese Spring seedlings has been released which includes a wide variety of epigenetic marks such as DNA methylation, seven histone modifications, and chromatin accessibility (Li et al., 2019). This data has been made publicly available through a bespoke genome browser (http://bioinfo.sibs.ac.cn/cs_epigenome) and can be readily accessed by researchers to gain insight into the epigenomic landscape surrounding their genes of interest.
Table 2. Natural variation resources available in wheat.
| Collection | Short description | Number of accessions | Genotyping | Data/seed availability | More information/Reference |
|---|---|---|---|---|---|
| Wild wheat relatives and progenitor species | |||||
| Seeds of Discovery | Wheat and wild relative accessions held by ICARDA and CIMMYT | 80,000 accessions: 56,342 domesticated hexaploid (eight taxa); 18,946 domesticated tetraploid (eight taxa); 3,903 crop wild relatives included all known 27 wild species from Aegilops-Triticum species complex and 11 genomic constitutions. | DArT-seq |
CIMMYT Dataverse
http://hdl.handle.net/11529/10548030
Germinate data warehouse http://germinate.cimmyt.org/wheat. Records for all germplasm accessions can also be accessed at https://ssl.fao.org/glis/ |
https://seedsofdiscovery.org/ |
| Open Wild Wheat | Accessions of Aegilops tauschii (D genome progenitor) | 265 accessions | Whole genome shotgun sequenced (10-30x) | Sequencing: https://opendata.earlham.ac.uk/wheat/under_license/toronto/; Seed: https://www.seedstor.ac.uk/search-browseaccessions.php?idCollection=38 | www.openwildwheat.org; Arora et al., 2019 |
| Wild wheat introgression lines | Introgression lines from Aegilops caudata, Aegilops speltoides, Amblyopyrum muticum, Thinopyrum bessarabicum, Thinopyrum elongatum, Thinopyrum intermedium, Thinopyrum ponticum, Triticum timopheevii, Triticum urartu, rye and wheat cultivars (Chinese Spring, Higbury, Paragon, Pavon 76) | 153 stable homozygous introgression lines available | 35K Axiom Wheat Relative Genotyping array + 710 KASP markers (Grewal et al., 2020) | Genotype: https://www.nottingham.ac.uk/wrc/germplasm-resources/genotyping.aspx; Seed: https://www.seedstor.ac.uk/ (accessions WR0001-WR0155) | www.nottingham.ac.uk/WISP; Grewal et al., 2018a; Grewal et al., 2018b, King et al., 2018, King et al., 2017 |
| Synthetic hexaploid wheat | |||||
| Synthetic hexaploid wheat | Sythetic hexaploid wheats generated using Aegilops tauschii (DD) + European tetraploid (AABB) wheat | 50 synthetic hexaploid wheats + pre-breeding accessions; backcross populations with Robigus and Paragon also available | 35K Axiom breeders array |
Genotype: https://www.cerealsdb.uk.net/cerealgenomics/CerealsDB/axiom_download.php
Seed: https://www.seedstor.ac.uk/ (store codes WS0001-WS0232) |
https://www.niab.com/research/research-projects/designing-future-wheat |
| Wheat diversity panels | |||||
| Watkins historic collection of landrace wheats | World collection of wheat landraces grown as farmer saved seed before the 1930s. Genetically stable collection developed by two generations of single seed descent | 829 accessions (core set of 119 represent majority of assayed genotypic variation). F4:5 mapping populations against Paragon, mainly for the core set. | 35K Axiom breeders array (Allen et al., 2017); subset exome sequenced (Gardiner et al., 2018) |
Genotype: https://www.cerealsdb.uk.net/cerealgenomics/CerealsDB/axiom_download.php
Seed: https://www.seedstor.ac.uk/ (store codes WATDE0001-WATDE1063) |
http://wisplandracepillar.jic
.ac.uk/results_resources.htm ; Wingen et al., 2014; Wingen et al., 2017 |
| GEDIFLUX (Genetic Diversity Flux) collection | Western European winter wheat varieties that individually occupied over 5% of national acreage from 1945 to 2000. Bi-parental populations with Paragon (ongoing) | 479 accessions | 35K Axiom breeders array |
Genotype: https://www.cerealsdb.uk.net/cerealgenomics/CerealsDB/axiom_download.php; Seed: https://www.seedstor.ac.uk/ (store codes WGED0001- WGED0729) |
http://wisplandracepillar.jic.ac.uk/results_resources.htm; Wingen et al., 2014 |
| NIAB wheat association mapping panel | Bread wheat varieties released between 1916–2007. Predominantly UK varieties (68%), also other North Western European countries e.g. France (10%) and Germany (8%) | 480 accessions | 90K SNP array |
Seed, Genotype and Pedigree: https://www.niab.com/research/research-projects/resources |
Fradgley et al., 2019 |
| OzWheat diversity panel | Genetic diversity in Australian wheat breeding (colonial landraces 1860s, first Australian-bred cultivars 1890s, CIMMYT-derived semi dwarfs 1960s, post 2000 wheat) | 285 accessions | 90K SNP array + additional 26K SNPs from transcriptome data |
Seed and Genotype: contact Shannon Dillon from CSIRO (Shannon.Dillon@csiro.au) |
|
| Vavilov wheat collection | Hexaploid wheat accessions including landraces, historic breeding lines and cultivars. Pure lines generated by single seed descent | 295 accessions | DArtT-seq (34,311 polymorphic markers) |
Genotype: Lee Hickey at The University of Queensland (l.hickey@uq.edu.au) Seed: Australian Grains Genebank (sally.norton@ecodev.vic.gov.au) |
Riaz et al., 2017 |
| WHEALBI wheat panel | Worldwide wheat accessions including diploid and tetraploid wild relatives, old hexaploid landraces and modern elite cultivars | 487 accessions | Exome capture (~600,000 genetic variants in ~40,000 genes; 12,000 genes identified as putative presence/absence variation compared to RefSeqv1.0) |
Genotype:https://urgi.versailles.inra.fr/download/iwgsc/IWGSC_RefSeq_Annotations/v1.0/iwgsc_refseqv1.0_Whealbi_GWAS.zip; Seed: https://www.gbif.org/dataset/a52ca10a-136a-4072-a6de-3ec6e7852365 |
Pont et al., 2019 |
| Global Durum Wheat (GDP) panel | Diversity used in durum wheat breeding programs globally, including landraces and modern varieties | 1,056 accessions | 90K SNP array |
Genotype: ms in preparation; Seed: ICARDA genebank http://indms.icarda.org
Filippo Bassi, F.Bassi@cgiar.org |
|
| Tetraploid wheat Global Collection (TGC) | Wild emmer wheat, domesticated emmer, durum wheat landraces and other tetraploid wheat sub-species (Triticum aethiopicum, Triticum carthlicum, Triticum polonicum, Triticum turanicum, Triticum turgidum, Triticum karamyschevii and Triticum petropavlovsky) | 1,856 accessions | 90K SNP array | Genotype: GrainGenes; Seed: on request for non-commercial use from University of Bologna (marco.maccaferri@unibo.it and roberto.tuberosa@unibo.it) | Maccaferri et al., 2019 |
| MAGIC populations | |||||
| CSIRO, Aus | 4-way (parents Baxter, Chara, Westonia, Yitpi); 8-way (parents Baxter, Westonia, Yitpi, AC Barrie (Canada), Xiaoya54 (China), Volcani (Israel), Pastor (Mexico), Alsen (USA)) | 1,500 (4-way) and 3,000 (8-way) RILs | 90K SNP array, microsatellite and DArT markers > 20,000 SNPs mapped in each population | Seed and Genotype: on request from CSIRO (Bill.Bovill@csiro.au) | Huang et al., 2012; Shah et al., 2019 |
| NIAB, UK | 8-way (parents Alchemy, Brompton, Claire, Hereward, Rialto, Robigus, Xi19, Soissions); 16-way (Banco, Bersee, Brigadier, Copain, Cordiale, Flamingo, Gladiator, Holdfast, Kloka, Maris Fundin, Robigus, Slejpner, Soissons, Spark, Steadfast, Stetson) | NIAB 8-way MAGIC:>1,000 RILs; NIAB 16-way MAGIC: ~600 RILs | 35K Axiom breeders array. Genome sequence (Claire, Robigus, others underway). Exome capture sequence of 16-way parents. Skim-seq of all RILs underway. | Claire and Robigus genomes: https://opendata.earlham.ac.uk/opendata/data/Triticum_aestivum/EI/v1.1/ Genotyping and Seed: https://www.niab.com/research/research-projects/resources | Mackay et al., 2014; Gardner et al., 2016 |
| Germany | 8-way (Event, Format, BAYP4535, Potenzial, Ambition, Bussard, Firl3565, Julius) | 394 F6:8 RILs | 5,435 SNPs from SNP array | Genotype and pedigree: http://doi.org/10.14459/2018mp1435172 (click the ‘open attachment browser’ link); Seed: Bavarian State Research Centre for Agriculture (Freising, Germany) | Stadlmeier et al., 2018 |
| Germany | WM-800, 8-way (Patras, Meister, Linus, JB Asano, Tobak, Bernstein, Safari, Julius) | 910 F4:6 RILs | 15K Infinium iSelect SNP array | Genotype and pedigree: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6069784; Seed: on request from Martin Luther University, Germany (klaus.pillen@landw.uni-halle.de) | Sannemann et al., 2018 |
| Durum | 4-way (Claudio (Italy), Colosseo (Italy), Neodur (France), Rascon/2*Tarro (advanced CIMMYT line)) | 334 F7:8 RILs | 90K SNP array | Genotype and pedigree: https://onlinelibrary.wiley.com/doi/full/10.1111/pbi.12424; Seed: on request for non-commercial use from University of Bologna (marco.maccaferri@unibo.it and roberto.tuberosa@unibo.it) | Milner et al., 2016 |
Functional studies
After identifying a gene of interest there are now several options and resources available for functional characterisation and validation in wheat (Figure 2). These include resources based both on natural and induced variation and can involve both transgenic and non-transgenic approaches. It is important to remember that due to the polyploid nature of wheat, there is often functional redundancy between homoeologs (Borrill et al., 2015). This means that it may be necessary to manipulate all homoeologs and paralogs simultaneously to measure a strong phenotypic effect (see the ‘Strategies for Use’ section below for more information).
Induced variation
TILLING
Polyploid species, such as wheat, are well suited to mutational approaches as the functional redundancy in their genomes allows for the tolerance of a higher mutational load compared with diploid species (Tsai et al., 2013; Uauy et al., 2017). Bespoke mutant populations can be developed and screened for desired mutations in a gene of interest, though the screening process is arduous and time-consuming. To overcome this barrier, an in silico wheat TILLING resource has been developed (Krasileva et al., 2017). This resource consists of two ethyl methanesulphonate (EMS) mutagenised populations: 1,535 lines of the tetraploid durum wheat variety ‘Kronos’ and 1,200 lines of the hexaploid bread wheat variety ‘Cadenza’. Exome capture and Illumina sequencing of these 2,735 mutant lines was then carried out. The raw data was originally aligned to the CSS reference, mutations were identified, and their effects predicted based on the CSS gene models (Krasileva et al., 2017). Alleles predicted in silico to be deleterious (e.g. premature stop codons, splice site mutations, non-synonymous amino acid substitutions with SIFT score <0.05), were identified for ~90% of the captured wheat genes (Krasileva et al., 2017), thus making this a powerful resource for rapidly identifying mutations in a gene of interest (Figure 2). The raw data has now been aligned to the RefSeqv1.0 genome, allowing mutation identification and effect prediction based on the RefSeqv1.1 gene models. These updated data are publicly available on Ensembl Plants (see Case Study for details). For legacy purposes, the mutations called against the CSS reference remain available via www.wheat-tilling.com. However, caution should be exercised as the mutation effects here are predicted based on the CSS gene models, which are known to be less reliable than the RefSeq gene models (Brinton et al., 2018).
There are several important considerations when selecting a mutant line for characterisation. First, it is essential to check the predicted effect of mutations in the context of a complete and experimentally validated gene model. Second, in most cases, crossing is necessary to combine mutations in homoeologous genes in order to generate a complete null individual. Third, mutant lines will contain a high level of background mutations: a typical mutant line has between 50 (tetraploid) and 110 (hexaploid) mutations predicted to result in a truncated protein. Depending on the phenotype of interest (i.e. qualitative vs. quantitative) several rounds of backcrossing may be required before the phenotype can be assessed (see ‘Strategies for Use’). Lastly, if the gene of interest is missing or is already a null allele in Kronos or Cadenza (which can be determined using the full genome sequences of the two cultivars), mutant populations of other genotypes are available (e.g. Dong et al., 2009; Chen et al., 2012; Bovina et al., 2014; Sestili et al., 2015; Colasuonno et al., 2016), although these would need to be screened using conventional PCR-based approaches. Additional practical information about selecting mutant lines and downstream analyses can be found at www.wheat-training.com/functional-studies and in Uauy et al. (2017).
Transgenic approaches
Stable transformation of wheat is possible and can be performed using a variety of methods including both particle bombardment (Vasil et al., 1992; Sparks and Jones, 2009) and Agrobacterium-mediated transformation (Cheng et al., 1997; Sparks et al., 2014). Generating stable transgenic lines in wheat most commonly involves transforming immature wheat embryos and subsequent callus regeneration (Harwood, 2012). Reports in the literature of Agrobacterium-mediated wheat transformation generally describe low transformation efficiencies with average efficiencies of around 5%. An efficient, but patented, transformation system is available through licence from Japan Tobacco (www.jti.co.jp). Transformation by overexpression of transcription factors such as maize Baby Boom and Wuschel2 has also yielded improved transformation efficiencies in monocots (Lowe et al., 2016), although there are no formal reports yet in wheat. Recently, an open-access wheat transformation system with transformation efficiencies of up to 25% was published (Hayta et al., 2019), albeit for a single cultivar.
Using transgenic approaches, gene expression can be altered in a variety of ways such as overexpressing or ectopically expressing the gene of interest using either constitutive, tissue-specific or inducible promoters (Hensel et al., 2011). Similarly, RNA-interference (RNAi) has been used successfully in wheat to reduce gene expression with the added benefit that constructs can be designed to target all homoeologous genes simultaneously, thereby overcoming the potential drawback of functional redundancy among homoeologs (Fu et al., 2007). In addition to altering expression patterns, modified proteins can also be introduced (e.g. including tags) for downstream experiments such as ChIP-Seq (Deng et al., 2015) or localisation studies (Harwood et al., 2005). However, these are still not commonly employed in wheat research. As transformation methods have only been optimised for a limited number of wheat varieties (e.g. Richardson et al., 2014), it is important to understand whether the gene is expressed/functional in the chosen variety when defining transgenic strategies (see ‘Strategies for Use’).
Recent developments in genome editing technologies provide new opportunities for manipulating genes in wheat. TALEN and CRISPR/Cas9-mediated genome editing has been successfully demonstrated in wheat both in transient expression systems (Shan et al., 2014) and stably transformed plants (Wang et al., 2014b; Luo et al., 2019), using a range of methods reviewed in Uauy et al. (2017). Currently, most studies have introduced specific point mutations or small deletions leading to subsequent protein disruption, although the technology holds the potential for complex applications such as allele swapping or gene insertion, as reviewed by Puchta (2017). Similar to RNAi, constructs for Cas9-mediated gene editing can be designed to target all homoeologs simultaneously (Zhang et al., 2016; Howells et al., 2018). Due to the current efficiency of genome editing however, the likelihood of obtaining mutations in all homoeologs in a single T0 plant remains low (0.9%; Zhang et al., 2016) and subsequent crosses to combine multiple edited targets are likely to be required.
A major limitation of using transgenic approaches to manipulate agronomically relevant traits is the associated legal and regulatory constraints. To overcome these, the nuclease transgene can be segregated away from the edited gene(s) in subsequent generations. However, in Europe, and in contrast to many other countries in the world, the resulting plants would be regulated as transgenics due to the 2018 ruling on genome editing by the European Court of Justice (ECJ). Some studies have documented CRISPR/Cas9-editing in wheat without transgene integration, for example, by delivering the CRISPR/Cas9 components as ribonucleoproteins (RNPs). As no foreign DNA is used in CRISPR/Cas9 RNP-mediated genome editing, the wheat mutants obtained are completely transgene free (Liang et al., 2017), although still not exempt from the ECJ regulation.
Virus-Induced Gene Silencing
Virus-Induced Gene Silencing (VIGS) involves transient knock-down of expression of target genes followed by assessment of the resulting phenotype (Lee et al., 2012). The most widely used vectors for VIGS in wheat are those derived from barley stripe mosaic virus (BSMV), a plant virus with a tripartite RNA genome that readily spreads throughout tissues following mechanical rub-inoculation onto the leaves. All three BSMV genomic RNAs, RNAα, RNAβ and RNAγ, are required to cause infection. RNAγ has been modified to allow insertion of short (up to 350 bp) plant mRNA derived sequences. Infection of plants with the resulting recombinant virus induces a natural post-transcriptional gene silencing defence mechanism that targets the viral RNA, but also the endogenous plant mRNA having high level (>70%) nucleotide identity with the plant sequence inserted into RNAγ, for degradation. A detailed protocol for VIGS is available at www.wheat-training.com (Figure 2).
VIGS in wheat has been used primarily to investigate disease resistance in a range of varieties, and has been restricted to a few tissue types such as leaf (Lee et al., 2015), young seedlings (Zhang et al., 2017a) and spikes (Ma et al., 2012). However, in principle, BSMV-mediated VIGS can be applied to any wheat genotype and to almost any gene of interest. This functional genomics tool is particularly useful when analysing multiple candidate genes, for example in map-based cloning projects (i.e. when physical intervals contain several candidate genes), or from RNA-Seq differentially expressed datasets. VIGS is also useful in wheat genotypes that are difficult to transform and in those for which mutant/TILLING populations are unavailable. VIGS can be used for simultaneous silencing of all homoeologs or, in principle, entire small gene families without the need for further genetic crosses.
Natural Variation
Although using induced variation presents a clear route to understand the function of specific genes in wheat, the wealth of natural variation in wheat lines, and populations based on this variation, presents an alternative route to discover genes and correlate them with function. For example, populations differing for alleles of the gene of interest could be used to rapidly infer the role of the gene. In order to capture the diversity within wheat and create populations to test gene function, natural variation has been extensively documented. Most studies have focused on SNPs between varieties that can be quickly assayed through SNP arrays designed from gene coding sequences and untranslated regions (UTRs) (Wang et al., 2014a; Winfield et al., 2016; Allen et al., 2017), described in Borrill et al. (2015) and www.wheat-training.com. Thousands of varieties and landraces have been processed using these arrays and datasets are available through websites such as TCAP (https://triticeaetoolbox.org/wheat) (Blake et al., 2016) and CerealsDB (http://www.cerealsdb.uk.net/cerealgenomics/CerealsDB) (Wilkinson et al., 2016). Given that all SNPs from the latter have been incorporated into Ensembl Plants, this means that large in silico allelic series are readily available for many genes of interest.
Beyond SNP variation, two recent studies (He et al., 2019; Pont et al., 2019) applied exome capture to diverse wheat lines to characterise the natural variation throughout the coding regions of wheat. These studies identified millions of SNPs within coding sequences in over 1,000 wheat lines, including hexaploid cultivars and landraces, and tetraploid and diploid relatives. The data (available at http://wheatgenomics.plantpath.ksu.edu/1000EC and https://urgi.versailles.inra.fr) will allow rapid characterisation of the extent of variation within genes of interest. These changes in coding sequences may have direct phenotypic consequences, however the impact of most of these variants remains unknown.
Therefore, despite this wealth of data, the challenge remains to define the functional significance of this variation. Traditionally, mapping populations or association panels would need to be developed or assembled, and then genotyped, to assess how particular SNPs or haplotypes affect the trait of interest. In wheat, many of these resources are now publicly available (Figure 2), thus facilitating the functional characterisation of genes of interest. We describe some of these resources below and include links to access genotypes, sequences and seeds in Table 2. Further details are available at www.wheat-training.com.
Wild wheat relatives and progenitor species
There is relatively low genetic variation in elite bread wheat varieties, especially on the D genome. This typically reflects adaptation and selection from landraces over a long time period, combined with the genetic bottleneck effects associated with the rare natural hybridisation events between the diploid and tetraploid ancestral wheat species that led to the evolution of hexaploid wheat. Wheat is related to several other grass species, many of which are wild and uncultivated. These wild relatives provide a vast and largely untapped reservoir of genetic variation for many agronomically important traits. A wealth of cytogenetic stocks for these wild relatives have been created over the last 100 years by researchers globally, reviewed by Mujeeb-Kazi et al. (2013). The recent genotyping and sequencing of some of these resources makes them especially suitable for gene functional characterisation (Table 2).
Synthetic hexaploid wheat
Another approach to capture variation in wheat progenitors is via ‘re-synthesis’, the process used to create synthetic hexaploid wheat (SHW). SHWs are typically created by crossing tetraploid durum wheat with the diploid D-genome progenitor Aegilops tauschii. Approximately 400 SHWs were developed at CIMMYT in Mexico during the 1990s (Mujeeb-Kazi et al., 1996) and these have been extensively utilised in CIMMYT and international wheat breeding programmes (e.g. Gororo et al., 2002; Ogbonnaya et al., 2007). More recently, NIAB (UK) have developed a new SHW resource encompassing 50 SHWs along with pre-breeding derivatives. This germplasm, alongside marker data, is publicly available (Table 2).
Wheat diversity panels
Numerous collections of wheat landraces, varieties and breeders’ lines are available from research centres around the world. These panels represent valuable sources of potential genetic variation for targeted exploitation within wheat research and pre-breeding pipelines, especially when associated with existing genotypic and phenotypic datasets (Table 2). Further details are available at www.wheat-training.com.
Multiparent Advanced Generation Inter-Cross (MAGIC) populations
MAGIC populations have been developed for many crop species (Huang et al., 2015; Cockram and Mackay, 2018). The multiple generations of inter-crossing required to create MAGIC populations results in highly recombined chromosomes, which enables the use of approaches such as genome wide association scans (GWAS) and whole-genome average interval mapping (WGAIM; Verbyla et al., 2007) to define small genetic intervals for traits of interest as reviewed by Verbyla et al. (2014). Likewise, the use of multiple parents allows more allelic variation to be examined compared to typical bi-parental populations (Cockram and Mackay, 2018). In wheat, seven MAGIC populations are currently publicly available which are constructed from 4, 8 or 16 founders. Parent information and further details can be found in Table 2.
Combining induced and natural variation for a holistic picture of gene function
To date, natural variation has largely been used for forward genetics approaches such as mapping genetic regions underlying a phenotypic trait of interest. However, there is now an opportunity to apply natural variation in wheat for reverse genetics studies to complement transgenic, gene editing and induced variation approaches. For example, the pre-harvest sprouting locus Phs-A1 was reported by two independent studies to be underpinned by different genes: in one case by a pair of tandem duplicated Plasma Membrane 19 (PM19-A1 and PM19-A2) genes (Barrero et al., 2015), and in the other by a mitogen-activated protein kinase kinase 3 (TaMKK3-A) gene (Torada et al., 2016). Transgenic approaches seemed to validate the role of both PM19 and TaMKK3-A to influence pre-harvest sprouting. However, by using eleven bi-parental populations and a MAGIC population segregating for the Phs-A1 locus, it was possible to break the linkage with the polymorphism in PM19 and confirm that the causal gene in all populations was TaMKK3-A (Shorinola et al., 2017). This example illustrates the power of natural variation to validate the causal variants underpinning phenotypes in wheat.
Populations exploiting natural variation can also be used to validate gene function. For example, TEOSINTE BRANCHED1 (TB1) was identified as a regulator of wheat spike architecture using a 4-parent Australian MAGIC population, and this function was confirmed using induced variation (TILLING and transgenic overexpression) and natural variation in the 8-parent UK MAGIC population (Dixon et al., 2018). Interestingly, whilst TB1 was important in both MAGIC populations, different homoeologs underpinned the variation: TB1-D1 in the Australian population and TB1-B1 in the UK population. This study suggests that by using natural variation, we can start to understand the nuanced regulation of phenotypes in wheat elicited by individual homoeologs. Together, these examples show that researchers now have at their disposal a powerful toolkit to combine induced and natural variation to study gene function in wheat.
Moving towards a wheat pangenome
Increases in DNA sequencing outputs and related technologies have allowed the assembly of chromosome scale assemblies for multiple cultivars in major crops such as maize (https://nam-genomes.org/), rice (Zhou et al., 2019) or oilseed rape (Song et al., 2020). For wheat, 16 hexaploid (eight with spring habit, and eight with winter habit), and three tetraploid varieties/accessions have been assembled, several to a similar standard as the reference Chinese Spring genome (Table 3). Annotation of most of these varieties is ongoing through the 10+ Wheat Genomes Project (http://www.10wheatgenomes.com) and will provide information on the core (genes shared by all assembled varieties) and dispensable genes (genes shared among a few varieties). In addition, presence absence variation, copy number variation, structural rearrangements (inversions/translocations), and variation across non-coding regions are being quantified. Importantly, several of these genotypes are part of the resources outlined above, for example sequenced TILLING populations (Kronos and Cadenza). These assemblies will be integrated into Ensembl Plants and are available for download under Toronto Agreement (https://wheat.ipk-gatersleben.de/).
Table 3. Tetraploid and hexaploid wheat genome assemblies that are currently available, in addition to the Chinese Spring reference hexaploid genome.
| Variety | Habit | Origin | Availability † |
|---|---|---|---|
| Hexaploid wheat | |||
| CDC Landmark | spring | Canada | 10+ Genome Project |
| CDC Stanley | spring | Canada | 10+ Genome Project |
| Paragon | spring | UK | 10+ Genome Project |
| Cadenza | spring | UK | 10+ Genome Project |
| LongReach Lancer | spring | Australia | 10+ Genome Project |
| Mace | spring | Australia | 10+ Genome Project |
| Synthetic W7984 | spring | Mexico | Chapman et al. (2015) |
| Weebill | spring | Mexico | 10+ Genome Project |
| ArinaLrFor | winter | Switzerland | 10+ Genome Project |
| Julius | winter | Germany | 10+ Genome Project |
| Jagger | winter | US | 10+ Genome Project |
| Robigus | winter | UK | 10+ Genome Project |
| Claire | winter | UK | 10+ Genome Project |
| Norin61 | winter | Japan | 10+ Genome Project |
| SY Mattis | winter | France | 10+ Genome Project |
| Spelt (PI190962) | winter | Europe | 10+ Genome Project |
| Tetraploid wheat | |||
| Zavitan* | - | Israel | Avni et al. (2017) |
| Svevo | spring | Italy | Maccaferri et al., 2019 |
| Kronos | spring | US | 10+ Genome Project |
*‘Zavitan’ is a tetraploid wild emmer (T. dicoccoides) accession.
†Varieties included within the 10+ Wheat Genomes Project can be accessed through the Earlham Grassroot Genomics portal (https://wheatis.tgac.ac.uk/grassroots-portal/blast) and the 10+ Wheat Genomes project portal (http://webblast.ipk-gatersleben.de/wheat_ten_genomes) (subset of varieties in each). The ‘Svevo’ genome can be accessed through https://www.interomics.eu/durum-wheat-genome and Ensembl Plants. ‘Synthetic W7984’ and ‘Zavitan’ can be accessed through the Grassroot Genomics, and Ensembl Plants, respectively.
Strategies for use
Variety selection and growth conditions
Whilst resources are now available for the functional validation of target genes in wheat, practical knowledge is also required to maximise the value of these resources. Firstly, wheat varieties are adapted to different growing conditions (e.g. daylength and vernalisation requirements) making it important to consider the conditions under which functional validation will be conducted. If phenotyping will be undertaken in greenhouse or controlled environment conditions then most varieties will be suitable, although varieties without vernalisation requirements are faster to grow (details on wheat growth conditions at www.wheat-training.com). If field trials are required for phenotypic characterisation (e.g. yield-related traits), local adaptation is often necessary for correct interpretation of results given genotype x environment interactions. For example, the sequenced TILLING populations (Kronos and Cadenza) do not require vernalisation, facilitating greenhouse experiments, and originate from different regions of the world, allowing field trials under different environments (Kronos is a Californian variety adapted to warm dry weather whereas Cadenza is a UK variety adapted to cooler conditions).
For CRISPR/Cas9 and other non-transient transgenic approaches several varieties may be used, although relatively few wheat varieties have been shown to display high enough transformation efficiencies to be practical. This means that traditionally most transgenic studies in wheat have been limited to a few varieties, such as ‘Fielder’, Cadenza, ‘Bobwhite’, ‘Kenong 199’ and Kronos (Li et al., 2012; Richardson et al., 2014; Liang et al., 2017; Hayta et al., 2019). This is now changing thanks to work by groups at NIAB (UK), CAAS (China) and CSIRO (Australia) who have successfully transformed 39 (Wallington, 2015), 15 (Wang et al., 2017) and six (Richardson et al., 2014) varieties, respectively. However, the Agrobacterium-mediated transformation efficiencies in all these studies still differ between varieties. Correct varietal selection for transformation is critical for functional studies, given that some varieties might not be suitable to study a particular phenotype (e.g. if the variety is resistant to a disease and hence cannot be used to test a candidate resistance gene). Similarly, it is important to assess whether the gene of interest is present/functional in the chosen variety, for example through PCR amplification and sequencing of the gene. For several varieties this can now be done quickly by direct examination of their genome sequence (Table 3).
Combining mutations for complete knock-outs in polyploid wheat
As we noted earlier, the polyploid nature of wheat means that it normally has multiple homoeologous copies of every gene. These copies typically have highly similar coding DNA sequence and may have redundant functions (Borrill et al., 2015). Therefore, to characterise the function of a gene in wheat it is often necessary to knock out all three homoeologs. This may be achieved by simultaneously targeting all three copies using either RNAi (e.g. Uauy et al., 2006) or CRISPR/Cas9 (e.g. Zhang et al., 2017b). A large number of transformants need to be screened to identify a null in all three genomes from a CRISPR construct (Zhang et al., 2017b; Howells et al., 2018). If the targets are more divergent it may not even be possible to use a single guide RNA to target all three homoeologs, in which case several guides may be used through multiplexing. Alternatively, separate knock-outs for each homoeolog can be generated by CRISPR/Cas9 or identified in TILLING populations. The mutations in each homoeolog can be combined by crossing (for details see www.wheat-training.com), with two crosses necessary to combine knock-out mutations in each of the three homoeologs in hexaploid wheat (Figure 4). Tetraploid wheat, with only two homoeologs, can be used to accelerate functional characterisation as it requires just one cross to create complete knock-out mutants (Figure 4). After self-pollination of this F1, phenotyping of the trait of interest can be initiated in the F2 generation by comparing homozygous double knock-out mutants to the sibling wild type plants. It is important to note that TILLING lines contain many background mutations and backcrossing may be required to overcome the confounding effects of background mutations on target phenotype. More details on these strategies are published in Uauy et al. (2017).
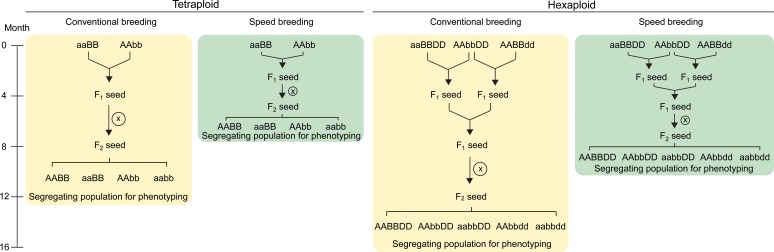
Figure 4. Crossing scheme to combine TILLING or CRISPR/Cas9 single mutants in wheat.
In tetraploid wheat, mutations in the A and B genome homoeologs can be combined through a single cross. The F1 plants are self-pollinated to produce a segregating F2 population which contains homozygous double and single mutants, as well as wild type plants (screening using molecular markers required; only four genotypes shown). These F2 progeny can be characterised for the phenotype of interest. The use of ‘speed breeding’ (Watson et al., 2018), reduces the time taken to reach this phenotyping stage from 12 (yellow) to 7.5 months (green). In hexaploid wheat, a second round of crossing is required to combine the mutant alleles from all three homoeologs. The F2 progeny segregating for the three mutant alleles can be genotyped using molecular markers to select the required combination of mutant alleles (only five genotypes shown; all factorial combinations are possible). Speed breeding reduces the time taken to generate triple homozygous mutants for phenotyping to 10 months (green), compared to 16 months in conventional conditions (yellow). Self-pollination is represented by an X inside a circle. Combinations of wild type alleles from the A (AA), B (BB) and D (DD) genomes, as well as the mutant alleles from each genome (aa, bb and dd, respectively) are indicated.
Accelerating crossing, generation time, and phenotyping
The need to combine multiple mutations/alleles and carry out backcrossing to remove background mutations takes a considerable amount of time, with at least four months required per generation in a spring wheat genetic background. Recently, the ‘speed breeding’ technique has been implemented in wheat (and other crops such as barley, canola and chickpea), which uses extended day lengths of 22 hr and improved light quality to accelerate the generation time in wheat (Ghosh et al., 2018; Watson et al., 2018). Reduction of generation times to 8–10 weeks is achieved through an accelerated growth rate and harvesting of immature seeds 2–3 weeks post anthesis. The immature seeds are dried and then imbibed in the cold, resulting in nearly 100% germination. Incorporating speed breeding within crossing programmes can reduce the time required to produce and phenotype double mutants in tetraploid wheat to less than 7.5 months and triple mutants in hexaploid wheat to less than 10 months (Figure 4). In addition to reducing generation times, it has been shown that several traits of interest such as disease resistance, height and flowering time can be properly characterised under speed breeding conditions (Watson et al., 2018).
Homoeolog-specific PCR markers
To carry out the crossing schemes described above, it is essential to be able to select for the mutations of interest. In polyploid wheat it is necessary to track mutations in each homoeolog separately, which can be achieved using homoeolog-specific genetic markers. Primers can be designed to include a homoeolog-specific SNP at the 3’ end of the primer. The primer will amplify the targeted homoeolog more efficiently than the non-targeted homoeolog(s) resulting in genome-specific amplification. Rapid design of homoeolog-specific primers can be achieved using the PolyMarker pipeline (Ramirez-Gonzalez et al., 2015) and webserver (http://www.polymarker.info/). Routinely, genotyping of SNPs is carried out using Kompetitive Allele Specific PCR (KASP) markers which are relatively high throughput, inexpensive and can be used in individual lab settings equipped with PCR machines and widely available fluorescence plate readers (Allen et al., 2011). The SNP to be genotyped (e.g. between mutant and wild type) is located at the 3’ end of the two alternative allele-specific primers used in the KASP reaction (one for the mutant and one for the wild type allele), whilst the homoeolog-specific SNP is located at the 3’ end of the common primer. Amplification should thus be both homoeolog-specific and allele-specific. Further guidance on the design of genome-specific primers and KASP markers is available at www.wheat-training.com.
Case study
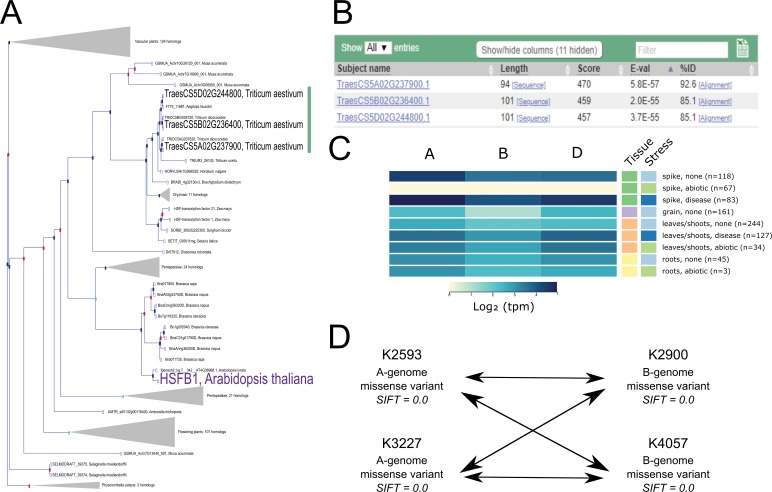
To put the previous resources into context, we present a case study for obtaining wheat mutants and expression data using a gene of interest from Arabidopsis thaliana. The heat shock factor-like transcription factor TBF1, also known as HsfB1, is a critical regulator of the plant growth-to-defence transition (Pajerowska-Mukhtar et al., 2012), and the response to heat stress (Guo et al., 2016). We therefore hypothesise that its wheat orthologs may have a similar role in regulating defence and/or abiotic stress responses (Ikeda et al., 2011). The first step to test this hypothesis is to identify wheat TBF1 orthologs, which can be done using the Ensembl Plants Gene Tree (Bolser et al., 2015), which displays predicted orthologs for all species included in Ensembl Plants. TBF1 is one of five HSFB orthologs, named HSFB1, 2A, 2B, 4, and 5, respectively. Examination of the Ensembl Plants Gene Tree shows a single wheat triad that falls within the HSFB1 clade, located on the group five chromosomes (Figure 5A). It is important to note that most gene models were annotated in an automated manner and hence gene structures are likely to contain some errors, pending manual curation. We would thus recommend that researchers manually inspect the annotation of their genes of interest before proceeding further with their analyses.
Figure 5. Case study exemplifying use of available resources for gene functional characterisation in wheat.
(A). The Ensembl Plants Gene Tree illustrates the identification of the wheat triad (green bar) most closely related to AtHSFB1 (shown in purple). (B) Using Os09g0456800 (the rice ortholog of AtHSFB1) as a BLASTp query against wheat predicted proteins independently identifies the same wheat triad. (C) Examination of RNA expression data from www.wheat-expression.com shows that the wheat triad is most highly expressed in the spike, with differential expression in abiotic and disease stress conditions. The samples are identified by tissue of origin (spike, green; grain, purple; leaves/shoots, orange; roots, yellow) and stress (none, light blue; abiotic, green; disease, dark blue) as they are on the website. (D) After identification of suitable wheat TILLING mutants, A and B genome homoeologs are combined via this example crossing scheme, demonstrating the four crosses required between the two selected mutations in each homoeolog. Note that the functional validation proposed in (D) is carried out using the tetraploid mutant population.
To support the predicted Arabidopsis-wheat orthologs obtained from Ensembl Plants, we recommend carrying out comparisons between wheat and rice to establish orthology between these cereal species. Both the wheat homoeologs and the rice gene model Os09g0456800 have the same gene structure, consisting of two exons with a conserved intron/exon boundary position. To further support the relationship of the rice gene to the wheat homoeologs, the predicted rice protein can be used as a query for BLASTp analysis of the wheat proteome in Ensembl Plants; the expected wheat orthologs are the top three hits for the A, B, and D genomes (Figure 5B).
Having identified the wheat orthologs of Arabidopsis TBF1, we can examine and compare expression profiles using the expVIP browser (www.wheat-expression.com) (Borrill et al., 2016; Ramírez-González et al., 2018; Figure 5C). All three wheat homoeologs have similar expression profiles, with expression changes in the spike under disease and abiotic stress. This is consistent with the eFP browser data which shows high expression in the spikelet and awns of the non-stressed plants, as well as in more mature leaf tissues (Winter et al., 2007; Ramírez-González et al., 2018). The expression data suggests that the wheat TBF1 homoeologs are most strongly expressed in the spike and may have differential expression in response to biotic and abiotic stress. We can also explore the epigenetic environment of the three homoeologs using the bread wheat epigenomic map (http://bioinfo.sibs.ac.cn/cs_epigenome; Li et al., 2019). A large peak for the H3K9ac histone modification at the 5′ end of the homoeologs is indicative of active transcription from the promoter, corresponding with the observed gene expression. In contrast, the A-homoeolog TraesCS5A02G237900 is flanked by two genes which have low expression at the seedling stage, and correspondingly low levels of H3K9ac modifications in their promoters. It is worth noting that the epigenomic browser uses RefSeqv1.0 gene models, rather than the RefSeqv1.1 gene models used on Ensembl Plants.
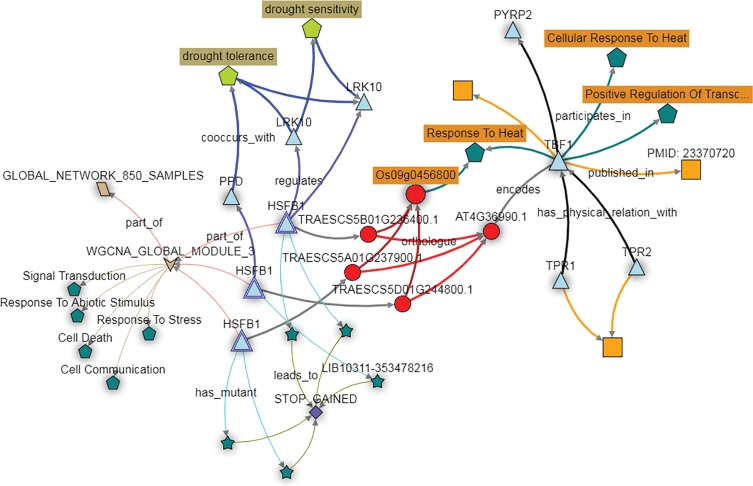
Further investigation of these homoeologs can be performed using the KnetMiner knowledge network. For wheat TBF1 orthologs, this includes homology, co-expression data, and associated TILLING mutants, combined with other wheat-specific information such as GENIE3 networks, wheat related publications, gene-phenotype relations extracted from the literature, GWAS data and Arabidopsis protein-protein interactions. Here the wheat genes, referred to as HSFB1, are orthologous to the Arabidopsis gene TBF1 as demonstrated earlier, and the three wheat homoeologs fall into a module associated with responses to abiotic stresses (Figure 6). In addition, the HSFB1 B and D homoeologs are predicted in the GENIE3 network to target the LRK10 and PPD genes, which have known links to drought tolerance and sensitivity (Figure 6). The KnetMiner database also recapitulates the relationship between the wheat HSFB1 homoeologs and their rice and Arabidopsis orthologs, which regulate heat stress responses (Figure 6). Considered as a whole, these data support the hypothesis that the HSFB1 wheat genes are involved in the response to abiotic stress, perhaps specifically in drought response.
Figure 6. The KnetMiner network illustrates the putative role of the wheat TBF1 orthologs in responding to abiotic stress.
The wheat orthologs of the Arabidopsis gene TBF1, here depicted as three copies of the gene HSFB1 (light blue triangles) fall in expression module three (brown arrow; WGCNA module 3). The genes in this module are enriched for GO terms such as ‘Response to Stress’ and ‘Response to Abiotic Stimulus’ (dark green pentagons). The HFSB1 homoeologs are predicted to regulate other genes (blue triangles) in the GENIE3 network (purple connecting arrows) which are associated with the drought tolerance trait ontology terms (light green pentagon). PTC mutations are available for all three HFSB1 homoeologs (dark green stars connecting with STOP GAINED SNP effect) in the Cadenza population.
After evaluating in silico expression levels, we can then characterise the phenotype of wheat TBF1 mutants using the exome-sequenced wheat TILLING mutant populations (Figure 2). We suggest to initially use the Kronos population, as it is based on a tetraploid line and thus contains only two copies of the gene (A and B homoeologs). This means that only two mutants need to be crossed to generate a full knockout. The hexaploid Cadenza TILLING population could also be used, but this would require an additional generation to combine mutant alleles across all three homoeologs (Figure 4).
All TILLING mutations identified against the more recent RefSeqv1.0 genome can be accessed directly from Ensembl Plants in the ‘Genetic Variation’ section. Available mutations in the gene of interest can be visualised as a table or positioned along the gene using the ‘Variant Image’ or ‘Variant Table’ option. We can thus rapidly identify mutations that are predicted to lead to a premature termination codon (PTC). However, if no appropriate PTC mutations are available, splice-site mutations predicted to lead to downstream frameshifts, or missense mutations in highly conserved amino acid residues with low SIFT (Sorting Intolerant from Tolerant; Ng and Henikoff, 2003) scores are good alternatives. SIFT scores predict the effect of a mutation on protein function and are based on the physical properties of the alternative amino acid as well as sequence homology.
For both the A and the B genome TBF1 homoeologs in Kronos, no PTC mutations are available. However, we identified missense mutations in highly conserved residues with SIFT scores of 0 suggesting that these mutations are likely to have a deleterious effect on protein function (Figure 5D). In addition to SIFT, we also recommend using the PSSM viewer (https://www.ncbi.nlm.nih.gov/Class/Structure/pssm/pssm_viewer.cgi) to help predict the effect of specific missense mutations on conserved protein domains.
TILLING lines from both populations can be ordered via the GRU (https://www.seedstor.ac.uk/shopping-cart-tilling.php) in the UK or from the Dubcovsky lab (https://dubcovskylab.ucdavis.edu/wheat-tilling) in the USA. To maximise the chance of having selected functionally important mutants, we recommend choosing two independent mutant lines for each homoeolog and carrying out crosses between each mutant in the A and B genomes (four crosses shown in Figure 5D). Detailed guides on growing wheat plants, genotyping TILLING mutants, and crossing mutants can be found on www.wheat-training.com.
Seedlings are genotyped to confirm that the correct mutation is present and to select for homozygous individuals for crossing. To do this, we design genome-specific primers to use in a KASP assay as outlined above and on www.wheat-training.com. For most TILLING mutations genome-specific primers have been predesigned and are available in Ensembl Plants. If there are no suitable predesigned primers, online tools such as PolyMarker can be used (Ramirez-Gonzalez et al., 2015), or if needed, can be designed manually. After carrying out the initial cross, we grow the F1 individuals under speed breeding conditions, and self-pollinate to obtain the F2 seed. We then grow F2 individuals and select via genetic markers individuals homozygous for one or both mutant alleles, as well as homozygous wild type control individuals (Figure 4). We can then carry out our first phenotypic evaluation on the F2 plants using the homozygous wild type lines as controls without the need for backcrossing to Kronos. We can do this because the background mutations in the chosen lines will be segregating within both the mutant and the wild type lines, leading to an equivalent background mutation load between the sibling genotypes (Uauy et al., 2017). Backcrossing to Kronos can be started either with the single mutants while carrying out the initial cross and/or with the F2 double mutant at a later stage. Backcrossing to remove background mutations is especially important when studying quantitative traits, such as yield components (Simmonds et al., 2016), and when plants are intended for field phenotyping.
Concluding remarks
In the last few years there has been a dramatic expansion in the resources available to carry out functional genomics in wheat, largely based upon improvements in the available reference sequences. Within a few years a step-change has been achieved from a highly fragmented assembly with incomplete gene models to a full pseudomolecule reference sequence alongside a detailed gene model annotation. This reference sequence allows the physical anchoring of genes in complete chromosomal order and provides improved gene models facilitating the design of transgenic constructs and primers. Most resources described in this review are integrated with the recent bread wheat reference genome sequence including the expVIP and eFP expression browsers, TILLING mutants, and Ensembl Plants sequence analyses and display tools. As a result, it is now easier to use these resources as they are unified by a common reference genome and gene models. Furthermore, a pan-genome of wheat is being produced which will provide high quality genome sequences for multiple varieties of wheat. These genomes will facilitate functional studies in a range of different genetic backgrounds and enhance the value of the populations containing natural variation captured from diverse wheat varieties.
Future directions
Whilst many major advances have been made in the last five years to lay the groundwork for gene discovery and functional characterisation in polyploid wheat, looking to the future several key challenges remain.
Polyploidy is a common challenge amongst crop species. In wheat, we frequently assume that due to functional redundancy it will be necessary to knock out all three homoeologs of a gene to assess its phenotypic impact. Yet the extent of homoeolog functional redundancy is still unclear (Borrill et al., 2019). Transcriptomics and proteomics approaches will help generate hypotheses as to the extent of homoeolog redundancy in wheat and allow researchers to specifically target the most phenotypically relevant homoeolog for genetic manipulation.
Defining accessible (open) chromatin regions allows the identification of cis-regulatory sequences of potential functional significance. In animals and plants, genetic variants associated with quantitative traits are significantly enriched in these open chromatin sequences (Maurano et al., 2012; Rodgers-Melnick et al., 2016). In wheat, where over 98% of the genome is non-coding, it will be critical to identify open chromatin regions to more precisely define non-coding variation that may be of functional relevance. Work in tomato has elegantly shown how a wide range of phenotypic variation for quantitative traits can be engineered by genome editing of cis-regulatory regions of transcription factors (Rodríguez-Leal et al., 2017).
To more readily test these hypotheses, increased transformation efficiency and reduced costs will also reshape the future of wheat research, perhaps one day becoming as accessible for wheat researchers as floral dip transformation is for Arabidopsis. It is becoming clear from research in wheat and other species that genetic background can have a strong influence on gene function. Therefore, it is essential to develop new protocols to transform multiple wheat varieties to account for these effects and to ensure that the potential of gene editing approaches is fulfilled.
Genomic databases have been powerful in integrating data from multiple studies and international efforts are now bringing together phenotypic data alongside genotypic data (e.g. Blake et al., 2016 and Howe et al., 2020). Challenges remain to standardise phenotype collection protocols and ontologies, which will realise the full power of this information (e.g. Papoutsoglou et al., 2020). Expanding these databases to include environmental conditions will allow assessments of interactions between genotypes, phenotypes and the environment.
High quality genome sequences facilitate moving beyond gene-based analysis, revealing the effects of non-genic regions on phenotype. Whilst working in crops with complex genomes will remain challenging, the advance of genomic techniques has enabled the wheat community to leverage lessons learnt in model species. The approaches taken in wheat provide a framework to understand biologically important traits in other species with large genomes.
Acknowledgements
This work was supported by the UK Biotechnology and Biological Sciences Research Council (BBSRC) through the Designing Future Wheat (BB/P016855/1) and GEN (BB/P013511/1) ISPs, grants BB/M008908/1, BB/M011666/1 and BB/P010741/1, an Anniversary Future Leader Fellowship BB/M014045/1, and funding for the Ensembl4breeders workshop (to BCM), and the European Molecular Biology Laboratory (BCM, GN). This work was also supported by the Rank Prize Funds New Lecturer Award (to PB) and a Royal Society Research Grant (RGS\R1\191163). Support was also received from the John Innes Centre Institute Strategic Fund (to SAH), the John Innes Foundation (to SAH and SG), the 2Blades Foundation (to SG and BBHW) and a Monsanto Beachell-Borlaug International Scholarship (to SG).
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Cristobal Uauy, Email: cristobal.uauy@jic.ac.uk.
Christian S Hardtke, University of Lausanne, Switzerland.
Christian S Hardtke, University of Lausanne, Switzerland.
Funding Information
This paper was supported by the following grants:
Biotechnology and Biological Sciences Research Council BB/P016855/1 to Nikolai M Adamski, Jemima Brinton, Sophie A Harrington, Clémence Marchal, Ricardo H Ramirez-Gonzalez, Luzie U Wingen, Brande BH Wulff, Cristobal Uauy.
Biotechnology and Biological Sciences Research Council BB/P013511/1 to Nikolai M Adamski, Jemima Brinton, Sophie A Harrington, Clémence Marchal, Sreya Ghosh, Wendy Harwood, Sadiye Hayta, Cristobal Uauy.
Biotechnology and Biological Sciences Research Council BB/M008908/1 to James Cockram.
Biotechnology and Biological Sciences Research Council BB/M011666/1 to Alison R Bentley, James Cockram.
Biotechnology and Biological Sciences Research Council BB/P010741/1 to James Cockram.
Biotechnology and Biological Sciences Research Council BB/M014045/1 to Philippa Borrill.
Rank Prize Funds to Philippa Borrill.
Royal Society RGS\R1\191163 to Philippa Borrill.
John Innes Foundation to Sophie A Harrington, Sreya Ghosh.
European Molecular Biology Laboratory to Bruno Contreras-Moreira, Guy Naamati.
Monsanto-Beachell Borlaug International Scholarship MBBIS 06-400258-12580 to Sreya Ghosh.
2Blades Foundation Wheat rust to Sreya Ghosh, Brande BH Wulff.
John Innes Centre Institute Strategic Fund to Sophie A Harrington.
Additional information
Competing interests
No competing interests declared.
References
- Alaux M, Rogers J, Letellier T, Flores R, Alfama F, Pommier C, Mohellibi N, Durand S, Kimmel E, Michotey C, Guerche C, Loaec M, Lainé M, Steinbach D, Choulet F, Rimbert H, Leroy P, Guilhot N, Salse J, Feuillet C, Paux E, Eversole K, Adam-Blondon AF, Quesneville H, International Wheat Genome Sequencing Consortium Linking the international wheat genome sequencing consortium bread wheat reference genome sequence to wheat genetic and phenomic data. Genome Biology. 2018;19:111. doi: 10.1186/s13059-018-1491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen AM, Barker GL, Berry ST, Coghill JA, Gwilliam R, Kirby S, Robinson P, Brenchley RC, D'Amore R, McKenzie N, Waite D, Hall A, Bevan M, Hall N, Edwards KJ. Transcript-specific, single-nucleotide polymorphism discovery and linkage analysis in hexaploid bread wheat (Triticum aestivum L.) Plant Biotechnology Journal. 2011;9:1086–1099. doi: 10.1111/j.1467-7652.2011.00628.x. [DOI] [PubMed] [Google Scholar]
- Allen AM, Winfield MO, Burridge AJ, Downie RC, Benbow HR, Barker GL, Wilkinson PA, Coghill J, Waterfall C, Davassi A, Scopes G, Pirani A, Webster T, Brew F, Bloor C, Griffiths S, Bentley AR, Alda M, Jack P, Phillips AL, Edwards KJ. Characterization of a wheat breeders' Array suitable for high-throughput SNP genotyping of global accessions of hexaploid bread wheat (Triticum aestivum) Plant Biotechnology Journal. 2017;15:390–401. doi: 10.1111/pbi.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora S, Steuernagel B, Gaurav K, Chandramohan S, Long Y, Matny O, Johnson R, Enk J, Periyannan S, Singh N, Asyraf Md Hatta M, Athiyannan N, Cheema J, Yu G, Kangara N, Ghosh S, Szabo LJ, Poland J, Bariana H, Jones JDG, Bentley AR, Ayliffe M, Olson E, Xu SS, Steffenson BJ, Lagudah E, Wulff BBH. Resistance gene cloning from a wild crop relative by sequence capture and association genetics. Nature Biotechnology. 2019;37:139–143. doi: 10.1038/s41587-018-0007-9. [DOI] [PubMed] [Google Scholar]
- Avni R, Nave M, Barad O, Baruch K, Twardziok SO, Gundlach H, Hale I, Mascher M, Spannagl M, Wiebe K, Jordan KW, Golan G, Deek J, Ben-Zvi B, Ben-Zvi G, Himmelbach A, MacLachlan RP, Sharpe AG, Fritz A, Ben-David R, Budak H, Fahima T, Korol A, Faris JD, Hernandez A, Mikel MA, Levy AA, Steffenson B, Maccaferri M, Tuberosa R, Cattivelli L, Faccioli P, Ceriotti A, Kashkush K, Pourkheirandish M, Komatsuda T, Eilam T, Sela H, Sharon A, Ohad N, Chamovitz DA, Mayer KFX, Stein N, Ronen G, Peleg Z, Pozniak CJ, Akhunov ED, Distelfeld A. Wild Emmer genome architecture and diversity elucidate wheat evolution and domestication. Science. 2017;357:93–97. doi: 10.1126/science.aan0032. [DOI] [PubMed] [Google Scholar]
- Barrero JM, Cavanagh C, Verbyla KL, Tibbits JF, Verbyla AP, Huang BE, Rosewarne GM, Stephen S, Wang P, Whan A, Rigault P, Hayden MJ, Gubler F. Transcriptomic analysis of wheat near-isogenic lines identifies PM19-A1 and A2 as candidates for a major dormancy QTL. Genome Biology. 2015;16:93. doi: 10.1186/s13059-015-0665-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake VC, Birkett C, Matthews DE, Hane DL, Bradbury P, Jannink J-L. The triticeae toolbox: combining phenotype and genotype data to advance Small-Grains breeding. The Plant Genome. 2016;9:0099. doi: 10.3835/plantgenome2014.12.0099. [DOI] [PubMed] [Google Scholar]
- Blake VC, Woodhouse MR, Lazo GR, Odell SG, Wight CP, Tinker NA, Wang Y, Gu YQ, Birkett CL, Jannink JL, Matthews DE, Hane DL, Michel SL, Yao E, Sen TZ. GrainGenes: centralized small grain resources and digital platform for geneticists and breeders. Database : The Journal of Biological Databases and Curation. 2019;2019:baz065. doi: 10.1093/database/baz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolser DM, Kerhornou A, Walts B, Kersey P. Triticeae resources in ensembl plants. Plant and Cell Physiology. 2015;56:e3. doi: 10.1093/pcp/pcu183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrill P, Adamski N, Uauy C. Genomics as the key to unlocking the polyploid potential of wheat. New Phytologist. 2015;208:1008–1022. doi: 10.1111/nph.13533. [DOI] [PubMed] [Google Scholar]
- Borrill P, Ramirez-Gonzalez R, Uauy C. expVIP: a customizable RNA-seq data analysis and visualization platform. Plant Physiology. 2016;170:2172–2186. doi: 10.1104/pp.15.01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrill P, Harrington SA, Uauy C. Applying the latest advances in genomics and phenomics for trait discovery in polyploid wheat. The Plant Journal : For Cell and Molecular Biology. 2019;97:56–72. doi: 10.1111/tpj.14150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrill P. Blurring the boundaries between cereal crops and model plants. New Phytologist. 2020;9:16229. doi: 10.1111/nph.16229. [DOI] [PubMed] [Google Scholar]
- Bovina R, Brunazzi A, Gasparini G, Sestili F, Palombieri S, Botticella E, Lafiandra D, Mantovani P, Massi A. Development of a TILLING resource in durum wheat for reverse- and forward-genetic analyses. Crop and Pasture Science. 2014;65:112–124. doi: 10.1071/CP13226. [DOI] [Google Scholar]
- Brenchley R, Spannagl M, Pfeifer M, Barker GL, D'Amore R, Allen AM, McKenzie N, Kramer M, Kerhornou A, Bolser D, Kay S, Waite D, Trick M, Bancroft I, Gu Y, Huo N, Luo MC, Sehgal S, Gill B, Kianian S, Anderson O, Kersey P, Dvorak J, McCombie WR, Hall A, Mayer KF, Edwards KJ, Bevan MW, Hall N. Analysis of the bread wheat genome using whole-genome shotgun sequencing. Nature. 2012;491:705–710. doi: 10.1038/nature11650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton J, Simmonds J, Uauy C. Ubiquitin-related genes are differentially expressed in isogenic lines contrasting for pericarp cell size and grain weight in hexaploid wheat. BMC Plant Biology. 2018;18:22. doi: 10.1186/s12870-018-1241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JA, Mascher M, Buluç A, Barry K, Georganas E, Session A, Strnadova V, Jenkins J, Sehgal S, Oliker L, Schmutz J, Yelick KA, Scholz U, Waugh R, Poland JA, Muehlbauer GJ, Stein N, Rokhsar DS. A whole-genome shotgun approach for assembling and anchoring the hexaploid bread wheat genome. Genome Biology. 2015;16:26. doi: 10.1186/s13059-015-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Huang L, Min D, Phillips A, Wang S, Madgwick PJ, Parry MA, Hu YG. Development and characterization of a new TILLING population of common bread wheat (Triticum aestivum L.) PLOS ONE. 2012;7:e41570. doi: 10.1371/journal.pone.0041570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiology. 1997;115:971–980. doi: 10.1104/pp.115.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavijo BJ, Venturini L, Schudoma C, Accinelli GG, Kaithakottil G, Wright J, Borrill P, Kettleborough G, Heavens D, Chapman H, Lipscombe J, Barker T, Lu FH, McKenzie N, Raats D, Ramirez-Gonzalez RH, Coince A, Peel N, Percival-Alwyn L, Duncan O, Trösch J, Yu G, Bolser DM, Namaati G, Kerhornou A, Spannagl M, Gundlach H, Haberer G, Davey RP, Fosker C, Palma FD, Phillips AL, Millar AH, Kersey PJ, Uauy C, Krasileva KV, Swarbreck D, Bevan MW, Clark MD. An improved assembly and annotation of the allohexaploid wheat genome identifies complete families of agronomic genes and provides genomic evidence for chromosomal translocations. Genome Research. 2017;27:885–896. doi: 10.1101/gr.217117.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockram J, Mackay I. Genetic mapping populations for conducting high-resolution trait mapping in plants. In: Varshney R. K, Pandey M. K, Chitikineni A, editors. Plant Genetics and Molecular Biology. Springer International Publishing; 2018. pp. 109–138. [DOI] [Google Scholar]
- Colasuonno P, Incerti O, Lozito ML, Simeone R, Gadaleta A, Blanco A. DHPLC technology for high-throughput detection of mutations in a durum wheat TILLING population. BMC Genetics. 2016;17:43. doi: 10.1186/s12863-016-0350-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Casao MC, Wang P, Sato K, Hayes PM, Finnegan EJ, Trevaskis B. Direct links between the vernalization response and other key traits of cereal crops. Nature Communications. 2015;6:5882. doi: 10.1038/ncomms6882. [DOI] [PubMed] [Google Scholar]
- Devos KM, Dubcovsky J, Dvořák J, Chinoy CN, Gale MD. Structural evolution of wheat chromosomes 4A, 5A, and 7B and its impact on recombination. Theoretical and Applied Genetics. 1995;91:282–288. doi: 10.1007/BF00220890. [DOI] [PubMed] [Google Scholar]
- Dixon LE, Greenwood JR, Bencivenga S, Zhang P, Cockram J, Mellers G, Ramm K, Cavanagh C, Swain SM, Boden SA. TEOSINTE BRANCHED1 Regulates Inflorescence Architecture and Development in Bread Wheat ( Triticum aestivum ) The Plant Cell. 2018;30:563–581. doi: 10.1105/tpc.17.00961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Dalton-Morgan J, Vincent K, Sharp P. A modified TILLING method for wheat breeding. The Plant Genome. 2009;2:39–47. doi: 10.3835/plantgenome2008.10.0012. [DOI] [Google Scholar]
- Fradgley N, Gardner KA, Cockram J, Elderfield J, Hickey JM, Howell P, Jackson R, Mackay IJ. A large-scale pedigree resource of wheat reveals evidence for adaptation and selection by breeders. PLOS Biology. 2019;17:e3000071. doi: 10.1371/journal.pbio.3000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu D, Uauy C, Blechl A, Dubcovsky J. RNA interference for wheat functional gene analysis. Transgenic Research. 2007;16:689–701. doi: 10.1007/s11248-007-9150-7. [DOI] [PubMed] [Google Scholar]
- Gardiner LJ, Quinton-Tulloch M, Olohan L, Price J, Hall N, Hall A. A genome-wide survey of DNA methylation in hexaploid wheat. Genome Biology. 2015;16:273. doi: 10.1186/s13059-015-0838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner LJ, Joynson R, Omony J, Rusholme-Pilcher R, Olohan L, Lang D, Bai C, Hawkesford M, Salt D, Spannagl M, Mayer KFX, Kenny J, Bevan M, Hall N, Hall A. Hidden variation in polyploid wheat drives local adaptation. Genome Research. 2018;28:1319–1332. doi: 10.1101/gr.233551.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner KA, Wittern LM, Mackay IJ. A highly recombined, high-density, eight-founder wheat MAGIC map reveals extensive segregation distortion and genomic locations of introgression segments. Plant Biotechnology Journal. 2016;14:1406–1417. doi: 10.1111/pbi.12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garsmeur O, Droc G, Antonise R, Grimwood J, Potier B, Aitken K, Jenkins J, Martin G, Charron C, Hervouet C, Costet L, Yahiaoui N, Healey A, Sims D, Cherukuri Y, Sreedasyam A, Kilian A, Chan A, Van Sluys MA, Swaminathan K, Town C, Bergès H, Simmons B, Glaszmann JC, van der Vossen E, Henry R, Schmutz J, D'Hont A. A mosaic monoploid reference sequence for the highly complex genome of sugarcane. Nature Communications. 2018;9:2638. doi: 10.1038/s41467-018-05051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng S, Kong X, Song G, Jia M, Guan J, Wang F, Qin Z, Wu L, Lan X, Li A, Mao L. DNA methylation dynamics during the interaction of wheat progenitor Aegilops tauschii with the obligate biotrophic fungus Blumeria graminis f. sp. tritici. The New Phytologist. 2019;221:1023–1035. doi: 10.1111/nph.15432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Watson A, Gonzalez-Navarro OE, Ramirez-Gonzalez RH, Yanes L, Mendoza-Suárez M, Simmonds J, Wells R, Rayner T, Green P, Hafeez A, Hayta S, Melton RE, Steed A, Sarkar A, Carter J, Perkins L, Lord J, Tester M, Osbourn A, Moscou MJ, Nicholson P, Harwood W, Martin C, Domoney C, Uauy C, Hazard B, Wulff BBH, Hickey LT. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research . bioRxiv. 2018 doi: 10.1101/369512. [DOI] [PubMed]
- Gororo NN, Eagles HA, Eastwood RF, Nicolas ME, Flood RG. Use of Triticum tauschii to improve yield of wheat in low-yielding environments. Euphytica. 2002;123:241–254. doi: 10.1023/A:1014910000128. [DOI] [Google Scholar]
- Grewal S, Hubbart-Edwards S, Yang C, Scholefield D, Ashling S, Burridge A, Wilkinson PA, King IP, King J. Detection of T. urartu introgressions in wheat and development of a panel of interspecific introgression lines. Frontiers in Plant Science. 2018a;9:1565. doi: 10.3389/fpls.2018.01565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S, Yang C, Edwards SH, Scholefield D, Ashling S, Burridge AJ, King IP, King J. Characterisation of Thinopyrum bessarabicum chromosomes through genome-wide introgressions into wheat. Theoretical and Applied Genetics. 2018b;131:389–406. doi: 10.1007/s00122-017-3009-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal S, Hubbart-Edwards S, Yang C, Devi U, Baker L, Heath J, Ashling S, Scholefield D, Howells C, Yarde J, Isaac P, King IP, King J. Rapid identification of homozygosity and site of wild relative introgressions in wheat through chromosome-specific KASP genotyping assays. Plant Biotechnology Journal. 2020;18:743–755. doi: 10.1111/pbi.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Liu JH, Ma X, Luo DX, Gong ZH, Lu MH. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Frontiers in Plant Science. 2016;7:114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington SA, Backhaus AE, Singh A, Hassani-Pak K, Uauy C. Validation and characterisation of a wheat GENIE3 network using an independent RNA-Seq dataset. bioRxiv. 2019 doi: 10.1101/684183. [DOI]
- Harwood WA, Bilham LJ, Travella S, Salvo-Garrido H, Snape JW. Fluorescence in situ hybridization to localize transgenes in plant chromosomes. Methods in Molecular Biology. 2005;286:327–340. doi: 10.1385/1-59259-827-7:327. [DOI] [PubMed] [Google Scholar]
- Harwood WA. Advances and remaining challenges in the transformation of barley and wheat. Journal of Experimental Botany. 2012;63:1791–1798. doi: 10.1093/jxb/err380. [DOI] [PubMed] [Google Scholar]
- Hassani-Pak K, Castellote M, Esch M, Hindle M, Lysenko A, Taubert J, Rawlings C. Developing integrated crop knowledge networks to advance candidate gene discovery. Applied & Translational Genomics. 2016;11:18–26. doi: 10.1016/j.atg.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayta S, Smedley MA, Demir SU, Blundell R, Hinchliffe A, Atkinson N, Harwood WA. An efficient and reproducible Agrobacterium-mediated transformation method for hexaploid wheat (Triticum aestivum L.) Plant Methods. 2019;15:121. doi: 10.1186/s13007-019-0503-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, Pasam R, Shi F, Kant S, Keeble-Gagnere G, Kay P, Forrest K, Fritz A, Hucl P, Wiebe K, Knox R, Cuthbert R, Pozniak C, Akhunova A, Morrell PL, Davies JP, Webb SR, Spangenberg G, Hayes B, Daetwyler H, Tibbits J, Hayden M, Akhunov E. Exome sequencing highlights the role of wild-relative introgression in shaping the adaptive landscape of the wheat genome. Nature Genetics. 2019;51:896–904. doi: 10.1038/s41588-019-0382-2. [DOI] [PubMed] [Google Scholar]
- Hensel G, Himmelbach A, Chen W, Douchkov DK, Kumlehn J. Transgene expression systems in the triticeae cereals. Journal of Plant Physiology. 2011;168:30–44. doi: 10.1016/j.jplph.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Herrero J, Muffato M, Beal K, Fitzgerald S, Gordon L, Pignatelli M, Vilella AJ, Searle SMJ, Amode R, Brent S, Spooner W, Kulesha E, Yates A, Flicek P. Ensembl comparative genomics resources. Database. 2016;20:bav096. doi: 10.1093/database/bav096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe KL, Contreras-Moreira B, De Silva N, Maslen G, Akanni W, Allen J, Alvarez-Jarreta J, Barba M, Bolser DM, Cambell L, Carbajo M, Chakiachvili M, Christensen M, Cummins C, Cuzick A, Davis P, Fexova S, Gall A, George N, Gil L, Gupta P, Hammond-Kosack KE, Haskell E, Hunt SE, Jaiswal P, Janacek SH, Kersey PJ, Langridge N, Maheswari U, Maurel T, McDowall MD, Moore B, Muffato M, Naamati G, Naithani S, Olson A, Papatheodorou I, Patricio M, Paulini M, Pedro H, Perry E, Preece J, Rosello M, Russell M, Sitnik V, Staines DM, Stein J, Tello-Ruiz MK, Trevanion SJ, Urban M, Wei S, Ware D, Williams G, Yates AD, Flicek P. Ensembl genomes 2020-enabling non-vertebrate genomic research. Nucleic Acids Research. 2020;48:D689–D695. doi: 10.1093/nar/gkz890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells RM, Craze M, Bowden S, Wallington EJ. Efficient generation of stable, heritable gene edits in wheat using CRISPR/Cas9. BMC Plant Biology. 2018;18:215. doi: 10.1186/s12870-018-1433-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang BE, George AW, Forrest KL, Kilian A, Hayden MJ, Morell MK, Cavanagh CR. A multiparent advanced generation inter-cross population for genetic analysis in wheat. Plant Biotechnology Journal. 2012;10:826–839. doi: 10.1111/j.1467-7652.2012.00702.x. [DOI] [PubMed] [Google Scholar]
- Huang BE, Verbyla KL, Verbyla AP, Raghavan C, Singh VK, Gaur P, Leung H, Varshney RK, Cavanagh CR. MAGIC populations in crops: current status and future prospects. Theoretical and Applied Genetics. 2015;128:999–1017. doi: 10.1007/s00122-015-2506-0. [DOI] [PubMed] [Google Scholar]
- Huynh-Thu VA, Irrthum A, Wehenkel L, Geurts P. Inferring regulatory networks from expression data using tree-based methods. PLOS ONE. 2010;5:e12776. doi: 10.1371/journal.pone.0012776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda M, Mitsuda N, Ohme-Takagi M. Arabidopsis HsfB1 and HsfB2b act as repressors of the expression of heat-inducible hsfs but positively regulate the acquired thermotolerance. Plant Physiology. 2011;157:1243–1254. doi: 10.1104/pp.111.179036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J, Grewal S, Yang CY, Hubbart S, Scholefield D, Ashling S, Edwards KJ, Allen AM, Burridge A, Bloor C, Davassi A, da Silva GJ, Chalmers K, King IP. A step change in the transfer of interspecific variation into wheat from Amblyopyrum muticum. Plant Biotechnology Journal. 2017;15:217–226. doi: 10.1111/pbi.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J, Grewal S, Yang CY, Hubbart Edwards S, Scholefield D, Ashling S, Harper JA, Allen AM, Edwards KJ, Burridge AJ, King IP. Introgression of Aegilops speltoides segments in Triticum aestivum and the effect of the gametocidal genes. Annals of Botany. 2018;121:229–240. doi: 10.1093/aob/mcx149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasileva KV, Vasquez-Gross HA, Howell T, Bailey P, Paraiso F, Clissold L, Simmonds J, Ramirez-Gonzalez RH, Wang X, Borrill P, Fosker C, Ayling S, Phillips AL, Uauy C, Dubcovsky J. Uncovering hidden variation in polyploid wheat. PNAS. 2017;114:E913–E921. doi: 10.1073/pnas.1619268114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Hammond-Kosack KE, Kanyuka K. Barley stripe mosaic virus-mediated tools for investigating gene function in cereal plants and their pathogens: virus-induced gene silencing, host-mediated gene silencing, and virus-mediated overexpression of heterologous protein. Plant Physiology. 2012;160:582–590. doi: 10.1104/pp.112.203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Rudd JJ, Kanyuka K. Virus induced gene silencing (VIGS) for functional analysis of wheat genes involved in Zymoseptoria tritici susceptibility and resistance. Fungal Genetics and Biology. 2015;79:84–88. doi: 10.1016/j.fgb.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ye X, An B, Du L, Xu H. Genetic transformation of wheat: current status and future prospects. Plant Biotechnology Reports. 2012;6:183–193. doi: 10.1007/s11816-011-0213-0. [DOI] [Google Scholar]
- Li Z, Wang M, Lin K, Xie Y, Guo J, Ye L, Zhuang Y, Teng W, Ran X, Tong Y, Xue Y, Zhang W, Zhang Y. The bread wheat epigenomic map reveals distinct chromatin architectural and evolutionary features of functional genetic elements. Genome Biology. 2019;20:139. doi: 10.1186/s13059-019-1746-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Chen K, Li T, Zhang Y, Wang Y, Zhao Q, Liu J, Zhang H, Liu C, Ran Y, Gao C. Efficient DNA-free genome editing of bread wheat using CRISPR/Cas9 ribonucleoprotein complexes. Nature Communications. 2017;8:14261. doi: 10.1038/ncomms14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling H-Q, Ma B, Shi X, Liu H, Dong L, Sun H, Cao Y, Gao Q, Zheng S, Li Y, Yu Y, Du H, Qi M, Li Y, Lu H, Yu H, Cui Y, Wang N, Chen C, Wu H, Zhao Y, Zhang J, Li Y, Zhou W, Zhang B, Hu W, van Eijk MJT, Tang J, Witsenboer HMA, Zhao S, Li Z, Zhang A, Wang D, Liang C. Genome sequence of the progenitor of wheat A subgenome Triticum urartu. Nature. 2018;557:424–428. doi: 10.1038/s41586-018-0108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe K, Wu E, Wang N, Hoerster G, Hastings C, Cho MJ, Scelonge C, Lenderts B, Chamberlin M, Cushatt J, Wang L, Ryan L, Khan T, Chow-Yiu J, Hua W, Yu M, Banh J, Bao Z, Brink K, Igo E, Rudrappa B, Shamseer PM, Bruce W, Newman L, Shen B, Zheng P, Bidney D, Falco C, Register J, Zhao ZY, Xu D, Jones T, Gordon-Kamm W. Morphogenic regulators Baby boom and Wuschel Improve Monocot Transformation. The Plant Cell. 2016;28:1998–2015. doi: 10.1105/tpc.16.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MC, Gu YQ, Puiu D, Wang H, Twardziok SO, Deal KR, Huo N, Zhu T, Wang L, Wang Y, McGuire PE, Liu S, Long H, Ramasamy RK, Rodriguez JC, Van SL, Yuan L, Wang Z, Xia Z, Xiao L, Anderson OD, Ouyang S, Liang Y, Zimin AV, Pertea G, Qi P, Bennetzen JL, Dai X, Dawson MW, Müller HG, Kugler K, Rivarola-Duarte L, Spannagl M, Mayer KFX, Lu FH, Bevan MW, Leroy P, Li P, You FM, Sun Q, Liu Z, Lyons E, Wicker T, Salzberg SL, Devos KM, Dvořák J. Genome sequence of the progenitor of the wheat D genome Aegilops tauschii. Nature. 2017;551:498–502. doi: 10.1038/nature24486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo M, Li H, Chakraborty S, Morbitzer R, Rinaldo A, Upadhyaya N, Bhatt D, Louis S, Richardson T, Lahaye T, Ayliffe M. Efficient TALEN-mediated gene editing in wheat. Plant Biotechnology Journal. 2019;17:2026–2028. doi: 10.1111/pbi.13169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Yan Y, Huang L, Chen M, Zhao H. Virus-induced gene-silencing in wheat spikes and grains and its application in functional analysis of HMW-GS-encoding genes. BMC Plant Biology. 2012;12:141. doi: 10.1186/1471-2229-12-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Stiller J, Berkman PJ, Wei Y, Rogers J, Feuillet C, Dolezel J, Mayer KF, Eversole K, Zheng YL, Liu C. Sequence-based analysis of translocations and inversions in bread wheat (Triticum aestivum L.) PLOS ONE. 2013;8:e79329. doi: 10.1371/journal.pone.0079329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccaferri M, Harris NS, Twardziok SO, Pasam RK, Gundlach H, Spannagl M, Ormanbekova D, Lux T, Prade VM, Milner SG, Himmelbach A, Mascher M, Bagnaresi P, Faccioli P, Cozzi P, Lauria M, Lazzari B, Stella A, Manconi A, Gnocchi M, Moscatelli M, Avni R, Deek J, Biyiklioglu S, Frascaroli E, Corneti S, Salvi S, Sonnante G, Desiderio F, Marè C, Crosatti C, Mica E, Özkan H, Kilian B, De Vita P, Marone D, Joukhadar R, Mazzucotelli E, Nigro D, Gadaleta A, Chao S, Faris JD, Melo ATO, Pumphrey M, Pecchioni N, Milanesi L, Wiebe K, Ens J, MacLachlan RP, Clarke JM, Sharpe AG, Koh CS, Liang KYH, Taylor GJ, Knox R, Budak H, Mastrangelo AM, Xu SS, Stein N, Hale I, Distelfeld A, Hayden MJ, Tuberosa R, Walkowiak S, Mayer KFX, Ceriotti A, Pozniak CJ, Cattivelli L. Durum wheat genome highlights past domestication signatures and future improvement targets. Nature Genetics. 2019;51:885–895. doi: 10.1038/s41588-019-0381-3. [DOI] [PubMed] [Google Scholar]
- Mackay IJ, Bansept-Basler P, Barber T, Bentley AR, Cockram J, Gosman N, Greenland AJ, Horsnell R, Howells R, O'Sullivan DM, Rose GA, Howell PJ. An Eight-Parent multiparent advanced generation Inter-Cross population for Winter-Sown wheat: creation, properties, and validation. G3: Genes|Genomes|Genetics. 2014;4:1603–1610. doi: 10.1534/g3.114.012963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Muehlbauer GJ, Rokhsar DS, Chapman J, Schmutz J, Barry K, Muñoz-Amatriaín M, Close TJ, Wise RP, Schulman AH, Himmelbach A, Mayer KF, Scholz U, Poland JA, Stein N, Waugh R. Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ) The Plant Journal. 2013;76:718–727. doi: 10.1111/tpj.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y. Evolution of polyploid Triticum wheats under cultivation: the role of domestication, natural hybridization and allopolyploid speciation in their diversification. Plant and Cell Physiology. 2011;52:750–764. doi: 10.1093/pcp/pcr018. [DOI] [PubMed] [Google Scholar]
- Maurano MT, Humbert R, Rynes E, Thurman RE, Haugen E, Wang H, Reynolds AP, Sandstrom R, Qu H, Brody J, Shafer A, Neri F, Lee K, Kutyavin T, Stehling-Sun S, Johnson AK, Canfield TK, Giste E, Diegel M, Bates D, Hansen RS, Neph S, Sabo PJ, Heimfeld S, Raubitschek A, Ziegler S, Cotsapas C, Sotoodehnia N, Glass I, Sunyaev SR, Kaul R, Stamatoyannopoulos JA. Systematic localization of common disease-associated variation in regulatory DNA. Science. 2012;337:1190–1195. doi: 10.1126/science.1222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KFX, Rogers J, Dole el J, Pozniak C, Eversole K, Feuillet C, Gill B, Friebe B, Lukaszewski AJ, Sourdille P, Endo TR, Kubalakova M, ihalikova J, Dubska Z, Vrana J, perkova R, imkova H, Febrer M, Clissold L, McLay K, Singh K, Chhuneja P, Singh NK, Khurana J, Akhunov E, Choulet F, Alberti A, Barbe V, Wincker P, Kanamori H, Kobayashi F, Itoh T, Matsumoto T, Sakai H, Tanaka T, Wu J, Ogihara Y, Handa H, Maclachlan PR, Sharpe A, Klassen D, Edwards D, Batley J, Olsen O-A, Sandve SR, Lien S, Steuernagel B, Wulff B, Caccamo M, Ayling S, Ramirez-Gonzalez RH, Clavijo BJ, Wright J, Pfeifer M, Spannagl M, Martis MM, Mascher M, Chapman J, Poland JA, Scholz U, Barry K, Waugh R, Rokhsar DS, Muehlbauer GJ, Stein N, Gundlach H, Zytnicki M, Jamilloux V, Quesneville H, Wicker T, Faccioli P, Colaiacovo M, Stanca AM, Budak H, Cattivelli L, Glover N, Pingault L, Paux E, Sharma S, Appels R, Bellgard M, Chapman B, Nussbaumer T, Bader KC, Rimbert H, Wang S, Knox R, Kilian A, Alaux M, Alfama F, Couderc L, Guilhot N, Viseux C, Loaec M, Keller B, Praud S, International Wheat Genome Sequencing Consortium (IWGSC) A chromosome-based draft sequence of the hexaploid bread wheat (Triticum aestivum) genome. Science. 2014;345:1251788. doi: 10.1126/science.1251788. [DOI] [PubMed] [Google Scholar]
- Miki Y, Yoshida K, Mizuno N, Nasuda S, Sato K, Takumi S. Origin of wheat B-genome chromosomes inferred from RNA sequencing analysis of leaf transcripts from section sitopsis species of Aegilops. DNA Research. 2019;26:171–182. doi: 10.1093/dnares/dsy047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner SG, Maccaferri M, Huang BE, Mantovani P, Massi A, Frascaroli E, Tuberosa R, Salvi S. A multiparental cross population for mapping QTL for agronomic traits in durum wheat (Triticum turgidum ssp. durum) Plant Biotechnology Journal. 2016;14:735–748. doi: 10.1111/pbi.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujeeb-Kazi A, Rosas V, Roldan S. Conservation of the genetic variation of Triticum tauschii (Coss.) Schmalh. (Aegilops squarrosa auct. non L.) in synthetic hexaploid wheats (T. turgidum L. s.lat. x T. tauschii; 2n=6x=42, AABBDD) and its potential utilization for wheat improvement. Genetic Resources and Crop Evolution. 1996;43:129–134. doi: 10.1007/BF00126756. [DOI] [Google Scholar]
- Mujeeb-Kazi A, Kazi AG, Dundas I, Rasheed A, Ogbonnaya F, Kishii M, Bonnett D, Wang RRC, Xu S, Chen P, Mahmood T, Bux H, Farrakh S. Chapter Four - Genetic diversity for wheat improvement as a conduit to food security. In: Sparks D. L, editor. Advances in Agronomy. Vol. 122. Academic Press; 2013. pp. 179–257. [DOI] [Google Scholar]
- Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Research. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogbonnaya FC, Ye G, Trethowan R, Dreccer F, Lush D, Shepperd J, van Ginkel M. Yield of synthetic backcross-derived lines in rainfed environments of Australia. Euphytica. 2007;157:321–336. doi: 10.1007/s10681-007-9381-y. [DOI] [Google Scholar]
- Pajerowska-Mukhtar KM, Wang W, Tada Y, Oka N, Tucker CL, Fonseca JP, Dong X. The HSF-like transcription factor TBF1 is a major molecular switch for plant growth-to-defense transition. Current Biology. 2012;22:103–112. doi: 10.1016/j.cub.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papoutsoglou EA, Faria D, Arend D, Arnaud E, Athanasiadis IN, Chaves I, Coppens F, Cornut G, Costa BV, Ćwiek‐Kupczyńska H, Droesbeke B, Finkers R, Gruden K, Junker A, King GJ, Krajewski P, Lange M, Laporte MA, Michotey C, Oppermann M, Ostler R, Poorter H, Ramírez‐Gonzalez R, Ramšak Živa, Reif JC, Rocca‐Serra P, Sansone SA, Scholz U, Tardieu F, Uauy C, Usadel B, Visser RGF, Weise S, Kersey PJ, Miguel CM, Adam‐Blondon AF, Pommier C. Enabling reusability of plant phenomic datasets with MIAPPE 1.1. New Phytologist. 2020:16544. doi: 10.1111/nph.16544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S, Vazquez-Gross H, Herin SY, Hane D, Wang Y, Gu YQ, Dubcovsky J. WheatExp: an RNA-seq expression database for polyploid wheat. BMC Plant Biology. 2015;15:299. doi: 10.1186/s12870-015-0692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont C, Leroy T, Seidel M, Tondelli A, Duchemin W, Armisen D, Lang D, Bustos-Korts D, Goué N, Balfourier F, Molnár-Láng M, Lage J, Kilian B, Özkan H, Waite D, Dyer S, Letellier T, Alaux M, Russell J, Keller B, van Eeuwijk F, Spannagl M, Mayer KFX, Waugh R, Stein N, Cattivelli L, Haberer G, Charmet G, Salse J, Wheat and Barley Legacy for Breeding Improvement (WHEALBI) consortium Tracing the ancestry of modern bread wheats. Nature Genetics. 2019;51:905–911. doi: 10.1038/s41588-019-0393-z. [DOI] [PubMed] [Google Scholar]
- Puchta H. Applying CRISPR/Cas for genome engineering in plants: the best is yet to come. Current Opinion in Plant Biology. 2017;36:1–8. doi: 10.1016/j.pbi.2016.11.011. [DOI] [PubMed] [Google Scholar]
- Ramirez-Gonzalez RH, Uauy C, Caccamo M. PolyMarker: a fast polyploid primer design pipeline. Bioinformatics. 2015;31:2038–2039. doi: 10.1093/bioinformatics/btv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-González RH, Borrill P, Lang D, Harrington SA, Brinton J, Venturini L, Davey M, Jacobs J, van Ex F, Pasha A, Khedikar Y, Robinson SJ, Cory AT, Florio T, Concia L, Juery C, Schoonbeek H, Steuernagel B, Xiang D, Ridout CJ, Chalhoub B, Mayer KFX, Benhamed M, Latrasse D, Bendahmane A, Wulff BBH, Appels R, Tiwari V, Datla R, Choulet F, Pozniak CJ, Provart NJ, Sharpe AG, Paux E, Spannagl M, Bräutigam A, Uauy C, International Wheat Genome Sequencing Consortium The transcriptional landscape of polyploid wheat. Science. 2018;361:eaar6089. doi: 10.1126/science.aar6089. [DOI] [PubMed] [Google Scholar]
- Riaz A, Hathorn A, Dinglasan E, Ziems L, Richard C, Singh D, Mitrofanova O, Afanasenko O, Aitken E, Godwin I, Hickey L. Into the vault of the Vavilov wheats: old diversity for new alleles. Genetic Resources and Crop Evolution. 2017;64:531–544. doi: 10.1007/s10722-016-0380-5. [DOI] [Google Scholar]
- Richardson T, Thistleton J, Higgins TJ, Howitt C, Ayliffe M. Efficient Agrobacterium transformation of elite wheat germplasm without selection. Plant Cell, Tissue and Organ Culture. 2014;119:647–659. doi: 10.1007/s11240-014-0564-7. [DOI] [Google Scholar]
- Rodgers-Melnick E, Vera DL, Bass HW, Buckler ES. Open chromatin reveals the functional maize genome. PNAS. 2016;113:E3177–E3184. doi: 10.1073/pnas.1525244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Leal D, Lemmon ZH, Man J, Bartlett ME, Lippman ZB. Engineering quantitative trait variation for crop improvement by genome editing. Cell. 2017;171:470–480. doi: 10.1016/j.cell.2017.08.030. [DOI] [PubMed] [Google Scholar]
- Sannemann W, Lisker A, Maurer A, Léon J, Kazman E, Cöster H, Holzapfel J, Kempf H, Korzun V, Ebmeyer E, Pillen K. Adaptive selection of founder segments and epistatic control of plant height in the MAGIC winter wheat population WM-800. BMC Genomics. 2018;19:559. doi: 10.1186/s12864-018-4915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestili F, Palombieri S, Botticella E, Mantovani P, Bovina R, Lafiandra D. TILLING mutants of durum wheat result in a high amylose phenotype and provide information on alternative splicing mechanisms. Plant Science. 2015;233:127–133. doi: 10.1016/j.plantsci.2015.01.009. [DOI] [PubMed] [Google Scholar]
- Shah R, Huang BE, Whan A, Newberry M, Verbyla K, Morell MK, Cavanagh CR. The complex genetic architecture of recombination and structural variation in wheat uncovered using a large 8-founder MAGIC population. bioRxiv. 2019 doi: 10.1101/594317. [DOI]
- Shan Q, Wang Y, Li J, Gao C. Genome editing in rice and wheat using the CRISPR/Cas system. Nature Protocols. 2014;9:2395–2410. doi: 10.1038/nprot.2014.157. [DOI] [PubMed] [Google Scholar]
- Shorinola O, Balcárková B, Hyles J, Tibbits JFG, Hayden MJ, Holušova K, Valárik M, Distelfeld A, Torada A, Barrero JM, Uauy C. Haplotype analysis of the Pre-harvest sprouting resistance locus Phs-A1 Reveals a Causal Role of TaMKK3-A in Global Germplasm. Frontiers in Plant Science. 2017;8:1555. doi: 10.3389/fpls.2017.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds J, Scott P, Brinton J, Mestre TC, Bush M, Del Blanco A, Dubcovsky J, Uauy C. A splice acceptor site mutation in TaGW2-A1 increases thousand grain weight in tetraploid and hexaploid wheat through wider and longer grains. Theoretical and Applied Genetics. 2016;129:1099–1112. doi: 10.1007/s00122-016-2686-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JM, Guan Z, Hu J, Guo C, Yang Z, Wang S, Liu D, Wang B, Lu S, Zhou R, Xie WZ, Cheng Y, Zhang Y, Liu K, Yang QY, Chen LL, Guo L. Eight high-quality genomes reveal pan-genome architecture and ecotype differentiation of Brassica napus. Nature Plants. 2020;6:34–45. doi: 10.1038/s41477-019-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks CA, Doherty A, Jones HD. Genetic transformation of wheat via Agrobacterium-mediated DNA delivery. In: Henry R. J, Furtado A, editors. Cereal Genomics. Totowa, NJ: Humana Press; 2014. pp. 235–250. [DOI] [PubMed] [Google Scholar]
- Sparks CA, Jones HD. Biolistics transformation of wheat. In: Jones HD, Shewry PR, editors. Transgenic Wheat, Barley and Oats: Production and Characterization Protocols. Totowa, NJ: Humana Press; 2009. pp. 71–92. [DOI] [Google Scholar]
- Stadlmeier M, Hartl L, Mohler V. Usefulness of a multiparent advanced generation intercross population with a greatly reduced mating design for genetic studies in winter wheat. Frontiers in Plant Science. 2018;9:1825. doi: 10.3389/fpls.2018.01825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The International Wheat Genome Sequencing Consortium (IWGSC) Appels R, Eversole K, Feuillet C, Keller B, Rogers J, Stein N, Pozniak CJ, Stein N, Choulet F, Distelfeld A, Eversole K, Poland J, Rogers J, Ronen G, Sharpe AG, Pozniak C, Ronen G, Stein N, Barad O, Baruch K, Choulet F, Keeble-Gagnère G, Mascher M, Sharpe AG, Ben-Zvi G, Josselin AA, Stein N, Mascher M, Himmelbach A, Choulet F, Keeble-Gagnère G, Mascher M, Rogers J, Balfourier F, Gutierrez-Gonzalez J, Hayden M, Josselin AA, Koh C, Muehlbauer G, Pasam RK, Paux E, Pozniak CJ, Rigault P, Sharpe AG, Tibbits J, Tiwari V, Choulet F, Keeble-Gagnère G, Mascher M, Josselin AA, Rogers J, Spannagl M, Choulet F, Lang D, Gundlach H, Haberer G, Keeble-Gagnère G, Mayer KFX, Ormanbekova D, Paux E, Prade V, Šimková H, Wicker T, Choulet F, Spannagl M, Swarbreck D, Rimbert H, Felder M, Guilhot N, Gundlach H, Haberer G, Kaithakottil G, Keilwagen J, Lang D, Leroy P, Lux T, Mayer KFX, Twardziok S, Venturini L, Appels R, Rimbert H, Choulet F, Juhász A, Keeble-Gagnère G, Choulet F, Spannagl M, Lang D, Abrouk M, Haberer G, Keeble-Gagnère G, Mayer KFX, Wicker T, Choulet F, Wicker T, Gundlach H, Lang D, Spannagl M, Lang D, Spannagl M, Appels R, Fischer I, Uauy C, Borrill P, Ramirez-Gonzalez RH, Appels R, Arnaud D, Chalabi S, Chalhoub B, Choulet F, Cory A, Datla R, Davey MW, Hayden M, Jacobs J, Lang D, Robinson SJ, Spannagl M, Steuernagel B, Tibbits J, Tiwari V, van Ex F, Wulff BBH, Pozniak CJ, Robinson SJ, Sharpe AG, Cory A, Benhamed M, Paux E, Bendahmane A, Concia L, Latrasse D, Rogers J, Jacobs J, Alaux M, Appels R, Bartoš J, Bellec A, Berges H, Doležel J, Feuillet C, Frenkel Z, Gill B, Korol A, Letellier T, Olsen OA, Šimková H, Singh K, Valárik M, van der Vossen E, Vautrin S, Weining S, Korol A, Frenkel Z, Fahima T, Glikson V, Raats D, Rogers J, Tiwari V, Gill B, Paux E, Poland J, Doležel J, Číhalíková J, Šimková H, Toegelová H, Vrána J, Sourdille P, Darrier B, Appels R, Spannagl M, Lang D, Fischer I, Ormanbekova D, Prade V, Barabaschi D, Cattivelli L, Hernandez P, Galvez S, Budak H, Steuernagel B, Jones JDG, Witek K, Wulff BBH, Yu G, Small I, Melonek J, Zhou R, Juhász A, Belova T, Appels R, Olsen OA, Kanyuka K, King R, Nilsen K, Walkowiak S, Pozniak CJ, Cuthbert R, Datla R, Knox R, Wiebe K, Xiang D, Rohde A, Golds T, Doležel J, Čížková J, Tibbits J, Budak H, Akpinar BA, Biyiklioglu S, Muehlbauer G, Poland J, Gao L, Gutierrez-Gonzalez J, N'Daiye A, Doležel J, Šimková H, Číhalíková J, Kubaláková M, Šafář J, Vrána J, Berges H, Bellec A, Vautrin S, Alaux M, Alfama F, Adam-Blondon AF, Flores R, Guerche C, Letellier T, Loaec M, Quesneville H, Pozniak CJ, Sharpe AG, Walkowiak S, Budak H, Condie J, Ens J, Koh C, Maclachlan R, Tan Y, Wicker T, Choulet F, Paux E, Alberti A, Aury JM, Balfourier F, Barbe V, Couloux A, Cruaud C, Labadie K, Mangenot S, Wincker P, Gill B, Kaur G, Luo M, Sehgal S, Singh K, Chhuneja P, Gupta OP, Jindal S, Kaur P, Malik P, Sharma P, Yadav B, Singh NK, Khurana J, Chaudhary C, Khurana P, Kumar V, Mahato A, Mathur S, Sevanthi A, Sharma N, Tomar RS, Rogers J, Jacobs J, Alaux M, Bellec A, Berges H, Doležel J, Feuillet C, Frenkel Z, Gill B, Korol A, van der Vossen E, Vautrin S, Gill B, Kaur G, Luo M, Sehgal S, Bartoš J, Holušová K, Plíhal O, Clark MD, Heavens D, Kettleborough G, Wright J, Valárik M, Abrouk M, Balcárková B, Holušová K, Hu Y, Luo M, Salina E, Ravin N, Skryabin K, Beletsky A, Kadnikov V, Mardanov A, Nesterov M, Rakitin A, Sergeeva E, Handa H, Kanamori H, Katagiri S, Kobayashi F, Nasuda S, Tanaka T, Wu J, Appels R, Hayden M, Keeble-Gagnère G, Rigault P, Tibbits J, Olsen OA, Belova T, Cattonaro F, Jiumeng M, Kugler K, Mayer KFX, Pfeifer M, Sandve S, Xun X, Zhan B, Šimková H, Abrouk M, Batley J, Bayer PE, Edwards D, Hayashi S, Toegelová H, Tulpová Z, Visendi P, Weining S, Cui L, Du X, Feng K, Nie X, Tong W, Wang L, Borrill P, Gundlach H, Galvez S, Kaithakottil G, Lang D, Lux T, Mascher M, Ormanbekova D, Prade V, Ramirez-Gonzalez RH, Spannagl M, Stein N, Uauy C, Venturini L, Stein N, Appels R, Eversole K, Rogers J, Borrill P, Cattivelli L, Choulet F, Hernandez P, Kanyuka K, Lang D, Mascher M, Nilsen K, Paux E, Pozniak CJ, Ramirez-Gonzalez RH, Šimková H, Small I, Spannagl M, Swarbreck D, Uauy C. Shifting the limits in wheat research and breeding using a fully annotated reference genome. Science. 2018;361:eaar7191. doi: 10.1126/science.aar7191. [DOI] [PubMed] [Google Scholar]
- Thole V, Bassard JE, Ramírez-González R, Trick M, Ghasemi Afshar B, Breitel D, Hill L, Foito A, Shepherd L, Freitag S, Nunes Dos Santos C, Menezes R, Bañados P, Naesby M, Wang L, Sorokin A, Tikhonova O, Shelenga T, Stewart D, Vain P, Martin C. RNA-seq, de novo transcriptome assembly and flavonoid gene analysis in 13 wild and cultivated berry fruit species with high content of phenolics. BMC Genomics. 2019;20:995. doi: 10.1186/s12864-019-6183-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torada A, Koike M, Ogawa T, Takenouchi Y, Tadamura K, Wu J, Matsumoto T, Kawaura K, Ogihara Y. A causal gene for seed dormancy on wheat chromosome 4A encodes a MAP kinase kinase. Current Biology. 2016;26:782–787. doi: 10.1016/j.cub.2016.01.063. [DOI] [PubMed] [Google Scholar]
- Tsai H, Missirian V, Ngo KJ, Tran RK, Chan SR, Sundaresan V, Comai L. Production of a high-efficiency TILLING population through polyploidization. Plant Physiology. 2013;161:1604–1614. doi: 10.1104/pp.112.213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J. A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science. 2006;314:1298–1301. doi: 10.1126/science.1133649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uauy C, Wulff BBH, Dubcovsky J. Combining traditional mutagenesis with new High-Throughput sequencing and genome editing to reveal hidden variation in polyploid wheat. Annual Review of Genetics. 2017;51:435–454. doi: 10.1146/annurev-genet-120116-024533. [DOI] [PubMed] [Google Scholar]
- Vasil V, Castillo AM, Fromm ME, Vasil IK. Herbicide resistant fertile transgenic wheat plants obtained by microprojectile bombardment of regenerable embryogenic callus. Nature Biotechnology. 1992;10:667–674. doi: 10.1038/nbt0692-667. [DOI] [Google Scholar]
- Verbyla AP, Cullis BR, Thompson R. The analysis of QTL by simultaneous use of the full linkage map. Theoretical and Applied Genetics. 2007;116:95–111. doi: 10.1007/s00122-007-0650-x. [DOI] [PubMed] [Google Scholar]
- Verbyla AP, George AW, Cavanagh CR, Verbyla KL. Whole-genome QTL analysis for MAGIC. Theoretical and Applied Genetics. 2014;127:1753–1770. doi: 10.1007/s00122-014-2337-4. [DOI] [PubMed] [Google Scholar]
- Vilella AJ, Severin J, Ureta-Vidal A, Heng L, Durbin R, Birney E. EnsemblCompara GeneTrees: complete, duplication-aware phylogenetic trees in vertebrates. Genome Research. 2009;19:327–335. doi: 10.1101/gr.073585.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallington E. NIAB Crop Transformation. NIAB; 2015. https://www.niab.com/services/plant-breeding/crop-transformation [Google Scholar]
- Wang S, Wong D, Forrest K, Allen A, Chao S, Huang BE, Maccaferri M, Salvi S, Milner SG, Cattivelli L, Mastrangelo AM, Whan A, Stephen S, Barker G, Wieseke R, Plieske J, Lillemo M, Mather D, Appels R, Dolferus R, Brown-Guedira G, Korol A, Akhunova AR, Feuillet C, Salse J, Morgante M, Pozniak C, Luo MC, Dvorak J, Morell M, Dubcovsky J, Ganal M, Tuberosa R, Lawley C, Mikoulitch I, Cavanagh C, Edwards KJ, Hayden M, Akhunov E, International Wheat Genome Sequencing Consortium Characterization of polyploid wheat genomic diversity using a high-density 90,000 single nucleotide polymorphism array. Plant Biotechnology Journal. 2014a;12:787–796. doi: 10.1111/pbi.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cheng X, Shan Q, Zhang Y, Liu J, Gao C, Qiu J-L. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nature Biotechnology. 2014b;32:947–951. doi: 10.1038/nbt.2969. [DOI] [PubMed] [Google Scholar]
- Wang K, Liu H, Du L, Ye X. Generation of marker-free transgenic hexaploid wheat via an Agrobacterium-mediated co-transformation strategy in commercial chinese wheat varieties. Plant Biotechnology Journal. 2017;15:614–623. doi: 10.1111/pbi.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey MD, Asyraf Md Hatta M, Hinchliffe A, Steed A, Reynolds D, Adamski NM, Breakspear A, Korolev A, Rayner T, Dixon LE, Riaz A, Martin W, Ryan M, Edwards D, Batley J, Raman H, Carter J, Rogers C, Domoney C, Moore G, Harwood W, Nicholson P, Dieters MJ, DeLacy IH, Zhou J, Uauy C, Boden SA, Park RF, Wulff BBH, Hickey LT. Speed breeding is a powerful tool to accelerate crop research and breeding. Nature Plants. 2018;4:23–29. doi: 10.1038/s41477-017-0083-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson PA, Winfield MO, Barker GL, Tyrrell S, Bian X, Allen AM, Burridge A, Coghill JA, Waterfall C, Caccamo M, Davey RP, Edwards KJ. CerealsDB 3.0: expansion of resources and data integration. BMC Bioinformatics. 2016;17:256. doi: 10.1186/s12859-016-1139-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield MO, Allen AM, Burridge AJ, Barker GL, Benbow HR, Wilkinson PA, Coghill J, Waterfall C, Davassi A, Scopes G, Pirani A, Webster T, Brew F, Bloor C, King J, West C, Griffiths S, King I, Bentley AR, Edwards KJ. High-density SNP genotyping array for hexaploid wheat and its secondary and tertiary gene pool. Plant Biotechnology Journal. 2016;14:1195–1206. doi: 10.1111/pbi.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingen LU, Orford S, Goram R, Leverington-Waite M, Bilham L, Patsiou TS, Ambrose M, Dicks J, Griffiths S. Establishing the A. E. Watkins landrace cultivar collection as a resource for systematic gene discovery in bread wheat. Theoretical and Applied Genetics. 2014;127:1831–1842. doi: 10.1007/s00122-014-2344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingen LU, West C, Leverington-Waite M, Collier S, Orford S, Goram R, Yang CY, King J, Allen AM, Burridge A, Edwards KJ, Griffiths S. Wheat landrace genome diversity. Genetics. 2017;205:1657–1676. doi: 10.1534/genetics.116.194688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter D, Vinegar B, Nahal H, Ammar R, Wilson GV, Provart NJ. An "Electronic Fluorescent Pictograph" browser for exploring and analyzing large-scale biological data sets. PLOS ONE. 2007;2:e718. doi: 10.1371/journal.pone.0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liang Z, Zong Y, Wang Y, Liu J, Chen K, Qiu JL, Gao C. Efficient and transgene-free genome editing in wheat through transient expression of CRISPR/Cas9 DNA or RNA. Nature Communications. 2016;7:12617. doi: 10.1038/ncomms12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yu D, Zhang Y, Liu K, Xu K, Zhang F, Wang J, Tan G, Nie X, Ji Q, Zhao L, Li C. Vacuum and Co-cultivation agroinfiltration of (Germinated) Seeds results in tobacco rattle virus (TRV) Mediated Whole-Plant Virus-Induced gene silencing (VIGS) in wheat and maize. Frontiers in Plant Science. 2017a;8:393. doi: 10.3389/fpls.2017.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Bai Y, Wu G, Zou S, Chen Y, Gao C, Tang D. Simultaneous modification of three homoeologs of TaEDR1 by genome editing enhances powdery mildew resistance in wheat. The Plant Journal. 2017b;91:714–724. doi: 10.1111/tpj.13599. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zou C, Li K, Wang K, Li T, Gao L, Zhang X, Wang H, Yang Z, Liu X, Jiang W, Mao L, Kong X, Jiao Y, Jia J. The Aegilops tauschii genome reveals multiple impacts of transposons. Nature Plants. 2017;3:946–955. doi: 10.1038/s41477-017-0067-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Chebotarov D, Kudrna D, Llaca V, Lee S, Rajasekar S, Mohammed N, Al-Bader N, Sobel-Sorenson C, Parakkal P, Arbelaez LJ, Franco N, Alexandrov N, Hamilton NRS, Leung H, Mauleon R, Lorieux M, Zuccolo A, McNally K, Zhang J, Wing RA. Twelve Platinum-Standard reference genomes sequences (PSRefSeq) that complete the full range of genetic diversity of asian rice. bioRxiv. 2019 doi: 10.1101/2019.12.29.888347. [DOI]
- Zimin AV, Puiu D, Hall R, Kingan S, Clavijo BJ, Salzberg SL. The first near-complete assembly of the hexaploid bread wheat genome, Triticum aestivum. GigaScience. 2017;6:1–7. doi: 10.1093/gigascience/gix097. [DOI] [PMC free article] [PubMed] [Google Scholar]