Abstract
Copper is one of the essential micronutrients, and copper-containing enzymes contribute to crucial functions in the body. Lysyl oxidase is a copper-containing enzyme that remodels the extracellular matrix by cross-linking collagen and elastin. The overexpression of lysyl oxidase was recently shown to promote tumor metastasis. M2-like macrophages were also found to significantly accumulate in the tumor microenvironment, and correlated with a poor patient’s outcome. We speculate that M2-like macrophages promote tumor progression via lysyl oxidase expression. Epigenetics, a mitotically heritable change in gene expression without any change in DNA sequencing, is also associated with tumor progression. However, the relationship between lysyl oxidase expression in M2-like macrophages and epigenetics remains unclear. Lysyl oxidase expression was significantly induced in human leukemic THP-1 cell-derived M2-like macrophages. Furthermore, the level of histone H3 tri-methylation at lysine 27 was decreased, and a pre-treatment with a H3K27 demethylase inhibitor notably suppressed lysyl oxidase expression in M2-like macrophages. Lysyl oxidase derived from M2-like macrophages also enhanced breast cancer cell migration, and this was suppressed by a H3K27 demethylase inhibitor. The present results suggest the mechanism of lysyl oxidase expression in M2-like macrophages as an aspect of epigenetics, particularly histone methylation.
Keywords: lysyl oxidase, M2-like macrophages, epigenetics
Introduction
Macrophages are important components of blood vessels and participate in immune responses. Pro-inflammatory M1-like macrophages accumulate in artery plaque,(1) and also exacerbate inflammatory responses through the production of reactive oxygen species (ROS) and some inflammatory cytokines.(2) Anti-inflammatory M2-like macrophages accumulate in the tumor microenvironment,(3) and exacerbate tumor progression through the secretion of cytokines such as transforming growth factor-β (TGF-β).(4) M2-like macrophages have been implicated in angiogenesis, epithelial-to-mesenchymal transition (EMT), and metastasis through the release of promoting factors such as matrix metalloproteinases (MMPs) and vascular endothelial growth factor (VEGF).(5,6)
Copper, an essential micronutrient, plays a key role in several physiological processes including cell proliferation and cell differentiation.(7) Recent studies revealed that copper levels were elevated in tumor tissues, which facilitated tumor cell growth.(8) High expression levels of copper transporter 1 (CTR1) have been associated with tumor progression.(9) Lysyl oxidase (LOX) is a copper-containing amine oxidase that catalyzes the cross-linking collagen and elastin, and plays an important role in maintaining homeostasis in the extracellular matrix (ECM). However, accumulated evidence has implicated LOX in tumor metastasis.(10) In the tumor microenvironment, LOX regulates EMT molecules, such as snail and twist,(11–13) and creates pre-metastatic niches by increasing the stiffness of tissue. Accordingly, elucidating the molecular mechanisms governing LOX expression may clarify the pathological biochemistry of metastasis.(14)
Epigenetics is generally referred to as a mitotically heritable change in gene expression without any change in DNA sequencing. Epigenetics, DNA methylation and histone modifications, is associated with tumor progression through the silencing of tumor suppressor genes and promotion of oncogenes.(15,16) A recent study reported that the polarization of M2-like macrophages was closely related to epigenetics, and, the histone demethylase, Jmjd3, in particular, plays a critical role in this process.(17) In order to identify the relationship between LOX expression and epigenetics, we investigated the mechanisms of LOX expression in M2-like macrophages as an aspect of epigenetic factors.
Materials and Methods
Reagents
12-O-tetradecanoylphorbol-13-acetate (TPA), a Jmjd3 inhibitor (GSK-J4), and actinomycin D (ActD) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Interleukin (IL)-4 and IL-13 were purchased from PeproTech (Rocky Hill, NJ). β-Aminopropionitrile (BAPN) and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (A6154) and -mouse IgG (A4416) were purchased from Sigma Aldrich Co. (St. Louis, MO). Anti-LOX (#58135) and anti-histone H3 tri-methylation at lysine 27 (H3K27me3) (#9733) rabbit monoclonal antibodies were purchased from Cell Signaling Technology (Danvers, MA). An anti-actin (MAB1501) mouse monoclonal antibody was purchased from Millipore Co. (Billerica, MA). Trans IT-LT1 (MIR2300) was purchased from Mirus Bio LLC. (Madison, WI). The TRI reagent was purchased from Molecular Research Center Inc. (Cincinnati, OH).
Cell culture
Human leukemic THP-1 cells were cultured in RPMI 1640 medium containing 10% (v/v) heat-inactivated fetal calf serum (FCS), 100 units/ml penicillin, and 100 µg/ml streptomycin. Human breast cancer MDA-MB-231 cells were cultured in DMEM (D6429, Sigma-Aldrich Co.) containing 10% (v/v) FCS, 100 units/ml penicillin, and 100 µg/ml streptomycin. Cells were maintained at 37°C in a humidified 5% CO2 incubator. THP-1 cells were differentiated into M0 macrophages with TPA (100 nM) for 24 h. M0 macrophages were differentiated into M2-like macrophages by incubating them with IL-4 (20 ng/ml) and IL-13 (20 ng/ml) for 24 h.
Reverse transcription-polymerase chain reaction (RT-PCR) analysis
Cells were washed with ice-cold phosphate-buffered saline (PBS) and total RNA was extracted with 1 ml TRI reagent. The synthesis of cDNA was performed using the ReverTra Ace® qPCR RT kit (Toyobo, Osaka, Japan) according to the manufacturer’s protocol. Quantitative RT-PCR was performed by the methods using ThunderbirdTM SYBR qPCR Mix (Toyobo) as described in our previous study.(18) The primer sequences used in the present study were as follows: LOX, sense 5'-CCT ACT ACA TCC AGG CGT CCA-3'; antisense 5'-CAT AAT CTC TGA CAT CTG CCC CTG T-3': arginase-1, sense 5'-TCA TCT GGG TGG ATG CTC ACA C-3'; antisense 5'-GAG AAT CCT GGC ACA TCG GGA A-3': CD206, sense 5'-AGC CAA CAC CAG CTC CTC AAG A-3'; antisense 5'-CAA AAC GCT CGC GCA TTG TCC A-3': 18S rRNA, sense 5'-CGG CTA CCA CAT CCA AGG AA-3'; antisense 5'-GCT GGA ATT ACC GCG GCT-3'.
Histone preparation
Core histone was isolated from macrophages as described below. After cells had been treated, they were lysed in extraction buffer [0.1 M Tris-HCl, pH 7.5, containing 0.15 M NaCl, 1.5 mM MgCl2, 0.65% NP-40, 10 mM NaF, 1 mM Na3VO4, 20 mM β-glycerophosphate, 1 mM dithiothreitol (DTT), and 1 mM phenylmethanesulfonyl fluoride (PMSF)]. After centrifugation at 13,200 × g for 10 s, the pellets were mixed with 0.2 M H2SO4 followed by centrifugation at 13,200 × g for 20 min. The supernatant was mixed with 100% trichloroacetic acid and centrifuged at 13,200 × g for 20 min. Pellets were washed with acetone and centrifuged again at 13,200 × g for 5 min. The remaining histone was dissolved in sodium dodecyl sulfate (SDS) buffer (0.45 M Tris-HCl, pH 8.8 containing 2% SDS, 6% 2-mercaptoethanol, and 0.01% bromophenol blue).
Western blotting
Whole cell extracts were prepared in radio-immunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl, pH 8.0, containing 150 mM sodium chloride, 0.5% sodium deoxycholate, 0.1% SDS, 0.1 mM NaF, 1 mM Na3VO4, 20 mM β-glycerophosphate, 5 mg/ml leupeptin, 1 mM DTT, 1 mM PMSF, and 1% NP-40). After centrifugation at 14,000 × g for 10 min, the protein concentration of the resulting supernatant was measured with the Bio-Rad protein assay (BioRad, Hercules, CA). Whole cell protein extracts protein or histone extracts were boiled with SDS sample buffer, separated by SDS-PAGE, and transferred electrophoretically onto PVDF membranes. The membranes were then incubated with the respective specific primary antibodies (1:1,000). The blots were incubated with HRP-conjugated antibodies (1:5,000). Bands were detected using SuperSignalTM West Pico Plus (Thermo Scientific, Rockford, IL) or ImmunoStar® LD, and imaged using a Chemi Doc Touch Imaging System (BioRad, Hercules, CA).
Chromatin immunoprecipitation (ChIP) assay
ChIP assays were performed as described in our previous study with minor modifications.(19) Sheared genomic DNA was immunoprecipitated with primary antibodies overnight, and this was followed by an incubation with Dynabeads® Protein G (Invitrogen, Carlsbad) for 2 h. The abundance of lox promoter regions in ChIP precipitates was quantified using a PCR analysis with Taq DNA polymerase (Toyobo). The primer sequences used in the ChIP assay were as follows: sense 5'-TGG CAT TGC TTG GTG GAG A-3', antisense 5'-TTT TGC CAG ATT GAC CCC G-3' (141 bp). After amplification, these PCR products were loaded onto a 2% (w/v) agarose gel for electrophoresis and visualized using FLA5100 (Fuji Film, Tokyo, Japan). A densitometric analysis of PCR products was performed with Multi-Gauge ver. 3.0 (Fuji Film, Tokyo, Japan).
Transwell migration assay
MDA-MB-231 cell invasion was evaluated by a 24-well transwell co-culture system (Corning, Corning, NY) with an 8-µm-pore polycarbonate filter membrane. THP-1 cells (5 × 105 cells/well) were added to the lower chamber and cultured with 100 nM TPA for 24 h. After the incubation, M0 macrophages were differentiated into M2-like macrophages in the presence or absence of GSK-J4 (10 µM). After differentiation, MDA-MB-231 cells (1 × 104 cells/well) were added to the upper chamber, and were co-cultured for 24 h with or without BAPN (500 µM). MDA-MB-231 cells inside the chamber were removed by cotton swabs and the cells passing through the membrane of the upper chamber were stained with 0.5% crystal violet and evaluated under a microscope.
Statistical analysis
Data are expressed as the means ± SE of three independent experiments. Statistical evaluations of data were performed using ANOVA followed by post hoc Bonferroni tests. A p value less than 0.05 was considered to be significant.
Results
Confirmation of THP-1 cell differentiation into M2-like macrophages
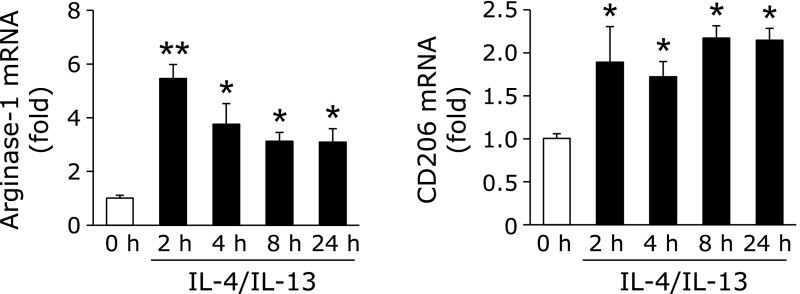
To examine the expression of LOX in monocytic THP-1 cell-derived M2-like macrophages, we initially investigated the expression of the M2-like macrophages differentiation markers, arginase-1 and CD206.(20) THP-1 cell-derived M2-like macrophages were prepared by the treatment with TPA for 24 h (M0 macrophages), and a subsequent treatment with IL-4 and IL-13 for the indicated times. As shown in Fig. 1, the expression of arginase-1 and CD206 was significantly increased.
Fig. 1.
Confirmation of THP-1 cell differentiation into M2-like macrophages. THP-1 cells were incubated with TPA (100 nM) for 24 h (M0 macrophages), and then treated with IL-4 and IL-13 (20 ng/ml each) for the indicated times. After the treatment, the expression of M2 markers was detected by real-time RT-PCR (n = 3) (*p<0.05, **p<0.01 vs 0 h). Graphs show data as the mean ± SD.
Expression of LOX in M2-like macrophages
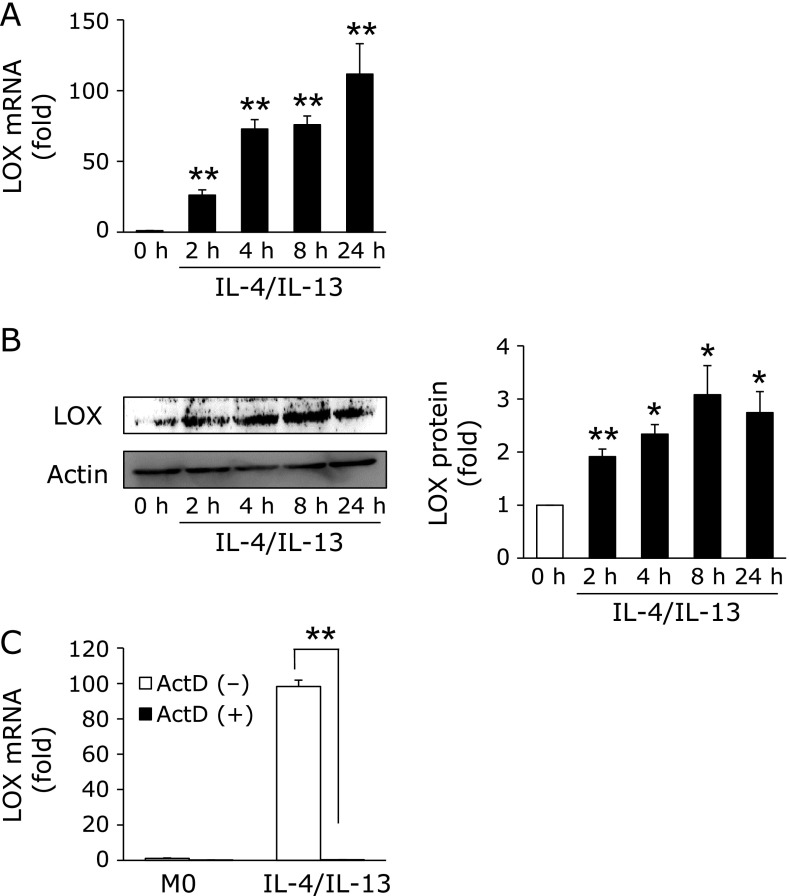
We investigated the expression of LOX in M2-like macrophages. As shown in Fig. 2A and B, LOX mRNA and protein levels significantly increased in M2-like macrophages in a time-dependent manner. We then examined whether the induction of LOX is regulated at the transcription level using actinomycin D (ActD), an RNA polymerase inhibitor. As expected, the induction of LOX in M2-like macrophages was completely suppressed in the presence of ActD (Fig. 2C).
Fig. 2.
Increasing LOX expression following differentiation into M2-like macrophages. (A and B) M0 macrophages were treated with IL-4 and IL-13 (20 ng/ml each) for the indicated times. The expression of LOX was detected by real-time RT-PCR (n = 3) and Western blotting (n = 3) (*p<0.05, **p<0.01 vs 0 h). (C) M0 macrophages were pre-treated with or without ActD (2 ng/ml) for 30 min before the treatment with IL-4 and IL-13 (20 ng/ml each). After the treatment, LOX expression was detected by real-time RT-PCR (n = 3) (**p<0.01). Graphs show data as the mean ± SD.
H3K27me3 levels contribute to LOX expression in M2-like macrophages
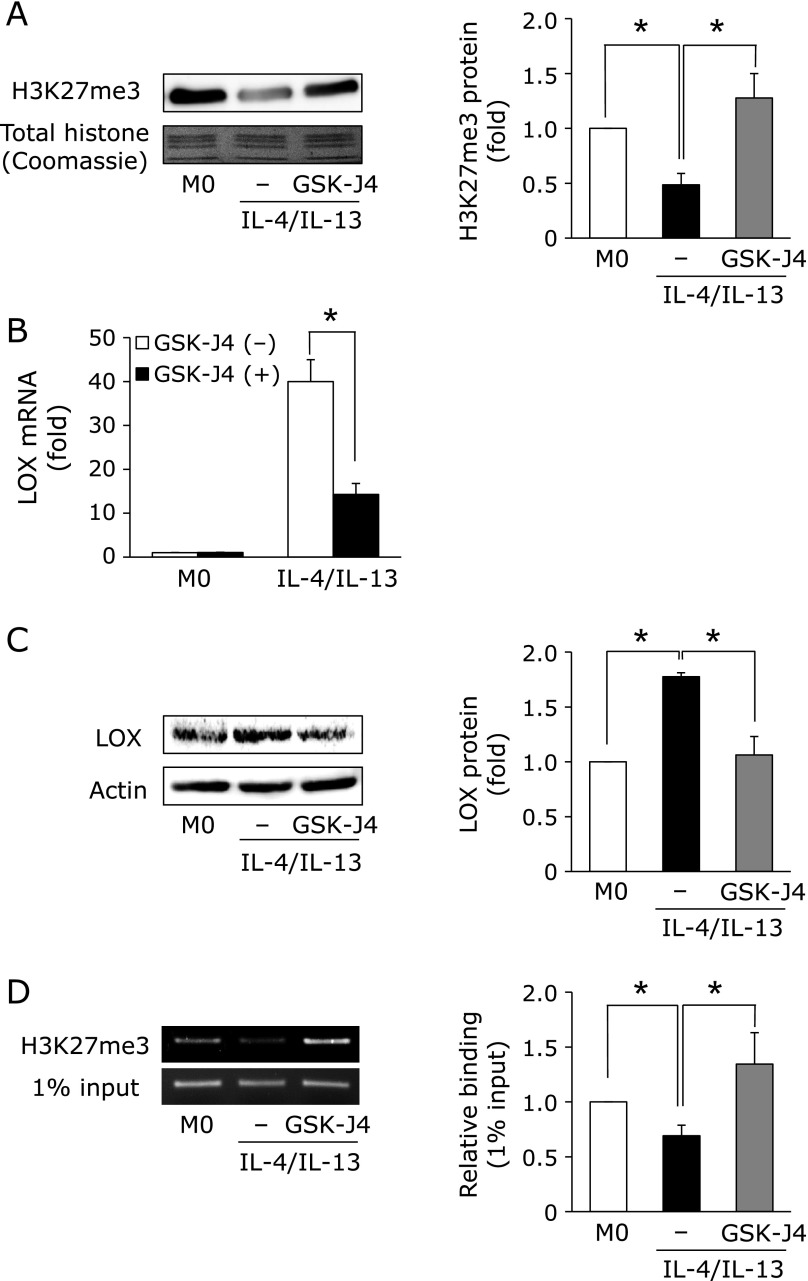
Considerable evidence has indicated that epigenetic gene modifications significantly contribute to tumorigenesis and tumor progression.(15,16) In previous studies, the Jmjd3-mediated demethylation of histone H3 tri-methylation at lysine 27 (H3K27me3) was associated with differentiation into M2-like macrophages.(17) To confirm whether H3K27me3 correlates with the induction of LOX in M2-like macrophages, we investigated H3K27me3 levels in M2-like macrophages. As shown in Fig. 3A, H3K27me3 levels significantly decreased in M2-like macrophages. Moreover, the decrease in H3K27me3 levels was suppressed in the presence of GSK-J4, an inhibitor of Jmjd3, suggesting that Jmjd3 plays a key role in differentiation-mediated H3K27 demethylation. We then investigated the effects of GSK-J4 on LOX expression. As expected, LOX mRNA and protein induction were significantly suppressed in the presence of GSK-J4 (Fig. 3B and C). Additionally, our ChIP results clearly indicated that the level of H3K27me3 within the lox promoter was decreased by the treatment with IL-4 and IL-13, and its decrease was recovered in the presence of GSK-J4 (Fig. 3D).
Fig. 3.
H3K27me3 levels contribute to LOX expression in M2-like macrophages. (A) M0 macrophages were pre-treated with or without GSK-J4 (10 µM) for 30 min. Cells were then treated with IL-4 and IL-13 (20 ng/ml) for 4 h. Histone was extracted from cells, and the expression of H3K27me3 was detected by Western blotting (n = 3) (*p<0.05). (B and C) LOX mRNA and protein expression levels in M2-like macrophages pre-treated with or without GSK-J4 were assessed using real-time RT-PCR (n = 3) and Western blotting (n = 3) (*p<0.05). (D) H3K27me3 levels within the lox promoter region of M2-like macrophages were detected by a ChIP assay (n = 3) (*p<0.05). Graphs show data as the mean ± SD.
LOX derived from M2-like macrophages promotes human breast cancer cell MDA-MB-231 migration
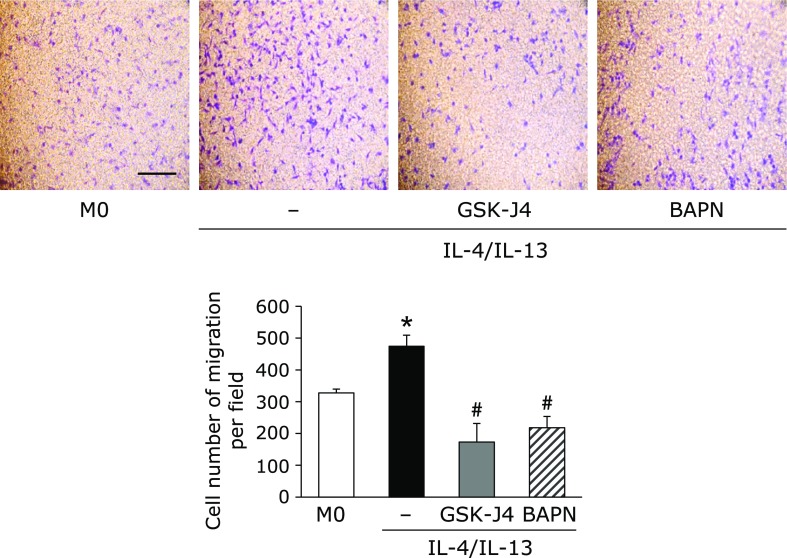
We investigated whether LOX derived from M2-like macrophages promotes cancer cell migration. To assess the involvement of LOX in human breast cancer MDA-MB-231 cell migration, we used the LOX-specific inhibitor, BAPN. As shown in Fig. 4, the number of MDA-MB-231 migrating cells which co-cultured with M2-like macrophages increased. A pre-treatment with GSK-J4 or BAPN suppressed the number of migrating cells.
Fig. 4.
LOX derived from M2-like macrophages promotes human breast cancer cell MDA-MB-231 migration. THP-1 cells were seeded in the lower chamber and incubated with 100 nM TPA for 24 h. After the incubation, M0 macrophages were pre-treated with or without GSK-J4 for 30 min, and then treated with IL-4 and IL-13 (20 ng/ml each) for 24 h. MDA-MB-231 breast cancer cells (1 × 104 cells/well) were seeded in the upper chamber, co-cultured with macrophages for 24 h with or without BAPN (500 µM), and the migratory effects of LOX were then evaluated. Migrated cells were stained and counted under a microscope (n = 3) (*p<0.05 vs M0 macrophages, #p<0.05 vs untreated M2-like macrophages). The scale bar shows 100 µm.
Discussion
M2-like macrophages accumulate in the tumor microenvironment, and this has been correlated with a poor patient outcome.(3) Accumulated evidence revealed that stromal cells and tumor cells release factors that promote metastasis, such as TGF-β and MMPs.(21,22) TGF-β has the potential to promote the EMT process and facilitate tumor cell progression in several tumors.(23,24) We previously reported that the oxidative stress-mediated EMT process plays a critical role in breast cancer progression.(25) On the other hand, decomposition of the basal membrane by MMPs induces tumor cell migration from a primary tumor site to a metastatic site,(26) indicating that MMPs play a key role in tumor metastasis. The induction of MMP2 and MMP9 expression was significantly stronger in M0 macrophages than monocytes (unpublished data), but was not significantly different between M2-like macrophages and M0 macrophages (data not shown). Therefore, other factors may be contributing to M2-like macrophage-derived tumor progression.
Copper, an essential micronutrient, plays an important role in physiological processes including wound repair and angiogenesis.(27,28) Since copper may exist in oxidized and reduced states in the body, this metal have functions as co-factor in redox enzymes.(29) We previously reported that the loss of superoxide dismutase 3, a copper-containing secretory antioxidant enzyme, may result in the accumulation of intracellular ROS in tumor cells, and ultimately exacerbate tumor progression.(30) Copper is also required for characteristic phenomena involved in cancer progression such as proliferative immortality, angiogenesis, and metastasis.(31) Cancer tissues and the sera of cancer patients have been shown to contain elevated copper levels,(32) suggesting that a copper imbalance facilitates tumor progression. LOX, a copper-containing enzyme, cross-links the ECM to create pre-metastatic niches to which bone marrow-derived and tumor cells are recruited.(33) In the present study, we examined the significant induction of LOX in THP-1 cell-derived M2-like macrophages (Fig. 2A and B), which are accompanied by the induction of CD206. Additionally, our results clearly indicated that BAPN suppressed M2-like macrophage-derived breast cancer cell migration (Fig. 4). This result is consistent with previous findings showing that BAPN suppresses hypoxia-induced tumor cell metastasis.(34) Therefore, LOX appears to play an important role in M2-like macrophage-derived tumor progression.
Epigenetics are essential for the development and maintenance of gene expression in mammals. Along with epigenetic alterations, epigenetic abnormalities may be induced, which are closely involved in tumorigenesis and tumor progression.(15) The promoters of various tumor suppressor genes, including Rb and BRCA1, are methylated in ovarian carcinoma.(35–37) Histone modifications, particularly, alternations in H3K27 methylation patterns contribute to aberrant genes, such as PD-1 and CTLA-4, in various tumors.(38) For example, H3K27 methyltransferase is overexpressed in breast cancer.(39) On the other hand, several histone lysine demethylases including Jmjd3, are up-regulated in prostate cancer.(40) These enzymes cooperatively regulate to promote oncogenes and attenuate tumor suppressor genes in malignant cancer. Based on a previous study in which macrophage differentiation into the M2 type is regulated by Jmjd3-mediated H3K27 demethylation,(17) we investigated the involvement of Jmjd3 in LOX induction in M2-like macrophages. As expected, the induction of LOX was significantly suppressed in the presence of GSK-J4, an inhibitor of Jmjd3 (Fig. 3B and C), and this was accompanied by H3K27 demethylation within the lox promoter region (Fig. 3D). These results suggest that Jmjd3-mediated H3K27 plays a key role in the induction of LOX in THP-1 cell-derived M2-like macrophages.
In the present study, we identified the expression of LOX in M2-like macrophages in terms of epigenetic regulation. We also demonstrated that LOX derived from M2-like macrophages promoted breast cancer cell migration. One approach in anti-metastatic therapies to prevent LOX expression by M2-like macrophages may be its inhibition by H3K27 demethylase. We have not yet identified a transcription factor that governs the induction of LOX in M2-like macrophages; therefore, further experiments are needed to elucidate the exact molecular mechanisms involved in LOX expression in M2-like macrophages. Overall, the present results will contribute to our understanding of the role of epigenetics in LOX expression as well as the physiological role of LOX in tumor metastasis.
Author Contributions
RT and TK wrote the manuscript. RT carried out the experiments. RT and TK designed the study. HH and TA supervised the study. All authors interpreted the results, commented on the manuscript, and approved submission of this paper.
Acknowledgments
The present study was supported in part by JSPS KAKENHI (to TK) (Grant Number 17K08277) and the Sasakawa Scientific Research Grant from The Japan Science Society (to TK) (Grant Number 2018-4007).
Abbreviations
- ActD
actinomycin D
- BAPN
β-aminopropionitrile
- ChIP
chromatin immunoprecipitation
- CTR1
copper transporter 1
- DTT
dithiothreitol
- EMT
epithelial-to-mesenchymal transition
- FCS
fetal calf serum
- H3K27me3
histone H3 tri-methylation at lysine 27
- HRP
horseradish peroxidase
- IL
interleukin
- LOX
lysyl oxidase
- MMP
matrix metalloproteinase
- PBS
phosphate-buffered saline
- PMSF
phenylmethanesulfonyl fluoride
- RIPA
radio-immunoprecipitation assay
- ROS
reactive oxygen species
- RT-PCR
reverse transcription-polymerase chain reaction
- SDS
sodium dodecyl sulfate
- TGF
transforming growth factor
- TPA
12-O-tetradecanoylphorbol-13-acetate
- VEGF
vascular endothelial growth factor
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Shaikh S, Brittenden J, Lahiri R, Brown PA, Thies F, Wilson HM. Macrophage subtypes in symptomatic carotid artery and femoral artery plaques. Eur J Vasc Endovasc Surg 2012; 44: 491–497. [DOI] [PubMed] [Google Scholar]
- 2.He C, Carter AB. The metabolic prospective and redox regulation of macrophage polarization. J Clin Cell Immunol 2015; 6. pii: 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res 2006; 66: 605–612. [DOI] [PubMed] [Google Scholar]
- 4.Liu Z, Kuang W, Zhou Q, Zhang Y. TGF-β1 secreted by M2 phenotype macrophages enhances the stemness and migration of glioma cells via the SMAD2/3 signalling pathway. Int J Mol Med 2018; 42: 3395–3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinnakota K, Zhang Y, Selvanesan BC, et al. M2-like macrophages induce colon cancer cell invasion via matrix metalloproteinases. J Cell Physiol 2017; 232: 3468–3480. [DOI] [PubMed] [Google Scholar]
- 6.Qi L, Yu H, Zhang Y, et al. IL-10 secreted by M2 macrophage promoted tumorigenesis through interaction with JAK2 in glioma. Oncotarget 2016; 7: 71673–71685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodríguez JP, Ríos S, González M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J Cell Biochem 2002; 85: 92–100. [PubMed] [Google Scholar]
- 8.Ishida S, Andreux P, Poitry-Yamate C, Auwerx J, Hanahan D. Bioavailable copper modulates oxidative phosphorylation and growth of tumors. Proc Natl Acad Sci U S A 2013; 110: 19507–19512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xia Y, Liu L, Bai Q, et al. Prognostic value of copper transporter 1 expression in patients with clear cell renal cell carcinoma. Oncol Lett 2017; 14: 5791–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao Q, Ge G. Lysyl oxidase, extracellular matrix remodeling and cancer metastasis. Cancer Microenviron 2012; 5: 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasashima H, Yashiro M, Kinoshita H, et al. Lysyl oxidase is associated with the epithelial–mesenchymal transition of gastric cancer cells in hypoxia. Gastric Cancer 2016; 19: 431–442. [DOI] [PubMed] [Google Scholar]
- 12.Wei L, Song XR, Sun JJ, Wang XW, Xie L, Lv LY. Lysyl oxidase may play a critical role in hypoxia-induced NSCLC cells invasion and migration. Cancer Biother Radiopharm 2012; 27: 672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El-Haibi CP, Bell GW, Zhang J, et al. Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy. Proc Natl Acad Sci U S A 2012; 109: 17460–17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox TR, Gartland A, Erler JT. Lysyl oxidase, a targetable secreted molecule involved in cancer metastasis. Cancer Res 2016; 76: 188–192. [DOI] [PubMed] [Google Scholar]
- 15.Jones PA, Baylin SB. The fundamental role of epigenetic events in cancer. Nat Rev Genet 2002; 3: 415–428. [DOI] [PubMed] [Google Scholar]
- 16.Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res 2006; 8: R38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Satoh T, Takeuchi O, Vandenbon A, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 2010; 11: 936–944. [DOI] [PubMed] [Google Scholar]
- 18.Ichihara M, Kamiya T, Hara H, Adachi T. The MEF2A and MEF2D function as scaffold proteins that interact with HDAC1 or p300 in SOD3 expression in THP-1 cells. Free Radic Res 2008; 52: 799–807. [DOI] [PubMed] [Google Scholar]
- 19.Kamiya T, Machiura M, Makino J, Hara H, Hozumi I, Adachi T. Epigenetic regulation of extracellular-superoxide dismutase in human monocytes. Free Radic Biol Med 2013; 61: 197–205. [DOI] [PubMed] [Google Scholar]
- 20.Toda M, Mizuguchi S, Minamiyama Y, et al. Pirfenidone suppresses polarization to M2 phenotype macrophages and the fibrogenic activity of rat lung fibroblasts. J Clin Biochem Nutr 2018; 63: 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krstic J, Santibanez JF. Transforming growth factor-β and matrix metalloproteinases: functional interactions in tumor stroma-infiltrating myeloid cells. ScientificWorldJournal 2014; 2014: 521754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto H, Itoh F, Iku S, et al. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human pancreatic adenocarcinomas: clinicopathologic and prognostic significance of matrilysin expression. J Clin Oncol 2001; 19: 1118–1127. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Lamouille S, Derynck R. TGF-β-induced epithelial to mesenchymal transition. Cell Res 2009; 19: 156–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang L, Pang Y, Moses HL. TGF-β and immune cells: an important regulatory axis in the tumor microenvironment and progression. Trends Immunol 2010; 31: 220–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kamiya T, Goto A, Kurokawa E, Hara H, Adachi T. Cross talk mechanism among EMT, ROS, and histone acetylation in phorbol ester-treated human breast cancer MCF-7 cells. Oxid Med Cell Longev 2016; 2016: 1284372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehner C, Hockla A, Miller E, Ran S, Radisky DC, Radisky ES. Tumor cell-produced matrix metalloproteinase 9 (MMP-9) drives malignant progression and metastasis of basal-like triple negative breast cancer. Oncotarget 2014; 5: 2736–2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Das A, Sudhahar V, Chen GF, et al. Endothelial antioxidant-1: a key mediator of copper-dependent wound healing in vivo. Sci Rep 2016; 6: 33783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris ED. A requirement for copper in angiogenesis. Nutr Rev 2004; 62: 60–64. [DOI] [PubMed] [Google Scholar]
- 29.Blockhuys S, Wittung-Stafshede P. Roles of copper-binding proteins in breast cancer. Int J Mol Sci 2017; 18. pii: E871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamiya T, Hara H, Yamada H, Imai H, Inagaki N, Adachi T. Cobalt chloride decreases EC-SOD expression through intracellular ROS generation and p38-MAPK pathways in COS7 cells. Free Radic Res 2008; 42: 949–956. [DOI] [PubMed] [Google Scholar]
- 31.Pan Q, Kleer CG, van Golen KL, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res 2002; 62: 4854–4859. [PubMed] [Google Scholar]
- 32.Gupta SK, Shukla VK, Vaidya MP, Roy SK, Gupta S. Serum trace elements and Cu/Zn ratio in breast cancer patients. J Surg Oncol 1991; 46: 178–181. [DOI] [PubMed] [Google Scholar]
- 33.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009; 15: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bondareva A, Downey CM, Ayres F, et al. The lysyl oxidase inhibitor, β-aminopropionitrile, diminishes the metastatic colonization potential of circulating breast cancer cells. PLoS One 2009; 4: e5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stirzaker C, Millar DS, Paul CL, et al. Extensive DNA methylation spanning the Rb promoter in retinoblastoma tumors. Cancer Res 1997; 57: 2229–2237. [PubMed] [Google Scholar]
- 36.Merlo A, Herman JG, Mao L, et al. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med 1991; 1: 686–692. [DOI] [PubMed] [Google Scholar]
- 37.Rice JC, Ozcelik H, Maxeiner P, Andrulis I, Futscher BW. Methylation of the BRCA1 promoter is associated with decreased BRCA1 mRNA levels in clinical breast cancer specimens. Carcinogenesis 2000; 21: 1761–1765. [DOI] [PubMed] [Google Scholar]
- 38.Sasidharan Nair V, El Salhat H, Taha RZ, John A, Ali BR, Elkord E. DNA methylation and repressive H3K9 and H3K27 trimethylation in the promoter regions of PD-1, CTLA-4, TIM-3, LAG-3, TIGIT, and PD-L1 genes in human primary breast cancer. Clin Epigenetics 2018; 10: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bracken AP, Pasini D, Capra M, Prosperini E, Colli E, Helin K. EZH2 is downstream of the pRB-E2F pathway, essential for proliferation and amplified in cancer. EMBO J 2003; 22: 5323–5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD. JMJD3 is a histone H3K27 demethylase. Cell Res 2007; 17: 850–857. [DOI] [PubMed] [Google Scholar]