Abstract
The interaction between endothelial cells and vascular smooth muscle cells (VSMC) plays an important role in regulating cardiovascular homeostasis. Endothelial cells synthesize and release endothelium-derived relaxing factors (EDRFs), including vasodilator prostaglandins, nitric oxide (NO), and endothelium-dependent hyperpolarization (EDH) factors. Importantly, the contribution of EDRFs to endothelium-dependent vasodilatation markedly varies in a vessel size-dependent manner; NO mainly mediates vasodilatation of relatively large vessels, while EDH factors in small resistance vessels. We have previously identified that endothelium-derived hydrogen peroxide (H2O2) is an EDH factor especially in microcirculation. Several lines of evidence indicate the importance of the physiological balance between NO and H2O2/EDH factor. Rho-kinase was identified as the effectors of the small GTP-binding protein, RhoA. Both endothelial NO production and NO-mediated signaling in VSMC are targets and effectors of the RhoA/Rho-kinase pathway. In endothelial cells, the RhoA/Rho-kinase pathway negatively regulates NO production. On the contrary, the pathway enhances VSMC contraction with resultant occurrence of coronary artery spasm and promotes the development of oxidative stress and vascular remodeling. In this review, I will briefly summarize the current knowledge on the regulatory roles of endothelium-derived relaxing factors, with special references to NO and H2O2/EDH factor, in relation to Rho-kinase, in cardiovascular health and disease.
Keywords: endothelium, endothelium-derived relaxing factors, nitric oxide, hydrogen peroxide, Rho-kinase
Introduction
The endothelium plays important roles in modulating the tonus of underlying vascular smooth muscle cells (VSMC) by synthesizing and releasing endothelium-derived relaxing factors (EDRFs), including vasodilator prostaglandins (e.g., prostacyclin), nitric oxide (NO), and endothelium-dependent hyperpolarization (EDH) factors (Fig. 1).(1,2) Since the discovery of endothelium-dependent hyperpolarization in 1988,(3,4) several candidates have been proposed as the nature of EDH factors. Importantly, the contribution of EDRFs to endothelium-dependent vasodilatations markedly varies as a function of vessel size; endothelium-derived NO mainly mediates vasodilatation of relatively large, conduit vessels, while EDH factors that of resistance arteries (Fig. 2).(1,5) This vessel-size-dependent contribution of NO and EDH factors is well preserved among species, from rodents to humans, in order to maintain a physiological balance between them.(1,2) Endothelial dysfunction is characterized by impaired production and/or action of EDRFs, reflecting the hallmark and potential predictor for atherosclerotic cardiovascular diseases.(2) Various risk factors (e.g., smoking, diabetes mellitus, hypertension, and dyslipidemia) cause endothelial dysfunction, initiating the step toward atherosclerotic cardiovascular diseases.(1,2)
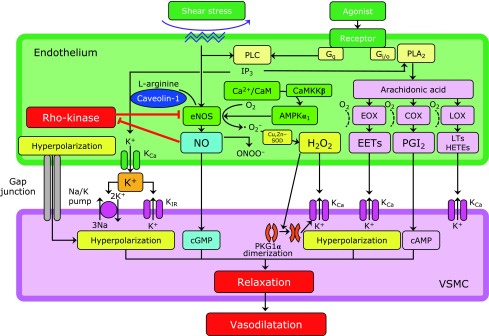
Fig. 1.
Endothelium-derived relaxing factors. Endothelial cells synthesize and release nitric oxide and endothelium-dependent hyperpolarization (EDH) factors. Endothelium-derived H2O2 is one of the major EDH factors. AMPKα1, α1-subunit of AMP-activated protein kinase; CaM, calmodulin; CaMKKβ, Ca2+/CaM-dependent protein kinase β; cAMP, cyclic AMP; cGMP, cyclic GMP; COX, cyclooxygenase; EETs, epoxyeicosatrienoic acids; eNOS, endothelial NO synthase; EOX, epoxygenase; HETEs, hydroxyeicosatetraenoic acids; H2O2, hydrogen peroxide; IP3, inositol trisphosphate; KCa, calcium-activated potassium channel; KIR, inwardly rectifying potassium channel; LOX, lipoxygenase; LTs, leukotrienes; NO, nitric oxide; ONOO−, peroxynitrite; PGI2, prostacyclin; PKG1α, 1α-subunit of protein kinase G; PLA2, phospholipase A2; PLC, phospholipase C; SOD, superoxide dismutase.
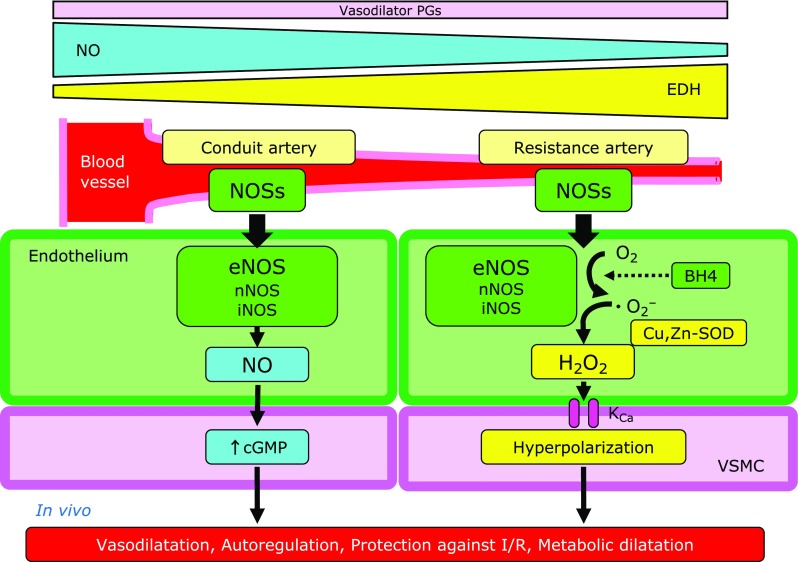
Fig. 2.
Vessel size-dependent roles of endothelial nitric oxide synthases system. The contribution of EDRFs to endothelium-dependent vasodilatations markedly varies as a function of vessel size; endothelium-derived NO mainly mediates vasodilatation of relatively large, conduit vessels, while EDH factors that of resistance arteries. BH4, tetrahydrobiopterin; cGMP, cyclic GMP; Cu,Zn-SOD, copper-zinc superoxide dismutase; eNOS, endothelial nitric oxide synthase; H2O2, hydrogen peroxide; iNOS, inducible NOS; I/R, ischemia-reperfusion; KCa, calcium-activated potassium channel; nNOS, neuronal NOS; NO, nitric oxide; NOSs, nitric oxide synthases; VSMC, vascular smooth muscle cells.
Rho-kinases (Rho-kinase α/ROKα/ROCK2 and Rho-kinase β/ROKβ/ROCK1) were identified as the effector of the small GTP-binding protein, RhoA, independently by 3 research groups in 1996.(6–8) Hereafter, both Rho-kinase α/ROKα/ROCK2 and Rho-kinase β/ROKβ/ ROCK1 are collectively referred to as Rho-kinase.(1) Accumulating evidence indicates that Rho-kinase plays important roles in the pathogenesis of oxidative stress and cardiovascular diseases.(1,9,10)
In this review, I will briefly summarize the current knowledge on the two endothelium-derived relaxing factors, NO and H2O2/EDH factor, and Rho-kinase in cardiovascular health and disease.
Physiological Balance between NO and EDH
EDH factors cause hyperpolarization and subsequent relaxation of underlying VSMC with resultant vasodilatation of small resistance vessels, finely tuning blood pressure and tissue perfusion instantaneously in response to diverse physiological demands.(1,2) As mentioned above, endothelium-derived NO and EDH factors share the important roles in modulating vascular tonus in a distinct vessel size-dependent manner (Fig. 2).(1,5) In this scope, vasodilator prostaglandins play a small but constant role, independent of vessel size in general. In contrast, NO predominantly regulates the tonus of relatively large conduit vessels (e.g., aorta and epicardial coronary arteries), while the importance of EDH factors increases as vessel size decreases (e.g., small mesenteric arteries and coronary microvessels).(1,5) Thus, EDH-mediated vasodilatation is especially important in microcirculation, where blood pressure and tissue perfusion are critically determined. Moreover, such redundant mechanisms in endothelium-dependent vasodilatations are advantageous for maintaining cardiovascular homeostasis with compensatory interactions.(11–14) Indeed, in various pathological conditions with atherosclerotic risk factors, NO-mediated relaxations are easily impaired, where EDH-mediated responses are fairly preserved or even enhanced to serve as a compensatory vasodilator system.(11–14) Multiple mechanisms appear to be involved in the enhanced EDH-mediated responses in small resistance vessels as discussed later.(15)
Endothelium-derived H2O2 as an EDH Factor
Identification of endothelium-derived H2O2 as an EDH factor
Several EDH factors appear to exist depending on the vascular bed, vessel size, and species studied, including epoxyeicosatrienoic acids, metabolites of arachidonic P450 epoxygenase pathway,(16,17) electrical communication through gap junctions,(18) K+ ions,(19) and as we demonstrated in 2000, endothelium-derived hydrogen peroxide (H2O2) (Fig. 1).(20,21) Indeed, endothelium-derived H2O2 at physiologically low concentrations is one of the major EDH factors in mouse and human small mesenteric arteries and human, porcine, and canine coronary arteries.(20–27) Thus, endothelium-derived H2O2 attracts increasing attention in view of its emerging relevance for cardiovascular disease.(1,2,20–29) In the clinical settings, it has been repeatedly reported that chronic nitrate therapy has neutral or even harmful effects in patients with cardiovascular diseases and that antioxidant treatments are also ineffective to prevent cardiovascular events.(30–33) These lines of evidence indicate the importance of the physiological balance between NO and EDH factors in maintaining cardiovascular homeostasis and in curing diseases associated with endothelial dysfunction.(1,11)
H2O2 is an important physiological signaling molecule serving especially in microcirculation, for blood pressure, coronary microcirculation, and metabolic functions.(25–27,34–36) Reactive oxygen species (ROS) have been considered to be harmful in general because of their highly-damaging effects on cells and tissues and pathological implications in various cardiovascular diseases, including atherosclerosis, hypertension, heart failure, and coronary artery disease.(34–37) However, as exemplified by endothelium-derived H2O2/EDH factor, a growing evidence has demonstrated that physiological levels of ROS can serve as crucial signaling molecules in health and disease.(38) The following 4 sets of early observations led us to hypothesize that a putative EDH factor might be a non-NO vasodilator substance (likely ROS) derived from endothelial NO synthases (NOSs) system. First, both NO-mediated and EDH-mediated responses are susceptible to vascular injuries caused by various atherosclerotic factors, and conversely, the treatment of those risk factors can restore both responses.(1,2,39) Second, it was previously demonstrated that endothelium-derived free radicals exert relaxing or contracting effects in an endothelium-dependent manner in canine coronary arteries.(40) Third, both endothelial NOS (eNOS)-derived NO generation and EDH-mediated responses are dependent on calcium/calmodulin.(41) Fourth, a simple molecule (like NO) rather than complex substances may be favorable in instantaneously modulating vascular tone in response to physiological demands in the body. Finally, in 2000, we were able to demonstrate for the first time that eNOS-derived H2O2 is an EDH factor in mouse mesenteric arteries, using eNOS-knockout (KO) mice.(20) Subsequently, this was also confirmed in other blood vessels, including human mesenteric and coronary arteries, porcine and canine coronary arteries, and piglet pial arterioles.(21–29,39,42,43)
Endothelial source of H2O2/EDH factor
Endothelium-derived H2O2 could be generated by the dismutation of superoxide anions, which are derived from various sources in the endothelium, including eNOS, NADPH oxidase, mitochondrial electron transport chain, xanthine oxidase, and lipoxygenase (Fig. 1).(44) There are 3 NOS isoforms; eNOS (NOS3), neuronal NOS (nNOS, NOS1), and inducible NOS (iNOS, NOS2). Using singly-eNOS-KO, doubly-n/eNOS-KO, and triply-n/i/eNOS-KO mice, we have previously demonstrated that EDH-mediated relaxations are progressively reduced as the number of deleted NOS genes increased.(45) Collectively, these results indicate that the 3 NOSs isoforms compensate each other to maintain H2O2-mediated EDH-type relaxations (Fig. 2). Thus, in large conduit vessels, NOSs mainly serve as a NO-generating system to cause soluble guanylate cyclase (sGC)-cyclic guanosine monophosphate (cGMP)-mediated vasodilatation, whereas in small resistance vessels, they act as a superoxide-generating system to evoke H2O2/EDH factor-mediated responses (Fig. 2).(45–47) In addition, among superoxide dismutase (SOD) isoforms, Cu,Zn-SOD plays a key role in the synthesis of H2O2/EDH factor in the endothelium (Fig. 1).(48) Indeed, eNOS produces superoxide anions under physiological conditions when synthesizing NO from l-arginine and oxygen, while Cu,Zn-SOD dismutates those superoxide anions into H2O2. Moreover, Cu,Zn-SOD-KO mice show markedly impaired EDH-mediated hyperpolarizations and relaxations in mesenteric arteries and coronary circulation without VSMC dysfunction.(48) Importantly, superoxide anions relevant to H2O2/EDH factor are not derived from pathologically uncoupled eNOS because H2O2-mediated EDH-type responses are not suppressed by NOS inhibitors and upregulation of eNOS co-factor tetrahydrobiopterin had no effects on the responses.(44) Other sources of superoxide anions in H2O2-mediated vasodilatation, such as mitochondrial respiratory chain and NADPH oxidase, have also been identified in human coronary arterioles.(49,50)
A recent study has demonstrated the potential regulatory mechanisms underlying the physiologically relevant H2O2 in the endothelium.(51,52) Indeed, local subcellular concentrations at microdomains rather than net cellular concentrations may be critical to determine whether the effects of ROS can be detrimental or beneficial for cellular signaling and co-localization of the source and target of H2O2 may help to avoid non-specific harmful oxidations.(51,52) One good example of this notion is that only a minor increase in ROS caused by caveolar localization of NADPH oxidase-1 in hypertension is enough to interfere with NO-mediated signaling.(53)
Mode of action of H2O2/EDH factor
Oxidative modification of cGMP-dependent protein kinase G (PKG) is a central mechanism by which H2O2 induces hyperpolarization and relaxation of underlying VSMC,(43,54) although other modes of action of H2O2/EDH factor have also been proposed (Fig. 1).(55) Briefly, H2O2 induces dimerization of 1α-isoforms of PKG (PKG1α) through an interprotein disulfide bond formation between them to enhance the kinase activity through phosphorylation. The activated PKG1α subsequently stimulates K+ channels with resultant hyperpolarization and vasodilatation in mouse mesenteric arteries and human coronary arterioles.(43,54,56) H2O2 also promotes the translocation of PKG1α from cytoplasm to membrane in human and porcine coronary arteries.(54,57) Such reversible post-translational modification, like phosphorylation, may be favorable for the fine control of vascular tone in response to demand fluctuation in vivo.(58)
Mechanisms for the dominant role of H2O2/EDH factor in microcirculation
Accumulating evidence has provided the mechanistic insights into vessel size-dependent contribution of NO and H2O2/EDH factor (Fig. 3). Previous studies have shown that pretreatment with NO donors attenuates EDH-mediated vasodilatation in porcine coronary arteries in vitro and canine coronary microcirculation in vivo and that NO exerts a negative-feedback effect on endothelium-dependent vasodilatation through cGMP-mediated desensitization in canine coronary arteries ex vivo.(59–61) Multiple mechanisms have been proposed for the dominant role of H2O2/EDH factor in microcirculation (Fig. 3). Among them, cGMP-dependent activation of PKG desensitizes VSMC to H2O2 by inhibiting H2O2-induced PKG1α dimerization, a central mechanism of H2O2/EDH factor-mediated vasodilatation, and in turn, pharmacological inhibition of sGC sensitizes conduit vessels, but not resistance vessels, to H2O2-induced vasodilatation in mice.(62) Furthermore, mouse resistance vessels have less NO production and less antioxidant capacity, predisposing PKG1α to be more sensitive to H2O2-induced activation.(62,63) Other key players for the dominant role of H2O2/EDH factor in resistance vessels include endothelial caveolin-1 (a negative regulator of eNOS) and α1-subunit of endothelial AMP-activated protein kinase (Fig. 3).(63,64) In contrast, phosphorylation at Tyr657 of eNOS in response to H2O2 leads to reduction in eNOS activity with resultant reduced NO production.(65) Taken together, these mechanisms are in line with the widely accepted notion that EDH-mediated responses function as a compensatory vasodilator system when NO-mediated relaxations are compromised.(1,2,11) It is important to maintain the vessel size-dependent contribution of NO and EDH factors because excessive endothelial NO production by either caveolin-1 deficiency or eNOS overexpression disrupts the physiological balance between NO and H2O2/EDH factors in endothelium-dependent vasodilatation, resulting in impaired cardiovascular homeostasis associated with enhanced nitrative stress in mice in vivo.(11,63,66)
Fig. 3.
Molecular mechanisms of enhanced H2O2/EDH factor-mediated responses in microvesseles. Multiple mechanisms are involved in the enhanced EDH-mediated responses in microvessels. AMPKα1, α1-subunit of AMP-activated protein kinase; CaM, calmodulin; CaMKKβ, Ca2+/CaM-dependent protein kinase β; CaMK2, Ca2+/CaM dependent protein kinase II; cGMP, cyclic GMP; Cu,Zn-SOD, copper-zinc superoxide dismutase; EDH, endothelium-dependent hyperpolarization; H2O2, hydrogen peroxide; IP3, inositol trisphosphate; I/R, ischemia-reperfusion; KCa, calcium-activated potassium channel; NO, nitric oxide; NOSs, NO synthases; P, phosphorylation; PKG1α, 1α-subunit of protein kinase G; PLC, phospholipase C; sGC, soluble guanylate cyclase; TRPV4, transient receptor potential vanilloid 4, VSMC; vascular smooth muscle cells.
Clinical significance of H2O2/EDH factor
Endothelium-derived H2O2 plays an important role in blood pressure regulation. Pharmacological inhibition of catalase, which decomposes H2O2 into O2 and H2O2, decreases arterial blood pressure associated with enhanced PKG1α dimerization in vivo.(57) Moreover, the ‛redox-dead’ knock-in mice of Cys42Ser PKG1α, whose mutant PKG1α is unable to be activated by H2O2-induced dimerization due to the deletion in its redox-sensitive sulfur, exhibit markedly impaired EDH-mediated hyperpolarization and relaxation in resistance arteries associated with systemic arterial hypertension.(35) Furthermore, H2O2 has potent vasodilator properties in coronary resistance vessels and plays important roles in coronary autoregulation,(25) cardioprotection against myocardial ischemia/reperfusion injury,(26) and tachycardia-induced metabolic coronary vasodilatations(27) in dogs in vivo. Since coronary vascular resistance is mainly determined by the prearterioles and arterioles,(67) where the effect of EDH-mediated relaxations outweigh that of NO-mediated ones, it is important to maintain the vessel size-dependent contribution of NO and EDH factors for the treatment of coronary artery disease (CAD). Thus, endothelium-derived H2O2 functions as an important endogenous second messenger at its physiological low concentrations to elicit EDH-meditated vasodilatations and to maintain vascular homeostasis in the coronary circulation.(1,11,21,39,46,47)
Clinical Implications for Endothelial Functions (H2O2/EDH)
Endothelial function tests
Assessment of endothelial functions has been acknowledged as a useful surrogate marker of cardiovascular events in many clinical settings, although it is challenging to accurately assess EDH-mediated responses, especially in humans in vivo, because the contribution of EDH factors could be determined only after the blockade of both vasodilator prostaglandins and NO by its definition.(1,2) EDH-mediated vasodilatation can be enhanced to compensate for impaired NO-mediated responses in the early stage of atherosclerotic conditions.(13,21) However, after prolonged exposure to atherosclerotic risk factors, this compensatory role of EDH-mediated responses is finally disrupted to cause metabolic disturbance.(68) Indeed, endothelial dysfunction, as reflected by impaired flow-mediated dilatation (FMD) of the brachial artery or digital reactive hyperemia index (RHI) in peripheral arterial tonometry, is associated with future cardiovascular events in patients with coronary artery disease and one SD decrease in FMD or RHI is associated with doubling of cardiovascular event risk.(69)
H2O2/EDH factor and coronary artery disease
Previous studies focused structural and functional abnormalities of “epicardial” coronary arteries in CAD patients because they are easily visible on coronary angiography and amenable to procedural intervention (e.g., percutaneous coronary intervention). However, those of coronary microvasculature, referred to as coronary microvascular dysfunction (CMD), have recently attracted increasing attention due to their unexpectedly high prevalence and significant prognostic impacts in this population.(70) The etiologies of CMD still remain largely unknown and may be heterogeneous, for which several structural (e.g., vascular remodeling, vascular rarefaction, and extramural compression) and functional abnormalities (e.g., endothelial dysfunction, VSMC dysfunction, and microvascular spasm) have been demonstrated.(67,71) Given that H2O2 has potent vasodilator properties in coronary resistance vessels where EDH factors play relatively dominant roles than NO, it is highly possible that impaired H2O2/EDH factor-mediated vasodilatation is involved in the pathogenesis of CMD. Indeed, in eNOS-KO mice, CMD caused by reduced H2O2/EDH factor is substantially involved in the pathogenesis of cardiac diastolic dysfunction.(66) Thus, for the treatment of CAD, it is essential to maintain the physiological balance between NO and H2O2/EDH factor, which notion is supported by the fact that significant negative interactions exist between NO and several EDH factors and that nitrates as NO donors are not beneficial for the treatment of CMD.(11,63,66)
Lessons from clinical trials targeting NO: it is a time to change our mind
Although the role of CMD has been implicated in patients with obstructive CAD who underwent successful revascularization,(72) the effects of isosorbide-5-mononitrate were unexpectedly neutral in patients with microvascular ischemia despite successful percutaneous coronary intervention.(33) Besides CAD, recent studies highlighted the high prevalence and pathophysiological relevance of CMD in patients with heart failure with preserved ejection fraction (HFpEF).(73–75) Contrary to the premise that enhancing NO-mediated vasodilatation should exert beneficial effects on patients with HFpEF, the results of systemic and long-term administrations of inorganic nitrite in those patients were disappointing or even harmful in randomized clinical trials.(76) In a recent animal study, genetic ablation of endothelial arginase-1, an inhibitor of NO production, did not improve vasomotor function of resistance arteries in diabetic mice.(77) Similarly, antioxidant therapies for patients with cardiovascular diseases had no benefits.(78) These lines of evidence indicate that we need to change our mind to avoid excessive NO supplementation and to pay more attention to the potential harmful effects of non-specific elimination of ROS by antioxidants.(79,80) Multiple mechanisms may be involved in the failure of antioxidant therapies, including inadequate dose, short treatment duration, and pro-oxidant effects of antioxidants upon supplementation and thus so-called “antioxidant paradox” in clinical trials requires further investigations.(81) An alternative explanation for such “paradox” of NO-targeted therapy may be nitrosative stress induced by an excessive amount of NO,(63,81) again suggesting the importance of physiological balance between NO and EDH factors in endothelium-dependent vasodilatation. Further research is warranted to address how to modulate CMD to improve clinical outcomes of patients with cardiovascular diseases.
Roles of Rho-kinase in the Cardiovascular System
Molecular regulation of Rho-kinase
Rho-kinase (ROCKs) is an important downstream effector of the small GTP-binding protein RhoA (Fig. 4). During the past 20 years, significant progress has been made regarding the molecular mechanisms and therapeutic importance of Rho-kinase in cardiovascular medicine.(1,82–87) The Rho family of small G proteins includes 20 members of ubiquitously expressed proteins, including RhoA, Rac1, and Cdc42.(1,82–87) Among them, RhoA acts as a molecular switch that cycles between an inactive GDP-bound and an active GTP-bound conformation interacting with downstream targets (Fig. 4). RhoA is activated by the guanine nucleotide exchange factors (GEFs) that catalyze exchange of GDP for GTP and is inactivated by the GTPase activating proteins (GAPs).(88) There are 2 isoforms of Rho-kinase, Rho-kinase α/ROCK α/ROCK2 and Rho-kinase β/ROCK β/ROCK1, which were identified as the effector of Rho and have been shown to play important roles in the pathogenesis of cardiovascular diseases.(1,9,10) Phosphorylation of myosin light chain (MLC) is crucial for VSMC contraction. MLC is phosphorylated by Ca2+/calmodulin-activated MLC kinase (MLCK) and is dephosphorylated by MLC phosphatase (MLCP) (Fig. 4).(89) Agonists bind to G-protein–coupled receptors and induce contraction by increasing both cytosolic Ca2+ concentration and ROCK activity through mediating GEF.(88–92) The substrates of ROCK include MLC, myosin phosphatase target subunit (MYPT)-1, ezrin/radixin/moesin family, adducin, phosphatase and tensin homolog, eNOS, Tau, and LIM-kinases (Fig. 4).(88–92) Recently, functional differences between ROCK1 and ROCK2 have been reported in vitro. ROCK1 is specifically cleaved by caspase-3, whereas granzyme B cleaves ROCK2.(93,94)
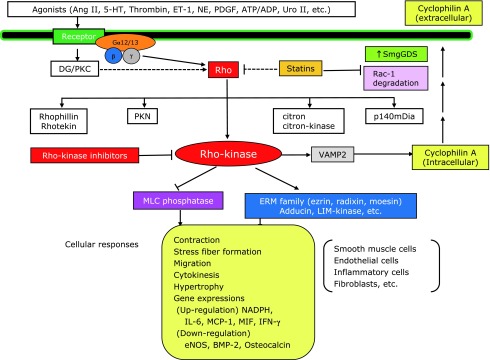
Fig. 4.
Role of Rho/Rho-kinase pathway in the pathogenesis of cardiovascular diseases. Rho/Rho-kinase–mediated pathway plays an important role in the signal transduction initiated by many agonists, including angiotensin II (Ang II), serotonin (5-HT), thrombin, endothelin-1 (ET-1), norepinephrine (NE), platelet-derived growth factor (PDGF), adenosine triphosphate (ATP)/adenosine diphosphate (ADP), and urotensin II (Uro II). Through the modulation of its target effectors, Rho-kinase is substantially involved in vascular smooth muscle contraction (via inhibition of myosin phosphatase) and in the pathogenesis of arteriosclerosis (via activation of ERM, adducin, and other effectors). Whereas statins inhibit Rho at their relatively higher concentrations, they simultaneously inhibit pathways mediated by other G proteins, such as Ras and Rac. By contrast, Rho-kinase inhibitors selectively inhibit Rho-kinase pathway. Solid line indicates proven pathway and dashed line proposed pathway. DG, diacylglycerol; MLC, myosin light chain; PKC, protein kinase C; SmgGDS, small GTP-binding protein dissociation stimulator.
Negative interactions between NO and Rho-kinase
The RhoA/Rho-kinase pathway negatively regulates NO production in endothelial cells, while it enhances contraction of VSMC by MLC phosphorylation through inhibition of MYPT-1 of MLCP and promotes VSMC contraction (Fig. 4).(82–87) Rho-kinase has opposing activities in the regulation of the endothelial barrier function at the cell margins and contractile F-actin stress fibers.(95) Thus, disruption of the endothelial barrier results in increased endothelial permeability, promoting organ damage in various diseases.(1,86,87) The RhoA/Rho-kinase signaling pathway is involved in the mechano-transduction mechanism for the adherence junction strengthening at endothelial contacts.(96) This endothelial mechanosensing is important for endothelial alignment along the flow direction, which contributes to vascular homeostasis. Indeed, a disturbed flow promotes endothelial dysfunction and the development of atherosclerosis.(97,98) Several studies demonstrated that NO and Rho-kinase have opposing effects.(99,100) Rho-kinase-KO mice showed preserved endothelial functions in a diabetic model.(100) Moreover, NO and Rho-kinase exert opposing effects on the phosphorylation of AMP-activated protein kinase in lipid metabolism and the insulin receptor substrate-1 in insulin signaling.(101–103) Statins upregulate eNOS by cholesterol-independent mechanisms, involving the inhibition of Rho geranyl-geranylation and hydroxyfasudil reversed hypoxia-induced upregulation of Rho-kinase and eNOS downregulation in human endothelial cells.(104,105) In addition, small GTP-binding protein dissociation stimulator (SmgGDS) plays a central role in the pleiotropic effects of statins, independently of the Rho-kinase pathway, through Rac1 degradation (Fig. 4).(106) Thus, we need to consider the complex interactions between Rho-kinase and NO signaling for vascular homeostasis in vivo.
Role of Rho-kinase on vascular reactive oxygen species
The balance between oxidants and antioxidants maintains redox status equilibrium in the cardiovascular system.(107) The RhoA/Rho-kinase pathway is one of the major intracellular pathways that enhance the expressions of molecules for oxidative stress (NADPH, IL-6, MCP-1, MIF, and IFN-γ), thrombosis (PAI-1 and tissue factor), and tissue fibrosis (TGF-β1 and Bcl-2), while the pathway also markedly downregulates eNOS and osteogenesis-related molecules (BMP-2 and osteocalcin) (Fig. 1 and 4).(85–87,105,108–112) Oxidative stress by excessive ROS damages mitochondrial proteins and further increase intracellular ROS, thus forming a vicious cycle of ROS augmentation. In addition to ROS generation in mitochondria, several enzymes also generate intracellular ROS, including NADPH that produce O2− and H2O2. Importantly, enhanced Rho-kinase activity downregulates eNOS, resulting in impaired endothelial responses to NO and EDH (Fig. 1 and 4).(1,86,87) eNOS produces NO with resultant production of cGMP, and NO can react with O2− to produce peroxynitrite (ONOO−).(113) Among ROS, H2O2 can easily penetrate the cell membrane and act as a second messenger. Peroxiredoxin is regenerated by the antioxidant protein thioredoxin 1 and reduces H2O2 levels, thus balancing the intracellular redox state.(114) The details of the interactions between Rho-kinase and NO/H2O2 as an EDH factor remain to be fully elucidated in future studies.
Conclusions
This review highlights the potential importance of the physiological balance between NO and H2O2/EDH factor in a distinct vessel size-dependent manner through the diverse functions of endothelial NOSs system in maintaining cardiovascular homeostasis. It remains an open question how to improve endothelial functions without affecting the delicate balance between NO and EDH factors. Further characterization and better understanding of endothelium-dependent vasodilatations are important to this end, which helps us develop novel therapeutic strategies in cardiovascular medicine. The identification of Rho-kinase as an important mediator of oxidative stress in cardiovascular health and disease provides insight into the development of new therapies. Indeed, accumulating evidence indicates that Rho-kinase is substantially involved in the pathogenesis of a wide variety of cardiovascular diseases, suggesting that it is an important therapeutic target in cardiovascular medicine.
Acknowledgments
This work was supported in part by the grants-in-aid for scientific research from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Tokyo, Japan, and the grants-in-aid for scientific research from the Japanese Ministry of Health, Labour, and Welfare, Tokyo, Japan. I am deeply grateful to the collaborators at the Mayo Clinic, Kyushu University, and Tohoku University, especially Prof. Paul M. Vanhoutte and Prof. Akira Takeshita for their life-long supports for me. Finally, I would like to greatly appreciate the Society for Free Radical Research JAPAN for giving me a chance for this invited review article to memorize the 2019 Award of the Society.
Abbreviations
- BMP-2
bone morphogenetic protein-2
- cGMP
cyclic guanosine monophosphate
- CMD
coronary microvascular dysfunction
- EDH
endothelium-dependent hyperpolarization
- EDRF(s)
endothelium-derived relaxing factor(s)
- eNOS
endothelial nitric oxide synthase
- GAPs
GTPase activating proteins
- GEFs
guanine nucleotide exchange factors
- H2O2
hydrogen peroxide
- MCP-1
macrophage chemoattractant protein-1
- MIF
macrophage inhibitory factor
- MLC
myosin light chain
- MLCK
myosin light chain kinase
- MLCP
myosin light chain phosphatase
- MYPT-1
myosin phosphatase target subunit-1
- NADPH
nicotinamide adenine dinucleotide phosphate
- NO
nitric oxide
- NOS
nitric oxide synthase
- PAI-1
plasminogen activator inhibitor-1
- PKG
cGMP-dependent protein kinase
- ROS
reactive oxygen species
- sGC
soluble guanylate cyclase
- SmgGDS
small GTP-binding protein dissociation stimulator
- SOD
superoxide dismutase
- TGF-β1
transforming growth factor-β1
- VSMC
vascular smooth muscle cell
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Shimokawa H. 2014 Williams Harvey Lecture: importance of coronary vasomotion abnormalities—from bench to bedside. Eur Heart J 2014; 35: 3180–3193. [DOI] [PubMed] [Google Scholar]
- 2.Vanhoutte PM, Shimokawa H, Feletou M, Tang EH. Endothelial dysfunction and vascular disease—a 30th anniversary update. Acta Physiol (Oxf) 2017; 219: 22–96. [DOI] [PubMed] [Google Scholar]
- 3.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. Br J Pharmacol 1988; 93: 515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen G, Suzuki H, Weston AH. Acetylcholine releases endothelium-derived hyperpolarizing factor and EDRF from rat blood vessels. Br J Pharmacol 1988; 95: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shimokawa H, Yasutake H, Fujii K, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol 1996; 28: 703–711. [DOI] [PubMed] [Google Scholar]
- 6.Matsui T, Amano M, Yamamoto T, et al. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for small GTP binding protein Rho. EMBO J 1996; 15: 2208–2216. [PMC free article] [PubMed] [Google Scholar]
- 7.Leung T, Chen XQ, Manser E, Lim L. The p160 RhoA-binding kinase ROK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol 1996; 16: 5313–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizaki T, Maekawa M, Fujisawa K, et al. The small GTP-binding protein Rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO J 1996; 15: 1885–1893. [PMC free article] [PubMed] [Google Scholar]
- 9.Kimura K, Ito M, Amano M, et al. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase). Science 1996; 273: 245–248. [DOI] [PubMed] [Google Scholar]
- 10.Amano M, Ito M, Kimura K, et al. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J Biol Chem 1996; 271: 20246–20249. [DOI] [PubMed] [Google Scholar]
- 11.Godo S, Sawada A, Saito H, et al. Disruption of physiological balance between nitric oxide and endothelium-dependent hyperpolarization impairs cardiovascular homeostasis in mice. Arterioscler Thromb Vasc Biol 2016; 36: 97–107. [DOI] [PubMed] [Google Scholar]
- 12.Urakami-Harasawa L, Shimokawa H, Nakashima M, Egashira K, Takeshita A. Importance of endothelium-derived hyperpolarizing factor in human arteries. J Clin Invest 1997; 100: 2793–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Félétou M, Vanhoutte PM. EDHF: an update. Clin Sci (Lond) 2009; 117: 139–155. [DOI] [PubMed] [Google Scholar]
- 14.Ozkor MA, Murrow JR, Rahman AM, et al. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation 2011; 123: 2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohashi J, Sawada A, Nakajima S, Noda K, Takaki A, Shimokawa H. Mechanisms for enhanced endothelium-derived hyperpolarizing factor-mediated responses in microvessels in mice. Circ J 2012; 76: 1768–1779. [DOI] [PubMed] [Google Scholar]
- 16.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res 1996; 78: 415–423. [DOI] [PubMed] [Google Scholar]
- 17.Fisslthaler B, Popp R, Kiss L, et al. Cytochrome P450 2C is an EDHF synthase in coronary arteries. Nature 1999; 401: 493–497. [DOI] [PubMed] [Google Scholar]
- 18.Griffith TM, Chaytor AT, Edwards DH. The obligatory link: role of gap junctional communication in endothelium-dependent smooth muscle hyperpolarization. Pharmacol Res 2004; 49: 551–564. [DOI] [PubMed] [Google Scholar]
- 19.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature 1998; 396: 269–272. [DOI] [PubMed] [Google Scholar]
- 20.Matoba T, Shimokawa H, Nakashima M, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest 2000; 106: 1521–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch 2010; 459: 915–922. [DOI] [PubMed] [Google Scholar]
- 22.Matoba T, Shimokawa H, Kubota H, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun 2002; 290: 909–913. [DOI] [PubMed] [Google Scholar]
- 23.Miura H, Bosnjak JJ, Ning G, Saito T, Miura M, Gutterman DD. Role for hydrogen peroxide in flow-induced dilation of human coronary arterioles. Circ Res 2003; 92: e31–e40. [DOI] [PubMed] [Google Scholar]
- 24.Matoba T, Shimokawa H, Morikawa K, et al. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol 2003; 23: 1224–1230. [DOI] [PubMed] [Google Scholar]
- 25.Yada T, Shimokawa H, Hiramatsu O, et al. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation 2003; 107: 1040–1045. [DOI] [PubMed] [Google Scholar]
- 26.Yada T, Shimokawa H, Hiramatsu O, et al. Cardioprotective role of endogenous hydrogen peroxide during ischemia-reperfusion injury in canine coronary microcirculation in vivo. Am J Physiol 2006; 291: H1138–H1146. [DOI] [PubMed] [Google Scholar]
- 27.Yada T, Shimokawa H, Hiramatsu O, et al. Important role of endogenous hydrogen peroxide in pacing-induced metabolic coronary vasodilation in dogs in vivo. J Am Coll Cardiol 2007; 50: 1272–1278. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Bubolz AH, Mendoza S, Zhang DX, Gutterman DD. H2O2 is the transferrable factor mediating flow-induced dilation in human coronary arterioles. Circ Res 2011; 108: 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Félétou M. The Endothelium: Part 2: EDHF-Mediated Responses “The Classical Pathway”. San Rafael, CA: Morgan & Claypool Life Sciences Publisher, 2011. [PubMed] [Google Scholar]
- 30.Russo G, Di Franco A, Lamendola P, et al. Lack of effect of nitrates on exercise stress test results in patients with microvascular angina. Cardiovasc Drugs Ther 2013; 27: 229–234. [DOI] [PubMed] [Google Scholar]
- 31.Redfield MM, Anstrom KJ, Levine JA, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med 2015; 373: 2314–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi J, Nihei T, Takagi Y, et al. Prognostic impact of chronic nitrate therapy in patients with vasospastic angina: multicentre registry study of the Japanese coronary spasm association. Eur Heart J 2015; 36: 228–237. [DOI] [PubMed] [Google Scholar]
- 33.Golino M, Spera FR, Manfredonia L, et al. Microvascular ischemia in patients with successful percutaneous coronary intervention: effects of ranolazine and isosorbide-5-mononitrate. Eur Rev Med Pharmacol Sci 2018; 22: 6545–6550. [DOI] [PubMed] [Google Scholar]
- 34.Vanhoutte PM. Endothelium-derived free radicals: for worse and for better. J Clin Invest 2001; 107: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med 2012; 18: 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakajima S, Ohashi J, Sawada A, Noda K, Fukumoto Y, Shimokawa H. Essential role of bone marrow for microvascular endothelial and metabolic functions in mice. Circ Res 2012; 111: 87–96. [DOI] [PubMed] [Google Scholar]
- 37.Reddi AR, Culotta VC. SOD1 integrates signals from oxygen and glucose to repress respiration. Cell 2013; 152: 224–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holmström KM, Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat Rev Mol Cell Biol 2014; 15: 411–421. [DOI] [PubMed] [Google Scholar]
- 39.Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol 2005; 39: 725–732. [DOI] [PubMed] [Google Scholar]
- 40.Rubanyi GM, Vanhoutte PM. Oxygen-derived free radicals, endothelium, and responsiveness of vascular smooth muscle. Am J Physiol 1986; 250 (5 Pt 2): H815–H821. [DOI] [PubMed] [Google Scholar]
- 41.Nagao T, Illiano S, Vanhoutte PM. Calmodulin antagonists inhibit endothelium- dependent hyperpolarization in the canine coronary artery. Br J Pharmacol 1992; 107: 382–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lacza Z, Puskar M, Kis B, Perciaccante JV, Miller AW, Busija DW. Hydrogen peroxide acts as an EDHF in the piglet pial vasculature in response to bradykinin. Am J Physiol Heart Circ Physiol 2002; 283: H406–H411. [DOI] [PubMed] [Google Scholar]
- 43.Burgoyne JR, Oka S, Ale-Agha N, Eaton P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid Redox Signal 2013; 18: 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takaki A, Morikawa K, Murayama Y, et al. Roles of endothelial oxidases in endothelium-derived hyperpolarizing factor responses in mice. J Cardiovasc Pharmacol 2008; 52: 510–517. [DOI] [PubMed] [Google Scholar]
- 45.Takaki A, Morikawa K, Tsutsui M, et al. Crucial role of nitric oxide synthases system in endothelium-dependent hyperpolarization in mice. J Exp Med 2008; 205: 2053–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimokawa H, Godo S. Diverse functions of endothelial NO synthases system: NO and EDH. J Cardiovasc Pharmacol 2016; 67: 361–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Godo S, Shimokawa H. Divergent roles of endothelial nitric oxide synthases system in maintaining cardiovascular homeostasis. Free Radic Biol Med 2017; 109: 4–10. [DOI] [PubMed] [Google Scholar]
- 48.Morikawa K, Shimokawa H, Matoba T, et al. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. J Clin Invest 2003; 112: 1871–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. Circ Res 2003; 93: 573–580. [DOI] [PubMed] [Google Scholar]
- 50.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA Jr, Gutterman DD. Bradykinin-induced dilation of human coronary arterioles requires NADPH oxidase-derived reactive oxygen species. Arterioscler Thromb Vasc Biol 2009; 29: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sartoretto JL, Kalwa H, Pluth MD, Lippard SJ, Michel T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc Natl Acad Sci U S A 2011; 108: 15792–15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shiroto T, Romero N, Sugiyama T, et al. Caveolin-1 is a critical determinant of autophagy, metabolic switching, and oxidative stress in vascular endothelium. PLoS One 2014; 9: e87871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lobysheva I, Rath G, Sekkali B, et al. Moderate caveolin-1 downregulation prevents NADPH oxidase-dependent endothelial nitric oxide synthase uncoupling by angiotensin II in endothelial cells. Arterioscler Thromb Vasc Biol 2011; 31: 2098–2105. [DOI] [PubMed] [Google Scholar]
- 54.Burgoyne JR, Madhani M, Cuello F, et al. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 2007; 317: 1393–1397. [DOI] [PubMed] [Google Scholar]
- 55.Chidgey J, Fraser PA, Aaronson PI. Reactive oxygen species facilitate the EDH response in arterioles by potentiating intracellular endothelial Ca2+ release. Free Rad Biol Med 2016; 97: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang DX, Borbouse L, Gebremedhin D, et al. H2O2-induced dilation in human coronary arterioles: role of protein kinase G dimerization and large-conductance Ca2+-activated K+ channel activation. Circ Res 2012; 110: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dou D, Zheng X, Liu J, Xu X, Ye L, Gao Y. Hydrogen peroxide enhances vasodilatation by increasing dimerization of cGMP-dependent protein kinase type Ia. Circ J 2012; 76: 1792–1798. [DOI] [PubMed] [Google Scholar]
- 58.Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying NO: sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res 2016; 119: 375–396. [DOI] [PubMed] [Google Scholar]
- 59.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation 1996; 94: 3341–3347. [DOI] [PubMed] [Google Scholar]
- 60.Nishikawa Y, Stepp DW, Chilian WM. Nitric oxide exerts feedback inhibition on EDHF-induced coronary arteriolar dilation in vivo. Am J Physiol 2000; 279: H459–H465. [DOI] [PubMed] [Google Scholar]
- 61.Olmos L, Mombouli JV, Illiano S, Vanhoutte PM. cGMP mediates the desensitization to bradykinin in isolated canine coronary arteries. Am J Physiol 1995; 268 (2 Pt 2): H865–H870. [DOI] [PubMed] [Google Scholar]
- 62.Burgoyne JR, Prysyazhna O, Rudyk O, Eaton P. cGMP-dependent activation of protein kinase G precludes disulfide activation: implications for blood pressure control. Hypertension 2012; 60: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 63.Saito H, Godo S, Sato S, et al. Important role of endothelial caveolin-1 in the protective role of endothelium-dependent hyperpolarization against nitric oxide-mediated nitrative stress in microcirculation in mice. J Cardiovasc Pharmacol 2018; 71: 113–126. [DOI] [PubMed] [Google Scholar]
- 64.Enkhjargal B, Godo S, Sawada A, et al. Endothelial AMP-activated protein kinase regulates blood pressure and coronary flow responses through hyperpolarization mechanism in mice. Arterioscler Thromb Vasc Biol 2014; 34: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 65.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch 2010; 459: 793–806. [DOI] [PubMed] [Google Scholar]
- 66.Ikumi Y, Shiroto T, Godo S, et al. Important roles of endothelium-dependent hyperpolarization in coronary microcirculation and cardiac diastolic function in mice. J Cardiovasc Pharmacol 2020; 75: 31–40. [DOI] [PubMed] [Google Scholar]
- 67.Crea F, Lanza G, Camici P. Mechanisms of coronary microvascular dysfunction. In: Coronary Microvascular Dysfunction. Springer Milan, Italy: 2014; 31–47. [Google Scholar]
- 68.Chadderdon SM, Belcik JT, Bader L, et al. Temporal changes in skeletal muscle capillary responses and endothelial-derived vasodilators in obesity-related insulin resistance. Diabetes 2016; 65: 2249–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc 2015; 4. pii: e002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murthy VL, Naya M, Taqueti VR, et al. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014; 129: 2518–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suda A, Takahashi J, Hao K, et al. Coronary functional abnormalities in patients with angina and non-obstructive coronary artery disease. J Am Coll Cardiol 2019; 74: 2350–2360. [DOI] [PubMed] [Google Scholar]
- 72.Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018; 391: 31–40. [DOI] [PubMed] [Google Scholar]
- 73.Crea F, Bairey Merz CN, Beltrame JF, et al. The parallel tales of microvascular angina and heart failure with preserved ejection fraction: a paradigm shift. Eur Heart J 2016; 38: 473–477. [DOI] [PubMed] [Google Scholar]
- 74.Dryer K, Gajjar M, Narang N, et al. Coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Am J Physiol 2018; 314: H1033–H1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shah SJ, Lam CSP, Svedlund S, et al. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018; 39: 3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borlaug BA, Anstrom KJ, Lewis GD, et al. Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: the INDIE-HFpEF randomized clinical trial. JAMA 2018; 320: 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chennupati R, Meens MJ, Janssen BJ, et al. Deletion of endothelial arginase 1 does not improve vasomotor function in diabetic mice. Physiol Rep 2018; 6: e13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. JAMA 2007; 297: 842–857. [DOI] [PubMed] [Google Scholar]
- 79.Satoh K, Godo S, Saito H, Enkhjargal S, Shimokawa H. Dual roles of vascular-derived reactive oxygen species—With a special reference to hydrogen peroxide and cyclophilin A—. J Mol Cell Cardiol 2014; 73: 50–56. [DOI] [PubMed] [Google Scholar]
- 80.Shimokawa H, Satoh K. Light and dark of reactive oxygen species for vascular function: 2014 ASVB (Asian Society of Vascular Biology). J Cardiovasc Pharmacol 2015; 65: 412–418. [DOI] [PubMed] [Google Scholar]
- 81.Schiattarella GG, Altamirano F, Tong D, et al. Nitrosative stress drives heart failure with preserved ejection fraction. Nature 2019; 568: 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fukata Y, Amano M, Kaibuchi K. Rho-Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharmacol Sci 2001; 22: 32–39. [DOI] [PubMed] [Google Scholar]
- 83.Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev 2001; 81: 153–208. [DOI] [PubMed] [Google Scholar]
- 84.Rikitake Y, Liao JK. ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther 2005; 3: 441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimokawa H, Takeshita A. Rho-kinase is an important therapeutic target in cardiovascular medicine. Arterioscler Thromb Vasc Biol 2005; 25: 1767–1775. [DOI] [PubMed] [Google Scholar]
- 86.Shimokawa H, Satoh K. 2015 ATVB Plenary Lecture: Translational research on Rho-kinase in cardiovascular medicine. Arterioscler Thromb Vasc Biol 2015; 35: 1756–1769. [DOI] [PubMed] [Google Scholar]
- 87.Shimokawa H, Sunamura K, Satoh K. RhoA/Rho-kinase in the cardiovascular system. Circ Res 2016; 118: 352–366. [DOI] [PubMed] [Google Scholar]
- 88.Bernards A. GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim Biophys Acta 2003; 1603: 47–82. [DOI] [PubMed] [Google Scholar]
- 89.Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature 1994; 372: 231–236. [DOI] [PubMed] [Google Scholar]
- 90.Hirata K, Kikuchi A, Sasaki T, et al. Involvement of rho p21 in the GTP-enhanced calcium ion sensitivity of smooth muscle contraction. J Biol Chem 1992; 267: 8719–8722. [PubMed] [Google Scholar]
- 91.Gong MC, Iizuka K, Nixon G, et al. Role of guanine nucleotide-bindingproteins—ras-family or trimeric proteins or both—in Ca2+ sensitization of smooth muscle. Proc Natl Acad Sci U S A 1996; 93: 1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nishioka T, Shohag MH, Amano M, Kaibuchi K. Developing novel methods to search for substrates of protein kinases such as Rho-kinase. Biochim Biophys Acta 2015; 1854 (10 Pt B): 1663–1666. [DOI] [PubMed] [Google Scholar]
- 93.Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol 2001; 3: 339–345. [DOI] [PubMed] [Google Scholar]
- 94.Sebbagh M, Hamelin J, Bertoglio J, Solary E, Breard J. Direct cleavage of ROCK II by granzyme B induces target cell membrane blebbing in a caspase-independent manner. J Exp Med 2005; 201: 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.van Nieuw Amerongen GP, Beckers CM, Achekar ID, Zeeman S, Musters RJ, van Hinsbergh VW. Involvement of Rho-kinases in endothelial barrier maintenance. Arterioscler Thromb Vasc Biol 2007; 27: 2332–2339. [DOI] [PubMed] [Google Scholar]
- 96.Bell RD, Winkler EA, Singh I, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012; 485: 512–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Berk BC. Atheroprotective signaling mechanisms activated by steady laminar flow in endothelial cells. Circulation 2008; 117: 1082–1089. [DOI] [PubMed] [Google Scholar]
- 98.Nigro P, Abe J, Berk BC. Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal 2011; 15: 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou Q, Liao JK. Rho-kinases: an important mediator of atherosclerosis and vascular disease. Curr Pharm Des 2009; 15: 3108–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yao L, Chandra S, Toque HA, et al. Prevention of diabetes-induced arginase activation and vascular dysfunction by Rho-kinases (ROCK) knockout. Cardiovasc Res 2013; 97: 509–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Noda K, Godo S, Saito H, Tsutsui M, Shimokawa H. Opposing roles of nitric oxide and Rho-kinase in lipid metabolism in mice. Tohoku J ExpMed 2015; 235: 171–183. [DOI] [PubMed] [Google Scholar]
- 102.Noda K, Nakajima S, Godo S, et al. Rho-kinase inhibition ameliorates metabolic disorders through activation of AMPK pathway in mice. PLoS One 2014; 9: e110446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-α) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem 2002; 277: 6214–6222. [DOI] [PubMed] [Google Scholar]
- 104.Takemoto M, Liao JK. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors. Arterioscler Thromb Vasc Biol 2001; 21: 1712–1719. [DOI] [PubMed] [Google Scholar]
- 105.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation 2002; 106: 57–62. [DOI] [PubMed] [Google Scholar]
- 106.Tanaka S, Fukumoto Y, Nochioka K, et al. Statins exert the pleiotropic effects through small GTP-binding protein dissociation stimulator upregulation with a resultant Rac1 degradation. Arterioscler Thromb Vasc Biol 2013; 33: 1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shao D, Oka S, Brady CD, Haendeler J, Eaton P, Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol 2012; 52: 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Higashi M, Shimokawa H, Hattori T, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res 2003; 93: 767–775. [DOI] [PubMed] [Google Scholar]
- 109.Hattori T, Shimokawa H, Higashi M, et al. Long-term treatment with a specific Rho-kinase inhibitor suppresses cardiac allograft vasculopathy in mice. Circ Res 2004; 94: 46–52. [DOI] [PubMed] [Google Scholar]
- 110.Funakoshi Y, Ichiki T, Shimokawa H, et al. Rho-kinase mediates angiotensin II-induced monocyte chemoattractant protein-1 expression in rat vascular smooth muscle cells. Hypertension 2001; 38: 100–104. [DOI] [PubMed] [Google Scholar]
- 111.Takeda K, Ichiki T, Tokunou T, et al. Critical role of Rho-kinase and MEK/ERK pathways for angiotensin II-induced plasminogen activator inhibitor type-1 gene expression. Arterioscler Thromb Vasc Biol 2001; 21: 868–873. [DOI] [PubMed] [Google Scholar]
- 112.Ohnaka K, Shimoda S, Nawata H, et al. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun 2001; 287: 337–342. [DOI] [PubMed] [Google Scholar]
- 113.Cohen RA, Adachi T. Nitric-oxide-induced vasodilatation: regulation by physiologic s-glutathiolation and pathologic oxidation of the sarcoplasmic endoplasmic reticulum calcium ATPase. Trends Cardiovasc Med 2006; 16: 109–114. [DOI] [PubMed] [Google Scholar]
- 114.Wu C, Parrott AM, Fu C, et al. Thioredoxin 1-mediated post-translational modifications: reduction, transnitrosylation, denitrosylation, and related proteomics methodologies. Antioxid Redox Signal 2011; 15: 2565–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]