Abstract
The placenta is a crucial organ for supporting a healthy pregnancy, and defective development or function of the placenta is implicated in a number of complications of pregnancy that affect both maternal and fetal health, including maternal preeclampsia, fetal growth restriction, and spontaneous pre-term birth. In this review, we highlight the role of the placental genome in mediating fetal and maternal health by discussing the impact of a variety of genetic alterations, from large whole-chromosome aneuploidies to single-nucleotide variants, on placental development and function. We also discuss the placental methylome in relation to its potential applications for refining diagnosis, predicting pathology, and identifying genetic variants with potential functional significance. We conclude that understanding the influence of the placental genome on common placental-mediated pathologies is critical to improving perinatal health outcomes.
Keywords: Placenta, genetics, genomics, DNA methylation, epigenetics, pregnancy
Introduction
The placenta is the key mediator of maternal and fetal health during pregnancy. It is a highly adaptive organ that responds to maternal and fetal signals, shows remarkable variability in size and shape, and can tolerate some degree of localized pathology while still able to support fetal growth to term. Nevertheless, abnormal development or function of the placenta underlies many complications of pregnancy including miscarriage, maternal preeclampsia (PE), fetal growth restriction (FGR), preterm birth (PTB), and fetal malformation (Burton and Jauniaux 2018; Morgan 2016). Chromosome imbalance, genetic variation, and epigenetic changes have been implicated and/or associated with these conditions, although our knowledge of normal and abnormal genomic variation in the placenta is still maturing.
The placental genome is normally identical to that of the fetus, as both are developmentally derived from the conceptus. However this can differ due to mosaicism (or more rarely, chimerism) discussed further below. The placenta also interfaces directly with the maternal decidua, which derives from a thickening and modification of the uterine wall during pregnancy and is shed along with the placenta at parturition. Within the decidua, there is an intermingling of placental-derived cells and maternal cells, as a subset of placental trophoblast cells migrate into the maternal tissue both to anchor the placenta into the uterus and to remodel the maternal uterine spiral arteries—opening up blood flow to the placenta (Figure 1). The maternal blood comes into direct contact with the syncytiotrophoblast, a multinucleated syncytium that forms the outer layer of the placental chorionic villi. This syncytium not only provides a protective sheath, but can selectively uptake nutrients and oxygen from maternal blood and transport these into the fetal circulation.
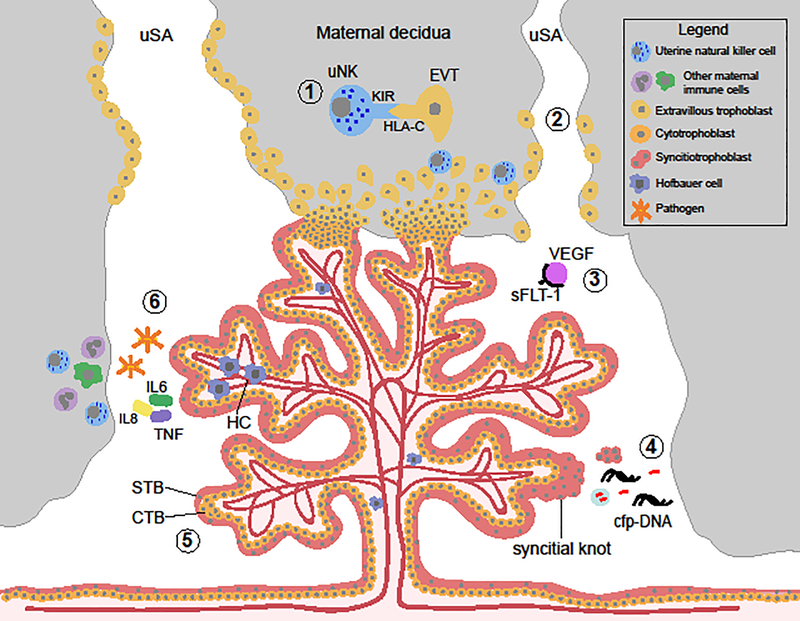
Figure 1.
The placenta’s role in mediating fetal and maternal health. Schematic representation of a placental villous tree, the functional unit of the placenta, and its interaction with the maternal uterus. 1) Interactions between placental extravillous trophoblast cells (EVT) and maternal uterine natural killer cells (uNK) in the maternal decidua promote immune tolerance of the mother to the developing fetus, mediate EVT invasion into the maternal uterus, and aid in resource allocation to the fetus. 2) Deficiencies in remodeling of the uterine spiral arteries by EVTs are associated with health complications of maternal preeclampsia and fetal growth restriction. 3) Placental sFLT1 in maternal blood binds with placental growth factor and vascular endothelial growth factor (VEGF). Altered levels of secretion of sFLT1 by the syncitiotrophoblast (STB) has been implicated in defective endothelial dysfunction and alterations in angiogenesis associated with PE and FGR. 4) The STB is in direct contact with maternal blood in the intervillous space, thus debris shed from this trophoblast cell layer composes the majority of “fetal” material in maternal blood, including cell-free placental DNA used in non-invasive prenatal testing. 5) Defects in differentiation of cytotrophoblast (CTB) cells to form the STB is seen in placentas associated with trisomy 21. 6) Microbial infection results in altered number of placental macrophages, Hofbauer cells (HC), and triggers an innate immune response in the trophoblast cells. uNK, uterine natural killer cell; EVT, extravillous trophoblast; STB, syncitiotrophoblast; CTB, cytotrophoblast; cfp-DNA cell-free placental DNA; HC, Hofbauer cell.
In contras, to the genome, the epigenome undergoes substantia, remodeling throughout development (Hanna et al. 2018), such that the placental and embryonic epigenomes are very distinct. Much of our knowledge of epigenomic reprogramming in human development comes from studies of DNAme, which reaches its lowest level in the blastocyst around the time of implantation. However, the early passive loss of DNAme on the maternal genome is less extensive in human as compared to mouse embryos, which may explain the large number of imprinted (parent-of-origin specific) gene expression and DNAme in the human placenta (Hamada et al. 2016; Hanna et al. 2018). After implantation, de novo DNAme continues as cells differentiate and, while the placenta retains a lower level of DNAme than the rest of the embryo, it also undergoes continued changes in DNAme throughout gestation (Novakovic et al. 2011). Factors such as embryo culture, maternal nutrition, and chemical exposures, may impact placental and fetal health in part via effects on both imprinted and non-imprinted epigenetic marks. However, the evidence for this in humans is still under development and discussed below.
Early events in placentation set the stage for fetal growth and pregnancy health
During the initial stages of implantation, the trophoblast cells produce substances that allow for adhesion and invasion into the uterine wall, alter maternal immune cell phenotype to prevent embryo rejection, and prevent menstruation. Defects in these processes due to chromosomal abnormality in the conceptus are the most common causes of early pregnancy loss, with 70% of early miscarriages having an abnormal karyotype, of which ~80% are whole chromosome aneuploidies (Soler et al. 2017). In a recent study in mouse, it was demonstrated that the vast majority of gene knockouts showing an embryonic lethal effect caused defects in placentation, suggesting that an abnormal placenta is often an unappreciated cause of early lethality (Perez-Garcia et al. 2018).
Trophoblast invasion and maternal spiral artery remodeling is also dependent on maintaining a delicate balance between maternal and placental immune signaling, which can be affected by both maternal and placental/fetal genetic make-up (Moffett et al. 2015). Insufficiency of this remodeling process underlies most cases of severe PE and/or FGR. Furthermore, the placenta also acts to some degree as a barrier that can protect the developing fetus from pathogens and other adverse environmental exposures. Inflammation of the placenta due to maternal anti-fetal rejection or infection can lead to a breakdown of this barrier and increase risk for infection which can lead to inflammation of fetal membranes commonly linked to PTB (Kim et al. 2015). Both PTB and presence of placental inflammatory lesions have significant recurrence risks and this is at least partly genetically influenced, though environmental factors such as smoking, maternal obesity, and maternal infection can also be important.
Placental genetics in maternal and fetal medicine
Large-scale chromosome abnormalities in the placenta have significant impact on fetal and maternal health outcomes
Spontaneously-arising de novo chromosome imbalances have clear and well-established roles in poor pregnancy outcomes. One of the more extreme examples of this is the imbalance of whole haploid complements of chromosomes. Most commonly, this presents as triploidy, the presence of an extra set of chromosomes that could be maternal (digyny) or paternal (diandry) in origin. Triploidy is present in ~8% of miscarriages, but contributes to ~22% of miscarriages occurring after 17 weeks of gestational age (Hardy et al. 2016). Phenotypes differ depending on the parental origin of the extra chromosome complement, highlighting the importance of genomic imprinting. Digynic triploids have small placentas that are generally insufficient to support fetal growth to term, and are associated with severe growth restriction and congenital malformations in the fetus (McFadden and Robinson 2006). Diandric triploid fetuses can be normally-sized or growth restricted, with congenital malformations, and have placentas often presenting as a partial hydatidiform mole (PHM) (McFadden and Pantzar 1996; McFadden and Robinson 2006). This placental phenotype of a PHM overlaps that of a complete hydatidiform mole (CHM), both being characterized by villous trophoblast hyperplasia and hydropic degeneration of the chorionic villi. CHMs are typically due to an androgenetic conceptus (diploid, but chromosomes are entirely paternally-derived), though rarely may be biparental with loss of maternal methylation at imprinted loci (Sanchez-Delgado et al. 2015). Distinguishing these with genetic testing is relevant, as recurrence risk in the latter case is high, since biparental CHMs can be due to rare maternal mutations that appear to affect imprint setting in the oocyte (discussed below). In CHM and PHM, not only is placental development severely abnormal, but embryonic development is abnormal (PHM) or lacking (CHM) and there is an increased risk for PE and trophoblastic tumors, though risk is much lower in cases of partial compared to complete moles (Jauniaux. 1999; Petts et al. 2014).
Chromosome aneuploidy (monosomy or trisomy) is the most common cause of miscarriage, and is detectable in approximately 50–70% of early embryos (Baart et al. 2006). Early miscarriage involving aneuploidy is likely caused by defects in trophoblast functions that are crucial for implantation and establishment of exchange of materials. Although trisomy 13, 18, 21 and sex chromosome aneuploidies can be present in a viable fetus, the role of the placenta in intrauterine survival and phenotypes associated with these aneuploidies is under-appreciated. The vast majority of embryos with trisomy 13 and 18 are spontaneously aborted, but the presence of normal diploid cells in the placental trophoblast cells may allow survival of these fetuses to term (Kalousek et al. 1989). Similar placental mosaicism is not common for viable trisomy 21, suggesting that the additional chromosome 21 has less of a detrimental impact on placental function, and thus embryo survival, as for chromosomes 13 and 18. Despite this, placental development is impacted in the case of trisomy 21, as there is defective formation of the syncytiotrophoblast (Frendo et al. 2000). This affects placental secretion and transfer of nutrients, and is the mechanism underlying the increased levels of human chorionic gonadotropin (hCG) in maternal blood used for prenatal screening for trisomy 21 (Pidoux et al. 2007).
In addition to impacts on the fetus, defective placental development and function caused by aneuploidy can impact maternal health. For example, trisomy 13 is associated with increased incidence of maternal PE (Dotters-Katz et al. 2018; Tuohy and James 1992). Of the many genes on chromosome 13 with increased dosage, FLT1 is of particular interest, as levels of soluble FLT1 (sFLT1) in maternal serum have been associated with PE in multiple independent studies (Maynard et al. 2003; Robinson et al. 2006). FLT1 is expressed mainly in the syncytiotrophoblast and released into maternal circulation where it binds to placental growth factor (PlGF) and vascular endothelial growth factor (VEGF), blocking them from binding to receptors on endothelial cells (Jim and Karumanchi 2017). High levels of sFLT1 lead to endothelial damage in the mother (Maynard et al. 2003), thus directly implicating it in the pathogenesis of PE.
While we tend to focus on the impacts of aneuploidy in the fetus, aneuploidy confined to extraembryonic lineages, i.e. confined placental mosaicism (CPM), can also impact fetal development. Placental mosaicism is identified in approximately 1–2% prenatal chorionic villus samples (CVS), however it is only confirmed upon amniocentesis approximately 10% of the time (Ledbetter et al. 1992; Phillips et al. 1996; Wang et al. 1993). Furthermore, even non-mosaic trisomy detected on CVS is commonly confined to the placenta when involving a non-viable trisomy diagnosed in ongoing pregnancies. Although the fetus may not carry detectable levels of abnormal cells, CPM detected prenatally has been associated with numerous complications, including stillbirth, FGR, PTB, and congenital malformations (Johnson et al. 1990; Kalousek et al. 1991; Toutain et al. 2018; Yong et al. 2003). In particular, CPM may account for a significant proportion of cases of idiopathic FGR, being present in the placentas of approximately 10% of growth restricted newborns but at much lower rates in controls (Robinson et al. 2010; Stipoljev et al. 2001; Wilkins-Haug et al. 1995). Outcomes of prenatally-detected CPM depend on the chromosome involved and levels of placental trisomy, with more severe outcomes and higher levels of trisomy associated from trisomy arising from a meiotic rather than post-zygotic error (Robinson et al. 1997; Wolstenholme. 1996). For example, trisomy 16 is almost always due to maternal meiotic nondisjunction with the diploid cell population arising from a post-zygotic rescue, thus tends to be associated with high levels of trisomy in the placenta, and newborn birth weights are nearly always below the population mean. Placental trisomy 16 is also associated with an increased risk of maternal PE (Yong et al. 2006), and higher levels of trisomy 16 on direct CVS (trophoblast) are associated with increased risk of congenital malformations (Yong et al. 2003). Follow-up of infants with CPM of trisomy 16 show the majority of infants with FGR have catch-up growth, and though there are few reports of global developmental delay, these are only in the subset of cases where mosaicism was also detected in amniotic fluid (Langlois et al. 2006).
Placental mosaicism in the age of NIPT
In addition to the risks for fetal and maternal health, it is increasingly important to consider the possibility of abnormalities being confined to the placenta as we move toward widespread use of noninvasive prenatal testing (NIPT) to diagnose aneuploidy and other genetic abnormalities of the fetus. In NIPT, the “fetal fraction” of circulating cell-free DNA in maternal blood is evaluated, but this “fetal” DNA is largely of placental trophoblast origin (Alberry et al. 2007). It may derive from cells (extravillous trophoblast) and trophoblast-derived microvessicles/exosomes that are actively released into the maternal space, however a substantial portion derives from syncytiotrophoblast debris resulting from apoptosis/necrosis and release of multicellular fragments. There is growing evidence of CPM contributing to false positive and false negative NIPT results; in a recent meta-analysis, 33% of false-positives due to a biological or technical reason were attributed to CPM (Hartwig et al. 2017). Currently, confirmation of any positive screening result from NIPT using invasive diagnostic methods, such as amniocentesis, is recommended by the Society of Obstetricians & Gynecologists of Canada, the Canadian College of Medical Genetics, and the American College of Medical Genetics (Audibert et al. 2017; Gregg et al. 2016). As NIPT potentially moves into testing for chromosome abnormalities beyond common trisomies and sex chromosome aneuploidies, CPM becomes more relevant to consider. Currently, NIPT has very low positive predictive value for rare autosomal trisomies (Pescia et al. 2017), potentially in part due to the higher likelihood of the aneuploid cell population being confined to the placenta. Furthermore, some trisomies, such as trisomy 8, may persist only when trisomy is absent from the trophoblast (Wolstenholme 1996), thus leading to increased false negatives. Should testing for rare autosomal trisomies using NIPT move forward, CPM may contribute to significantly more false-positives in these cases. Recently, methods to predict placental mosaicism from NIPT results have been developed (Brison et al. 2018), which have the potential to both improve predictive value of NIPT and better our understanding of the prevalence and impact of placental mosaicism in ongoing pregnancies.
Placental copy number variants: another avenue to investigate placental genetics in fetal and maternal health?
Though the study of placental aneuploidies has revealed significant associations with pregnancy complications, there have been few studies investigating the role of submicroscopic genetic imbalances, copy number variants (CNVs), in the placenta in association with perinatal health outcomes. A number of rare pathogenic CNVs have been identified in fetal samples from miscarriage and stillbirths (Ernst et al. 2015; Rajcan-Separovic et al. 2010), however, to date, results concerning the role of CNVs in the placenta are conflicting. It has been suggested that there is an increased load of CNVs in the placenta, and that this load is highest in healthy term pregnancies compared to numerous pregnancy complications, including PE, large- and small-for-gestational age babies, gestational diabetes, and recurrent pregnancy loss (Kasak et al. 2015; Kasak et al. 2017). However, another study found the opposite, that more CNVs were present in placentas associated with FGR and PE compared to healthy controls, and that load correlated with the severity of the pathology (Biron-Shental et al. 2016). Such discrepancies may be due to small sample sizes, different analysis methods, or factors such as DNA quality and cell composition that vary depending on source or gestational age, and can influence detection of CNVs. Additionally, it is yet unknown if CPM of CNVs is a common occurrence, and whether it may contribute to pregnancy complications. At least one case of a submicroscopic CNV of clinical relevance detected by CVS and confined to placental tissue in a healthy live born male has been reported (Karampetsou et al. 2014). While exciting, the investigation of placental CNVs is still in its infancy, and will require studies with larger sample sizes and careful attention to data quality and rigorous analysis to establish the associations with pregnancy complications in both the fetus and the mother.
Rare genetic variants affecting placental function can contribute to pregnancy complications
Common pregnancy complications such as PE, FGR, and PTB are heterogeneous conditions, and while it seems likely that rare genetic mutations may lead to these disorders, their total contribution is yet undetermined. From genetic linkage studies of familial PE, rare maternal mutations in genes relevant to placental function have been reported, including CORIN, which facilitates proper trophoblast invasion and spiral artery remodeling (Cui et al. 2012), and STOX1, hypothesized to be important in recruitment of uterine natural killer cells (uNK) and monocytes by placental EVTs for successful interaction at the maternal-placental interface (Dunk et al. 2016). Though promising, such findings have failed to replicate in some cohorts (Iglesias-Platas et al. 2007; Kivinen et al. 2007). Rare variants in genes known to be involved in growth have been associated with FGR. For example, a rare report of a paternally-inherited IGF2 nonsense mutation in a family associated with severe fetal and postnatal growth restriction with a Silver-Russell-like phenotype was documented (Begemann et al. 2015). While loss of function of IGF2 has known implications on fetal growth, it may also impact function in placental cells, as IGF2 promotes cytotrophoblast proliferation in vitro (Forbes et al. 2008) and trophoblast-specific loss of Igf2 in mice generates placental and fetal growth restriction (Fowden et al. 2002). Maternal mutations in NLRP7 and KHDC3L have been reported to cause recurrent biparental CHM due to abnormal setting or maintenance of maternal imprints in the oocyte, leading to widespread impacts on imprinting in the placenta (Djuric et al. 2006; Parry et al. 2011; Sanchez-Delgado et al. 2015). Mutations in these genes are not commonly associated with androgenetic CHM, triploidy, recurrent pregnancy loss or infertility (Aghajanova et al. 2015; Mahadevan et al. 2013; Manokhina et al. 2013), thus alternate genetic variants may be involved (Nguyen et al. 2018).
Common genetic variants associated with pregnancy complications converge on a few major pathways of placental function
While the importance of maternal genetic effects in pregnancy complications is well-established, the significance of fetal/placental genetic factors cannot be ignored. In fact, fetal (placental) genetic effects explain 20% of the variation in PE (Cnattingius et al. 2004). Despite the compelling evidence from epidemiological studies supporting fetal contribution to the heritability of PE, genome-wide association studies (GWAS) investigating fetal (placental) single nucleotide polymorphisms (SNPs) in relation to PE are limited. A SNP near the FLT1 locus (rs4769613) is the only PE-associated risk variant that convincingly replicated in an independent European cohort in a GWAS (Gray et al. 2018; McGinnis et al. 2017). This result is convincing both due to its reproducibility and its biological relevance, as sFLT1 levels have been extensively associated with PE. Interestingly, these SNPs were also found to be associated with red blood cell count (McGinnis et al. 2017) and may influence erythropoiesis in the placenta.
A few additional studies have investigated the association between SNPs in candidate genes and PE, though rarely is the placental genotype investigated. Placental genotype at a common SNP in MTHFR (rs1801133) has been associated with PE in one study (Cheaui et al. 2015), and though this failed to replicate in a separate study, there was a trend of increased “TT” genotypes in the combined PE and/or FGR cohort (Del Gobbo et al. 2018). Differences between studies may depend on geographical location, as MTHFR variants may play a less significant role in populations such as North America where there is folate supplementation. Other candidate studies have identified an association between placental variants in immune system genes such as HLA-G and IL10 and an increased susceptibility to PE (Makris et al. 2006; Moreau et al. 2008), but these findings have not yet been replicated in larger cohorts. While rarely are the same variants identified to be associated with PE, alterations in gene pathways involved in angiogenesis, hypoxia and immune responses are often demonstrated, suggesting dysregulation of common biological processes in PE.
Similarly, although few reproducible SNP associations with spontaneous PTB have been reported, common biological pathways underlying spontaneous PTB have been implicated based on genetic association studies. The infection-inflammation response network is the most consistent pathway associated with spontaneous PTB (Uzun et al. 2013). Histologic evidence of inflammation in the placenta, fetal membranes, or umbilical cord is commonly observed in association with spontaneous PTB (Andrews et al. 2006; Salafia et al. 1991). Placental inflammatory lesions are also frequently observed, and a high recurrence risk for these lesions has been well-demonstrated (Ghidini and Salafia 2005), supporting the role of the placenta in genetic susceptibility for spontaneous PTB. Additionally, genetic variation in inflammatory genes in the placenta such as TLR and TNF is associated with preterm rupture of membranes (Rey et al. 2008), which leads to PTB.
A few studies have examined the interaction between maternal and fetal/placental genotypes, identifying compounded risk when the mother and the infant carry specific combinations of genetic variants. For example, maternal IL1B “GG” genotype (rs16944) and fetal IL6 “GC” genotype (rs1800795) in a South American population was associated with increased risk of PTB (Pereyra et al. 2016). No significant association was observed when the variants were analyzed independently, likely because both alleles were predisposing in individuals with European ancestry. Further, it has been shown that the maternal KIR activating “AA” genotype in combination with a fetal HLA-C “C2” allele is associated with an increased risk of placental insufficiency leading to PE and/or low birth weight (Hiby et al. 2014). The risk is greater if the placental C2 is expressed from the paternal chromosome. The KIR receptors on uNK cells bind directly to the HLA-C molecules expressed on trophoblast cells and these interactions likely play an important role in regulating the degree of invasion and allocating resources between the fetus and the mother.
Placental epigenetics in maternal and fetal medicine
While the study of genetic variation in the placenta is important to understanding how some pregnancies may be predisposed to adverse reproductive outcome, there is much information to be leveraged from the placental epigenome as well. Epigenetic modifications such as DNA methylation (DNAme) and histone modifications (e.g. methylation, phosphorylation, acetylation, and ubiquitinylation) are stable marks that are involved in the regulation of gene expression that is key to cell differentiation. Histone modifications and DNAme tend to occur in tandem, working to recruit the other modification to stably activate or repress genes (Cedar and Bergman 2009; Kondo. 2009). Several studies in model organisms and stem cells in vitro, have established the crucial role for histone modifications in early cell fate of the blastocyst extraembryonic lineage and in the development and differentiation of placental cell types (Rugg-Gunn et al. 2010; Saha et al. 2013; Torres-Padilla et al. 2007). For example, the trophectoderm initially has lower levels of H3K27me3, a repressive mark at gene promoters and enhancers, as compared to the inner cells mass (Saha et al. 2013); although this changes dynamically in development and does not correlate with typical patterns of chromatin condensation in trophoblast populations, suggesting unusual properties of histone methylation in these cells (Fogarty et al. 2015). Histone modifications and the enzymes catalyzing them are necessary for the regulation of expression of key placental proteins, including syncytin (Chuang et al. 2006), maspin (Dokras et al. 2006), pregnancy-specific glycoproteins (Camolotto et al. 2013), and the human growth hormone protein family (Ganguly et al. 2015). Altered expression of these genes or in resulting histone modifications have been associated with pregnancy complications including PE, FGR, and gestational diabetes (Alahari et al. 2018; Paauw et al. 2018; Xie et al. 2019).
Due to the ease of its assessment, DNAme has been more extensively studied in human populations and has potential applications to maternal and fetal medicine. Characterization of DNAme in the placenta can improve our current understanding of disease pathogenesis and help identify biomarkers for various placental-mediated pregnancy outcomes. Although a subset of DNAme changes can reflect changes to gene expression in specific cell types, the information obtained from these molecular processes is not identical. For example, DNAme may reflect more stable long-term changes to gene regulation that might be present from earlier in development and therefore represent very early changes predictive of the disease. It might also reveal pathology-associated changes to cell ratios, which can be informative in sub-classifying placentas by phenotype. Additionally, genetic alterations may be reflected in DNAme and therefore help to identify functional genetic variation that may explain disease susceptibility (Figure 2).
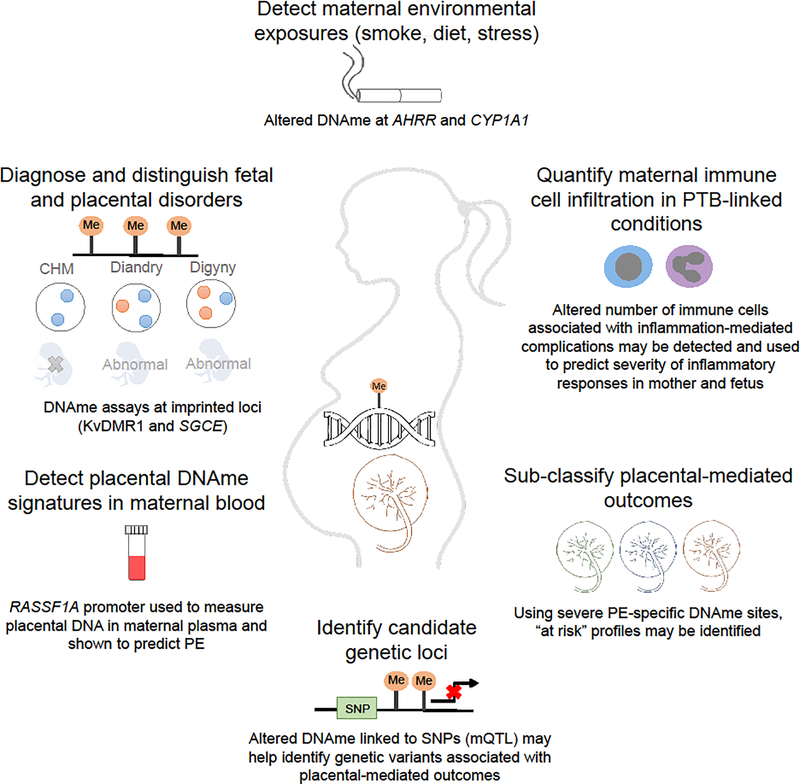
Figure 2.
Examples of applications of placental DNAme in assessing and improving maternal and fetal health. These include: detecting altered DNAme linked to in utero exposures; detecting placental-specific DNAme signatures in maternal blood; quantifying maternal immune cells infiltrating the placenta; subclassifying placental phenotypes into more homogenous groups; diagnosing chromosomal imbalance or imprinting errors; and using methylation changes linked to disease to identify nearby predisposing SNPs (mQTLs).
Placental DNAme is unique
The DNAme profile of the placenta is distinct compared to somatic tissues, offering an avenue for development of biomarkers for assessing placental health, as this property can be used to distinguish placental from maternal DNA. Both the trophoblast and the mesenchyme core of the placental chorionic villi have a unique DNAme landscape compared with maternal decidua, fetal membranes (chorion and amnion), and embryonic tissues (brain, kidney, muscle, spinal cord) (Robinson and Price 2015). While on average the placenta is hypomethylated compared to somatic tissues (Ehrlich et al. 1982; Novakovic et al. 2010; Schroeder et al. 2013), this largely reflects that approximately 40% of the placental genome comprises large blocks (>100kb) of low to intermediate methylation, termed partially methylated domains (PMDs) (Schroeder et al. 2013). In contrast to the bimodal distribution of DNAme in somatic tissues, where most CpG sites are either highly (>90%) or lowly (<10%) methylated, the presence of PMDs and imprinted genes in the placenta results in a unique trimodal distribution of DNAme measures. Hypomethylation is also reported for several families of retrotransposable elements (REs) in the placenta compared to other somatic tissues, though the degree of this varies depending on the RE family and on the evolutionary age of the sequence (Chatterjee et al. 2016; Price et al. 2012; Reiss et al. 2007). Furthermore, the DNAme level of LINE-1 elements is highly correlated with location within versus outside of a PMD, rather than being an independent property of these repeat elements themselves (Schroeder et al. 2013). Hypomethylation of RE-derived promoters has been shown to contribute to placental-specific expression of genes or certain gene isoforms important for placental development and function, including: INSL4, involved in placental apoptosis (Macaulay et al. 2011); ILR2B, which contributes to immune communication at the maternal-fetal interface via proliferation and differentiation of uNK cells (Cohen et al. 2011); and expression of endogenous retroviral envelope proteins syntytin-1 and −2, which are involved in the fusion of cytotrophoblast cells to form the syncytiotrophoblast layer (Macaulay et al. 2011; Vargas et al. 2009).
Applications of placental DNAme in maternal & fetal health
Diagnosis and sub-classification of placental-mediated pregnancy complications
In genetic disorders linked to epigenetic dysregulation, specific patterns of DNAme in blood are diagnostic of the underlying epigenetic errors (Butcher et al. 2017). In the placenta, rapid DNAme-based assays at imprinted loci can be used for the diagnosis of parental genetic imbalances, such as those in CHM or triploidy, or to screen for abnormal imprint setting as observed in biparental CHMs (Bourque et al. 2011). Accurate diagnosis of the underlying mechanisms for placentas with mole-like features is important for assessing risk for choriocarcinoma, as altered DNAme patterns are associated with invasive choriocarcinomal cell lines (Novakovic et al. 2008). Further, DNAme at imprinted loci is sensitive enough to provide quantitative estimates of the level of normal and abnormal cells in cases of mosaicism or chimerism (Bourque et al. 2011). Loss of methylation at several imprinted genes has also been observed in a subgroup of placentas associated with infertility and/or assisted reproduction (Choufani et al. 2018).
Widespread DNAme changes are reported in placentas from pregnancies with severe PE, with a subset of these PE-associated DNAme sites being reproduced in multiple studies (Wilson et al. 2018; Yeung et al. 2016). Reproducible DNAme alterations can potentially be used to diagnose cases with characteristic pathology of placental insufficiency, i.e. advanced villous maturation and distal villous hypoplasia. Genes associated with these DNAme changes include those implicated in PE pathogenesis, such as FLT1, CXCL9, an inflammatory chemokine that inhibits angiogenesis and may contribute to inadequate vascular remodeling; JUNB, a transcription factor with altered placental expression in response to hypoxia in trophoblasts (Yuen et al. 2013); and INHBA, a subunit of Activin A, primarily produced by the placenta during pregnancy, that shows increased serum levels in PE-affected women. Clustering placentas from diverse outcomes using independently-validated PE-associated DNAme sites identified an additional cluster of placentas that largely included PTBs with a putative “inflammation” phenotype (Wilson et al. 2018), a key risk factor for many pregnancy complications. A similar clustering pattern with at least 5 distinct sub clusters was observed using gene expression profiling of healthy and affected placentas of mixed etiologies (Leavey et al. 2018). By integrating pathology information, histological lesions of the placenta have been linked to the gene expression-defined subclasses, providing improved characterization of PE subtypes (Benton et al. 2018). For example, one cluster was most highly associated with maternal vascular malperfusion, while another was strongly correlated with histological chorioamnionitis. Such analyses can be used to identify more homogeneous patient populations to improve biomarker development and understanding of the underlying factors contributing to pregnancy complications.
Quantify altered cell ratios in placental-mediated pregnancy complications
As DNAme is cell-specific, pathology-associated DNAme changes in the placenta may be in part indicative of changes to cell composition. For example, PE-associated DNAme changes may reflect an increase in syncytiotrophoblast mass, as well as its dysfunction. While placental cell-specific DNAme signatures are still being developed, testing for cell-specific changes may have applications in diagnosis. This may provide an approach to quantify low levels of maternal immune cell infiltration in various inflammation-mediated placental pathologies. An increase in infiltration of maternal neutrophils is common in acute chorioamnionitis, a commonly reported inflammatory lesion in placentas from spontaneous PTBs. Using neutrophil-specific DNAme sites, acute chorioamnionitis affected placentas could be largely seperated from non-affected placentas, suggesting some DNAme changes associated with the placental pathology may be attributed to an increase in neutrophils as a response to inflammation (Konwar et al. 2018). Similarly, an increased number of nucleated red blood cells in umbilical cord blood of PE-affected pregnancies is observed (Aali et al. 2007; Hebbar et al. 2014), suggesting enhanced erythropoiesis as a fetal response to a hypoxic environment. Cell-specific DNAme levels may potentially be useful to predict the severity of immune-specific responses both in the mother and fetus. While the utility of measuring this in the placenta is not yet explored, microchimeric maternal cells in cord blood have been shown to predict neonatal complications (Harrington et al. 2017).
Biomarker in maternal blood for placental-mediated pregnancy complications
In addition to its role in genetic diagnosis, placental DNA that is released into maternal blood during pregnancy may also be used to assess placental health based on DNAme profiles. Direct quantification of methylated placental DNA in maternal circulation is most feasible at sites where DNAme is exclusive to the placenta and absent in maternal blood, or vice versa. One study identified 958 CpGs that are consistently hypomethylated (β<0.25; β: methylation value ranging from 0–1) or hypermethylated (β>0.75) between maternal blood and placenta, thus providing a list of candidate CpGs with diagnostic potential (Hatt et al. 2015). For instance, the RASSF1A gene promoter is only methylated in placenta and has been used to directly measure placental DNA in maternal plasma, thus may aid in prediction of complications such as PE which is associated with increased trophoblast apoptosis and release of placental DNA into maternal circulation (Zhao et al. 2010). Alternatively, changes in placental DNAme at term may reflect altered protein levels earlier in gestation, providing another route of investigation for identifying biomarkers for placental insufficiency conditions (Wilson et al. 2015).
Sensor of environmental exposures
There has been widespread interest in using DNAme as a sensor of maternal environmental exposures during pregnancy. The most reproduced finding is an association between maternal smoking during pregnancy and altered placental DNAme at AHRR and CYP1A1 (Suter et al. 2010; van Otterdijk et al. 2017), genes reported to mediate toxic effects of nicotine metabolism. Interestingly, pesticide exposure has also been found to be associated with fewer PMDs with increased DNAme (Schmidt et al. 2016), with a greater impact than a number of other environmental exposures measured. In addition, altered placental DNAme at genes such as HTR2A and HSD11B2, which are involved in serotonin and glucocorticoid signaling in the placenta, in response to exposure to maternal stress has been associated with birth weight and neurobehavioural outcomes in infants (Green et al. 2015; Marsit et al. 2012). In another study, sites of altered placenta DNAme were linked to future development of autism; interestingly these overlapped genetic risk loci for autism, and their methylation was affected by prenatal vitamins (Zhu et al. 2018). Further, maternal health conditions such as gestational diabetes mellitus is also associated with altered DNAme in the placenta (Binder et al. 2015; Ruchat et al. 2013). As expected, these DNAme changes are enriched in metabolic and immune response pathways, supporting that a pro-inflammatory maternal environment likely alters the inflammatory profile in the placenta. It is important to note, however, that differences in placental DNAme related to inflammation-mediated diseases such as gestational diabetes may also reflect pathology-linked changes in immune cell populations in the placenta and the changes noted in such studies tend to be small.
Help identify genetic loci associated with poor maternal and fetal health outcomes
Emerging evidence shows that DNAme patterns are strongly influenced by underlying genetic variation. Based on studies in other tissues, approximately 20–80% of DNAme variance within a tissue can be attributed to genetic variants, referred to as methylation-quantitative trait loci (mQTL) (Do et al. 2016; Gertz et al. 2011). For example, a placental mQTL located in the enhancer region of IL6 is associated with both changes in IL6 expression and with acute chorioamnionitis in individuals of Asian ancestry (Konwar et al. 2019). In a comprehensive study of 300 human placentas comparing Illumina DNAme array to SNP array findings, 4,342 placental mQTLs were identified (Delahaye et al. 2018). Pathway analysis of placental mQTLs revealed an enrichment of inflammation-associated pathways primarily involving HLA- related genes that are known to regulate immunological responses during pregnancy. Similar to other tissues, placental mQTLs tended to be located in gene enhancer regions and were associated with transcription factor binding sites. This suggests a plausible impact of mQTLs on gene regulation, which in turn may affect susceptibility to various pregnancy complications. Interestingly, mQTLs identified in blood are frequently enriched among risk loci identified in association with complex diseases (McRae et al. 2018), aiding in prioritization of candidates implicated in disease pathogenesis. It is reasonable to speculate that placental-specific mQTLs may be used to refine GWAS signals for various placental- mediated complex outcomes such as PE, FGR, and spontaneous PTB.
Conclusion & Future Directions
Pregnancy health is currently routinely assessed by a combination of ultrasound, protein markers in serum, and biophysical measurements. As genetic diagnosis using placental cell free DNA circulating in maternal plasma has advanced, expanded opportunities for DNA-based assessment placental health can be considered. Although the role of chromosomal aneuploidy has been known for over half a century and has been the focus of much prenatal testing, the role of rare and common genetic variants, including copy number variants, in placental and fetal development is only beginning to be elucidated. Whether these types of errors may also commonly be mosaic and confined to the placenta, remains to be determined. In addition to diagnosing genetic errors, one can imagine identifying epigenetic biomarkers of risk. However, the application is limited largely by major gaps in our knowledge of placental health and the connections between epigenetic changes in the placenta at delivery and factors in maternal circulation. Understanding the underlying biology of the placenta and integrating pathological features with genetic and epigenetic changes is important in the development of new tools to predict maternal and fetal complications of pregnancy. An integrated assessment of the placenta at delivery also has potential for predicting future cognitive, immune, and metabolic development of the newborn.
A unique challenge to studies of the placenta is that we are not only trying to predict heterogeneous conditions, such as FGR and PTB, that clearly have both genetic and environmental contributions, but the interaction between maternal and placental/fetal genome is also critical. While obtaining large sample sizes is one approach to improve genetic association studies, there may be more power in focusing on improved phenotyping using pathology and molecular profiling, in combination with models that integrate genetic variation in mother and placenta along with common environmental exposures.
Acknowledgements
This work was supported by grants from the Canadian Institutes of Health Research (CIHR) [WPR; FRN 49520] and National Institutes of Health (WPR; RFN 5R01HD089713-04). WPR receives salary support through an award from the BC Children’s Hospital Research Institute. GFDG receives support from a CIHR Doctoral Fellowship.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of interest:
The authors declare that they have no conflict of interest.
Bibliography
- Aali BS, Malekpour R, Sedig F, Safa A (2007) Comparison of maternal and cord blood nucleated red blood cell count between pre-eclamptic and healthy women. The journal of obstetrics and gynaecology research 33:274–278. 10.1111/j.1447-0756.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Aghajanova L, Mahadevan S, Altmäe S, Stavreus-Evers A, Regan L, Sebire N, Dixon P, Fisher RA, Van den Veyver IB (2015) No evidence for mutations in NLRP7, NLRP2 or KHDC3L in women with unexplained recurrent pregnancy loss or infertility. Human reproduction (Oxford, England) 30:232–238. 10.1093/humrep/deu296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alahari S, Post M, Rolfo A, Weksberg R, Caniggia I (2018) Compromised JMJD6 Histone Demethylase Activity Affects VHL Gene Repression in Preeclampsia. J Clin Endocrinol Metab 103:1545–1557. 10.1210/jc.2017-02197. [DOI] [PubMed] [Google Scholar]
- Alberry M, Maddocks D, Jones M, Abdel Hadi M, Abdel-Fattah S, Avent N, Soothill PW (2007) Free fetal DNA in maternal plasma in anembryonic pregnancies: confirmation that the origin is the trophoblast. Prenatal diagnosis 27:415–418. 10.1002/pd.1700. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Goldenberg RL, Faye-Petersen O, Cliver S, Goepfert AR, Hauth JC (2006) The Alabama Preterm Birth study: polymorphonuclear and mononuclear cell placental infiltrations, other markers of inflammation, and outcomes in 23- to 32-week preterm newborn infants. American Journal of Obstetrics and Gynecology 195:803–808. 10.1016/j.ajog.2006.06.083. [DOI] [PubMed] [Google Scholar]
- Audibert F, De Bie I, Johnson J, Okun N, Wil son RD, Armour C, Chitayat D, Kim R (2017) No. 348-Joint SOGC-CCMG Guideline: Update on Prenatal Screening for Fetal Aneuploidy, Fetal Anomalies, and Adverse Pregnancy Outcomes. Journal of Obstetrics and Gynaecology Canada 39:805–817. 10.1016/j.jogc.2017.01.032. [DOI] [PubMed] [Google Scholar]
- Baart EB, Martini E, van den Berg I, Macklon NS, Galjaard RH, Fauser BCJM, Van Opstal D (2006) Preimplantation genetic screening reveals a high incidence of aneuploidy and mosaicism in embryos from young women undergoing IVF. Human reproduction (Oxford, England) 21:223–233. 10.1093/humrep/dei291. [DOI] [PubMed] [Google Scholar]
- Begemann M, Zirn B, Santen G, Wirthgen E, Soellner L, Büttel H, Schweizer R, van Workum W, Binder G, Eggermann T (2015) Paternally Inherited IGF2 Mutation and Growth Restriction. The New England journal of medicine 373:349–356. 10.1056/NEJMoa1415227. [DOI] [PubMed] [Google Scholar]
- Benton SJ, Leavey K, Grynspan D, Cox BJ, Bainbridge SA (2018) The clinical heterogeneity of preeclampsia is related to both placental gene expression and placental histopathology. Am J Obstet Gynecol 219:604.e–604.e25. [DOI] [PubMed] [Google Scholar]
- Binder AM, LaRocca J, Lesseur C, Marsit CJ, Michels KB (2015) Epigenome-wide and transcriptome-wide analyses reveal gestational diabetes is associated with alterations in the human leukocyte antigen complex. Clinical epigenetics 7:79 10.1186/s13148-015-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron-Shental T, Sharony R, Shtorch-Asor A, Keiser M, Sadeh-Mestechkin D, Laish I, Amiel A (2016) Genomic Alterations Are Enhanced in Placentas from Pregnancies with Fetal Growth Restriction and Preeclampsia: Preliminary Results. Molecular Syndromology 6:276–280. 10.1159/000444064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque D, Peñaherrera M, Yuen R, Van Allen M, McFadden D, Robinson W (2011) The utility of quantitative methylation assays at imprinted genes for the diagnosis of fetal and placental disorders. Clinical Genetics 79:169–175. 10.1111/j.1399-0004.2010.01443.x. [DOI] [PubMed] [Google Scholar]
- Brison N, Neofytou M, Dehaspe L, Bayindir B, Van Den Bogaert K, Dardour L, Peeters H, Van Esch H, Van Buggenhout G, Vogels A, Ravel T, Legius E, Devriendt K, Vermeesch JR (2018) Predicting fetoplacental chromosomal mosaicism during non-invasive prenatal testing. Prenatal Diagnosis 38:258–266. 10.1002/pd.5223. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E (2018) Pathophysiology of placental-derived fetal growth restriction. American Journal of Obstetrics and Gynecology 218:S74–S761. 10.1016/j.ajog.2017.11.577. [DOI] [PubMed] [Google Scholar]
- Butcher DT, Cytrynbaum C, Turinsky AL, Siu MT, Inbar-Feigenberg M, Mendoza-Londono R, Chitayat D, Walker S, Machado J, Caluseriu O, Dupuis L, Grafodatskaya D, Reardon W, Gilbert-Dussardier B, Verloes A, Bilan F, Milunsky JM, Basran R, Papsin B, Stockley TL, Scherer SW, Choufani S, Brudno M, Weksberg R (2017) CHARGE and Kabuki Syndromes: Gene-Specific DNA Methylation Signatures Identify Epigenetic Mechanisms Linking These Clinically Overlapping Conditions. Am J Hum Genet 100:773–788. 10.1016/j.ajhg.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camolotto SA, Racca AC, Ridano ME, Genti-Raimondi S, Panzetta-Dutari GM (2013) PSG gene expression is up-regulated by lysine acetylation involving histone and nonhistone proteins. PLoS One 8:e55992 10.1371/journal.pone.0055992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H, Bergman Y (2009) Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet 10:295–304. 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Macaulay EC, Rodger EJ, Stockwell PA, Parry MF, Roberts HE, Slatter TL, Hung NA, Devenish CJ, Morison IM (2016) Placental Hypomethylation Is More Pronounced in Genomic Loci Devoid of Retroelements. G3 (Bethesda) 6:1911–1921. 10.1534/g3.116.030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheaui P, Anade ME, Salazar-Pousada D, Escobar GS, Hidalgo L, Ramirez C, Spaanderman MEA, Kramer BW, Gavilanes AWD (2015) Polymorphisms of the methylenetetrahydrofolate reductase gene (C677T and A1298C) in the placenta of pregnancies complicated with preeclampsia. Gynecological Endocrinology 31:569–572. 10.3109/09513590.2015.1031104. [DOI] [PubMed] [Google Scholar]
- Choufani S, Turinsky AL, Melamed N, Greenblatt E, Brudno M, Bérard A, Fraser WD, Weksberg R, Trasler J, Monnier P, Study Group, For The D Cohort (2018) Impact of Assisted Reproduction, Infertility, Sex, and Paternal Factors on the Placental DNA Methylome. Hum Mol Genet. 10.1093/hmg/ddy321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HC, Chang CW, Chang GD, Yao TP, Chen H (2006) Histone deacetylase 3 binds to and regulates the GCMa transcription factor. Nucleic Acids Res 34:1459–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnattingius S, Reilly M, Pawitan Y, Lichtenstein P (2004) Maternal and fetal genetic factors account for most of familial aggregation of preeclampsia: A population-based Swedish cohort study. American Journal of Medical Genetics Part A 130A:365–371. 10.1002/ajmg.a.30257. [DOI] [PubMed] [Google Scholar]
- Cohen CJ, Rebollo R, Babovic S, Dai EL, Robinson WP, Mager DL (2011) Placenta-specific expression of the interleukin-2 (IL-2) receptor beta subunit from an endogenous retroviral promoter. J Biol Chem 286:35543–35552. https://doi.org/jbc.M111.227637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Wang W, Dong N, Lou J, Srinivasan DK, Cheng W, Huang X, Liu M, Fang C, Peng J, Chen S, Wu S, Liu Z, Dong L, Zhou Y, Wu Q (2012) Role of corin in trophoblast invasion and uterine spiral artery remodelling in pregnancy. Nature 484:246–250. 10.1038/nature10897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Gobbo GF, Price EM, Hanna CW, Robinson WP (2018) No evidence for association of MTHFR 677C>T and 1298A>C variants with placental DNA methylation. Clin Epigen 10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahaye F, Do C, Kong Y, Ashkar R, Salas M, Tycko B, Wapner R, Hughes F (2018) Genetic variants influence on the placenta regulatory landscape. PLoS genetics 14:e1007785 10.1371/journal.pgen.1007785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric U, Bagga R, Kircheisen R, Rouleau GA, Kuick R, Ao A, Ratti B, Murdoch S, Slim R, Mazhar B, Seoud M, Khan R, Hanash S (2006) Mutations in NALP7 cause recurrent hydatidiform moles and reproductive wastage in humans. Nature Genetics 38:300–302. 10.1038/ng1740. [DOI] [PubMed] [Google Scholar]
- Do C, Lang C, Lin J, Darbary H, Krupska I, Gaba A, Petukhova L, Vonsattel J, Gallagher M, Goland R, Clynes R, Dwork A, Kral J, Monk C, Christiano A, Tycko B (2016) Mechanisms and Disease Associations of Haplotype-Dependent Allele-Specific DNA Methylation. American J Hum Genet 98:934–955. 10.1016/j.ajhg.2016.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokras A, Coffin J, Field L, Frakes A, Lee H, Madan A, Nelson T, Ryu GY, Yoon JG, Madan A (2006) Epigenetic regulation of maspin expression in the human placenta. Mol Hum Reprod 12:611–617. [DOI] [PubMed] [Google Scholar]
- Dotters-Katz SK, Humphrey WM, Senz KL, Lee VR, Shaffer BL, Kuller JA, Caughey AB (2018) Trisomy 13 and the risk of gestational hypertensive disorders: a population-based study. J Matern Fetal Neonatal Med 31:1951–1955. 10.1080/14767058.2017.1332037. [DOI] [PubMed] [Google Scholar]
- Dunk C, Van Dijk M, Choudhury R, Harris L, Lee Jones R, Lye S (2016) The preeclampsia susceptibility gene STOX1 mutation results in altered EVT chemokine profiles and defective maternal leukocyte recruitment. Placenta 45:116–117. 10.1016/j.placenta.2016.06.193. [DOI] [Google Scholar]
- Ehrlich M, Gama-Sosa MA, Huang LH, Midgett RM, Kuo KC, McCune RA, Gehrke C (1982) Amount and distribution of 5-methylcytosine in human DNA from different types of tissues of cells. Nucleic Acids Res 10:2709–2721. 10.1093/nar/10.8.2709 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst LM, Rand CM, Bao R, Andrade J, Linn RL, Minturn L, Zhang C, Kang W, Weese-Mayer DE (2015) Stillbirth: Genome-wide copy number variation profiling in archived placental umbilical cord samples with pathologic and clinical correlation. Placenta 36:783–789. 10.1016/j.placenta.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Fogarty NM, Burton GJ, Ferguson-Smith AC (2015) Different epigenetic states define syncytiotrophoblast and cytotrophoblast nuclei in the trophoblast of the human placenta. Placenta 36:796–802. 10.1016/j.placenta.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Forbes K, Westwood M, Baker PN, Aplin JD (2008) Insulin-like growth factor I and II regulate the life cycle of trophoblast in the developing human placenta. American Journal of Physiology - Cell Physiology 294:1313–1322. 10.1152/ajpcell.00035.2008. [DOI] [PubMed] [Google Scholar]
- Fowden A, Hughes J, Ferguson-Smith A, Fundele R, Sibley C, Stewart F, Constancia M, Hemberger M, Kelsey G, Reik W, Dean W (2002) Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417:945–948. 10.1038/nature00819. [DOI] [PubMed] [Google Scholar]
- Frendo JL, Vidaud M, Guibourdenche J, Luton D, Muller F, Bellet D, Giovagrandi Y, Tarrade A, Porquet D, Blot P, Evain-Brion D (2000) Defect of villous cytotrophoblast differentiation into syncytiotrophoblast in Down’s syndrome. The Journal of clinical endocrinology and metabolism 85:3700–3707. 10.1210/jc.85.10.3700. [DOI] [PubMed] [Google Scholar]
- Ganguly E, Bock ME, Cattini PA (2015) Expression of Placental Members of the Human Growth Hormone Gene Family Is Increased in Response to Sequential Inhibition of DNA Methylation and Histone Deacetylation. Biores Open Access 4:446–456. 10.1089/biores.2015.0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertz J, Varley K, Reddy T, Bowling K, Pauli F, Parker S, Kucera K, Willard H, Myers R (2011) Analysis of DNA Methylation in a three-generation family reveals widespread genetic influence on epigenetic regulation. PLoS Genetics 7 10.1371/journal.pgen.1002228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghidini A, Salafia CM (2005) Histologic placental lesions in women with recurrent preterm delivery. Acta Obstetricia et Gynecologica Scandinavica 84:547–550. 10.1111/j.00016349.2005.00694.x. [DOI] [PubMed] [Google Scholar]
- Gray KJ, Saxena R, Karumanchi SA (2018) Genetic predisposition to preeclampsia is conferred by fetal DNA variants near FLT1, a gene involved in the regulation of angiogenesis. American Journal of Obstetrics and Gynecology 218:211–218. 10.1016/j.ajog.2017.11.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BB, Armstrong DA, Lesseur C, Paquette AG, Guerin DJ, Kwan LE, Marsit CJ (2015) The Role of Placental 11-Beta Hydroxysteroid Dehydrogenase Type 1 and Type 2 Methylation on Gene Expression and Infant Birth Weight. Biology of reproduction 92:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG, Klugman S, Watson MS (2016) Noninvasive prenatal screening for fetal aneuploidy, 2016 update: a position statement of the American College of Medical Genetics and Genomics. Genet Med 18:1056–1065. 10.1038/gim.2016.97. [DOI] [PubMed] [Google Scholar]
- Hamada H, Okae H, Toh H, Chiba H, Hiura H, Shirane K, Sato T, Suyama M, Yaegashi N, Sasaki H, Arima T (2016) Allele-Specific Methylome and Transcriptome Analysis Reveals Widespread Imprinting in the Human Placenta. Am J Hum Genet 99:1045–1058. 10.1016/j.ajhg.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna CW, Demond H, Kelsey G (2018) Epigenetic regulation in development: is the mouse a good model for the human?. Human reproduction update 24:556–576. 10.1093/humupd/dmy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K, Hardy PJ, Jacobs PA, Lewallen K, Hassold TJ (2016) Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis. Am J Med Genet 170:2671–2680. 10.1002/ajmg.a.37795. [DOI] [PubMed] [Google Scholar]
- Harrington WE, Kanaan SB, Muehlenbachs A, Morrison R, Stevenson P, Fried M, Duffy PE, Lee Nelson J (2017) Maternal Microchimerism Predicts Increased Infection but Decreased Disease due to Plasmodium falciparum During Early Childhood. The Journal of infectious diseases 215:1445–1451. 10.1093/infdis/jix129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig TS, Ambye L, Sørensen S, Jørgensen FS (2017) Discordant non-invasive prenatal testing (NIPT) - a systematic review. Prenatal Diagnosis 37:527–539. 10.1002/pd.5049. [DOI] [PubMed] [Google Scholar]
- Hatt L, Aagaard MM, Graakjaer J, Bach C, Sommer S, Agerholm IE, Kølvraa S, Bojesen A (2015) Microarray-Based Analysis of Methylation Status of CpGs in Placental DNA and Maternal Blood DNA - Potential New Epigenetic Biomarkers for Cell Free Fetal DNA-Based Diagnosis. PLoS One 10:e0128918 10.1371/journal.pone.0128918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebbar S, Misha M, Rai L (2014) Significance of Maternal and Cord Blood Nucleated Red Blood Cell Count in Pregnancies Complicated by Preeclampsia. Journal of pregnancy 2014:496416–7. 10.1155/2014/496416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby SE, Apps R, Chazara O, Farrell LE, Magnus P, Trogstad L, Gjessing HK, Carrington M, Moffett A (2014) Maternal KIR in combination with paternal HLA-C2 regulate human birth weight. J Immunol 192:5069–5073. 10.4049/jimmunol.1400577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Platas I, Monk D, Jebbink J, Buimer M, Boer K, van der Post J, Hills F, Apostolidou S, Ris-Stalpers C, Stanier P, Moore GE (2007) STOX1 is not imprinted and is not likely to be involved in preeclampsia. Nature genetics 39:279–280. 10.1038/ng0307-279. [DOI] [PubMed] [Google Scholar]
- Jauniaux E (1999) Partial Moles: from Postnatal to Prenatal Diagnosis. Placenta 20:379–388. 10.1053/plac.1999.0390. [DOI] [PubMed] [Google Scholar]
- Jim B, Karumanchi SA (2017) Preeclampsia: Pathogenesis, Prevention, and Long-Term Complications. Seminars in Nephrology 37:386–397. 10.1016/j.semnephrol.2017.05.011. [DOI] [PubMed] [Google Scholar]
- Johnson A, Wapner R, Davis G, Jackson L (1990) Mosaicism in Chorionic Villus Sampling: An Association With Poor Perinatal Outcome. Obstetrics & Gynecology 75:573–577. [PubMed] [Google Scholar]
- Kalousek DK, Barrett IJ, McGillivray BC (1989) Placental mosaicism and intrauterine survival of trisomies 13 and 18. Am J Hum Genet 44:338. [PMC free article] [PubMed] [Google Scholar]
- Kalousek DK, Howard-Peebles PN, Olson SB, Barrett IJ, Dorfmann A, Black SH, Schulman JD, Wilson RD (1991) Confirmation of CVS mosaicism in term placentae and high frequency of intrauterine growth retardation association with confined placental mosaicism. Prenat Diagn 11:743–750. 10.1002/pd.1970111002. [DOI] [PubMed] [Google Scholar]
- Karampetsou E, Morrogh D, Ballard T, Waters JJ, Lench N, Chitty LS (2014) Confined placental mosaicism: implications for fetal chromosomal analysis using microarray comparative genomic hybridization. Prenatal Diagnosis 34:98–101. 10.1002/pd.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasak L, Rull K, Sober S, Laan M (2017) Copy number variation profile in the placental and parental genomes of recurrent pregnancy loss families. Scientific reports 7:45327 10.1038/srep45327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasak L, Rull K, Vaas P, Teesalu P, Laan M (2015) Extensive load of somatic CNVs in the human placenta. Scientific reports 5:8342 10.1038/srep08342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P, Kim JS (2015) Chronic inflammation of the placenta: definition, classification, pathogenesis, and clinical significance. Am J Obstet Gynecol 213:53 10.1016/j.ajog.2015.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivinen K, Peterson H, Hiltunen L, Laivuori H, Heino S, Tiala I, Knuutila S, Rasi V, Kere J (2007) Evaluation of STOX1 as a preeclampsia candidate gene in a population-wide sample. Eur J Hum Genet 15:494–497. 10.1038/sj.ejhg.5201788. [DOI] [PubMed] [Google Scholar]
- Kondo Y (2009) Epigenetic cross-talk between DNA methylation and histone modifications in human cancers. Yonsei Med J 50:455–463. 10.3349/ymj.2009.50.4.455 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konwar C, Del Gobbo GF, Terry J, Robinson WP (2019) Association of a placental Interleukin-6 genetic variant (rs1800796) with DNA methylation, gene expression and risk of acute chorioamnionitis. BMC Med Genet 20:3–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konwar C, Price M, Wang Li Qing, Wilson S, Terry J, Robinson W (2018) DNA methylation profiling of acute chorioamnionitis-associated placentas and fetal membranes: insights into epigenetic variation in spontaneous preterm births. Epigenetics and Chromatin 11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois S, Yong PJ, Yong SL, Barrett I, Kalousek DK, Miny P, Exeler R, Morris K, Robinson WP (2006) Postnatal follow-up of prenatally diagnosed trisomy 16 mosaicism. Prenat Diagn 26:548–558. 10.1002/pd.1457. [DOI] [PubMed] [Google Scholar]
- Leavey K, Wilson SL, Bainbridge SA, Robinson WP, Cox BJ (2018) Epigenetic regulation of placental gene expression in transcriptional subtypes of preeclampsia. Clin Epigen 10:28 10.1186/s13148-018-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter DH, Zachary JM, Simpson JL, Golbus MS, Pergament E, Jackson L, Mahoney MJ, Desnick RJ, Schulman J, Copeland KL (1992) Cytogenetic results from the U.S. Collaborative Study on CVS. Prenat Diagn 12:317–345. 10.1002/pd.1970120503. [DOI] [PubMed] [Google Scholar]
- Macaulay EC, Weeks RJ, Andrews S, Morison IM (2011) Hypomethylation of functional retrotransposon- derived genes in the human placenta. Mamm Genome 22:722–735. 10.1007/s00335-011-9355-1. [DOI] [PubMed] [Google Scholar]
- Mahadevan S, Wen S, Balasa A, Fruhman G, Mateus J, Wagner A, Al-Hussaini T, Van den Veyver, Ignatia B (2013) No evidence for mutations in NLRP7 and KHDC3L in women with androgenetic hydatidiform moles. Prenat Diagn 33:1242–1247. 10.1002/pd.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makris A, Xu B, Yu B, Thornton C, Hennessy A (2006) Placental Deficiency of Interleukin-10 (IL-10) in Preeclampsia and its Relationship to an IL10 Promoter Polymorphism. Placenta 27:445–451. 10.1016/j.placenta.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Manokhina I, Hanna CW, Stephenson MD, McFadden DE, Robinson WP (2013) Maternal NLRP7 and C6orf221 variants are not a common risk factor for androgenetic moles, triploidy and recurrent miscarriage. Mol Hum Reprod 19:539–544. 10.1093/molehr/gat019. [DOI] [PubMed] [Google Scholar]
- Marsit CJ, Maccani MA, Padbury JF, Lester BM (2012) Placental 11-Beta Hydroxysteroid Dehydrogenase Methylation Is Associated with Newborn Growth and a Measure of Neurobehavioral Outcome. PLoS One 7:e33794 10.1371/journal.pone.0033794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard SE, Min J, Merchan J, Lim K, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA (2003) Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. Journal of Clinical Investigation 111:649–658. 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DE, Pantzar JT (1996) Placental pathology of triploidy. Human Pathology 27:1018–1020. 10.1016/S0046-8177(96)90277-4. [DOI] [PubMed] [Google Scholar]
- McFadden DE, Robinson WP (2006) Phenotype of triploid embryos. Journal of medical genetics 43:609–612. 10.1136/jmg.2005.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis R, Steinthorsdottir V, William NO, Thorleifsson G, Shooter S, Hjartardottir S, Bumpstead S, Stefansdottir L, Hildyard L, Sigurdsson JK, et al. (2017) Variants in the fetal genome near FLT1 are associated with risk of preeclampsia. Nature Genetics 49:1255–1260. 10.1038/ng.3895. [DOI] [PubMed] [Google Scholar]
- McRae AF, Marioni RE, Shah S, Yang J, Powell JE, Harris SE, Gibson J, Henders AK, Bowdler L, Painter JN, Murphy L, Martin NG, Starr JM, Wray NR, Deary IJ, Visscher PM, Montgomery GW (2018) Identification of 55,000 Replicated DNA Methylation QTL. Scientific Reports 8:17605 10.1038/s41598-018-35871-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffett A, Hiby SE, Sharkey AM (2015) The role of the maternal immune system in the regulation of human birthweight. Philos Trans Royal Soc B 370:20140071 10.1098/rstb.2014.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P, Contu L, Alba F, Lai S, Simoes R, Orrù S, Carcassi C, Roger M, Rabreau M, Carosella ED (2008) HLA-G Gene Polymorphism in Human Placentas: Possible Association of G0106 Allele with Preeclampsia and Miscarriage. Biology of Reproduction 79:459–467. 10.1095/biolreprod.108.068874. [DOI] [PubMed] [Google Scholar]
- Morgan T (2016) Role of the Placenta in Preterm Birth: A Review. Amer J Perinatol 33:258–266. 10.1055/s-0035-1570379. [DOI] [PubMed] [Google Scholar]
- Nguyen NMP, Ge Z, Reddy R, Fahiminiya S, Sauthier P, Bagga R, Sahin FI, Mahadevan S, Osmond M, Breguet M, Rahimi K, Lapensee L, Hovanes K, Srinivasan R, Van den Veyver, Ignatia B, Sahoo T, Ao A, Majewski J, Taketo T, Slim R (2018) Causative Mutations and Mechanism of Androgenetic Hydatidiform Moles. Am J Hum Genet 103:740–751. 10.1016/j.ajhg.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic B, Wong NC, Sibson M, Ng HK, Morley R, Manuelpillai U, Down T, Rakyan VK, Beck S, Hiendleder S, Roberts CT, Craig JM, Saffery R (2010) DNA methylation-mediated down-regulation of DNA methyltransferase-1 (DNMT1) is coincident with, but not essential for, global hypomethylation in human placenta. J Biol Chem 285:9583–9593. 10.1074/jbc.M109.064956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic B, Rakyan V, Ng HK, Manuelpillai U, Dewi C, Wong NC, Morley R, Down T, Beck S, Craig JM, Saffery R (2008) Specific tumour-associated methylation in normal human term placenta and first-trimester cytotrophoblasts. Molecular Human Reproduction 14:547–554. 10.1093/molehr/gan046. [DOI] [PubMed] [Google Scholar]
- Novakovic B, Yuen RK, Gordon L, Penaherrera MS, Sharkey A, Moffett A, Craig JM, Robinson WP, Saffery R (2011) Evidence for widespread changes in promoter methylation profile in human placenta in response to increasing gestational age and environmental/stochastic factors. BMC Genomics 12:529 10.1186/1471-2164-12-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paauw ND, Lely AT, Joles JA, Franx A, Nikkels PG, Mokry M, van Rijn BB (2018) H3K27 acetylation and gene expression analysis reveals differences in placental chromatin activity in fetal growth restriction. Clin Epigenetics 10:8–x. eCollection 2018. 10.1186/s13148-018-0508-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry D, Logan C, Hayward B, Shires M, Landolsi H, Diggle C, Carr I, Rittore C, Touitou I, Philibert L, Fisher R, Fallahian M, Huntriss J, Picton H, Malik S, Taylor G, Johnson C, Bonthron D, Sheridan E (2011) Mutations Causing Familial Biparental Hydatidiform Mole Implicate C6orf221 as a Possible Regulator of Genomic Imprinting in the Human Oocyte. Am J Hum Genet 89:451–458. 10.1016/j.ajhg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra S, Bertoni B, Sapiro R (2016) Interactions between environmental factors and maternal-fetal genetic variations: strategies to elucidate risks of preterm birth. European Journal of Obstetrics & Gynecology and Reproductive Biology 202:20–25. 10.1016/j.ejogrb.2016.04.030. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia V, Fineberg E, Wilson R, Murray A, Mazzeo CI, Tudor C, Sienerth A, White JK, Tuck E, Ryder EJ, Gleeson D, Siragher E, Wardle-Jones H, Staudt N, Wali N, Collins J, Geyer S, Busch-Nentwich EM, Galli A, Smith JC, Robertson E, Adams DJ, Weninger WJ, Mohun T, Hemberger M (2018) Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 555:46–468Q. 10.1038/nature26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pescia G, Guex N, Iseli C, Brennan L, Osteras M, Xenarios I, Farinelli L, Conrad B (2017) Cell-free DNA testing of an extended range of chromosomal anomalies: clinical experience with 6,388 consecutive cases. Genet Med 19:169–175. 10.1038/gim.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petts G, Fisher RA, Short D, Lindsay I, Seckl MJ, Sebire NJ (2014) Histopathological and immunohistochemical features of early hydatidiform mole in relation to subsequent development of persistent gestational trophoblastic disease. The Journal of reproductive medicine 59:213. [PubMed] [Google Scholar]
- Phillips OP, Tharapel AT, Lerner JL, Park VM, Wachtel SS, Shulman LP (1996) Risk of fetal mosaicism when placental mosaicism is diagnosed by chorionic villus sampling. American Journal of Obstetrics and Gynecology 174:850–855. 10.1016/S0002-9378(96)70312-5. [DOI] [PubMed] [Google Scholar]
- Pidoux G, Gerbaud P, Marpeau O, Guibourdenche J, Ferreira F, Badet J, Evain-Brion D, Frendo J (2007) Human Placental Development Is Impaired by Abnormal Human Chorionic Gonadotropin Signaling in Trisomy 21 Pregnancies. Endocrinology 148:5403–5413. 10.1210/en.2007-0589. [DOI] [PubMed] [Google Scholar]
- Price EM, Cotton AM, Peñaherrera MS, McFadden DE, Kobor MS, Robinson W (2012) Different measures of “genome-wide” DNA methylation exhibit unique properties in placental and somatic tissues. Epigenetics 7:652–663. 10.4161/epi.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajcan-Separovic E, Diego-Alvarez D, Robinson WP, Tyson C, Qiao Y, Harvard C, Fawcett C, Kalousek D, Philipp T, Somerville MJ, Stephenson MD (2010) Identification of copy number variants in miscarriages from couples with idiopathic recurrent pregnancy loss. Hum Reprod 25:2913–2922. 10.1093/humrep/deq202. [DOI] [PubMed] [Google Scholar]
- Reiss D, Zhang Y, Mager DL (2007) Widely variable endogenous retroviral methylation levels in human placenta. Nucleic Acids Res 35:4743–4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey G, Skowronek F, Alciaturi J, Alonso J, Bertoni B, Sapiro R (2008) Toll receptor 4 Asp299Gly polymorphism and its association with preterm birth and premature rupture of membranes in a South American population. Mol Hum Reprod 14:555–559. 10.1093/molehr/gan049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W (2006) Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. American Journal of Obstetrics and Gynecology 195:255–259. 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- Robinson WP, Barrett IJ, Bernard L, Telenius A, Bernasconi F, Wilson RD, Best RG, Howard-Peebles PN, Langlois S, Kalousek DK (1997) Meiotic origin of trisomy in confined placental mosaicism is correlated with presence of fetal uniparental disomy, high levels of trisomy in trophoblast, and increased risk of fetal intrauterine growth restriction. Am J Hum Genet 60:917. [PMC free article] [PubMed] [Google Scholar]
- Robinson WP, Peñaherrera MS, Jiang R, Avila L, Sloan J, McFadden DE, Langlois S, von Dadelszen P (2010) Assessing the role of placental trisomy in preeclampsia and intrauterine growth restriction. Prenatal diagnosis 30:1. [DOI] [PubMed] [Google Scholar]
- Robinson WP, Price EM (2015) The human placental methylome. Cold Spring Harbor perspectives in medicine 5:a023044 10.1101/cshperspect.a023044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchat S, Houde A, Voisin G, St-Pierre J, Perron P, Baillargeon J, Gaudet D, Hivert M, Brisson D, Bouchard L (2013) Gestational diabetes mellitus epigenetically affects genes predominantly involved in metabolic diseases. Epigenetics 8:935–943. 10.4161/epi.25578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg-Gunn PJ, Cox BJ, Ralston A, Rossant J (2010) Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc Natl Acad Sci U S A 107:10783–10790. 10.1073/pnas.0914507107 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha B, Home P, Ray S, Larson M, Paul A, Rajendran G, Behr B, Paul S (2013) EED and KDM6B coordinate the first mammalian cell lineage commitment to ensure embryo implantation. Mol Cell Biol 33:2691–2705. 10.1128/MCB.00069-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salafia CM, Vogel CA, Vintzileos AM, Bantham KF, Pezzullo J, Silberman L (1991) Placental pathologic findings in preterm birth. American Journal of Obstetrics and Gynecology 165:934–938. 10.1016/0002-9378(91)90443-U [DOI] [PubMed] [Google Scholar]
- Sanchez-Delgado M, Martin-Trujillo A, Tayama C, Vidal E, Esteller M, Iglesias-Platas I, Deo N, Barney O, Maclean K, Hata K, Nakabayashi K, Fisher R, Monk D (2015) Absence of Maternal Methylation in Biparental Hydatidiform Moles from Women with NLRP7 Maternal-Effect Mutations Reveals Widespread Placenta-Specific Imprinting. PLoS genetics 11:e1005644 10.1371/journal.pgen.1005644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Schroeder DI, Crary-Dooley FK, Barkoski JM, Tancredi DJ, Walker CK, Ozonoff S, Hertz-Picciotto I, LaSalle JM (2016) Self-reported pregnancy exposures and placental DNA methylation in the MARBLES prospective autism sibling study. Environmental epigenetics 2:dvw024 10.1093/eep/dvw024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder DI, Blair JD, Lott P, Yu HOK, Hong D, Crary F, Ashwood P, Walker C, Korf I, Robinson WP, LaSalle JM (2013) The human placenta methylome. Proc Natl Acad Sci U S A 110:6037–6042. 10.1073/pnas.1215145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler A, Morales C, Mademont-Soler I, Margarit E, Borrell A, Borobio V, Muñoz M, Sánchez A (2017) Overview of Chromosome Abnormalities in First Trimester Miscarriages: A Series of 1,011 Consecutive Chorionic Villi Sample Karyotypes. Cytogenetic and genome research 152:81–89. 10.1159/000477707. [DOI] [PubMed] [Google Scholar]
- Stipoljev F, Latin V, Kos M, Miskovic B, Kurjak A (2001) Correlation of confined placental mosaicism with fetal intrauterine growth retardation. A case control study of placentas at delivery. Fetal diagnosis and therapy 16:4. [DOI] [PubMed] [Google Scholar]
- Suter M, Abramovici A, Showalter L, Hu M, Shope CD, Varner M, Aagaard-Tillery K (2010) In utero tobacco exposure epigenetically modifies placental CYP1A1 expression. Metabolism 59:1481–1490. 10.1016/j.metabol.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M (2007) Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature 445:214–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toutain J, Goutte-Gattat D, Horovitz J, Saura R (2018) Confined placental mosaicism revisited: Impact on pregnancy characteristics and outcome. PLoS One 13:e0195905 10.1371/journal.pone.0195905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuohy JF, James DK (1992) Pre-eclampsia and trisomy 13. British journal of obstetrics and gynaecology 99:891–894. 10.1111/j.1471-0528.1992.tb14436.x. [DOI] [PubMed] [Google Scholar]
- Uzun A, Dewan AT, Istrail S, Padbury JF (2013) Pathway-based genetic analysis of preterm birth. Genomics 101:163–170. 10.1016/j.ygeno.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Otterdijk SD, Binder AM, Michels KB (2017) Locus-specific DNA methylation in the placenta is associated with levels of pro-inflammatory proteins in cord blood and they are both independently affected by maternal smoking during pregnancy. Epigenetics 12:875–885. 10.1080/15592294.2017.1361592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas A, Moreau J, Landry S, LeBellego F, Toufaily C, Rassart E, Lafond J, Barbeau B (2009) Syncytin-2 plays an important role in the fusion of human trophoblast cells. J Mol Biol 392:301–318. 10.1016/j.jmb.2009.07.025 [doi]. [DOI] [PubMed] [Google Scholar]
- Wang BB, Rubin CH, Williams J3 (1993) Mosaicism in chorionic villus sampling: an analysis of incidence and chromosomes involved in 2612 consecutive cases. Prenat Diagn 13:179–190. 10.1002/pd.1970130305. [DOI] [PubMed] [Google Scholar]
- Wilkins-Haug L, Roberts DJ, Morton CC (1995) Confined placental mosaicism and intrauterine growth retardation: A case-control analysis of placentas at delivery. American Journal of Obstetrics and Gynecology 172:44–50. 10.1016/0002-9378(95)90082-9. [DOI] [PubMed] [Google Scholar]
- Wilson SL, Blair JD, Hogg K, Langlois S, von Dadelszen P, Robinson WP (2015) Placental DNA methylation at term reflects maternal serum levels of INHA and FN1, but not PAPPA, early in pregnancy. BMC medical genetics 16:111 10.1186/s12881-015-0257-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SL, Leavey K, Cox BJ, Robinson WP (2018) Mining DNA methylation alterations towards a classification of placental pathologies. Human Molecular Genetics 27:135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme J (1996) Confined placental mosaicism for trisomies 2, 3, 7, 8, 9, 16, and 22: their incidence, likely origins, and mechanisms for cell lineage compartmentalization. Prenat Diagn 16:511–524. [DOI] [PubMed] [Google Scholar]
- Xie D, Zhu J, Liu Q, Li J, Song M, Wang K, Zhou Q, Jia Y, Li T (2019) Dysregulation of HDAC9 Represses Trophoblast Cell Migration and Invasion Through TIMP3 Activation in Preeclampsia. Am J Hypertens 32:515–523. 10.1093/ajh/hpz006. [DOI] [PubMed] [Google Scholar]
- Yeung KR, Chiu CL, Pidsley R, Makris A, Hennessy A, Lind JM (2016) DNA methylation profiles in preeclampsia and healthy control placentas. American journal of physiology. Heart and circulatory physiology 310:H1295. [DOI] [PubMed] [Google Scholar]
- Yong PJ, Barrett IJ, Kalousek DK, Robinson WP (2003) Clinical aspects, prenatal diagnosis, and pathogenesis of trisomy 16 mosaicism. J Med Genet 40:175–182. 10.1136/jmg.40.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong PJ, Langlois S, Dadelszen Pv, Robinson W (2006) The association between preeclampsia and placental trisomy 16 mosaicism. Prenat Diagn 26:956–961. 10.1002/pd.1534. [DOI] [PubMed] [Google Scholar]
- Yuen RK, Chen B, Blair JD, Robinson WP, Nelson DM (2013) Hypoxia alters the epigenetic profile in cultured human placental trophoblasts. Epigenetics 8:192–202. 10.4161/epi.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Wang J, Liu R, Yang J, Cui K, Wu Y, Guo J, Mu Y, Wang X (2010) Quantification and application of the placental epigenetic signature of the RASSF1A gene in maternal plasma. Prenat Diagn 30:778–782. 10.1002/pd.2546. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Mordaunt CE, Yasui DH, Marathe R, Coulson R, Dunaway K, Walker C, Ozonoff S, Hertz-Picciotto I, Schmidt RJ, LaSalle JM (2018) Placental DNA methylation levels at CYP2E1 and IRS2 are associated with child outcome in a prospective autism study. bioRxiv :501007 10.1101/501007. [DOI] [PMC free article] [PubMed] [Google Scholar]