Abstract
Engagement in HIV care reduces HIV-related health disparities that persist across racial/ethnic and gender lines, yet African American (AA) women face multiple challenges to remaining engaged in care, including HIV-related stigma. We analyzed longitudinal data from 239 participants in the Unity Health Study to estimate associations between HIV-related stigma and engagement in care among AA women linked to HIV care. In adjusted Poisson regression analyses, engagement in care was not associated with HIV-related stigma, but was associated with older age (IRR = 1.01, 95% CI = [1.00, 1.01], p = .01), higher levels of education (IRR = 1.18, 95% CI = [1.02, 1.35], p = .03), and higher levels of social support (IRR = 1.05, 95% CI = [1.01–1.09], p = 0.04). Our findings suggest the need for targeted interventions to enhance engagement in care and to incorporate social support into health promotion programming for AA women living with HIV.
Keywords: African American, engagement in care, health disparities, HIV, HIV-related stigma, women
Despite improvements in HIV testing, prevention and treatment strategies, HIV-related health disparities persist across racial/ethnic and gender lines in the United States (Geter, Sutton, Armon, et al., 2018; Chapin-Bardales, Rosenberg, & Sullivan, 2017). In 2015, approximately 50% of new HIV diagnoses occurred in racial/ethnic minorities, yet they accounted for less than 30% of the the total U.S. population. In 2016, when African American (AA) women comprised only 13% of the U.S. female population, they represented 59% of HIV prevalence and 61% of HIV incidence in women living in the United States (Centers for Disease Control and Prevention [CDC], 2017). During that same year, the rate of death among AA women living with HIV (WLWH) was approximately 16 times higher than the rate of death among White WLWH (CDC, 2017). These observed disparities were at least partly attributable to suboptimal engagement in HIV care (Walcott, Kempf, Merlin, & Turan, 2016), an established predictor of HIV-related mortality (Mugavero et al., 2009).
Optimal engagement in HIV care entails timely linkage to HIV health care services after receiving an HIV diagnosis and being retained in health care over time (Mugavero, Davila, Nevin, & Giordano, 2010). When people living with HIV (PLWH) are consistently engaged in care, they are more likely to (a) obtain a prescription for antiretroviral therapy (ART), (b) adhere to ART regimens, (c) be followed by an HIV care provider to ensure effective treatment (Mugavero et al., 2012), and (d) achieve viral suppression (Mugavero et al., 2012), the ultimate goal of HIV care. However, racial/ethnic minorities and women often experience delays in the receipt of ART, suboptimal ART adherence, and poor viral suppression (Dasgupta, Oster, Li, & Hall, 2016), indicating that AA women may be at especially high risk for poor engagement in HIV care.
HIV-related stigma poses a significant challenge to engaging people in HIV care (Earnshaw, Smith, Chaudoir, Amico, & Copenhaver, 2013). HIV stigma is defined as negative attitudes directed toward PLWH (Parker & Aggleton, 2003). These attitudes contribute to feelings of being ostracized, isolated, lonely, and/or rejected (Fekete, Williams, & Skinta, 2018), and HIV-related stigma is associated with low rates of HIV testing, delayed linkages to HIV care, decreased adherence to ART and poor retention in care (Sangaramoorthy, Jamison, & Dyer, 2017). Furthermore, enacted stigma and internalized stigma are two distinct domains of HIV-related stigma that contribute to the negative feelings (Earnshaw, Smith, Chaudoir, Amico, & Copenhaver, 2013). Enacted stigma refers to perceived and/or experienced acts of prejudice or discrimination from others (Lekas, Siegel, & Schrimshaw, 2006). Alternatively, internalized stigma refers to feelings that evolve from one’s own acceptance of negative beliefs related to their HIV status (Link & Phelan, 2001).
Research has indicated that HIV-related stigma may differentially predict HIV-related health outcomes among AA women (Rueda et al., 2016). Indeed, AA WLWH may be at particularly high risk for poor engagement in HIV care due to their repeated exposure to HIV-related stigma within the larger context of intersecting stigmas related to their race, gender, and socioeconomic class (Geter, Sutton, & McCree, 2018; Leddy et al., 2019; Sangaramoorthy, et al., 2017). Recent findings indicated that HIV-related health inequities among racial/ethnic and gender minorities have emerged due to complex economic, social, and structural vulnerabilities that contribute to the health inequities that influence the engagement of AA women in the HIV care continuum (Chapin-Bardales, Rosenberg, & Sullivan, 2017; Geter, Sutton, & McCree, 2018). These vulnerabilities include living below the poverty level, having less than a high school education, living in unstable housing, having limited access to quality health care, exposure to higher sexually transmitted infection incidence rates, and repeated exposure to discrimination and/or HIV-related stigma (CDC, 2018; Chapin-Bardales et al., 2017; Geter, Sutton, & McCree, 2018). HIV-related stigma, in particular, may exacerbate existing sources of vulnerability among PLWH (Parker & Aggleton, 2003), yet relatively few studies of the impact of stigma on HIV outcomes are based on longitudinal data (Turan et al., 2019) and fewer studies have examined retention in care (as compared to adherence) as an outcome of stigma (Rice et al., 2017). Furthermore, little is known regarding the influence of each distinct domain of HIV-related stigma on the engagement of AA women in HIV care.
The purpose of our study was to examine how different dimensions of HIV-related stigma are associated with engagement in HIV care among AA WLWH. We hypothesized that higher levels of internalized stigma and enacted stigma would each be associated with lower engagement in care.
Methods
Study Design
We conducted a secondary analysis of longitudinal data from participants in the Unity Health Study (Rao, Kemp, et al., 2018), a randomized controlled trial that tested the effectiveness of a peer-led HIV-related stigma reduction intervention among 239 AA WLWH. Of those, 237 had at least one HIV medical care appointment scheduled during the study period and were included in our secondary analysis. Participants in the Unity Health Study were recruited from three non-profit HIV specialty clinics, including two in Chicago, Illinois (a private university-based clinic and a public hospital-affiliated clinic) and one in Birmingham, Alabama (a public university-based clinic). Recruitment took place from May 2013 to October 2015, with the last follow-up assessment completed in December 2016. Women were considered eligible if they: (a) self-identified as having an AA racial/ethnic background, (b) were ages 18 or older, and (c) had a documented HIV seropositive status. Individuals who identified as being African born, of Afro-Caribbean descent, or as Black Latina were excluded from the study. The Unity Health Study was approved by the institutional review boards at each of the participating study sites, and each study participant provided written informed consent prior to participating in study activities. Comprehensive details of the Unity Health Study are reported in Rao, Kemp, et al. (2018).
Data Collection
Demographic and psychosocial data were self-reported via tablet-based audio computer assisted self-interview (ACASI). Demographic information was collected at baseline and psychosocial data were collected at baseline, immediately after the Unity intervention (a peer-led behavioral intervention which used a workshop format to test the efficacy of a stigma reduction intervention among AA WLWH), 4 months post-intervention, immediately after the 6-month post-intervention booster session, 8 months post-intervention, and 12 months post-intervention. Appointment adherence data were abstracted by a research coordinator from clinic records continuously from 12 months pre baseline to 12 months post workshop.
Measures
Engagement in care.
The outcome of interest was engagement in HIV care (i.e., appointment adherence). Because numerous factors (e.g., viral load, substance use, disease progression) may influence the number of appointments a PLWH may require (Mugavero et al., 2009), we operationalized engagement in care as the number of attended HIV medical care appointments per number of scheduled HIV medical care appointments per person in a given time period between data collection visits (see Figure 1. The approach to linking engagement in HIV care with study visits; Mugavero, Amico, Horn, & Thompson, 2013). As such, each participant could have multiple observations. Scheduled HIV care appointments were designated as attended, missed, or rescheduled (canceled appointments were also documented, but counted as rescheduled, because they showed active engagement in the scheduling process). Engagement in care could not be calculated for time periods when there were no scheduled visits (i.e., the denominator would be 0), so person-time points without scheduled visits were excluded from the analysis.
Figure 1.
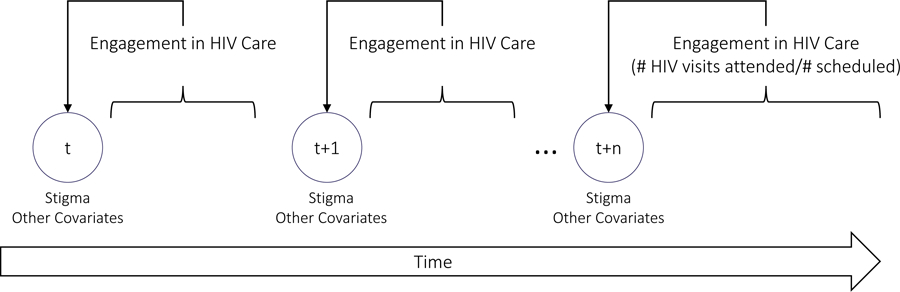
The approach to linking engagement in HIV care with study visits.
HIV-related stigma.
The predictor of interest, HIV-related stigma, was assessed using the 14-item Stigma Scale for Chronic Illness (SSCI; Rao et al., 2009). The SSCI evaluates two different dimensions of stigma: (a) internalized stigma and (b) enacted stigma, and includes items such as, I felt embarrassed about my illness and Because of my illness, I felt emotionally distant from other people. Using a Likert-type scale participants responded from 1 = never to 5 = always. Higher scores indicated higher levels of HIV-related stigma. This scale has been shown to be a valid measure of both internalized and enacted stigma among AA PLWH with high internal consistency, Cronbach’s α = 0.93 (Rao et al., 2016).
Covariates.
Covariates were chosen a priori based on their potential as confounders of the association between HIV-related stigma and engagement in care (Haley et al., 2014) and included: clinic type (private, university-based, public hospital-affiliated, public university-based), age in years, marital status (never been married, married or living with partner, separated/divorced/widowed), number of children (0, 1 to 3, 4 or more), education level (< high school, high school degree or equivalent, some college/AA/technical degree, college degree or higher), occupation (employed, homemaker, student, other), number of years since HIV diagnosis, and treatment group (treatment vs. control). Finally, we chose to include social support in our analysis as our research team recently found an association between increased social support and decreased HIV-related stigma over time among AA women enrolled in the Unity Health Study (Rao, Kemp, et al., 2018).
Social support was measured using two select subscales (emotional/informational support and positive social interaction subscales) from the Medical Outcomes Study Social Support Survey (MOS-SSS; Sherbourne & Stewart, 1991). The MOS-SSS has been used to assess perceived social support related to other chronic diseases (Ware & Sherbourne, 1992). The emotional/informational support subscale includes 8 items and has an internal reliability of Cronbach’s α = 0.97. The positive social interaction subscale has 3 items (Sherbourne & Stewart, 1991) and an internal reliability of Cronbach’s α = 0.96. For both subscales, participants answer questions regarding how often certain types of social support (e.g., someone to give you good advice about a crisis) are available by responding on a 5-point Likert scale from 1 = none of the time to 5 = all of the time. Higher scores indicated higher perceived social support.
Data Analysis
First, missing items from scale measures were mean imputed. Next, to describe the study sample, participant characteristics were summarized overall and then stratified by women with 100% appointment adherence and women with less than 100% appointment adherence. To test for differences across groups, we conducted chi-square tests to compare categorical variables and t tests to compare continuous variables. Next, to estimate the association between HIV-related stigma and appointment adherence, we used a Poisson generalized estimating equation approach to model the rate of attendance with the number of attended visits as the outcome and the number of scheduled visits as the exposure variable (Mugavero, et al., 2010). The model was specified with a log link function, exchangeable correlation structure, and robust (sandwich estimator) standard errors, with results reported as incidence rate ratios (IRR) with 95% confidence intervals (CI). The primary model was fully adjusted for all covariates (regardless of significance in univariate analysis) and included clinic type, age, marital status, number of children, education level, occupation, years since HIV diagnosis, social support, treatment group, time, and the treatment group by time interaction (to adjust for potential treatment effects over time). We also evaluated unadjusted and partially adjusted (adjusted for treatment and time only) models for reference. HIV-related stigma and social support scores were standardized prior to their inclusion in the regression analysis. The statistical analyses were conducted using version 3.4.1 of R statistical software (R Foundation for Statistical Computing Platform, 2017) and version 4.13-19 of the generalized estimating equation solver package (Carey, Lumley, & Ripley, 2015).
Results
Characteristics of Study Participants
Baseline demographic and clinical characteristics of study participants, stratified by attendance at baseline (less than 100% attendance vs. 100% attendance), are displayed in Table 1. Women who did not have perfect attendance were more likely to be younger (p < .01), to have lived with HIV for a shorter amount of time (p = .04), and to report lower levels of social support (p = .05). Women who missed at least one scheduled appointment also differed from those who were 100% adherent in terms of education (p = .04), marital status (p = .03), and clinic type (p = .01). There were no differences with respect to number of children, occupation, or HIV-related stigma.
Table 1.
Characteristics of Study Participants at Baseline by Appointment Attendance
| < 100% attendance (n = 80) | 100% attendance (n = 157) | p values | |
|---|---|---|---|
| Age, M (SD) a | 44.0 (10.7) | 48.1 (10.2) | .05 |
| Marital status b | .03 | ||
| Never been married | 34 (43.0%) | 50 (32.1%) | |
| Married or living with partner | 22 (27.8%) | 33 (21.2%) | |
| Separated, divorced, widowed | 23 (29.1%) | 73 (46.8%) | |
| Number of children b | .29 | ||
| No children | 41 (53.2%) | 93 (60.4%) | |
| 1–3 children | 28 (36.4%) | 53 (34.4%) | |
| 4+ children | 8 (10.4%) | 8 (5.2%) | |
| Education Level b | .04 | ||
| < HS education | 34 (43.6%) | 53 (34.9%) | |
| HS diploma or equivalent | 21 (26.9%) | 34 (22.4%) | |
| Some college/AA/technical degree | 22 (28.2%) | 48 (31.6%) | |
| College degree or higher | 1 (1.3%) | 17 (11.2%) | |
| Occupation b | .97 | ||
| Employed | 32 (45.1%) | 66 (44.9%) | |
| Homemaker | 22 (31.0%) | 46 (31.3%) | |
| Student | 6 (8.5%) | 10 (6.8%) | |
| Other | 11 (15.5%) | 25 (17.0%) | |
| Years since HIV diagnosis, M (SD) a | 12.7 (7.1) | 14.8 (7.3) | .04 |
| Social support, M (SD) a | 28.8 (11.9) | 32.1 (11.8) | .05 |
| HIV-related Stigma, M (SD) a | |||
| Internalized stigma | 19.4 (6.6) | 18.4 (7.1) | .27 |
| Enacted stigma | 13.7 (6.4) | 13.5 (6.9) | .82 |
| Clinic Type b | .01 | ||
| Private university-based | 11 (13.8%) | 35 (22.3%) | |
| Public hospital-affiliated | 39 (48.8%) | 46 (29.3%) | |
| Public university-based | 30 (37.5%) | 76 (48.4%) |
Note. HS = high school; AA = Associate’s Degree; SD = standard deviation.
t-tests were conducted to compare continuous variables.
Chi-squared tests were conducted to compare categorical variables.
Poisson Regression Analyses
As seen in Table 2, the association between HIV-related stigma and engagement in HIV health care was consistent across levels of adjustment. Adjusted for covariates, neither internalized stigma (incident rate ratio [IRR] = 1.04, 95% CI = [1.98, 1.09]) nor enacted stigma (IRR = 1.00, 95% CI = [0.96, 1.04]) were associated with engagement in care. However, engagement in care was associated with certain covariates.
Table 2.
Adjusted Associations Between HIV-Related Stigma, Covariates, and Engagement in Care (N = 679)
| Unadjusted | Partially adjusted | Fully adjusted | ||||
|---|---|---|---|---|---|---|
| IRR [95% CI] | p | IRR [95% CI] | p | IRR [95% CI] | p | |
| HIV-related stigma (SSCI) | ||||||
| Internalized stigma | 1.01 [0.96, 1.06] | .65 | 1.04 [0.98, 1.09] | .19 | 1.04 [0.98, 1.09] | .19 |
| Enacted stigma | 0.99 [0.95, 1.03] | .59 | 1.00 [0.96, 1.04] | .86 | 1.00 [0.96, 1.04] | .86 |
| Treatment vs. Control | 1.02 [0.92, 1.13] | .71 | 1.01 [0.92, 1.11] | .81 | ||
| Time | 1.00 [0.99, 1.01] | .93 | 1.00 [0.99, 1.01] | .83 | ||
| Treatment*Time | 1.00 [0.99, 1.01] | .94 | 1.00 [0.99, 1.01] | .98 | ||
| Age (years) | 1.01 [1.00, 1.01] | .01 | ||||
| Marital status | ||||||
| Married/living with partner vs. never married | 1.04 [0.94, 1.17] | .45 | ||||
| Separated/divorced/widowed vs. never married | 1.02 [0.92, 1.12) | .73 | ||||
| Number of children | ||||||
| 1–3 children vs. no children | 1.01 [0.91, 1.11] | .92 | ||||
| 4 or more children vs. no children | 0.85 [0.70, 1.05] | .13 | ||||
| Education Level | ||||||
| HS diploma or equivalent vs. < HS | 1.02 [0.91, 1.14] | .77 | ||||
| Some college/AA/technical degree | 1.13 [1.02, 1.25] | .02 | ||||
| College degree or higher | 1.18 [1.02, 1.35] | .03 | ||||
| Occupation | ||||||
| Homemaker vs. employed | 1.06 [0.95, 1.18] | .30 | ||||
| Student vs. employed | 1.02 [0.86, 1.20] | .84 | ||||
| Other vs. employed | 1.03 [0.90, 1.19] | .65 | ||||
| Time since HIV diagnosis (years) | 1.00 [1.00, 1.01] | .41 | ||||
| Social Support (MOS-SSS) | 1.05 [1.00, 1.09] | .04 | ||||
| Clinic Type | ||||||
| Public hospital-affiliated clinic vs. private University-based clinic |
0.75 [0.67, 0.85] | < .01 | ||||
| Public university-based clinic vs. private University-based clinic |
0.92 [0.83, 1.01] | .08 | ||||
Note. IRR = incident rate ratio; CI = confidence interval; HS = high school; AA = Associate’s Degree; SSCI = Stigma Scale for Chronic Illness; MOS-SSS = Medical Outcome Study Social Support Scale. Bold face font = p value < 0.05
Compared to women at the private university-based clinic, women at the public hospital-affiliated clinic had a 25% lower rate of engagement in HIV care (IRR = 0.75, 95% CI = [0.67, 0.85]) and, while not statistically significant, women at the public university-based clinic had an 8% lower rate of engagement in care (IRR = 0.92, 95% CI = [0.83, 1.01]). Second, older age was also significantly associated with engagement in care. Specifically, each additional year of age was associated with a 1% greater rate of engagement in HIV care (IRR = 1.01, 95% CI = [1.00, 1.01]). Additionally, when compared to women with less than a high school education, women who had some college/associate’s degree/technical degree had a 13% greater rate of engagement in care (IRR = 1.13, 95% CI = [1.02, 1.25]), and those that had obtained a college degree or higher had an 18% higher rate of engagement in care (IRR = 1.18, 95% CI = [1.02, 1.35]). Lastly, a one-unit higher level of social support was associated with a 5% higher rate of engagement in HIV care (IRR = 1.05, 95% CI = [1.00, 1.09]). Engagement in care was not significantly associated with marital status, number of children, occupation, or time since receiving an HIV diagnosis.
Discussion
We examined HIV-related stigma as a predictor of engagement in HIV care over time in a sample of AA WLWH receiving HIV care. Based on previous research findings (McDoom, Bokhour, Sullivan, & Drainoni, 2015), we hypothesized that higher levels of internalized and enacted HIV-related stigma would be associated with lower engagement of AA women in HIV care (Sangaramoorthy et al., 2017). However, in our analysis, neither internalized stigma nor enacted stigma was associated with ongoing engagement in care as predicted, after controlling for the sociodemographic characteristics of the participants. Instead, our analysis identified other variables that were predictive of ongoing engagement in care including older age, higher education level, higher social support, and the type of HIV clinic.
Although prior evidence indicated that HIV-related stigma was a risk factor for poorer engagement in medical care (Turan et al., 2017) among PLWH, we did not observe an association between HIV-related stigma and engagement in care in this sample of AA WLWH. These null findings may be explained by several factors. First, because HIV-related stigma has often been associated with social support (McDoom et al., 2015), it is possible that including social support as a covariate may have inhibited our ability to detect an association between HIV-related stigma and engagement in care. However, results from the unadjusted model did not indicate that there was a relationship between HIV-related stigma and engagement in care, even without adjustment for social support. An alternative explanation is that AA women often experience intersecting stigmas related to their race, gender and socioeconomic class (Sangaramoorthy et al., 2017; Turan, et al., 2017). For example, when Hargreaves, Busza, Mushati, Fearon, and Cowan (2017) examined stigma among approximately 1,000 female sex workers in Zimbabwe, they found the rate of sex-work stigma was significantly higher than the rate of HIV-related stigma in this population of women. Thus, it might be possible that the effect of HIV-related stigma on engagement in care may have been confounded by other types of stigma (i.e., the other non-HIV-related stigmas these AA women experience work in the opposite way), thereby enhancing engagement, which in turn would negate the detrimental effects of HIV stigma (Dale et al., 2019; Leddy et al., 2019). Comparing the association between HIV-related stigma and engagement in care among AA WLWH to the association between HIV-related stigma and engagement in care for other populations (e.g., White women, AA men, the general population) may provide further clarity. Second, it is possible that HIV-related stigma is a barrier to initial linkage to care but may be less influential on retention in care among AA women. If this is true, we may not expect to see an association between HIV-related stigma and engagement in care in a sample of women who are already linked to care (e.g., women participating in the Unity Health Study). Evaluating the association between HIV-related stigma and engagement in care (both linkage and retention) in a general sample of AA WLWH but prior to being linked to HIV care (Lima, Jeffries, Kudon, Wang, & McCree, 2018), may provide additional insights. Further exploration of the role of HIV-related stigma in engagement in care in this population is warranted.
Our findings did indicate that older age was associated with greater engagement in HIV care. These results were consistent with findings indicating that older age in WLWH facilitated acceptance of personal HIV status (Psaros et al., 2015). Specifically, in a study of WLWH, age buffered the relationship between stigma, ART adherence and HIV-related clinical outcomes (McCoy, Higgins, Zuniga, & Holstad, 2015). This trend is encouraging as individuals 50 years of age and older now account for approximately 45% of all PLWH in the United States, but these findings also emphasized a need to provide additional support for young AA PLWH.
Next, our analysis revealed that having a higher education level was associated with greater engagement in care. This finding was consistent with other studies that have found higher education levels associated with HIV-related health behaviors, including HIV testing and clinical appointment attendance (Wawrzyniak et al., 2015), and reinforced the need to facilitate engagement in care for AA WLWH with lower levels of education. Furthermore, these findings highlighted an opportunity to invest in education attainment as a means of improving HIV outcomes. Moreover, because education is considered a fundamental cause of health disparities (Assari, 2018), bolstering education attainment for AA women, in general, may be a means of improving health outcomes overall.
We also demonstrated a positive association between perceived social support and engagement in care among AA WLWH. Research has shown that formal and informal social support facilitated HIV testing, treatment adherence, and engagement in care by PLWH (Geter, Sutton, & McCree, 2018). Similarly, a qualitative study of AA WLWH highlighted social support as an important facilitator of engagement in care (McDoom et al., 2015). However, to our knowledge, ours is the first study to quantitatively demonstrate this association specifically among AA WLWH and to reiterate the potential for social support to be used in interventions to improve engagement in care in this population. Thus, future studies that enhance social support (or social networks) as a key tenet of stigma reduction interventions may have the potential to improve engagement in HIV care.
Finally, our study indicated that engagement in HIV care differed by the type of clinical site in which study participants received their care. Comparing WLWH located in Chicago, we found women at the public hospital-affiliated clinic had lower rates of engagement in HIV care compared to women at the private university-based clinic. These findings align closely with recent reports, which have indicated that the clinical environment can influence engagement in care and viral suppression (Geter, Sutton, & McCree, 2018; McDoom et al., 2015; Walcott et al., 2016). Additional research is needed to understand which clinic-level factors (e.g., patient-provider relationship, clinic location, available wraparound services) would better facilitate engagement in HIV care by AA women.
Our study had several limitations. First, because participants were enrolled in a randomized trial in which they were repeatedly questioned about engagement in HIV care, they may have developed an enhanced awareness of the importance of engagement in care due to repeated exposure to the clinical study, which, in turn, may have influenced their behavior related to engagement in care (Rao, Kemp, et al., 2018). Second, the association between type of clinical site and engagement in care data may have been partially due to variability in the way each clinic site documented clinic scheduling and attendance, because this information was abstracted from existing medical records. However, we anticipated this data collection method would be more accurate than self-report (Mugavero et al., 2010). Third, we may have been limited in our measures. For example, we might have had more nuanced findings if we had included individual social support subscales in our models. Researchers should explore the relationships between HIV-related stigma and social support (and their subscales) as they relate to engagement in care to more fully understand these dynamics. Additionally, because some health and social service factors were not collected as part of the Unity Study, our data might have been susceptible to residual confounding. Future studies should consider all variables of interest and potential confounders when deciding which data to collect from participants. In terms of scope, we chose to focus on individual-level factors associated with engagement in care. Research is needed to explore other levels (e.g., clinic level factors) that may influence AA women to engage in HIV care. Finally, because participants in our study were already engaged in care, our study did not elucidate the effects of stigma on engagement in care among patients not already linked to care.
Developing strategies to improve the engagement of AA women in HIV care is critical to improving health outcomes in this vulnerable group and reducing HIV-related disparities. AA WLWH face multiple barriers to remaining engaged in care. Although our study did not support the hypothesized association between HIV-related stigma and low engagement in care, the analysis did identify age, clinic type, education, and social support as key predictors of engagement in HIV care by AA women. Specifically, our findings suggest a need for focused interventions within and beyond the clinic (e.g., for younger women who have lower levels of education) and an opportunity to incorporate social support into programming for AA WLWH. Emerging evidence has indicated that social support may serve as a conduit to improve linkages to HIV care, increase HIV treatment adherence, and, thus, improve the quality of life for AA women living with HIV (McDoom et al., 2015). However, further research is needed to fully develop and evaluate tailored interventions that build on social support as a central facet to improve engagement of AA women in HIV care.
Key Considerations.
Social support was associated with increased engagement in HIV care by African American women living with HIV.
Level of engagement in HIV care may differ by type of clinical setting.
More tailored approaches are needed to improve engagement of young African American women in HIV care.
Gender-specific interventions are needed to address specific social determinants of health that may influence the engagement of African American women in HIV care.
Acknowledgements
This study was funded in part by the National Institutes of Health grant #R01MH098675-04 (PI: Deepa Rao), and the University of Washington/Fred Hutch Center for AIDS Research grant #AI027757 (PI: Jared Baeten). The authors would also like to thank the study participants for their contributions to the Unity Health Study.
Footnotes
Disclosures
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Contributor Information
Katryna McCoy, School of Nursing & Health Studies, University of Washington Bothell, Bothell, Washington, USA..
Lauren Lipira, Regional Research Institute, School of Social Work, Portland State University, Portland, Oregon, USA..
Christopher G. Kemp, Department of Global Health, University of Washington, Seattle, Washington, USA..
Paul E. Nevin, Department of Global Health, University of Washington, Seattle, Washington, USA..
David Huh, Indigenous Wellness Research Institute, School of Social Work, University of Washington, Seattle, Washington, USA..
Janet M. Turan, Department of Health Care Organization and Policy, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Michael J. Mugavero, Department of Medicine, University of Alabama at Birmingham, Birmingham, Alabama, USA..
Susan E. Cohn, Division of Infectious Diseases, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA..
Mieoak Bahk, Ruth M. Rothstein CORE Center, Chicago, Illinois, USA..
Jane M. Simoni, Department of Psychology, University of Washington, Seattle, Washington, USA..
Michele P. Andrasik, Fred Hutchinson Vaccine and Infectious Disease Division, Seattle, Washington, USA..
Deepa Rao, Department of Global Health, Department of Psychiatry, University of Washington, Seattle, Washington, USA..
References
- Assari S (2018). Life expectancy gain due to employment status depends on race, gender, education, and their intersections. Journal of Racial and Ethnic Health Disparities, 5(2), 375–386. doi: 10.1007/s40615-017-0381-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey VJ,Lumley T,&Ripley BD(2015).gee:Generalized estimation equationsolver(Version4.13-19)[Computersoftware]. Retrieved fromhttps://CRAN.R-project.org/package5gee
- Centers for Disease Control and Prevention. (2017). HIV surveillance in women (through 2017). Retrieved from https://www.cdc.gov/hiv/library/slideSets/index.html
- Centers for Disease Control and Prevention. (2018). Social determinants of health among adults with diagnosed HIV infection, 2016. Part A: Census tract-level social determinants of health among adults with diagnosed HIV infection—13 states, the District of Columbia, and Puerto Rico. Retrieved from https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-23-6.pdf
- Chapin-Bardales J, Rosenberg ES, & Sullivan PS (2017). Trends in racial/ethnic disparities of new AIDS diagnoses in the United States, 1984–2013. Annals of Epidemiology, 27(5), 329–334. doi: 10.1016/j.annepidem.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Oster AM, Li J, & Hall HI (2016). Disparities in consistent retention in HIV Care−-11 states and the District of Columbia, 2011–2013. Retrieved from https://www.cdc.gov/mmwr/volumes/65/wr/mm6504a2.htm [DOI] [PubMed]
- Earnshaw VA, Smith LR, Chaudoir SR, Amico KR, & Copenhaver MM (2013). HIV stigma mechanisms and well-being among PLWH: A test of the HIV stigma framework. AIDS and Behavior, 17(5), 1785–1795. doi: 10.1007/s10461-013-0437-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Williams SL, & Skinta MD (2018). Internalised HIV-stigma, loneliness, depressive symptoms and sleep quality in people living with HIV. Psychology and Health, 33(3), 398–415. doi: 10.1080/08870446.2017.1357816 [DOI] [PubMed] [Google Scholar]
- Geter A, Sutton MY, Armon C, Durham MD, Palella FJ Jr., Tedaldi E, … Buchacz K (2018). Trends of racial and ethnic disparities in virologic suppression among women in the HIV Outpatient Study, USA, 2010–2015. PLoS One, 13(1), e0189973. doi: 10.1371/journal.pone.0189973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geter A, Sutton MY, & McCree DH (2018). Social and structural determinants of HIV treatment and care among Black women living with HIV infection: A systematic review: 2005–2016. AIDS Care, 30(4), 409–416. doi: 10.1080/09540121.2018.1426827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley DF, Lucas J, Golin CE, Wang J, Hughes JP, Emel L, … Hodder SL (2014). Retention strategies and factors associated with missed visits among low income women at increased risk of HIV acquisition in the US (HPTN 064). AIDS Patient Care and STDs, 28(4), 206–217. doi: 10.1089/apc.2013.0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargreaves JR, Busza J, Mushati P, Fearon E, & Cowan FM (2017). Overlapping HIV and sex-work stigma among female sex workers recruited to 14 respondent-driven sampling surveys across Zimbabwe, 2013. AIDS Care, 29(6), 675–685. doi: 10.1080/09540121.2016.1268673 [DOI] [PubMed] [Google Scholar]
- Leddy AM, Turan JM, Johnson MO, Neilands TB, Kempf MC, Konkle-Parker D, … Turan B (2019). Poverty stigma is associated with suboptimal HIV care and treatment outcomes among women living with HIV in the U.S. AIDS. doi: 10.1097/qad.0000000000002189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekas H-M, Siegel K, & Schrimshaw EW (2006). Continuities and discontinuities in the experiences of felt and enacted stigma among women with HIV/AIDS. Qualitative Health Research, 16(9), 1165–1190. doi: 10.1177/1049732306292284 [DOI] [PubMed] [Google Scholar]
- Lima AC, Jeffries W. L. t., Kudon HZ, Wang G, & McCree DH (2018). HIV testing, positivity, and receipt of services among Black, White, and Hispanic women participating in HIV prevention programs funded by the Centers for Disease Control and Prevention, 2015. Womens Health Issues, 28(4), 358–366. doi: 10.1016/j.whi.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Link BG, & Phelan JC (2001). Conceptualizing stigma. Annual Review of Sociology, 27, 363–385. [Google Scholar]
- McCoy K, Higgins M, Zuniga JA, & Holstad MM (2015). Age, Stigma, adherence and clinical indicators in HIV-infected women. HIV/AIDS Research and Treatment, 2015(SE3), S1–S8. doi: 10.17140/hartoj-se-3-101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDoom MM, Bokhour B, Sullivan M, & Drainoni ML (2015). How older black women perceive the effects of stigma and social support on engagement in HIV care. AIDS Patient Care and STDs, 29(2), 95–101. doi: 10.1089/apc.2014.0184 [DOI] [PubMed] [Google Scholar]
- Mugavero MJ, Amico KR, Horn T, & Thompson MA (2013). The state of engagement in HIV care in the United States: From cascade to continuum to control. Clinical Infectious Diseases, 57(8), 1164–1171. doi: 10.1093/cid/cit420 [DOI] [PubMed] [Google Scholar]
- Mugavero MJ, Amico KR, Westfall AO, Crane HM, Zinski A, Willig JH, … Saag MS (2012). Early retention in HIV care and viral load suppression: Implications for a test and treat approach to HIV prevention. Journal of Acquired Immune Deficiency Syndromes, 59(1), 86–93. doi: 10.1097/QAI.0b013e318236f7d2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Davila JA, Nevin CR, & Giordano TP (2010). From access to engagement: Measuring retention in outpatient HIV clinical care. AIDS Patient Care and STDs, 24(10), 607–613. doi: 10.1089/apc.2010.0086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, … Allison JJ (2009). Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clinical Infectious Diseases, 48(2), 248–256. doi: 10.1086/595705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R, & Aggleton P (2003). HIV and AIDS-related stigma and discrimination: A conceptual framework and implications for action. Social Science & Medicine, 57(1), 13–24. doi: 10.1016/S0277-9536(02)00304-0 [DOI] [PubMed] [Google Scholar]
- Psaros C, Barinas J, Robbins GK, Bedoya CA, Park ER, & Safren SA (2015). Reflections on living with HIV over time: Exploring the perspective of HIV-infected women over 50. Aging & Mental Health, 19(2), 121–128. doi: 10.1080/13607863.2014.917608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D, Choi SW, Victorson D, Bode R, Peterman A, Heinemann A, & Cella D (2009). Measuring stigma across neurological conditions: The development of the Stigma Scale for Chronic Illness (SSCI). Quality of Life Research, 18(5), 585–595. doi: 10.1007/s11136-009-9475-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D, Kemp CG, Huh D, Nevin PE, Turan J, Cohn SE, … French AL (2018). Stigma reduction among African American women with HIV: UNITY Health Study. Journal of Acquired Immune Deficiency Syndromes, 78(3), 269–275. doi: 10.1097/qai.0000000000001673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D, Molina Y, Lambert N, & Cohn SE (2016). Assessing stigma among African Americans living with HIV. Stigma and Health, 1(3), 146–155. doi: 10.1037/sah0000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WS, Crockett KB, Mugavero MJ, Raper JL, Atkins GC, & Turan B (2017). Association between internalized HIV-related stigma and HIV care visit adherence. Journal of Acquired Immune Deficiency Syndromes, 76(5), 482–487. doi: 10.1097/qai.0000000000001543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda S, Mitra S, Chen S, Gogolishvili D, Globerman J, Chambers L, … Rourke SB (2016). Examining the associations between HIV-related stigma and health outcomes in people living with HIV/AIDS: A series of meta-analyses. BMJ Open, 6(7), e011453. doi: 10.1136/bmjopen-2016-011453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangaramoorthy T, Jamison AM, & Dyer TV (2017). HIV stigma, retention in care, and adherence among older Black women living with HIV. Journal of the Association of Nurses in AIDS Care, 28(4), 518–531. doi: 10.1016/j.jana.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Sherbourne CD, & Stewart AL (1991). The MOS social support survey. Social Science and Medicine, 32(6), 705–714. doi: 10.1016/0277-9536(91)90150-b [DOI] [PubMed] [Google Scholar]
- Turan B, Hatcher AM, Weiser SD, Johnson MO, Rice WS, & Turan JM (2017). Framing mechanisms linking HIV-related stigma, adherence to treatment, and health outcomes. American Journal of Public Health, 107(6), 863–869. doi: 10.2105/ajph.2017.303744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan B, Rice WS, Crockett KB, Johnson M, Neilands TB, Ross SN, … Turan JM (2019). Longitudinal association between internalized HIV stigma and antiretroviral therapy adherence for women living with HIV: The mediating role of depression. AIDS, 33(3), 571–576. doi: 10.1097/qad.0000000000002071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walcott M, Kempf MC, Merlin JS, & Turan JM (2016). Structural community factors and sub-optimal engagement in HIV care among low-income women in the Deep South of the USA. Culture Health & Sexuality, 18(6), 682–694. doi: 10.1080/13691058.2015.1110255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE Jr., & Sherbourne CD (1992). The MOS 36-Item Short-Form Health Survey (SF-36): I. Conceptual framework and item selection. Medical Care, 30(6), 473–483. doi: 10.2307/3765916 [DOI] [PubMed] [Google Scholar]
- Wawrzyniak AJ, Rodríguez AE, Falcon AE, Chakrabarti A, Parra A, Park J, … Metsch LR (2015). The association of individual and systemic barriers to optimal medical care in people living with HIV/AIDS (PLWHA) in Miami-Dade County. Journal of Acquired Immune Deficiency Syndromes, 69(0 1), S63–S72. doi: 10.1097/qai.0000000000000572 [DOI] [PMC free article] [PubMed] [Google Scholar]


