Abstract
Background.
Neurodevelopmental impairment is an important challenge for survivors after neonatal surgery with cardiopulmonary bypass (CPB). The subventricular zone, where most neural stem/progenitors originate, plays a critical role in cortical maturation of the frontal lobe. Promoting neurogenesis in the subventricular zone is therefore a potential therapeutic target for preserving cortical growth. Mesenchymal stromal cells (MSCs) promote endogenous regeneration in the rodent brain. We investigated the impact of MSC delivery through CPB on neural stem/progenitor cells and neuroblasts (i.e. young neurons) in the piglet subventricular zone.
Methods.
Two-week-old piglets (n=12) were randomly assigned to one of three groups: (1) Control, (2) Deep hypothermic circulatory-arrest, and (3) Circulatory-arrest followed by MSC administration. MSCs (10×106 per kg) were delivered through CPB during the rewarming period. Neural stem/progenitors, proliferating cells and neuroblasts were identified using immunohistochemistry at three hours after CPB.
Results.
CPB-induced insults caused an increased proliferation of neural stem/progenitors (p<.05). MSC delivery reduced the acute proliferation. MSC treatment increased the number of neuroblasts in the outer region of the subventricular zone (p<.05) where they form migrating chains toward the frontal lobe. Conversely, the thickness of the neuroblast-dense band along the lateral ventricle was reduced after treatment (p<.05). These findings suggest that MSC treatment changes neuroblasts distribution within the subventricular zone.
Conclusions.
MSC delivery through CPB has the potential to mitigate effects of CPB on neural stem/progenitor cells and promote migration of neuroblasts. Further investigation is necessary to determine the long-term effect of MSC treatment during CPB on postnatal neurogenesis.
The mortality of complex congenital heart disease (CHD) such as hypoplastic left heart syndrome has been dramatically improved in the last 2–3 decades1. However, many complex CHD patients who require surgical correction with cardiopulmonary bypass (CPB) during the early postnatal period suffer developmental delay, neurological impairment or behavioral problems2,3. Recent clinical studies have demonstrated that reduced oxygen delivery due to CHD in utero results in subnormal brain development at birth4,5. Additional brain damage also occurs after cardiac surgery in neonates who have brain immaturity due to fetal hypoxia6,7.
Within the postnatal human brain, the subventricular zone (SVZ) represents the largest source of neural stem/progenitor cells (NSPCs)8. These SVZ-derived NSPCs retain regenerative capacity throughout life, and in various pathological situations including traumatic and ischemic brain injury, NSPCs can proliferate, migrate to the site of injury, differentiate into the appropriate cell-type and replace damaged or lost neurons9,10,11. It has been recently shown that in the human brain young neurons migrate from the SVZ into the frontal lobe for several months after birth under normal physiological conditions12. In addition our previous study has demonstrated that restoration of SVZ NSPCs’ neurogenic potential is a possible therapy for improving cortical growth in CHD13.
Mesenchymal stromal cells (MSCs) are multipotent, non-hematopoietic cells that possess both immunomodulatory and regenerative properties and can treat a wide range of disease including hypoxic brain injury14,15. Various rodent studies have shown that MSCs in the brain promote neurogenesis from SVZ NSPCs16,17. Multiple clinical trials have also established the safety of MSC-based therapy18,19. These findings led to our overall hypothesis that MSC treatment protects neurogenic activity during cardiac surgery and promotes cortical regeneration through the action of endogenous SVZ NSPCs. The aim of the present study is to assess the acute effect of MSC delivery through CPB on postnatal neurogenesis in the porcine SVZ.
Material and Methods
Experimental Model
This study involved a total of 12 Yorkshire piglets (4.2 ± 0.8 kg) at two weeks of age (Archer Farms, Inc., Darlington, MD). Animals were randomly assigned to 3 groups (Figure-supplement 1A): i) Control (No surgery, n=4); ii) 15°C deep hypothermic circulatory arrest (DHCA, n=4); and iii) 15°C DHCA with MSC treatment (DHCA+MSC, n=4). CPB was established with ascending aortic perfusion and right atrial drainage via median sternotomy. After initial perfusion animals were cooled to a temperature of 15°C and then underwent DHCA for 60 minutes (Figure-supplement 1B). After 20 minutes of rewarming normal saline (DHCA group) or MSCs (10 × 106 cells/kg, DHCA+MSC group) were administered through the aortic cannula for 10 minutes according to the protocol. After rewarming (40 minutes total), animals were weaned from CPB. The hematocrit level of 30% was maintained and pH-stat strategy was performed to employ the current standard CPB technique. Animals were continuously sedated under general anesthesia after surgery until tissue harvest. At 3 hours after CPB, the brain was harvested after transarterial infusion of normal saline followed by 4% paraformaldehyde in phosphate buffered saline through the common carotid artery20,21. We performed all experiments in compliance with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals.” The study was approved by the Animal Care and Use Committee of the Children’s National Medical Center.
Cellular Analysis
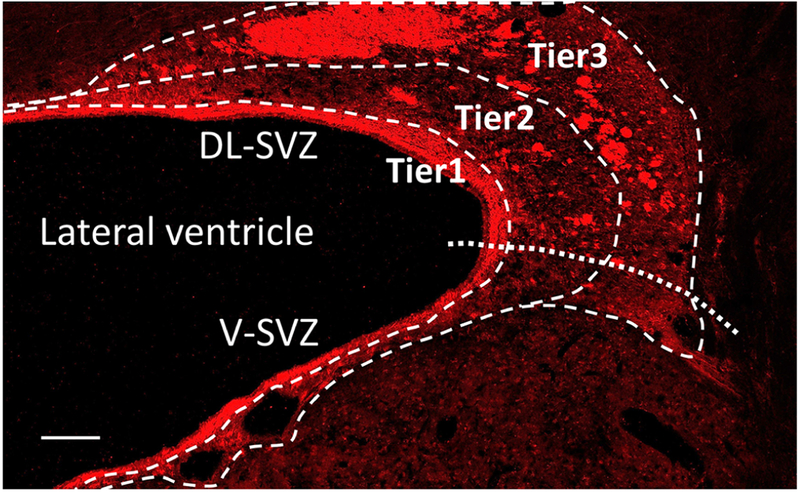
Cellular analysis was performed in coronal section of the brain including the SVZ around the anterior lateral ventricle, which has been shown to be the most active neurogenic niche in the postnatal porcine SVZ13 (Figure-supplement 1C, D). NSPCs and proliferating cells in the SVZ were identified using specific antibodies to SOX2 and Ki67. An anti-doublecortin (Dcx) antibody was used to identify young neurons (neuroblasts). Radial-glia like cells (i.e. neural stem cells) were identified using an anti-glial fibrillary acidic protein (GFAP) antibody to assess neurogenic activity close to the ventricular wall. For cellular analysis the porcine SVZ was divided into three tiers as previously described in the human SVZ12 (Figure 1). Tier 1 represents a cell-dense band of Dcx+ young neurons along the walls of the lateral ventricle. Tier 2 contains a more dispersed collection of migrating young neurons from the wall of the lateral ventricle. Tier 3 contains many Dcx+ young neurons within clusters frequently located around blood vessels, with dispersed migrating young neurons around these clusters. The SVZ is further subdivided into dorsolateral (DL) and ventral (V) regions as previously described13 (Figure 1). Images were acquired on a Leica TCS SP8 confocal microscope (Leica Microsystems, Exton, PA). To determine cell density the antibody-positive cells were blindly quantified in 3 microscopic fields from each region. The process length of GFAP+ radial-glia like cells was co-labeled with Dcx to determine the thickness of a cell-dense band of young neurons around the lateral ventricle (Tier 1) in each SVZ region.
Figure 1.
Magnified image of coronal section of the SVZ. Doublecortin stain. Scale bar, 200μm. SVZ, subventricular zone; DL-SVZ, dorsolateral-SVZ; V-SVZ, ventral-SVZ.
Statistical Analysis
A two-tailed, unpaired Student’s t test was performed for single comparisons. For multiple comparisons, a one-way or two-way analysis of variance (ANOVA) was applied with Bonferroni post hoc test. Statistical analysis was performed using the PRISM6 software package (GraphPad Software, Inc., La Jolla, CA). P values less than 0.05 were considered statistically significant.
The methods of MSC development was described in the supplemental materials.
Results
CPB-induced insults result in an increased proliferation of SVZ NSPCs during the acute period after surgery. MSC delivery mitigates the acute proliferation
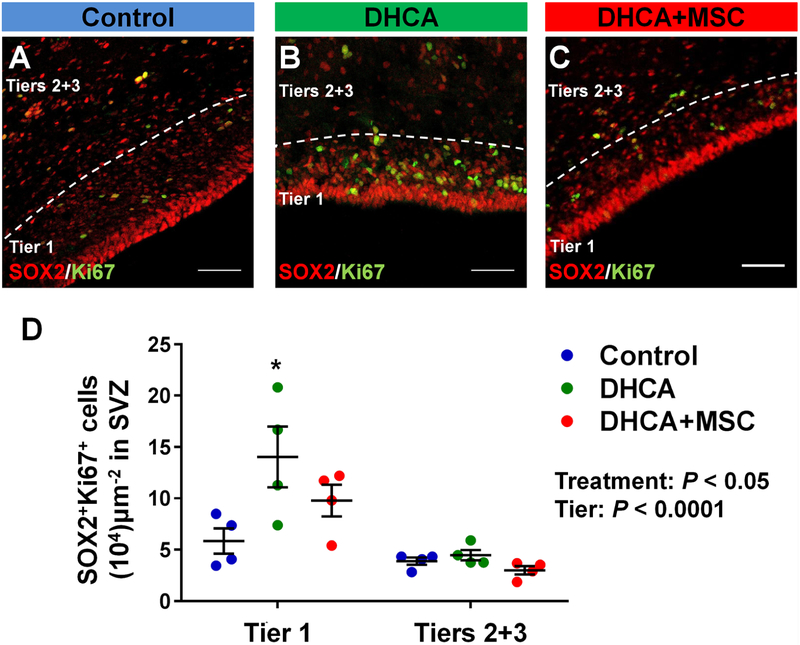
There were no differences in peri-operative conditions and biomarkers between DHCA and DHCA+MSC groups (Supplemental table 1). Significant regional differences were found in the number of SOX2+ NSPCs between the tiers assessed (Figure-supplement 2A, P<.0001). Similar to the human SVZ, tier 1 contained a larger number of NSPCs compared to tiers 2 and 313. Both DHCA and MSC treatment did not change the number at 3 hours after CPB (Figure-supplement 2A). After determining the number of SOX2+Ki67+ proliferating NSPCs in the entire SVZ, there were significant differences between the two regions and three treatment groups (Figure 2A–D). CPB/DHCA-induced insults significantly increased the proliferation of SVZ NSPCs in tier 1 compared with control (Figure 2A,B,D). When the SVZ was subdivided between DL-and V-SVZ, the number of proliferating NSPCs after DHCA was significantly higher than control in both SVZ regions (Figure-supplement 2B,C), indicating that CPB/DHCA-induced insults cause an increased proliferation of SVZ NSPCs in the acute period after cardiac surgery. There were no significant differences in the SOX2+Ki67+ proliferating NSPC number between control and DHCA+MSC groups as well as between DHCA and DHCA+MSC groups in the entire SVZ (Figure 2A–D) and both DL-and V-SVZ (Figure-supplement 2B,C). Altogether the results suggest that MSC delivery during the rewarming period mitigates CPB-induced acute proliferation of SVZ NSPCs.
Figure 2. CPB-induced insults result in an increased proliferation of SVZ NSPCs. MSC delivery mitigates acute proliferation. A-D.
, SOX2+Ki67+ cells in dorsolateral-SVZ (A-C) and the entire SVZ (D). P value was determined by two-way ANOVA with Bonferroni comparisons.*p<0.05 vs. Control by unpaired Student’s t test. Data are shown as mean ± standard error of mean (n=4 each). Scale bar, 50μm. DHCA, deep hypothermic circulatory arrest; MSC, mesenchymal stromal cell; SVZ, subventricular zone.
MSC treatment changes distribution of young neurons within the SVZ
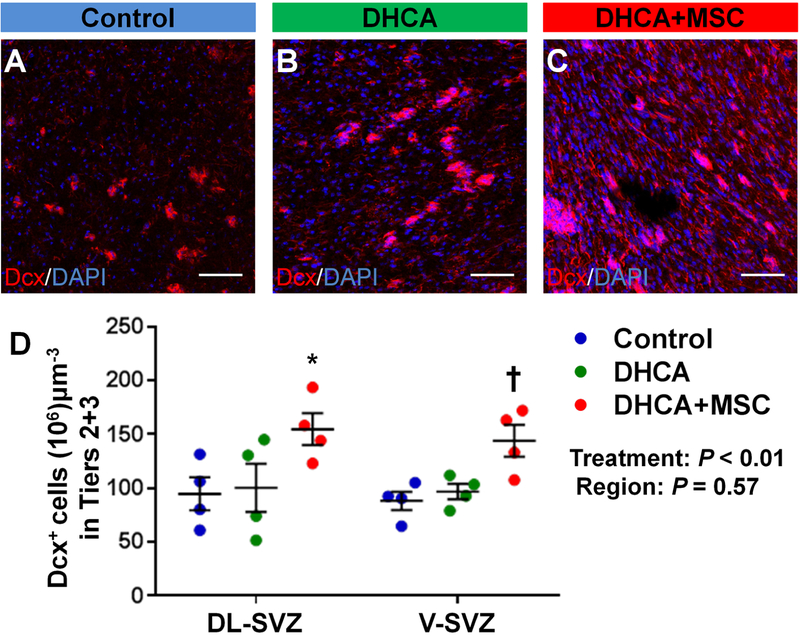
Recently it has been observed that SVZ neuroblasts in the human infant brain form a chain-like structure around blood vessels in the outer region and potentially migrate tangentially toward the frontal lobe12. When the neuroblast number was assessed in the outer region (Tier 2 and 3), there were no differences between control and DHCA groups (Figure 3A,B,D). A significant increase was seen in the number after MSC treatment compared with control (Figure 3A,C,D, Figure-supplement 3A). No differences in the increase of neuroblasts were observed between DL-and V-SVZ following MSC delivery (Figure 3D).
Figure 3. MSC treatment increases the number of neuroblasts within the outer SVZ (Tiers 2 and 3). A-D.
, Dcx+ neuroblasts in Tier 2 and 3 within DL-SVZ (A-C) and both of DL and V-SVZ (D). P value was determined by two-way ANOVA with Bonferroni comparisons. *p<0.05 vs Control and DHCA; †p<0.05 vs Control by unpaired Student’s t test. Data are shown as mean ± standard error of mean (n=4 each). Scale bar, 50μm. DHCA, deep hypothermic circulatory arrest; MSC, mesenchymal stromal cell; Dcx, doublecortin; DL-SVZ, dorsolateral-subventricular zone; V-SVZ, ventral-subventricular zone.
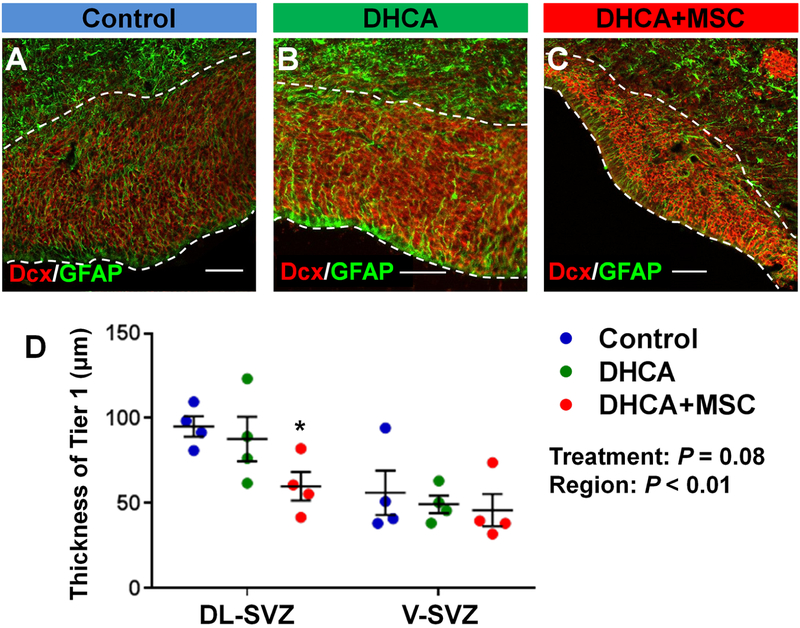
The processes of GFAP+ SVZ neural stem-like cells were co-labelled with Dcx+ cells to determine the thickness of tier 1, which is a dense cell-band of neuroblasts along the lateral ventricle (Figure 4A–C). There were no differences in the thickness between control and DHCA groups in the three SVZ regions analyzed (Figure 4A,B,D, Figure-supplement 3B). The thickness of the neuroblast band in DL-SVZ region was significantly reduced after MSC treatment compared with control (Figure 4A,C,D). Given the acute phase after proliferation or possible caspase activation in this study, the findings may indicate that MSC therapy alters the distribution of neuroblasts within the SVZ, namely the lateral migration from the dense neuroblast band toward tiers 2 and 3.
Figure 4. MSC treatment reduces the thickness of the dense neuroblasts band within the DL-SVZ. A-D.
, Cell-dense band of Dcx+ neuroblasts along the walls of the lateral ventricle (dotty line) in DL-SVZ (A-C) and both of DL and V-SVZ (D). P value was determined by two-way ANOVA with Bonferroni comparisons. *p<0.05 vs Control by unpaired Student’s t test. Data are shown as mean ± standard error of mean (n=4 each). Scale bar, 50μm. DHCA, deep hypothermic circulatory arrest; MSC, mesenchymal stromal cell; Dcx, doublecortin; GFAP, glial fibrillary acidic protein; DL-SVZ, dorsolateral-subventricular zone; V-SVZ, ventral-subventricular zone.
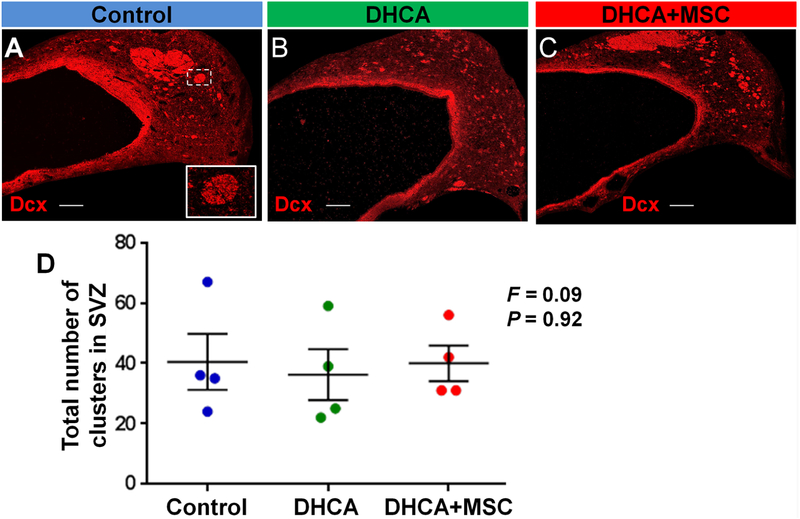
In order to determine whether the potential migration from tier 1 that was observed affects neuroblast clusters (i.e., the coronal section of chain-like migratory structures. Figure 5A box), the number and surface area of the clusters were analyzed in tiers 2 and 3 of the SVZ. There were no changes in the number among the three treatment groups (Figure 5A–D). Similarly no changes in size of neuroblast clusters were observed at 3 hours following DHCA and MSC treatment compared with control (Figure-supplement 4A,B).
Figure 5. The number of neuroblast clusters are not altered at 3 hours after surgery. A-C.
, Dcx+ neuroblast clusters in SVZ. D, Cluster number. P value was determined by one-way ANOVA with Bonferroni comparisons. Data are shown as mean ± standard error of mean (n=4 each). Scale bar, 200μm. DHCA, deep hypothermic circulatory arrest; MSC, mesenchymal stromal cell; Dcx, doublecortin; SVZ, subventricular zone; F, F ratio in the ANOVA.
Comment
This study using a porcine model has identified the impact of MSC delivery through CPB on acute cellular events within the SVZ, the largest source of stem and progenitor cells in the infant brain. Our analysis has shown that brain insults associated with CPB and DHCA result in an increased proliferation of SVZ NSPCs during the acute period after surgery. In addition we have demonstrated that MSC delivery mitigates the CPB-induced acute proliferation of NSPCs. Finally our results suggest that MSC therapy changes neuroblast distribution within the SVZ from a medial cell-dense band to outer regions of the SVZ where neuroblasts form migration chains moving tangentially toward the frontal lobe.
One of the most important current challenges in pediatric cardiac surgery is to reduce neurodevelopment deficits22. Complex CHD such as hypoplastic left heart syndrome and d-transposition of the great arteries alter fetal cerebral oxygen delivery and cause immature and delayed brain development at birth23,24. Additional brain damage commonly occurs after surgery in neonates who have brain immaturity due to fetal hypoxia6,7. Therefore to reduce neurological deficits in CHD patients it will be necessary to promote recovery from hypoxia-induced immature brain development and to mitigate brain damage associated with cardiac surgery.
We have demonstrated that MSC therapy increased the number of neuroblasts in the outer SVZ (Tiers 2 and 3) and reduced the thickness of a neuroblast-dense band (Tier 1). It has been known that the alteration of cell number (i.e. increases/decreases of the cell density) after initial proliferation or caspase activation is a long process in in vivo brain tissues. On the other hand the dynamic motility of neuroblasts in the postnatal SVZ is well-characterized including the capacity of traveling a long distance during a relatively short period (approximately 40–80 μm/hr)25. Since the present study assessed the SVZ at 3 hours after CPB, our results might capture the unique migration of neuroblasts in tier 1 toward outer regions of the SVZ after MSC treatment. The outer SVZ (Tiers 2 and 3) contains many neuroblasts within clusters, coronal section of migratory chains (Figure 5A Box), and dispersed Dcx+ cells around these clusters (Figure 1). Recent studies indicate that new neurons migrate along the migratory chain to the frontal lobe and integrate into the frontal cortex during the early postnatal period12,13, ultimately contributing to maturation of the frontal cortex24. Our previous study has shown that preoperative chronic hypoxia in the piglet model reduces the number of neuroblasts in the frontal cortex13. The current findings therefore suggest that MSC treatment during pediatric cardiac surgery has the potential to promote migration of SVZ neuroblasts toward the frontal lobe thereby improving cortical maturation in the neonatal and infant brain with CHD. Further investigation is necessary to determine the long-term effect of MSC treatment on the migration of SVZ neuroblasts and cortical maturation. Functions of the chain-like structure in the human outer SVZ (Tiers 2 and 3) are largely unknown. Since the unique signature of human cortical development is also displayed in the piglet brain13, future studies using the model will provide novel insights of cellular and molecular mechanisms of postnatal neurogenesis in the human infant cortex.
The present study shows that CPB/DHCA-induced insults increased proliferating NSPCs in the SVZ. The results are consistent with our previous findings of CPB-induced proliferation of oligodendrocyte progenitor cells in white matter within the acute period after surgery20. In our previous studies increased proliferation of these progenitors did not improve white matter recovery but rather resulted in oligodendrocyte dysmaturaiton and delayed myelination in the 4 weeks after CPB20. The current study suggests that CPB-induced acute proliferation of SVZ NSPCs is reduced after MSC delivery. MSCs are remarkable cells that are presently being studied for the treatment of a wide variety of diseases with their beneficial effects including delivery of trophic factors and immune modulation16,26. In the brain MSCs sense the danger in the cerebral environment, communicate with resident cells, and react to the needs of the hypoxic/ischemic microenvironment through secretion of a wide number of neuroprotective and neurotrophic factors15,17. Results from initial clinical trials support the concept that MSCs promote endogenous recovery in the damaged brain after stroke18,19. Rapid cellular responses to systemic infusion of MSCs have been well described27,28. For instance, MSCs induced inflammatory T cell apoptosis via FAS/FAS ligand pathway starting from 1.5 hours after the infusion27. It has been well known that the migration of SVZ neuroblasts is facilitated by neurotrophic factors29,30. Some chemoattractants secreted by MSCs therefore may contribute to the rapid migration of SVZ neuroblasts observed in the present study. Future studies using a porcine model will assist in our understanding of development of SVZ NSPCs after cardiac surgery and the effects of MSC treatment on CPB-induced dysmaturation of progenitor cells.
MSCs can be enriched and expanded from multiple sources31,32. Since the majority of clinical trials in stroke have used bone marrow-derived MSCs33, the safety of bone marrow-derived MSCs has been well established compared with other sources. MSC treatment may be either autologous or allogenic. Indeed autologous transplantation has little risk of immunoreaction but requires a longer period to expand cells for transplant34, limiting the use for neonates. Allogeneic bone marrow-derived MSCs therefore could be the most clinically-relevant cell type for neonates with CHD. Allogeneic MSCs may have cellular and humoral alloreactivity. MSC immunogenicity however is still considerably attenuated compared with other allogeneic cell types because of their intrinsic anti-inflammatory and immunomodulatory properties and absent to low major histocompatibility class II antigen expression35,36. These properties also contribute to their hypo-immunogenicity after xeno-transplantation. The present study is indeed xeno-transplantation: however allergic reactions have not seen after human MSC delivery in the piglet CPB model. Since CPB and surgery cause various systemic inflammatory responses, further study is necessary to determine the anti-inflammatory and immunomodulatory effects of MSCs after surgery.
Our previous study has shown that the density of cell populations in the SVZ varies according to the age and region13. The present study used the same age and region for assessment: however limitations to our data include the small sample size. Another limitation is that our studies only assessed the acute effect of MSC treatment in the SVZ. It is also possible that acute reactions of MSC may be altered according to different CPB conditions such as temperature and duration and between DHCA and low-cerebral perfusion. In addition, there are various opportunities of MSC administration during CPB. Repeated cell administration as well as different dosages of MSC should be tested to optimize MSC treatment for children with CHD. Our previous studies have demonstrated that CPB and circulatory arrest-induced brain insults resulted in apoptosis and dysmaturation of oligodendrocyte lineage and microglia expansion20,21,37. In the damaged rodent white matter, MSCs promote endogenous proliferation and differentiation of oligodendrocyte progenitors17,38. Administration of MSCs also regulates microglia activation after hypoxic-ischemic brain injury39,40. Future studies using the piglet model will allow us to determine the effects of MSCs on surgery-induced developmental alterations in various cell populations and assist in establishing the optimal regimen of MSC delivery through CPB during pediatric cardiac surgery.
In conclusion, MSC delivery though CPB has the potential to mitigate effects of CPB on SVZ NSPCs and promote migration of neuroblasts. Further investigation is necessary to determine the long-term effect of MSC treatment during pediatric cardiac surgery on postnatal neurogenesis.
Supplementary Material
Abbreviations and Acronyms
- ANOVA
analysis of variance
- CHD
congenital heart disease
- CPB
cardiopulmonary bypass
- Dcx
doublecortin
- DHCA
deep hypothermic circulatory arrest
- DL
dorsolateral
- GFAP
glial fibrillary acidic protein
- MSC
mesenchymal stromal cell
- NSPC
neural stem/progenitor cell
- SVZ
subventricular zone
- V
ventral
Footnotes
This article will be presented at the Oral Session of the Fifty-fifth Annual Meeting of The Society of Thoracic Surgeons, San Diego, CA, Jan 27–29, 2019
References
- 1.Khairy P, Ionescu-lttu R, Mackie AS, Abrahamowicz M, Pilote L and Marelli AJ. Changing mortality in congenital heart disease. J Am Coll Cardiol 2010;56:1149–57. [DOI] [PubMed] [Google Scholar]
- 2.Bellinger DC, Wypij D, Rivkin MJ, Demaso DR, et al. Adolescents With d-Transposition of the Great Arteries Corrected With the Arterial Switch Procedure: Neuropsychological Assessment and Structural Brain Imaging. Circulation 2011;124:1361–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wernovsky G and Licht DJ. Neurodevelopmental Outcomes in Children with Congenital Heart Disease – What can we impact? Pediatr Crit Care Med 2016; 17(8 Suppl 1): S232–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Macgowan CK, Sled JG, Yoo SJ, et al. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation 2015;131:1313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peyvandi S, Latal B, Miller SP, McQuillen PS. The neonatal brain in critical congenital heart disease: Insights and future directions. Neuroimage 2019;185:776–82. [DOI] [PubMed] [Google Scholar]
- 6.Andropoulos DB, Hunter JV, Nelson DP, et al. Brain immaturity is associated with brain injury before and after neonatal cardiac surgery with high-flow bypass and cerebral oxygenation monitoring. J Thorac Cardiovasc Surg 2010;139:543–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beca J, Gunn JK, Coleman L, et al. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation 2013;127:971–9 [DOI] [PubMed] [Google Scholar]
- 8.Sanai N, Alvarez-Buylla A, Berger MS. Neural stem cells and the origin of gliomas. N Engl J Med 2005;353:811–22. [DOI] [PubMed] [Google Scholar]
- 9.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med 2002;8:963–970. [DOI] [PubMed] [Google Scholar]
- 10.Christie KJ, Turnley AM. Regulation of endogenous neural stem/progenitor cells for neural repairfactors that promote neurogenesis and gliogenesis in the normal and damaged brain. Frontiers in cellular neuroscience 2012;6:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo CT, Mirzadeh Z, Soriano-Navarro M, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell 2006;127:1253–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paredes MF, James D, Gil-Perotin S,et al. Extensive migration of young neurons into the infant human frontal lobe. Science 2016;354:aaf7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morton PD, Korotcova L, Lewis BK et al. Abnormal neurogenesis and cortical growth in congenital heart disease. Sci Transl. Med 2017;9(374):eaah7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernardo ME and Fibbe WE. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013;13:392–402. [DOI] [PubMed] [Google Scholar]
- 15.Eckert MA, Vu Q, Xie K, et al. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab 2013;33:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kan I, Barhum Y, Melamed E, et al. Mesenchymal stem cells stimulate endogenous neurogenesis in the subventricular zone of adult mice. Stem Cell Rev 2011;7:404–12. [DOI] [PubMed] [Google Scholar]
- 17.van Velthoven CT, Kavelaars A and Heijnen CJ. Mesenchymal stem cells as a treatment for neonatal ischemic brain damage. Pediatr Res 2012;71:474–81. [DOI] [PubMed] [Google Scholar]
- 18.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells 2010;28:1099–106. [DOI] [PubMed] [Google Scholar]
- 19.Bhasin A, Srivastava MV, Mohanty S, et al. Stem cell therapy: a clinical trial of stroke. Clinical neurology and neurosurgery 2013;115:1003–8. [DOI] [PubMed] [Google Scholar]
- 20.Ishibashi N, Scafidi J, Murata A, et al. White matter protection in congenital heart surgery. Circulation 2012;125:859–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korotcova L, Kumar S, Agematsu K, et al. Prolonged White Matter Inflammation After Cardiopulmonary Bypass and Circulatory Arrest in a Juvenile Porcine Model. Ann Thorac Surg 2015;100:1030–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaltman JR, Andropoulos DB, Checchia PA, et al. Report of the pediatric heart network and national heart, lung, and blood institute working group on the perioperative management of congenital heart disease. Circulation 2010;121:2766–72. [DOI] [PubMed] [Google Scholar]
- 23.Morton PD, Ishibashi N, Jonas RA. Neurodevelopmental Abnormalities and Congenital Heart Disease: Insights Into Altered Brain Maturation. Circulation Res 2017;120:960–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leonetti C, Back SA, Gallo V, Ishibashi N. Cortical Dysmaturation in Congenital Heart Disease. Trends Neurosci 2019;S0166–2236(18)30316–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nam SC, kim Y, Dryanovski D et al. Dynamic features of postnatal subventricular zone cell motility: a two-photon time-lapse study. J Comp Neurol. 2007;505(2):190–208. [DOI] [PubMed] [Google Scholar]
- 26.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair–current views. Stem Cells 2007;25:2896–2902. [DOI] [PubMed] [Google Scholar]
- 27.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell 2012;10(5):544–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kB signaling in resident macrophages. Blood 2011;118(2):330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Snapyan M, Lemasson M, Brill MS et al. Vasculature guides migrating neuronal precursors in the adult mammalian forebrain via brain-derived neurotrophic factor signaling. J Neurosci 2009;29:4172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bath KG, Lee FS. Neurotrophic factor control of adult SVZ neurogenesis. Dev Neurobiol.2010;70:339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Najar M, Raicevic G, Boufker HI, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton’s Jelly and bone marrow sources. Cell Immunol 2010;264:171–9. [DOI] [PubMed] [Google Scholar]
- 32.Yust-Katz S, Fisher-Shoval Y, Barhum Y, et al. Placental mesenchymal stromal cells induced into neurotrophic factor-producing cells protect neuronal cells from hypoxia and oxidative stress. Cytotherapy 2012;14:45–55. [DOI] [PubMed] [Google Scholar]
- 33.Eckert MA, Vu Q, Xie K, et al. Evidence for High Translational Potential of Mesenchymal Stromal Cell Therapy to Improve Recovery from Ischemic Stroke. Journal of Cerebral Blood Flow & Metabolism 2013;33:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koç ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol 2000; 18:307–316. [DOI] [PubMed] [Google Scholar]
- 35.Griffin MD, Ritter T and Mahon BP. Immunological aspects of allogeneic mesenchymal stem cell therapies. Hum Gene Ther 2010;21:1641–55 [DOI] [PubMed] [Google Scholar]
- 36.Le Blanc K, Pittenger M. Mesenchymal stem cells: progress toward promise. Cytotherapy 2005;7:36–45. [DOI] [PubMed] [Google Scholar]
- 37.Stinnett GR, Lin S, Korotcov AV, et al. Microstructural Alterations and Oligodendrocyte Dysmaturation in White Matter After Cardiopulmonary Bypass in a Juvenile Porcine Model. J Am Heart Assoc 2017;6:e005997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaramillo-Merchan J, Jones J, Ivorra JL, et al. Mesenchymal stromal-cell transplants induce oligodendrocyte progenitor migration and remyelination in a chronic demyelination model. Cell Death Dis 2013;4:e779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ishizaka S, Horie N, Satoh K, Fukuda Y, Nishida N and Nagata I. Intra-arterial cell transplantation provides timing-dependent cell distribution and functional recovery after stroke. Stroke 2013;44:720–6. [DOI] [PubMed] [Google Scholar]
- 40.Giunti D, Parodi B, Usai C, et al. Mesenchymal stem cells shape microglia effector functions through the release of CX3CL1. Stem Cells 2012;30:2044–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.