Abstract
Myocardial infarction is characterized by sudden ischemia and cardiomyocyte death. Mitochondria have critical roles in regulating cardiomyocyte viability and can sustain damage under ischemic conditions. Mitophagy is a mechanism by which damaged mitochondria are removed by autophagy to maintain mitochondrial structure and function. We investigated the role of the dynamin-like GTPase optic atrophy 1 (Opa1) in mitophagy following myocardial infarction. Opa1 expression was downregulated in infarcted hearts in vivo and in hypoxia-treated cardiomyocytes in vitro. We found that Opa1 overexpression protected cardiomyocytes against hypoxia-induced damage and enhanced cell viability by inducing mitophagy. Opa1-induced mitophagy was activated by treatment with irisin, which protected cardiomyocytes from further damage following myocardial infarction. Opa1 knockdown abolished the cardioprotective effects of irisin resulting in an enhanced inflammatory response, increased oxidative stress, and mitochondrial dysfunction in cardiomyocytes. Our data indicate that Opa1 plays an important role in maintaining cardiomyocyte viability and mitochondrial function following myocardial infarction by inducing mitophagy. Irisin can activate Opa1-induced mitophagy and protect against cardiomyocyte injury following myocardial infarction.
Keywords: Opa1, irisin, myocardial infarction, mitophagy, mitochondria
INTRODUCTION
Myocardial infarction is characterized by sudden ischemia, cardiomyocyte death, and fibrosis [1, 2]. Many signaling pathways can cause cardiomyocyte death including the mitochondrial apoptosis pathway and the inflammatory response [3]. Antiapoptotic and antioxidative agents have been shown to reduce cardiomyocyte damage [4]. Additionally, therapeutic strategies that target the mitochondria in damaged cardiomyocytes may protect against cardiomyocyte death following myocardial infarction [5, 6]. Damaged mitochondria release reactive oxygen species (ROS) into the cytoplasm, which can lead to apoptosis [7]. Mitochondria are important for producing ATP to sustain cardiomyocyte contractility and function. They also release pro-apoptotic factors to initiate programmatic cell death and are therefore critical regulators of cell face and function [8, 9].
Mitophagy is a mechanism by which damaged mitochondria are removed by autophagy to maintain mitochondrial structure and function. Mitophagy can block the release of ROS release from mitochondria and prevent apoptosis following mitochondrial damage in a lysosome-dependent process [10, 11]. Optic atrophy 1 (Opa1) is a dynamin-like GTPase that regulates fusion of the mitochondrial inner membrane [12]. Opa1 has been shown to regulate mitophagy through fusion-dependent and -independent mechanisms [13]. Interestingly, Opa1 has been shown to have cardioprotective effects [14]. Increased Opa1 expression was shown to reduce oxidative stress in cardiomyocytes in hypoxia-reperfusion injury through Ca2+/calmodulin-dependent protein kinase II (CaMKII signaling) [15]. Additionally, Opa1 upregulation reduced myocardial ischemia through activation of the Brain-derived neurotrophic factor (BDNF)/tropomyosin-related kinase B (TrkB) pathway [16]. Reduced Opa1 expression inhibited mitophagy resulting in myocardial ischemia-reperfusion injury [17]. Finally, inhibition of mitophagy through PTEN-dependent upregulation of Opa1 protected cardiomyocytes against lipopolysaccharide (LPS)-mediated inflammation in a model of septic cardiomyopathy [18–20]. Thus, Opa1 may play a role in protecting cardiomyocytes from damage following myocardial infarction by regulating mitophagy [21, 22].
Mitophagy is important for regulating mitochondrial energy metabolism, oxidative stress, and apoptosis [23, 24]. Here, we investigated whether Opa1 promotes mitophagy to protect cardiomyocytes following myocardial infarction. Additionally, we explored whether irisin, a drug that has been shown to regulate mitochondrial function and suppress septic cardiomyopathy, could regulate Opa1-induced mitophagy following myocardial infarction [25–28].
RESULTS
Opa1 is downregulated in the infarcted heart and in hypoxia-treated cardiomyocytes
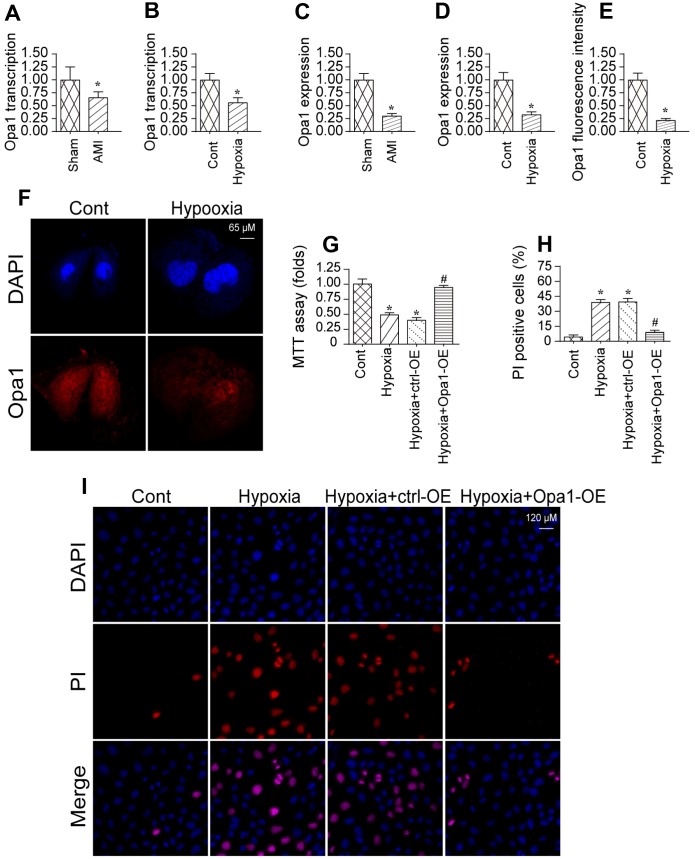
We previously established an in vivo model of myocardial infarction [29, 30]. Using that model, we analyzed Opa1 expression in infarcted hearts compared to controls using quantitative real-time PCR and western blotting. Opa1 expression was lower in infarcted hearts compared to controls (sham) indicating Opa1 expression is downregulated following myocardial infarction (Figure 1A–1D). A reduction in Opa1 expression was also observed by immunofluorescence.
Figure 1.
Opa1 is downregulated in the infarcted heart and in hypoxia-treated cardiomyocytes. (A–D) Quantitative real-time PCR and western blot analysis of Opa1 expression. (E, F) Analysis of Opa1 expression in cardiomyocytes in vitro by immunofluorescence. (G) MTT assays of cardiomyocyte viability. (H, I) Analysis of cardiomyocyte apoptosis by PI staining. *P < 0.05 vs. the control group; #P < 0.05 vs. the hypoxia + ctrl-OE group.
We also established an in vitro model of myocardial infarction in which cardiomyocytes were subjected to hypoxic conditions. Opa1 expression was reduced in cardiomyocytes cultured under hypoxic conditions for 48 hours compared to controls (Figure 1E, 1F), which is consistent with those of a previous findings [31]. Overexpression of Opa1 increased the viability hypoxia-treated cardiomyocytes as compared to untransfected controls (Figure 1G). Correspondingly, Opa1 overexpression in reduced the incidence of apoptosis among hypoxia-treated cardiomyocytes (Figure 1H–1I). These data suggest that Opa1 is important for protecting cardiomyocytes against hypoxia-induced damage.
Opa1 mediates mitophagy in the infarcted heart
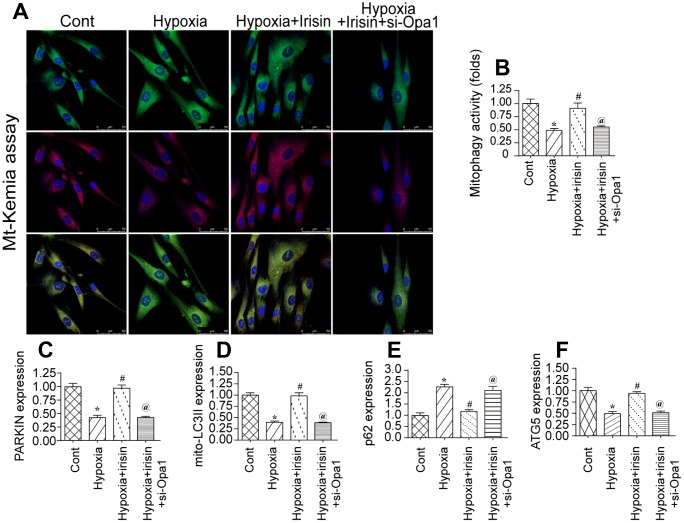
Previous studies demonstrated that Opa1 can promote mitophagy [32, 33]. We therefore investigated the effect of hypoxia on mitophagy in cardiomyocytes by flow cytometry using the fluorescent reporter mt-Keima. We observed a reduction in mitophagy in hypoxia-treated cardiomyocytes compared to controls (Figure 2A, 2B). Interestingly, Opa1 overexpression resulted in an increase in mitophagy in hypoxia-treated cardiomyocytes, suggesting it may induce mitophagy in the infarcted heart [34, 35].
Figure 2.
Irisin activates Opa1-induced mitophagy. (A, B) Flow cytometry analysis of mitophagy using the fluorescent probe mt-Keima. (C–F) Analysis of the expression of mitophagy-associated proteins by western blotting. *P < 0.05 vs. the control group; #P < 0.05 vs. the hypoxia + irisin group.
We previously demonstrated that irisin modulated mitochondrial function in a model of septic cardiomyopathy. We therefore hypothesized that irisin could modulate Opa1-induced mitophagy in hypoxia-treated cardiomyocytes following myocardial infarction. Interestingly, we observed a decrease in the levels of various mitophagy-associated proteins under hypoxic conditions by western blotting. This effect was reversed by treatment with irisin, suggesting that irisin can activate Opa1-induced mitophagy in cardiomyocytes under hypoxic stress (Figure 2C–2F).
Irisin activates Opa1-induced mitophagy and restores mitochondrial energy metabolism
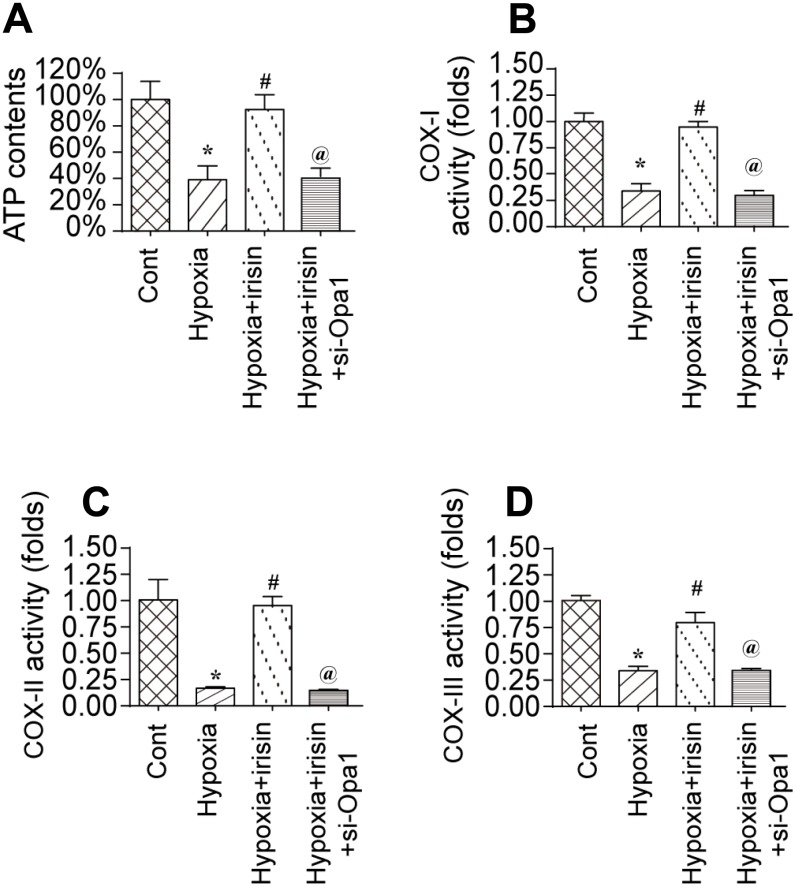
To investigate the mechanisms underlying the protective effects of Opa1-induced mitophagy, we evaluated the alterations in mitochondrial function [36]. A reduction in the levels of mitochondria-derived ATP was observed in hypoxia-treated cardiomyocytes. Treatment with irisin resulted in an increase in ATP levels in hypoxia-treated cardiomyocytes compared to controls (Figure 3A) [37, 38]. The increase in ATP was inhibited by knockdown of Opa1 by siRNA (si-Opa1) (Figure 3A). We also observed downregulation of the levels of the mitochondrial respiratory complex in response to hypoxia, which was reversed by treatment with irisin (Figure 3B–3D). Knockdown of Opa1 by siRNA abolished the irisin-mediated protective effects on the mitochondrial respiratory complex (Figure 3B–3D). These results indicate that irisin exerts cardioprotective effects by activating Opa1-induced mitophagy.
Figure 3.
Irisin activates Opa1-induced mitophagy to restore mitochondrial energy metabolism. (A) Measurement of ATP production by ELISA. (B–D) Measurement of mitochondrial respiratory complex activity by ELISA. *P < 0.05 vs. the control group; #P < 0.05 vs. the hypoxia + irisin group.
Opa1-induced mitophagy maintains mitochondrial function and reduces oxidative stress
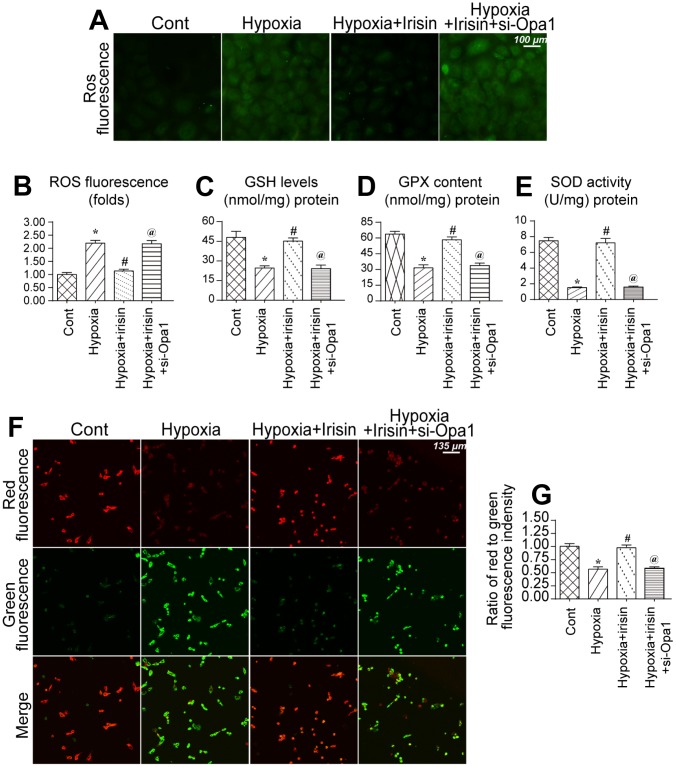
We further analyzed the protective effects of irisin and Opa1-induced mitophagy following myocardial infarction [39, 40]. An increase in ROS in mitochondria was observed in hypoxia-treated cardiomyocytes (Figure 4A, 4B). Irisin reduced the levels of ROS whereas Opa1 knockdown by siRNA suppressed the antioxidative effects of irisin in hypoxia-treated cardiomyocytes (Figure 4A, 4B). Additionally, we found that the levels of components of the antioxidative system including glutathione (GSH), superoxide dismutase (SOD), and glutathione peroxidase (GPX), were reduced under conditions of hypoxic stress (Figure 4C–4E). Interestingly, irisin promoted Opa1-induced mitophagy and increased the levels of GSH, SOD, and GPX (Figure 4C–4E). Thus, irisin promotes Opa1-induced mitophagy, which reduces oxidative stress.
Figure 4.
Opa1-induced mitophagy maintains mitochondrial function and reduces oxidative stress. (A, B) Analysis of ROS levels in cardiomyocytes. (C–E) ELISA assays to evaluate the levels of antioxidants. (F, G) Measurement of alterations in the mitochondrial membrane potential using a JC-1 probe. *P < 0.05 vs. the control group; #P < 0.05 vs. the hypoxia + irisin group.
We also found that the mitochondrial membrane potential, a marker of mitochondrial function, was disrupted by hypoxic stress (Figure 4F, 4G) [41, 42]. Irisin treatment restored the mitochondrial membrane potential in hypoxia-treated cardiomyocytes whereas Opa1 knockdown disrupted the mitochondrial membrane potential in irisin-treated cardiomyocytes (Figure 4F, 4G). These data indicate Opa1-induced mitophagy is important for maintaining mitochondrial function and reducing oxidative stress in cardiomyocytes.
Opa1-induced mitophagy inhibits apoptosis
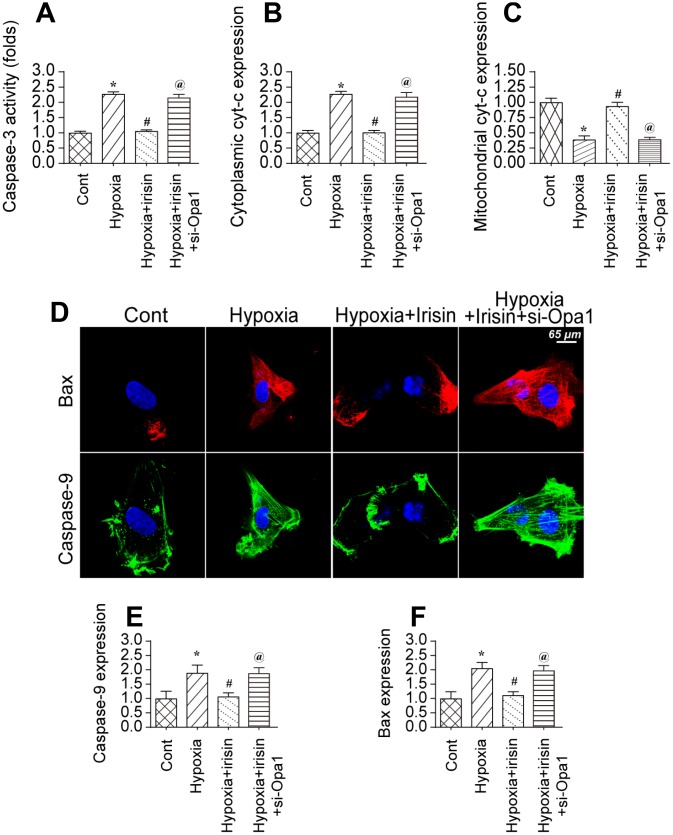
We investigated whether Opa1-induced mitophagy could protect cardiomyocytes against hypoxia-induced apoptosis [43]. Caspase-3 activity increased in hypoxia-treated cardiomyocytes, which was indicative of activation of apoptotic cell death (Figure 5A) [44]. Treatment of hypoxia-treated cardiomyocytes with irisin inhibited caspase-3 activation. Finally, knockdown of Opa1 enhanced caspase-3 activation in irisin-treated cardiomyocytes (Figure 5A). These data indicate that the antiapoptotic effects of irisin are mediated by Opa1-induced mitophagy.
Figure 5.
Opa1-induced mitophagy inhibits the mitochondrial apoptosis pathway. (A) Analysis of caspase-3 activity in cardiomyocytes by ELISA. (B, C) Western blot analysis of cyt-c levels in the cytoplasm and mitochondria. (D–F) Immunofluorescence analysis of Bax and caspase-9 expression in cardiomyocytes. *P < 0.05 vs. the control group; #P < 0.05 vs. the hypoxia + irisin group.
The mitochondrial apoptotic pathway is characterized by cytochrome C (cyt-c) translocation to the cytoplasm. We observed an increase in cytoplasmic cyt-c levels by western blotting in hypoxia-treated cardiomyocytes (Figure 5B, 5C). Irisin treatment resulted in a decrease in the levels of cytoplasmic cyt-c in an Opa1-dependent manner (Figure 5B, 5C). The mitochondrial apoptotic pathway is also characterized by upregulation of caspase-9 and Bax [45]. We observed upregulation of both caspase-9 and Bax in hypoxia-treated cardiomyocytes by immunofluorescence (Figure 5D–5F). Treatment with irisin resulted in downregulation of caspase-9 and Bax in hypoxia-treated cardiomyocytes (Figure 5D–5F). Knockdown of Opa1 by siRNA resulted in an increase in caspase-9 and Bax levels in irisin- and hypoxia-treated cardiomyocytes (Figure 5D–5F). These data suggest that the anti-apoptotic effects of irisin require activation of Opa1-induced mitophagy in hypoxia-treated cardiomyocytes.
DISCUSSION
Cardiomyocyte damage and death contributes to the pathogenesis of myocardial infarction [46, 47]. Myocardial infarction is caused by the sudden loss of blood supply to the heart and leads to cardiomyocyte death [48–50]. Death of cardiomyocytes results in activation of the inflammatory response [51, 52]. Additionally, the lack of blood flow and nutrients activates the transcription of pro-inflammatory factors that further enhance inflammation of the myocardium [53, 54]. Excessive inflammation can lead to the accumulation of pro-inflammatory cells such as white blood cells [55]. Although white blood cells are involved in repairing damage to the myocardium, they can also induce oxidative stress [56, 57]. The accumulation of these pro-inflammatory factors increases cardiomyocyte damage [58, 59].
Both mitochondria-dependent and -independent apoptotic pathways can lead to cardiomyocyte death [60, 61]. The mitochondria-dependent pathway is initiated in response to unrepaired mitochondrial damage. In contrast, the mitochondria-independent pathway is regulated by the endoplasmic reticulum [62, 63], lysosomes [64], and other factors. There are several differences between the mitochondria-dependent and -independent apoptosis pathways [65, 66]. The mitochondria-dependent pathway involves a reduction in mitochondrial membrane potential and activation of caspase-9 whereas the mitochondria-independent pathway is characterized by activation of caspase-3 and cellular membrane rupture [67]. We found that mitochondria-dependent apoptosis occurs during the progression of myocardial infarction. However, we could not exclude the possibility of mitochondria-independent apoptosis [68, 69]. We also found that the inflammatory response and oxidative stress were activated following myocardial infarction and could trigger cardiomyocyte death through the mitochondria-dependent apoptosis pathway [70].
Several studies have explored approaches for blocking mitochondria-dependent apoptosis in cardiomyocytes [71]. Mitophagy is a protective mechanism that involves the selective removal of dysfunctional mitochondria thereby allowing cellular repair [72]. Several proteins that are important for autophagy also play a role in mitophagy [73]. However, there are some proteins that are only involved in mitophagy including FUNDC1, BNIP3, PARK2, and NIX [74]. These proteins may have roles in cardiovascular diseases including myocardial infarction.
We previously demonstrated that the inner mitochondrial membrane protein Opa1 has an important role in promoting mitophagy [75, 76]. Consistent with previous studies, we found that irisin could activate Opa1-induced mitophagy in cardiomyocytes [77, 78]. Irisin treatment was associated with an increase in Opa1 expression. However, we did not evaluate whether there are differences in the expression of the Opa1 isoforms (L-Opa1 and S-Opa1) [79, 80]. Interestingly, Opa1 knockdown suppressed mitophagy and abolished the cardioprotective effects of irisin, suggesting that the protective effects of irisin on cardiomyocytes following myocardial infarction are dependent upon Opa1-induced mitophagy [81, 82].
Collectively, our data indicate that Opa1 plays an important role in regulating cardiomyocyte viability following myocardial infarction by activating mitophagy. Irisin can activate Opa1-induced mitophagy in the infarcted heart and could have therapeutic efficacy in patients with acute myocardial injury.
MATERIALS AND METHODS
Cell culture and animal models
Primary cardiomyocytes were isolated from and a model of myocardial infarction established as described [83, 84]. Cells were cultured under hypoxic conditions for 48 hours to mimic myocardial infarction [24, 85]. Cardiomyocytes were cultured in Dulbecco’s Modified Eagle Medium (DMEM, Sigma, St. Louis, MO, USA) supplemented with 10% fetal bovine serum (Sigma, St. Louis, MO, USA) in a humidified atmosphere of 5% CO2 at 37°C. Irisin treatment was performed as described previously [86, 87].
Immunofluorescence staining
Cells seeded in plates were fixed with 4% paraformaldehyde and then blocked and permeabilized in solution containing 3% BSA (Sigma Aldrich, St. Louis, MO, USA), 10% normal goat serum (Vector Laboratories, Burlingame, CA, USA), and 0.3% Triton X-100 (Sigma Aldrich, St. Louis, MO, USA). The cells were then incubated with the indicated primary antibodies (Cell Signaling Technology, Danvers, MA, USA; Abcam, Cambridge, MA, USA) [88, 89]. Following the incubation, the cells were washed and incubated with corresponding Alexa Fluor secondary antibodies (Life Technologies, Carlsbad, CA, USA) [90, 91]. Lipid droplets and nuclei were stained with Hoechst 33342 prior to imaging the cells by confocal microscopy [92].
ROS measurement
Intracellular ROS levels were measured in cells plated in 6-well dishes at an equal density. The cells were trypsinized, washed with phosphate-buffered saline (PBS) [93, 94], and then stained with 2 μmol/L chloromethyl-20,70-dichlorodihydrofluorescein diacetate (CM-H2-DCFDA) (Life Technologies, Carlsbad, CA, USA). Relative cell counts were determined using a FACSCanto II Analyzer flow cytometer (BD Biosciences, San Jose, CA, USA). Data were analyzed using the FlowJo software (FlowJo, LLC, Ashland, OR, USA) [95].
Opa1 knockdown and overexpression
Opa1 knockdown was performed using siRNA as described [96–99]. Briefly, cardiomyocytes were cultured in 6-well plates (1 x 106 / well), 6 cm dishes (3 x 106 / dish), or petri dishes (3 x 105 / well) [100]. The siRNA dose was the following: 50 pmol / petri dish, 100 pmol / 6-well plates, 166 pmol / 6 cm dish, and 434 pmol / 10 cm dish. Cell transfection with siRNA was performed using the Lipofectamine RNAiMAX Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions [101]. Knockdown efficiency was evaluated 36 hours after transfection by quantitative real-time PCR. Adenovirus overexpression assays were performed as described previously [102].
Western blotting
Cells were washed with PBS and lysed in cold lysis buffer containing a protease inhibitor cocktail as described [103, 104]. Cell lysates were centrifuged at 13,000 g at 4 °C for 40 min. The supernatants were collected and total protein quantified [105]. Equal quantities of total protein were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes (Millipore, Bedford, MA, USA). The membranes were incubated with primary antibodies at 4°C for 15 hours [106]. Following the incubation, the membranes were incubated with the respective secondary antibodies at 25°C for 90 minutes. Proteins were visualized using the ECL substrate (Thermo Fisher Scientific, Waltham, MA, USA). GAPDH was used as a loading control [107, 108].
Quantitative real-time PCR
RNA from serum was extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol [109, 110]. The mRNA was reverse transcribed into cDNA using the First Strand Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) [111]. Real-time PCR was performed using an ABI7900 Real-time PCR system (Applied Biosystems, Foster City, CA, USA) using the SYBR Green Master Mix Kit (Takara, Dalian, China) [112]. We normalize mRNA expression to that of GAPDH. Relative gene expression was calculated using the comparative Ct method [113].
Enzyme-linked immunosorbent assays
The levels of antioxidants such as GSH, SOD, and GPX were evaluated using enzyme-linked immunosorbent assay (ELISA) kits (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions [112, 114].
Statistical analysis
Quantitative real-time PCR and ELISAs data were analyzed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA) [115]. The data are presented as the mean ± standard deviation. Differences between groups were analyzed using unpaired t-tests. *A P < 0.05 was considered statistically significant [116].
Footnotes
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
REFERENCES
- 1.Abukar Y, Ramchandra R, Hood SG, McKinley MJ, Booth LC, Yao ST, May CN. Increased cardiac sympathetic nerve activity in ovine heart failure is reduced by lesion of the area postrema, but not lamina terminalis. Basic Res Cardiol. 2018; 113:35. 10.1007/s00395-018-0695-9 [DOI] [PubMed] [Google Scholar]
- 2.Aluja D, Inserte J, Penela P, Ramos P, Ribas C, Iñiguez MA, Mayor F Jr, Garcia-Dorado D. Calpains mediate isoproterenol-induced hypertrophy through modulation of GRK2. Basic Res Cardiol. 2019; 114:21. 10.1007/s00395-019-0730-5 [DOI] [PubMed] [Google Scholar]
- 3.Amanakis G, Kleinbongard P, Heusch G, Skyschally A. Attenuation of ST-segment elevation after ischemic conditioning maneuvers reflects cardioprotection online. Basic Res Cardiol. 2019; 114:22. 10.1007/s00395-019-0732-3 [DOI] [PubMed] [Google Scholar]
- 4.Audia JP, Yang XM, Crockett ES, Housley N, Haq EU, O’Donnell K, Cohen MV, Downey JM, Alvarez DF. Caspase-1 inhibition by VX-765 administered at reperfusion in P2Y12 receptor antagonist-treated rats provides long-term reduction in myocardial infarct size and preservation of ventricular function. Basic Res Cardiol. 2018; 113:32. 10.1007/s00395-018-0692-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ham SW, Jeon HY, Jin X, Kim EJ, Kim JK, Shin YJ, Lee Y, Kim SH, Lee SY, Seo S, Park MG, Kim HM, Nam DH, Kim H. TP53 gain-of-function mutation promotes inflammation in glioblastoma. Cell Death Differ. 2019; 26:409–25. 10.1038/s41418-018-0126-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill SM, Wrobel L, Rubinsztein DC. Post-translational modifications of Beclin 1 provide multiple strategies for autophagy regulation. Cell Death Differ. 2019; 26:617–29. 10.1038/s41418-018-0254-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, Lindner D. Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol. 2019; 114:19. 10.1007/s00395-019-0722-5 [DOI] [PubMed] [Google Scholar]
- 8.Basalay MV, Davidson SM, Gourine AV, Yellon DM. Neural mechanisms in remote ischaemic conditioning in the heart and brain: mechanistic and translational aspects. Basic Res Cardiol. 2018; 113:25. 10.1007/s00395-018-0684-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hockings C, Alsop AE, Fennell SC, Lee EF, Fairlie WD, Dewson G, Kluck RM. Mcl-1 and Bcl-xL sequestration of Bak confers differential resistance to BH3-only proteins. Cell Death Differ. 2018; 25:721–34. 10.1038/s41418-017-0010-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang S, Li Y, Yuan X, Zhao M, Wang J, Li Y, Li Y, Lin H, Zhang Q, Wang W, Li D, Dong X, Li L, et al. The UbL-UBA Ubiquilin4 protein functions as a tumor suppressor in gastric cancer by p53-dependent and p53-independent regulation of p21. Cell Death Differ. 2019; 26:516–30. 10.1038/s41418-018-0141-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker HE, Kiel AM, Luebbe ST, Simon BR, Earl CC, Regmi A, Roell WC, Mather KJ, Tune JD, Goodwill AG. Inhibition of sodium-glucose cotransporter-2 preserves cardiac function during regional myocardial ischemia independent of alterations in myocardial substrate utilization. Basic Res Cardiol. 2019; 114:25. 10.1007/s00395-019-0733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beckendorf J, van den Hoogenhof MM, Backs J. Physiological and unappreciated roles of CaMKII in the heart. Basic Res Cardiol. 2018; 113:29. 10.1007/s00395-018-0688-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knupp J, Arvan P, Chang A. Increased mitochondrial respiration promotes survival from endoplasmic reticulum stress. Cell Death Differ. 2019; 26:487–501. 10.1038/s41418-018-0133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bøtker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, Efentakis P, Femminò S, García-Dorado D, et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol. 2018; 113:39. 10.1007/s00395-018-0696-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo H, Song S, Chen Y, Xu M, Sun L, Meng G, Zhang W. Inhibitor 1 of Protein Phosphatase 1 Regulates Ca2+/Calmodulin-Dependent Protein Kinase II to Alleviate Oxidative Stress in Hypoxia-Reoxygenation Injury of Cardiomyocytes. Oxid Med Cell Longev. 2019; 2019:2193019. 10.1155/2019/2193019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Z, Wang SP, Shao Q, Li PF, Sun Y, Luo LZ, Yan XQ, Fan ZY, Hu J, Zhao J, Hang PZ, Du ZM. Brain-derived neurotrophic factor mimetic, 7,8-dihydroxyflavone, protects against myocardial ischemia by rebalancing optic atrophy 1 processing. Free Radic Biol Med. 2019; 145:187–97. 10.1016/j.freeradbiomed.2019.09.033 [DOI] [PubMed] [Google Scholar]
- 17.Guan L, Che Z, Meng X, Yu Y, Li M, Yu Z, Shi H, Yang D, Yu M. MCU Up-regulation contributes to myocardial ischemia-reperfusion Injury through calpain/OPA-1-mediated mitochondrial fusion/mitophagy Inhibition. J Cell Mol Med. 2019; 23:7830–43. 10.1111/jcmm.14662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain R, Mintern JD, Tan I, Dewson G, Strasser A, Gray DH. How do thymic epithelial cells die? Cell Death Differ. 2018; 25:1002–04. 10.1038/s41418-018-0093-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jilg A, Bechstein P, Saade A, Dick M, Li TX, Tosini G, Rami A, Zemmar A, Stehle JH. Melatonin modulates daytime-dependent synaptic plasticity and learning efficiency. J Pineal Res. 2019; 66:e12553. 10.1111/jpi.12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Shi W, Zhang J, Ren L. To Explore the Protective Mechanism of PTEN-Induced Kinase 1 (PINK1)/Parkin Mitophagy-Mediated Extract of Periplaneta Americana on Lipopolysaccharide-Induced Cardiomyocyte Injury. Med Sci Monit. 2019; 25:1383–91. 10.12659/MSM.912980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao T, Fan S, Zheng D, Wang G, Yu Y, Chen R, Song LS, Fan GC, Zhang Z, Peng T. Increased calpain-1 in mitochondria induces dilated heart failure in mice: role of mitochondrial superoxide anion. Basic Res Cardiol. 2019; 114:17. 10.1007/s00395-019-0726-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeelani R, Maitra D, Chatzicharalampous C, Najeemuddin S, Morris RT, Abu-Soud HM. Melatonin prevents hypochlorous acid-mediated cyanocobalamin destruction and cyanogen chloride generation. J Pineal Res. 2018; 64:64. 10.1111/jpi.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coverstone ED, Bach RG, Chen L, Bierut LJ, Li AY, Lenzini PA, O’Neill HC, Spertus JA, Sucharov CC, Stitzel JA, Schilling JD, Cresci S. A novel genetic marker of decreased inflammation and improved survival after acute myocardial infarction. Basic Res Cardiol. 2018; 113:38. 10.1007/s00395-018-0697-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang CC, Chiou CH, Liu SC, Hu SL, Su CM, Tsai CH, Tang CH. Melatonin attenuates TNF-α and IL-1β expression in synovial fibroblasts and diminishes cartilage degradation: implications for the treatment of rheumatoid arthritis. J Pineal Res. 2019; 66:e12560. 10.1111/jpi.12560 [DOI] [PubMed] [Google Scholar]
- 25.Curley D, Lavin Plaza B, Shah AM, Botnar RM. Molecular imaging of cardiac remodelling after myocardial infarction. Basic Res Cardiol. 2018; 113:10. 10.1007/s00395-018-0668-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Bradley MD, Wagner BA, Buettner GR, Monga V, Milhem M, Spitz DR, Allen BG. Redox active metals and H2O2 mediate the increased efficacy of pharmacological ascorbate in combination with gemcitabine or radiation in pre-clinical sarcoma models. Redox Biol. 2018; 14:417–22. 10.1016/j.redox.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dassanayaka S, Brittian KR, Jurkovic A, Higgins LA, Audam TN, Long BW, Harrison LT, Militello G, Riggs DW, Chitre MG, Uchida S, Muthusamy S, Gumpert AM, Jones SP. E2f1 deletion attenuates infarct-induced ventricular remodeling without affecting O-GlcNAcylation. Basic Res Cardiol. 2019; 114:28. 10.1007/s00395-019-0737-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serrato AJ, Romero-Puertas MC, Lázaro-Payo A, Sahrawy M. Regulation by S-nitrosylation of the Calvin-Benson cycle fructose-1,6-bisphosphatase in Pisum sativum. Redox Biol. 2018; 14:409–16. 10.1016/j.redox.2017.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heusch G. Coronary microvascular obstruction: the new frontier in cardioprotection. Basic Res Cardiol. 2019; 114:45. 10.1007/s00395-019-0756-8 [DOI] [PubMed] [Google Scholar]
- 30.Zhou L, Zhang H, Davies KJ, Forman HJ. Aging-related decline in the induction of Nrf2-regulated antioxidant genes in human bronchial epithelial cells. Redox Biol. 2018; 14:35–40. 10.1016/j.redox.2017.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ciani E, Fontaine R, Maugars G, Mizrahi N, Mayer I, Levavi-Sivan B, Weltzien FA. Melatonin receptors in Atlantic salmon stimulate cAMP levels in heterologous cell lines and show season-dependent daily variations in pituitary expression levels. J Pineal Res. 2019; 67:e12590. 10.1111/jpi.12590 [DOI] [PubMed] [Google Scholar]
- 32.Chen Y, Liu K, Shi Y, Shao C. The tango of ROS and p53 in tissue stem cells. Cell Death Differ. 2018; 25:639–41. 10.1038/s41418-018-0062-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou YQ, Liu DQ, Chen SP, Sun J, Zhou XR, Rittner H, Mei W, Tian YK, Zhang HX, Chen F, Ye DW. Reactive oxygen species scavengers ameliorate mechanical allodynia in a rat model of cancer-induced bone pain. Redox Biol. 2018; 14:391–97. 10.1016/j.redox.2017.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cecon E, Ivanova A, Luka M, Gbahou F, Friederich A, Guillaume JL, Keller P, Knoch K, Ahmad R, Delagrange P, Solimena M, Jockers R. Detection of recombinant and endogenous mouse melatonin receptors by monoclonal antibodies targeting the C-terminal domain. J Pineal Res. 2019; 66:e12540. 10.1111/jpi.12540 [DOI] [PubMed] [Google Scholar]
- 35.Chen YT, Yang CC, Shao PL, Huang CR, Yip HK. Melatonin-mediated downregulation of ZNF746 suppresses bladder tumorigenesis mainly through inhibiting the AKT-MMP-9 signaling pathway. J Pineal Res. 2019; 66:e12536. 10.1111/jpi.12536 [DOI] [PubMed] [Google Scholar]
- 36.Hofmann F. A concise discussion of the regulatory role of cGMP kinase I in cardiac physiology and pathology. Basic Res Cardiol. 2018; 113:31. 10.1007/s00395-018-0690-1 [DOI] [PubMed] [Google Scholar]
- 37.Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase 1: beyond repair of the oxidatively modified base lesions. Redox Biol. 2018; 14:669–78. 10.1016/j.redox.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brazão V, Colato RP, Santello FH, Vale GT, Gonzaga NA, Tirapelli CR, Prado JC Jr. Effects of melatonin on thymic and oxidative stress dysfunctions during Trypanosoma cruzi infection. J Pineal Res. 2018; 65:e12510. 10.1111/jpi.12510 [DOI] [PubMed] [Google Scholar]
- 39.Honda T, He Q, Wang F, Redington AN. Acute and chronic remote ischemic conditioning attenuate septic cardiomyopathy, improve cardiac output, protect systemic organs, and improve mortality in a lipopolysaccharide-induced sepsis model. Basic Res Cardiol. 2019; 114:15. 10.1007/s00395-019-0724-3 [DOI] [PubMed] [Google Scholar]
- 40.Chen H, Kankel MW, Su SC, Han SW, Ofengeim D. Exploring the genetics and non-cell autonomous mechanisms underlying ALS/FTLD. Cell Death Differ. 2018; 25:648–62. 10.1038/s41418-018-0060-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hou L, Guo J, Xu F, Weng X, Yue W, Ge J. Cardiomyocyte dimethylarginine dimethylaminohydrolase1 attenuates left-ventricular remodeling after acute myocardial infarction: involvement in oxidative stress and apoptosis. Basic Res Cardiol. 2018; 113:28. 10.1007/s00395-018-0685-y [DOI] [PubMed] [Google Scholar]
- 42.Bellot GL, Dong X, Lahiri A, Sebastin SJ, Batinic-Haberle I, Pervaiz S, Puhaindran ME. MnSOD is implicated in accelerated wound healing upon Negative Pressure Wound Therapy (NPWT): A case in point for MnSOD mimetics as adjuvants for wound management. Redox Biol. 2019; 20:307–20. 10.1016/j.redox.2018.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang Q, Xiang B, Wang H, Huang K, Kong H, Hu S. Remote ischaemic preconditioning ameliorates sinus rhythm restoration rate through Cox maze radiofrequency procedure associated with inflammation reaction reduction. Basic Res Cardiol. 2019; 114:14. 10.1007/s00395-019-0723-4 [DOI] [PubMed] [Google Scholar]
- 44.Carè A, Bellenghi M, Matarrese P, Gabriele L, Salvioli S, Malorni W. Sex disparity in cancer: roles of microRNAs and related functional players. Cell Death Differ. 2018; 25:477–85. 10.1038/s41418-017-0051-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Biernacki M, Ambrożewicz E, Gęgotek A, Toczek M, Bielawska K, Skrzydlewska E. Redox system and phospholipid metabolism in the kidney of hypertensive rats after FAAH inhibitor URB597 administration. Redox Biol. 2018; 15:41–50. 10.1016/j.redox.2017.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cabon L, Bertaux A, Brunelle-Navas MN, Nemazanyy I, Scourzic L, Delavallée L, Vela L, Baritaud M, Bouchet S, Lopez C, Quang Van V, Garbin K, Chateau D, et al. AIF loss deregulates hematopoiesis and reveals different adaptive metabolic responses in bone marrow cells and thymocytes. Cell Death Differ. 2018; 25:983–1001. 10.1038/s41418-017-0035-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heppner DE, Hristova M, Ida T, Mijuskovic A, Dustin CM, Bogdándi V, Fukuto JM, Dick TP, Nagy P, Li J, Akaike T, van der Vliet A. Cysteine perthiosulfenic acid (Cys-SSOH): A novel intermediate in thiol-based redox signaling? Redox Biol. 2018; 14:379–85. 10.1016/j.redox.2017.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinbongard P, Skyschally A, Gent S, Pesch M, Heusch G. STAT3 as a common signal of ischemic conditioning: a lesson on “rigor and reproducibility” in preclinical studies on cardioprotection. Basic Res Cardiol. 2017; 113:3. 10.1007/s00395-017-0660-z [DOI] [PubMed] [Google Scholar]
- 49.Bramasole L, Sinha A, Gurevich S, Radzinski M, Klein Y, Panat N, Gefen E, Rinaldi T, Jimenez-Morales D, Johnson J, Krogan NJ, Reis N, Reichmann D, et al. Proteasome lid bridges mitochondrial stress with Cdc53/Cullin1 NEDDylation status. Redox Biol. 2019; 20:533–43. 10.1016/j.redox.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatori Y, Inouye S, Akagi R, Seyama T. Local redox environment beneath biological membranes probed by palmitoylated-roGFP. Redox Biol. 2018; 14:679–85. 10.1016/j.redox.2017.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bittremieux M, La Rovere RM, Akl H, Martines C, Welkenhuyzen K, Dubron K, Baes M, Janssens A, Vandenberghe P, Laurenti L, Rietdorf K, Morciano G, Pinton P, et al. Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2. Cell Death Differ. 2019; 26:531–47. 10.1038/s41418-018-0142-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiri F, Pirhadi S, Rahmani A. Identification of new potential HIV-1 reverse transcriptase inhibitors by QSAR modeling and structure-based virtual screening. J Recept Signal Transduct Res. 2018; 38:37–47. 10.1080/10799893.2017.1414844 [DOI] [PubMed] [Google Scholar]
- 53.Capece D, D’Andrea D, Verzella D, Tornatore L, Begalli F, Bennett J, Zazzeroni F, Franzoso G. Turning an old GADDget into a troublemaker. Cell Death Differ. 2018; 25:642–44. 10.1038/s41418-018-0087-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandra M, Escalante-Alcalde D, Bhuiyan MS, Orr AW, Kevil C, Morris AJ, Nam H, Dominic P, McCarthy KJ, Miriyala S, Panchatcharam M. Cardiac-specific inactivation of LPP3 in mice leads to myocardial dysfunction and heart failure. Redox Biol. 2018; 14:261–71. 10.1016/j.redox.2017.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandrasekharan A, Varadarajan SN, Lekshmi A, Lupitha SS, Darvin P, Chandrasekhar L, Pillai PR, Santhoshkumar TR, Pillai MR. A high-throughput real-time in vitro assay using mitochondrial targeted roGFP for screening of drugs targeting mitochondria. Redox Biol. 2019; 20:379–89. 10.1016/j.redox.2018.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou L, Wang K, Zhang C, Sun F, Che Y, Zhao X, Zhang D, Li H, Wang Q. Complement receptor 3 mediates NADPH oxidase activation and dopaminergic neurodegeneration through a Src-Erk-dependent pathway. Redox Biol. 2018; 14:250–60. 10.1016/j.redox.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saba Khan N, Verma R, Pradhan D, Nayek A, Bhuyan R, Kumar Sahu T, Kumar Jain A. Analysis of interleukin 23 and 7G10 interactions for computational design of lead antibodies against immune-mediated inflammatory diseases. J Recept Signal Transduct Res. 2018; 38:327–34. 10.1080/10799893.2018.1511729 [DOI] [PubMed] [Google Scholar]
- 58.Chang HC, Kao CH, Chung SY, Chen WC, Aninda LP, Chen YH, Juan YA, Chen SL. Bhlhe40 differentially regulates the function and number of peroxisomes and mitochondria in myogenic cells. Redox Biol. 2019; 20:321–33. 10.1016/j.redox.2018.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hao L, Sun Q, Zhong W, Zhang W, Sun X, Zhou Z. Mitochondria-targeted ubiquinone (MitoQ) enhances acetaldehyde clearance by reversing alcohol-induced posttranslational modification of aldehyde dehydrogenase 2: A molecular mechanism of protection against alcoholic liver disease. Redox Biol. 2018; 14:626–36. 10.1016/j.redox.2017.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koentges C, Pepin ME, Müsse C, Pfeil K, Alvarez SV, Hoppe N, Hoffmann MM, Odening KE, Sossalla S, Zirlik A, Hein L, Bode C, Wende AR, Bugger H. Gene expression analysis to identify mechanisms underlying heart failure susceptibility in mice and humans. Basic Res Cardiol. 2017; 113:8. 10.1007/s00395-017-0666-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruiz-Hernández A, Romero-Nava R, Huang F, Hong E, Villafaña S. Altered function and expression of the orphan GPR135 at the cardiovascular level in diabetic Wistar rats. J Recept Signal Transduct Res. 2018; 38:484–91. 10.1080/10799893.2019.1597116 [DOI] [PubMed] [Google Scholar]
- 62.Bellomo C, Caja L, Fabregat I, Mikulits W, Kardassis D, Heldin CH, Moustakas A. Snail mediates crosstalk between TGFβ and LXRα in hepatocellular carcinoma. Cell Death Differ. 2018; 25:885–903. 10.1038/s41418-017-0021-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheignon C, Tomas M, Bonnefont-Rousselot D, Faller P, Hureau C, Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018; 14:450–64. 10.1016/j.redox.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guidarelli A, Fiorani M, Cerioni L, Cantoni O. Calcium signals between the ryanodine receptor- and mitochondria critically regulate the effects of arsenite on mitochondrial superoxide formation and on the ensuing survival vs apoptotic signaling. Redox Biol. 2019; 20:285–95. 10.1016/j.redox.2018.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cremonini E, Wang Z, Bettaieb A, Adamo AM, Daveri E, Mills DA, Kalanetra KM, Haj FG, Karakas S, Oteiza PI. (-)-Epicatechin protects the intestinal barrier from high fat diet-induced permeabilization: implications for steatosis and insulin resistance. Redox Biol. 2018; 14:588–99. 10.1016/j.redox.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ranhotra HS. The estrogen-related receptors in metabolism and cancer: newer insights. J Recept Signal Transduct Res. 2018; 38:95–100. 10.1080/10799893.2018.1456552 [DOI] [PubMed] [Google Scholar]
- 67.Afonso MB, Rodrigues PM, Simão AL, Gaspar MM, Carvalho T, Borralho P, Bañales JM, Castro RE, Rodrigues CM. miRNA-21 ablation protects against liver injury and necroptosis in cholestasis. Cell Death Differ. 2018; 25:857–72. 10.1038/s41418-017-0019-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Espinosa-Díez C, Miguel V, Vallejo S, Sánchez FJ, Sandoval E, Blanco E, Cannata P, Peiró C, Sánchez-Ferrer CF, Lamas S. Role of glutathione biosynthesis in endothelial dysfunction and fibrosis. Redox Biol. 2018; 14:88–99. 10.1016/j.redox.2017.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Han SJ, Choi HS, Kim JI, Park JW, Park KM. IDH2 deficiency increases the liver susceptibility to ischemia-reperfusion injury via increased mitochondrial oxidative injury. Redox Biol. 2018; 14:142–53. 10.1016/j.redox.2017.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aalto AL, Mohan AK, Schwintzer L, Kupka S, Kietz C, Walczak H, Broemer M, Meinander A. M1-linked ubiquitination by LUBEL is required for inflammatory responses to oral infection in Drosophila. Cell Death Differ. 2019; 26:860–76. 10.1038/s41418-018-0164-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flórido A, Saraiva N, Cerqueira S, Almeida N, Parsons M, Batinic-Haberle I, Miranda JP, Costa JG, Carrara G, Castro M, Oliveira NG, Fernandes AS. The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: impact on doxorubicin-treated cells. Redox Biol. 2019; 20:367–78. 10.1016/j.redox.2018.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hung CH, Cheng SS, Cheung YT, Wuwongse S, Zhang NQ, Ho YS, Lee SM, Chang RC. A reciprocal relationship between reactive oxygen species and mitochondrial dynamics in neurodegeneration. Redox Biol. 2018; 14:7–19. 10.1016/j.redox.2017.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Álvarez-Fernández M, Sanz-Flores M, Sanz-Castillo B, Salazar-Roa M, Partida D, Zapatero-Solana E, Ali HR, Manchado E, Lowe S, VanArsdale T, Shields D, Caldas C, Quintela-Fandino M, Malumbres M. Therapeutic relevance of the PP2A-B55 inhibitory kinase MASTL/Greatwall in breast cancer. Cell Death Differ. 2018; 25:828–40. 10.1038/s41418-017-0024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hysi PG, Khawaja AP, Menni C, Tamraz B, Wareham N, Khaw KT, Foster PJ, Benet LZ, Spector TD, Hammond CJ. Ascorbic acid metabolites are involved in intraocular pressure control in the general population. Redox Biol. 2019; 20:349–53. 10.1016/j.redox.2018.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderton H, Bandala-Sanchez E, Simpson DS, Rickard JA, Ng AP, Di Rago L, Hall C, Vince JE, Silke J, Liccardi G, Feltham R. RIPK1 prevents TRADD-driven, but TNFR1 independent, apoptosis during development. Cell Death Differ. 2019; 26:877–89. 10.1038/s41418-018-0166-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Avalle L, Camporeale A, Morciano G, Caroccia N, Ghetti E, Orecchia V, Viavattene D, Giorgi C, Pinton P, Poli V. STAT3 localizes to the ER, acting as a gatekeeper for ER-mitochondrion Ca2+ fluxes and apoptotic responses. Cell Death Differ. 2019; 26:932–42. 10.1038/s41418-018-0171-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuhrmann DC, Wittig I, Brüne B. TMEM126B deficiency reduces mitochondrial SDH oxidation by LPS, attenuating HIF-1α stabilization and IL-1β expression. Redox Biol. 2019; 20:204–16. 10.1016/j.redox.2018.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel H, Ansari A, Pawara R, Ansari I, Jadhav H, Surana S. Design and synthesis of novel 2,4-disubstituted aminopyrimidines: reversible non-covalent T790M EGFR inhibitors. J Recept Signal Transduct Res. 2018; 38:393–412. 10.1080/10799893.2018.1557207 [DOI] [PubMed] [Google Scholar]
- 79.Bagati A, Bianchi-Smiraglia A, Moparthy S, Kolesnikova K, Fink EE, Kolesnikova M, Roll MV, Jowdy P, Wolff DW, Polechetti A, Yun DH, Lipchick BC, Paul LM, et al. FOXQ1 controls the induced differentiation of melanocytic cells. Cell Death Differ. 2018; 25:1040–49. 10.1038/s41418-018-0066-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frandsen JR, Narayanasamy P. Neuroprotection through flavonoid: enhancement of the glyoxalase pathway. Redox Biol. 2018; 14:465–73. 10.1016/j.redox.2017.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Battistelli C, Sabarese G, Santangelo L, Montaldo C, Gonzalez FJ, Tripodi M, Cicchini C. The lncRNA HOTAIR transcription is controlled by HNF4α-induced chromatin topology modulation. Cell Death Differ. 2019; 26:890–901. 10.1038/s41418-018-0170-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng J, Li Y, Zhou Y, Zhang L, Liu X, Zuo Z. Pharmacophore modeling, molecular docking and molecular dynamics studies on natural products database to discover novel skeleton as non-purine xanthine oxidase inhibitors. J Recept Signal Transduct Res. 2018; 38:246–55. 10.1080/10799893.2018.1476544 [DOI] [PubMed] [Google Scholar]
- 83.Davidson SM, Arjun S, Basalay MV, Bell RM, Bromage DI, Bøtker HE, Carr RD, Cunningham J, Ghosh AK, Heusch G, Ibanez B, Kleinbongard P, Lecour S, et al. The 10th Biennial Hatter Cardiovascular Institute workshop: cellular protection-evaluating new directions in the setting of myocardial infarction, ischaemic stroke, and cardio-oncology. Basic Res Cardiol. 2018; 113:43. 10.1007/s00395-018-0704-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hobson SR, Gurusinghe S, Lim R, Alers NO, Miller SL, Kingdom JC, Wallace EM. Melatonin improves endothelial function in vitro and prolongs pregnancy in women with early-onset preeclampsia. J Pineal Res. 2018; 65:e12508. 10.1111/jpi.12508 [DOI] [PubMed] [Google Scholar]
- 85.Staudacher V, Trujillo M, Diederichs T, Dick TP, Radi R, Morgan B, Deponte M. Redox-sensitive GFP fusions for monitoring the catalytic mechanism and inactivation of peroxiredoxins in living cells. Redox Biol. 2018; 14:549–56. 10.1016/j.redox.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Goiran T, Duplan E, Rouland L, El Manaa W, Lauritzen I, Dunys J, You H, Checler F, Alves da Costa C. Nuclear p53-mediated repression of autophagy involves PINK1 transcriptional down-regulation. Cell Death Differ. 2018; 25:873–84. 10.1038/s41418-017-0016-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Han YS, Kim SM, Lee JH, Jung SK, Noh H, Lee SH. Melatonin protects chronic kidney disease mesenchymal stem cells against senescence via PrPC -dependent enhancement of the mitochondrial function. J Pineal Res. 2019; 66:e12535. 10.1111/jpi.12535 [DOI] [PubMed] [Google Scholar]
- 88.Tabish TA, Zhang S, Winyard PG. Developing the next generation of graphene-based platforms for cancer therapeutics: the potential role of reactive oxygen species. Redox Biol. 2018; 15:34–40. 10.1016/j.redox.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gul-Kahraman K, Yilmaz-Bozoglan M, Sahna E. Physiological and pharmacological effects of melatonin on remote ischemic perconditioning after myocardial ischemia-reperfusion injury in rats: Role of Cybb, Fas, NfκB, Irisin signaling pathway. J Pineal Res. 2019; 67:e12589. 10.1111/jpi.12589 [DOI] [PubMed] [Google Scholar]
- 90.DeLeon-Pennell KY, Mouton AJ, Ero OK, Ma Y, Padmanabhan Iyer R, Flynn ER, Espinoza I, Musani SK, Vasan RS, Hall ME, Fox ER, Lindsey ML. LXR/RXR signaling and neutrophil phenotype following myocardial infarction classify sex differences in remodeling. Basic Res Cardiol. 2018; 113:40. 10.1007/s00395-018-0699-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gobert F, Luauté J, Raverot V, Cotton F, Dailler F, Claustrat B, Perrin F, Gronfier C. Is circadian rhythmicity a prerequisite to coma recovery? Circadian recovery concomitant to cognitive improvement in two comatose patients. J Pineal Res. 2019; 66:e12555. 10.1111/jpi.12555 [DOI] [PubMed] [Google Scholar]
- 92.Gandarillas A, Molinuevo R, Sanz-Gómez N. Mammalian endoreplication emerges to reveal a potential developmental timer. Cell Death Differ. 2018; 25:471–76. 10.1038/s41418-017-0040-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018; 25:486–541. 10.1038/s41418-017-0012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gobbi G, Comai S. Sleep well. Untangling the role of melatonin MT1 and MT2 receptors in sleep. J Pineal Res. 2019; 66:e12544. 10.1111/jpi.12544 [DOI] [PubMed] [Google Scholar]
- 95.Edwards KS, Ashraf S, Lomax TM, Wiseman JM, Hall ME, Gava FN, Hall JE, Hosler JP, Harmancey R. Uncoupling protein 3 deficiency impairs myocardial fatty acid oxidation and contractile recovery following ischemia/reperfusion. Basic Res Cardiol. 2018; 113:47. 10.1007/s00395-018-0707-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Frank D, Vince JE. Pyroptosis versus necroptosis: similarities, differences, and crosstalk. Cell Death Differ. 2019; 26:99–114. 10.1038/s41418-018-0212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fernández Vázquez G, Reiter RJ, Agil A. Melatonin increases brown adipose tissue mass and function in Zücker diabetic fatty rats: implications for obesity control. J Pineal Res. 2018; 64:e12472. 10.1111/jpi.12472 [DOI] [PubMed] [Google Scholar]
- 98.Tan SW, Yip GW, Suda T, Baeg GH. Small Maf functions in the maintenance of germline stem cells in the Drosophila testis. Redox Biol. 2018; 15:125–34. 10.1016/j.redox.2017.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galano A, Reiter RJ. Melatonin and its metabolites vs oxidative stress: from individual actions to collective protection. J Pineal Res. 2018; 65:e12514. 10.1111/jpi.12514 [DOI] [PubMed] [Google Scholar]
- 100.Eid RA, Alkhateeb MA, Eleawa S, Al-Hashem FH, Al-Shraim M, El-Kott AF, Zaki MS, Dallak MA, Aldera H. Cardioprotective effect of ghrelin against myocardial infarction-induced left ventricular injury via inhibition of SOCS3 and activation of JAK2/STAT3 signaling. Basic Res Cardiol. 2018; 113:13. 10.1007/s00395-018-0671-4 [DOI] [PubMed] [Google Scholar]
- 101.Wang B, Yee Aw T, Stokes KY. N-acetylcysteine attenuates systemic platelet activation and cerebral vessel thrombosis in diabetes. Redox Biol. 2018; 14:218–28. 10.1016/j.redox.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eiringhaus J, Herting J, Schatter F, Nikolaev VO, Sprenger J, Wang Y, Köhn M, Zabel M, El-Armouche A, Hasenfuss G, Sossalla S, Fischer TH. Protein kinase/phosphatase balance mediates the effects of increased late sodium current on ventricular calcium cycling. Basic Res Cardiol. 2019; 114:13. 10.1007/s00395-019-0720-7 [DOI] [PubMed] [Google Scholar]
- 103.Denton D, Xu T, Dayan S, Nicolson S, Kumar S. Dpp regulates autophagy-dependent midgut removal and signals to block ecdysone production. Cell Death Differ. 2019; 26:763–78. 10.1038/s41418-018-0154-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Erland LA, Yasunaga A, Li IT, Murch SJ, Saxena PK. Direct visualization of location and uptake of applied melatonin and serotonin in living tissues and their redistribution in plants in response to thermal stress. J Pineal Res. 2019; 66:e12527. 10.1111/jpi.12527 [DOI] [PubMed] [Google Scholar]
- 105.Yang T, Cao C, Yang J, Liu T, Lei XG, Zhang Z, Xu S. miR-200a-5p regulates myocardial necroptosis induced by Se deficiency via targeting RNF11. Redox Biol. 2018; 15:159–69. 10.1016/j.redox.2017.11.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gebhard C, Maafi F, Stähli BE, Dang J, Nachar W, de Oliveira Moraes AB, Kernaleguen AE, Lavoie V, Mecteau M, Mihalache-Avram T, Shi Y, Chabot-Blanchet M, Busseuil D, et al. Apolipoprotein A-I proteolysis in aortic valve stenosis: role of cathepsin S. Basic Res Cardiol. 2018; 113:30. 10.1007/s00395-018-0689-7 [DOI] [PubMed] [Google Scholar]
- 107.Denton D, Kumar S. Autophagy-dependent cell death. Cell Death Differ. 2019; 26:605–16. 10.1038/s41418-018-0252-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ding S, Lin N, Sheng X, Zhao Y, Su Y, Xu L, Tong R, Yan Y, Fu Y, He J, Gao Y, Yuan A, Ye L, et al. Melatonin stabilizes rupture-prone vulnerable plaques via regulating macrophage polarization in a nuclear circadian receptor RORα-dependent manner. J Pineal Res. 2019; 67:e12581. 10.1111/jpi.12581 [DOI] [PubMed] [Google Scholar]
- 109.Zhang B, Hou R, Zou Z, Luo T, Zhang Y, Wang L, Wang B. Mechanically induced autophagy is associated with ATP metabolism and cellular viability in osteocytes in vitro. Redox Biol. 2018; 14:492–98. 10.1016/j.redox.2017.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ding M, Ning J, Feng N, Li Z, Liu Z, Wang Y, Wang Y, Li X, Huo C, Jia X, Xu R, Fu F, Wang X, Pei J. Dynamin-related protein 1-mediated mitochondrial fission contributes to post-traumatic cardiac dysfunction in rats and the protective effect of melatonin. J Pineal Res. 2018; 64:64. 10.1111/jpi.12447 [DOI] [PubMed] [Google Scholar]
- 111.Darido C, Georgy SR, Cullinane C, Partridge DD, Walker R, Srivastava S, Roslan S, Carpinelli MR, Dworkin S, Pearson RB, Jane SM. Stage-dependent therapeutic efficacy in PI3K/mTOR-driven squamous cell carcinoma of the skin. Cell Death Differ. 2018; 25:1146–59. 10.1038/s41418-017-0032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang YH, Wang DW, Xu SF, Zhang S, Fan YG, Yang YY, Guo SQ, Wang S, Guo T, Wang ZY, Guo C. α-Lipoic acid improves abnormal behavior by mitigation of oxidative stress, inflammation, ferroptosis, and tauopathy in P301S Tau transgenic mice. Redox Biol. 2018; 14:535–48. 10.1016/j.redox.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hadebe N, Cour M, Lecour S. The SAFE pathway for cardioprotection: is this a promising target? Basic Res Cardiol. 2018; 113:9. 10.1007/s00395-018-0670-5 [DOI] [PubMed] [Google Scholar]
- 114.Herzog J, Schmidt FP, Hahad O, Mahmoudpour SH, Mangold AK, Garcia Andreo P, Prochaska J, Koeck T, Wild PS, Sørensen M, Daiber A, Münzel T. Acute exposure to nocturnal train noise induces endothelial dysfunction and pro-thromboinflammatory changes of the plasma proteome in healthy subjects. Basic Res Cardiol. 2019; 114:46. 10.1007/s00395-019-0753-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Heusch G. 25 years of remote ischemic conditioning: from laboratory curiosity to clinical outcome. Basic Res Cardiol. 2018; 113:15. 10.1007/s00395-018-0673-2 [DOI] [PubMed] [Google Scholar]
- 116.Cieri D, Vicario M, Giacomello M, Vallese F, Filadi R, Wagner T, Pozzan T, Pizzo P, Scorrano L, Brini M, Calì T. SPLICS: a split green fluorescent protein-based contact site sensor for narrow and wide heterotypic organelle juxtaposition. Cell Death Differ. 2018; 25:1131–45. 10.1038/s41418-017-0033-z [DOI] [PMC free article] [PubMed] [Google Scholar]