Abstract
Transplant recipients are vulnerable to a higher risk of malignancy after solid organ transplantation and allogeneic hematopoietic stem-cell transplant. Post-transplant lymphoproliferative disorders (PTLD) include a wide spectrum of diseases ranging from benign proliferation of lymphoid tissues to frank malignancy with aggressive behavior. Two main risk factors of PTLD are: Firstly, the cumulative immunosuppressive burden, and secondly, the oncogenic impact of the Epstein-Barr virus. The latter is a key pathognomonic driver of PTLD evolution. Over the last two decades, a considerable progress has been made in diagnosis and therapy of PTLD. The treatment of PTLD includes reduction of immunosuppression, rituximab therapy, either isolated or in combination with other chemotherapeutic agents, adoptive therapy, surgical intervention, antiviral therapy and radiotherapy. In this review we shall discuss the prevalence, clinical clues, prophylactic measures as well as the current and future therapeutic strategies of this devastating disorder.
Keywords: Lymphoproliferative disorders, Epstein-Barr virus, Solid organ transplant, Hematopoietic stem cell transplant, Post-transplant lymphoproliferative disorder prevention, Future therapies
Core tip: Post-transplant lymphoproliferative disorders (PTLD) is a serious complication related to the intensity of post-transplant immunosuppression. The role of Epstein-Barr virus (EBV) in PTLD evolution is well established; however, development of PTLD in EBV negative patients is not uncommon. The key step in the management of PTLD is to reduce the immunosuppressive load. Transplant clinicians should be vigilant to the possibility of this complication, particularly in patients with past history of exposure to immunosuppression during treatment of the primary renal disease. High index of suspicion is crucial for timely diagnosis. Therapeutic options include rituximab, chemotherapy, antivirals, adoptive therapy and surgery.
INTRODUCTION
Post-transplant lymphoproliferative disorders (PTLD) are one of the most important malignancies after solid organ transplantation (SOT) and hematopoietic stem-cell transplant (HSCT)[1], and it develops as a result of uncontrolled B cell proliferation due to blunted immunological surveillance. B cells may get infected by Epstein-Barr virus (EBV) either by: (1) Post-transplant viral reactivation; and (2) Primary EBV infection, through the donated organ or via environmental exposure. The majority of PTLD cases (> 85%) are usually observed in the first post-transplant year. On the other hand, PTLD as a result of T-cell proliferation is seen much less commonly and is mostly EBV-negative. The magnitude of cumulative immunosuppressive burden has a crucial role in PTLD evolution[2]. Lymphoma accounts for 21% of all malignancies in SOT recipients as compared to 4% and 5% in immunocompetent individuals, respectively in men and women[3,4]. Clinically, PTLD may manifest either as localized lesion or as systemic disease. Lowering the clinical threshold of PTLD diagnosis is fundamental. Transplant clinicians should be vigilant to this serious disorder. Tissue diagnosis (histopathology) is crucial for PTLD diagnosis, in addition to a clear evidence of EBV DNA, RNA, or protein material[2].
The mainstay of PTLD primary management is reduction of immunosuppression (RI). Complete cessation of the immunosuppressive drugs may be necessary to stop the disease progression. However, RI is not always feasible; a potential risk of allograft loss or graft dysfunction has to be considered particularly for vital organ transplants (e.g., heart transplant). A variety of therapeutic options include surgical clearance, anti-viral agents, local radiotherapy, intravenous immunoglobulin (IVIG), chemotherapeutic agents, monoclonal antibodies and cytotoxic T lymphocytes with variable success[2]. A combination of the above treatment modalities offers better results rather than when used in isolation.
Epidemiology of PTLD
Penn et al[5] described five cases of PTLD in 1969 for the first time. Since that time, an increased recognition of PTLD has been observed in both SOT as well as in HSCT[6,7]. Many explanations have been suggested to elucidate the increased awareness of PTLD prevalence e.g., better diagnostic technology, older age of donors and recipients, increased awareness of this disorder, the advent of new immunosuppressive strategies and introduction of the haplo-identical (HSCT).
The increased risk is expressed as “standardized incidence ratios” (SIRs) i.e., the incidence of lymphoma in transplant cohort divided by its incidence in general population (non-transplant cohort)[8]. SIRs of 10 (non-Hodgkin’s lymphoma) and 4 (Hodgkin’s lymphoma) have been reported among SOT recipients[8]. On the other hand, a reported incidence of PTLD in 3.2% of HSCT recipients has been observed in multicenter studies[7].
Risk factors
Risk factors are, reportedly, varied according to the type of the transplant organ:
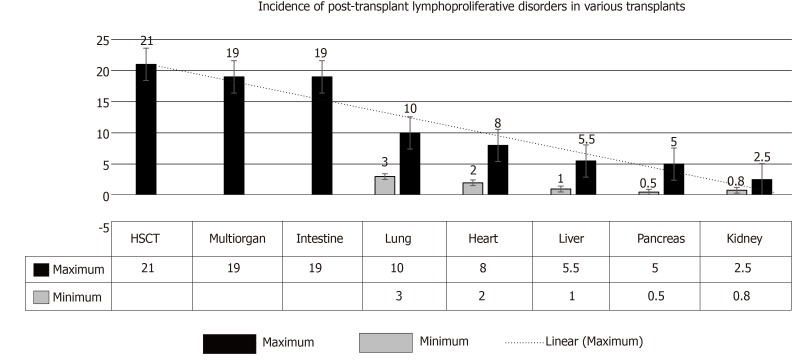
(1) SOT: In adults, the incidence of PTLD has been reported to range from 0.8%-2.5% in kidney transplant recipients (KTR), 0.5%-5.0% in pancreatic TRs, 1.0%-5.5% in liver TRs, 2.0-8.0% in heart TRs, 3.0-10.0% in lung TRs, and ≤ 20% in multi-organ and intestinal TRs[9,10] (Figure 1). These figures suggest that the amount of lymphatic tissue in an allograft and the degree of immunosuppression are key factors.
Figure 1.
The range increased incidence of post-transplant lymphoproliferative disorders in various transplants. Incidence in intestinal transplant and in multi-organ transplants it is < 20%, while in hematopoietic stem-cell transplant it is > 20% with selective T-cell depletion[4]. HSCT: Haplo-identical allogeneic hematopoietic stem-cell transplant.
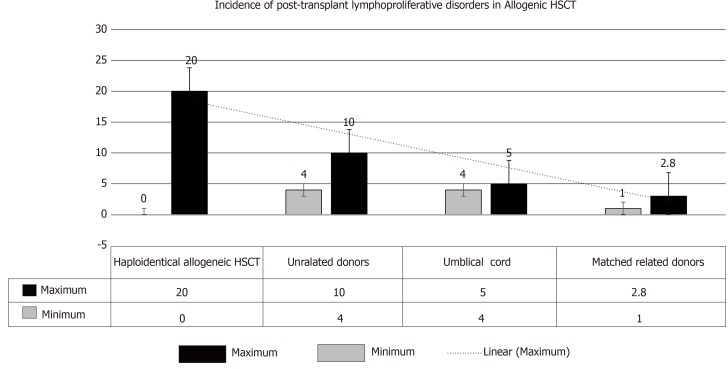
(2) Allogenic HSCT: PTLD incidence is primarily related to the degree of HLA matching with consequent introduction of T-cell depleting agents prior to transplant. Higher risk, however, has been observed with particular T-cell depleting strategies (relative risk: 8.4-15.8). On the other hand, its incidence has been relatively lower with the use of non-specific broad lymphocyte depleting agents (T- and B-cells) (relative risk = 3.1)[11]. Hence, the magnitude of increased risk of PTLD can be graded as follows: (1) HSCT (zero in patients who received cyclophosphamide for GVHD and > 20% with selective T-cell depletion); (2) Umbilical-cord transplantation (4%-5%); (3) Transplant from unrelated donors (4%-10%); and (4) Transplant from matched, related donors (1%-3%)[7,10-13] (Figure 2).
Figure 2.
Incidence of post-transplant lymphoproliferative disorders after allogenic hematopoietic stem-cell transplant. An additional risk factor in hematopoietic stem-cell transplantation is: recipient age of > 50 yr[4]. HSCT: Haplo-identical allogeneic hematopoietic stem-cell transplant.
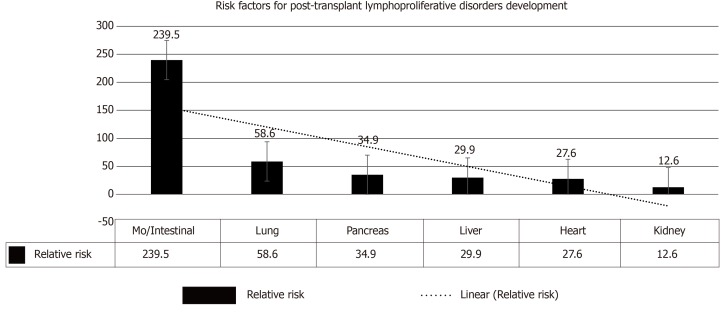
Impaired immune surveillance has been considered to be the explanation for infection-related malignancy a phenomenon similar to the predisposition of malignancy in patients with human immunodeficiency virus[14]. The role of immunosuppressive agents is less clear due to variability in timing, duration, and dosage in different immunosuppressive strategies. Whereas the type of induction therapy has a fundamental role in the early developed PTLD, the one that develops late PTLD is largely determined by cumulative immunosuppressive burden. A number of PTLDs in allogeneic HSCT are donor-driven (EBV-infected lymphocytes) and are usually observed in 1st post-transplant year, with almost 100% being EBV positive. The most crucial contributing factors for PTLD evolution were the “donor type” as well as the “T-cell depleting strategy”[11]. However, sharing role of other factors is less evident (Figure 3).
Figure 3.
Risk factors for the development of post-transplant lymphoproliferative disorders after solid-organ transplantation[4]. MO: Multi-organ.
The lack of long-term follow up of TRs may result in underestimation of actual incidence of PTLD. On the other hand, the registry data might result in overestimation of this cohort of patients[15]. Compared to EBV seropositive TRs, the seronegative patients in SOT are more vulnerable to develop PTLD with an increased estimated risk of 10-75[16,17]. This observation explains the high prevalence of PTLD in pediatric TRs. By far, the primary EBV infection is considered the most effective factor triggering PTLD development in pediatric age group. Considering the improving patient and allograft survival, two peaks of PTLD incidence have been observed, first peak: In the first post-transplant year (mostly EBV seropositive), and, second peak: Usually present 5-15 years after transplant (mostly EBV seronegative). Furthermore, the evolution of the late PTLD (> 20 years post-transplant) has been on rise[10,18].
(3) It is noteworthy to mention that the presence of previous exposure to the immunosuppressive load during treatment of the primary renal disease in the native kidney is an unnoticed risk factor for PTLD evolution.
(4) Oncogenic EBV: EBV may alter cell growth via several mechanisms: (1) With lack of immune recognition, EBV may induce highly regulated growth transformation with expression of all of its growth inducing proteins. (2) Induction of the potent oncogenes e.g., LMP1 and LMP2 via environmental factors. (3) EBV induced proliferating cells as well as EBV variant/HLA types combination may permit these proteins to by-pass immune control and go unrecognized. And (4) Growth alterations with the right levels of expression of cell targets and viral and cellular mRNA[19].
Serology via viral capsid antigens (VCA-IgG) antibody detection is the best solitary serological test to indicate previous EBV exposure. Molecular testing: essential diagnostic technique in immunocompromised TR, where serology can be confusing and unclear owing to the erratic humoral response. Consequently, (molecular plus serological methods) combination may allow early detection of EBV with prompt diagnosis of infection[19]. Healthy donors may carry the high-risk variants of LMP-1 that predispose to malignant evolution. Understanding EBV molecular epidemiology in various populations and recognition of virulent strains can help in institution of a robust preventive strategies of PTLD[20]. In view of the better understanding of these underlying mechanisms, each one may admit a potential therapeutic target, e.g., cytotoxic T-cell immunotherapeutic agents targeting EBV proteins. Critical pathways (activated by EBV) blockers e.g., NFκB, PI3kinase, EGFR, can also block critical activation locations of EBV oncogenes[19].
Pathogenesis
Role of EBV: For decades, PTLD development was attributed mainly to EBV infection, however, recent reports suggest that as many as 50% PTLD in SOT are not accompanied by EBV infection[21]. For EBV-positive TRs, the development of PTLD can be attributed to immunosuppressive-induced decline in the T-cell immune-surveillance. EBV can integrate into normal B-cell program leading to proliferation and transformation of these cells. Normally, these antigens would trigger a T-cell response capable of destruction of most of the EBV-infected B cells. However, this immune defense mechanism has been compromised in TRs leading to unlimited B-cell transformation and the evolution of lymphoma[22]. On the other hand, pathogenesis of PTLD in EBV-negative patients is less evident. Several hypotheses have been postulated e.g., CMV or another viral infection, prolonged immunosuppression, allograft-driven persistent antigenic triggering, hit-and-run hypothesis i.e., EBV commences the pathogenic process leading to the development of PTLD and then vanishes.
EBV-positive vs EBV-negative PTLD: In the light of molecular-genomic data of diffuse large B-cell lymphoma subtype, a range of distinguishing features have been identified to discriminate between EBV+ve and EBV-ve PTLD (Table 1)[25]. However, there is a lack of clear distinction between clinical consequences of different EBV serotypes and their response to therapy. Further studies are warranted to recognize more precise molecular-genomic classification of both types.
Table 1.
Epstein-Barr virus-positive vs Epstein-Barr virus-negative post-transplant lymphoproliferative disorders[25]
| EBV-positive PTLD | EBV-negative PTLD | |
| Molecular-genomic studies | Fewer genomic abnormalities | Share many genomic/ transcriptmic features with diffuse large B-cell lymphoma in IC patients |
| Origin | Mostly B-cell proliferative lesions | Mostly T-cell proliferative lesions |
| Gene-expression | “Non-germinal” center B-cell | “Germinal center B-cell type”[4] |
| Prevalence | More common (first peak) | Less common (second peak) |
| Risk of PTLD | Less risk compared to seronegative TR | Seronegative SOT pediatric TR are more vulnerable to develop PTLD with increased estimated risk of 10-75[16,17] |
| SOT vs HSCT | Almost all cases of HSCT (100%) are EBV positive | In SOT, both EBV positive and negative are present |
| Clinical consequences of EBV status | Less clear | Less clear |
| Prognosis/response to therapy in adults. | Not prognostic/predictive of response to therapy[21,23] | |
| Common criteria | A considerable proportion of both EBV+ve and -ve PTLD respond to RI as a sole intervention[24] | |
| Future studies | Whole-exome/genome wide sequencing and studies of role of EBV-associated microRNAs, may further define PTLD pathogenesis with more precise molecular-genomic classification of both EBV+ve and EBV-ve PTLD | |
RI: Reduction of immunosuppression; IC: Immunocompetent; PTLD: Post-transplant lymphoproliferative disorders; EBV: Epstein-Barr virus; HSCT: Haplo-identical allogeneic hematopoietic stem-cell transplant; KTR: Kidney transplant recipients; TR: Transplant recipients; SOT: Solid organ transplantation.
T-cell subtype PTLD (usually EBV-ve), a rare tumor, and presents with manifestations that are dissimilar to those of T-cell lymphoma in immunocompetent subjects[25]. However, molecular-genomic information would help to define best therapeutic strategies for both types[24].
Classification: The main differences between early and late onset PTLD have been shown in Tables 2 and 3. However, depending mainly on histopathological classification, diagnosis of PTLD can be categorized according to WHO 2017 Classification, as follows: (1) Three nondestructive PTLD: plasmacytic hyperplasia, florid follicular hyperplasia, and infectious mononucleosis-like PTLD. (2) Polymorphic PTLD. (3) Monomorphic PTLD (B-cell, T-cell, or natural killer-cell types). And (4) classic Hodgkin’s lymphoma-like PTLD.
Table 2.
Early vs late onset post-transplant lymphoproliferative disorders in adults[4]
| Early PTLD | Late onset PTLD | |
| General characteristics | EBV positivity | Frequent EBV negative tumors |
| Graft involvement | Less often graft involvement[3] | |
| Less often: Extranodal disease | Extra-nodal disease: Common | |
| Nondestructive PTLD1: Present early | High incidence of late onset Hodgkin’s lymphoma after allogeneic HSCT | |
| Less often: Monomorphic subtype[3] | Specific tumorigenic events: C-myc translocations | |
| Origin: higher % of donor-derived PTLD especially in 1st post-tx year) | Elevated LDH level | |
| Risk factors | Same | Same |
| Response to therapy | Same | Same |
| Patient survival (at 1- and 5- yr) | 65% and 46%, (In adult heart/lung tx)[1,45] | 53% and 41% (In adult heart/lung tx)[1,45] |
| Future therapy | Proteasome inhibition (bortezomib) may be useful after allogeneic HSCT[3] | |
| Role of immun-osuppression | Induction therapy has a role | Cumulative immunosuppression is crucial |
| Prevalence | Majority of PTLD cases | Less prevalent |
1Non-destructive post-transplant lymphoproliferative disorders includes plasmacytic hyperplasia post-transplant (according to the Classification of PTLD by the WHO). Tx: Transplantation; PTLD: Post-transplant lymphoproliferative disorders; EBV: Epstein-Barr virus; HSCT: Haplo-identical allogeneic hematopoietic stem-cell transplant; LDH: Lactate dehydrogenase.
Table 3.
Early vs late onset post-transplant lymphoproliferative disorders in pediatrics[46]
| Early PTLD | Late PTLD | |
| General criteria | Diffuse large B-cell or other B-cell lymphoma | Burkitt’s lymphoma and Hodgkin’s disease are late events[47] |
| Atypical presentation (graft dysfunction, abdominal pain, frequent extra-nodal involvement in > 80% of TR)[46] | Frequent EBV negative tumors. Specific tumorigenic events e.g., C-myc translocations are restricted to late PTLDs | |
| Time to PTLD | Shortest for lung, heart/lung TR. Early PTLD is quite frequent in liver TR (Late PTLD beyond 5 yr is rare, immunosuppression can be tapered/hold due to tolerance) | Longest for the heart TR and at risk for late PTLD even > 10 yr after trans-plantation |
| Patient survival | No significant difference in most published studies[20,47-49] | |
| Distinct criteria | B-cell origin, almost exclusively EBV+ve, reflecting reduced immunosurv-eillance as major pathogenetic factor | Resembles tumors with distinct pathogenetic alterations and nodal appearance[46] |
| Role of immunos-uppression | Induction therapy has a role. More likely to develop graft rejection and switch to Tac before PTLD diagnosis | Cumulative immunosuppression is crucial |
Tx: Transplantation; TR: Transplant recipient; PTLD: Post-transplant lymphoproliferative disorders; EBV: Epstein-Barr virus; HSCT: Haplo-identical allogeneic hematopoietic stem-cell transplant; LDH: Lactate dehydrogenase.
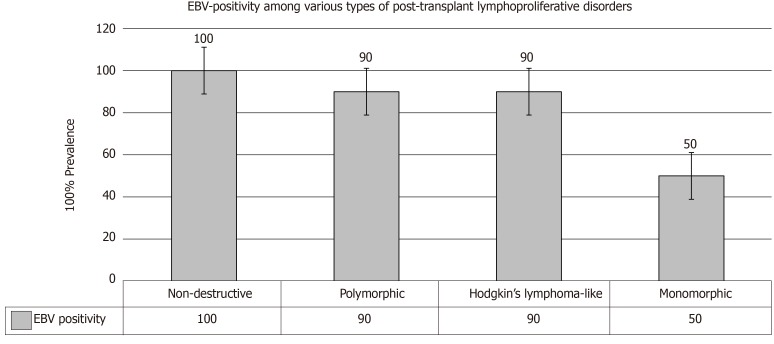
An associated EBV infection could be currently seen in almost all TRs with non-destructive PTLD, in > 90% of patients with polymorphic PTLD and Hodgkin’s lymphoma–like PTLD, and in only 50% of monomorphic PTLD (Figure 4). Pathologically, monomorphic PTLD cannot be discriminated from lymphomas in immunocompetent patients[26,27].
Figure 4.
Epstein-Barr virus positivity among various types of post-transplant lymphoproliferative disorders[4]. EBV: Epstein-Barr virus.
Gene-expression profile and immunohistochemical staining have been used to classify the diffuse large B-cell lymphoma in immunocompetent subjects depending on the cell of origin into “germinal center” B cell or “non–germinal” center B cell[28-30]. In PTLD, EBV+ve cases are mostly non-germinal center B-cell type, in contrary to the EBV-ve cases that are more likely to be “germinal center B-cell type”[31,32]. The presence of EBV infection is not necessary for PTLD diagnosis; however, the EBV-encoded RNA (EBER) in-situ hybridization assessment is mandatory for all the cases[33]. Despite wide-spread application of preemptive monitoring of peripheral-blood EBV viral load, it seems to be devoid of any diagnostic benefit. The pathological classification by the WHO aims for more consistency for better PTLD diagnosis, however, several aspects are currently missing: EBV sero-status, molecular-genomic criteria and transplant organ type (SOT vs HSCT)[34]. Once the histopathologic configuration is confirmed, prompt staging for PTLD is obtained via application of the currently used staging for lymphoma.
Clinical presentation: Clinically, PTLD manifestations vary from symptomless lesions to fulminating disease with multi-organ failure.
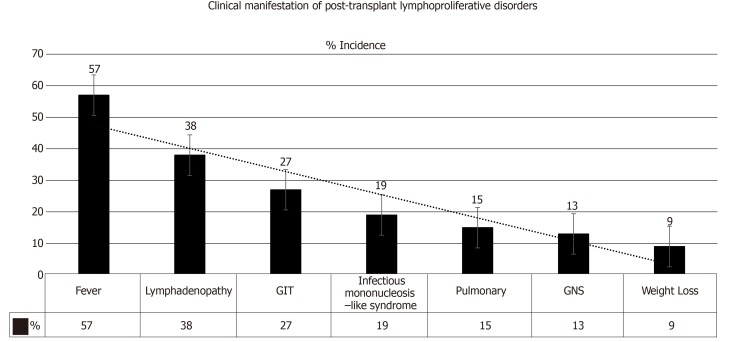
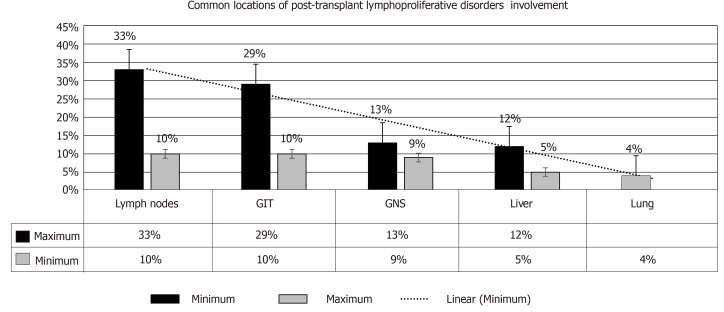
Salient features: PTLD may present as a local or disseminated disease. In either form, the tumor can behave aggressively in a rapidly progressive manner. Clinical manifestations include: Pyrexia (57%)[1], weight loss (9%)[35], neurological manifestations (13%)[36], nodal lesions (38%)[37], gastrointestinal manifestations (27%)[27], pulmonary manifestations (15%)[38] and infectious mononucleosis-like syndrome that could be fulminant (19%)[39], refer to Figure 5. An allograft dysfunction may ensue due to graft involvement. Lowering the threshold for PTLD diagnosis is crucial, as TR may present with nonspecific symptoms (e.g., fever, asthenia). An associated high EBV viral load by PCR should make one suspect PTLD[40-42]. The most common locations of PTLD involvement are as follows[43,44]: Lymph nodes, liver, lung, kidney, bone marrow, gastrointestinal tract (GIT), spleen, central nervous system (CNS), tonsils and salivary glands, refer to Figure 6[1,2].
Figure 5.
Clinical manifestations of post-transplant lymphoproliferative disorders[2]. GIT: Gastrointestinal tract; CNS: Central nervous system.
Figure 6.
Common locations of post-transplant lymphoproliferative disorder involvement[1]. GIT: Gastrointestinal tract; CNS: Central nervous system.
Differential diagnosis: Any high-risk TR who presents with pyrexia, pharyngitis and cervical lymphadenopathy would make one consider other diagnoses e.g., streptococcal infections or Infectious mononucleosis[2].
Time to PTLD for different transplanted organs: The time to PTLD is longest for the heart recipients and shortest for the lung and heart/lung in pediatric TR. Early PTLD is often of diffuse large B-cell or other B-cell lymphoma histology; whereas Burkitt’s lymphoma and Hodgkin’s disease are late events[46] (Table 3).
EBV monitoring for preemptive therapy: The risk of EBV+ve PTLD has been postulated to be related to three factors: Type of transplant organ, time elapsed until diagnosis of post-transplant PTLD and EBV serological status of both recipient and donor before transplant[16]. An estimation of the viral load via PCR amplification of peripheral blood EBV DNA is mandated to monitor preemptive PTLD therapy. It has been observed that TR with PTLD usually expresses an increased EBV viral load as compared to PTLD free TR. This higher viral load invites more risk for PTLD evolution[50-52]. However, several pitfalls have emerged in preemptive strategy monitoring: First, cut-off values are not clear, second, sources of samples are not universal and third, absence of standard points of time to perform the monitoring.
This disparity, however, has been reflected in positive and negative predictive of EBV viral load values for both SOT (28%-100% and 75%-100%, respectively) and allogeneic HSCT (25%-40% and 67%-86%, respectively)[53-56]. Compared to the reliability of EBV DNA via peripheral-blood mononuclear cells, the “cell-free plasma EBV DNA” has been reported as a better marker of EBV activity[41,57]. In order to limit the risk of PTLD development in SOT and HSCT, a variety of preemptive strategies have been suggested[58,59], e.g., RI, rituximab therapy, and adoptive transfer of EBV-specified T cells. Considering a suitable preemptive approach should be confined to the high-risk group of PTLD patients, however, the precise definition of the cohort of patients at high risk has not been established yet[3].
Prophylaxis: In order to limit the risk of developing PTLD, it is worthwhile quoting a consensus statement on classification and risk factors for PTLD[39]. Primarily, EBV sero-status of both donor and recipient should be recognized before donor selection. EBV-negative TR is better receiving grafts from EBV-negative donors whenever available. A fine-tuning the immunosuppressive burden to as low as clinically possible. Reactivation of other viruses, e.g., CMV or BK should trigger initiation of RI since viral application of other viruses might herald over-immunosuppression. Preemptive/prophylactic antiviral therapy in potentially high-risk groups should be also considered. Maintenance of high titers anti-EBV antibodies via IVIG/CytoGam administration is also recommended. The preemptive therapy should be considered in select groups that are at high-risk for developing PTLD. Furthermore, monitoring EBV viral load in a high-risk case and considering preemptive RI with rising titers, and close monitoring of allograft function have been also recommended[2].
TREATMENT OF PTLD
RI
The mainstay of primary PTLD management is to ameliorate the immunosuppressive burden, so that EBV-specific cellular immunity can be partially restored with no additional risk of acute rejection. RI can reverse 20%-80% of patients with PTLD[60-62]. RI plan includes 50% reduction of calcineurin inhibitors (CNI), either tacrolimus (Tac) and cyclosporine (CyA) doses in addition to withdrawal of the antimetabolites such as azathioprine or mycophenolate mofetil (MMF), despite the lack of evidence demonstrating any relation between MMF and PTLD development[62]. With the exception of glucocorticoids, withdrawal of all immunosuppressive medications in critically ill cases should be considered.
Considering their early response, TR can be restaged within two to four weeks in contrary to lymphoma staging in immunocompetent patients. Monitoring allograft function is mandated during the trial of RI to recognize any manifestations of early rejection. An acute rejection rate of 37% has been observed in a prospective trial entailed the RI strategy as a sequential plan for post-SOT PTLD therapy[61]. Compared to EBV positive disease, the EBV negative cases are less responsive to RI[10,24]. However, a complete lack of response to RI has been observed in old aged patients (> 50 years), bulky lesions (> 7 cm), as well as in advanced stages of the disease (Ann Arbor stage III/IV)[60].
Rituximab therapy
Rituximab (Rtx) is a potent chimeric anti-CD20 monoclonal antibody that binds CD-20 antigen, leading to B cell depletion via several mechanisms e.g., phagocytosis (macrophages), complement mediated cytotoxicity, and through natural killer cells (antibody-dependent cell-mediated toxicity)[63]. Of note, CD20-positivity in B-cell PTLD approached 75% of TR in the prospective phase 2 trial (largest subgroup)[64].
However, Rtx has been approved as a standard therapeutic agent in PTLD for three types of the WHO classification: (1) Nondestructive PTLD, (2) Polymorphic PTLD, and (3) Monomorphic diffuse large B-cell lymphoma-like PTLD not responding to RI. The overall response to Rtx monotherapy (375 mg/m2 body-surface area, weekly for 4 wk, single agent) in addition to RI, approached 44%-79% with a complete remission has been observed in 20%-55% of cases[23,65-68]. Adding 4 doses of Rtx, can raise the rate of complete remission to 34%-60.5%[66]. In the PTLD-1 trial (prospective, multicenter trial including post-SOT PTLD), the complete remission rate approached 25% after standard induction augmented by another four doses of 3 weekly Rtx (low-risk patients)[23]. The complete response can be interpreted as three associated benefits: Better overall survival, extended time to progression, and better progression-free survival.
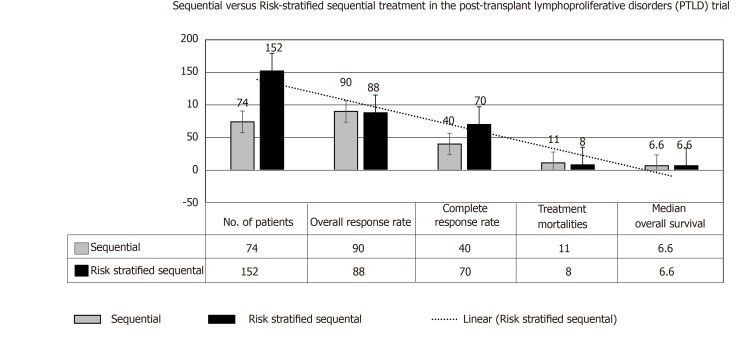
Furthermore, in comparison with the group of TRs with complete remission with Rtx followed by CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), the low-risk group in the cohort receiving risk-stratified sequential expressed longer disease-free survival at 3 year, despite no change in overall survival[68,69], please see Figure 7. More recent prospective trial PTLD-2 is registering TRs with the “risk stratification” based on the following parameters: Type of the allograft, response to Rtx therapy, and international prognostic index (IPI) scoring[4].
Figure 7.
Development of rituximab-based treatment strategies for post-transplant lymphoproliferative disorders after solid organ transplantation: Sequential (2002-2008) vs risk-stratified sequential (2006-2014) treatment[23,65].
Chemotherapy
Indications of Immunochemotherapy include: Burkitt’s lymphoma, Hodgkin’s lymphoma, peripheral T-cell lymphoma, primary CNS lymphoma and other uncommon lymphomas, and B-cell PTLD unresponsive to Rtx and RI[23].
Considering the standard-of-care approaches related to specific histologic features in the rare subtype lymphomas[4,5], have mostly improved patient’s outcome[43,65,70-75]. Despite unproven efficacy, a reduction of the immunosuppressive burden should be evaluated by transplant physicians in view of the immunosuppressive effect of chemotherapy agents and their toxicity. In all CD20+ve subtypes (75% or more), Rtx should be included. The poor outcome of chemotherapy-treated PTLD patients between 1980 and 1990 was partially attributed to the high rates of therapy related mortalities[76]. However, their outcomes greatly improved after the advent of the proper supportive care and administration of granulocyte colony-stimulating factors (G-CSF). Safety and efficacy of Rtx (375 mg/square meter/week/4 wk), followed by CHOP regimen every 3 wk and G-CSF support have been elucidated in the PTLD-1 trial[68].
A risk-stratified sequential therapeutic approach has been admitted in the second part of this trial as follows: Rtx + CHOP (R-CHOP) given over 3 wk for 4 cycles with G-CSF support in cases with no complete response to isolated Rtx therapy. Overall response rate approached 88%, with 70% of cases with any response achieved a complete response at the end of therapeutic program. Of note, post-R-CHOP supportive G-CSF was mandated in all patients with anti-Pneumocystis jirovecii prophylactic therapy[23]. Considering an excellent outcome reported of this trial, a reduction of the immunosuppressive load and risk-stratified sequential therapy are widely considered the standardized care of polymorphic and monomorphic diffuse large B-cell lymphoma-like PTLD (regardless to EBV status) after SOT.
Adoptive immunotherapy
Infusion of donor lymphocytes, to achieve adoptive immunotherapy, has been shown to manage PTLD in HSCT patients that is primarily originating from donor cells. This situation is in contrast to PTLD developing in TRs of SOT. A robust EBV-specific cellular immune response is induced by EBV-specific cytotoxic lymphocytes (CTLs)[22,77]. The major risk of this therapeutic modality, however, is GVHD development[77,78].
Expanded EBV-specific CTLs have been an effective therapeutic option in autologous (recipient-derived PTLD) as well as in donor-derived PTLD[79]. A variety of recent approaches e.g., adoptive transfer of “pamidronate-expanded Vγ9Vδ2 T cells” and Tac-resistant, engineered CTLs has been admitted as new therapeutic options for PTLD with no need to decrease the immunosuppressive load[80].
Outpatient care
In light of serial follow up of the EBV viral load in identifying the patients at risk and in monitoring the response to therapy, the following steps have been suggested: (1) Weekly monitoring of EBV viral titers[81] in higher risk patients. Monthly monitoring initially followed by three monthly monitoring for low risk groups. (2) Whilst viral load drop denotes a response to therapy, persistently high or continuous rise in viral load indicates disease development or progression. (3) Serial physical examination, radiology testing and monitoring allograft function should be viewed as a part of comprehensive clinical picture that includes EBV viral load assessment. The latter does not necessarily correlate with PTLD status. (4) Optimum balance between PTLD management and avoidance of allograft acute rejection is crucial. (5) Therapeutic options should be tailored as per multidisciplinary team discussion. And (6) The initial therapeutic step is RI or cessation of immunosuppression, after which further therapeutic options is tailored according to the response and clonality[2].
Future strategies
A list of newer therapeutic medications has been proposed[80-87]. However, their efficacy remains to be validated via randomized controlled trials: (1) Bruton’s tyrosine kinase (BTK) inhibition[80] (Ibrutinib): Virtually active in GVHD and allograft rejection; remarkably active in activated B cells (ABC) type diffuse large B cell lymphoma (DLBCL). (2) Inhibition of PI3K and mTORi[82] [Idelalisib (PI3K inhibitor)]; SRL and everolimus: Evident - in vitro evidence - of involved pathways; mTORi also have robust immunosuppressive impact, introduction in PTLD therapy still controversial. (3) Proteasome inhibition[83] (Bortezomib): Particularly efficacious in the early presented PTLD post allogeneic HSCT. (4) Radioimmunotherapy[84], (90Yibritumomab, tiuxetan): Apparent efficacy seen only in a small pilot trial. (5) Checkpoint inhibitors[85] (Pembrolizumab, nivolumab): Cytotoxic T lymphocyte-associated antigen 4 pathway: Contraindication, given high risk of (fatal) acute rejection; programmed death 1 (PD1) or programmed death ligand 1 (PDL1) pathway: Lower risk of acute rejection; recommended only in clinical trials. And (6) Anti-CD30 therapy[86] (Brentuximab vedotin): Expression of CD30 in 85% of all PTLD subtypes; the given effects is only limited to case reports.
To summarize
Reduction of immunosuppression is the cornerstone of PTLD management. Rituximab therapy is indicated in nondestructive PTLD, polymorphic PTLD, and, monomorphic diffuse large B-cell lymphoma-like PTLD not responding to RI. Chemotherapy is indicated for: Burkitt’s lymphoma, Hodgkin’s lymphoma, peripheral T-cell lymphoma, primary CNS lymphoma, and B-cell PTLD unresponsive to Rtx/RI with variable results. However, “risk-stratified sequential” therapeutic approach seems to be promising. Other modalities may include adoptive immunotherapy and outpatient care. Investigational agents that’re currently under trials have been shown above.
Prognosis
Outcome of PTLD patients has greatly improved owing to the advent of new lymphoma-specific protocols as well as to the better supportive care. Seventy percent of the PTLD-1 patients had achieved a complete remission with median survival of approximately 6.6 years[23,74,75]. IPI has been universally applied by most hematologists and oncologists to recognize the prognostic attitude in aggressive lymphoma[88]. IPI is a prognostic scoring system that includes the following: Patient’s age, performance attitude, current stage, lactate dehydrogenase (LDH), and number of extra-nodal locations. Another scoring system has been also given in a French registry system that relies primarily upon patient’s age, serum creatinine concentration, LDH level, PTLD localization, and histopathologic criteria[89], however, it is not superior to the IPI[90].
The PTLD-1 trial has settled the prognostic validity of IPI[69]. However, PTLD-2 trial is currently in progress to optimize the role of these prognostic factors. Evens et al[72], concluded that hypoalbuminemia is a robust prognostic factor in a multicenter study. Khedmat et al[91] reported that CD20-positivity in PTLD indicates poorer outcome. LeBlond et al[92], on the other hand, applied IPI to adult TR with PTLD following SOT to identify criteria for poor survival. Using univariate analysis, the poor prognostic criteria have been postulated[44,91,92] that include the following: Monoclonality, negative EBV serology, primary CNS involvement, tumor originated from T-cell, performance status ≥ 2, chemotherapy-based therapy (plus RI), and, multiple involved locations (i.e., > 1 vs 1).
Re-transplantation and PTLD recurrence
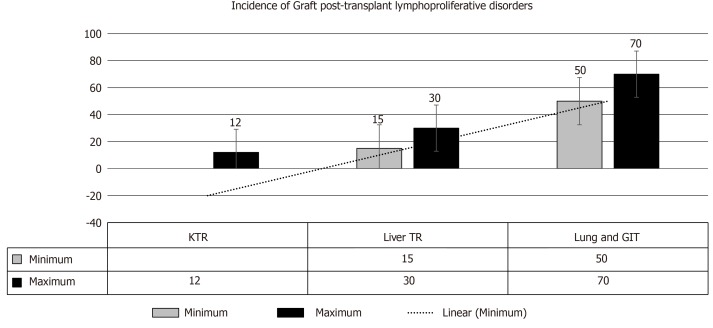
Feasibility of re-transplantation after successful management of PTLD has been reported in particular cases; however, one-year disease free survival is necessary after control of PTLD before re-transplantation[93]. In one French study involving 55 cases with re-transplantation, average time between PTLD recognition and re-transplants was 90 mo. Fortunately, PTLD recurrence has been reported in only one case[94]. An anti-EBV partially acquired immunity has been proposed as a potential protective mechanism[94]. To limit the possibility of PTLD recurrence the following recommendations are worth noting[95]: (1) Time to retransplant: Approximately two years of time should elapse after successful PTLD management. Many transplant physicians recommend 12 to 24 mo after complete PTLD remission, before commencing a new kidney transplant. Dierickx et al[4] reported a mean time of 76 mo for registration in waiting list and a mean of 99 mo between disease remission and the retransplantation. (2) EBV: The following recommendations is currently suggested in the literature: (a) TR should experience Epstein–Barr nuclear antigen IgG positivity (an anti-EBV indicator of robust cytotoxic response) before retransplantation. (b) Low/absent EBV viral load is recommended at the time of retransplantation. (c) Close monitoring of TRs with persistently high EBV viral load is advised. (d) Anti-viral therapy: Long-term prophylactic antiviral therapy with serial estimation of EBV viral load is crucial to limit the incidence of PTLD recurrence[96]. Ganciclovir has been suggested for this purpose[97]. (3) Role of immunosuppression: There is general consensus that PTLD is disease of post-transplant immunosuppression. However, it is the magnitude of immunosuppressive intensity that is the fundamental trigger for PTLD evolution. Of note, the intensity of immunosuppression cannot be calculated as a priori information[98]. Consequently, RI/withdrawal of immunosuppression has been the cornerstone of PTLD management. Retransplantation after PTLD cure remains controversial due to the re-exposure of immunosuppression. (4) Induction therapy: The following agents are considered: (a) ATG vs IL-2 receptor antagonists: The T cell-depleting agents should be excluded from the induction strategies with IL-2 receptor antagonists appeared to have the first priority. Of note, ATG induction significantly triggers the risk of lymphoma evolution as compared to other agents[99]. The latter agents, however, may provide two benefits, first, a lower risk of PTLD development, and, second, TRs are more amenable to avoid long-term excessive immunosuppression after retransplantation. (b) Rituximab in induction therapy: Rtx may be introduced as an element of desensitization regimen in high-risk TR. Rtx has been used before in bone marrow or heart TR with seriously high EBV loads in order to inhibit EBV proliferation within lymphocytes, consequently limiting the risk of PTLD development[58,100]. (5) Maintenance immunosuppression: The fundamental target in regard to maintaining immunosuppression is to avoid the intense state of immunosuppression so that the recovered immune system can promote the evolution of the anti-EBV cytotoxic T lymphocyte, thereby, hampering EBV-triggered B cell proliferation[101]. However, the potential risk of PTLD development should not impede/interfere with our choice of proper immunosuppressive regimen (grade B, level 3)[58]: (a) Triple therapy (CNI, MMF and steroids) use is very common in the current post-transplant maintenance therapy, therefore, the lowest safe dosages monitored by target trough levels should be considered. (b) MMF: Considering the safety of MMF in regard to PTLD evolution, MMF can be included safely in the immunosuppressive protocols with no more added risk[102]. (c) mTOR inhibitors: Their role in PTLD development remains debatable. These agents may inhibit the development of lymphomas in vitro, but their clinical application in human still warrant the proper evidence[103]. (d) Graft PTLD: Is very intriguing (Figure 8) and usually has a good prognostic outcome, furthermore, graft nephrectomy is almost curative[91,104]. (6) Monoclonal gammopathy: Whilst the presence of monoclonal gammopathy may indicate incompletely remitted PTLD, its complete resolution is an obvious indicator of complete remission. And (7) Origin of PTLD (donor vs recipient): Identification of the tumor source is crucial for future therapeutic plans and recognition of the biology of the next PTLD, if any[28]. Of note, Olagne et al[101], reported an obvious trend to a better outcome in TRs with “donor” lymphomas. Clinical clues about the origin of lymphoma cell line (i.e., either donor derived or of recipient origin) is an important therapeutic guide in using cytotoxic T‐cell infusions in PTLD management.
Figure 8.
Incidence of graft post-transplant lymphoproliferative disorder involvement[4,27]. KTR: Kidney transplant recipients; GIT: Gastrointestinal tract; TR: Transplant recipients.
CONCLUSION
PTLD is a disease of immunosuppression. Recent progress in our understanding of the underlying pathophysiology of PTLD as well as the role of EBV has led to a better management. PTLD recurrence has been rarely reported after re-transplantation that requires careful planning of immunosuppression. An ever-improving molecular-genomic technology has had its impact on upgrading our diagnostic and therapeutic strategies that will be reflected in improved recipient’s outcome. However, close liaison with hemato-oncology team of key importance since the lessons learnt from lymphoma management in the general population can be applied to the management of patients who develop PTLD.
Footnotes
Conflict-of-interest statement: No conflict of interest.
Manuscript source: Unsolicited manuscript
Peer-review started: September 17, 2019
First decision: October 14, 2019
Article in press: December 13, 2019
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hibberd AD, Kupeli S, Law MF S-Editor: Ma RY L-Editor: A E-Editor: Xing YX
Contributor Information
Fedaey Abbas, Nephrology Department, Jaber El Ahmed Military Hospital, Safat 13005, Kuwait; Faculty of Health and Science, University of Liverpool, Institute of Learning and Teaching, School of Medicine, Liverpool L69 3GB, United Kingdom.
Mohsen El Kossi, Faculty of Health and Science, University of Liverpool, Institute of Learning and Teaching, School of Medicine, Liverpool L69 3GB, United Kingdom; Doncaster Royal Infirmary, Doncaster DN2 5LT, United Kingdom.
Ihab Sakr Shaheen, Faculty of Health and Science, University of Liverpool, Institute of Learning and Teaching, School of Medicine, Liverpool L69 3GB, United Kingdom; Department of Paediatric Nephrology, Royal Hospital for Children, Glasgow G51 4TF, United Kingdom.
Ajay Sharma, Faculty of Health and Science, University of Liverpool, Institute of Learning and Teaching, School of Medicine, Liverpool L69 3GB, United Kingdom; Department of Transplant Surgery, Royal Liverpool University Hospitals, Liverpool L7 8XP, United Kingdom.
Ahmed Halawa, Faculty of Health and Science, University of Liverpool, Institute of Learning and Teaching, School of Medicine, Liverpool L69 3GB, United Kingdom; Department of Transplantation, Sheffield Teaching Hospitals, Sheffield S57AU, United Kingdom. ahmed.halawa@liverpool.ac.uk.
References
- 1.Dharnidharka VR. Comprehensive review of post-organ transplant hematologic cancers. Am J Transplant. 2018;18:537–549. doi: 10.1111/ajt.14603. [DOI] [PubMed] [Google Scholar]
- 2.Medscape. Post-transplant Lymphoproliferative Disease. Available from: URL: https://emedicine.medscape.com/article/431364-overview#showall. [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Dierickx D, Habermann TM. Post-Transplantation Lymphoproliferative Disorders in Adults. N Engl J Med. 2018;378:549–562. doi: 10.1056/NEJMra1702693. [DOI] [PubMed] [Google Scholar]
- 5.Penn I, Hammond W, Brettschneider L, Starzl TE. Malignant lymphomas in transplantation patients. Transplant Proc. 1969;1:106–112. [PMC free article] [PubMed] [Google Scholar]
- 6.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, Wolfe RA, Goodrich NP, Bayakly AR, Clarke CA, Copeland G, Finch JL, Fleissner ML, Goodman MT, Kahn A, Koch L, Lynch CF, Madeleine MM, Pawlish K, Rao C, Williams MA, Castenson D, Curry M, Parsons R, Fant G, Lin M. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Styczynski J, Gil L, Tridello G, Ljungman P, Donnelly JP, van der Velden W, Omar H, Martino R, Halkes C, Faraci M, Theunissen K, Kalwak K, Hubacek P, Sica S, Nozzoli C, Fagioli F, Matthes S, Diaz MA, Migliavacca M, Balduzzi A, Tomaszewska A, Camara Rde L, van Biezen A, Hoek J, Iacobelli S, Einsele H, Cesaro S Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Response to rituximab-based therapy and risk factor analysis in Epstein Barr Virus-related lymphoproliferative disorder after hematopoietic stem cell transplant in children and adults: a study from the Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Clin Infect Dis. 2013;57:794–802. doi: 10.1093/cid/cit391. [DOI] [PubMed] [Google Scholar]
- 8.de Fijter JW. Cancer and mTOR Inhibitors in Transplant Recipients. Transplantation. 2017;101:45–55. doi: 10.1097/TP.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 9.Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 10.Dierickx D, Tousseyn T, Sagaert X, Fieuws S, Wlodarska I, Morscio J, Brepoels L, Kuypers D, Vanhaecke J, Nevens F, Verleden G, Van Damme-Lombaerts R, Renard M, Pirenne J, De Wolf-Peeters C, Verhoef G. Single-center analysis of biopsy-confirmed posttransplant lymphoproliferative disorder: incidence, clinicopathological characteristics and prognostic factors. Leuk Lymphoma. 2013;54:2433–2440. doi: 10.3109/10428194.2013.780655. [DOI] [PubMed] [Google Scholar]
- 11.Landgren O, Gilbert ES, Rizzo JD, Socié G, Banks PM, Sobocinski KA, Horowitz MM, Jaffe ES, Kingma DW, Travis LB, Flowers ME, Martin PJ, Deeg HJ, Curtis RE. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanakry JA, Kasamon YL, Bolaños-Meade J, Borrello IM, Brodsky RA, Fuchs EJ, Ghosh N, Gladstone DE, Gocke CD, Huff CA, Kanakry CG, Luznik L, Matsui W, Mogri HJ, Swinnen LJ, Symons HJ, Jones RJ, Ambinder RF. Absence of post-transplantation lymphoproliferative disorder after allogeneic blood or marrow transplantation using post-transplantation cyclophosphamide as graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant. 2013;19:1514–1517. doi: 10.1016/j.bbmt.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunstein CG, Weisdorf DJ, DeFor T, Barker JN, Tolar J, van Burik JA, Wagner JE. Marked increased risk of Epstein-Barr virus-related complications with the addition of antithymocyte globulin to a nonmyeloablative conditioning prior to unrelated umbilical cord blood transplantation. Blood. 2006;108:2874–2880. doi: 10.1182/blood-2006-03-011791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet. 2007;370:59–67. doi: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 15.Maksten EF, Vase MØ, Kampmann J, d'Amore F, Møller MB, Strandhave C, Bendix K, Bistrup C, Thiesson HC, Søndergaard E, Hamilton-Dutoit S, Jespersen B. Post-transplant lymphoproliferative disorder following kidney transplantation: a population-based cohort study. Transpl Int. 2016;29:483–493. doi: 10.1111/tri.12744. [DOI] [PubMed] [Google Scholar]
- 16.Walker RC, Marshall WF, Strickler JG, Wiesner RH, Velosa JA, Habermann TM, McGregor CG, Paya CV. Pretransplantation assessment of the risk of lymphoproliferative disorder. Clin Infect Dis. 1995;20:1346–1353. doi: 10.1093/clinids/20.5.1346. [DOI] [PubMed] [Google Scholar]
- 17.Cockfield SM. Identifying the patient at risk for post-transplant lymphoproliferative disorder. Transpl Infect Dis. 2001;3:70–78. doi: 10.1034/j.1399-3062.2001.003002070.x. [DOI] [PubMed] [Google Scholar]
- 18.Morton M, Coupes B, Roberts SA, Klapper PE, Byers RJ, Vallely PJ, Ryan K, Picton ML. Epidemiology of posttransplantation lymphoproliferative disorder in adult renal transplant recipients. Transplantation. 2013;95:470–478. doi: 10.1097/TP.0b013e318276a237. [DOI] [PubMed] [Google Scholar]
- 19.Raab-Traub N. Novel mechanisms of EBV-induced oncogenesis. Curr Opin Virol. 2012;2:453–458. doi: 10.1016/j.coviro.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smatti MK, Al-Sadeq DW, Ali NH, Pintus G, Abou-Saleh H, Nasrallah GK. Epstein-Barr Virus Epidemiology, Serology, and Genetic Variability of LMP-1 Oncogene Among Healthy Population: An Update. Front Oncol. 2018;8:211. doi: 10.3389/fonc.2018.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luskin MR, Heil DS, Tan KS, Choi S, Stadtmauer EA, Schuster SJ, Porter DL, Vonderheide RH, Bagg A, Heitjan DF, Tsai DE, Reshef R. The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am J Transplant. 2015;15:2665–2673. doi: 10.1111/ajt.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorley-Lawson DA, Gross A. Persistence of the Epstein-Barr virus and the origins of associated lymphomas. N Engl J Med. 2004;350:1328–1337. doi: 10.1056/NEJMra032015. [DOI] [PubMed] [Google Scholar]
- 23.Trappe RU, Dierickx D, Zimmermann H, Morschhauser F, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Reinke P, Verhoef G, Subklewe M, Hüttmann A, Tousseyn T, Salles G, Kliem V, Hauser IA, Tarella C, Van Den Neste E, Gheysens O, Anagnostopoulos I, Leblond V, Riess H, Choquet S. Response to Rituximab Induction Is a Predictive Marker in B-Cell Post-Transplant Lymphoproliferative Disorder and Allows Successful Stratification Into Rituximab or R-CHOP Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol. 2017;35:536–543. doi: 10.1200/JCO.2016.69.3564. [DOI] [PubMed] [Google Scholar]
- 24.Reshef R, Vardhanabhuti S, Luskin MR, Heitjan DF, Hadjiliadis D, Goral S, Krok KL, Goldberg LR, Porter DL, Stadtmauer EA, Tsai DE. Reduction of immunosuppression as initial therapy for posttransplantation lymphoproliferative disorder(*) Am J Transplant. 2011;11:336–347. doi: 10.1111/j.1600-6143.2010.03387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Margolskee E, Jobanputra V, Jain P, Chen J, Ganapathi K, Nahum O, Levy B, Morscio J, Murty V, Tousseyn T, Alobeid B, Mansukhani M, Bhagat G. Genetic landscape of T- and NK-cell post-transplant lymphoproliferative disorders. Oncotarget. 2016;7:37636–37648. doi: 10.18632/oncotarget.9400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz AD, Jaffe ES. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swerdlow SH, Webber SA, Chadburn A, Ferry JA. WHO classification of tumors of hematopoietic and lymphoid tissues, 4th ed. In: Swerdlow SH, Campo E, Harris NL, editors. Lyon, France: IARC Press; 2017. pp. 453–462. [Google Scholar]
- 28.Alizadeh AA, Eisen MB, Davis RE, Ma C, Lossos IS, Rosenwald A, Boldrick JC, Sabet H, Tran T, Yu X, Powell JI, Yang L, Marti GE, Moore T, Hudson J, Jr, Lu L, Lewis DB, Tibshirani R, Sherlock G, Chan WC, Greiner TC, Weisenburger DD, Armitage JO, Warnke R, Levy R, Wilson W, Grever MR, Byrd JC, Botstein D, Brown PO, Staudt LM. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403:503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 29.Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E, Braziel RM, Jaffe ES, Pan Z, Farinha P, Smith LM, Falini B, Banham AH, Rosenwald A, Staudt LM, Connors JM, Armitage JO, Chan WC. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 30.Lenz G, Wright G, Dave SS, Xiao W, Powell J, Zhao H, Xu W, Tan B, Goldschmidt N, Iqbal J, Vose J, Bast M, Fu K, Weisenburger DD, Greiner TC, Armitage JO, Kyle A, May L, Gascoyne RD, Connors JM, Troen G, Holte H, Kvaloy S, Dierickx D, Verhoef G, Delabie J, Smeland EB, Jares P, Martinez A, Lopez-Guillermo A, Montserrat E, Campo E, Braziel RM, Miller TP, Rimsza LM, Cook JR, Pohlman B, Sweetenham J, Tubbs RR, Fisher RI, Hartmann E, Rosenwald A, Ott G, Muller-Hermelink HK, Wrench D, Lister TA, Jaffe ES, Wilson WH, Chan WC, Staudt LM Lymphoma/Leukemia Molecular Profiling Project. Stromal gene signatures in large-B-cell lymphomas. N Engl J Med. 2008;359:2313–2323. doi: 10.1056/NEJMoa0802885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morscio J, Dierickx D, Ferreiro JF, Herreman A, Van Loo P, Bittoun E, Verhoef G, Matthys P, Cools J, Wlodarska I, De Wolf-Peeters C, Sagaert X, Tousseyn T. Gene expression profiling reveals clear differences between EBV-positive and EBV-negative posttransplant lymphoproliferative disorders. Am J Transplant. 2013;13:1305–1316. doi: 10.1111/ajt.12196. [DOI] [PubMed] [Google Scholar]
- 32.Courville EL, Yohe S, Chou D, Nardi V, Lazaryan A, Thakral B, Nelson AC, Ferry JA, Sohani AR. EBV-negative monomorphic B-cell post-transplant lymphoproliferative disorders are pathologically distinct from EBV-positive cases and frequently contain TP53 mutations. Mod Pathol. 2016;29:1200–1211. doi: 10.1038/modpathol.2016.130. [DOI] [PubMed] [Google Scholar]
- 33.Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, Marcus R, Parameshwar J, Ramsay A, Newstead C Haemato-oncology Task Force of the British Committee for Standards in Haematology and British Transplantation Society. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010;149:675–692. doi: 10.1111/j.1365-2141.2010.08161.x. [DOI] [PubMed] [Google Scholar]
- 34.Weisenburger DD, Gross TG. Post-transplant lymphoproliferative disorder: a heterogeneous conundrum. Br J Haematol. 2017;179:854–856. doi: 10.1111/bjh.14274. [DOI] [PubMed] [Google Scholar]
- 35.Cohen JI. Epstein-Barr virus infection. N Engl J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 36.Capello D, Rossi D, Gaidano G. Post-transplant lymphoproliferative disorders: molecular basis of disease histogenesis and pathogenesis. Hematol Oncol. 2005;23:61–67. doi: 10.1002/hon.751. [DOI] [PubMed] [Google Scholar]
- 37.Green M, Webber S. Posttransplantation lymphoproliferative disorders. Pediatr Clin North Am. 2003;50:1471–1491. doi: 10.1016/s0031-3955(03)00127-5. [DOI] [PubMed] [Google Scholar]
- 38.Bakker NA, van Imhoff GW. Post-transplant lymphoproliferative disorders: from treatment to early detection and prevention? Haematologica. 2007;92:1447–1450. doi: 10.3324/haematol.11272. [DOI] [PubMed] [Google Scholar]
- 39.Glotz D, Chapman JR, Dharnidharka VR, Hanto DW, Castro MC, Hirsch HH, Leblond V, Mehta AK, Moulin B, Pagliuca A, Pascual J, Rickinson AB, Russo FP, Trappe RU, Webster AC, Zuckermann AO, Gross TG. The Seville expert workshop for progress in posttransplant lymphoproliferative disorders. Transplantation. 2012;94:784–793. doi: 10.1097/TP.0b013e318269e64f. [DOI] [PubMed] [Google Scholar]
- 40.Meerbach A, Wutzler P, Häfer R, Zintl F, Gruhn B. Monitoring of Epstein-Barr virus load after hematopoietic stem cell transplantation for early intervention in post-transplant lymphoproliferative disease. J Med Virol. 2008;80:441–454. doi: 10.1002/jmv.21096. [DOI] [PubMed] [Google Scholar]
- 41.Lee TC, Savoldo B, Rooney CM, Heslop HE, Gee AP, Caldwell Y, Barshes NR, Scott JD, Bristow LJ, O'Mahony CA, Goss JA. Quantitative EBV viral loads and immunosuppression alterations can decrease PTLD incidence in pediatric liver transplant recipients. Am J Transplant. 2005;5:2222–2228. doi: 10.1111/j.1600-6143.2005.01002.x. [DOI] [PubMed] [Google Scholar]
- 42.Riddler SA, Breinig MC, McKnight JL. Increased levels of circulating Epstein-Barr virus (EBV)-infected lymphocytes and decreased EBV nuclear antigen antibody responses are associated with the development of posttransplant lymphoproliferative disease in solid-organ transplant recipients. Blood. 1994;84:972–984. [PubMed] [Google Scholar]
- 43.Buell JF, Gross TG, Hanaway MJ, Trofe J, Muthiak C, First MR, Alloway RR, Woodle ES. Chemotherapy for posttransplant lymphoproliferative disorder: the Israel Penn International Transplant Tumor Registry experience. Transplant Proc. 2005;37:956–957. doi: 10.1016/j.transproceed.2004.12.124. [DOI] [PubMed] [Google Scholar]
- 44.Jagadeesh D, Woda BA, Draper J, Evens AM. Post transplant lymphoproliferative disorders: risk, classification, and therapeutic recommendations. Curr Treat Options Oncol. 2012;13:122–136. doi: 10.1007/s11864-011-0177-x. [DOI] [PubMed] [Google Scholar]
- 45.Khedmat H, Taheri S. Early versus late outset of lymphoproliferative disorders post-heart and lung transplantation: the PTLD. Int Survey Hematol Oncol Stem Cell Ther. 2011;4:10-16. doi: 10.5144/1658-3876.2011.10. [DOI] [PubMed] [Google Scholar]
- 46.Schober T, Framke T, Kreipe H, Schulz TF, Großhennig A, Hussein K, Baumann U, Pape L, Schubert S, Wingen AM, Jack T, Koch A, Klein C, Maecker-Kolhoff B. Characteristics of early and late PTLD development in pediatric solid organ transplant recipients. Transplantation. 2013;95:240–246. doi: 10.1097/TP.0b013e318277e344. [DOI] [PubMed] [Google Scholar]
- 47.Webber SA, Naftel DC, Fricker FJ, Olesnevich P, Blume ED, Addonizio L, Kirklin JK, Canter CE Pediatric Heart Transplant Study. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. 2006;367:233–239. doi: 10.1016/S0140-6736(06)67933-6. [DOI] [PubMed] [Google Scholar]
- 48.Maecker B, Jack T, Zimmermann M, Abdul-Khaliq H, Burdelski M, Fuchs A, Hoyer P, Koepf S, Kraemer U, Laube GF, Müller-Wiefel DE, Netz H, Pohl M, Toenshoff B, Wagner HJ, Wallot M, Welte K, Melter M, Offner G, Klein C. CNS or bone marrow involvement as risk factors for poor survival in post-transplantation lymphoproliferative disorders in children after solid organ transplantation. J Clin Oncol. 2007;25:4902–4908. doi: 10.1200/JCO.2006.10.2392. [DOI] [PubMed] [Google Scholar]
- 49.Ghobrial IM, Habermann TM, Macon WR, Ristow KM, Larson TS, Walker RC, Ansell SM, Gores GJ, Stegall MD, McGregor CG. Differences between early and late posttransplant lymphoproliferative disorders in solid organ transplant patients: are they two different diseases? Transplantation. 2005;79:244–247. doi: 10.1097/01.tp.0000144335.39913.5c. [DOI] [PubMed] [Google Scholar]
- 50.Wagner HJ, Wessel M, Jabs W, Smets F, Fischer L, Offner G, Bucsky P. Patients at risk for development of posttransplant lymphoproliferative disorder: plasma versus peripheral blood mononuclear cells as material for quantification of Epstein-Barr viral load by using real-time quantitative polymerase chain reaction. Transplantation. 2001;72:1012–1019. doi: 10.1097/00007890-200109270-00006. [DOI] [PubMed] [Google Scholar]
- 51.Stevens SJ, Verschuuren EA, Pronk I, van Der Bij W, Harmsen MC, The TH, Meijer CJ, van Den Brule AJ, Middeldorp JM. Frequent monitoring of Epstein-Barr virus DNA load in unfractionated whole blood is essential for early detection of posttransplant lymphoproliferative disease in high-risk patients. Blood. 2001;97:1165–1171. doi: 10.1182/blood.v97.5.1165. [DOI] [PubMed] [Google Scholar]
- 52.Cho YU, Chi HS, Jang S, Park SH, Park CJ. Pattern analysis of Epstein-Barr virus viremia and its significance in the evaluation of organ transplant patients suspected of having posttransplant lymphoproliferative disorders. Am J Clin Pathol. 2014;141:268–274. doi: 10.1309/AJCP9WYEXKOL9YUV. [DOI] [PubMed] [Google Scholar]
- 53.Semenova T, Lupo J, Alain S, Perrin-Confort G, Grossi L, Dimier J, Epaulard O, Morand P, Germi R. Multicenter Evaluation of Whole-Blood Epstein-Barr Viral Load Standardization Using the WHO International Standard. J Clin Microbiol. 2016;54:1746–1750. doi: 10.1128/JCM.03336-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Styczynski J, van der Velden W, Fox CP, Engelhard D, de la Camara R, Cordonnier C, Ljungman P Sixth European Conference on Infections in Leukemia, a joint venture of the Infectious Diseases Working Party of the European Society of Blood and Marrow Transplantation (EBMT-IDWP), the Infectious Diseases Group of the European Organization for Research and Treatment of Cancer (EORTC-IDG), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN) Management of Epstein-Barr Virus infections and post-transplant lymphoproliferative disorders in patients after allogeneic hematopoietic stem cell transplantation: Sixth European Conference on Infections in Leukemia (ECIL-6) guidelines. Haematologica. 2016;101:803–811. doi: 10.3324/haematol.2016.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kanakry JA, Hegde AM, Durand CM, Massie AB, Greer AE, Ambinder RF, Valsamakis A. The clinical significance of EBV DNA in the plasma and peripheral blood mononuclear cells of patients with or without EBV diseases. Blood. 2016;127:2007–2017. doi: 10.1182/blood-2015-09-672030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worth A, Conyers R, Cohen J, Jagani M, Chiesa R, Rao K, Goulden N, Veys P, Amrolia PJ. Pre-emptive rituximab based on viraemia and T cell reconstitution: a highly effective strategy for the prevention of Epstein-Barr virus-associated lymphoproliferative disease following stem cell transplantation. Br J Haematol. 2011;155:377–385. doi: 10.1111/j.1365-2141.2011.08855.x. [DOI] [PubMed] [Google Scholar]
- 57.Tsai DE, Douglas L, Andreadis C, Vogl DT, Arnoldi S, Kotloff R, Svoboda J, Bloom RD, Olthoff KM, Brozena SC, Schuster SJ, Stadtmauer EA, Robertson ES, Wasik MA, Ahya VN. EBV PCR in the diagnosis and monitoring of posttransplant lymphoproliferative disorder: results of a two-arm prospective trial. Am J Transplant. 2008;8:1016–1024. doi: 10.1111/j.1600-6143.2008.02183.x. [DOI] [PubMed] [Google Scholar]
- 58.Choquet S, Varnous S, Deback C, Golmard JL, Leblond V. Adapted treatment of Epstein-Barr virus infection to prevent posttransplant lymphoproliferative disorder after heart transplantation. Am J Transplant. 2014;14:857–866. doi: 10.1111/ajt.12640. [DOI] [PubMed] [Google Scholar]
- 59.Rasche L, Kapp M, Einsele H, Mielke S. EBV-induced post transplant lymphoproliferative disorders: a persisting challenge in allogeneic hematopoetic SCT. Bone Marrow Transplant. 2014;49:163–167. doi: 10.1038/bmt.2013.96. [DOI] [PubMed] [Google Scholar]
- 60.Tsai DE, Hardy CL, Tomaszewski JE, Kotloff RM, Oltoff KM, Somer BG, Schuster SJ, Porter DL, Montone KT, Stadtmauer EA. Reduction in immunosuppression as initial therapy for posttransplant lymphoproliferative disorder: analysis of prognostic variables and long-term follow-up of 42 adult patients. Transplantation. 2001;71:1076–1088. doi: 10.1097/00007890-200104270-00012. [DOI] [PubMed] [Google Scholar]
- 61.Swinnen LJ, LeBlanc M, Grogan TM, Gordon LI, Stiff PJ, Miller AM, Kasamon Y, Miller TP, Fisher RI. Prospective study of sequential reduction in immunosuppression, interferon alpha-2B, and chemotherapy for posttransplantation lymphoproliferative disorder. Transplantation. 2008;86:215–222. doi: 10.1097/TP.0b013e3181761659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, Marcus R, Parameshwar J, Ramsay A, Newstead C Haemato-oncology Task Force of the British Committee for Standards in Haematology and British Transplantation Society. Management of post-transplant lymphoproliferative disorder in adult solid organ transplant recipients - BCSH and BTS Guidelines. Br J Haematol. 2010;149:693–705. doi: 10.1111/j.1365-2141.2010.08160.x. [DOI] [PubMed] [Google Scholar]
- 63.Khalil MAM, Khalil MAU, Khan TF, Tan J. Drug-Induced Hematological Cytopenia in Kidney Transplantation and the Challenges It Poses for Kidney Transplant Physicians. J Transplantation. 2018 doi: 10.1155/2018/9429265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zimmermann H, Trappe RU. EBV and posttransplantation lymphoproliferative disease: what to do? Hematology Am Soc Hematol Educ Program. 2013;2013:95–102. doi: 10.1182/asheducation-2013.1.95. [DOI] [PubMed] [Google Scholar]
- 65.Choquet S, Leblond V, Herbrecht R, Socié G, Stoppa AM, Vandenberghe P, Fischer A, Morschhauser F, Salles G, Feremans W, Vilmer E, Peraldi MN, Lang P, Lebranchu Y, Oksenhendler E, Garnier JL, Lamy T, Jaccard A, Ferrant A, Offner F, Hermine O, Moreau A, Fafi-Kremer S, Morand P, Chatenoud L, Berriot-Varoqueaux N, Bergougnoux L, Milpied N. Efficacy and safety of rituximab in B-cell post-transplantation lymphoproliferative disorders: results of a prospective multicenter phase 2 study. Blood. 2006;107:3053–3057. doi: 10.1182/blood-2005-01-0377. [DOI] [PubMed] [Google Scholar]
- 66.González-Barca E, Domingo-Domenech E, Capote FJ, Gómez-Codina J, Salar A, Bailen A, Ribera JM, López A, Briones J, Muñoz A, Encuentra M, de Sevilla AF GEL/TAMO (Grupo Español de Linfomas); GELCAB (Grupo para el Estudio de los Linfomas Catalano-Balear); GOTEL (Grupo Oncológico para el Tratamiento y Estudio de los Linfomas) Prospective phase II trial of extended treatment with rituximab in patients with B-cell post-transplant lymphoproliferative disease. Haematologica. 2007;92:1489–1494. doi: 10.3324/haematol.11360. [DOI] [PubMed] [Google Scholar]
- 67.Choquet S, Oertel S, LeBlond V, Riess H, Varoqueaux N, Dörken B, Trappe R. Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol. 2007;86:599–607. doi: 10.1007/s00277-007-0298-2. [DOI] [PubMed] [Google Scholar]
- 68.Trappe R, Oertel S, Leblond V, Mollee P, Sender M, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Salles G, Morschhauser F, Jaccard A, Lamy T, Leithäuser M, Zimmermann H, Anagnostopoulos I, Raphael M, Riess H, Choquet S German PTLD Study Group; European PTLD Network. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13:196–206. doi: 10.1016/S1470-2045(11)70300-X. [DOI] [PubMed] [Google Scholar]
- 69.Trappe RU, Choquet S, Dierickx D, Mollee P, Zaucha JM, Dreyling MH, Dührsen U, Tarella C, Shpilberg O, Sender M, Salles G, Morschhauser F, Jaccard A, Lamy T, Reinke P, Neuhaus R, Lehmkuhl H, Horst HA, Leithäuser M, Schlattmann P, Anagnostopoulos I, Raphael M, Riess H, Leblond V, Oertel S German PTLD Study Group and the European PTLD Network. International prognostic index, type of transplant and response to rituximab are key parameters to tailor treatment in adults with CD20-positive B cell PTLD: clues from the PTLD-1 trial. Am J Transplant. 2015;15:1091–1100. doi: 10.1111/ajt.13086. [DOI] [PubMed] [Google Scholar]
- 70.Dierickx D, Tousseyn T, Gheysens O. How I treat posttransplant lymphoproliferative disorders. Blood. 2015;126:2274–2283. doi: 10.1182/blood-2015-05-615872. [DOI] [PubMed] [Google Scholar]
- 71.Batchelor TT, Thye LS, Habermann TM. Current Management Concepts: Primary Central Nervous System Lymphoma, Natural Killer T-Cell Lymphoma Nasal Type, and Post-transplant Lymphoproliferative Disorder. Am Soc Clin Oncol Educ Book. 2016;35:e354–e366. doi: 10.1200/EDBK_159030. [DOI] [PubMed] [Google Scholar]
- 72.Evens AM, Choquet S, Kroll-Desrosiers AR, Jagadeesh D, Smith SM, Morschhauser F, Leblond V, Roy R, Barton B, Gordon LI, Gandhi MK, Dierickx D, Schiff D, Habermann TM, Trappe R. Primary CNS posttransplant lymphoproliferative disease (PTLD): an international report of 84 cases in the modern era. Am J Transplant. 2013;13:1512–1522. doi: 10.1111/ajt.12211. [DOI] [PubMed] [Google Scholar]
- 73.Mahale P, Shiels MS, Lynch CF, Engels EA. Incidence and outcomes of primary central nervous system lymphoma in solid organ transplant recipients. Am J Transplant. 2018;18:453–461. doi: 10.1111/ajt.14465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rosenberg AS, Klein AK, Ruthazer R, Evens AM. Hodgkin lymphoma post-transplant lymphoproliferative disorder: A comparative analysis of clinical characteristics, prognosis, and survival. Am J Hematol. 2016;91:560–565. doi: 10.1002/ajh.24346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenberg AS, Ruthazer R, Paulus JK, Kent DM, Evens AM, Klein AK. Survival Analyses and Prognosis of Plasma-Cell Myeloma and Plasmacytoma-Like Posttransplantation Lymphoproliferative Disorders. Clin Lymphoma Myeloma Leuk. 2016;16:684–692.e3. doi: 10.1016/j.clml.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Habermann TM. Posttransplant lymphoproliferative disorders. Cancer Treat Res. 2008;142:273–292. doi: 10.1007/978-0-387-73744-7_12. [DOI] [PubMed] [Google Scholar]
- 77.Perica K, Varela JC, Oelke M, Schneck J. Adoptive T cell immunotherapy for cancer. Rambam Maimonides Med J. 2015;6:e0004. doi: 10.5041/RMMJ.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papadopoulos EB, Ladanyi M, Emanuel D, Mackinnon S, Boulad F, Carabasi MH, Castro-Malaspina H, Childs BH, Gillio AP, Small TN. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med. 1994;330:1185–1191. doi: 10.1056/NEJM199404283301703. [DOI] [PubMed] [Google Scholar]
- 79.Merlo A, Turrini R, Dolcetti R, Martorelli D, Muraro E, Comoli P, Rosato A. The interplay between Epstein-Barr virus and the immune system: a rationale for adoptive cell therapy of EBV-related disorders. Haematologica. 2010;95:1769–1777. doi: 10.3324/haematol.2010.023689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dharnidharka VR, Mohanakumar T. New approaches to treating B-cell cancers induced by Epstein-Barr virus. N Engl J Med. 2015;372:569–571. doi: 10.1056/NEJMcibr1415117. [DOI] [PubMed] [Google Scholar]
- 81.Green M. Management of Epstein-Barr virus-induced post-transplant lymphoproliferative disease in recipients of solid organ transplantation. Am J Transplant. 2001;1:103–108. [PubMed] [Google Scholar]
- 82.Schutt SD, Fu J, Nguyen H, Bastian D, Heinrichs J, Wu Y, Liu C, McDonald DG, Pidala J, Yu XZ. Inhibition of BTK and ITK with Ibrutinib Is Effective in the Prevention of Chronic Graft-versus-Host Disease in Mice. PLoS One. 2015;10:e0137641. doi: 10.1371/journal.pone.0137641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Furukawa S, Wei L, Krams SM, Esquivel CO, Martinez OM. PI3Kδ inhibition augments the efficacy of rapamycin in suppressing proliferation of Epstein-Barr virus (EBV)+ B cell lymphomas. Am J Transplant. 2013;13:2035–2043. doi: 10.1111/ajt.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morscio J, Finalet Ferreiro J, Vander Borght S, Bittoun E, Gheysens O, Dierickx D, Verhoef G, Wlodarska I, Tousseyn T. Identification of distinct subgroups of EBV-positive post-transplant diffuse large B-cell lymphoma. Mod Pathol. 2017;30:370–381. doi: 10.1038/modpathol.2016.199. [DOI] [PubMed] [Google Scholar]
- 85.Rossignol J, Terriou L, Robu D, Willekens C, Hivert B, Pascal L, Guieze R, Trappe R, Baillet C, Huglo D, Morschhauser F. Radioimmunotherapy ((90) Y-Ibritumomab Tiuxetan) for Posttransplant Lymphoproliferative Disorders After Prior Exposure to Rituximab. Am J Transplant. 2015;15:1976–1981. doi: 10.1111/ajt.13244. [DOI] [PubMed] [Google Scholar]
- 86.Barnett R, Barta VS, Jhaveri KD. Preserved Renal-Allograft Function and the PD-1 Pathway Inhibitor Nivolumab. N Engl J Med. 2017;376:191–192. doi: 10.1056/NEJMc1614298. [DOI] [PubMed] [Google Scholar]
- 87.Vase MØ, MØ EF, Bendix K, Hamilton-Dutoit S, Andersen C, Møller MB, Sørensen SS, Jespersen B, Kampmann J, Søndergård E, Nielsen PS, D'amore F. Occurrence and prognostic relevance of CD30 expression in post-transplant lymphoproliferative disorders. Leuk Lymphoma. 2015;56:1677–1685. doi: 10.3109/10428194.2014.966242. [DOI] [PubMed] [Google Scholar]
- 88.International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 89.Caillard S, Porcher R, Provot F, Dantal J, Choquet S, Durrbach A, Morelon E, Moal V, Janbon B, Alamartine E, Pouteil Noble C, Morel D, Kamar N, Buchler M, Mamzer MF, Peraldi MN, Hiesse C, Renoult E, Toupance O, Rerolle JP, Delmas S, Lang P, Lebranchu Y, Heng AE, Rebibou JM, Mousson C, Glotz D, Rivalan J, Thierry A, Etienne I, Moal MC, Albano L, Subra JF, Ouali N, Westeel PF, Delahousse M, Genin R, Hurault de Ligny B, Moulin B. Post-transplantation lymphoproliferative disorder after kidney transplantation: report of a nationwide French registry and the development of a new prognostic score. J Clin Oncol. 2013;31:1302–1309. doi: 10.1200/JCO.2012.43.2344. [DOI] [PubMed] [Google Scholar]
- 90.Dierickx D, Tousseyn T, Morscio J, Fieuws S, Verhoef G. Validation of prognostic scores in post-transplantation lymphoproliferative disorders. J Clin Oncol. 2013;31:3443–3444. doi: 10.1200/JCO.2013.50.3326. [DOI] [PubMed] [Google Scholar]
- 91.Khedmat H, Taheri S. Lymphoproliferative disorders in pediatric liver allograft recipients: a review of 212 cases. Hematol Oncol Stem Cell Ther. 2012;5:84–90. doi: 10.5144/1658-3876.2012.84. [DOI] [PubMed] [Google Scholar]
- 92.Leblond V, Dhedin N, Mamzer Bruneel MF, Choquet S, Hermine O, Porcher R, Nguyen Quoc S, Davi F, Charlotte F, Dorent R, Barrou B, Vernant JP, Raphael M, Levy V. Identification of prognostic factors in 61 patients with posttransplantation lymphoproliferative disorders. J Clin Oncol. 2001;19:772–778. doi: 10.1200/JCO.2001.19.3.772. [DOI] [PubMed] [Google Scholar]
- 93.Johnson SR, Cherikh WS, Kauffman HM, Pavlakis M, Hanto DW. Retransplantation after post-transplant lymphoproliferative disorders: an OPTN/UNOS database analysis. Am J Transplant. 2006;6:2743–2749. doi: 10.1111/j.1600-6143.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 94.Caillard S, Cellot E, Dantal J, Thaunat O, Provot F, Janbon B, Buchler M, Anglicheau D, Merville P, Lang P, Frimat L, Colosio C, Alamartine E, Kamar N, Heng AE, Durrbach A, Moal V, Rivalan J, Etienne I, Peraldi MN, Moreau A, Moulin B French PTLD Registry. A French Cohort Study of Kidney Retransplantation after Post-Transplant Lymphoproliferative Disorders. Clin J Am Soc Nephrol. 2017;12:1663–1670. doi: 10.2215/CJN.03790417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Karras A, Thervet E, Le Meur Y, Baudet-Bonneville V, Kessler M, Legendre C. Successful renal retransplantation after post-transplant lymphoproliferative disease. Am J Transplant. 2004;4:1904–1909. doi: 10.1111/j.1600-6143.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 96.Hanto DW. Retransplantation after post-transplant lymphoproliferative diseases (PTLD): when is it safe? Am J Transplant. 2004;4:1733–1734. doi: 10.1111/j.1600-6143.2004.00623.x. [DOI] [PubMed] [Google Scholar]
- 97.Funch DP, Walker AM, Schneider G, Ziyadeh NJ, Pescovitz MD. Ganciclovir and acyclovir reduce the risk of post-transplant lymphoproliferative disorder in renal transplant recipients. Am J Transplant. 2005;5:2894–2900. doi: 10.1111/j.1600-6143.2005.01115.x. [DOI] [PubMed] [Google Scholar]
- 98.Sampaio MS, Cho YW, Shah T, Bunnapradist S, Hutchinson IV. Association of immunosuppressive maintenance regimens with posttransplant lymphoproliferative disorder in kidney transplant recipients. Transplantation. 2012;93:73–81. doi: 10.1097/TP.0b013e31823ae7db. [DOI] [PubMed] [Google Scholar]
- 99.Opelz G, Naujokat C, Daniel V, Terness P, Döhler B. Disassociation between risk of graft loss and risk of non-Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation. 2006;81:1227–1233. doi: 10.1097/01.tp.0000219817.18049.36. [DOI] [PubMed] [Google Scholar]
- 100.van der Velden WJ, Mori T, Stevens WB, de Haan AF, Stelma FF, Blijlevens NM, Donnelly JP. Reduced PTLD-related mortality in patients experiencing EBV infection following allo-SCT after the introduction of a protocol incorporating pre-emptive rituximab. Bone Marrow Transplant. 2013;48:1465–1471. doi: 10.1038/bmt.2013.84. [DOI] [PubMed] [Google Scholar]
- 101.Olagne J, Caillard S, Gaub MP, Chenard MP, Moulin B. Post-transplant lymphoproliferative disorders: determination of donor/recipient origin in a large cohort of kidney recipients. Am J Transplant. 2011;11:1260–1269. doi: 10.1111/j.1600-6143.2011.03544.x. [DOI] [PubMed] [Google Scholar]
- 102.Robson R, Cecka JM, Opelz G, Budde M, Sacks S. Prospective registry-based observational cohort study of the long-term risk of malignancies in renal transplant patients treated with mycophenolate mofetil. Am J Transplant. 2005;5:2954–2960. doi: 10.1111/j.1600-6143.2005.01125.x. [DOI] [PubMed] [Google Scholar]
- 103.Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472–4480. [PubMed] [Google Scholar]
- 104.Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, Velten M, Moulin B French Transplant Centers. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682–693. doi: 10.1111/j.1600-6143.2011.03896.x. [DOI] [PubMed] [Google Scholar]