Abstract
Objective:
To evaluate the relationship between medication adherence and visual field progression in participants randomized to the medication arm of the Collaborative Initial Glaucoma Treatment Study (CIGTS).
Design:
The CIGTS was a randomized, multi-center clinical trial comparing initial treatment with topical medications to trabeculectomy for 607 participants with newly diagnosed glaucoma.
Participants:
307 participants randomized to the medication arm of the CIGTS.
Methods:
Participants were followed at 6 month intervals for up to 10 years. Self-reported medication adherence and visual fields were measured. Medication adherence was assessed by telephone from responses to “Did you happen to miss any dose of your medication yesterday?” The impact of medication adherence on mean deviation (MD) over time was assessed with a linear mixed regression model adjusting for the effects of baseline MD and age, cataract extraction, interactions and time (through year 8, excluding time after crossover to surgery). Medication adherence was modeled as a cumulative sum of the number of prior visits where a missed dose of medication was reported.
Main outcome measure:
MD over time.
Results:
307 subjects (306 with adherence data) were randomized to treatment with topical medications and followed for an average of 7.3 years (standard deviation=2.3). 142 subjects (46%) reported never missing a dose of their medication over all available follow-up, 112 (37%) reported missing medication for up to 1/3 of their visits, 31 (10%) reported missing medication for 1/3 to 2/3 of their visits, and 21 (7%) reporting missing medication at >2/3 of their visits. Worse medication adherence was associated with loss of MD over time (p=0.005). For subjects who reported never missing a dose of medication, the average predicted MD loss over 8 years was 0.62 dB, consistent with age-related loss (95% confidence interval, CI=0.17−1.06; p=0.007); subjects reporting missing medication doses at 1/3 of visits had a loss of 1.42 dB (CI, 0.86−1.98; p<0.0001); subjects reporting missing medication doses at 2/3 of visits had a loss of 2.23 dB (CI, 1.19−3.26; p<0.0001).
Conclusion:
This longitudinal assessment demonstrated a statistically and clinically significant association between medication non-adherence and glaucomatous vision loss.
Precis
We found a statistically and clinically significant relationship between medication adherence and glaucomatous vision loss over 8 years among the 306 Collaborative Initial Glaucoma Treatment Study participants randomized to the medication arm of the trial.
Glaucoma is a leading cause of irreversible blindness world-wide. In the United States (US) it affects 2.9 million people,1 of whom 120,000 are blind.2 The prevalence of glaucoma is rising, with 64.3 million people affected globally in 2013 and a projection of 112 million people affected by 2040.3
Lowering intraocular pressure (IOP) via medical or surgical therapy is a proven treatment to slow or halt glaucomatous progression.1–4 Medical therapy is the most common approach to treatment5 and costs from glaucoma medications account for 54% of Medicare Part D prescribing costs by US eye care providers.6 However, cross sectional analyses of glaucoma medication-taking behavior, including medication refill data,7,8 estimate that rates of medication adherence in the US are about 50%. Rates of persistence with glaucoma medications, or the continued use of prescribed medication over the long term, are even lower. A retrospective cohort study of 1,234 patients newly diagnosed with open-angle glaucoma found that only 15% had persistently good adherence over four years of follow-up.9 In another study, even when patients knew their adherence was being electronically monitored, only 83% took their once-daily dosed medication correctly >75% of days during a three-month period.10
The impact of non-adherence on clinical outcomes has gained increasing attention in recent years, with almost no publications in 1970, about 5,000 in 1980, and nearly 40,000 in 2007.11 Non-adherence leading to hospitalization has been documented in decompensated heart failure, where 57% of hospitalized patients reported poor medication adherence,12 and in schizophrenia, where 40% of re-hospitalizations were due to poor medication adherence.13
In glaucoma, patients who took ≥80% of their prescribed medications had an 86% reduced odds of having a moderate or severe visual field defect compared to those who took <80% of their prescribed medications.14 Though it may seem intuitive that poor medication adherence will lead to poor outcomes, questions remain as to the efficacy of partial adherence. To date, only one small study (n=35) has assessed the impact of glaucoma medication adherence on glaucomatous progression.15 Few available data sources capture both longitudinal measures of medication adherence and repeated assessment of visual field status.
The Collaborative Initial Glaucoma Treatment Study (CIGTS) was a randomized multi-center clinical trial of initial treatment with medications or trabeculectomy in which self-reported medication adherence and visual field tests were measured over follow-up in a standardized fashion. Additionally, CIGTS data includes participants with high rates of longitudinal follow-up: 79% of participants had 5 years of follow-up data. Thus, the CIGTS data offer unique insights into the association between medication taking behavior and glaucomatous progression. The purpose of this study is to evaluate the longitudinal relationship between medication adherence and visual field progression among participants enrolled in the medication arm of the CIGTS.
Methods
In the CIGTS (Clinical-Trials.gov Identifier # NCT00000149), 607 subjects with newly-diagnosed, open-angle glaucoma were enrolled at 14 centers across the US and randomized to either initial treatment with surgery (trabeculectomy, n=300) or topical medications (n=307). Informed consent was obtained from all participants. Eligibility of an eye included a combination of elevated IOP, visual field changes, and optic disc findings.16 For unilaterally eligible subjects, the study eye was designated at baseline as the only eligible eye; for bilaterally eligible subjects, the study eye was determined by physician discretion. The study eye was the worse eye by either IOP, mean deviation (MD), or cup-disc ratio in 99.3% of subjects.” This analysis includes the 306 participants in the medication arm of the CIGTS who had at least one follow-up visit.
Socio-demographic factors and assessment of depression were obtained at the baseline study visit. Depression was assessed based on participants’ responses to the eight-item Center for Epidemiologic Studies Depression Scale (CES-D 8).17 The scale was scored with a possible range from 0–24, where higher scores indicated more depressive symptoms. A CES-D score ≥ 7 was used as an indicator of mild or worse depression.18
Follow-up study visits were conducted at 3 months, 6 months, and every 6 months thereafter up to 10 years after randomization. The visits included a thorough clinical examination and visual field assessment using the Humphrey Visual Field Analyzer 24–2 full threshold test (Zeiss-Humphrey Systems, Dublin, CA). The visual field summary measure used for this analysis was the MD in decibels (dB). A telephone interview assessing quality of life and other self-reported measures was conducted within a ±45-day window of the exam. Although the clinical examination and quality of life telephone interviews followed the same 6-month intervals, the two study components were scheduled independently and could occur in either order.19 The interviews included questions about medication adherence, assessed by asking participants to respond to the following question for each medication prescribed, “Everyone finds it difficult to take all of their medications exactly as the doctor prescribed. There are lots of reasons for this. Sometimes the instructions are complicated or hard to read or understand, especially when there are several different ones to take. Sometimes the medications have to be taken at inconvenient times; often it’s hard to remember to take them; sometimes they have unpleasant side effects. We want to get an idea of what medication taking is like for you. Did you happen to miss any dose of your [name of medication] yesterday (yes/no)?” Single-item tools to assess medication adherence have been found to be valid ways to assess adherence with low patient burden.20,21 Participants were classified as non-adherent when reporting a missed dose for one or more of their prescribed medications.
Approval for this analysis of data collected on CIGTS participants was granted by the University of Michigan Institutional Review Board. The study was conducted in accordance with Health Insurance Portability and Accountability Act regulations and adhered to the tenets of the Declaration of Helsinki.
Statistical Methods
Baseline demographics and clinical characteristics are summarized with descriptive statistics. For each participant, medication adherence was categorized as: 1) no visits with a report of a missed dose of medication; 2) at least one but ≤1/3 of visits with a report of a missed dose of medication; 3) >1/3 of visits but <2/3 of visits with a report of a missed dose of medication; 4) ≥2/3 of visits with a report of a missed dose of medication. In different analyses, medication adherence was summarized over all follow-up visits, or calculated as a cumulative measure over the follow-up visits.
Potential baseline predictors of non-adherence (the patient-specific proportion of non-adherent visits over all follow-up), were individually tested with linear regression: age, sex, race, IOP, and MD. These regressions test slope for continuous variables (age, IOP, MD) or differences for dichotomous variables. Additionally, the linear relationship of non-adherence with possible psychosocial antecedents of non-adherence (education, marital status, and depression) were similarly tested.
The association of medication adherence with visual field progression over time, measured by MD, was assessed using a linear mixed regression model. This model, built on previous work also investigating predictors of visual field progression19 included the following covariates (fixed effects): baseline MD and age, time from enrollment, a time-varying indicator for a visit one year prior to cataract extraction, and two interactions (baseline MD by the cataract indicator and age by time from enrollment). These variables, used to adjust for confounding effects, were previously identified using best-subset and backward selection procedures. In both the previous and current model, repeated measures of MD within a subject over time were modeled with a heterogenous Toeplitz correlation structure,22 which provided a better fit to the data than including random subject effects. Modifications to the original model included restricting the sample to those subjects randomized to medication, including follow-up visits from 3 months through 8 years (with small sample sizes thereafter limiting our ability to obtain robust estimates), and excluding any follow-up visit information after a participant failed the medication arm of the study and crossed over to receive either laser trabeculoplasty or incisional surgery, as these could change their medication regimen. Medication adherence was then added to the model to test for an association with visual field progression. Medication adherence was modeled as a time-varying covariate, cumulatively summing the number of prior visits where a missed dose of medication was reported. After dropping non-significant variables, all variables retained in the final model were independently associated with the outcome, MD, at p<0.05. Variables previously assessed and found unrelated to visual field progression included clinical center, gender, education, current smoking status, alcohol intake, family history of glaucoma, type of glaucoma, pupil response, iris color, optic disc hemorrhage, corneal thickness, and systemic hypertension. SAS version 9.4 (Cary, NC) was used for all statistical analyses.
Results
The 306 participants in the medication arm of the CIGTS trial who had at least one follow-up visit had a mean (±SD) age at enrollment of 57.4±11.2 years (range, 29 to 76 years) and 54% were male. Approximately half (55%) self-reported their race as White, 39% as Black, and 6% as another race. Nearly half (46%) had completed less than or equal to a high school education and 54% had completed at least some college. 57% were married. The mean baseline CESD-8 depression score was 2.4 ± 3.8 and 14% of participants had mild or worse depression.
Participants entered the study with a mean deviation of −5.2 ± 4.3 dB (range, −23.5 to +3.4 dB) and a mean IOP of 27.6 ± 5.5 mmHg (range, 19 to 48 mmHg). Participants were followed for a mean of 7.3 ± 2.3 years.
The distributions across the four medication adherence categories, cumulatively over time from enrollment, are displayed in Figure 1. Because adherence is cumulative, the number of subjects reporting that they never missed a dose of their medication can only remain constant or decrease. Some fluctuation is due to changing sample sizes from missed visits or participant dropout. As expected, the percentage of participants reporting no missed doses of medication decreased over time, with 58% in year 3, 49% in year 5, and 39% in year 8. Conversely, the percentage of participants reporting a missed dose of medication at 1/3 − 2/3 of visits increased from 11% in year 3 to 17% in year 8.
Figure 1.
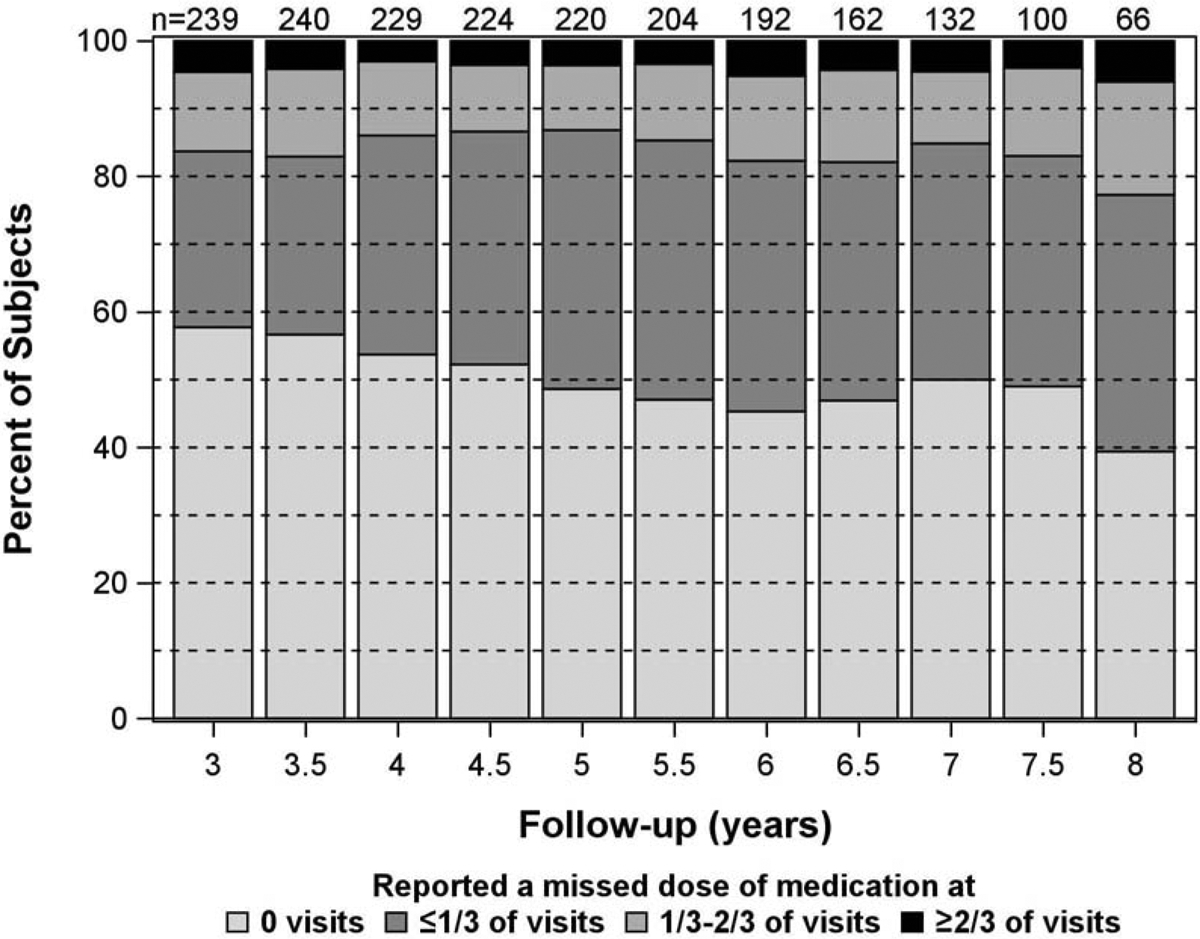
Stacked bar charts showing the percentage of patients in each of the 4 categories of medication adherence over time from enrollment. For each patient at each visit, the category is assigned based on the cumulative number of visits with adherence divided by the total number of visits up to that point.
Over all available follow-up, 46% of participants (n = 142) reported never missing a dose of medication, 37% of participants (n = 112) reported a missed dose at up to 1/3 of visits, 10% of participants (n = 31) reported a missed dose for between 1/3 − 2/3 of visits, and 7% of participants (n = 21 (6.9%) reported a missed dose for >2/3 of visits. Participants reporting more missed doses of medication over follow-up (missing >2/3 of doses versus no missing doses) were on average 10 years younger (p=0.0001), had more severe visual field loss at baseline by 1.6 dB (p=0.006, Table 1), a greater proportion were of Black race (67% versus 33%, respectively; p=0.027), a greater proportion were not married (57% versus 39%, respectively; p=0.007), and a greater proportion had a high school or less education (66.7% versus 47.9%, respectively; p=0.023). Additionally, participants reporting more missed doses of medication (missing >2/3 of doses versus no missing doses) had higher average depression scores on the CESD-8 (4.3 versus 2.2, respectively; p<0.0001), and higher proportions of mild or worse depression (29% versus 12%, respectively; p=0.0004). Baseline IOP and gender did not show an association with missed doses of medication over follow-up (Table 1).
Table 1.
Baseline patient demographics and clinical characteristics by adherence levels for 306 patients in the medication arm of the Collaborative Initial Glaucoma Treatment Study (CIGTS)
| 0 Missed (n=142) |
≤⅓ Missed (n=112) |
⅓-⅔ Missed (n=31) |
≥⅔ Missed (n=21) |
||
|---|---|---|---|---|---|
| Baseline Variables | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | P-value* |
| Age (years) | 58.5 (11.0) | 57.9 (11.3) | 56.4 (9.7) | 48.4 (10.8) | 0.0001 |
| MD (dB) | −4.7 (4.1) | −5.2 (3.9) | −6.7 (5.3) | −6.3 (5.3) | 0.0057 |
| IOP (mmHg) | 27.7 (5.5) | 26.7 (5.0) | 29.7 (6.8) | 28.2 (5.8) | 0.2388 |
| CESD Score | 2.1 (3.4) | 2.0 (3.4) | 4.5 (4.6) | 4.3 (5.2) | <0.0001 |
| # (%) | # (%) | # (%) | # (%) | P-value* | |
| Gender | |||||
| Male | 82 (57.8) | 58 (51.8) | 15 (48.4) | 9 (42.9) | 0.3904 |
| Female | 60 (42.2) | 54 (48.2) | 16 (51.6) | 12 (57.1) | |
| Race | |||||
| White/Other | 92 (64.8) | 70 (62.5) | 17 (54.8) | 7 (33.3) | 0.0268 |
| Black | 50 (35.2) | 42 (37.5) | 14 (45.2) | 14 (66.7) | |
| Marital Status | |||||
| Married | 86 (60.6) | 67 (59.8) | 12 (38.7) | 9 (42.9) | 0.0065 |
| Not Married | 56 (39.4) | 45 (40.2) | 19 (61.3) | 12 (57.1) | |
| Education | |||||
| ≤HS | 68 (47.9) | 65 (58.0) | 17 (54.8) | 14 (66.7) | 0.0229 |
| Some College+ | 74 (52.1) | 47 (42.0) | 14 (45.2) | 7 (33.3) | |
| Depression | |||||
| CESD<7 | 121 (87.7) | 99 (91.7) | 21 (67.7) | 15 (71.4) | 0.0004 |
| CESD≥7 | 17 (12.3) | 9 (8.3) | 10 (32.3) | 6 (28.6) |
SD, standard deviation; MD, mean deviation; dB, decibels; IOP, intraocular pressure; mmHg, millimeters of mercury; CESD, Center for Epidemiologic Studies Depression Scale; HS, high school
Regression analysis to test for a linear relationship (for continuous variables) or difference (for dichotomous variables)
In the linear mixed regression model of visual field progression, an increase in the number of visits where a patient reported a missed dose of medication was significantly associated with a decrease (worsening) in mean deviation (estimate=−0.14 dB per visit with missed dose, p=0.0054, Figure 2), after adjusting for significant confounding variables. For a subject who reported never missing a dose of medication, the average predicted loss in MD over 8 years was −0.62 dB (95% confidence interval (CI) −1.06 to −0.17, p=0.007; Table 2). In comparison, for a subject reporting missed medication doses at 1/3 of visits over 8 years, the average predicted loss in MD was −1.42 dB (95% CI −1.98 to −0.86, p<0.0001); for a subject reporting missed medication doses at 1/2 of visits over 8 years, the average predicted loss in MD was −1.82 dB (95% CI −2.61 to −1.04, p<0.0001); and, for a subject reporting missed medication doses at 2/3 of visits over 8 years, the average predicted loss in MD was −2.23 dB (95% CI −3.26 to −1.19, p<0.0001).
Figure 2.
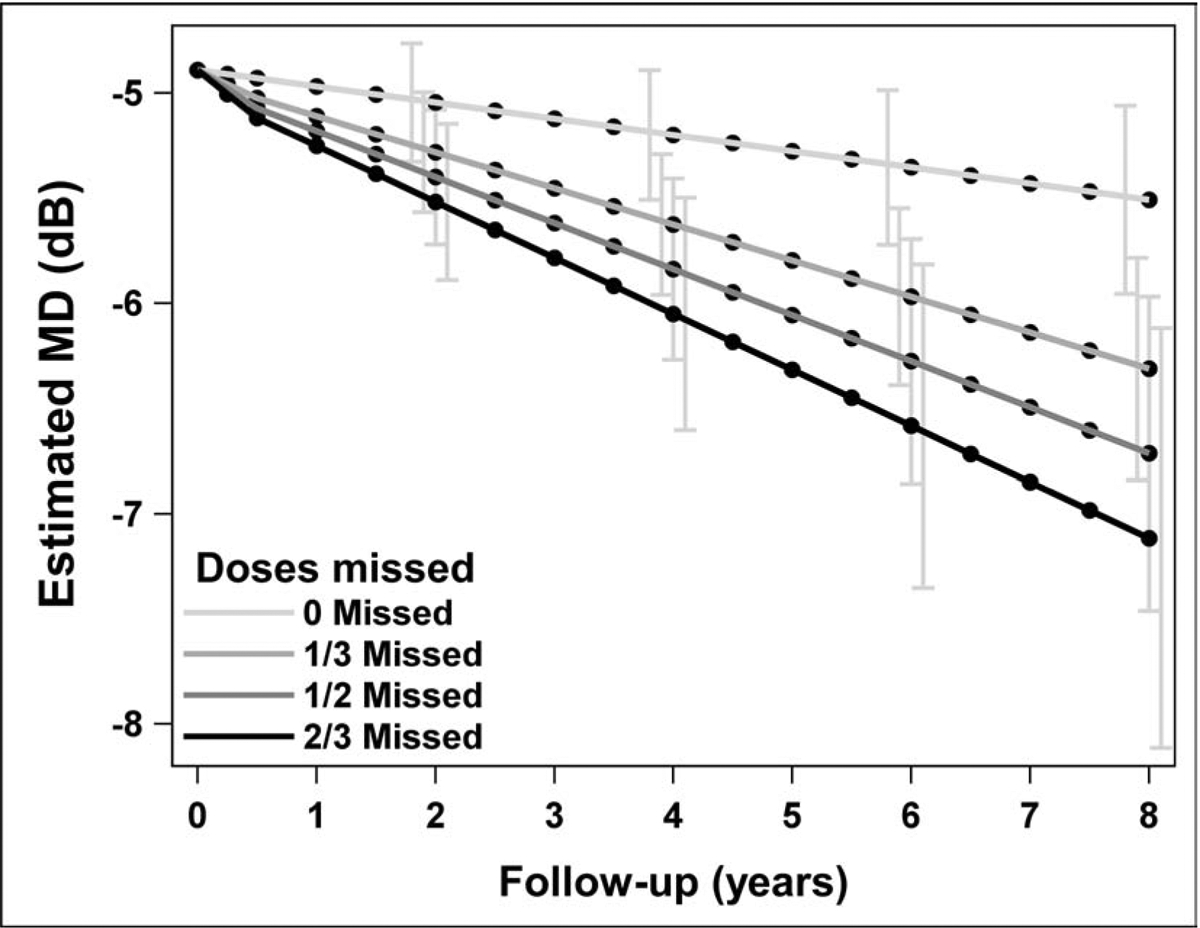
For different levels of adherence, the mean deviation (MD) over time from enrollment was estimated using a linear mixed regression model. For this display, the covariates baseline MD and age were set to their mean values, and cataract extraction was set to ‘no’; 95% confidence intervals are displayed at years 2, 4, 6, and 8.
Table 2.
Estimated mean deviation (MD) from a linear mixed regression model*, by adherence and follow-up
| 0 Missed (n=142) |
≤⅓ Missed (n=112) |
⅓-⅔ Missed (n=31) |
≥⅔ Missed (n=21) |
|
|---|---|---|---|---|
| Years From Enrollment | Estimate (CI) |
Estimate (CI) |
Estimate (CI) |
Estimate (CI) |
| 2 | −5.04 | −5.28 | −5.40 | −5.52 |
| (−5.34, −4.75) | (−5.58, −4.99) | (−5.73, −5.07) | (−5.90, −5.14) | |
| 4 | −5.20 | −5.62 | −5.84 | −6.05 |
| (−5.52, −4.88) | (−5.97, −5.28) | (−6.28, −5.40) | (−6.61, −5.49) | |
| 6 | −5.35 | −5.97 | −6.28 | −6.58 |
| (−5.73, −4.98) | (−6.40, −5.54) | (−6.87, −5.69) | (−7.36, −5.81) | |
| 8 | −5.51 | −6.31 | −6.71 | −7.12 |
| (−5.96, −5.05) | (−6.85, −5.78) | (−7.47, −5.96) | (−8.12, −6.11) | |
| MD Loss | −0.62 | −1.42 | −1.82 | −2.23 |
| 0 vs 8 | (−1.06, −0.17) | (−1.98, −0.86) | (−2.61, −1.04) | (−3.26, −1.19) |
| P-value | 0.0065 | <0.0001 | <0.0001 | <0.0001 |
For different levels of adherence, the mean deviation (MD) over time from enrollment was estimated using a linear mixed regression model. For this display, the covariates baseline MD and age were set to their mean values, and cataract extraction was set to ‘no’; 95% confidence intervals (CI) are provided.
Discussion
The CIGTS trial is one of few studies to have captured longitudinal measures of both medication adherence and visual field assessment. This longitudinal analysis demonstrated a statistically and clinically significant association between medication adherence and glaucomatous loss of visual function among the participants randomized to the medication arm of the trial over 8 years of follow-up. The estimated loss of visual field for a participant who did not report having missed any doses of prescribed glaucoma medication over 8 years of follow-up was 0.62 dB, after adjusting for significant confounding variables. This is similar to other estimates of age-related visual field loss with stable glaucoma that range between 0.05 dB per year23 and 0.1 dB per year,24 which would produce a range of 0.4 to 0.8 dB of MD loss over 8 years. Conversely, a participant who reported missing medication doses at 1/3 of visits lost an average of 1.42 dB of MD over 8 years and those who reported missing medication doses at 2/3 of visits lost an average of 2.23 dB of MD over 8 years. These data display a dose-response relationship between the extent of medication adherence and glaucomatous visual field loss, in line with the remark made in 1985 by C. Everett Coop, the US Surgeon General between 1982–1989, that “Drugs don’t work in patients who don’t take them.”25
The single previous longitudinal study to assess the relationship between medication adherence and visual field progression took place in Italy between 2005–2009 by Rossi and colleagues.26 The study was an observational case series that assessed 35 out of 59 participants originally enrolled in a study assessing medication adherence to monotherapy with either travaprost or combination travaprost/timolol. These 35 participants had completed at least 12 months of follow-up wherein their medication adherence was monitored electronically and the percent of prescribed doses taken was calculated. Visual fields (7–8 per participant) were obtained before and after the monitoring period. A visual field expert masked to participants’ adherence status judged whether there was visual field progression. Mean deviation, pattern standard deviation and glaucoma stage were plotted over time and if the slope of the plotted line was flat or positive, the field was classified as stable and if the slope was negative the field was classified as progressed. In 72.4% of participants, the visual field was classified as stable and median adherence was 85% (interquartile range (IQR) 75%−97%). In 27.6% of participants, the visual field was classified as having progressed and median adherence was 21% (IQR 9%−45%) (p<0.0001). Rossi and colleagues also demonstrated a dose-response effect of medication adherence on visual field progression. Of the 12 (34.3%) participants with >90% adherence, none progressed; of the 14 participants with 50%−90% adherence, 14.3% (n = 2) progressed, and of the 9 participants with <50% adherence, 88.9% (n = 8) progressed. This dose response effect, where worsening adherence levels were associated with increased visual field progression over time, is similar to what was seen in our CIGTS population of 306 participants.
The dose-response relationship we found between medication adherence and visual field progression was significant even though the assessment of medication adherence – self-report – is notorious for overestimating true adherence.27 In 1986, Kass and colleagues27 developed an electronic eye drop adherence monitor that was shaped like a bottle of pilocarpine. They gave this monitor to 184 patients who were prescribed pilocarpine three or four times daily under the guise that this new bottle was merely different from the usual pilocarpine bottle because it contained a free sample of medication. Therefore, patients did not know that their medication adherence was being electronically monitored. Patients reported taking 97.1 ± 5.9% of prescribed pilocarpine doses, whereas the medication adherence monitor data indicated that patients actually administered no more than 76.0 ± 24.3% of pilocarpine doses. Accordingly, the magnitude of the association between medication adherence and glaucomatous visual field progression that we found in this study is likely an underestimate because CIGTS participants were more likely to have overestimated their true medication adherence.
In this study, we also examined baseline antecedents, or predictors, of poor medication adherence. We found that CIGTS participants who were younger, were not married, had worse baseline mean deviation, were of Black race, had ≤High School education or were depressed were more likely to have poor glaucoma medication adherence. The association between poor glaucoma medication adherence and younger age,28,29 less education,30 and minority race31 has been demonstrated in previous analyses. The association of depression with poorer medication adherence has also been demonstrated in a number of studies.32–34 Jayawant and colleagues reported that living alone was another important risk factor for poor persistence with glaucoma medication.33 People with co-morbid mental illness and/or poor social support will need increased personalized support from the health care system to improve their chronic disease self-management and thereby improve outcomes.
While people with glaucoma spend most of their time outside of their eye care providers’ offices, they nonetheless require daily, devoted attention to taking their medications as prescribed, which often requires administering topical medications several times daily. Enabling a person’s adherence behavior requires identifying ways that the person can integrate medication-taking into their daily routine. This becomes even more important when aging begins to impact short term memory.35–38 Since each patient has a unique set of barriers to optimal glaucoma medication adherence,29 and people who live alone or have depression may have even more barriers to optimal adherence, any systems-level approach to improving glaucoma self-management support must be personalized. Personalized counseling has shown promise in multiple randomized controlled trials in improving medication adherence for glaucoma patients,39–41 and creating a standardized way to deliver this type of support42 has potential to improve adherence. Providing between visit support with reminders and alerts provided electronically43,44 and by phone calls from a dedicated “glaucoma coach” or health navigator may improve adherence and thus minimize glaucomatous field loss.
This study has a number of strengths. We had access to longitudinal information on adherence and key clinical variables collected on a large number (n=306) of people with newly diagnosed glaucoma followed for up to 8 years, which increased our ability to detect effects. Our source of data on visual field and self-reported adherence were systematically captured by certified staff following a uniform protocol as part of a randomized controlled clinical trial. The study had also had limitations. Though single-item measures of medication adherence have had reasonable reliability in comparison to biological outcomes such as viral load and CD4 count in patients with AIDS,20 they still overestimate medication adherence compared to more objective adherence measures, such as un-announced pill counts.21 Though our methodology likely underestimated true levels of poor medication adherence,21,27 having the research associate normalize the behavior in the phone script may have helped participants answer more truthfully. Had participants been continuously assessed throughout the study period with electronic medication monitoring for a more objective assessment of adherence, we may have seen an even more robust association between medication adherence and visual field progression. Additionally, because this was not a randomized controlled trial of the impact of medication adherence on visual field progression, it is possible that the non-adherent subjects had worsening field loss due to factors other than medication adherence. As such, this manuscript details only the association between medication taking behavior and visual field progression, and not any causative link. Like most clinical trial participants, CIGTS participants were quite adherent both to their medications and to follow-up, and exhibited a fairly small amount of visual field progression overall throughout the follow-up period. Given these factors, the current study likely underestimates the true magnitude of the association between medication adherence and visual field progression.
In 2003, the World Health Organization stated that “increasing the effectiveness of adherence interventions may have a far greater impact on the health of the population than any improvement in specific medical treatments.”45 To increase the effectiveness of our current glaucoma treatments, there is a critical need to focus on helping support patients in improving their glaucoma medication adherence.
Funding Agency:
National Eye Institute (K23EY025320, PANC; R21EY028997 DCM) and Research to Prevent Blindness Career Development Award (PANC). The funding organizations had no role in the design or conduct of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was presented in part at: The American Glaucoma Society Annual Meeting, San Francisco, CA, March 16, 2019.
Conflict of Interest: No conflicting relationships exist for any author.
References
- 1.Heijl A et al. EMGT. Arch Ophthalmol. 2002:1268–1279. [DOI] [PubMed] [Google Scholar]
- 2.Collaborative Normal-tension Glaucoma Group Study. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126:498–505. [DOI] [PubMed] [Google Scholar]
- 3.Gaasterland DE, Ederer F, Beck A, et al. The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000. [DOI] [PubMed] [Google Scholar]
- 4.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): A randomised, multicentre, placebo-controlled trial. Lancet. 2015. [DOI] [PubMed] [Google Scholar]
- 5.Devgan U, Yu F, Kim E, Coleman AL. Surgical undertreatment of glaucoma in black beneficiaries of Medicare. Arch Ophthalmol. 2000. [DOI] [PubMed] [Google Scholar]
- 6.Newman-Casey PA, Woodward MA, Niziol LM, Lee PP, De Lott LB. Brand Medications and Medicare Part D: How Eye Care Providers’ Prescribing Patterns Influence Costs. Ophthalmology. 2018;125:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feehan M, Munger M, Cooper D, et al. Adherence to Glaucoma Medications Over 12 Months in Two US Community Pharmacy Chains. J Clin Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheer R, Bunniran S, Uribe C, Fiscella RG, Patel VD, Chandwani HS. Predictors of Nonadherence to Topical Intraocular Pressure Reduction Medications Among Medicare Members: A Claims-Based Retrospective Cohort Study. J Manag Care Spec Pharm. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman-Casey PA, Blachley T, Lee PP, Heisler M, Farris KB, Stein JD. Patterns of Glaucoma Medication Adherence over Four Years of Follow-Up. Ophthalmology. 2015;122:2010–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boland MV., Chang DS, Frazier T, Plyler R, Friedman DS. Electronic monitoring to assess adherence with once-daily glaucoma medications and risk factors for nonadherence: The automated dosing reminder study. JAMA Ophthalmol. 2014. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg EL, Dekoven M, Schabert VF, Coyle A. Patient medication adherence: the forgotten aspect of biologics. Biotechnol Healthc. 2009;6:39–44. [PMC free article] [PubMed] [Google Scholar]
- 12.Schiff GD, Fung S, Speroff T, McNutt RA. Decompensated heart failure: symptoms, patterns of onset, and contributing factors. Am J Med. 2003;114:625–630. [DOI] [PubMed] [Google Scholar]
- 13.Misdrahi D, Llorca PM, Lançon C, Bayle FJ. Compliance in schizophrenia: predictive factors, therapeutical considerations and research implications. Encephale. 2002;28:266–272. [PubMed] [Google Scholar]
- 14.Sleath B, Blalock S, Covert D, et al. The Relationship between Glaucoma Medication Adherence, Eye Drop Technique, and Visual Field Defect Severity. Ophthalmology. 2011;118:2398–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossi GCM, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do Adherence Rates and Glaucomatous Visual Field Progression Correlate? Eur J Ophthalmol. 2011;21:410–414. [DOI] [PubMed] [Google Scholar]
- 16.Musch D, LIchter P, Guire K, CL S. The Collaborative Initial Glaucoma Treatment Study: study design, methods, and baseline characteristics of enrolled patients. Ophthalmology. 1999;106:653–662. [DOI] [PubMed] [Google Scholar]
- 17.Karim J, Weisz R, Bibi Z, ur Rehman S. Validation of the Eight-Item Center for Epidemiologic Studies Depression Scale (CES-D) Among Older Adults. Curr Psychol. 2015;34:681–692. [Google Scholar]
- 18.Musch DC, Niziol LM, Janz NK, Gillespie BW. Trends in and Predictors of Depression Among Participants in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2019;197:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janz NK, Wren PA, Lichter PR, Musch DC, Gillespie BW, Guire KE. Quality of life in newly diagnosed glaucoma patients. Ophthalmology. 2001;108:887–897. [DOI] [PubMed] [Google Scholar]
- 20.Feldman BJ, Fredericksen RJ, Crane PK, et al. Evaluation of the Single-Item Self-Rating Adherence Scale for Use in Routine Clinical Care of People Living with HIV. AIDS Behav. 2013;17:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalichman SC, Amaral CM, Swetzes C, et al. A Simple Single-Item Rating Scale to Measure Medication Adherence: Further Evidence for Convergent Validity. J Int Assoc Physicians AIDS Care. 2009;8:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfinger RD. Heterogeneous Variance: Covariance Structures for Repeated Measures. J Agric Biol Environ Stat. 1996;1:205. [Google Scholar]
- 23.Chauhan BC, Malik R, Shuba LM, Rafuse PE, Nicolela MT, Artes PH. Rates of Glaucomatous Visual Field Change in a Large Clinical Population. Investig Opthalmology Vis Sci. 2014;55:4135. [DOI] [PubMed] [Google Scholar]
- 24.Rabiolo A, Morales E, L M. COMPARISON OF METHODS TO DETECT AND MEASURE GLAUCOMATOUS PERIMETRIC PROGRESSION In: American Academy of Ophthalmology Annual Meeting. Chicago, IL; 2019. [Google Scholar]
- 25.Lindenfeld J, Jessup M. ‘Drugs don’t work in patients who don’t take them’ (Everett Koop C, MD, US Surgeon General, 1985; ). Eur J Heart Fail. 2017;19:1412–1413. [DOI] [PubMed] [Google Scholar]
- 26.Rossi GCM, Pasinetti GM, Scudeller L, Radaelli R, Bianchi PE. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011. [DOI] [PubMed] [Google Scholar]
- 27.Kass MA, Meltzer DW, Gordon M, Cooper D, Goldberg J. Compliance with Topical Pilocarpine Treatment. Am J Ophthalmol. 1986;101:515–523. [DOI] [PubMed] [Google Scholar]
- 28.Chang DS, Friedman DS, Frazier T, Plyler R, Boland MV. Development and Validation of a Predictive Model for Nonadherence with Once-Daily Glaucoma Medications. Ophthalmology. 2013;120:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman-Casey PA, Robin AL, Blachley T, et al. The Most Common Barriers to Glaucoma Medication Adherence: A Cross-Sectional Survey. Ophthalmology. 2015;122:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boland MV., Chang DS, Frazier T, Plyler R, Friedman DS. Electronic Monitoring to Assess Adherence With Once-Daily Glaucoma Medications and Risk Factors for Nonadherence. JAMA Ophthalmol. 2014;132:838–844. [DOI] [PubMed] [Google Scholar]
- 31.Friedman DS, Hahn SR, Gelb L, et al. Doctor–Patient Communication, Health-Related Beliefs, and Adherence in Glaucoma. Ophthalmology. 2008;115:1320–1327.e3. [DOI] [PubMed] [Google Scholar]
- 32.Lim MC, Watnik MR, Imson KR, Porter SM, Granier AM. Adherence to Glaucoma Medication: The effect of interventions and association with personality type. J Glaucoma. 2013;22:439–446. [DOI] [PubMed] [Google Scholar]
- 33.Jayawant SS, Bhosle MJ, Anderson RT, Balkrishnan R. Depressive Symptomatology, Medication Persistence, and Associated Healthcare Costs in Older Adults With Glaucoma. J Glaucoma. 2007;16:513–520. [DOI] [PubMed] [Google Scholar]
- 34.Pappa C, Hyphantis T, Pappa S, et al. Psychiatric manifestations and personality traits associated with compliance with glaucoma treatment. J Psychosom Res. 2006;61:609–617. [DOI] [PubMed] [Google Scholar]
- 35.Gollwitzer PM. Implementation intentions: Strong effects of simple plans. Am Psychol. 1999. [Google Scholar]
- 36.Gollwitzer PM, Brandstätter V. Implementation Intentions and Effective Goal Pursuit. J Pers Soc Psychol. 1997. [DOI] [PubMed] [Google Scholar]
- 37.Liu LL, Park DC. Aging and medical adherence: The use of automatic processes to achieve effortful things. Psychol Aging. 2004. [DOI] [PubMed] [Google Scholar]
- 38.Nadkarni A, Kucukarslan SN, Bagozzi RP, Yates JF, Erickson SR. Examining determinants of self management behaviors in patients with diabetes: An application of the Theoretical Model of Effortful Decision Making and Enactment. Patient Educ Couns. 2011. [DOI] [PubMed] [Google Scholar]
- 39.Gray TA, Fenerty C, Harper R, et al. Individualised patient care as an adjunct to standard care for promoting adherence to ocular hypotensive therapy: an exploratory randomised controlled trial. Eye. 2012;26:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okeke CO, Quigley HA, Jampel HD, et al. Interventions Improve Poor Adherence with Once Daily Glaucoma Medications in Electronically Monitored Patients. Ophthalmology. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norell SE. Improving medication compliance: A randomised clinical trial. Br Med J. 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alía JM, Edwards HG. Ion solvation and ion association in lithium trifluoromethanesulfonate solutions in three aprotic solvents. An FT-Raman spectroscopic study. Vib Spectrosc. 2000;24:185–200. [Google Scholar]
- 43.Boland MV., Chang DS, Frazier T, Plyler R, Jefferys JL, Friedman DS. Automated telecommunication-based reminders and adherence with once-daily glaucoma medication dosing: The automated dosing reminder study. JAMA Ophthalmol. 2014. [DOI] [PubMed] [Google Scholar]
- 44.Varadaraj V, Friedman DS, Boland MV. Association of an Electronic Health Record–Linked Glaucoma Medical Reminder With Patient Satisfaction. JAMA Ophthalmol. December 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haynes RB, McDonald H, Garg AX MP. World Health Organization. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf.


