Abstract
The role of enterobacterial flora in the onset and progression of inflammatory bowel diseases is a topic of considerable interest. Here, we assessed the association among enterobacterial flora, dietary factors, and ulcerative colitis (UC) progression. Forty-six patients with UC who were diagnosed as being in remission were enrolled. We collected each patient’s stool sample one or two days before diagnostic colonoscopy. After colonoscopy, we observed the patients for one year and then retrospectively divided them into two groups: remission (n = 39) and relapse (n = 7) groups, depending on whether the relapse occurred during the follow-up period, and analyzed the relationship among patient characteristics, dietary factors, enterobacterial flora, and UC relapse. Overall, there were no significant differences in bacterial community populations between the remission and relapse groups, except that the order Lactobacillales was detected at a significantly higher rate in the relapse than in the remission group (100% vs 71.4%, p<0.05). Vitamin C intake was significantly higher in the remission than in the relapse group (p<0.05). Although there were no obvious differences in enterobacterial flora between the remission and relapse groups, there was a relationship among enterobacterial flora, diet, and UC progression. Given that the enterobacterial flora was only analyzed at the initiation of the study, we conclude that in future analyses, enterobacterial flora should be sampled at numerous time points to examine its role in UC progression. Further long-term longitudinal studies examining enterobacterial flora, dietary factors, and UC progression are also required.
Keywords: ulcerative colitis, enterobacterial flora, dietary factor
Introduction
Inflammatory bowel disease (IBD), which includes Crohn’s disease and ulcerative colitis (UC), is a chronic and refractory disease of the gastrointestinal tract with unclear cause(1–4) Environmental parameters such as meals, genetics, and abnormalities in the immune system are regarded as causes of IBD; however, further details are unclear. There have also been numerous reports describing the prognosis of patients with IBD,(5–7) but here also the details are unclear. In Japan, the number of patients with IBD increases year by year most likely as a result of the westernization of eating habits. Moreover, IBD appears most commonly in young people, causing a great social burden because of its negative effect on the ability to study and work, and a decline in the quality of life.
Current research has suggested that enterobacterial flora may play a role in the onset and progression of IBD. Detailed gene analysis studies have been carried out using next generation sequencing to provide molecular details about such enterobacterial flora. For example, there is a report that the genus Clostridium causes an enteric-mediated decrease in T cells(8) in IBD patients compared with that in healthy people.(9) There is also a report that a decrease in the genus Clostridium causes enteric inflammation.(10) Andoh et al.(11) reported that fecal microbial communities in patients with IBD are different from those in healthy individuals. On the other hand, several studies examined the relationship between dietary factors and the progression of UC. Keshteli et al.(12) reported that a higher intake of poultry and maltose is related to a decreased risk of UC relapse. Jowett et al.(13) reported that the consumption of meat, red meat, processed meat, protein, and alcohol is related to an increased risk of UC relapse. In contrast, Brotherton et al.(14) did not find any association between fiber intake and disease relapse. The aim of this study was to examine the association among enterobacterial flora, dietary factors, and the progression of UC, which to the best of our knowledge has not been previously explored.
Materials and Methods
Patients and study design
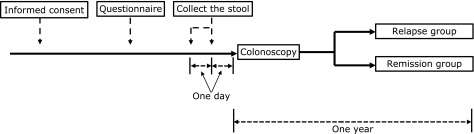
This was a single center, retrospective cohort study. Between December 2015 and May 2017, we enrolled 46 patients with UC at the Aichi Medical University School of Medicine who had been diagnosed as being in remission using the total Mayo score.(15) The protocol (Fig. 1) was reviewed and approved by the Ethics Committees at Aichi Medical University School of Medicine (No. 15-042). All patients gave their written informed consent prior to study inclusion. First, patients answered a questionnaire on their dietary and alcohol habits. Second, we collected patient stool samples the morning before colonoscopy. Third, we performed a diagnostic colonoscopy examination and then observed the patients for one year after the colonoscopy. After observation, we retrospectively divided the patients into two groups (a remission group or a relapse group) depending on whether a relapse occurred during the follow-up period. We then analyzed the relationships among patient characteristics, dietary factors, enterobacterial flora, and UC relapse. UC relapse was defined as a switch of medicine, in addition to other drugs for induction of remission, a total Mayo score of greater than 3, or if either Mayo sub-score was greater than 2. Moreover, we measured fecal calprotectin (FCP) and short chain fatty acids (SCFAs) in the patients for whom we collected an adequately sized stool. Following this, we analyzed the FCP and SCFA levels between the two groups.
Fig. 1.
Flowchart for this study.
Food and alcohol questionnaire
We asked the patients questions about their intake of food, drink, seasonings, and pastry during the last year using a self-administered short food frequency questionnaire (FFQ).(16) In the questionnaire sheet, the intake frequencies were categorized as never or seldom, 1–3 times/month, 1–2 times/week, 3–4 times/week, 5–6 times/week, once/day, twice/day, and >3 times/day. Following this, we scored the questionnaire results and analyzed the data.
Fecal DNA extraction
Stool samples (0.2–0.5 g) were collected a day or two before colonoscopy and were then washed three times with sterile distilled water, suspended in 4 M guanidinium thiocyanate, 100 mM Tris-HCl (pH 9.0), and 40 mM EDTA, and beaten with glass beads using a mini-bead beater (BioSpec Products Inc., Bartlesville, OK). Thereafter, the DNA was extracted from the bead-treated suspension using benzyl chloride, as described by Zhu et al. The DNA extract was then purified using a GFXTM PCR DNA and Gel Band Purification Kit (GE Healthcare, Chicago, IL). The final concentration of each DNA sample was then adjusted to 10 ng/µl.
Terminal restriction fragment length polymorphism (T-RFLP) analysis
Amplification of 16S rDNA and its digestion with restriction enzymes, size fractionation of the terminal restriction fragments (T-RFs), and the analysis of the T-RF length polymorphisms (T-RFLPs) were performed according to the protocol described by Nagashima et al.(17) Briefly, polymerase chain reaction (PCR) was performed using a total fecal DNA (10 ng/µl) sample and Escherichia coli-specific primer pairs for amplification of 16S rDNA (16f: 5'-TGCCAGCAGCCGCGGTA-3' for E. coli positions 516–532 and 1510r: 5'-GGTTACCTTGTTACGACTT-3' for E. coli positions 1510–1492). The 5'-end of the forward primer was labeled with 6'-carboxyfluorescein, which was synthesized by Applied Biosystems Inc. (Foster City, CA). The amplified 16S rDNA genes were purified using a GFXTM PCR DNA and Gel Band Purification Kit (GE Healthcare) and dissolved in 30 µl of distilled water. The purified PCR products (2 µl) were digested with Bsl I (10 U) at 55°C for 3 h. The lengths of the T-RFs were determined using an ABI Prism® 3130xl genetic analyzer (Applied Biosystems Inc.) in GeneScan mode with standard size markers (MapMarker® X-Rhodamine Labeled 50–1,000 bp; BioVentures, Inc., Murfreesboro, TN). The fragment sizes were estimated using the Local Southern Method sizing algorithm (GeneMapper®; Applied Biosystems Inc.).
The T-RFs were divided into 30 operational taxonomic units (OTUs) according to the method described by Nagashima et al.(17) Each OTU was quantified as a percentage of the total OTU area and expressed as the peak percent area under the curve (%). Cluster analyses based on the Bsl I T-RFLP patterns were performed using GeneMaths software (Applied Maths NV, Sint-Martens-Latem, Belgium).
Assessment of fecal calprotectin and short chain fatty acids
The stool samples (FCP: 1 g, SCFAs: 0.1 g) were collected a day or two before colonoscopy. FCP and SCFA levels were measured using the appropriate enzyme-linked immune sorbent assay kit: FCP (Thermo Fisher Diagnostics Co., Ltd., Tokyo, Japan) and SCFAs (Techno Suruga Laboratory Co., Ltd., Shizuoka, Japan).
Statistical analysis
Age, disease duration, Mayo score, the period to relapse, blood test results, the population of bacterial communities, diet comparison, FCP, and SCFAs are expressed as medians and interquartile ranges (IQRs). Categorical variables were compared between the remission group and the relapse group using a Chi-squared test. Continuous variables and ordinal variables were compared between the same two groups using a Mann-Whitney U test. The correlation between enterobacterial flora and clinical parameters [diet, endoscopic Mayo score, disease duration (≥10 years or <10 years), and treatment with azathioprine or steroids] was analyzed using a Spearman rank order correlation. The period to relapse was calculated using the Kaplan-Meier method and is expressed as the median. A p value <0.05 was considered significant. All statistical analyses were performed using BellCurve for Excel (Social Survey Research Information Co., Ltd., Tokyo, Japan).
Results
Patient characteristics
In this study, we analyzed a total of 46 patients with UC. The baseline characteristics of the patients are shown in Table 1. The remission group comprised 39 patients, and the relapse group comprised 7 patients. Twenty-one patients in the remission group were women, whereas three patients in the relapse group were women. The median age of the patients in the remission group was significantly higher than that in the relapse group (p<0.05). Moreover, disease duration in the remission group was significantly longer than that in the relapse group (p<0.05). However, there were no significant differences in disease location, medication, Mayo scores, and blood test results between both groups.
Table 1.
Baseline characteristics (n = 46)
| Remission group (n = 39) | Relapse group (n = 7) | p value | |
|---|---|---|---|
| Sex* | |||
| Male | 18 | 4 | |
| Female | 21 | 3 | NS |
| Age (median, years)** | 49 | 40 | <0.05 |
| Smoking | |||
| Nonsmokers | 25 | 5 | NS |
| Former smokers | 10 | 2 | NS |
| Current smokers | 4 | 0 | NA |
| Disease duration (median, years)** | 10.8 | 2.3 | <0.05 |
| Disease location* | |||
| Pancolitis (n) | 16 | 5 | |
| Left-sided colitis (n) | 19 | 2 | |
| Proctitis (n) | 4 | 0 | NS |
| Medication* | |||
| 5-Aminosalicylic acid (n) | 34 | 6 | NS |
| 5-Aminosalicylic acid enema (n) | 2 | 1 | NS |
| Prednisolone (n) | 1 | 0 | NS |
| Azathioprine (n) | 7 | 1 | NS |
| Anti-tumor necrosis factor-α (n) | 0 | 0 | NA |
| Probiotics (n) | 16 | 3 | NS |
| Others (n) | 1 | 0 | NA |
| Endoscopic Mayo score (median)** | 0 | 1 | NS |
| Total Mayo score (median)** | 1 | 1 | NS |
| Blood test** | |||
| White blood cell (median, /µl) | 5,500 | 4,500 | NS |
| Hemoglobin (median, g/dl) | 13.8 | 14.3 | NS |
| Platelet (median, 104/µl) | 23.4 | 26.8 | NS |
| C-reactive protein (median, mg/dl) | 0.07 | 0.05 | NS |
| Erythrocyte sedimentation rate (median, mm/h) | 5 | 4 | NS |
| Albmin (median, g/dl) | 4.3 | 4.4 | NS |
*Chi-squared test, **Mann-Whitney U test.
The rate of ulcerative colitis relapse
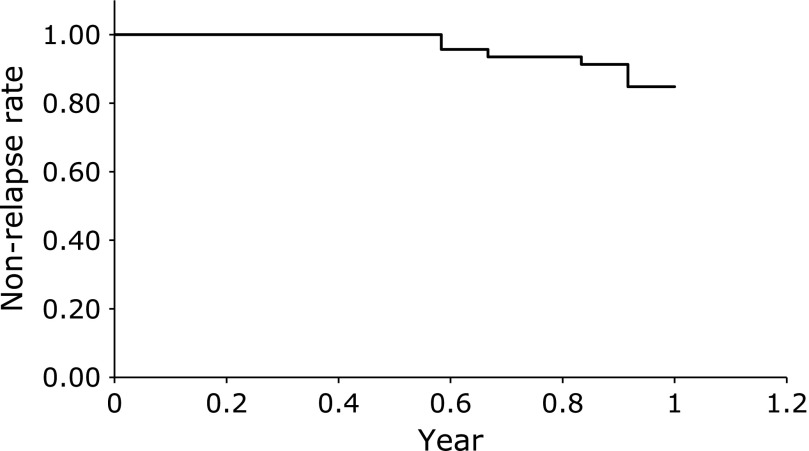
Figure 2 shows the rate of UC relapse. The relapse rate was 15.2% in one year. Moreover, the median time to relapse was 0.83 years.
Fig. 2.
The rate of ulcerative colitis relapse.
T-RFLP analysis of the fecal samples and dendrogram of the enterobacterial flora
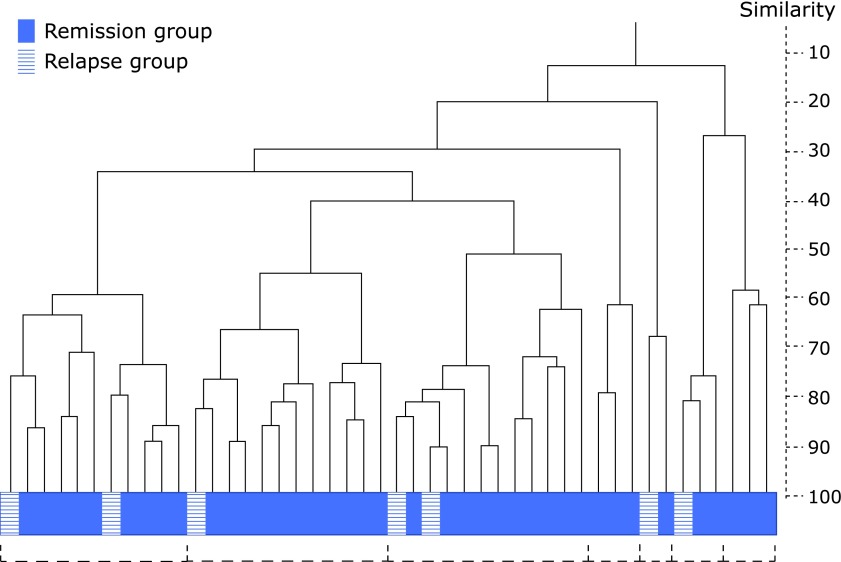
Table 2 shows the results of the T-RFLP analysis. Overall, there were no significant differences in the population of bacterial communities between the remission and relapse groups. However, in this analysis, the detection rate of the order Lactobacillales in the relapse group was significantly higher than that in the remission group (100% vs 71.4%, p<0.05). Figure 3 shows a dendrogram of the enterobacterial flora. The vertical axis expresses the degree of similarity. Based on this dendrogram, we were able to divide the enterobacterial flora into seven distinct clusters. However, even visually, we were unable to separate the two groups of patients into distinct categories.
Table 2.
Terminal restriction fragment length polymorphism analysis
| Remission group (n = 39) |
Relapse group (n = 7) |
||||
|---|---|---|---|---|---|
| Population** (% of total sequences) | Detection rate (%)* | Population** (% of total sequences) | Detection rate (%)* | ||
| Bifidobacterium spp. | 6.7 (2.1–25.0) | 100 | 12.1 (7.2–17.5) | 94.8 | |
| Order Lactobacillales | 4.1 (1.1–21.9) | 71.4# | 8.9 (2.2–13.9) | 100 | |
| Bacteroides spp. | 41.5 (28.1–53.5) | 100 | 40.2 (34.4–50.6) | 100 | |
| Clostridium cluster IV | 3.5 (1.3–5.4) | 100 | 6.8 (0.6–10.0) | 84.6 | |
| Clostridium cluster IX | 1.3 (1.2–4.1) | 85.7 | 1.8 (0.6–6.1) | 87.1 | |
| Clostridium cluster XI | 0.5 (0.3–0.5) | 85.7 | 0.0 (0.0–1.0) | 46.1 | |
| Clostridium subcluster XIVa | 9.5 (6.8–17.3) | 100 | 12.6 (8.2–20.0) | 100 | |
| Clostridium cluster XVIII | 0.7 (0.4–2.2) | 85.7 | 1.6 (0.9–2.1) | 94.8 | |
| Prevotella spp. | 0.0 (0.0–0.3) | 28.5 | 0.0 (0.0–0.8) | 30.7 | |
| Others | 5.3 (5.0–7.2) | 100 | 4.6 (3.3–7.4) | 100 | |
Data are median (interquartile range), #p<0.05 vs Relapse group. *Chi-squared test, **Mann-Whitney U test.
Fig. 3.
Dendrogram of the enterobacterial flora in patients with ulcerative colitis. The vertical axis expresses the similarity.
Diet and alcohol comparison
Table 3 shows a comparison of the diets between the two different groups of patients. The intake of vitamin C in the remission group was significantly higher than that in the relapse group (p<0.05). Moreover, the intake of fat and n-3 polyunsaturated fatty acids in the remission group tended to be higher than that in the relapse group (p = 0.07 and p = 0.08, respectively). There were no significant differences in alcohol intake between the two groups using a Mann-Whitney U test analysis. Similarly, when we analyzed alcohol intake using a Chi-squared test, we found no significant differences between the groups.
Table 3.
Diet comparison
| Remission group (n = 39) | Relapse group (n = 7) | p value | |
|---|---|---|---|
| Total energy (median, kcal) | 1,684.9 (1,542.8–1,911.0) | 1,683.7 (1,403.2–1,720.6) | 0.15 |
| Carbohydrate (median, g) | 233.8 (201.1–248.9) | 231.3 (185.7–251.0) | 0.58 |
| Fat (median, g) | 48.7 (42.0–56.5) | 41.9 (34.4–44.1) | 0.07 |
| Protein (median, g) | 54.1 (46.6–61.7) | 43.6 (42.5–50.3) | 0.14 |
| Vitamin C (median, mg) | 82 (70.2–99.2) | 63.7 (49.0–64.1) | <0.05 |
| SFA (median, g) | 11.7 (9.9–14.6) | 9.6 (9.1–11.3) | 0.13 |
| TDF (median, g) | 11.4 (8.8–12.2) | 9.5 (8.6–11.7) | 0.37 |
| n-3 PUFA (median, mg) | 2,119.7 (1,892.3–2,452.7) | 1,840.8 (1,723.6–1,898.5) | 0.08 |
| n-6 PUFA (median, mg) | 10,614.8 (9,702.5–12,681.4) | 9,613.5 (8,949.2–12,030.4) | 0.4 |
| Alcohol (median, kcal) | 0 (0–11.9) | 0 (0–7.3) | 0.64 |
Data are median (interquartile range). All analyses were performed using Mann-Whitney U test. SFA, saturated fatty acids; TDF, total dietary fiber; PUFA, polyunsaturated fatty acids.
Correlation between enterobacterial flora and clinical parameters
Table 4 shows the only correlations that tended to differ (p<0.1) between the different enterobacterial flora and clinical parameters. The order Lactobacillales and total dietary fiber, the order Lactobacillales and total energy, Clostridium cluster XIVa and carbohydrates, and the genus Prevotella and medication were all positively correlated with each other (r = 0.31; p = 0.03, r = 0.25; p = 0.08, r = 0.25; p = 0.08, r = 0.39; p<0.01, respectively). On the other hand, Clostridium cluster IV and carbohydrates, and Clostridium cluster XIVa and alcohol were both negatively correlated with each other (r = −0.27; p = 0.06, r = −0.28; p = 0.05, respectively).
Table 4.
Correlation of enterobacterial flora and clinical parameters (n = 46)
| Clinical parameters | r | p value | |
|---|---|---|---|
| Order Lactobacillales | TDF (g) | 0.31 | 0.03 |
| Order Lactobacillales | Total energy (kcal) | 0.25 | 0.08 |
| Clostridium cluster IV | Carbohydrate (g) | −0.27 | 0.06 |
| Clostridium subcluster XIVa | Carbohydrate (g) | 0.25 | 0.08 |
| Clostridium subcluster XIVa | Alcohol (kcal) | −0.28 | 0.05 |
| Prevotella | Treatment with AZA or steroids | 0.39 | <0.01 |
All analyses were performed using Spearman rank order correlation. TDF, total dietary fiber; AZA, azathioprine.
Fecal calprotectin and short chain fatty acids
We examined FCP levels in 35 patients in the remission group and in 6 patients in the relapse group. The median FCP levels (mg/kg, IQR) in both groups were 81.5 (28.4–486.0) and 61.0 (31.8–155.9), respectively. In the univariate analysis, there were no significant differences in the FCP levels between the two groups (p = 0.85). Moreover, we examined SCFA levels in 33 patients in the remission group and in 5 patients in the relapse group. The median acetic acid, propionic acid, n-butyric acid, iso-butyric acid, n-valeric acid, iso-valeric, and caproic acid levels (µmol/g, IQR) in the remission group were 36.2 (23.9–51.8), 14.4 (6.3–21.6), 7.6 (3.7–12.7), 1.8 (0.5–2.8), 0.4 (0.0–1.3), 2.5 (0.8–4.0), and 0.0 (0.0–0.0), respectively. The median acetic acid, propionic acid, n-butyric level, iso-butyric acid, n-valeric acid, iso-valeric, and caproic acid levels (µmol/g, IQR) in the relapse group were 42.3 (41.9–51.1), 12.8 (2.6–14.1), 1.8 (1.3–4.7), 1.3 (0.8–1.3), 0.4 (0.3–0.6), 1.5 (0.8–2.1), and 0.0 (0.0–0.0), respectively. Using a univariate analysis, there were no significant differences in these SCFA levels between the two groups (p = 0.53, 0.21, 0.06, 0.27, 0.67, 0.23, and 0.41, respectively).
Discussion
This is the first report exploring the association among enterobacterial flora, dietary factors, and UC progression. In this study, we showed that there was only a difference in the detection rate for the order Lactobacillales between the remission and relapse groups, and that overall, there were no obvious differences in enterobacterial flora populations between these two groups. However, we did show that the intake of vitamin C decreases the risk of UC relapse.
Currently, it is thought that enterobacterial flora is closely correlated with the pathophysiology of UC, and as such, there have been a few reports examining enterobacterial flora in UC. For example, Andoh et al.(11) reported that fecal microbial communities could be classified into five clusters, with almost all of the patients with inactive UC being classified into clusters I, II, and III. In contrast, they reported that almost all the patients with active UC could be classified into clusters IV and V. Fukuda et al.(18) also reported that there was a significant difference in the digitized scores of enterobacterial flora between patients with active UC and patients with quiescent UC. Based on these reports, it can be concluded that there is a significant difference in enterobacterial flora between active UC patients and inactive UC patients. In this study, we analyzed and compared fecal microbial communities between UC patients who were either in remission or who had experienced a relapse. We only found a difference in the detection rate for the order Lactobacillales between the two groups of patients. We think that the reason for this result is that, in this study, we performed only one measurement of the fecal microbial communities, which was at the beginning of the observation period. In addition, the time at which all the patients underwent relapse was in the latter half of the observation period. Therefore, our analysis did not reflect the enterobacterial flora at the time of UC relapse but rather reflected the enterobacterial flora present at some significant time before relapse. It is therefore not surprising that there were no obvious differences in the populations of enterobacterial flora between the remission group and the relapse group. Moreover, we think that the reason for the reported differences in the enterobacterial flora between active UC patients and inactive UC patients is due to UC relapse itself or due to changes in the enterobacterial flora just before UC relapse. We therefore need further studies to observe changes in the enterobacterial flora over time.
Previous studies have reported that there is a relationship between dietary factors and the progression of UC. For example, Keshteli et al.(12) compared the intake of nutrients and food between a UC remission group and a UC relapse group. They showed that a higher intake of poultry and maltose was related to a decreased risk of UC relapse. They also showed that there was no significant difference in the intake of vitamin C between the two groups. Vitamin C is a well-known antioxidant, and there have been several reports examining the relationship between antioxidants and UC therapy. In this regard, Kimura et al.(19,20) reported that 5-[4-(2-carboxyethylcarbamoyl) phenylazo] salicylic acid disodium salt dihydrate (CAS 80573-04-2, BX661A) is being developed as a new therapeutic drug for the treatment of UC. In addition, the gastro-protective drug rebamipide, an amino acid derivative of 2-(1H)-quinolinone, is also active as a hydroxyl radical scavenger in vitro.(21,22) In our study, vitamin C intake was found to be associated with a decreased risk of UC relapse. Since Heesook et al.(23) reported that nutrition intervention should be carried out to ensure better treatment efficacy in IBD patients, we think that an increased intake of vitamin C would play a role in preventing UC relapse. On the other hand, Sugihara et al.(24) reported that dietary phosphate exacerbates intestinal inflammation in experimental colitis. In this study, we were not able to examine the intake of phosphate. Thus, further prospective studies that consider the intake of phosphate in IBD patients are needed.
In this study, we also analyzed the correlation between enterobacterial flora and clinical parameters. Prior to this study, there were several reports examining the correlation between enterobacterial flora and dietary factors. The enterobacterial flora digests numerous dietary fibers, which are not able to be digested by the human gastrointestinal tract, and in the process, the levels of SCFAs such as butyric acid, propionic acid, and acetic acid increase. Butyric acid and propionic acid are important energy sources for the epithelium of the colonic mucosa. Moreover, butyric acid and propionic acid derive control-related T cells, and reduce colonic inflammation.(25,26) Consistent with a previous report, in our study, the order Lactobacillales and TDF were positively correlated with each other in UC patients. From the results of this study, we hypothesize that dietary factors can influence the enterobacterial flora in UC patients, and therefore, this requires further study.
In our study, we also found that there were no significant differences in FCP and SCFA levels between the remission and relapse groups. Recently, FCP has been reported to be a predictor of relapse in UC. Lasson et al.(27) reported that patients with elevated FCP levels are at risk of relapse within 3 months following FCP increase. Moreover, Ferreiro-Iglesias et al.(28) showed that high FCP levels predict a relapse occurring within the following 2 months in IBD patients undergoing infliximab maintenance therapy. In accordance with our data on enterobacterial flora, we think that the reason for the lack of significant differences in FCP SCFA levels between the remission and relapse groups in our study is that relapse times in all the patients in the relapse group were more than 6 months from the beginning of observation. As reported, we also think that FCP and SCFA levels allow for a prediction of relapse within a few months. Further studies are therefore required to address this.
There are some limitations in this study. First, this study included a relatively small number of UC patients in a single center. Second, we only analyzed the enterobacterial flora once at the beginning of the observation period. We recognize the need to analyze microbial communities in UC relapse patients over periods of time to confirm the usefulness of analyzing microbial communities as a prognostic factor in UC patients. Third, we were not able to obtain enough feces to measure FCP and SCFA levels in all patients.
In conclusion, there were no obvious differences in enterobacterial flora between the UC remission group and the UC relapse group. However, we did show that there was a relationship among enterobacterial flora, diet, and UC progression. In the future, a detailed analysis of enterobacterial flora taken over time in UC relapse should be conducted. Moreover, we think that further long-term longitudinal studies examining enterobacterial flora, dietary factors, and UC progression are needed.
Author Contributions
TS and MS designed the study, performed the statistical analysis, and wrote the manuscript. SN, SI, KA, TY, YY, YT, SI, YH, ME, MM, SY, YF, and NO acquired the data. CG performed the statistical analysis. KK interpreted the data. All authors drafted and gave final approval of the manuscript.
Acknowledgments
We would like to thank T. Yamauchi for assistance with collecting the fecal samples, and Editage (www.editage.jp) for English language editing.
Abbreviations
- FCP
fecal calprotectin
- FFQ
short food frequency questionnaire
- IBD
inflammatory bowel disease
- IQR
interquartile range
- OTU
operational taxonomic unit
- PCR
polymerase chain reaction
- SCFAs
short chain fatty acids
- T-RFLP
terminal restriction fragment length polymorphism
- UC
ulcerative colitis
Conflicts of Interest
KK received lecture fees from Daiichi Sankyo Co., Ltd., Astra Zeneca Co., Ltd., EA Pharma Co., Ltd., Mylan Co., Ltd., Sanwa Kagaku Kenkyusho Co., Ltd., and Takeda Pharmaceutical Co., Ltd. KK received research grants from Astellas Co., Ltd., Daiichi Sankyo Co., Ltd., EA Pharma Co., Ltd., Mitsubishi Tanabe Pharma Co., Ltd., and Takeda Pharmaceutical Co., Ltd. The other authors have no conflicts of interest to declare.
References
- 1.Hibi T, Ogata H. Novel pathophysiological concepts of inflammatory bowel disease. J Gastroenterol 2006; 41: 10–16. [DOI] [PubMed] [Google Scholar]
- 2.Sands BE. Inflammatory bowel disease: past, present, and future. J Gastroenterol 2007; 42: 16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayer L. Evolving paradigms in the pathogenesis of IBD. J Gastroenterol 2010; 45: 9–16. [DOI] [PubMed] [Google Scholar]
- 4.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347: 417–429. [DOI] [PubMed] [Google Scholar]
- 5.Frøslie KF, Jahnsen J, Moum BA, Vatn MH; IBSEN group. Mucosal healing in inflammatory bowel disease: results from a Norwegian population-based cohort. Gastroenterology 2007; 133: 412–422. [DOI] [PubMed] [Google Scholar]
- 6.Solem CA, Loftus EV, Tremaine WJ, Harmsen WS, Zinsmeister AR, Sandborn WJ. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis 2005; 11: 707–712. [DOI] [PubMed] [Google Scholar]
- 7.Chen JM, Liu T, Gao S, Tong XD, Deng FH, Nie B. Efficacy of noninvasive evaluations in monitoring inflammatory bowel disease activity: a prospective study in China. World J Gastroenterol 2017; 23: 8235–8247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atarashi K, Tanoue T, Shima T, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 2011; 331: 337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loh G, Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes 2012; 3: 544–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peloquin JM, Nguyen DD. The microbiota and inflammatory bowel disease: insights from animal models. Anaerobe 2013; 24: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andoh A, Imaeda H, Aomatsu T, et al. Comparison of the fecal microbiota profiles between ulcerative colitis and Crohn’s disease using terminal restriction fragment length polymorphism analysis. J Gastroenterol 2011; 46: 479–486. [DOI] [PubMed] [Google Scholar]
- 12.Keshteli AH, van den Brand FF, Madsen KL, et al. Dietary and metabolomic determinants of relapse in ulcerative colitis patients: a pilot prospective cohort study. World J Gastroenterol 2017; 23: 3890–3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jowett SL, Seal CJ, Pearce MS, et al. Influence of dietary factors on the clinical course of ulcerative colitis: a prospective cohort study. Gut 2004; 53: 1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brotherton CS, Martin CA, Long MD, Kappelman MD, Sandler RS. Avoidance of fiber is associated with greater risk of Crohn’s disease flare in a 6 month period. Clin Gastroenterol Hepatol 2016; 14: 1130–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med 1987; 317: 1625–1629. [DOI] [PubMed] [Google Scholar]
- 16.Tokudome S, Goto C, Imaeda N, Tokudome Y, Ikeda M, Maki S. Development of a data-based short food frequency questionnaire for assessing nutrient intake by middle-aged Japanese. Asian Pac J Cancer Prev 2004; 5: 40–43. [PubMed] [Google Scholar]
- 17.Nagashima K, Hisada T, Sato M, Mochizuki J. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol 2003; 69: 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukuda K, Fujita Y. Determination of the discriminant score of intestinal microbiota as a biomarker of disease activity in patients with ulcerative colitis. BMC Gastroenterol 2014; 14: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimura I, Kawasaki M, Matsuda A, Kataoka M, Kokurba Y. Effects of BX661A, a new therapeutic agent for ulcerative colitis, on chemotaxis and reactive oxygen species production in polymorphonuclear leukocytes in comparison with salazosulfapyridine and its metabolite sulfapyridine. Arzneimittelforschung 1998; 48: 1163–1167. [PubMed] [Google Scholar]
- 20.Kimura I, Kawasaki M, Nagahama S, Matsuda A, Kataoka M, Kokuba Y. Determination of the active moiety of BX661A, a new therapeutic agent for ulcerative colitis, by studying its therapeutic effects on ulcerative colitis induced by dextran sulfate sodium in rats. Arzneimittelforschung 1998; 48: 1091–1096. [PubMed] [Google Scholar]
- 21.Naito Y, Yoshikawa T, Tanigawa T, et al. Hydroxyl radical scavenging by rebamipide and related compounds: electron paramagnetic resonance study. Free Radic Biol Med 1995; 18: 117–123. [DOI] [PubMed] [Google Scholar]
- 22.Yoshikawa T, Naito Y, Tanigawa T, Kondo M. Free radical scavenging activity of the novel anti-ulcer agent rebamipide studied by electron spin resonance. Arzneimittelforschung 1993; 43: 363–366. [PubMed] [Google Scholar]
- 23.Lim H, Kim HJ, Hong SJ, Kim S. Nutrient intake and bone mineral density by nutritional status in patients with inflammatory bowel disease. J Bone Metab 2014; 21: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugihara K, Masuda M, Nakao M, et al. Dietary phosphate exacerbates intestinal inflammation in experimental colitis. J Clin Biochem Nutr 2017; 61: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013; 504: 446–450. [DOI] [PubMed] [Google Scholar]
- 26.Arpaia N, Campbell C, Fan X, et al. Metabolites produced by commensal bacteria promote peripheral regulatory T cell generation. Nature 2013; 504: 451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lasson A, Simrén M, Stotzer PO, Isaksson S, Öhman L, Strid H. Fecal calprotectin levels predict the clinical course in patients with new onset of ulcerative colitis. Inflamm Bowel Dis 2013; 19: 576–581. [DOI] [PubMed] [Google Scholar]
- 28.Ferreiro-Iglesias R, Barreiro-de Acosta M, Otero Santiago M, et al. Fecal calprotectin as predictor of relapse in patients with inflammatory bowel disease under maintenance Infliximab therapy. J Clin Gastroenterol 2016; 50: 147–151. [DOI] [PubMed] [Google Scholar]