Abstract
This study investigated the effect of a dietary supplement containing astaxanthin-rich extract derived from Paracoccus carotinifaciens (astaxanthin supplement) on the status of stress and sleep in individuals aged 20–64 years. Twenty-five subjects orally administered 12 mg astaxanthin/day of astaxanthin supplement for 8 weeks (astaxanthin group) and 29 subjects given a placebo (placebo group) were evaluated with Profile of Mood States 2nd Edition for stress and Oguri–Shirakawa–Azumi Sleep Inventory for Middle-aged and Aged version for sleep. We did not observe any significant intergroup differences in the stress and sleep. A subgroup analysis was performed after dividing the subjects into two groups: those who scored >65 and those who scored ≤65 in the “Depression–Dejection” dimension of Profile of Mood States 2nd Edition. The sleep of subjects who scored >65 (”Depression–Dejection”) showed significant improvement in the astaxanthin group compared with the placebo group, whereas no significant improvement was observed in stress and the other subjects. Our results indicate that people who tend to be strongly depressed may experience improved sleep after ingesting astaxanthin supplement. On the basis of the parameters tested, administration of astaxanthin supplement was not associated with any problems related to safety. Clinical registration: This study has been registered at the University Hospital Medical Information Network (https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000038619) on August 24, 2018 as “A study to evaluate the effect of intake of astaxanthin on the status of stress and sleep in adults,” Identification No. UMIN000033863.
Keywords: Paracoccus carotinifaciens, astaxanthin, mental stress, sleep, rare carotenoids
Introduction
Astaxanthin (3,3'-dihydroxy-β,β-carotene-4,4'-dione in the IUPAC nomenclature) is a red-tinged pigment that belongs to a group of chemicals known as carotenoids. It contains β-carotene, lycopene, and lutein, and is classified as a xanthophyll. It occurs naturally in aquatic creatures, such as fish and shrimp, and is examined widely as a functional food for human consumption because of its strong antioxidant ability. Cyclists who received supplements of 4 mg astaxanthin/day for 28 days showed significant improvement during the 20-km time trial compared with their counterparts in the placebo group.(1) The study conducted by Ito et al.(2) showed that a daily intake of 4 mg astaxanthin appeared to be helpful in reducing UV-induced skin damage. Meanwhile, the phonatory parameters of subjects who were prescribed 24 mg astaxanthin/day for 28 days were maintained after a 60-min vocal loading, whereas the parameters of subjects who did not receive this supplement deteriorated.(3) Taking 6 mg astaxanthin affected superoxide anion scavenging activity in the aqueous humor in females, and could be involved in the control of VEGF levels in the anterior eye.(4) In our previous study, subjects aged 45–54 years who took 8 mg astaxanthin daily for 8 weeks experienced improvement of cognitive function compared with subjects in the placebo group.(5) Thus, the reported effects of astaxanthin for human consumption are based on numerous clinical studies.
Astaxanthin intended for human use come from natural sources, whereas chemical synthetic astaxanthin is typically used in feeds, such as pigment for fish culture. Haematococcus pluvialis, which is a green alga, is the best source of astaxanthin for human applications; moreover, astaxanthin derived from H. pluvialis is used in most functional studies. Astaxanthin is the predominant component of the carotenoids derived from H. pluvialis, and only the optically pure (3S,3'S)-astaxanthin, more than 90% terminal hydroxyl group of which is mostly mono-esterified with fatty acids, has been isolated.(6) The red yeast Phaffia rhodozyma is also known to produce astaxanthin. The chemical composition of astaxanthin derived from this yeast is opposite that of the commonly occurring natural astaxanthin (3S,3'S-configuration), which is designated as the 3R,3'R-configuration, and the terminal ends have hydroxyl groups that are not esterified or modified in any way.(7) Paracoccus carotinifaciens is also known to produce carotenoid compounds that contain astaxanthin.(8) Astaxanthin derived from P. carotinifaciens is a “free” form devoid of terminal modification and has a 3S,3'S-configuration.(9) In addition to astaxanthin, 20–30% of the extract derived from P. carotinifaciens consisted of the potent antioxidants adonirubin and adonixanthin.(10) Thus, natural astaxanthin shows a wide variety of characteristics depending on their source.
The number of people who suffer from mental stress and take functional foods or dietary supplements to manage the stress is increasing because our society is changing as people strive to become more competitive and/or more controlled. Therefore, studies to prove the effect of functional food on mental stress have attracted attention. Middle-aged women who have psychological distress and depressive symptoms were administered with 1.05 g ethyl-eicosapentaenoic acid/day plus 0.15 g ethyl-docosahexaenoic acid/day, and results showed alleviation of psychological distress and improvement in depression scales in this group of individuals compared with those in the placebo group.(11) During basic combat training, female soldiers who took iron supplements showed significantly improved scores in the Vigor scale of the Profile of Mood States (POMS).(12) A probiotic supplement consisting of Lactobacillus acidophilus (2 × 109 CFU/g), Lactobacillus casei (2 × 109 CFU/g), and Bifidobacterium bifidum (2 × 109 CFU/g) reduced the Beck Depression Inventory scores of patients with depressive disorder.(13) There are also several studies that investigated the effects of astaxanthin on mood or mental state. The effect of the combination of 12 mg astaxanthin/day and 20 mg tocotrienol/day on daily fatigue was examined using the POMS questionnaire, and results showed that the astaxanthin group showed significant improvement in the Friendliness factor of POMS compared with the placebo group.(14) Moreover, astaxanthin and sesamin were provided to volunteers to evaluate the effect of the combination on mental fatigue, and the visual analogue scale of mental fatigue in people who took the combination decreased more significantly than that in people who took the placebo.(15) Hence, various functional foods have been investigated for their usefulness in reducing stress.
There are many reported cases of people with depression who have problems with sleeping as well. For example, Taylor et al.(16) reported that people with insomnia had higher depression and anxiety levels than people without insomnia. Murphy and Peterson(17) noted that major depressive disorder is frequently accompanied by subjective sleep disturbances and polysomnographic abnormalities. Therefore, there have been several clinical studies that not only focused on depression, but on sleep as well. Ghaderi et al.(18) reported that patients treated with methadone were supplemented with vitamin D and showed improvement in quality of sleep and depression level. In an open-label trail, l-theanine was administered to patients with depression disorder, and results showed that the supplementation led to the reduction of Hamilton Depression Rating Scale score and Pittsburgh Sleep Quality Index from baseline.(19) Another open-label trial showed that subjects administered with pyrroloquinoline quinone posted improved Depression scores in POMS and in four of five items in Oguri–Shirakawa–Azumi Sleep Inventory for Middle-aged and Aged version (OSA-MA) from baseline.(20) Thus, sleep quality, which is strongly related to depression or mental stress, is also important to manage mood, and an object to research with functional food.
In the present study, we compared the improvements in mental stress as the primary outcome using the Profile of Mood State 2nd Edition (POMS 2) and improvements in sleep as the secondary outcome using OSA-MA between subjects aged 20–64 years who were administered a dietary supplement containing astaxanthin-rich extract derived from P. carotinifaciens and subjects who received a placebo. The reports of clinical studies with astaxanthin-rich extract derived from P. carotinifaciens are limited, compared with the extract derived from H. pluvialis. The astaxanthin-rich extract derived from P. carotinifaciens has different features such as free-form astaxanthin, adonirubin, and adonixanthin, compared with that derived from H. pluvialis. Therefore, the present study suggests not only additional evidence of astaxanthin supplement but also efficacy of astaxanthin supplement differently derived, which will be helpful in developing dietary supplements for mental stress and sleep and promoting carotenoid research.
Materials and Methods
Study design
This study was designed as a randomized, double-blind, placebo-controlled, parallel intergroup comparison.
Subjects
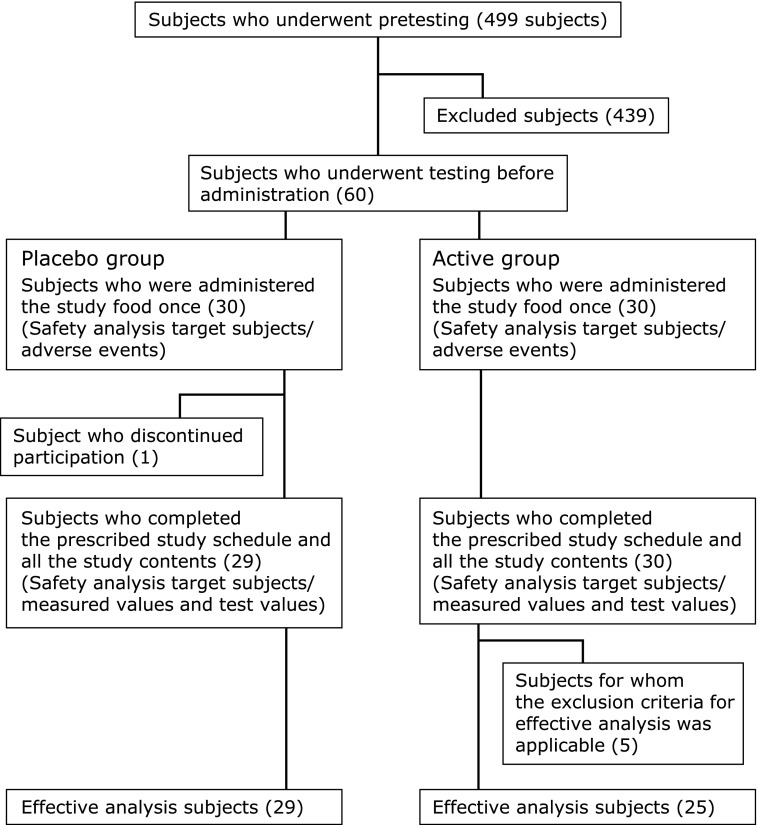
The classification and details of subjects who participated in this study are shown in Fig. 1. The placebo group was designated as group P and the active group was designated as group A. A total of 499 healthy subjects aged 20–64 years were initially identified as candidates for this study. On the basis of results of pre-study tests (physical tests and POMS2),(21,22) 60 subjects (group P, n = 30; group A, n = 30) who were healthy but scored high in the “Depression–Dejection” domain of POMS 2 were determined to be appropriate participants and were enrolled in this study.
Fig. 1.
Classifications and details of subjects.
Of the 60 subjects, one individual (ID 193010: group P) voluntarily discontinued participation in the study. The remaining 59 subjects completed the prescribed study schedule and performed all study tests. In addition, five in group A were excluded for per-protocol analysis because four of them could not be confirmed to have ingested the test supplement, and the other drank numerous times during this study. Thus, only the remaining 54 subjects (group P, n = 29; group A, n = 25) were available for efficacy analysis. In terms of safety, 60 adverse events (30 in group P, 30 in group A) were observed in subjects who were administered with the supplement preparations. Data from 59 of the original 60 subjects (group P, n = 29; group A, n = 30) who completed the study schedule and all study procedures were used in the safety analysis. The administration rate of the supplement was 87.7% in one subject (ID 193429, group A), 93% in another subject (ID 193173, group P), 98.2% in another subject (ID193078, group P), and 100% in the other 56 subjects.
Test food
We prepared 1% astaxanthin powder, which also contains 0.17% adonirubin and 0.18% adonixanthin, with an astaxanthin-rich extract from P. carotinifaciens using the method described in our previous study,(5) and produced a jelly containing 2 mg of astaxanthin with 1% astaxanthin powder, pH adjuster, sweetener, gelling agent, flavor (mixed berry), and water; it was later administered to all subjects in group A as astaxanthin supplement (Table 1). As a substitute, subjects in group P were given jelly containing artificial red coloring (Food Red No. 2, Amaranth), which was prepared using the same method (except for the 1% astaxanthin powder) used in the supplement provided to group A. The astaxanthin-rich extract from P. carotinifaciens and 1% astaxanthin powder were examined in terms of safety according to United States Food and Drug Administration protocols and have been filed as a New Dietary Ingredient.
Table 1.
Comparison of supplements per day
| Item | Astaxanthin supplement | Placebo | ||
|---|---|---|---|---|
| Form | 10 g of jelly × 6 pieces | 10 g of jelly × 6 pieces | ||
| Ingredients (mg) | 1% Astaxanthin powder | 1,200 | Food coloring | 30 |
| pH adjuster | 1,344 | pH adjuster | 1,344 | |
| Sweetener | 6,138 | Sweetener | 6,138 | |
| Gelling agent | 945 | Gelling agent | 942 | |
| Flavor | 300 | Flavor | 300 | |
| Water | 50,073 | Water | 51,246 | |
| Astaxanthin content (mg) | 12 | — | ||
| Energy (kJ) | 128 | 126 | ||
| Protein (g) | 0.06 | 0.06 | ||
| Fat (g) | 0.24 | <0.06 | ||
| Carbohydrate (g) | 8.46 | 9.12 | ||
| Sodium (mg) | 154 | 202 | ||
In previous studies with astaxanthin, a dose of 12 mg astaxanthin/day was administered to subjects for 8 weeks, and the Friendliness factor in POMS was observed to improve in the astaxanthin group.(14) Because the administration period of this study was 8 weeks, which is similar to that used in a previous study, we also used the same dosage of 12 mg astaxanthin/day in this investigation. All subjects were requested to ingest three jellies of their respective supplement preparations after breakfast and again after dinner for 8 weeks, for a total of six jellies (12 mg of astaxanthin or placebo) per day.
Institutional review board
This study was conducted according to the Declaration of Helsinki (October 2013 revision). The Ethical Guidelines for Biomedical Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology and the Ministry of Health, Labour and Welfare, December 22, 2014) and the Ethical Guidance for Biomedical Research Involving Human Subjects [Ministry of Education, Culture, Sports, Science, and Technology, and the Ministry of Health, Labour and Welfare, February 9, 2015 (May 29, 2017 partial revision)] were followed.
Physical measurements
We measured the height and weight of participants, calculated their body mass index (BMI), and conducted the following tests: systolic and diastolic blood pressure levels, heart rate, hematologic test, biological examination of blood, and urine test (protein, sugar, and occult blood).
Analysis of astaxanthin, adonirubin, and adonixanthin concentration in human serum
The concentration of astaxanthin, adonirubin, and adonixanthin (Fig. 2) in human serum was analyzed using the method described.(23) Briefly, 1 ml water and 1 ml ethanol, containing 0.85 µg of β-apo-8'-carotenal (internal standard) and 50 µg of 2,6-dibuthylhydroxytoluene, were added to 300 µl of serum. Next, 5 ml hexane was added to the mixture, which was then shaken for 20 min. Then the hexane layer was placed in another test tube after centrifugation [4°C, 3,000 rpm (1,464 × g), 10 min] and was dried over under nitrogen gas flow. The residue was dissolved in 100 µl chloroform/ethanol and subjected to ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis using Cadenza CD-C18 column with mixture of 85% acetonitrile in 2 mM ammonium acetate and acetonitrile/methanol/tetrahydrofuran (60:38:2) in 2 mM ammonium acetate as solvent. Detection and quantification were performed via selective reaction monitoring (SRM) of protonated molecule (MH+) of astaxanthin, adonirubin, and adonixanthin.
Fig. 2.
Structural formula of astaxanthin, adonirubin, and adonixanthin.
POMS 2 test
The subjects’ stress was assessed using the POMS 2 questionnaire(21,22) just before administration, after 4 weeks of administration, and 8 weeks of administration. The results were calculated as score of “Anger–Hostility” (AH), “Confusion–Bewilderment” (CB), “Depression–Dejection” (DD), “Fatigue–Inertia” (FI), “Tension–Anxiety” (TA), “Vigor–Activity” (VA), “Friendliness” (F), and “Total Mood Disturbance” (TMD). Decreased score in AH, CB, DD, FI, TA, and TMD, and increased score in VA and F were interpreted as indicative of improved mental states.
OSA-MA test
The subjects were asked to fill out the OSA-MA questionnaire(24) within 5 min of waking up to evaluate their sleep. The tests were completed in 5 days in the 10 days of working day before the POMS 2 test at 0 weeks, 4 weeks and 8 weeks of administration. The results recorded on the day closest to the POMS 2 test were calculated to be the score of “Sleepiness on rising,” “Initiation and maintenance of sleep,” “Frequent dreaming,” “Refreshing,” and “Sleep length.” Increased scores in all items were interpreted to indicate improved sleep quality.
Sample size
We estimated the sample size based on “F” of POMS 2 from the data presented by Hongo et al.(14) It was assumed that the mean difference would be 1.6, with a standard deviation (SD) of 4.4. To detect this difference, with a power of 80% and a significance level of 5% and taking into account that 10% of the patients will be lost to follow-up, it was calculated that 30 subjects would be needed to be studied in each group.
Statistical analysis
For the subject background factors, a chi-square test was used to compare sex (male/female) between groups A and P, and a two-sample t test was also used to compare the others between both groups. For the serum concentrations of carotenoid, POMS 2, and OSA-MA, a one-sample t test was used to compare the changes between pre-administration baseline values with those after administration in groups A and P. A two-sample t test was also used to compare the amount of change between both groups in the serum concentrations of carotenoid. For POMS 2 and OSA-MA, we used two-way repeated-measures analysis of variance (ANOVA) to compare the amount of change between both groups. Numerical values were expressed as the mean and SD, and the standard of significance for the tests was set at 5% on both sides. All statistical analysis was performed using IBM SPSS Statistics 25.
Results and Discussion
Background factors of subjects
The background factors of the 54 subjects who underwent effective analysis are listed in Table 2. The mean age of subjects in group P, which consisted of 12 men and 17 women, was 45.3 ± 9.3 years. Meanwhile, the mean age of subjects in group A, which consisted of 11 men and 14 women, was 45.8 ± 10.5 years. DD scores in POMS 2 were 64.1 ± 7.7 and 65.1 ± 8.0 points in groups P and A, respectively. No intergroup differences were observed in data pertaining to the subjects’ age, sex, POMS 2 score, physical measurement, or physical examination. In the previous study with astaxanthin supplementation, the DD scores (in POMS 2) of subjects, comprising male and female participants aged 20–64 years who were healthy but feeling fatigued, was 51.9 ± 8.1,(14) indicating that the subjects selected in the present study had a stronger tendency to be depressed.
Table 2.
Subject background factors
| Item | Group P (n = 29) |
Group A (n = 25) |
p value | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Age (years) | 45.3 | 9.3 | 45.8 | 10.5 | 0.866 | ||
| Sex (male/female) | Males: 12/Females: 17 | Males: 11/Females: 14 | 1.000 | ||||
| Height (cm) | 163.40 | 9.50 | 164.8 | 7.16 | 0.645 | ||
| Weight (kg) | 57.42 | 12.53 | 59.00 | 9.35 | 0.607 | ||
| BMI (kg/m2) | 21.30 | 2.89 | 21.75 | 2.73 | 0.557 | ||
| Systolic BP (mmHg) | 115.1 | 13.5 | 115.1 | 13.4 | 0.998 | ||
| Diastolic BP (mmHg) | 68.7 | 9.4 | 71.4 | 11.2 | 0.332 | ||
| Heart rate (bpm) | 69.0 | 8.4 | 70.6 | 9.7 | 0.527 | ||
| POMS2 | AH | 61.9 | 12.4 | 61.3 | 12.4 | 0.857 | |
| CB | 63.3 | 8.5 | 61.6 | 10.4 | 0.492 | ||
| DD | 64.1 | 7.7 | 65.1 | 8.0 | 0.649 | ||
| FI | 63.3 | 8.7 | 64.8 | 8.7 | 0.542 | ||
| TA | 65.1 | 9.2 | 64.6 | 9.7 | 0.847 | ||
| VA | 44.1 | 7.1 | 45.3 | 8.8 | 0.578 | ||
| F | 46.0 | 8.7 | 47.4 | 11.7 | 0.635 | ||
| TMD | 65.4 | 7.6 | 28.5 | 2.2 | 0.918 | ||
Inter-group comparisons with group P (sex: chi-square test; items other than sex: 2-sample t test).
Blood carotenoid levels
Table 3 presents changes in the measured values and the amount of change compared with the baseline values for blood astaxanthin, adonirubin, and adonixanthin levels. In group A, the values at 8 weeks after administration for all items were associated with significantly greater increases compared with group P (group P vs group A: astaxanthin, 0.001 ± 0.007 vs 0.168 ± 0.078 µg/ml; adonirubin: 0.000 ± 0.000 vs 0.036 ± 0.021 µg/ml; adonixanthin: 0.000 ± 0.000 vs 0.032 ± 0.015 µg/ml). In comparison with the baseline, although the values at 8 weeks after administration did not increase in group P, all values at 8 weeks after administration were significantly increased in group A. A study that focused on people aged 65 years and older reported that a higher total carotenoid level was associated with lower probability of depressed mood.(25) Although the age of subjects is different from that in the present study, the increase in serum carotenoid concentration in group A might have a good effect on depression. In addition, in our previous study, the serum concentration of astaxanthin in subjects who took 8 mg astaxanthin/day for 8 weeks was 0.173 ± 0.058 µg astaxanthin/ml.(5) Moreover, Nakagawa et al.(26) reported that astaxanthin concentrations in erythrocytes and plasma were not different between subjects administered with 6 mg and 12 mg astaxanthin daily. Therefore, although the intake of astaxanthin (12 mg astaxanthin/day) was higher in the present study compared with that used in our previous study (8 mg astaxanthin/day), the astaxanthin concentration in serum could not be different, and the appropriate intake might be 8 mg astaxanthin/day or less. Moreover, the carotenoid ratio in the test food (astaxanthin/adonirubin/adonixanthin = 1:0.17:0.18) changed to that in the serum of subjects (astaxanthin/adonirubin/adonixanthin = 1:0.21:0.19). Nishino et al.(27) examined serum carotenoid concentration in monkeys and showed that the carotenoid ratio of β-cryptoxanthin in diet increased more in plasma compared with lutein and zeaxanthin. Both ends in the molecular structure of adonirubin and adonixanthin have different functional groups like β-cryptoxanthin, and therefore, asymmetric xanthophylls might be easier to transfer to blood or remain in blood in primates.
Table 3.
Serum concentration of carotenoid
| Item | Numerical item | Group | Baseline |
Week 8 |
p value (Inter-group) | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||||
| Astaxanthin (µg/ml) | Measured value | P | 0.000 | 0.002 | 0.001 | 0.007 | ||
| A | 0.002 | 0.012 | 0.171** | 0.082 | ||||
| Amount of change | P | 0.001 | 0.007 | 0.000 | ||||
| A | 0.168 | 0.078 | ||||||
| Adonirubin (µg/ml) | Measured value | P | 0.000 | 0.000 | 0.000 | 0.000 | ||
| A | 0.000 | 0.002 | 0.037** | 0.022 | ||||
| Amount of change | P | 0.000 | 0.000 | 0.000 | ||||
| A | 0.036 | 0.021 | ||||||
| Adonixanthin (µg/ml) | Measured value | P | 0.000 | 0.000 | 0.000 | 0.000 | ||
| A | 0.000 | 0.000 | 0.032** | 0.015 | ||||
| Amount of change | P | 0.000 | 0.000 | 0.000 | ||||
| A | 0.032 | 0.015 | ||||||
Group P: n = 29, group A: n = 25. Intra-group comparison at baseline, *p<0.05 and **p<0.01 (1-sample t test). Inter-group comparison with group P (2-sample t test).
POMS 2 test
Table 4 presents changes in the measured values and the amount of change compared with the baseline for all items of POMS 2. There were no significant intergroup differences regarding the amount of change after administration for any of these items. In comparison with the baseline, AH, CB, DD, FI, TA, and TMD were significantly decreased, and VA and F were significantly increased at 4 and/or 8 weeks after administration in group P. Moreover, CB, DD, FI, TA, and TMD were significantly decreased, and VA was significantly increased at 4 and/or 8 weeks after administration in group A compared with the baseline. In the study of Nishioka et al.,(28) anxiety and depression in mice administered with astaxanthin were evaluated with elevated plus maze test and hole-board test for anxiety and forced swim test and tail suspension test for depression; their results suggested that astaxanthin exerted anxiolytic-like effects, but not antidepressant-like effects. In addition, Jiang et al.(29) demonstrated the anti-depressant effect of astaxanthin on mice using forced swim test and tail suspension test, and the putative mechanism of the serotonergic system. Furthermore, Zhou et al.(30) reported the anti-depressant effect of astaxanthin on diabetic mice, and the mechanism involved in the inhibition of inflammation, thereby protecting neurons in the hippocampus, amygdala, and hypothalamus against hyperglycemic damage. Moreover, Imai et al.(15) illustrated that supplementation astaxanthin and sesamin for human applications significantly improved recovery from fatigue-inducing mental tasks compared with placebo. Thus, although significant improvement with astaxanthin on depression was not observed in the present study, astaxanthin might have a good psychological effect by decreasing depression and/or anxiety.
Table 4.
Scores of POMS2
| Numerical item | Group | Baseline |
Week 4 |
Week 8 |
p value (Inter-group) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||||
| AH | Measured value | P | 61.9 | 12.4 | 57.1** | 14.0 | 54.3** | 12.6 | |||
| A | 61.3 | 12.4 | 58.8 | 13.1 | 58.4 | 14.0 | |||||
| Amount of change | P | −4.8 | 8.1 | −7.7 | 8.5 | 0.109 | |||||
| A | −2.6 | 7.4 | −2.9 | 9.8 | |||||||
| CB | Measured value | P | 63.3 | 8.5 | 58.1* | 11.8 | 55.8** | 10.6 | |||
| A | 61.6 | 10.4 | 57.8* | 11.2 | 57.12 | 11.5 | |||||
| Amount of change | P | −5.2 | 11.0 | −7.6 | 10.8 | 0.391 | |||||
| A | −3.7 | 8.7 | −4.4 | 10.8 | |||||||
| DD | Measured value | P | 64.1 | 7.7 | 60.3 | 11.0 | 57.3** | 10.7 | |||
| A | 65.1 | 8.0 | 60.2** | 9.2 | 59.8* | 9.7 | |||||
| Amount of change | P | −3.8 | 11.3 | −6.8 | 10.9 | 0.924 | |||||
| A | −4.8 | 8.4 | −5.3 | 9.6 | |||||||
| FI | Measured value | P | 63.3 | 8.7 | 59.4* | 10.5 | 55.8** | 11.5 | |||
| A | 64.8 | 8.7 | 59.5** | 10.7 | 57.9** | 11.0 | |||||
| Amount of change | P | −4.0 | 9.4 | −7.5 | 11.1 | 0.887 | |||||
| A | −5.3 | 8.4 | −6.9 | 7.8 | |||||||
| TA | Measured value | P | 65.1 | 9.2 | 58.2** | 12.5 | 56.0** | 12.5 | |||
| A | 64.6 | 9.7 | 60.2* | 11.5 | 58.8** | 10.6 | |||||
| Amount of change | P | −7.0 | 11.6 | −9.1 | 12.2 | 0.313 | |||||
| A | −4.4 | 9.9 | −5.8 | 10.2 | |||||||
| VA | Measured value | P | 44.1 | 7.1 | 47.8* | 10.5 | 48.4* | 11.4 | |||
| A | 45.3 | 8.8 | 48 | 8.8 | 49.3* | 8.9 | |||||
| Amount of change | P | 3.7 | 7.3 | 4.3 | 8.5 | 0.764 | |||||
| A | 2.8 | 7.2 | 4 | 9.5 | |||||||
| F | Measured value | P | 46.0 | 8.7 | 48.8* | 12.2 | 48.6 | 12.4 | |||
| A | 47.4 | 11.7 | 48.8 | 9.1 | 49.2 | 9.8 | |||||
| Amount of change | P | 2.8 | 6.8 | 2.6 | 9.2 | 0.633 | |||||
| A | 1.4 | 8.3 | 1.8 | 10.1 | |||||||
| TMD | Measured value | P | 65.4 | 7.6 | 59.5** | 12.1 | 56.4** | 11.9 | |||
| A | 65.2 | 7.6 | 60.2** | 10.2 | 59.2** | 11.2 | |||||
| Amount of change | P | −5.9 | 9.9 | −9.0 | 10.5 | 0.422 | |||||
| A | −5.0 | 7.7 | −6.0 | 9.1 | |||||||
Group P: n = 29, group A: n = 25. Intra-group comparison at baseline, *p<0.05 and **p<0.01 (1-sample t test). Inter-group comparison with group P (Two-way-repeated measures of ANOVA). AH, Anger-Hostility; CB, Confusion-Bewilderment; DD, Depression-Dejection; FI, Fatigue-Inertia; TA, Tension-Anxiety; VA, Vigor-Activity; F, Friendliness; TMD, Total Mood Disturbance. Decreased score in AH, CB, DD, FI, TA, and TMD, and increased score in VA and F indicated to improve metal states.
Regarding POMS, although F of POMS 2 in subjects who ingested astaxanthin improved significantly, DD or FI in the active group did not significantly change compared with the placebo group.(14) In addition, the scores of POMS in subjects administered with astaxanthin and sesamin did not improve, even though improvement was observed in the visual analogue scale of recovery from mental tasks.(15) Hence, it might be difficult to evaluate the effect of dietary supplementation on depression using POMS for a short period of administration such as 8 weeks and/or a small study sample (such as 60 subjects).
OSA-MA test
The results of OSA-MA in the evaluation of sleep quality are shown in Table 5. Although “Frequent dreaming” after administration in group A was significantly lower than that in group P, the values became crossed between 4 weeks and 8 weeks of administration, which is not thought to indicate any effect. In comparison with the baseline, “Sleepiness on rising” and “Refreshing” at 8 weeks after administration exhibited significant increases in group P, and “Sleepiness on rising” at 4 and 8 weeks after administration and “Refreshing” at 4 weeks after administration were significantly increased in group A. Imai et al.(15) did not observe any significant difference in OSA-MA between supplementation of astaxanthin and sesamin and placebo as well, in agreement with our results. On the other hand, Lin et al.(31) indicated that consumption of the antioxidant-rich kiwi fruit may improve sleep onset, duration, and efficiency in adults with self-reported sleep disturbances. In addition, in their study Milesi et al.(32) provided melon juice containing superoxide dismutase, which is the main enzyme of the enzymatic antioxidant defense system of the body, to healthy volunteers with sleep problems, and the authors noted that compared with placebo, the supplementation significantly improved the sleep quality of participants. Thus, although taking antioxidants or reducing oxidative stress could be beneficial to alleviate sleep problems, the supplementation of astaxanthin in the present study may not have enough antioxidant capability to improve sleep, or the supplementation period may not be of sufficient duration to exert the antioxidant effect on sleep.
Table 5.
Scores of OSA-MA
| Numerical item | Group | Baseline |
Week 4 |
Week 8 |
p value (Inter-group) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | ||||||
| Sleepiness on rising | Measured value | P | 12.6 | 7.9 | 14.7 | 8.3 | 16.3* | 7.1 | |||
| A | 12.4 | 7.3 | 16.7* | 6.7 | 17.3** | 6.5 | |||||
| Amount of change | P | 2.0 | 6.6 | 3.7 | 7.7 | 0.384 | |||||
| A | 4.3 | 8.0 | 4.9 | 8.3 | |||||||
| Initiation and maintenance of sleep | Measured value | P | 17.1 | 4.8 | 17.6 | 6.5 | 16.3 | 7.2 | |||
| A | 17.7 | 7.2 | 19.4 | 5.9 | 18.4 | 5.9 | |||||
| Amount of change | P | 0.5 | 6.6 | −0.8 | 6.6 | 0.412 | |||||
| A | 1.7 | 78 | 0.7 | 8.2 | |||||||
| Frequent dreaming | Measured value | P | 22.1 | 8.9 | 20.8 | 9.8 | 23.8 | 7.7 | |||
| A | 25.4 | 6.1 | 25.5 | 5.8 | 22.2 | 7.8 | |||||
| Amount of change | P | −1.3 | 7.5 | 1.7 | 7.7 | 0.010 | |||||
| A | 0.1 | 5.9 | −3.1 | 8.1 | |||||||
| Refreshing | Measured value | P | 13 | 6 | 15 | 7.1 | 16.1* | 6.7 | |||
| A | 14.5 | 8.1 | 18* | 6.6 | 17.2 | 6.4 | |||||
| Amount of change | P | 1.9 | 6.1 | 3.1 | 6.6 | 0.703 | |||||
| A | 3.5 | 6.7 | 2.7 | 7.1 | |||||||
| Sleep length | Measured value | P | 14.9 | 6.9 | 16 | 8.5 | 15.8 | 6.8 | |||
| A | 14.9 | 6.9 | 16.8 | 6.3 | 16.3 | 7.9 | |||||
| Amount of change | P | 1.1 | 8.4 | 0.8 | 8.2 | 0.698 | |||||
| A | 1.9 | 8.5 | 1.4 | 7.7 | |||||||
Group P: n = 29, group A: n = 25. Intra-group comparison at baseline, *p<0.05 and **p<0.01 (1-sample t test). Inter-group comparison with group P (Two-way-repeated measures of ANOVA). Increased scores in all items indicated to improve sleep quality.
Additional analysis of groups classified according to DD score
To perform an effective exploratory analysis, we divided the subjects into two groups based on their DD scores in POMS 2: (1) those who scored >65 and (2) those who scored ≤65. We then performed an additional analysis for POMS 2 and OSA-MA tests in these two groups. The background factors of subjects in these groups are shown in Table 6. No intergroup differences between group P and group A divided on the basis of DD score were observed in data pertaining to the subjects’ age, sex, POMS 2 score, physical measurement, or physical examination.
Table 6.
Subject background factors of subjects in group P and A divided by score 65 (median) of “DD” in POMS2 at baseline
| Item | >65 of “DD” |
≤65 of “DD” |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group P (n = 14) |
Group A (n = 12) |
p value | Group P (n = 15) |
Group A (n = 13) |
p value | |||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||||||
| Age (years) | 43.6 | 8.9 | 44.5 | 9.6 | 0.800 | 47 | 9.7 | 47 | 11.4 | 1.000 | ||||
| Sex (males/females) | Males: 7/Females: 7 | Males: 6/Females: 6 | 1.000 | Males: 5/Females: 10 | Males: 5/Females: 8 | 1.000 | ||||||||
| Height (cm) | 164.36 | 9.82 | 163.85 | 6.03 | 0.876 | 162.5 | 9.44 | 165.05 | 8.27 | 0.457 | ||||
| Weight (kg) | 59.15 | 13.88 | 61.33 | 8.75 | 0.643 | 55.8 | 11.37 | 56.84 | 9.71 | 0.799 | ||||
| BMI (kg/m2) | 21.68 | 3.36 | 22.78 | 2.54 | 0.363 | 20.94 | 2.43 | 20.8 | 2.63 | 0.887 | ||||
| Systolic pressure (mmHg) | 113.3 | 12.9 | 118.5 | 13.7 | 0.327 | 116.7 | 14.2 | 111.9 | 12.9 | 0.360 | ||||
| Diastolic pressure (mmHg) | 66.9 | 8.4 | 75.2 | 12.3 | 0.055 | 70.3 | 10.3 | 67.9 | 9.2 | 0.534 | ||||
| Heart rate (bpm) | 67.9 | 8.9 | 70.4 | 10.3 | 0.503 | 70.1 | 8 | 70.7 | 9.4 | 0.851 | ||||
| POMS2 | AH | 64.6 | 12.3 | 64.5 | 15.6 | 0.979 | 59.4 | 12.3 | 58.4 | 8.1 | 0.802 | |||
| CB | 64.5 | 5.2 | 67.2 | 11.2 | 0.433 | 62.3 | 10.8 | 56.4 | 6.5 | 0.100 | ||||
| DD | 69.6 | 2.1 | 71.8 | 4.2 | 0.147 | 58.7 | 7.1 | 58.9 | 5.0 | 0.937 | ||||
| FI | 64.4 | 8.5 | 67.3 | 8.4 | 0.392 | 62.4 | 9.1 | 62.5 | 8.6 | 0.967 | ||||
| TA | 67.1 | 8.5 | 67.9 | 10.2 | 0.834 | 63.3 | 9.6 | 61.6 | 8.6 | 0.639 | ||||
| VA | 42.1 | 5.8 | 44.9 | 10.5 | 0.390 | 45.9 | 7.9 | 45.6 | 7.4 | 0.914 | ||||
| F | 44.5 | 6.4 | 46.5 | 13.3 | 0.620 | 47.5 | 10.4 | 48.2 | 10.5 | 0.863 | ||||
| TMD | 68.8 | 5.5 | 70.0 | 5.1 | 0.566 | 62.3 | 8.1 | 60.8 | 6.9 | 0.607 | ||||
Inter-group comparisons with group P (sex: chi-square test; items other than sex: 2-sample t test).
Changes in the measured values and the amount of change for all items of the subjects who scored >65 and those who scored ≤65 in the DD subscale of POMS 2 in comparison to the baseline in POMS 2 and OSA-MA are presented in Table 7 and 8, respectively. In subjects who scored >65 in DD, although there was no significant intergroup difference in POMS 2, group A showed statistically significant improvement in “Sleepiness on rising” of OSA-MA compared to the placebo group. A significant difference in “Frequent dreaming” between these groups was likewise observed, but the values were crossed between 4 weeks and 8 weeks of administration, which did not indicate any effect of astaxanthin. Moreover, “Initiation and maintenance of sleep” and “Sleep length” in group A showed a better improvement than those in group P (p<0.1). Furthermore, there was no significant difference between these groups in subjects who scored ≤65. Based on these results, astaxanthin supplementation seems to have a good effect on sleep for people who have a stronger tendency to be depressed. Murphy and Peterson observed that problems with sleep regulation often precede depressive disorders.(17) Therefore, astaxanthin supplementation might be useful in preventing sleep problems before the situation could deteriorate further. The effect of astaxanthin on depression, which we expected, was not observed even with the subgroup analysis in the present study. As such, further studies would be necessary to confirm the effect of astaxanthin on depression and the relation between oxidative stress, depression, and sleep.
Table 7.
POMS 2 and OSA-MA of subjects who scored higher than 65 in “DD” at baseline
| Item | Numerical item | Group | Baseline |
Week 4 |
Week 8 |
p value (Intergroup) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||||||
| POMS2 | AH | Measured value | P | 64.6 | 12.3 | 60.9 | 14.6 | 56.9** | 12.7 | |||
| A | 64.5 | 15.6 | 61.9 | 15 | 61.4 | 16.2 | ||||||
| Amount of change | P | −3.7 | 8.6 | −7.7 | 8.5 | 0.395 | ||||||
| A | −2.6 | 8.4 | −3.1 | 10.6 | ||||||||
| CB | Measured value | P | 64.5 | 5.2 | 58.8 | 10.6 | 56.4** | 9.5 | ||||
| A | 67.2 | 11.2 | 62 | 14.1 | 59.1* | 12.1 | ||||||
| Amount of change | P | −5.7 | 10.6 | −8.1 | 8.8 | 0.945 | ||||||
| A | −5.2 | 10.7 | −8.1 | 10.9 | ||||||||
| DD | Measured value | P | 69.9 | 2.1 | 62.3* | 10.8 | 58.8** | 8.9 | ||||
| A | 71.8 | 4.2 | 65.3* | 8.6 | 62.5** | 9.3 | ||||||
| Amount of change | P | −7.6 | 10.4 | −11.1 | 8.5 | 0.684 | ||||||
| A | −6.5 | 9.6 | −9.3 | 9.2 | ||||||||
| FI | Measured value | P | 64.4 | 8.5 | 62.4 | 8.8 | 58.6 | 12.2 | ||||
| A | 67.3 | 8.4 | 62.7 | 11.8 | 61.3 | 11.2 | ||||||
| Amount of change | P | −2 | 6.7 | −5.8 | 11.2 | 0.702 | ||||||
| A | −4.6 | 10.4 | −5.9 | 9.6 | ||||||||
| TA | Measured value | P | 67.1 | 8.5 | 60.1* | 11.1 | 56.9** | 11.3 | ||||
| A | 67.9 | 10.2 | 63 | 12.9 | 61.5 | 12 | ||||||
| Amount of change | P | −7 | 11.8 | −10.2 | 11.8 | 0.491 | ||||||
| A | −4.9 | 10.1 | −6.4 | 10.6 | ||||||||
| VA | Measured value | P | 42.1 | 5.8 | 46.1 | 7.6 | 47.1* | 9 | ||||
| A | 44.9 | 10.5 | 47 | 10.6 | 48.8 | 10.6 | ||||||
| Amount of change | P | 4.1 | 7.6 | 5.1 | 8.4 | 0.659 | ||||||
| A | 2.1 | 9.1 | 3.9 | 12.5 | ||||||||
| F | Measured value | P | 44.5 | 6.4 | 47.4 | 12 | 47.9 | 12.3 | ||||
| A | 46.5 | 13.3 | 47.5 | 9 | 48.1 | 8.5 | ||||||
| Amount of change | P | 2.9 | 8.2 | 3.4 | 9.9 | 0.651 | ||||||
| A | 1 | 11.6 | 1.6 | 14 | ||||||||
| TMD | Measured value | P | 68.8 | 5.5 | 62.3* | 10.8 | 58.4** | 10.4 | ||||
| A | 70 | 5.1 | 64.5 | 10.4 | 62.4* | 11.1 | ||||||
| Amount of change | P | −6.5 | 9.5 | −10.4 | 10 | 0.607 | ||||||
| A | −5.5 | 9 | 7.6 | 9.6 | ||||||||
| OSA-MA | Sleepiness on rising | Measured value | P | 15.2 | 7.9 | 15.9 | 9.4 | 15.3 | 7.6 | |||
| A | 11.2 | 6.5 | 18.1** | 5.9 | 16.3 | 7.3 | ||||||
| Amount of change | P | 0.7 | 4.8 | 0.1 | 5.5 | 0.035 | ||||||
| A | 6.9 | 7.4 | 5.1 | 9.0 | ||||||||
| Initiation and maintenance of sleep | Measured value | P | 16.6 | 4.8 | 17.3 | 6.6 | 16.5 | 6.6 | ||||
| A | 14.7 | 5.9 | 20.3* | 6.3 | 16.3 | 5.4 | ||||||
| Amount of change | P | 0.8 | 5.5 | 0 | 5.1 | 0.066 | ||||||
| A | 5.6 | 6.3 | 1.6 | 7.8 | ||||||||
| Frequent dreaming | Measured value | P | 24.6 | 6.8 | 22.1* | 8.5 | 25.2 | 6.3 | ||||
| A | 24.9 | 6.9 | 27.6 | 3.6 | 22.8 | 7.6 | ||||||
| Amount of change | P | −2.5 | 3.3 | 0.7 | 6.4 | 0.024 | ||||||
| A | 2.7 | 5.9 | −2.1 | 7.9 | ||||||||
| Refreshing | Measured value | P | 12.8 | 5.3 | 15 | 7.3 | 15.7 | 5.9 | ||||
| A | 13.8 | 7.9 | 19.3* | 6.3 | 14.9 | 6.4 | ||||||
| Amount of change | P | 2.1 | 5.1 | 2.9 | 5.7 | 0.711 | ||||||
| A | 5.5 | 7.1 | 1.1 | 6.9 | ||||||||
| Sleep length | Measured value | P | 14.2 | 6.2 | 15 | 8.7 | 14.8 | 6.8 | ||||
| A | 12.5 | 6.6 | 17.9* | 6.6 | 14.8 | 7.7 | ||||||
| Amount of change | P | 0.8 | 3.8 | 0.6 | 8.4 | 0.077 | ||||||
| A | 5.3 | 6.9 | 2.3 | 7.0 | ||||||||
Group P, n = 14; group A, n = 12. Intragroup comparison at baseline, *p<0.05 and **p<0.01 (one-sample t test). Intergroup comparison with group P (two-way repeated-measures ANOVA). AH, Anger-Hostility; CB, Confusion-Bewilderment; DD, Depression-Dejection; FI, Fatigue-Inertia; TA, Tension-Anxiety; VA, Vigor-Activity; F, Friendliness; TMD, Total Mood Disturbance. Decreased score in AH, CB, DD, FI, TA, and TMD, and increased score in VA and F indicate improved mental state. Increased scores in all items of OSA-MA indicate improved sleep quality.
Table 8.
POMS2 and OSA-MA of subjects scored 65 of “DD” or lower at baseline
| Item | Numerical item | Group | Baseline |
Week 4 |
Week 8 |
p value (Inter-group) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||||||
| POMS2 | AH | Measured value | P | 59.4 | 12.3 | 53.5* | 12.9 | 51.8** | 12.4 | |||
| A | 58.4 | 8.1 | 55.8 | 10.8 | 55.6 | 11.7 | ||||||
| Amount of change | P | −5.9 | 7.7 | −7.6 | 8.7 | 0.168 | ||||||
| A | −2.5 | 6.7 | −2.8 | 9.4 | ||||||||
| CB | Measured value | P | 62.3 | 10.8 | 57.5 | 13.2 | 55.1* | 11.8 | ||||
| A | 56.4 | 6.5 | 54 | 6.2 | 55.3 | 11.1 | ||||||
| Amount of change | P | −4.8 | 11.9 | −7.1 | 12.6 | 0.279 | ||||||
| A | −2.4 | 6.7 | −1.1 | 10.0 | ||||||||
| DD | Measured value | P | 58.7 | 7.1 | 58.5 | 11.2 | 55.9 | 12.2 | ||||
| A | 58.9 | 5.0 | 55.6 | 7.4 | 57.3 | 9.7 | ||||||
| Amount of change | P | −0.2 | 11.2 | −2.9 | 11.6 | 0.801 | ||||||
| A | −3.3 | 7.1 | −1.6 | 8.7 | ||||||||
| FI | Measured value | P | 62.4 | 9.1 | 56.6 | 11.5 | 53.3** | 10.5 | ||||
| A | 62.5 | 8.6 | 56.6** | 9.1 | 54.8** | 10.2 | ||||||
| Amount of change | P | −5.8 | 11.4 | −9.1 | 11.1 | 0.851 | ||||||
| A | −5.9 | 6.3 | −7.8 | 6.0 | ||||||||
| TA | Measured value | P | 63.3 | 9.6 | 56.3* | 13.9 | 55.2* | 13.9 | ||||
| A | 61.6 | 8.6 | 57.7 | 9.9 | 56.4 | 8.9 | ||||||
| Amount of change | P | −6.9 | 11.9 | −8.1 | 12.9 | 0.482 | ||||||
| A | −3.9 | 10.2 | −5.2 | 10.2 | ||||||||
| VA | Measured value | P | 45.9 | 7.9 | 49.3 | 12.7 | 49.5 | 13.4 | ||||
| A | 45.6 | 7.4 | 49.0* | 7.1 | 49.7* | 7.5 | ||||||
| Amount of change | P | 3.4 | 7.3 | 3.6 | 8.9 | 0.929 | ||||||
| A | 3.4 | 5.1 | 4.1 | 6.2 | ||||||||
| F | Measured value | P | 47.5 | 10.4 | 50.1* | 12.7 | 49.3 | 12.9 | ||||
| A | 48.2 | 10.5 | 50.0 | 9.4 | 50.2 | 11.0 | ||||||
| Amount of change | P | 2.7 | 5.5 | 1.8 | 9.0 | 0.889 | ||||||
| A | 1.8 | 3.8 | 2.1 | 5.0 | ||||||||
| TMD | Measured value | P | 62.3 | 8.1 | 56.9 | 13.0 | 54.5* | 13.1 | ||||
| A | 60.8 | 6.9 | 56.3* | 8.5 | 56.3 | 10.9 | ||||||
| Amount of change | P | −5.3 | 10.5 | −7.7 | 11.1 | 0.552 | ||||||
| A | −4.5 | 6.6 | 4.5 | 8.8 | ||||||||
| OSA-MA | Sleepiness on rising | Measured value | P | 10.2 | 7.6 | 13.5 | 7.4 | 17.3** | 6.7 | |||
| A | 13.5 | 8.1 | 15.4 | 7.4 | 18.2 | 5.8 | ||||||
| Amount of change | P | 3.3 | 7.9 | 7.1 | 8.2 | 0.509 | ||||||
| A | 1.9 | 7.9 | 4.6 | 8.0 | ||||||||
| Initiation and maintenance of sleep | Measured value | P | 17.7 | 4.9 | 17.9 | 6.6 | 16.2 | 7.8 | ||||
| A | 20.5 | 7.4 | 18.7 | 5.7 | 20.4 | 5.9 | ||||||
| Amount of change | P | 0.2 | 7.7 | −1.5 | 8.0 | 0.916 | ||||||
| A | −1.8 | 7.9 | 0 | 8.8 | ||||||||
| Frequent dreaming | Measured value | P | 19.7 | 10.1 | 19.6 | 11 | 22.4 | 8.7 | ||||
| A | 25.8 | 5.5 | 23.6 | 6.8 | 21.7 | 8.3 | ||||||
| Amount of change | P | −0.2 | 9.9 | 2.7 | 8.9 | 0.113 | ||||||
| A | −2.2 | 5.2 | −4.1 | 8.4 | ||||||||
| Refreshing | Measured value | P | 13.2 | 6.8 | 15 | 7.2 | 16.4 | 7.5 | ||||
| A | 15.1 | 8.5 | 16.7 | 6.8 | 19.4 | 5.8 | ||||||
| Amount of change | P | 1.8 | 7.1 | 3.2 | 7.5 | 0.866 | ||||||
| A | 1.6 | 5.8 | 4.3 | 7.3 | ||||||||
| Sleep length | Measured value | P | 15.6 | 7.7 | 16.9 | 8.5 | 16.7 | 6.8 | ||||
| A | 17.1 | 6.6 | 15.7 | 6.1 | 17.7 | 8.2 | ||||||
| Amount of change | P | 1.3 | 11.2 | 1.0 | 8.3 | 0.593 | ||||||
| A | −1.4 | 8.7 | 0.5 | 8.4 | ||||||||
Group P: n = 15, group A: n = 13. Intra-group comparison at baseline, *p<0.05 and **p<0.01 (1-sample t test). Inter-group comparison with group P (Two-way-repeated measures of ANOVA). AH, Anger-Hostility; CB, Confusion-Bewilderment; DD, Depression-Dejection; FI, Fatigue-Inertia; TA, Tension-Anxiety; VA, Vigor-Activity; F, Friendliness; TMD, Total Mood Disturbance. Decreased score in AH, CB, DD, FI, TA, and TMD, and increased score in VA and F indicated to improve metal states. Increased scores in all items of OSA-MA indicated to improve sleep quality.
Regarding the difference between the groups divided according to DD score, Yanik et al.(33) showed that the severity of depression among patients with depression was correlated with the amount of oxidative stress. In addition, Hill et al.(34) suggested from the study of Drosophila that a key function of sleep is to defend against oxidative stress, and they also pointed to a reciprocal role for reactive oxygen species in neurons in the regulation of sleep. Based on these findings, the subjects who scored >65 (DD) may have been exposed to greater oxidative stress compared with other subjects, and are more likely to improve the quality of their sleep by reducing the high oxidative stress with astaxanthin.
In comparison with the study conducted by Imai et al.,(15) who observed a significant improvement in recovery from mental fatigue among subjects who were administered with astaxanthin and sesamin compared with placebo, there was no significant difference between the groups in OSA-MA. Adonirubin and adonixanthin, which are rare carotenoids, are also found in the astaxanthin-rich extract derived from P. carotinifaciens, and are reported to not only have the same effect as astaxanthin but also have a unique effect.(10,35,36) Therefore, the rare carotenoids might work synergistically with astaxanthin in the human body, which might result in improvement in sleep quality.
Safety assessment
There were 22 adverse events reported in 10 of 30 subjects in group P (9 cold, 3 tiredness, 1 stomachache, 1 headache, 1 shoulder stiffness, 1 gastroenteritis, 1 malaise, 1 difficulty falling asleep, 1 anorexia, 1 discomfort of stomach, 1 stomatitis and 1 backache) and 14 adverse events in 8 of 30 subjects in group A (9 cold, 1 drowsiness, 1 diarrhea, 1 tiredness, 1 runny nose and 1 headache). According to a doctor, all adverse events observed in both groups were not serious and “not related to test foods.” Therefore, we concluded that the supplement caused no adverse events (side effects). The results of our investigation regarding changes in numerical values for physical measurements (including blood and urine tests) in all groups indicated that although there were minor changes, none were determined to be clinically significant. Although the supplementation dose was higher, and the age range of subjects was wider than that in our previous study,(5) there were no adverse events; thus, we believe that the astaxanthin-rich extract derived from P. carotinifaciens can be used safely and has potentially broader applications.
Astaxanthin-rich extract derived from P. carotinifaciens was given to subjects aged 20–64 years at 12 mg astaxanthin/day for 8 weeks to evaluate its effect on the status of stress and sleep in adult subjects compared with placebo. Its efficacy was measured with POMS 2 (mental stress) as the primary outcome and OSA-MA (sleep) as the secondary outcome, and we also assessed its safety. After administration, no significant differences were observed between the astaxanthin group and the placebo group in POMS 2 and OSA-MA. A subgroup analysis was conducted by dividing the subjects into two groups based on their DD score in POMS 2. In subjects who scored >65 (DD), although there was no significant intergroup difference in POMS 2, the “Sleepiness on rising” factor of OSA-MA in the astaxanthin group improved significantly compared with that in the placebo group. In addition, “Initiation and maintenance of sleep” and “Sleep length” in group A showed improvement compared with those in group P (p<0.1). On the other hand, significant differences of POMS 2 and OSA-MA were not observed in subjects who scored ≤65 (DD). Based on these observations, astaxanthin-rich extract derived from P. carotinifaciens might be useful for people who have a stronger tendency to be depressed who want to improve the quality of their sleep. All adverse events observed in both groups were deemed slight and “not related to test foods”; therefore, we concluded that the supplement caused no adverse events.
Author Contributions
MH, MK, and YK designed the study and wrote the initial draft of the manuscript. TU and TM contributed to interpretation of data and assisted in the preparation of the manuscript.
Acknowledgments
The authors thank M. Fukuda in Kyoto Biseibutsu Kenkyusho for technical assistance with analysis of astaxanthin, adonixanthin, and adonirubin.
Conflict of Interest
MH, MK, YK and TU are employees of JXTG Nippon Oil & Energy Corporation, which provided the astaxanthin-rich extract.
References
- 1.Earnest CP, Lupo M, White KM, Church TS. Effect of astaxanthin on cycling time trial performance. Int J Sports Med 2011; 32: 882–888. [DOI] [PubMed] [Google Scholar]
- 2.Ito N, Seki S, Ueda F. The protective role of astaxanthin for UV-induced skin deterioration in healthy people—a randomized, double-blind, placebo-controlled trial. Nutrients 2018; 10. pii: E817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneko M, Kishimoto Y, Suzuki R, Kawai Y, Tateya I, Hirano S. Protective effect of astaxanthin on vocal fold injury and inflammation due to vocal loading: a clinical trial. J Voice 2017; 31: 352–358. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto H, Arai K, Takahashi J, Chikuda M. Effects of astaxanthin on VEGF level and antioxidation in human aqueous humor: difference by sex. J Clin Biochem Nutr 2019; 65: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayashi M, Ishibashi T, Maoka T. Effect of astaxanthin-rich extract derived from Paracoccus carotinifaciens on cognitive function in middle-aged and older individuals. J Clin Biochem Nutr 2018; 62: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah MM, Liang Y, Cheng JJ, Daroch M. Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci 2016; 7: 531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt I, Schewe H, Gassel S, et al. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl Microbiol Biotechnol 2011; 89: 555–571. [DOI] [PubMed] [Google Scholar]
- 8.Tsubokura A, Yoneda H, Mizuta H. Paracoccus carotinifaciens sp. nov., a new aerobic Gram-negative astaxanthin-producing bacterium. Int J System Bacteriol 1999; 49 Pt 1: 277–282. [DOI] [PubMed] [Google Scholar]
- 9.Katsumata T, Ishibashi T, Kyle D. A sub-chronic toxicity evaluation of a natural astaxanthin-rich carotenoid extract of Paracoccus carotinifaciens in rats. Toxicol Rep 2014; 1: 582–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maoka T, Yasui H, Ohmori A, et al. Anti-oxidative, anti-tumor-promoting, and anti-carcinogenic activities of adonirubin and adonixanthin. J Oleo Sci 2013; 62: 181–186. [DOI] [PubMed] [Google Scholar]
- 11.Lucas M, Asselin G, Mérette C, Poulin MJ, Dodin S. Ethyl-eicosapentaenoic acid for the treatment of psychological distress and depressive symptoms in middle-aged women: a double-blind, placebo-controlled, randomized clinical trial. Am J Clin Nutr 2009; 89: 641–651. [DOI] [PubMed] [Google Scholar]
- 12.McClung JP, Karl JP, Cable SJ, et al. Randomized, double-blind, placebo-controlled trial of iron supplementation in female soldiers during military training: effects on iron status, physical performance, and mood. Am J Clin Nutr 2009; 90: 124–131. [DOI] [PubMed] [Google Scholar]
- 13.Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, et al. Clinical and metabolic response to probiotic administration in patients with major depressive disorder: a randomized, double-blind, placebo-controlled trial. Nutrition 2016; 32: 315–320. [DOI] [PubMed] [Google Scholar]
- 14.Hongo N, Fujishita M, Takahashi Y, et al. Daily fatigue-reducing effect of astaxanthin—a randomized, placebo-controlled, Double-blind, Parallel-group study. Jpn Pharmacol Ther 2017; 45: 62–72. [Google Scholar]
- 15.Imai A, Oda Y, Ito N, et al. Effects of dietary supplementation of astaxanthin and sesamin on daily fatigue: a randomized, double-blind, placebo-controlled, two-way crossover study. Nutrients 2018; 10. pii: E281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep 2005; 28: 1457–1464. [DOI] [PubMed] [Google Scholar]
- 17.Murphy MJ, Peterson MJ. Sleep disturbances in depression. Sleep Med Clin 2015; 10: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghaderi A, Banafshe HR, Motmaen M, Rasouli-Azad M, Bahmani F, Asemi Z. Clinical trial of the effects of vitamin D supplementation on psychological symptoms and metabolic profiles in maintenance methadone treatment patients. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79 (Pt B): 84–89. [DOI] [PubMed] [Google Scholar]
- 19.Hidese S, Ota M, Wakabayashi C, et al. Effects of chronic l-theanine administration in patients with major depressive disorder: an open-label study. Acta Neuropsychiatr. 2017;29:72–79. doi: 10.1017/neu.2016.33. [DOI] [PubMed] [Google Scholar]
- 20.Nakano M, Yamamoto T, Okamura H, Tsuda A, Kowatari Y. Effects of oral supplementation with pyrroloquinoline quinone on stress, fatigue, and sleep. Funct Food Health Dis 2012; 2: 307–332. [Google Scholar]
- 21.Heuchert JP, McNair DM. Profile of Mood States 2nd Edition. North Tonawanda, NY: Multi-Health Systems, 2012; 1–114. [Google Scholar]
- 22.Yokoyama K, Watanabe K. Japanese Translation of POMS 2: Profile of Mood States 2nd Edition. Tokyo: Kaneko Shobo, 2015; 1–172. (in Japanese) [Google Scholar]
- 23.Fukuda M, Ishibashi T, Maoka T. Analysis of adonixanthin and adonirubin in human serum by ultra performance liquid chromatography (UPLC)-MS/MS. Carotenoid Sci 2017; 21: 42–46. [Google Scholar]
- 24.Yamamoto Y, Tanaka H, Takase M, Yamazaki K, Azumi K, Shirakawa S. Standardization of revised version of OSA sleep inventory for middle age and aged. Brain Science and Mental Disorders 1999; 10: 401–409. (in Japanese) [Google Scholar]
- 25.Milaneschi Y, Bandinelli S, Penninx BW, et al. The relationship between plasma carotenoids and depressive symptoms in older persons. World J Biol Psychiatry 2012; 13: 588–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakagawa K, Kiko T, Miyazawa T, et al. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br J Nutr 2011; 105: 1563–1571. [DOI] [PubMed] [Google Scholar]
- 27.Nishino A, Ichihara T, Sugimoto K, Kuriki T, Yasui H, Maoka T. Predicting organ carotenoids levels from analysis of plasma could lead to errors: a study in cynomolgus monkeys. Nutr Res 2019; 61: 95–101. [DOI] [PubMed] [Google Scholar]
- 28.Nishioka Y, Oyagi A, Tsuruma K, Shimazawa M, Ishibashi T, Hara H. The antianxiety-like effect of astaxanthin extracted from Paracoccus carotinifaciens. Biofactors 2011; 37: 25–30. [DOI] [PubMed] [Google Scholar]
- 29.Jiang X, Zhu K, Xu Q, et al. The antidepressant-like effect of trans-astaxanthin involves the serotonergic system. Oncotarget 2017; 8: 25552–25563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou XY, Zhang F, Hu XT, et al. Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res 2017; 1657: 262–268. [DOI] [PubMed] [Google Scholar]
- 31.Lin HH, Tsai PS, Fang SC, Liu JF. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac J Clin Nutr 2011; 20: 169–174. [PubMed] [Google Scholar]
- 32.Milesi MA, Lacan D, Brosse H, Desor D, Notin C. Effect of an oral supplementation with a proprietary melon juice concentrate (Extramel) on stress and fatigue in healthy people: a pilot, double-blind, placebo-controlled clinical trial. Nutr J 2009; 8: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanik M, Erel O, Kati M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatr 2004; 16: 200–203. [DOI] [PubMed] [Google Scholar]
- 34.Hill VM, O'Connor RM, Sissoko GB, et al. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol 2018; 16: e2005206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inoue Y, Shimazawa M, Nagano R, et al. Astaxanthin analogs, adonixanthin and lycopene, activate Nrf2 to prevent light-induced photoreceptor degeneration. J Pharmacol Sci 2017; 134: 147–157. [DOI] [PubMed] [Google Scholar]
- 36.Iwata S, Imai T, Shimazawa M, et al. Protective effects of the astaxanthin derivative, adonixanthin, on brain hemorrhagic injury. Brain Res 2018; 1698: 130–138. [DOI] [PubMed] [Google Scholar]