Abstract
Introduction
Type 1 GM1 gangliosidosis is an ultra-rare, rapidly fatal lysosomal storage disorder, with life expectancy of less than 3 years of age. To date, only one prospective natural history study of limited size has been reported. Thus, there is a need for additional research to provide a better understanding of the progression of this disease. We have leveraged the past two decades of medical literature to conduct the first comprehensive retrospective study characterizing the natural history of Type 1 GM1 gangliosidosis.
Objectives
The objectives of this study were to establish a large sample of patients from the literature in order to identify: 1) clinically distinguishing factors between Type 1 and Type 2 GM1 gangliosidosis, 2) age at first symptom onset, first hospital admission, diagnosis, and death, 3) time to onset of common clinical findings, and 4) timing of developmental milestone loss.
Methods
PubMed was searched with the key word “GM1 Gangliosidosis” and for articles from the year 2000 onwards. A preliminary review of these results was conducted to establish subtype classification criteria for inclusion of only Type 1 patients, resulting in 44 articles being selected to generate the literature dataset of 154 Type 1 GM1 gangliosidosis patients. Key clinical events of these patient cases were recorded from the articles.
Results
Comprehensive subtyping criteria for Type 1 GM1 gangliosidosis were created, and clinical events, including onset, diagnosis, death, and symptomology, were mapped over time. In this dataset, average age of diagnosis was 8.7 months, and average age of death was 18.9 months.
Discussion
This analysis demonstrates the predictable clinical course of this disease, as almost all patients experienced significant multi-organ system dysfunction and neurodevelopmental regression, particularly in the 6- to 18-month age range. Patients were diagnosed at a late age relative to disease progression, indicating the need for improved public awareness and screening.
Conclusion
This study highlights the significant burden of illness in this disease and provides critical natural history data to drive earlier diagnosis, inform clinical trial design, and facilitate family counseling.
Keywords: GM1 gangliosidosis, Type 1, natural history study, lysosomal storage disorder, meta-analysis, literature review
1. INTRODUCTION
GM1 gangliosidosis (MIM# 230500) is a neuronopathic lysosomal storage disorder caused by a deficiency in the enzyme β-galactosidase (βgal, E.C. 3.2.1.23) due to biallelic mutations in the GLB1 gene. The ubiquitous lysosomal enzyme βgal is responsible for the degradation of GM1 ganglioside, oligosaccharides, and keratan sulfate, all of which perform many cellular signaling and structural roles (Regier 2016). Deficiency of βgal results in toxic lysosomal storage of these substrates, leading to cellular apoptosis. GM1 ganglioside buildup occurs primarily in the central nervous system (CNS), the predominant area of ganglioside synthesis, and causes severe progressive neurological deficits. Systemic disease manifestations are also common due to keratan sulfate and oligosaccharide buildup in peripheral organs, such as the heart, bone, liver, and spleen (Brunetti-Pierri 2008).
The incidence of GM1 gangliosidosis has been reported to be 1 in 100,000–200,000 live births per year (Sinigerska 2006), with increased incidence in certain populations due to founder mutations (Coelho 1997, Lenicker 1997, Severini 1999, Georgiou 2005, Santamaria 2006). There are currently no FDA-approved treatments for this disease, and management of these patients is limited to symptomatic supportive care.
Three clinical forms of GM1 gangliosidosis have been described based on age of first symptom onset and severity of disease progression. The Type 1 infantile form is the most severe, with death often before 3 years of age. This subtype has traditionally been characterized by an age of first symptom onset between birth and 6 months, with clinical findings of hypotonia and developmental delay in almost all patients (Brunetti-Pierri 2008). Several cases of perinatal onset have been reported, including intrauterine growth retardation, hydrops fetalis, and placental vacuolization (Sinelli 2005). In addition to CNS symptoms, infants frequently have accompanying hepatosplenomegaly, skeletal dysplasia, cherry-red maculae, cardiomyopathy, and coarse facial features (Brunetti-Pierri 2008).
The Type 2 form has been subdivided into the late infantile (Type 2a) and juvenile (Type 2b) subtypes. Late infantile patients often have symptom onset between 7 months and 2 years of age, while juvenile patients typically develop symptoms between 2 and 3 years of age. Type 2 patients exhibit similar symptoms to Type 1 patients, including psychomotor regression and eye and bone abnormalities, but have an attenuated progression (Regier 2016). Lastly, the Type 3 variant (chronic or adult) is characterized by later symptom onset (between 3 and 30 years of age) and progressive extrapyramidal symptoms, including dystonia and gait disturbance (Suzuki 2001).
Disease severity is associated with the residual activity of the mutant βgal enzyme, with activity nearly absent in Type 1-associated mutations and a small amount of residual activity in Type 2 and 3 patients (Suzuki 1978, Brunetti-Pierri 2008, Yang 2010). Over 165 mutations have been reported in the GLB1 gene (ClinVar Database), and several genotype-phenotype analyses have been published (Brunetti-Pierri 2008). Common pathogenic mutations associated with Type 1 disease, including p.R59H and c.1622–1627insG, have been identified in patients from specific geographic regions, such as Brazil (Silva 1999, Santamaria 2007, Baiotto 2011), Spain, and among the Roma (Santamaria 2006, Sinigerska 2006, Santamaria 2007). Type 2 and 3 patients are usually compound heterozygotes, with one null allele producing a nonfunctional βgal enzyme and the other often a missense allele resulting in impaired but residual enzyme production (Chakraborty 1994, Caciotti 2003). p.I51H, p.T82M, and p.R201H are GLB1 mutations reported to cause low βgal activity and have been identified in multiple slower-progressing Type 2 or 3 patients (Yoshida 1992, Chakraborty 1994, Kaye 1997, Hofer 2009, Regier, 2016).
The ultra-rare and rapidly fatal nature of Type 1 disease presents significant challenges in understanding the natural history of this subtype. In this context, results from two clinical studies ( NCT00668187, NCT02030015) have been published (Utz 2015, Jarnes Utz 2017, Nestrasil 2018). NCT00668187 is the first prospective study in Type 1 GM1 gangliosidosis, and NCT02030015 is an interventional trial studying the Syner-G regimen (miglustat + ketogenic diet) in infantile gangliosidosis subjects. Both studies were conducted by one academic group at a single clinical site. Jarnes Utz et al reported pooled results from both studies, characterizing clinical changes over time and overall survival in eight Type 1 GM1 gangliosidosis patients (Jarnes Utz 2017). Three out of 8 of these patients were given the experimental Syner-G regimen, which may have confounded the reported observations of disease progression (Jarnes Utz 2017). Furthermore, two other longitudinal analyses derived from the prospective natural history study ( NCT00668187) have been published, but their scope was limited to tracking only biomarker and brain MRI changes over time (Utz 2015, Nestrasil 2018). Brunetti-Pierri and Scaglia published a GM1 gangliosidosis literature review over a decade ago, identifying a retrospective literature cohort of 130 infantile patients. However, although symptom prevalence was reported, timing of symptom onset or disease progression were not discussed (Brunetti-Pierri 2008). Taken together, there is a paucity of natural history data around this subtype. Given that multiple interventional clinical trials in Type 1 GM1 gangliosidosis are planned to begin in 2020, there is a need to further characterize disease progression to inform future research study design, identify clinical endpoints, and facilitate family counseling.
This literature-based meta-analysis of Type 1 GM1 gangliosidosis is the first comprehensive retrospective study analyzing the time course of this disease. The analysis was conducted using data aggregated from the last two decades of published reports, with the goal of contributing to the current body of available natural history data. We have established an improved classification for the Type 1 subtype and a mapping of clinical findings as the disease progresses.
2. OBJECTIVES
The objectives of this study were to establish a large sample of patients from the literature in order to identify: 1) clinically distinguishing factors between Type 1 and Type 2 GM1 gangliosidosis, 2) age at first symptom onset, first hospital admission, diagnosis, and death, 3) time to onset of common clinical findings, and 4) timing of developmental milestone loss.
3. METHODS
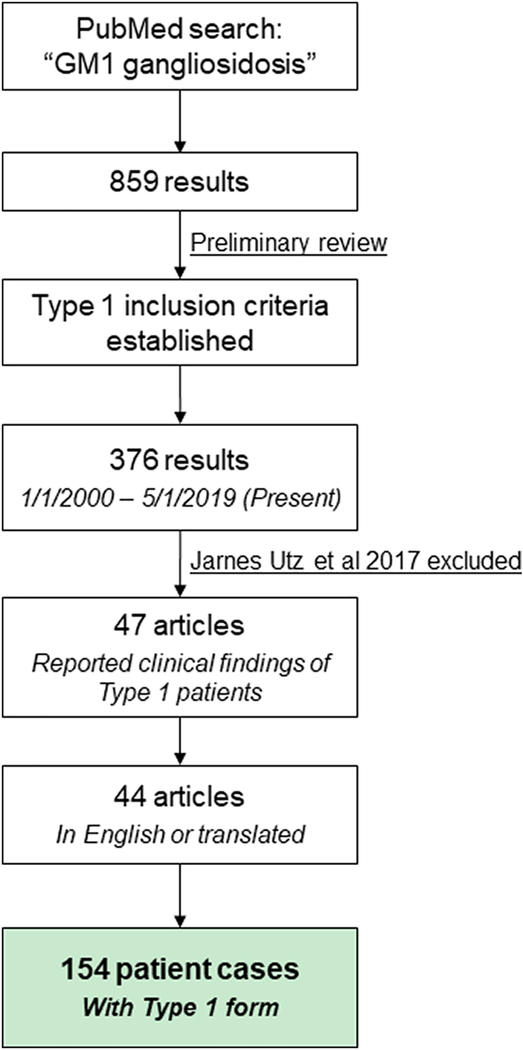
An outline of this literature-based meta-analysis is shown in Figure 1.
Figure 1: Generation of retrospective natural history cohort from literature.
PubMed was used to review all articles discussing GM1 gangliosidosis, and the inclusion criteria for Type 1 patients was established. 44 articles containing information from 154 patient cases were found.
PubMed was searched with the key word “GM1 gangliosidosis” and 859 results were obtained dating back to 1948. Given the overlapping symptoms, ages of onset, and enzyme activities between the Type 1 and Type 2 subtypes (Table 1), we conducted a preliminary review of these publications to establish standardized criteria for inclusion of only Type 1 cases into our analysis. This preliminary review yielded the Type 1 classification criteria discussed in the Results section (Figure 2). These criteria were used to generate our dataset of Type 1 patients. When this classification criteria could not be used due to insufficient reporting of clinical findings, the subtype diagnosis of the author was used. To ensure that the results of the meta-analysis reflected a contemporaneous patient population, publication cutoff years of 2000 to present day (analysis conducted in May 2019) were established for inclusion into the cohort. This cutoff criterion was based on the supportive standard of clinical care that has remained consistent over the past two decades for Type 1 patients. The PubMed search “Norman-Landing disease NOT GM1 gangliosidosis” was also used but yielded only two results from 1970.
Table 1: Significant overlap between Type 1 and Type 2 disease characteristics.
Review of the literature demonstrates that Type 1 and Type 2 patients have similar disease characteristics across multiple parameters, including frequency of certain symptomology (N = 130 Type 1, 23 Type 2, data taken from Brunetti-Pierri 2008), residual βgal activity (N = 89 Type 1, 55 Type 2), and age of first symptom onset (N = 109 Type 1, 61 Type 2) (Sinelli 2005, Santamaria 2006, Santamaria 2007, Caciotti 2007, Hofer 2010, Caciotti 2011, Bidchol 2015, Regier 2016, Feng 2018, Lee 2018).
| Type 1 | Type 2 | |
|---|---|---|
| Dysmorphic features | 87% | 66% |
| Hypotonia | 96% | 50% |
| Developmental delay / Mental retardation | 100% | 96% |
| Seizures | 9% | 18% |
| Cherry-red spot | 59% | 18% |
| Cardiomyopathy | 34% | 38% |
| Hepatosplenomegaly | 85% | 30% |
| Skeletal abnormalities | 82% | 69% |
| Extrapyramidal signs Observed in Type 3 | 0% | 0% |
| Dystonia Observed in Type 3 | 0% | 0% |
| Residual βgal activity | <1% to ~10% Assay-dependent |
<1% to ~25% Assay-dependent |
| Age of first symptom onset | Pre-birth to ~12 months | ~6 months to ~5 years |
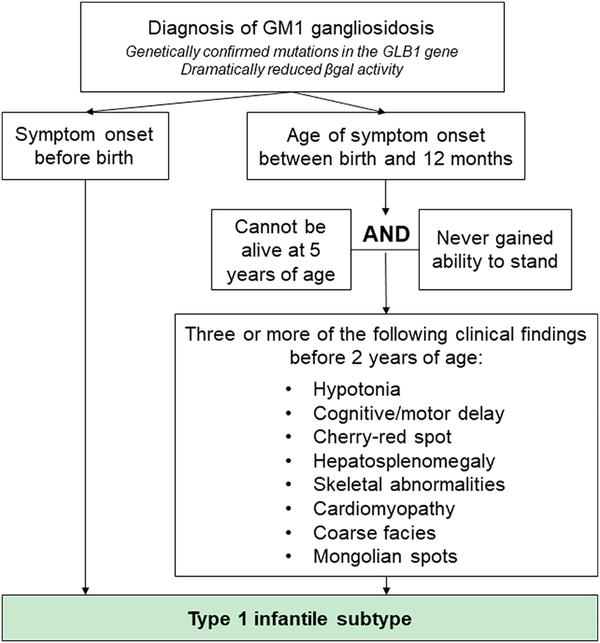
Figure 2: Algorithm to classify Type 1 GM1 gangliosidosis used as inclusion criteria for retrospective literature cohort.
Due to the overlapping disease characteristics between Type 1 and Type 2 patients, a classification scheme was created to identify patient cases that exhibited the Type 1 phenotype. This scheme was based on findings from the preliminary review of the GM1 gangliosidosis literature.
376 articles published after January 1, 2000 were identified and screened for case reports with descriptions of clinical findings of children with Type 1 GM1 gangliosidosis. The Jarnes Utz 2017 publication was excluded because it reported results from the only published prospective Type 1 GM1 gangliosidosis natural history study. Then, utilizing the subtyping criteria established from our preliminary review, 47 publications were found, with 44 articles in English or able to be translated to English (See Appendix). From these articles, 154 unique Type 1 GM1 gangliosidosis patient cases were identified, forming the retrospective natural history cohort (Figure 1). The age that key clinical events were first reported for each patient was recorded. These events included onset, diagnosis, and death as well as 35 signs and symptoms, such as hypotonia, hepatosplenomegaly, cherry-red spots, developmental delay, etc. An age of “0” was used for symptom onset at or before birth. Age of last follow-up was recorded to perform time-to-event analyses on certain parameters.
3.1. Statistics
Statistical analyses were performed using Microsoft Excel, R, and SAS®. Time-to-event analysis was performed for key outcomes (first symptom onset, first hospital admission, diagnosis of GM1 gangliosidosis, and death), with patients censored at their last known date of follow-up. For sign/symptom latency findings, only the sign/symptoms that were found in 5 or more patients were plotted. The mean (± standard error) age at which key signs/symptoms were first reported was calculated; only patients who experienced these clinical changes were included in the calculation. Similar outcomes were presented for the age at which developmental milestones were reported to be lost (or never attained).
4. RESULTS
4.1. Criteria to classify Type 1 infantile patients
The literature reports multiple methodologies used to define GM1 gangliosidosis subtypes (Brunetti-Pierri 2008, Weismann 2015, Regier 2016, Richter 2018). Preliminary review of the literature revealed similar symptoms, similar residual βgal activity, and similar ages of first symptom onset between Type 1- and Type 2-diagnosed individuals (Table 1). Interestingly, we found that Type 1 and Type 2 patients have overlapping ages of symptom onset at approximately 6 to 12 months of age (Caciotti 2007, Caciotti 2011, Lee 2018), and, often, Type 1 patients were reported to have higher βgal activity than Type 2 patients (Santamaria 2006, Santamaria 2007, Hofer 2010, Sperb 2013, Bidchol 2015, Feng 2018, Lee 2018). Nevertheless, the two subtypes were distinguishable based on symptom latency timing and speed of disease progression, with Type 1 patients exhibiting a noticeably faster progression than Type 2 patients.
These initial findings were consolidated to establish the comprehensive Type 1 criteria shown in Figure 2 to generate our retrospective dataset of 154 Type 1 patients. By requiring observation of multiple classic Type 1 symptoms early in life and excluding patients with long survival or who reached the later motor milestone of standing, the criteria seeks to only include patients exhibiting a disease course consistent with the rapidly progressive nature of the infantile phenotype (Figure 2, right). Further, because only Type 1 patients have been shown to exhibit signs before birth (Brunetti-Pierri 2008), any prenatal clinical findings allowed immediate classification into the Type 1 category (Figure 2, left). Importantly, many publications reported broad clinical findings from large groups of patients and did not specify timing of symptoms; our subtype classification system could not be used when assessing these articles. Despite this limitation, these publications were highly useful for our analysis, so the subtype diagnosis given by the author was used in these situations.
4.2. Time-to-event analyses of key events
As outlined in Figure 1, a dataset of 154 patients from the literature was generated. Key characteristics of this cohort are below:
Average age of first symptom onset: 2.8 months (Range: 0 – 11 months)
Average age of first hospital admission: 6.3 months (Range: 0 – 24 months)
Average age of diagnosis: 8.7 months (Range: 0 – 30 months)
Average age of death: 18.9 months (Range: 2 – 35 months)
These data reveal the lengthy time to diagnosis in this disease:
Time from first hospital admission to diagnosis: 2.4 months
Time from first symptom onset to diagnosis: 5.9 months
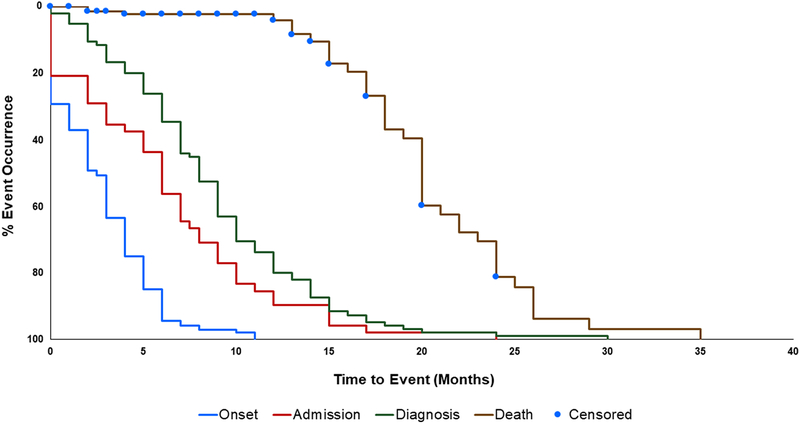
Figure 3 illustrates the time-to-event profile of disease progression from birth to each key event. Median time to onset was 2.5 months, median time to first hospital admission was 6.0 months, median time to diagnosis was 8.0 months, and median time to death was 20.0 months. Comparing the time to diagnosis curve to the time to death curve shows that patients are diagnosed approximately halfway through their lifespan. Over 25% of patients demonstrated symptom onset at or before birth. Although a <6-month symptom onset is often used to classify Type 1 patients, ~15% of patients had a symptom onset of ≥6 months. While 96% of patients were alive at 12 months and 63% of patients were alive at 18 months, only 19% of patients were alive at 24 months, indicating that mortality risk increases greatly for patients age 12 months and older. These data suggest that 12 months may be the time point at which irreversible neurodegeneration manifests (Figure 3).
Figure 3: Cumulative time-to-event analysis of key points during disease progression.
First symptom onset (blue line, N = 140), first hospital admission (red line, N = 48), diagnosis of GM1 gangliosidosis (green line, N = 95), and death (brown line, N = 154) were mapped over time, demonstrating diagnostic times that are high relative to patient survival. Patients were censored at last date of follow-up.
4.3. Sign and symptom latency findings
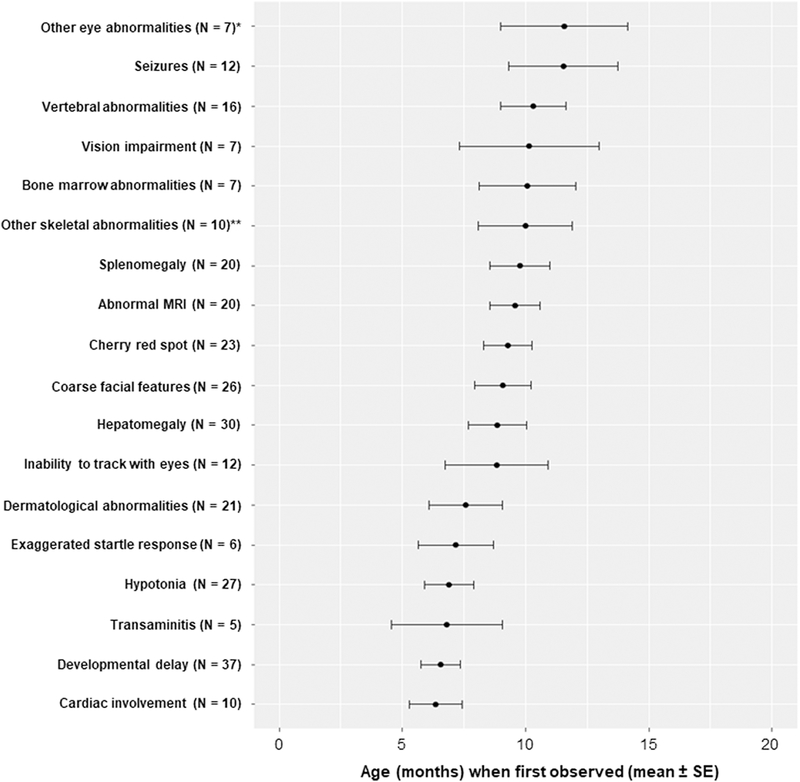
Data for specific timing of clinical findings were reported (in either a cross-sectional or longitudinal manner) for 39 of 154 patients. Figure 4 depicts the latency of 18 signs and symptoms (age at onset, in months) reported in these 39 patients and collectively illustrates the progression of Type 1 GM1 gangliosidosis. The average age of first observation of each of these 18 symptoms was prior to 12 months, reflecting the rapid onset of disease characteristics.
Figure 4: Age (months) at which signs and symptoms were first reported (N = 39).
Commonly observed symptomology in Type 1 GM1 gangliosidosis spans multiple organ systems and often occurs within the first year of life. *Eye abnormalities (e.g. strabismus, nystagmus) that do not include cherry red-spot or vision impairment. **Skeletal abnormalities (e.g. paddle-shaped ribs, osteoporosis) that do not include vertebral abnormalities or coarse facial features.
Corroborating the signs and symptoms previously reported by Brunetti-Pierri et al, these analyses demonstrated that patients experience widespread dysfunction of numerous organ systems, with developmental delay, hepatomegaly, coarse facial features, cherry-red spot, and hypotonia among the most common clinical findings reported in the contributing articles. In addition to these signs and symptoms, Type 1 patients often experienced dermatological abnormalities (i.e. Mongolian spots), bone marrow abnormalities (i.e. foamy cells), and vision impairment. Similar to infantile GM2 gangliosidosis (Bley 2011), seizures were associated with a later stage of disease, starting at approximately 10 to 12 months of age.
Of the initial presenting signs, dermatological abnormalities, coarse facies, developmental delay, and hypotonia were the most frequently reported. Together, these initial clinical findings should trigger the suspicion of GM1 gangliosidosis. This dermatological abnormality result corroborates that of Bersani et al, Abilkassem et al, Hackbart et al, and Ashrafi et al, who note that non-resolving Mongolian spots and skin hyperpigmentations can be considered an early sign of GM1 gangliosidosis, preceding the appearance of other disease features (Ashrafi 2006, Abilkassem 2013, Hackbart 2013, Bersani 2016).
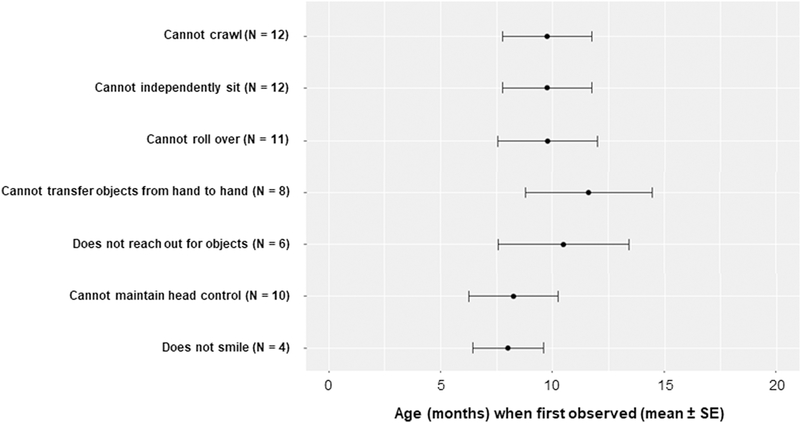
Data regarding the loss of specific developmental motor milestones was reported in 14 patient cases. For those with data reported, the loss (or lack of attainment) of these milestones (cannot roll over, cannot crawl, etc.) corresponded with the timing of significant clinical findings, with most milestones observed to be non-present by 12 months of age (Figure 5). Of the 154 cases, no patient was ever reported to be able to crawl, a developmental milestone that usually occurs between 7 and 9 months of age in normal infants.
Figure 5: Early developmental milestones are lost or never attained (N = 14).
Lack of these milestones at early timepoints demonstrates the rapid neurodevelopmental decline of Type 1 patients. In the absence of detailed reporting, it was generally assumed that reporting of non-presence on the earliest motor milestones (e.g. head control) meant there was also non-presence on the latest milestones (e.g. ability to sit independently, roll, and crawl).
5. DISCUSSION
5.1. Summary of natural history data in Type 1 GM1 gangliosidosis
Because Type 1 GM1 gangliosidosis is ultra-rare and rapidly fatal, published analyses from the sole prospective natural history study in this disease have been limited to sample sizes of 8 patients or less (Utz 2015, Jarnes Utz 2017, Nestrasil 2018). To add to this body of knowledge, the past two decades of medical literature have been leveraged to map a timeline of clinical changes in a large retrospective cohort of 154 patients. Despite the limitations of this approach, this literature meta-analysis has the advantage of including a much greater number of patients than would be possible in a prospective study.
The analysis elucidates multiple key components of the Type 1 GM1 gangliosidosis patient journey and disease progression. First, the various subtyping methodologies found in the literature and the overlapping features between Type 1 and Type 2 diseases demonstrate the need for clearer characterization of each subtype. Here, we have established comprehensive Type 1 subtyping criteria that was used as the inclusion criteria for our literature sample (Figure 2). Second, the disease is predictable and rapidly progressive, with death in the first few years of life (Figure 3). The analysis demonstrated a median survival of 20.0 months (N = 41), which was similar to the 19.1-month median survival of patients with feeding tubes and not on the Syner-G therapeutic regimen (ketogenic diet + miglustat) in the prospective study of Jarnes Utz et al (N = 3). Third, this analysis shows that diagnosis occurs extremely late in patients’ lifespans. The median age of diagnosis was 8.0 months (N = 95), similar to the 10.5-month median diagnosis age in the Jarnes Utz report (N = 8) (Jarnes Utz 2017). As shown in Figure 4, patients have already experienced significant symptomology at these timepoints. Fourth, the disease affects almost all organs and organ systems, including skin, eye, CNS, GI, heart, bone, liver, and spleen (Figure 4). These systemic manifestations contrast the disease features of the closely related GM2 gangliosidosis, where symptomology is generally limited to CNS degeneration (Bley 2011). Similar to what was reported by Jarnes Utz et al, most patients lost all early motor milestones by 2 years of age and no patient was noted to be able to crawl (Figure 5) (Jarnes Utz 2017).
Taken together, the totality of natural history data across both prospective and retrospective study types demonstrates that, over the first year of life, all Type 1 GM1 gangliosidosis individuals make limited developmental progress, briefly plateau then experience rapid neurodevelopmental decline, beginning at 6 months of age. The clinical course of Type 1 disease is rapid and predictable, with an extremely high burden of illness. Pooling the results of these studies, which have corroborating findings, will provide critical data to assist healthcare providers in making more timely diagnoses, formulating care plans, and aiding clinical researchers in designing interventional trials in Type 1 GM1 gangliosidosis.
5.2. Limitations of national history analyses in ultra-rare diseases
In this literature-based analysis, all publications were single case reports or studies of small case series, and many reported cross-sectional data from only a single timepoint. Therefore, it is important to note that our study reflects the potential bias that is characteristic of these types of meta-analyses. Specifically, publications often sought to report unique clinical findings, potentially biasing the sample to include more severe patients than would otherwise be expected. Another significant limitation of this retrospective study is the lack of detailed information within literature publications. For example, many patients were reported to have developmental regression, but the specific developmental milestones that had been gained or lost were not described. As a result, time-to-event analyses could not be performed on symptom latency (Figure 4) or developmental milestone loss (Figure 5).
Nevertheless, prospective studies in ultra-rare diseases also have inherent limitations, emphasizing the importance of generating comparator data from published literature sources. Specifically, while retrospective analyses may underestimate survival, prospective analyses may overestimate survival because patients are receiving constant clinical care and monitoring from multiple specialists and disease experts. Ultra-rare disease prospective analyses also have sample size limitations because large numbers of patients are difficult to find and recruit during the time of the study. Indeed, while our retrospective analysis includes 154 patients, the previously published prospective analyses are limited to sample sizes of 8 patients or less (Utz 2015, Jarnes Utz 2017, Nestrasil 2018). Finally, prospective studies could experience sampling bias, given that they are often run from single clinical sites, as is the case in the ongoing gangliosidosis natural history studies ( NCT00668187, NCT00029965, and NCT04041102). Given feasibility limitations, prospective studies may be limited to enrolling patients in certain geographic regions and at advanced ages (Jarnes Utz 2017). Such sampling bias could result in a more homogenous patient population, compared with a retrospective study that is able to include patients studied at all ages across the world (See Appendix).
Due to the limitations across study types, it is important to consider both retrospective and prospective natural history data when seeking to robustly characterize an ultra-rare disease like Type 1 GM1 gangliosidosis. Importantly, the data in our study closely corroborates that of previously published prospective analyses (Jarnes Utz 2017), providing confidence in the totality of natural history data gathered in this disease so far.
5.3. Considerations for future research
Due to the limited natural history data in Type 1 disease, this retrospective analysis can be used in conjunction with the previously published prospective natural history studies to serve as a comparator arm for future clinical trials (Utz 2015, Jarnes Utz 2017, Nestrasil 2018). This meta-analysis shows that 96% of patients are alive before 12 months, but that more than half of patients die between 12 and 24 months. Therefore, this age range may represent a period when significant and irreversible declines occur. In the context of rapid disease progression, early and frequent measurement of functional efficacy (i.e. at 3-month intervals) after the administration of an experimental intervention is possible to allow for detection of treatment benefit.
Strikingly, infantile patients are diagnosed quite late in their lifespan at a point where multi-organ system dysfunction is already evident. Slow diagnoses are a serious burden to patient families, causing them to spend substantial time and cost traveling to multiple specialists and to receive delayed access to helpful resources, such as patient advocacy groups. Delayed diagnosis and misdiagnosis may postpone initiation of palliative care (e.g. insertion of a gastric feeding tube and chest physiotherapy), which has been shown to improve quality of life and extend lifespan (Jarnes Utz 2017). Thus, improved public disease awareness and a better understanding of referral pathways are necessary to decrease time to diagnosis. Implementation of newborn screening and increased use of whole exome sequencing provide other avenues to improve diagnostic times (Pierson 2012, Burlina 2017, Bouhouche 2018, Lee 2018, Platt 2018).
6. CONCLUSION
This meta-analysis is the first to utilize retrospective clinical data to characterize the natural history timeline of Type 1 GM1 gangliosidosis. These data provide an improved understanding of disease subtyping; clinically distinguishing features of Type 1 disease include a lack of attainment of later motor milestones, such as crawling, standing, and walking, as well as appearance of significant systemic and neurological symptoms before 18 months of age. In this literature cohort, first symptom onset was often before 3 months of age, first hospital admission and diagnosis were before 9 months of age, and death usually occurred before 24 months. In particular, the 6- to 18-month range represented a period of significant neurodevelopmental regression and multi-organ system deterioration. Many early motor milestones, including head control, transferring objects, and sitting independently, were not present by one year of age.
The use of a retrospective meta-analysis dataset is an effective tool to describe the natural history of rare diseases, particularly those that face significant barriers to conducting robust prospective natural history studies. This analysis in a large global cohort of Type 1 patients (N = 154) provides critical data that both corroborates and adds to the existing prospective natural history dataset (N = 8). Specifically, this retrospective study shows similar timing of diagnosis, death, and symptomology when compared with previously published prospectively-derived data. These findings demonstrate the severe and predictable clinical course of Type 1 GM1 gangliosidosis and the need for earlier diagnosis as well as effective interventions to slow, halt, or prevent the rapid progression and terminal outcomes of patients. Together, prospective and retrospective natural history findings will inform key decisions around preclinical and clinical studies, setting the stage for the first approved treatment for GM1 gangliosidosis in the future.
ACKNOWLEDGEMENTS
We thank Dr. Gavin Corcoran for his useful suggestions during the course of data analysis and manuscript writing.
Financial Support: This study was funded by Axovant and the NIH Intramural Research Program.
7. APPENDIX
Sources used (N = 44) to generate literature cohort.
| Year | Author | Journal |
|---|---|---|
| 2019 | Kilic et al | Metabolic Brain Disease |
| 2018 | Zubaida et al | Journal of Genetics |
| 2018 | Ranjan and Patra | Journal of Pediatric Neurosciences |
| 2018 | Feng et al | Metabolic Brain Disease |
| 2018 | Kamate | Annals of Indian Academy of Neurology |
| 2018 | Sharawat et al | The Journal of Pediatrics |
| 2016 | Kumar Bharwaj and Khera | Indian Pediatrics |
| 2016 | Bersani et al | Journal of the German Society of Dermatology |
| 2015 | Papathemeli et al | JAMA Dermatology |
| 2015 | Vedak et al | Pediatric Dermatology |
| 2015 | Bidchol et al | Gene |
| 2015 | Padhi et al | Seminars in Ophthalmology |
| 2014 | Armstrong-Javors and Chu | Neurology |
| 2014 | Sidhu and Misra | The Journal of Pediatrics |
| 2014 | King et al | Journal of Genetic Counseling |
| 2013 | Abilkassem and Agadr | The Pan African Medical Journal |
| 2013 | Hackbart et al | The Journal of Pediatrics |
| 2013 | Sperb et al | Gene |
| 2012 | Lei et al | World Journal of Pediatrics |
| 2011 | Nada et al | Metabolic Brain Disease |
| 2011 | Dweikat et al | Indian Journal of Dermatology |
| 2011 | Caciotti et al | Biochimica et Biophysica Acta |
| 2010 | Yang et al | Journal of Biomedical Science |
| 2010 | Hofer et al | Clinical Genetics |
| 2009 | Khatiwada and Pokharel | Journal of the Nepal Medical Association |
| 2008 | Brunetti-Pierri et al | Journal of Child Neurology |
| 2007 | Brunetti-Pierri et al | Journal of Inherited Metabolic Disease |
| 2007 | Vattoth et al | The Neuroradiology Journal |
| 2007 | Santamaria et al | Clinical Genetics |
| 2007 | Caciotti et al | Human Mutation |
| 2006 | Erol et al | European Journal of Paediatric Neurology |
| 2006 | Pavlu et al | British Journal of Haematology |
| 2006 | Santamaria et al | Human Mutation |
| 2006 | Bloch et al | Acta Dermato-Venereologica |
| 2006 | Sinigerska et al | Molecular Genetics and Metabolism |
| 2006 | Ashrafi et al | Pediatric Neurology |
| 2005 | Sinelli et al | Acta Paediatrica |
| 2005 | Di Rocco et al | Neuropediatrics |
| 2005 | Georgiou et al | Genetic Testing |
| 2005 | Gururaj et al | Journal of Child Neurology |
| 2004 | Van der Voorn et al | Acta Neuropathologica |
| 2000 | Lin et al | Acta Paediatrica |
| 2000 | Morrone et al | Human Mutation |
| 2000 | Folkerth et al | Pediatric and Developmental Pathology |
Footnotes
Conflict of Interest Statement: PK and AK are employees of Axovant. FL and MH are paid consultants for Axovant. Axovant and NHGRI are collaborators in a clinical trial involving patients with GM1 gangliosidosis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8. REFERENCES
- Abilkassem R and Agadr A. (2013) Extensive Mongolian spot: a clinical sign that deserves attention. Pan Afr Med J. 16, 41 DOI: 10.11604/pamj.2013.16.41.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi MR, et al. (2006) Extensive Mongolian spots: a clinical sign merits special attention. Pediatr Neurol. 34, 143–145. DOI: 10.1016/j.pediatrneurol.2005.07.010 [DOI] [PubMed] [Google Scholar]
- Baiotto C, et al. (2011) Population analysis of the GLB1 gene in South Brazil. Genet Mol Biol. 34, 45–48. DOI: 10.1590/S1415-47572011000100009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani G, et al. (2016) Extensive irregular Mongolian blue spots as a clue for GM1 gangliosidosis type 1. J Dtsch Dermatol Ges. 14, 301–2. DOI: 10.1111/ddg.12755 [DOI] [PubMed] [Google Scholar]
- Bidchol AM, et al. (2015) Recurrent and novel GLB1 mutations in India. Gene. 567, 173–181. DOI: 10.1016/j.gene.2015.04.078 [DOI] [PubMed] [Google Scholar]
- Bley AE, et al. (2011) Natural history of infantile G(M2) gangliosidosis. Pediatrics. 128, e1233–1241. DOI: 10.1542/peds.2011-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhouche A, et al. (2018) Genetic analysis of undiagnosed juvenile GM1-gangliosidosis by microarray and exome sequencing. Case Rep Genet. 863598 DOI: 10.1155/2018/8635698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti-Pierri N and Scaglia F. (2008) GM1 gangliosidosis: review of clinical, molecular, and therapeutic aspects. Mol Genet Metab. 94, 391–396. DOI: 10.1016/j.ymgme.2008.04.012 [DOI] [PubMed] [Google Scholar]
- Burlina AB, et al. (2018) Newborn screening for lysosomal storage disorders by tandem mass spectrometry in North East Italy. J Inherit Metab Dis. 41, 209–219. DOI: 10.1007/s10545-017-0098-3 [DOI] [PubMed] [Google Scholar]
- Caciotti A, et al. (2003) Modulating action of the new polymorphism L436F detected in the GLB1 gene of a type-II GM1 gangliosidosis patient. Hum Genet. 113, 44–50. DOI: 10.1007/s00439-003-0930-8 [DOI] [PubMed] [Google Scholar]
- Caciotti A, et al. (2007) GM1 gangliosidosis: molecular analysis of nine patients and development of an RT-PCR assay for GLB1 gene expression profiling. Hum Mutat. 28, 204 DOI: 10.1002/humu.9475 [DOI] [PubMed] [Google Scholar]
- Caciotti A, et al. (2011) GM1 gangliosidosis and Morquio B disease: an update on genetic alterations and clinical findings. Biochim Biophys Acta. 1812, 782–790. DOI: 10.1016/j.bbadis.2011.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, et al. (1994) Mutations in the lysosomal beta-galactosidase gene that cause the adult form of GM1 gangliosidosis. Am J Hum Genet. 54, 1004–1013. [PMC free article] [PubMed] [Google Scholar]
- Coelho JC, et al. (1997) Selective screening of 10,000 high-risk Brazilian patients for the detection of inborn errors of metabolism. Eur J Pediatr. 156, 650–654. DOI: 10.1007/s004310050685 [DOI] [PubMed] [Google Scholar]
- Feng Y, et al. (2018) Clinical and molecular characteristics of 11 Chinese probands with GM1 gangliosidosis. Metab Brain Dis. 33, 2051–2057. DOI: 10.1007/s11011-018-0315-2 [DOI] [PubMed] [Google Scholar]
- Georgiou T, et al. (2005) The Arg482His mutation in the beta-galactosidase gene is responsible for a high frequency of GM1 gangliosidosis carrier in a Cypriot village. Genet Test. 9, 126–132. DOI: 10.1089/gte.2005.9.126 [DOI] [PubMed] [Google Scholar]
- Hackbart BA, et al. (2013) Mongolian spots are not always a benign sign. J Pediatr. 162, 1070 DOI: 10.1016/j.jpeds.2012.12.044 [DOI] [PubMed] [Google Scholar]
- Hofer D, et al. (2009) GM1 gangliosidosis and Morquio B disease: expression analysis of missense mutations affecting the catalytic site of acid beta-galactosidase. Hum Mutat. 30, 1241–1221. DOI: 10.1002/humu.21031 [DOI] [PubMed] [Google Scholar]
- Hofer D, et al. (2010) Phenotype determining alleles in GM1 gangliosidosis patients bearing novel GLB1 mutations. Clin Genet. 78, 236–246. DOI: 10.1111/j.1399-0004.2010.01379.x [DOI] [PubMed] [Google Scholar]
- Jarnes Utz JR, et al. (2017) Infantile gangliosidoses: Mapping a timeline of clinical changes. Mol Genet Metab. 121, 170–179. DOI: 10.1016/j.ymgme.2017.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye EM, et al. (1997) beta-Galactosidase gene mutations in patients with slowly progressive GM1 gangliosidosis. J Child Neurol. 12, 242–247. DOI: 10.1177/088307389701200404 [DOI] [PubMed] [Google Scholar]
- Lee JS, et al. (2018) Diagnostic challenge for the rare lysosomal storage disease: Late infantile GM1 gangliosidosis. Brain Dev. 40, 383–390. DOI: 10.1016/j.braindev.2018.01.009 [DOI] [PubMed] [Google Scholar]
- Lenicker HM, et al. (1997) Infantile generalized GM1 gangliosidosis: high incidence in the Maltese Islands. J Inherit Metab Dis. 20, 723–724. DOI: 10.1023/a:1005303332529 [DOI] [PubMed] [Google Scholar]
- Nestrasil I, et al. (2018) Distinct progression patterns of brain disease in infantile and juvenile gangliosidoses: Volumetric quantitative MRI study. Mol Genet Metab. 123, 97–104. DOI: 10.1016/j.ymgme.2017.12.432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson TM, et al. (2012) Exome sequencing as a diagnostic tool in a case of undiagnosed juvenile-onset GM1-gangliosidosis. Neurology. 79, 123–126. DOI: 10.1212/WNL.0b013e31825f047a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt FM, et al. (2018) Lysosomal storage diseases. Nat Rev Dis Primers. 4, 27 DOI: 10.1038/s41572-018-0025-4 [DOI] [PubMed] [Google Scholar]
- Regier DS, et al. (2016) MRI/MRS as a surrogate marker for clinical progression in GM1 gangliosidosis. Am J Med Genet A. 170, 634–644. DOI: 10.1002/ajmg.a.37468 [DOI] [PubMed] [Google Scholar]
- Richter JE, et al. (2018) Protein modeling and clinical description of a novel in-frame GLB1 deletion causing GM1 gangliosidosis type II. Mol Genet Genomic Med. 6, 1229–1235. DOI: 10.1002/mgg3.454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santamaria R, et al. (2006) Twenty-one novel mutations in the GLB1 gene identified in a large group of GM1-gangliosidosis and Morquio B patients: possible common origin for the prevalent p.R59H mutation among gypsies. Hum Mutat. 27, 1060 DOI: 10.1002/humu.9451 [DOI] [PubMed] [Google Scholar]
- Santamaria R, et al. (2007) Identification of 14 novel GLB1 mutations, including five deletions, in 19 patients with GM1 gangliosidosis from South America. Clin Genet, 71, 273–279. DOI: 10.1111/j.1399-0004.2007.00767.x [DOI] [PubMed] [Google Scholar]
- Severini MH, et al. (1999) High frequency of type 1 GM1 gangliosidosis in southern Brazil. J Inherit Metab Dis. 20, 723–724. DOI: 10.1034/j.1399-0004.1999.560215.x [DOI] [Google Scholar]
- Silva CM, et al. (1999) Six novel beta-galactosidase gene mutations in Brazilian patients with GM1-gangliosidosis. Hum Mutat. 13, 401–409. DOI: [DOI] [PubMed] [Google Scholar]
- Sinelli MT, et al. (2005) Fetal hydrops in GM(1) gangliosidosis: a case report. Acta Paediatr. 94, 1847–1849. DOI: 10.1111/j.1651-2227.2005.tb01867.x [DOI] [PubMed] [Google Scholar]
- Sinigerska I, et al. (2006) Founder mutation causing infantile GM1-gangliosidosis in the Gypsy population. Mol Genet Metab. 88, 93–95. DOI: 10.1016/j.ymgme.2005.12.009 [DOI] [PubMed] [Google Scholar]
- Sperb F, et al. (2013) Genotypic and phenotypic characterization of Brazilian patients with GM1 gangliosidosis. Gene. 512, 113–116. DOI: 10.1016/j.gene.2012.09.106 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, et al. (1978) GM1-gangliosidosis: accumulation of ganglioside GM1 in cultured skin fibroblasts and correlation with clinical types. Hum Genet. 43, 127–131. DOI: 10.1007/bf00293589 [DOI] [PubMed] [Google Scholar]
- Suzuki Y, et al. (2001) β-galactosidase deficiency (β-galactosidosis) GM1-gangliosidosis and Morquio B disease The Metabolic and Molecular Basis of Inherited Disease, McGraw-Hill, New York. [Google Scholar]
- Utz JR, et al. (2015) Biomarkers of central nervous system inflammation in infantile and juvenile gangliosidoses. Mol Genet Metab. 114, 274–280. DOI: 10.1016/j.ymgme.2014.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann CM, et al. (2015) Systemic AAV9 gene transfer in adult GM1 gangliosidosis mice reduces lysosomal storage in CNS and extends lifespan. Hum Mol Genet. 24, 4353–4364. DOI: 10.1093/hmg/ddv168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, et al. (2010) Three novel beta-galactosidase gene mutations in Han Chinese patients with GM1 gangliosidosis are correlated with disease severity. J Hum Genet. 60, 769–776. DOI: 10.1186/1423-0127-17-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, et al. (1992) GM1 gangliosidosis in adults: clinical and molecular analysis of 16 Japanese patients. Ann Neurol. 31, 328–332. DOI: 10.1002/ana.410310316 [DOI] [PubMed] [Google Scholar]