Abstract
BACKGROUND
Recently, as a possible therapy resolving solution, pentadecapeptide BPC 157 therapy, has been used in alleviating various vascular occlusion disturbances. BPC 157 was previously reviewed as novel mediator of Robert cytoprotection and endothelium protection in the stomach, and gut-brain axis, beneficial therapy in gastrointestinal tract, with particular reference to vascular recruitment, ulcerative colitis and tumor cachexia, and other tissues healing. Here we raised new hypothesis about BPC 157 therapy in the Budd-Chiari syndrome in rats, rapid bypassing of the suprahepatic inferior caval vein occlusion, and rats recovery with the active and effective pharmacotherapy treatment.
AIM
To investigate Budd-Chiari syndrome model (inferior caval vein suprahepatic occlusion) resolution, since BPC 157 resolves various rat vascular occlusion.
METHODS
We assessed the activated bypassing pathways between the inferior and superior caval veins and portocaval shunt, counteracted caval/portal hypertension, aortal hypotension, venous/arterial thrombosis, electrocardiogram disturbances, liver and gastrointestinal lesions (i.e., stomach and duodenum hemorrhages, in particular, congestion). Rats with suprahepatic occlusion of the inferior vena cava by ligation were medicated at 1 min, 15 min, 24 h, or 48 h post-ligation. Medication consisted of 10 µg/kg BPC 157, 10 ng BPC 157 or 5 mL/kg saline, administered once as an abdominal bath or intragastric application. Gross and microscopic observations were made, in addition to assessments of electrical activity of the heart (electrocardiogram), portal and caval hypertension, aortal hypotension, thrombosis, hepatomegaly, splenomegaly and venography. Furthermore, levels of nitric oxide, malondialdehyde in the liver and serum enzymes were determined.
RESULTS
BPC 157 counteracted increased P wave amplitude, tachycardia and ST-elevation, i.e., right heart failure from acute thrombotic coronary occlusion. The bypassing pathway of the inferior vena cava-azygos (hemiazygos) vein-superior vena cava and portocaval shunt occurred rapidly. Even with severe caval ˃ portal hypertension, BPC 157 antagonized portal and caval hypertension and aortal hypotension, and also reduced refractory ascites. Thrombosis of portal vein tributaries, inferior vena cava, and hepatic and coronary arteries was attenuated. In addition, there was reduced pathology of the lungs (severe capillary congestion) and liver (dilated central veins and terminal portal venules), decreased intestine hemorrhagic lesions (substantial capillary congestion, submucosal edema and architecture loss), and increased liver and spleen weight. During the period of ligation, nitric oxide- and malondialdehyde-levels in the liver remained within normal healthy values, and increases in serum enzymes were markedly reduced.
CONCLUSION
BPC 157 counteracts Budd Chiari syndrome in rats.
Keywords: BPC 157, Budd Chiari syndrome, Portal/caval hypertension
Core tip: To demonstrate that pentadecapeptide BPC 157 can resolve Budd Chiari syndrome in rats, we provided gross, microscopy, nitric oxide-, malondialdehyde-liver levels, serum enzymes, electrocardiogram, portal, caval hypertension, aortal hypotension, thrombosis, hepatomegaly, splenomegaly and venography assessments. BPC 157 counteracts increased P wave amplitude, tachycardia and ST-elevation (i.e., right heart failure; acute thrombotic coronary occlusion). Bypassing pathway (inferior caval vein-azygos (hemiazygos) vein-superior caval vein and portocaval shunt) rapidly appears. Even with the severe caval ˃ portal hypertension, BPC 157 counteracts portal and caval hypertension and aortal hypotension. BPC 157 counteracts refractory ascites. Portal vein tributaries, inferior caval vein, hepatic and coronary arteries thrombosis was counteracted. In addition, there are counteracted severe lung pathology, liver, intestine hemorrhagic lesion, increased liver and spleen weight. During ligation-period, nitric oxide- and malondialdehyde-level in liver remained within normal healthy values, and increased serum enzymes markedly lessened. In conclusion, BPC 157 counteracts Budd Chiari syndrome in rats.
INTRODUCTION
Here we raised new hypothesis about rapid bypassing of the suprahepatic inferior caval vein occlusion in the Budd-Chiari syndrome (BCS) in rats along with the active and effective pharmacotherapy treatment.
In this, we perceive BCS as originally suggested[1,2], a hepatic venous outflow obstruction and its manifestation, regardless of cause, but mostly attributable to thrombosis, which can be located anywhere from the small hepatic veins until the entrance of the inferior caval vena into the right atrium[1,2]. Thereby, with some limitations[3], since in the rat it is almost impossible to dissect the hepatic veins[3], few rats studies used occluding the inferior vena cava cranially to the hepatic veins[2,4-7], but bypassing of the occlusion in the rat BCS along with pharmacotherapy treatment was not considered.
Recently, as a possible therapy resolving solution, pentadecapeptide BPC 157 therapy[8-20], has been used in alleviating vascular occlusion disturbances[21-25]. BPC 157 was previously reviewed as novel mediator of Robert cytoprotection and endothelium protection in the stomach[8-11], and gut-brain axis[12], beneficial therapy in gastrointestinal tract[13,14], with particular reference to vascular recruitment[11,15-17], ulcerative colitis[14] and tumor cachexia[18], and other tissues healing[19,20].
Rapid activation of a bypassing loop[21-25] occurs in rats with an infrarenal ligation of the inferior vena cava, relieving a Virchow's triad situation[21], much like in rats with ischemic/reperfusion colitis[22], duodenal venous congestion lesions[23], perforated cecum[24], bile duct ligation induced liver cirrhosis and portal hypertension[25].
Specifically, there is prevention and reversal of both caval and portal hypertension and aortal hypotension, tachycardia, thrombosis and thrombocytopenia, and consequently reduced prolonged bleeding. In addition, there is both preserved and rescued intestinal mucosal integrity and vein integrity, reduced malondialdehyde (MDA), even to normal levels in both ischemic and reperfusion conditions in tissues (i.e., liver, colon, duodenum, cecum and veins) and plasma[21-25].
In this current study, we assessed whether BPC 157 therapy compensated for the complete suprahepatic occlusion of the inferior vena cava, an immediate obstructive inferior vena cava and portal syndrome. In these obstructions, it would activate an azygos/hemiazygos vein bypassing pathway, and upgrade an inadequate rescuing inferior-superior vena cava shunt to an adequate one, as well as a portocaval shunt. With BCS in rats, both caval and portal hypertension, and aortal hypertension occurred. BPC 157 therapy was assessed in counteracting rapid clot formation in the portal vein, superior mesenteric vein, splenic vein, inferior vena cava, hepatic artery, and coronary artery, as well as peaked P waves, significant ST-elevation, tachycardia, gross organ lesions, and liver and spleen weight increases.
Notably, BPC 157 was originally used as an anti-ulcer agent, being stable and maintaining a native configuration in human gastric juices for more than 24 h, as previously reviewed[8-20]. It has been used in trials for ulcerative colitis and multiple sclerosis, with a very safe profile (LD1 not achieved), as previously reviewed[8-20]. Furthermore, as an important conceptual and practical activity point, in previous studies[8-20], BPC 157 was thought to be a novel mediator of Robert’s cytoprotection, the concept of a compound providing epithelium and endothelium protection, maintaining gastrointestinal mucosal integrity and organoprotection, as previously reviewed[8-20]. Likely, these beneficial effects might additionally contribute to the suggested therapeutic effect related to vessel recruitment in bypassing occlusions. As such, we investigated pentadecapeptide BPC 157 in resolving short and long-lasting BCS in rats.
MATERIALS AND METHODS
Animals
This study was conducted with 12-wk-old, 200 g birth weight , male albino Wistar rats, randomly assigned at 6 rats/group/interval. Rats were bred in-house at the Pharmacology animal facility, School of Medicine, Zagreb, Croatia. The animal facility was registered by the Directorate of Veterinary (Reg. No: HR-POK-007). Laboratory rats were acclimated for five days and randomly assigned to their respective treatment groups. Laboratory animals were housed in polycarbonate (PC) cages under conventional laboratory conditions at 20–24 °C, relative humidity of 40%–70% and noise level 60 dB. Each cage was identified with dates, number of study, group, dose, number and sex of each animal. Fluorescent lighting provided illumination 12 hours per day. Standard good laboratory practice (GLP) diet and fresh water was provided ad libitum. Animal care was in compliance with standard operating procedures (SOPs) of the Pharmacology animal facility, and the European Convention for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes (ETS 123).
This study was approved by the local Ethic Committee. Ethical principles of the study complied with the European Directive 010/63/E, the Law on Amendments to the Animal Protection Act (Official Gazette 37/13), the Animal Protection Act (Official Gazette 135/06), the Ordinance on the protection of animals used for scientific purposes (Official Gazette 55/13), Federation of European Laboratory Animal Science Associations recommendations and the recommendations of the Ethics Committee of the School of Medicine, University of Zagreb. The experiments were assessed by observers blinded as to the treatment.
Drugs
Medication was administered as described previously[21-25], without use of a carrier or peptidase inhibitor, for stable gastric pentadecapeptide BPC 157, a partial sequence of the human gastric juice protein BPC, which was freely soluble in water at pH 7.0 and in saline. BPC 157 (GEPPPGKPADDAGLV, molecular weight 1419; Diagen, Slovenia) was prepared as a peptide with 99% high-performance liquid chromatography purity, with 1-des-Gly peptide being the main impurity. The dose and application regimens were as described previously[8-20].
Surgery
Rats were deeply anesthetized with intraperitoneal (ip) injected 40 mg/kg thiopental (Rotexmedica, Germany) and 10 mg/kg diazepam (Apaurin; Krka, Slovenia) The suprahepatic inferior vena cava was then exposed via a midline laparotomy, and was occluded by ligation. Rats were maintained for the next 15 min, 24 h or 48 h.
Medication
In rats with suprahepatic occlusion of the inferior vena cava, in evaluating lesions and blood vessels by gross and microscopic assessment, electrocardiogram (ECG), thrombosis, serum enzyme levels and oxidative stress (MDA and nitric oxide, NO levels) in liver tissue, rats were treated with 10 µg/kg BPC 157, 10 ng/kg BPC 157, or 5mL/kg saline as an abdominal bath at 1 min ligation-time. In addition, for portal vein, vena cava, and abdominal aorta pressure recordings 10 µg/kg BPC 157, 10 ng/kg BPC 157 or 5 mL/kg saline was applied in rats, either intragastrically or as an abdominal bath, at 15 minutes, 24 h or 48 h ligation-time. For venography, 10 µg/kg BPC 157, 10 ng/kg BPC 157 or 5 mL/kg saline was applied as an abdominal bath, at 15 minutes ligation-time, just before venography.
Portal and caval vein and abdominal aorta pressure recording
Recordings were made in deeply anesthetized and laparatomized rats, with a cannula (BD Neoflon™ Cannula) connected to a pressure transducer (78534C MONITOR/ TERMINAL; Hewlett Packard, United States) inserted into the portal vein, inferior vena cava and abdominal aorta at the level of the bifurcation at 15 min, 24 h or 48 h post-ligation. Each recording lasted five minutes, being assessed in one minute intervals.
Notably, normal rats exhibited a portal pressure of 3–5 mmHg[25] similar to that of the inferior vena cava, though with at least 1 mmHg higher values in the portal vein. By contrast, abdominal aorta blood pressure values were 100–120 mmHg at the level of the bifurcation[21].
ECG recording
ECGs were recorded continuously in deeply anesthetized rats for all three main leads, by positioning stainless steel electrodes on all four limbs using an ECG monitor with a 2090 programmer (Medtronic, United States) connected to a Waverunner LT342 digital oscilloscope (LeCroy, United States). This arrangement enabled precise recordings, measurements and analysis of ECG parameters[21].
Vessels, intestine, liver, spleen, ascites presentation
The presentation of the vessels was recorded in deeply anaesthetized rats, with a camera attached to a VMS-004 Discovery Deluxe USB microscope (Veho, United States). We assessed vessels, as to whether they had afilled or cleared out (hollow) appearance at the stomach, and for arcade vessels on the ventral and dorsal sides in 1 cm long segments of duodenum, jejunum, ascending colon, and for 10 vessels from the proximal to distal cecum throughout the experiment. The assessments occurred at selected time points before and after therapy, in rats with a suprahepatic occlusion of the inferior vena cava, at 5, 10 and 15 min post-ligation.
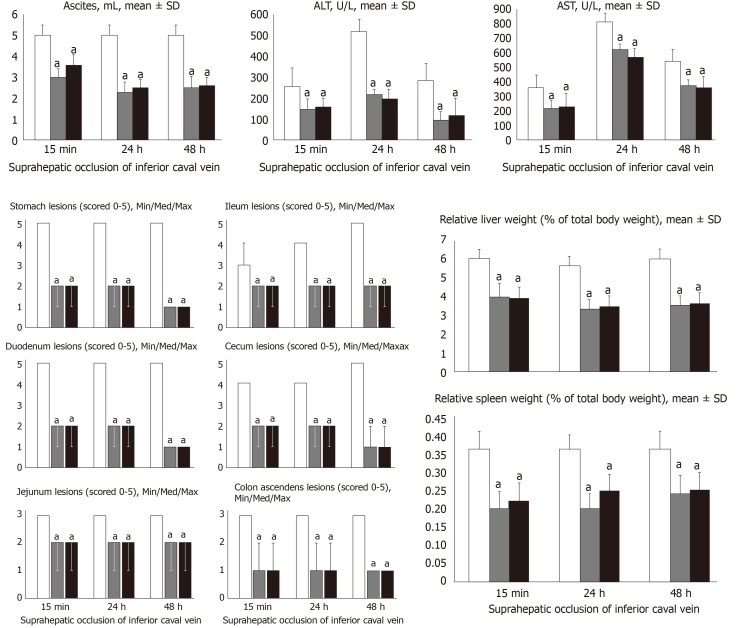
At 15 min, 24 h and 48 h post-ligation, we assessed hemorrhagic congestive areas in the stomach, duodenum, jejunum, cecum and ascending colon. Scoring was based on opening (1, normal mucosa presentation; 2, only small hemorrhagic areas; 3, advanced hemorrhagic areas; and 4, extensive and severe hemorrhagic areas) and azygos vein presentation (1, moderate decrease; 2, mild decrease; 3, not different from healthy; and 4, abundant increase). Liver and spleen weights were expressed as a percent of the total body weight (for normal rats, liver 3.2%–4.0% and spleen 0.20%–0.26%). Ascites (mL) was also assessed.
Venography
Venography was performed in rats with a suprahepatic occlusion of the inferior vena cava at 15 min post-ligation, using a C-VISION PLUS fluoroscopy unit (Shimadzu, Japan)[18]. Two ml (0.3 mL/s) warmed Omnipaque 350 (iohexol) non-ionic contrast medium (GE Healthcare, United States) was injected into the inferior vena cava at the level of bifurcation of rats with a suprahepatic occlusion of the inferior vena cava. The contrast medium was visualized under real-time to ensure adequate filling. A subtraction mode was used to record the images at 14 frames per second. At 15 minutes post-ligation, venograms were taken, captured, and digitized into files on a personal computer and were analyzed using ISSA image software (Vamstec, Croatia). Rats having a full presentation of the azygos vein and portal vein-superior mesenteric vein-inferior mesenteric vein-rectal vein-left iliac vein-inferior vena cava pathway were assessed.
Microscopy
Tissue specimens from liver, spleen, stomach, duodenum, ileum, cecum, ascending colon, cecum, lungs and heart were obtained from rats with suprahepatic occlusion of the inferior vena cava at 15 min, 24 h and 48 h post-ligation. These were fixed in buffered formalin (pH 7.4), for 24 h, dehydrated, and embedded in paraffin wax. The samples were stained with hematoxylin-eosin. Tissue injury was evaluated microscopically by a blinded examiner.
Thrombus assessment
On being euthanized, the portal vein, superior mesenteric vein (up to the inferior anterior pancreaticoduodenal vein), splenic vein and inferior vena cava, as well as the hepatic and coronary arteries were removed from the rats, and clots were weighed[21].
Bilirubin and enzyme activity
To determine the serum levels of aspartate transaminase, alanine transaminase (IU/L), and total bilirubin (μmol/L), blood samples were collected immediately after euthanasia and were centrifuged for 15 min at 3000 rpm. All tests were performed using an Olympus AU2700 analyzer with original test reagents (Olympus Diagnostics, Ireland)[26-32]. However, since there was no increase in bilirubin, the data were not shown.
Oxidative stress in liver
Oxidative stress was assessed in collected tissue samples at 15 min, 24 h and 48 h post-ligation, by quantifying thiobarbituric acid-reactive species (TBARS) as MDA equivalents, as previously described[21-25]. The tissue samples were homogenized in phosphate-buffered saline (PBS, pH 7.4) containing 0.1 mmol/L butylated hydroxytoluene (BHT, TissueRuptor; Qiagen, United States) and sonicated for 30 s in an ultrasonic ice bath (Branson, United States). Trichloroacetic acid (TCA, 10%) was added to the homogenate, the mixture was centrifuged at 3000 rpm for 5 min, and the supernatant was collected. Thiobarbituric acid (TBA, 1%) was added, and the samples were heated at 95 °C for 60 min. The tubes were then kept on ice for 10 min. Following centrifugation (14000 rpm, 10 min), the absorbance of the mixture was determined at a wavelength of 532 nm. The concentration of MDA was read from a standard calibration curve, plotted using 1,1,3,3’-tetraethoxy propane. The extent of lipid peroxidation was expressed as MDA equivalents, using a molar extinction coefficient for MDA of 1.56 × 105 mol/L/cm. Protein concentration was determined using a DC Protein Assay Kit, (Bio-Rad, United States). Results were expressed in nmol per mg protein.
NO determination in liver
NO- levels in liver tissue samples were determined at 15 min, 24 h and 48 h post-ligation, using the Griess reaction (Griess Reagent System; Promega, United States). Sulfanilamide was incubated with the homogenized tissue, and then, N-1-naphthyl ethylenediamine dihydrochloride was added. The Griess reaction was based on a diazotization reaction in which acidified nitrite reacted with diazonium ions and, in a further step, was coupled to N-1-naphthyl ethylenediamine dihydrochloride, forming a chromophoric azo derivate. Absorbance was measured at 540 nm, using sodium nitrite solution as a standard. NO-levels were reported in μmol/mg protein. The protein concentrations were determined using a DC Protein Assay Kit, (Bio-Rad, United States), as described previously[21-25].
Statistical analysis
Statistical analysis was performed by parametric one-way analysis of variance, with post-hoc Newman-Keuls test and non-parametric Kruskal-Wallis test and subsequently the Mann-Whitney U test to compare groups. Values were presented as the mean ± SD and as the minimum/median/maximum. To compare the frequency difference between groups, the χ2 test or Fischer's exact test was used. P < 0.05 was considered statistically significant.
RESULTS
All rats with suprahepatic occlusion of the inferior vena cava in BPC 157-treated groups exhibited a full presentation of the azygos vein and portal vein-superior mesenteric vein-inferior mesenteric vein-rectal vein-left iliac vein-inferior vena cava pathway, unlike the controls, (Fisher's exact probability test P < 0.05 compared to control; Figure 1). This justified the focus of this current study on the stable gastric pentadecapeptide BPC 157 and rapid recovery of all hemodynamic disturbances in Budd-Chiari-rats, the severe portal and caval hypertension and abdominal aorta hypotension and full course of BCS model.
Figure 1.
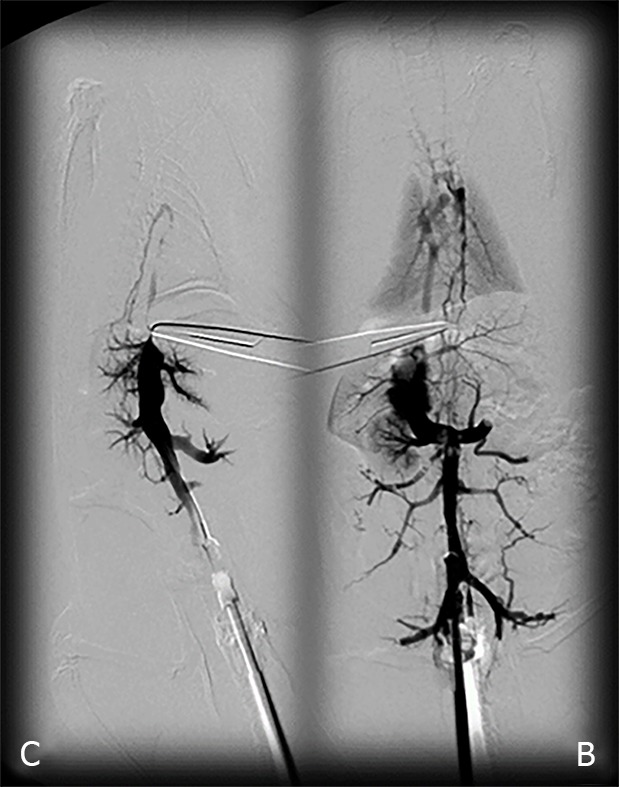
BPC 157 and venography assessment in rats with a suprahepatic occlusion of the inferior vena cava. Inferior vena cava venography at the level of the bifurcation at 15 minutes ligation time. Blood flow through the inferior vena cava through the azygos vein to the superior vena cava, portal vein to superior-inferior mesenteric vein-rectal vein-left iliac vein-inferior vena cava in BPC 157-treated rats (B), but not in the controls (C).
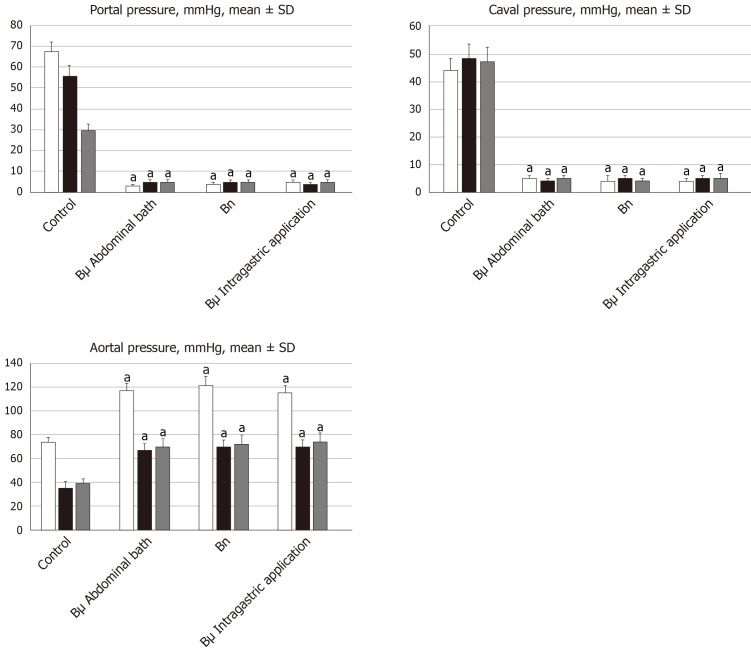
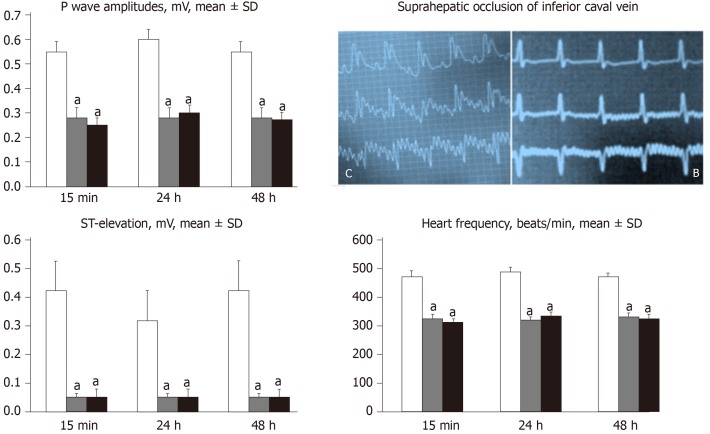
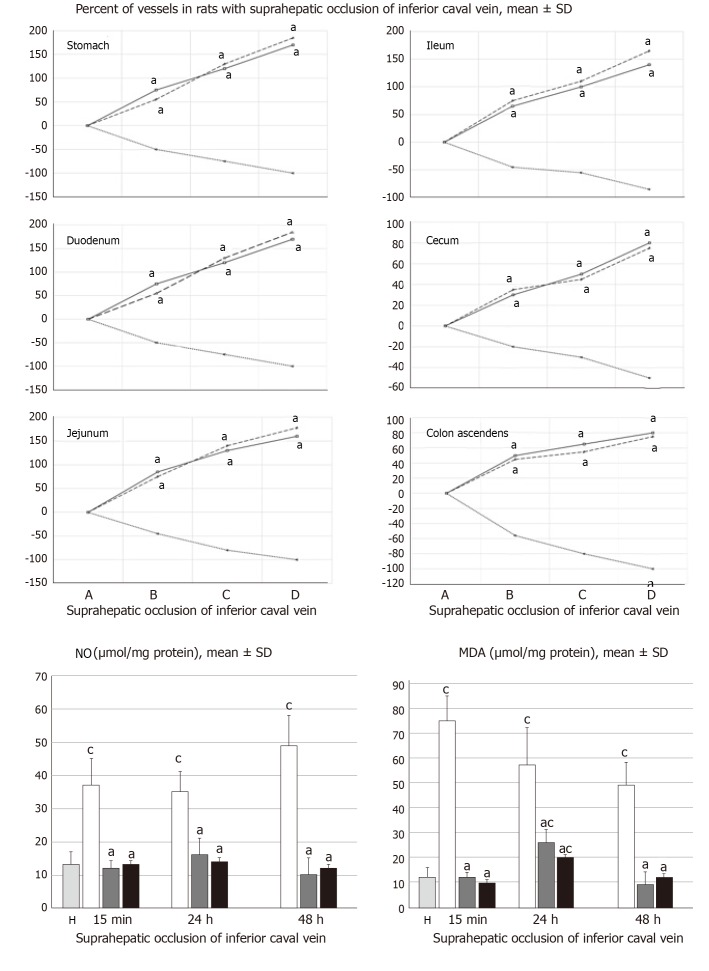
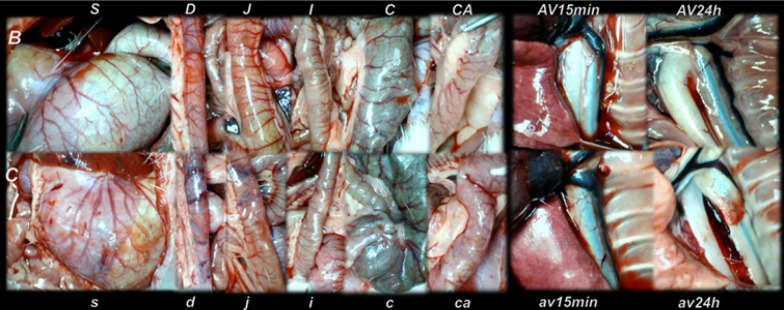
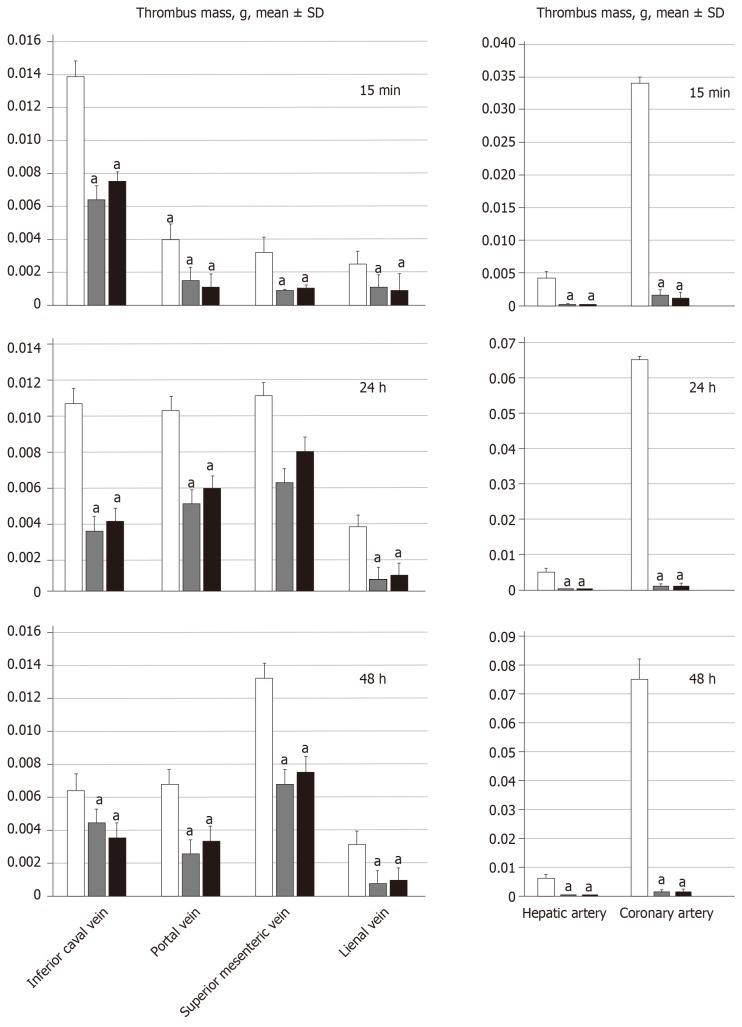
All BPC 157 administration regimens (10 µg/kg and 10 ng/kg, abdominal bath, and intragastric applications) were effective in rats with BCS (Figures 1-11). Indicative of how BPC 157 might affect the course of BCS (Figure 1) and reversed the course of disturbances, marked attenuation occurred when it was given at 15 min post-ligation, much as in rats with prolonged BCS (at 24 h and 48 h post-ligation). Therefore, preexisting severe portal and caval hypertension and systemic hypotension (seen in the abdominal aorta), either short-lasting or long-lasting, rapidly responded to any of the BPC 157 regimens (Figure 2). In addition, over prolonged periods, the worsening that simultaneously appeared and persisted, the high portal hypertension, and particularly, the caval hypertension and aortic hypotension were not compensated; however, these completely disappeared with BPC 157 medication (Figure 2). Similarly, controls presented with immediately peaked P values, significant ST-elevation and tachycardia, as identifiers of acute thrombotic coronary occlusion and right heart failure (Figure 3), which rapidly disappeared under all the BPC 157 regimens (Figure 3). As visualized grossly in the early and prolonged periods, with BPC 157 treatment, increased blood vessel branching rapidly appeared in the serosa of all organs affected (Figures 6 and 8), with hemorrhagic lesions being markedly attenuated in the stomach, duodenum, jejunum, ileum, cecum and ascending colon (Figures 5 and 7), as well as reducing hepatomegaly and splenomegaly (Figure 5). There was a particular presentation of the azygos vein as an indication of the counteraction of right heart malfunction and the reestablishing of blood flow continuity between caval veins (Figure 8). Indicative of a reversal of BCS, while there was progressive thrombosis in controls in the portal vein, splenic vein, superior mesenteric vein, and inferior vena cava, as well as in the hepatic and coronary arteries, strong attenuation occurred in the veins and arteries of BPC 157-treated rats, where considerably smaller clots were observed (Figure 4). In addition, BPC 157 rats had much less ascites (Figure 5), and while serum alanine transaminase and aspartate transaminase values increased in controls, they were lower in rats treated with BPC 157 at all time intervals (Figure 5).
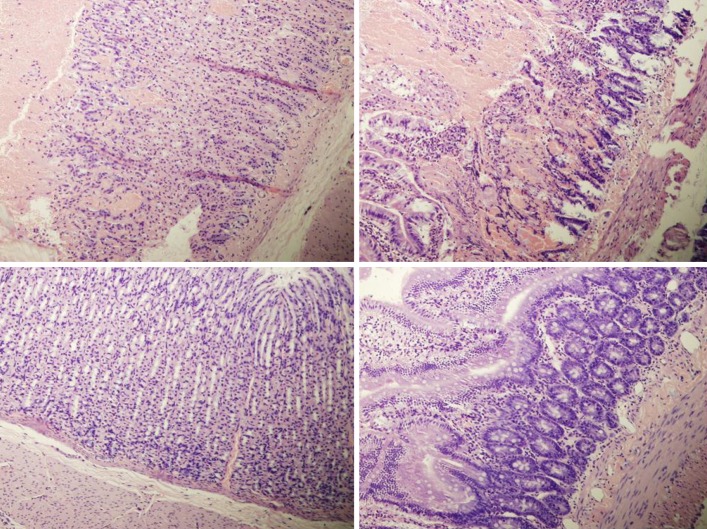
Figure 11.
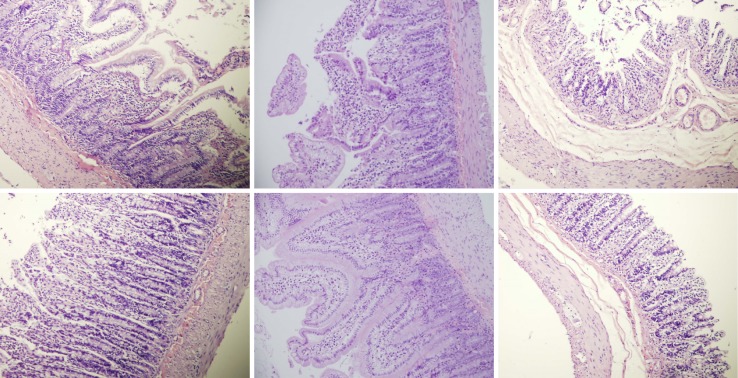
Histological presentation in rats with suprahepatic ligation of the inferior vena cava. Assessment (stained with hematoxylin-eosin, magnification x 10, scare bar: 50 µm) waste 48 h post-ligation, in controls (upper) and BPC 157-treated rats (lower), jejunum (left upper, control; lower, BPC 157), ileum (middle upper, control; lower, BPC 157), cecum (right upper, control; lower, BPC 157). Note, substantial capillary congestion with mild ischemic changes, loss of crypts with foci of haemorrhage, edema of the lamina propria and mild lymphocytic infiltration in controls (upper), which were markedly counteracted in BPC 157-treated rats (lower).
Figure 2.
Antagonizing effect of BPC 157 on portal and caval hypertension, and aortal hypotension. Portal, caval and aortal pressure (mmHg), mean ± SD. For portal vein, vena cava, and abdominal aorta pressure recordings, medication of BPC 157 [10 µg/kg (Bµg), 10 ng/kg (Bng)] or saline (5 mL/kg; control) was applied in rats intragastrically or as an abdominal bath, at 15 min (white bars), 24 h (black bars) or 48 h (gray bars) post-ligation. aP < 0.05 vs control.
Figure 3.
Counteracting effect of BPC 157 in rats with a suprahepatic occlusion of the inferior vena cava on peaked P waves, sinus tachycardia, ST-elevation 15 min post-ligation [controls (C), BPC 157 rats (B)] and assessment at 15 min, 24 h and 48 h ligation-time, mean ± SD. Treatment with 10 µg/kg BPC 157 (gray bars), 10 ng/kg BPC 157 (black bars), or saline (1 mL/rat; white bars) as a bath administration given at 1 min post-ligation. aP < 0.05 vs control.
Figure 6.
BPC 157 improves vessel presentation following suprahepatic occlusion of the inferior vena cava (upper), and eliminates or attenuates increased nitric oxide and malondialdehyde levels (nmol/mg protein) in liver. Vessels were assessed as to whether they were (filled or cleared out at the stomach, and between the arcade vessels on the ventral side for 1 cm long segments of duodenum, jejunum and ascending colon, and between 10 vessels from the proximal to distal cecum. Assessment was made throughout the experiment, with the point (A) immediately before therapy considered to be 100%, at selected time points before (A) and after therapy (B, C and D) in rats with suprahepatic occlusion of the inferior vena cava at 5 (B), 10 (C) and 15 (D) minutes post-ligation (mean ± SD). The gross appearance of the tissue was recorded using a USB microscope camera (upper). Counteraction of the increased nitric oxide and malondialdehyde levels in liver (lower) at 15 min, 24 h and 48 h post-ligation. Treatment with 10 µg/kg BPC 157 (gary bars), 10 ng/kg BPC 157 (black bars), or saline (1 mL/rat; white bars) as a bath administration given at 1 min post-ligation. aP < 0.05 vs saline; cP < 0.05 vs healthy liver (H). NO: Nitric oxide; MDA: Malondialdehyde.
Figure 8.
Gross vessel presentation in rats treated with saline (C, small letters, lower) and improvement in rats treated with BPC 157 (B, capitals, upper). Assessments were made using a camera attached to a USB microscope (Veho Discovery Deluxe VMS-004) in the stomach (s/S), duodenum (d/D), jejunum (j/J), ileum (i/I), cecum (c/C) and ascending colon (ca/CA) of rats with suprahepatic occlusion of the inferior vena cava at 15 min post-ligation (left), and azygos vein presentation at 15 min (av15min/AV15min) and 24 h (av24h/AV24h) post-ligation (right).
Figure 5.
BPC 157 counteracts ascites (mL), serum enzyme levels (U/L), hepatomegaly and splenomegaly (mean ± SD), stomach, duodenal, jejunal, ileal, cecal and ascending colon lesions (scored 0–5; Min/Med/Max), following suprahepatic occlusion of the inferior vena cava. Assessment at 15 min, 24 h and 48 h post-ligation. Treatment with 10 µg/kg BPC 157 (gray bars), 10 ng/kg BPC 157 (black bars), or saline (1 mL/rat; white bars) as a bath administration given at 1 min ligation. aP < 0.05 vs control.
Figure 7.
Gross presentation of lesions (C) and lesions attenuation (B) in rats with suprahepatic occlusion of inferior vena cava. Lesions were assessed using a camera attached to a USB microscope (Veho Discovery Deluxe VMS-004), at 15 min (upper, a), 24 h (middle, b), and 48 h (low, c) post-ligation in the gastrointestinal tract. From left to right, the stomach, duodenum, jejunum, ileum, cecum and ascending colon on treatment with saline (C) or BPC 157 (B).
Figure 4.
BPC 157 attenuates thrombus presentation (thrombus mass, g) in veins (inferior vena cava, portal vein, superior mesenteric vein, and splenic vein, SV) and arteries (hepatic artery and coronary artery) following suprahepatic occlusion of the inferior vena cava. Assessment at 15 min, 24 h and 48 h post-ligation; mean ± SD. Treatment with 10 µg/kg BPC 157 (gray bars), 10 ng/kg BPC 157 (black bars), or saline (1 mL/rat; white bars) as a bath administration given at 1 min post-ligation. aP < 0.05 vs control.
Increased MDA- and NO-levels in the liver may be indicative of BCS. BPC 157 administration resulted in MDA- and NO-levels in the liver, close to or within a normal healthy range in both early and prolonged periods of ligation (Figure 6).
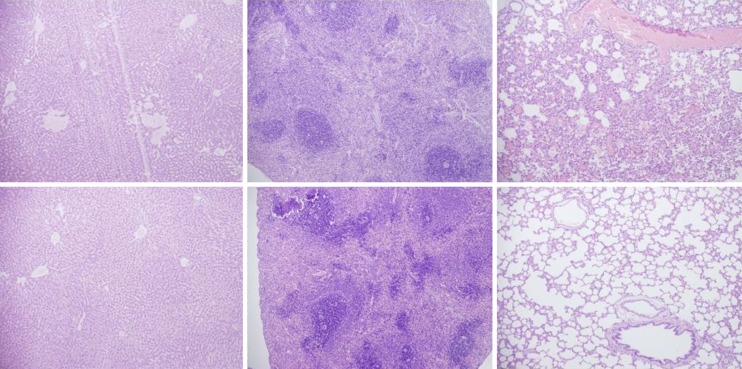
Rats with BCS regularly showed considerable lesions early post-ligation. For example, in liver, there was substantial congestion of the central vein, branches of the terminal portal venules, and sinusoidal dilatation in the controls, while there was far less congestion in BPC 157-treated rats (Figure 9). In the spleen, there was sinusoidal congestion, and dilatation and enlargement of red pulp leading to reduction of white pulp, features, which were less apparent in BPC 157-treated rats (Figure 9). In stomach of controls, there was substantial congestion and dilatation of mucosal and submucosal capillaries, submucosal edema, ischemic changes, such as architectural distortion and foci of hemorrhage with fibrin deposition. Duodenal lesions in controls were characterized by substantial capillary congestion with prominent ischemic changes, architecture distortion and loss of crypts, with foci of hemorrhage, edema of the lamina propria and mild lymphocytic infiltration and ischemic changes, such as architectural distortion and foci of hemorrhage with fibrin deposition (Figure 10). By contrast, only very mild capillary congestion was found in the stomach and duodenum of BPC 157-treated rats, with some reparatory changes to epithelium. In the jejunum, ileum, cecum and colon of control rats, substantial capillary congestion with mild ischemic changes, loss of crypts with foci of hemorrhage, edema of the lamina propria and mild lymphocytic infiltration were present. By contrast, these effects were much less expressed in BPC 157-treated rats; in particular, there was preservation of architecture and scattered lymphocytes with little to no ischemic changes, and some reparatory changes of epithelium (Figure 11). Finally, in the lungs, control rats showed edema of the interstitium, substantial dilatation and congestion of capillaries in the alveolar septum, with few to many lymphocytes, while in BPC 157-treated animals, we found little to no edema of the interstitium, very few lymphocytes, and only mild capillary congestion (Figure 9). In addition, as expected, no morphological changes were found in the myocardium due to the fact that changes found on ECGs were the result of acute right ventricular overload (data not shown).
Figure 9.
Histological presentation in rats with suprahepatic ligation of the inferior vena cava. Assessment (stained with hematoxylin-eosin, magnification x 10, scare bar: 50 µm) was made at 48 h post-ligation, in controls (upper) and BPC 157-treated rats (lower), liver (left upper, control; lower, BPC 157), spleen (middle upper, control; lower, BPC 157), and lungs (right upper, control; lower, BPC 157). Note, the substantial congestion of the central vein, branches of the terminal portal venules, and sinusoidal dilatation in liver, sinusoidal congestion, dilatation and enlargement of red pulp leading to reduction of white pulp in spleen, edema of the interstitium, substantial dilatation and congestion of capillaries in the alveolar septum in lungs (upper), which were markedly counteracted in BPC 157-treated rats (lower).
Figure 10.
Histological presentation in rats with suprahepatic ligation of the inferior vena cava. Assessment (stained with hematoxylin-eosin, magnification x 10, scare bar: 50 µm) was at 48 h post-ligation, in controls (upper) and BPC 157-treated rats (lower), stomach (left upper, control; lower, BPC 157), duodenum (right upper, control; lower, BPC 157). Note, substantial congestion and dilatation of mucosal and submucosal capillaries, submucosal edema, ischemic changes, such as architectural distortion and foci of hemorrhage with fibrin deposition in controls (upper), which were markedly counteracted in BPC 157-treated rats (lower).
DISCUSSION
We adopted that regardless of cause, Budd–Chiari syndrome hepatic venous outflow obstruction can be located anywhere from the small hepatic veins until the entrance of the inferior caval vena into the right atrium[1,2], as originally suggested[1,2]. Thereby, with the suprahepatic occlusion of the inferior caval vein, which was complete (and thereby, abrupt initiation), and its manifestations, as a model of the BCS in rats[2-7], and recovery in the BPC 157-treated rats, we extended evidence from previous vascular occlusion studies[21-25]. As a new point, BPC 157 therapy rapidly activated bypassing pathways a caval shunt (inferior vena cava-azygos vein-superior vena cava shunt) or a portocaval shunt (portal vein-superior-inferior mesenteric vein-rectal vein-inferior vena cava). Severe post-hepatic portal hypertension and caval hypertension was eliminated (and aortal hypotension as well), by BPC 157 given at 15 min, 24 h or 48 h, appearing to be successful as a deep vein thrombosis and ischemic/reperfusion therapy[21-25].
By contrast, in the control Budd-Chiari-rats, the failed vena cava shunt (i.e., azygos vein) ineffectively competes against resolving the condition of rats, with severe portal hypertension (> 60 mmHg) and caval hypertension (> 70 mmHg in the later period). A gradient of at least 15 mmHg as portal hypertension reflects a decrease in regular venous return to the heart and failure of spontaneous decompression of the portal system. These disturbances might be more than in humans, with the persistence of the left superior vena cava, the azygos vein on the left of the aorta and vertebral column, and the hemiazygos vein likely lacking[33]. For example, there is the higher range of this portal hypertension versus the long-lasting portal hypertension in rats drinking alcohol (> 25 mmHg)[26] or from bile duct ligation-induced liver cirrhosis (> 18 mmHg)[25]. Likewise, there is a higher range of caval hypertension versus inferior vena cava infrarenal occlusion-induced caval hypertension (> 25 mmHg)[21]. Severe aortal hypotension was not compensated, but progressed, unless BPC 157 was given.
Thus, the therapy effect overrode the high resistance to the therapy, which had to be more than the portal vein-stenosis with the slow progression of mild portal hypertension in rats (< 20 mmHg)[34-36]. Therefore, counteraction of the described abrupt BCS necessitated the pleiotropic beneficial effects of treatments such as BPC 157 that might be advantageous to counteract the whole syndrome, as previously reviewed[8-20].
Thereby, there were in particular beneficial effects on the liver, including on portal hypertension, and on the intestinal tract[26-32], lung[37-39], thrombosis[21,40], venous[21] and arterial[40], and heart disturbances[41-45], free radicals formation[46-49] and free radical-induced lesions[46-50]. These appear in addition to the described effect on vessel recruitment[21-25], with the azygos vein readily presented in BPC 157-treated rats compared to empty and tortuous examples in the controls (Figure 8).
For BPC 157-treated rats, in early and in late periods, the level of oxidative stress in the liver (MDA- and NO-levels, otherwise known to be increased and critical mediators of liver fibrosis[25]) was almost continuously within normal values as it was in rats with bile duct occlusion[25]. By contrast, along with previous studies[21-25,46-49], in control livers, high MDA- and NO-values during suprahepatic occlusion of the inferior vena cava persisted (Figure 6). Control rats exhibited rapid clot formation, generalized thrombosis (in the portal vein, superior mesenteric vein, splenic vein, inferior vena cava, hepatic artery and cardiac artery; Figure 4), and ascites (consistently present in the early and late period; Figure 5). BPC 157-treated rats exhibited counteraction of these effects, with less venous and arterial clots (as stasis is eliminated or largely attenuated) and reduced ascites, as had previously been emphasized[21-25,40].
In addition, controls presented with immediately peaked P values, significant ST-elevation and tachycardia, as identifiers of acute thrombotic coronary occlusion and right heart failure (Figure 3), and thereby, lung congestion (Figure 11). BPC 157-treated rats had the ECG disturbances completely abrogated, and less lung congestion. Therefore, the immediate presentation of adverse effects and the parallel therapeutic effects might suggest an essential cause-consequence chain of events of Budd-Chiari and BPC 157 in rats, and in particular, a relation to the liver as the prime organ affected[26-32] and failure of its circulation. All controls exhibited gross hemorrhagic lesion progression, hepatomegaly and splenomegaly (Figures 5 and 7), with gross bleeding in the stomach and duodenum, but also intestinal perforation, increased serum enzyme values (Figure 5), and a dilated central vein and terminal portal venules (Figure 9). This contrasted with the spared gross liver presentation, counteracted hepatomegaly and splenomegaly, reduced gross lesions in the stomach, duodenum and intestine, lower serum enzyme values, and no congestion of the central vein or branches of the terminal portal venules in BPC 157-treated rats. In particular, hepatic artery patency might be essential, and indicative for the thrombosis counteraction in all of the investigated veins and arteries, which were investigated[21,40]. Note, hepatic artery perfusion could be essential for recovery from hepatic venous outflow obstruction in rats, pointed out when usually occurring after 70% hepatectomy and right median hepatic vein ligation, in which the role of hepatic artery inflow is of particular importance, in the condition likely even more severe than the obstruction of the hepatic veins by ligation of the upper part of inferior caval vein[51].
Besides, BPC 157 therapy effects described in rats with bile duct cirrhosis and portal hypertension resulted in much less Ki-67- and alpha smooth muscle actin (α-SMA) staining, counteracted disturbed cell proliferation and cytoskeletal structure in hepatic stellate cells, and showed a less disturbed collagen presentation, and thereby, less Mallory staining, and more hepatocytes where fewer had double nuclei[25]. In the course of this cholestatic model of liver injury[25], BPC 157 also counteracted all characteristic structural, biochemical, and hemodynamic disturbances, with portal hypertension either not developed, portal pressure remaining normal (BPC 157 prophylactic regimen) or portal hypertension rapidly reversed (delayed post-treatment). In addition, further consideration might be given to the counteraction of increased liver values of interleukin-6, tumor necrosis factor alpha and interleukin-1β[25], and the relation with counteracted tumor cachexia[18], muscle wasting, increased pro-cachectic and pro-inflammatory mediators, and changes in the expression of forkhead box O3, phosphorylated protein kinase B, phosphorylated mammalian target of rapamycin, and phosphorylated glycogen synthase kinase 3β[18]. This indicates the interaction of BPC 157 with several molecular pathways[52-58], in particular with increased expression and internalization of vascular endothelial growth factor receptor 2 and the activation of the vascular endothelial growth factor receptor 2-Akt- endothelial NO synthase signaling pathway, without the need for other ligands or shear stress[54].
Finally, there is a “honeycomb” smaller vessel network, which appears at the intestinal serosa, which was also noticed in rats with ischemic/reperfusion colitis[23], duodenal venous congestion lesions[24], or inferior vena cava occlusion[21]. Thus, the counteraction of hemorrhagic intestinal lesions, from the stomach to the ascending colon, occurs as a very rapid activation of a bypassing loop, thereby reducing portal hypertension. Otherwise, intestinal ischemia[59] appears as the final consequence of blood pooling in the splanchnic bed, inducing portal hypertension, and multivisceral edema, and mesenterial venous occlusion, could also induce intestinal injury as early as within 5 min. Both of these effects are inflow and outflow alterations[60].
Concluding, BPC 157 therapy (intragastric or abdominal bath, 10 µg/kg and 10 ng/kg dosages), using a previously described protocol[21-25], could alleviate suprahepatic occlusion of the inferior caval vein, as model of the BCS in rats, activating bypassing pathways. BPC 157 likely counteracted Virchow's triad, as it did in rats with infrarenal inferior vena cava occlusion[21]. A particular effect on portal hypertension disturbances, by activation of bypassing pathways, might be envisaged that needs further elaboration. For example, BPC 157 might specifically interact with the NO-system, as previously reviewed[11,17]. In rats that received NO-agents, L-arginine methyl ester (L-NAME; a NOS blocker) or L-arginine (NOS-substrate), BPC 157 counteracted L-NAME-induced hypertension, as well as L-arginine-induced hypotension[61]. This effect appeared to counteract potassium overdose, severe hyperkalemia arrhythmias and hypertension[41] or doxorubicin induced chronic heart failure and hypotension[43]. Finally, as a novel understanding of the cytoprotective mechanisms that are essential for its activity, as previously reviewed[8-20], BPC 157 has recently been shown to increase the survival of cultured enteric neurons and the proliferation of cultured enteric glial cells; therefore healing of a damaged enteric nervous system might also contribute to the observed therapeutic effects[62]. These effects may together counteract a downward spiral in rats with BCS, in acute states, much as in prolonged ischemia.
ARTICLE HIGHLIGHTS
Research background
Resolving the Budd-Chiari syndrome (BCS) in rats follows the evidence that pentadecapeptide BPC 157 therapy alleviated various vascular occlusion disturbances. Rapid activation of a bypassing loop rescues rats with inferior vena cava infrarenal ligation, relieving a Virchow's triad situation, much like in rats with ischemic/reperfusion colitis, duodenal venous congestion lesions, perforated cecum, bile duct ligation induced liver cirrhosis and portal hypertension. BPC 157 was previously reviewed as novel mediator of Robert cytoprotection and endothelium protection in the stomach, and gut-brain axis, beneficial therapy in gastrointestinal tract, with particular reference to vascular recruitment, ulcerative colitis and tumor cachexia, and other tissues healing.
Research motivation
Against these obstructions, BPC 157 therapy rapidly activates an azygos/hemiazygos vein bypassing pathway, upgrading an inadequate rescuing inferior-superior vena cava shunt to an adequate one, as well as a portocaval shunt. With BCS in rats, both caval and portal hypertension, and aortal hypertension occurred and were largely eliminated by BPC 157 therapy. Likewise, BPC 157 therapy was shown in counteracting rapid clot formation in the portal vein, superior mesenteric vein, splenic vein, inferior vena cava, hepatic artery, and coronary artery, as well as peaked P waves, significant ST-elevation, tachycardia, gross organ lesions, and liver and spleen weight increases.
Research objectives
Occluding the inferior vena cava cranially to the hepatic veins, regardless of some limitations, was used for the Budd–Chiari syndrome and its manifestation in rat. To make the even more severe circumstances, with the complete occlusion of the suprahepatic inferior caval vein, Budd–Chiari syndrome has abrupt disturbances initiation. Thereby, BPC 157 rapid effect with the organized bypassing of the occlusion and Budd–Chiari syndrome manifestation reversal, may provide pharmacotherapy treatment in the rat BCS.
Research methods
In rats with occluded suprahepatic inferior caval vein, we assessed the activated bypassing pathways (inferior/superior caval veins; portocaval shunt), counteracted caval/portal hypertension, aortal hypotension, venous/arterial thrombosis, electrocardiogram disturbances, liver and gastrointestinal lesions (i.e., stomach and duodenum hemorrhages, in particular, congestion), hepatomegaly and splenomegaly. Medication (at 1 min, 15 min, 24 h, or 48 h) ligation time consisted of 10 µg/kg BPC 157, 10 ng BPC 157 or 5 mL/kg saline, administered once as an abdominal bath or intragastric application. Furthermore, levels of nitric oxide (NO), malondialdehyde (MDA) in the liver and serum enzymes were determined.
Research results
The bypassing pathways (the inferior vena cava-azygos (hemiazygos) vein-superior vena cava; portocaval shunt) occurred rapidly. BPC 157 antagonized portal/caval hypertension (caval ˃ portal hypertension), and aortal hypotension, refractory ascites and thrombosis (portal vein tributaries, inferior vena cava, hepatic and coronary arteries), pathology of the lungs (severe capillary congestion) and liver (dilated central veins and terminal portal venules), intestine hemorrhagic lesions (substantial capillary congestion, submucosal edema and architecture loss), increased liver and spleen weight serum and enzymes values, NO- and MDA-levels in the liver. BPC 157 counteracted increased P wave amplitude, tachycardia and ST-elevation, i.e., right heart failure from acute thrombotic coronary occlusion.
Research conclusions
Particular reversal of the regular threatening course otherwise arising in the rats with the occluded suprahepatic inferior caval vein. With application of the stable gastric pentadecapeptide BPC 157, we raised new hypothesis about rapid bypassing of the suprahepatic inferior caval vein occlusion in the BCS in rats, and mitigating its manifestations, along with the active and effective pharmacotherapy treatment. The beneficial BPC 157 effects, noted in the present study, may together counteract a downward spiral in rats with BCS, in acute states, much as in prolonged ischemia. In BCS research, rats studies used occluding the inferior vena cava cranially to the hepatic veins, but bypassing of the occlusion in the rat BCS along with pharmacotherapy treatment was not considered. BPC 157 therapy significance results with the rapidly activated azygos/hemiazygos vein bypassing pathway, upgrading an inadequate rescuing inferior-superior vena cava shunt to an adequate one, as well as a portocaval shunt. Consequently, the BCS-rats presented both caval and portal hypertension, and aortal hypertension, largely eliminated by BPC 157 therapy. Largely attenuated consequent disturbances (rapid clot formation in the portal vein, superior mesenteric vein, splenic vein, inferior vena cava, hepatic artery, and coronary artery, as well as peaked P waves, significant ST-elevation, tachycardia, gross organ lesions, and liver and spleen weight increases) all together support this contention. We used gross (USB camera) and microscopic observations, venography, blood pressure and electrocardiogram assessment, bilirubin and enzyme activity, levels of NO, MDA in the liver and serum enzymes assessment. As combined methods, they are together new methods to determine description of the Budd Chiari syndrome in rats, and the significance of the activated bypassing pathways between the inferior and superior caval veins and portocaval shunt, counteracted caval/portal hypertension, aortal hypotension, venous/arterial thrombosis, electrocardiogram disturbances, liver and gastrointestinal lesions (i.e., stomach and duodenum hemorrhages, in particular, congestion), hepatomegaly and splenomegaly. We should emphasize the rapidly activated azygos/hemiazygos vein bypassing pathway presented as an adequate rescuing inferior-superior vena cava shunt along with a portocaval shunt, both caval and portal hypertension, and aortal hypertension largely eliminated. As mentioned above, largely attenuated consequent disturbances (rapid clot formation in the portal vein, superior mesenteric vein, splenic vein, inferior vena cava, hepatic artery, and coronary artery, as well as peaked P waves, significant ST-elevation, tachycardia, gross organ lesions, and liver and spleen weight increases) all together support this contention. Recently, as a possible therapy resolving solution for Budd Chiari syndrome in rats, pentadecapeptide BPC 157 therapy has been used in alleviating vascular occlusion disturbances. Rapid activation of a bypassing loop occurs in rats with an infrarenal ligation of the inferior vena cava, relieving a Virchow's triad situation, much like in rats with ischemic/reperfusion colitis, duodenal venous congestion lesions, perforated cecum, bile duct ligation induced liver cirrhosis and portal hypertension. BPC 157 therapy (intragastric or abdominal bath, 10 µg/kg and 10 ng/kg dosages), using a previously described protocol, could alleviate suprahepatic occlusion of the inferior caval vein, as model of the BCS in rats, activating bypassing pathways. BPC 157 likely counteracted Virchow's triad, as it did in rats with infrarenal inferior vena cava occlusion. A particular effect on portal hypertension disturbances, by activation of bypassing pathways, might be envisaged that needs further elaboration.
Research perspectives
The bypassing pathway of the inferior vena cava-azygos (hemiazygos) vein-superior vena cava and portocaval shunt occurred rapidly. Even with severe caval/portal hypertension, BPC 157 antagonized portal and caval hypertension and aortal hypotension. Large extent of the consequent disturbances may verify the significance of the Budd Chiari syndrome in rats for the corresponding human condition. Likewise, the largely attenuated disturbances may indicate that the beneficial BPC 157 effects, noted in the present study, may likely counteract a downward spiral in the rats with the suprahepatic inferior caval vein ligation much like in the patients with BCS, as in prolonged ischemia. To further establish the proposed therapy solution for Budd Chiari syndrome, we continue research in this issue. Gross (USB camera) and microscopic observations, venography, blood pressure and electrocardiogram assessment, bilirubin and enzyme activity, levels of NO, MDA in the liver and serum enzymes as combined methods are best methods to determine description of the Budd Chiari syndrome in rats.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Department of Veterinary, Ministry of Agriculture, Republic of Croatia, No: UP/I 322-01/07-01/210.
Institutional animal care and use committee statement: Male Albino Wistar, body weight 200 g, 12 weeks old, randomly assigned, used in all of the experiments, 6 rats/group/interval, approved by local Ethic Committee.
Conflict-of-interest statement: The authors state that they have no conflicts of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Unsolicited manuscript
Peer-review started: October 7, 2019
First decision: November 27, 2019
Article in press: January 19, 2020
Specialty type: Gastroenterology and hepatology
Country of origin: Croatia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dragoteanu MN S-Editor: Ma YJ L-Editor: A E-Editor: Wu YXJ
Contributor Information
Slaven Gojkovic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Ivan Krezic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Borna Vrdoljak, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Dominik Malekinusic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Ivan Barisic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Andreja Petrovic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Katarina Horvat Pavlov, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Marijan Kolovrat, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Antonija Duzel, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Mario Knezevic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Katarina Kasnik Kovac, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Domagoj Drmic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Lovorka Batelja Vuletic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Antonio Kokot, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Alenka Boban Blagaic, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Sven Seiwerth, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia.
Predrag Sikiric, Departments of Pharmacology and Pathology, Medical Faculty University of Zagreb, Zagreb 10000, Croatia. sikiric@mef.hr.
Data sharing statement
No additional data are available.
References
- 1.Ludwig J, Hashimoto E, McGill DB, van Heerden JA. Classification of hepatic venous outflow obstruction: ambiguous terminology of the Budd-Chiari syndrome. Mayo Clin Proc. 1990;65:51–55. doi: 10.1016/s0025-6196(12)62109-0. [DOI] [PubMed] [Google Scholar]
- 2.Darwish Murad S, Dom VA, Ritman EL, de Groen PC, Beigley PE, Abraham SC, Zondervan PE, Janssen HL. Early changes of the portal tract on microcomputed tomography images in a newly-developed rat model for Budd-Chiari syndrome. J Gastroenterol Hepatol. 2008;23:1561–1566. doi: 10.1111/j.1440-1746.2008.05403.x. [DOI] [PubMed] [Google Scholar]
- 3.Abraldes JG, Pasarín M, García-Pagán JC. Animal models of portal hypertension. World J Gastroenterol. 2006;12:6577–6584. doi: 10.3748/wjg.v12.i41.6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akiyoshi H, Terada T. Centrilobular and perisinusoidal fibrosis in experimental congestive liver in the rat. J Hepatol. 1999;30:433–439. doi: 10.1016/s0168-8278(99)80102-0. [DOI] [PubMed] [Google Scholar]
- 5.Cheng DL, Zhu N, Li CL, Lv WF, Fang WW, Liu Y, Li CT. Significance of malondialdehyde, superoxide dismutase and endotoxin levels in Budd-Chiari syndrome in patients and a rat model. Exp Ther Med. 2018;16:5227–5235. doi: 10.3892/etm.2018.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orloff MJ, Daily PO, Girard B. Treatment of Budd-Chiari syndrome due to inferior vena cava occlusion by combined portal and vena caval decompression. Am J Surg. 1992;163:137–142; discussion 142-143. doi: 10.1016/0002-9610(92)90266-t. [DOI] [PubMed] [Google Scholar]
- 7.Hobbs KE. Budd-Chiari syndrome--transplant, meso-atrial shunt or combined portocaval shunt with cavo-atrial shunt. HPB Surg. 1993;6:331–333. doi: 10.1155/1993/75272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sikiric P, Seiwerth S, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D. Revised Robert's cytoprotection and adaptive cytoprotection and stable gastric pentadecapeptide BPC 157. Possible significance and implications for novel mediator. Curr Pharm Des. 2010;16:1224–1234. doi: 10.2174/138161210790945977. [DOI] [PubMed] [Google Scholar]
- 9.Sikiric P, Seiwerth S, Rucman R, Drmic D, Stupnisek M, Kokot A, Sever M, Zoricic I, Zoricic Z, Batelja L, Ziger T, Luetic K, Vlainic J, Rasic Z, Bencic ML. Stress in Gastrointestinal Tract and Stable Gastric Pentadecapeptide BPC 157. Finally, do we have a Solution? Curr Pharm Des. 2017;23:4012–4028. doi: 10.2174/1381612823666170220163219. [DOI] [PubMed] [Google Scholar]
- 10.Sikiric P, Hahm KB, Blagaic AB, Tvrdeic A, Pavlov KH, Petrovic A, Kokot A, Gojkovic S, Krezic I, Drmic D, Rucman R, Seiwerth S. Stable Gastric Pentadecapeptide BPC 157, Robert's Stomach Cytoprotection/Adaptive Cytoprotection/Organoprotection, and Selye's Stress Coping Response: Progress, Achievements, and the Future. Gut Liver. 2019 doi: 10.5009/gnl18490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sikiric P, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, Drmic D, Stupnisek M, Misic M, Vuletic LB, Pavlov KH, Barisic I, Kokot A, Peklic M, Strbe S, Blagaic AB, Tvrdeic A, Rokotov DS, Vrcic H, Staresinic M, Seiwerth S. Novel Cytoprotective Mediator, Stable Gastric Pentadecapeptide BPC 157. Vascular Recruitment and Gastrointestinal Tract Healing. Curr Pharm Des. 2018;24:1990–2001. doi: 10.2174/1381612824666180608101119. [DOI] [PubMed] [Google Scholar]
- 12.Sikiric P, Seiwerth S, Rucman R, Kolenc D, Vuletic LB, Drmic D, Grgic T, Strbe S, Zukanovic G, Crvenkovic D, Madzarac G, Rukavina I, Sucic M, Baric M, Starcevic N, Krstonijevic Z, Bencic ML, Filipcic I, Rokotov DS, Vlainic J. Brain-gut Axis and Pentadecapeptide BPC 157: Theoretical and Practical Implications. Curr Neuropharmacol. 2016;14:857–865. doi: 10.2174/1570159X13666160502153022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Vrcic H, Sebecic B. Stable gastric pentadecapeptide BPC 157: novel therapy in gastrointestinal tract. Curr Pharm Des. 2011;17:1612–1632. doi: 10.2174/138161211796196954. [DOI] [PubMed] [Google Scholar]
- 14.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Stambolija V, Zoricic Z, Vrcic H, Sebecic B. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Curr Med Chem. 2012;19:126–132. doi: 10.2174/092986712803414015. [DOI] [PubMed] [Google Scholar]
- 15.Seiwerth S, Brcic L, Vuletic LB, Kolenc D, Aralica G, Misic M, Zenko A, Drmic D, Rucman R, Sikiric P. BPC 157 and blood vessels. Curr Pharm Des. 2014;20:1121–1125. doi: 10.2174/13816128113199990421. [DOI] [PubMed] [Google Scholar]
- 16.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Aralica G, Safic H, Suran J, Rak D, Dzidic S, Vrcic H, Sebecic B. Toxicity by NSAIDs. Counteraction by stable gastric pentadecapeptide BPC 157. Curr Pharm Des. 2013;19:76–83. doi: 10.2174/13816128130111. [DOI] [PubMed] [Google Scholar]
- 17.Sikiric P, Seiwerth S, Rucman R, Turkovic B, Rokotov DS, Brcic L, Sever M, Klicek R, Radic B, Drmic D, Ilic S, Kolenc D, Aralica G, Stupnisek M, Suran J, Barisic I, Dzidic S, Vrcic H, Sebecic B. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr Pharm Des. 2014;20:1126–1135. doi: 10.2174/13816128113190990411. [DOI] [PubMed] [Google Scholar]
- 18.Kang EA, Han YM, An JM, Park YJ, Sikiric P, Kim DH, Kwon KA, Kim YJ, Yang D, Tchah H, Hahm KB. BPC157 as Potential Agent Rescuing from Cancer Cachexia. Curr Pharm Des. 2018;24:1947–1956. doi: 10.2174/1381612824666180614082950. [DOI] [PubMed] [Google Scholar]
- 19.Seiwerth S, Rucman R, Turkovic B, Sever M, Klicek R, Radic B, Drmic D, Stupnisek M, Misic M, Vuletic LB, Pavlov KH, Barisic I, Kokot A, Japjec M, Blagaic AB, Tvrdeic A, Rokotov DS, Vrcic H, Staresinic M, Sebecic B, Sikiric P. BPC 157 and Standard Angiogenic Growth Factors. Gastrointestinal Tract Healing, Lessons from Tendon, Ligament, Muscle and Bone Healing. Curr Pharm Des. 2018;24:1972–1989. doi: 10.2174/1381612824666180712110447. [DOI] [PubMed] [Google Scholar]
- 20.Gwyer D, Wragg NM, Wilson SL. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res. 2019;377:153–159. doi: 10.1007/s00441-019-03016-8. [DOI] [PubMed] [Google Scholar]
- 21.Vukojević J, Siroglavić M, Kašnik K, Kralj T, Stanćić D, Kokot A, Kolarić D, Drmić D, Sever AZ, Barišić I, Šuran J, Bojić D, Patrlj MH, Sjekavica I, Pavlov KH, Vidović T, Vlainić J, Stupnišek M, Seiwerth S, Sikirić P. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vascul Pharmacol. 2018;106:54–66. doi: 10.1016/j.vph.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 22.Duzel A, Vlainic J, Antunovic M, Malekinusic D, Vrdoljak B, Samara M, Gojkovic S, Krezic I, Vidovic T, Bilic Z, Knezevic M, Sever M, Lojo N, Kokot A, Kolovrat M, Drmic D, Vukojevic J, Kralj T, Kasnik K, Siroglavic M, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 in the treatment of colitis and ischemia and reperfusion in rats: New insights. World J Gastroenterol. 2017;23:8465–8488. doi: 10.3748/wjg.v23.i48.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amic F, Drmic D, Bilic Z, Krezic I, Zizek H, Peklic M, Klicek R, Pajtak A, Amic E, Vidovic T, Rakic M, Milkovic Perisa M, Horvat Pavlov K, Kokot A, Tvrdeic A, Boban Blagaic A, Zovak M, Seiwerth S, Sikiric P. Bypassing major venous occlusion and duodenal lesions in rats, and therapy with the stable gastric pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol. 2018;24:5366–5378. doi: 10.3748/wjg.v24.i47.5366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drmic D, Samara M, Vidovic T, Malekinusic D, Antunovic M, Vrdoljak B, Ruzman J, Milkovic Perisa M, Horvat Pavlov K, Jeyakumar J, Seiwerth S, Sikiric P. Counteraction of perforated cecum lesions in rats: Effects of pentadecapeptide BPC 157, L-NAME and L-arginine. World J Gastroenterol. 2018;24:5462–5476. doi: 10.3748/wjg.v24.i48.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sever AZ, Sever M, Vidovic T, Lojo N, Kolenc D, Vuletic LB, Drmic D, Kokot A, Zoricic I, Coric M, Vlainic J, Poljak L, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 in the therapy of the rats with bile duct ligation. Eur J Pharmacol. 2019;847:130–142. doi: 10.1016/j.ejphar.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Prkacin I, Separovic J, Aralicia G, Perovic D, Gjurasin M, Lovric-Bencic M, Stancic-Rokotov D, Staresinic M, Anic T, Mikus D, Sikiric P, Seiwerth S, Mise S, Rotkvic I, Jagic V, Rucman R, Petek M, Turkovic B, Marovic A, Sebecic B, Boban-Blagaic A, Kokic N. Portal hypertension and liver lesions in chronically alcohol drinking rats prevented and reversed by stable gastric pentadecapeptide BPC 157 (PL-10, PLD-116), and propranolol, but not ranitidine. J Physiol Paris. 2001;95:315–324. doi: 10.1016/s0928-4257(01)00044-4. [DOI] [PubMed] [Google Scholar]
- 27.Drmic D, Kolenc D, Ilic S, Bauk L, Sever M, Zenko Sever A, Luetic K, Suran J, Seiwerth S, Sikiric P. Celecoxib-induced gastrointestinal, liver and brain lesions in rats, counteraction by BPC 157 or L-arginine, aggravation by L-NAME. World J Gastroenterol. 2017;23:5304–5312. doi: 10.3748/wjg.v23.i29.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ilic S, Brcic I, Mester M, Filipovic M, Sever M, Klicek R, Barisic I, Radic B, Zoricic Z, Bilic V, Berkopic L, Brcic L, Kolenc D, Romic Z, Pazanin L, Seiwerth S, Sikiric P. Over-dose insulin and stable gastric pentadecapeptide BPC 157. Attenuated gastric ulcers, seizures, brain lesions, hepatomegaly, fatty liver, breakdown of liver glycogen, profound hypoglycemia and calcification in rats. J Physiol Pharmacol. 2009;60 Suppl 7:107–114. [PubMed] [Google Scholar]
- 29.Ilic S, Drmic D, Franjic S, Kolenc D, Coric M, Brcic L, Klicek R, Radic B, Sever M, Djuzel V, Filipovic M, Djakovic Z, Stambolija V, Blagaic AB, Zoricic I, Gjurasin M, Stupnisek M, Romic Z, Zarkovic K, Dzidic S, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and its effects on a NSAID toxicity model: diclofenac-induced gastrointestinal, liver, and encephalopathy lesions. Life Sci. 2011;88:535–542. doi: 10.1016/j.lfs.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Ilic S, Drmic D, Zarkovic K, Kolenc D, Brcic L, Radic B, Djuzel V, Blagaic AB, Romic Z, Dzidic S, Kalogjera L, Seiwerth S, Sikiric P. Ibuprofen hepatic encephalopathy, hepatomegaly, gastric lesion and gastric pentadecapeptide BPC 157 in rats. Eur J Pharmacol. 2011;667:322–329. doi: 10.1016/j.ejphar.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 31.Lojo N, Rasic Z, Zenko Sever A, Kolenc D, Vukusic D, Drmic D, Zoricic I, Sever M, Seiwerth S, Sikiric P. Effects of Diclofenac, L-NAME, L-Arginine, and Pentadecapeptide BPC 157 on Gastrointestinal, Liver, and Brain Lesions, Failed Anastomosis, and Intestinal Adaptation Deterioration in 24 Hour-Short-Bowel Rats. PLoS One. 2016;11:e0162590. doi: 10.1371/journal.pone.0162590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sikiric P, Seiwerth S, Grabarevic Z, Rucman R, Petek M, Rotkvic I, Turkovic B, Jagic V, Mildner B, Duvnjak M. Hepatoprotective effect of BPC 157, a 15-amino acid peptide, on liver lesions induced by either restraint stress or bile duct and hepatic artery ligation or CCl4 administration. A comparative study with dopamine agonists and somatostatin. Life Sci. 1993;53:PL291–PL296. doi: 10.1016/0024-3205(93)90589-u. [DOI] [PubMed] [Google Scholar]
- 33.Greene EC. Anatomy of the rat. Trans Am Philos Soc. 1935;27:ii. [Google Scholar]
- 34.Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999;30:1481–1489. doi: 10.1002/hep.510300622. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigues DA, da Silva AR, Serigiolle LC, Fidalgo Rde S, Favero SS, Leme PL. Constriction rate variation produced by partial ligation of the portal vein at pre-hepatic portal hypertension induced in rats. Arq Bras Cir Dig. 2014;27:280–284. doi: 10.1590/S0102-67202014000400012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen Z, Zhang JZ, Xia HM, Yang CX, Chen YJ. Stability of a rat model of prehepatic portal hypertension caused by partial ligation of the portal vein. World J Gastroenterol. 2009;15:4049–4054. doi: 10.3748/wjg.15.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabarevic Z, Tisljar M, Artukovic B, Bratulic M, Dzaja P, Seiwerth S, Sikiric P, Peric J, Geres D, Kos J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicks. J Physiol Paris. 1997;91:139–149. doi: 10.1016/s0928-4257(97)89478-8. [DOI] [PubMed] [Google Scholar]
- 38.Stancic-Rokotov D, Sikiric P, Seiwerth S, Slobodnjak Z, Aralica J, Aralica G, Perovic D, Anic T, Zoricic I, Buljat G, Prkacin I, Gjurasin M, Rucman R, Petek M, Turkovic B, Ivasovic Z, Jagic V, Staresinic M, Boban-Blagaic A. Ethanol gastric lesion aggravated by lung injury in rat. Therapy effect of antiulcer agents. J Physiol Paris. 2001;95:289–293. doi: 10.1016/s0928-4257(01)00040-7. [DOI] [PubMed] [Google Scholar]
- 39.Stancic-Rokotov D, Slobodnjak Z, Aralica J, Aralica G, Perovic D, Staresinic M, Gjurasin M, Anic T, Zoricic I, Buljat G, Prkacin I, Sikiric P, Seiwerth S, Rucman R, Petek M, Turkovic B, Kokic N, Jagic V, Boban-Blagaic A. Lung lesions and anti-ulcer agents beneficial effect: anti-ulcer agents pentadecapeptide BPC 157, ranitidine, omeprazole and atropine ameliorate lung lesion in rats. J Physiol Paris. 2001;95:303–308. doi: 10.1016/s0928-4257(01)00042-0. [DOI] [PubMed] [Google Scholar]
- 40.Hrelec M, Klicek R, Brcic L, Brcic I, Cvjetko I, Seiwerth S, Sikiric P. Abdominal aorta anastomosis in rats and stable gastric pentadecapeptide BPC 157, prophylaxis and therapy. J Physiol Pharmacol. 2009;60 Suppl 7:161–165. [PubMed] [Google Scholar]
- 41.Balenovic D, Bencic ML, Udovicic M, Simonji K, Hanzevacki JS, Barisic I, Kranjcevic S, Prkacin I, Coric V, Brcic L, Coric M, Brcic I, Borovic S, Radic B, Drmic D, Vrcic H, Seiwerth S, Sikiric P. Inhibition of methyldigoxin-induced arrhythmias by pentadecapeptide BPC 157: a relation with NO-system. Regul Pept. 2009;156:83–89. doi: 10.1016/j.regpep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Barisic I, Balenovic D, Klicek R, Radic B, Nikitovic B, Drmic D, Udovicic M, Strinic D, Bardak D, Berkopic L, Djuzel V, Sever M, Cvjetko I, Romic Z, Sindic A, Bencic ML, Seiwerth S, Sikiric P. Mortal hyperkalemia disturbances in rats are NO-system related. The life saving effect of pentadecapeptide BPC 157. Regul Pept. 2013;181:50–66. doi: 10.1016/j.regpep.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Lovric-Bencic M, Sikiric P, Hanzevacki JS, Seiwerth S, Rogic D, Kusec V, Aralica G, Konjevoda P, Batelja L, Blagaic AB. Doxorubicine-congestive heart failure-increased big endothelin-1 plasma concentration: reversal by amlodipine, losartan, and gastric pentadecapeptide BPC157 in rat and mouse. J Pharmacol Sci. 2004;95:19–26. doi: 10.1254/jphs.95.19. [DOI] [PubMed] [Google Scholar]
- 44.Strinic D, Belosic Halle Z, Luetic K, Nedic A, Petrovic I, Sucic M, Zivanovic Posilovic G, Balenovic D, Strbe S, Udovicic M, Drmic D, Stupnisek M, Lovric Bencic M, Seiwerth S, Sikiric P. BPC 157 counteracts QTc prolongation induced by haloperidol, fluphenazine, clozapine, olanzapine, quetiapine, sulpiride, and metoclopramide in rats. Life Sci. 2017;186:66–79. doi: 10.1016/j.lfs.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 45.Zivanovic-Posilovic G, Balenovic D, Barisic I, Strinic D, Stambolija V, Udovicic M, Uzun S, Drmic D, Vlainic J, Bencic ML, Sindic A, Seiwerth S, Sikiric P. Stable gastric pentadecapeptide BPC 157 and bupivacaine. Eur J Pharmacol. 2016;793:56–65. doi: 10.1016/j.ejphar.2016.10.035. [DOI] [PubMed] [Google Scholar]
- 46.Belosic Halle Z, Vlainic J, Drmic D, Strinic D, Luetic K, Sucic M, Medvidovic-Grubisic M, Pavelic Turudic T, Petrovic I, Seiwerth S, Sikiric P. Class side effects: decreased pressure in the lower oesophageal and the pyloric sphincters after the administration of dopamine antagonists, neuroleptics, anti-emetics, L-NAME, pentadecapeptide BPC 157 and L-arginine. Inflammopharmacology. 2017 doi: 10.1007/s10787-017-0358-8. [DOI] [PubMed] [Google Scholar]
- 47.Luetic K, Sucic M, Vlainic J, Halle ZB, Strinic D, Vidovic T, Luetic F, Marusic M, Gulic S, Pavelic TT, Kokot A, Seiwerth RS, Drmic D, Batelja L, Seiwerth S, Sikiric P. Cyclophosphamide induced stomach and duodenal lesions as a NO-system disturbance in rats: L-NAME, L-arginine, stable gastric pentadecapeptide BPC 157. Inflammopharmacology. 2017;25:255–264. doi: 10.1007/s10787-017-0330-7. [DOI] [PubMed] [Google Scholar]
- 48.Sucic M, Luetic K, Jandric I, Drmic D, Sever AZ, Vuletic LB, Halle ZB, Strinic D, Kokot A, Seiwerth RS, Zoricic I, Blagaic AB, Seiwerth S, Sikiric P. Therapy of the rat hemorrhagic cystitis induced by cyclophosphamide. Stable gastric pentadecapeptide BPC 157, L-arginine, L-NAME. Eur J Pharmacol. 2019;861:172593. doi: 10.1016/j.ejphar.2019.172593. [DOI] [PubMed] [Google Scholar]
- 49.Ilic S, Drmic D, Zarkovic K, Kolenc D, Coric M, Brcic L, Klicek R, Radic B, Sever M, Djuzel V, Ivica M, Boban Blagaic A, Zoricic Z, Anic T, Zoricic I, Djidic S, Romic Z, Seiwerth S, Sikiric P. High hepatotoxic dose of paracetamol produces generalized convulsions and brain damage in rats. A counteraction with the stable gastric pentadecapeptide BPC 157 (PL 14736) J Physiol Pharmacol. 2010;61:241–250. [PubMed] [Google Scholar]
- 50.Sikiric P, Marovic A, Matoz W, Anic T, Buljat G, Mikus D, Stancic-Rokotov D, Separovic J, Seiwerth S, Grabarevic Z, Rucman R, Petek M, Ziger T, Sebecic B, Zoricic I, Turkovic B, Aralica G, Perovic D, Duplancic B, Lovric-Bencic M, Rotkvic I, Mise S, Jagic V, Hahn V. A behavioural study of the effect of pentadecapeptide BPC 157 in Parkinson's disease models in mice and gastric lesions induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydrophyridine. J Physiol Paris. 1999;93:505–512. doi: 10.1016/s0928-4257(99)00119-9. [DOI] [PubMed] [Google Scholar]
- 51.Huang H, Deng M, Jin H, Liu A, Dirsch O, Dahmen U. Hepatic arterial perfusion is essential for the spontaneous recovery from focal hepatic venous outflow obstruction in rats. Am J Transplant. 2011;11:2342–2352. doi: 10.1111/j.1600-6143.2011.03682.x. [DOI] [PubMed] [Google Scholar]
- 52.Chang CH, Tsai WC, Lin MS, Hsu YH, Pang JH. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J Appl Physiol (1985) 2011;110:774–780. doi: 10.1152/japplphysiol.00945.2010. [DOI] [PubMed] [Google Scholar]
- 53.Chang CH, Tsai WC, Hsu YH, Pang JH. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules. 2014;19:19066–19077. doi: 10.3390/molecules191119066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang T, Zhang K, Sun L, Xue X, Zhang C, Shu Z, Mu N, Gu J, Zhang W, Wang Y, Zhang Y, Zhang W. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des Devel Ther. 2015;9:2485–2499. doi: 10.2147/DDDT.S82030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hsieh MJ, Liu HT, Wang CN, Huang HY, Lin Y, Ko YS, Wang JS, Chang VH, Pang JS. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J Mol Med (Berl) 2017;95:323–333. doi: 10.1007/s00109-016-1488-y. [DOI] [PubMed] [Google Scholar]
- 56.Tkalcević VI, Cuzić S, Brajsa K, Mildner B, Bokulić A, Situm K, Perović D, Glojnarić I, Parnham MJ. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur J Pharmacol. 2007;570:212–221. doi: 10.1016/j.ejphar.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 57.Chen Y, Wang W, Wang H, Li Y, Shi M, Li H, Yan J. Rapamycin Attenuates Splenomegaly in both Intrahepatic and Prehepatic Portal Hypertensive Rats by Blocking mTOR Signaling Pathway. PLoS One. 2016;11:e0141159. doi: 10.1371/journal.pone.0141159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cesarec V, Becejac T, Misic M, Djakovic Z, Olujic D, Drmic D, Brcic L, Rokotov DS, Seiwerth S, Sikiric P. Pentadecapeptide BPC 157 and the esophagocutaneous fistula healing therapy. Eur J Pharmacol. 2013;701:203–212. doi: 10.1016/j.ejphar.2012.11.055. [DOI] [PubMed] [Google Scholar]
- 59.Martell M, Coll M, Ezkurdia N, Raurell I, Genescà J. Physiopathology of splanchnic vasodilation in portal hypertension. World J Hepatol. 2010;2:208–220. doi: 10.4254/wjh.v2.i6.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuchida Y, Aoki N, Fukuda O, Nakano M, Igarashi H. Changes in hemodynamics in jejunal flaps of rabbits due to ischemia, venous congestion, and reperfusion as measured by means of colored microspheres. Plast Reconstr Surg. 1998;101:147–154. doi: 10.1097/00006534-199801000-00024. [DOI] [PubMed] [Google Scholar]
- 61.Sikirić P, Seiwerth S, Grabarević Z, Rucman R, Petek M, Jagić V, Turković B, Rotkvić I, Mise S, Zoricić I, Konjevoda P, Perović D, Jurina L, Separović J, Hanzevacki M, Artuković B, Bratulić M, Tisljar M, Gjurasin M, Miklić P, Stancić-Rokotov D, Slobodnjak Z, Jelovac N, Marović A. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur J Pharmacol. 1997;332:23–33. doi: 10.1016/s0014-2999(97)01033-9. [DOI] [PubMed] [Google Scholar]
- 62.Wang XY, Qu M, Duan R, Shi D, Jin L, Gao J, Wood JD, Li J, Wang GD. Cytoprotective Mechanism of the Novel Gastric Peptide BPC157 in Gastrointestinal Tract and Cultured Enteric Neurons and Glial Cells. Neurosci Bull. 2019;35:167–170. doi: 10.1007/s12264-018-0269-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.