Abstract
BACKGROUND
The exact mechanism of proton pump inhibitors (PPIs)-induced hypomagnesemia (PPIH) is largely unknown. Previous studies proposed that PPIH is a consequence of intestinal Mg2+ malabsorption. However, the mechanism of PPIs-suppressed intestinal Mg2+ absorption is under debate.
AIM
To investigate the effect of 12-wk and 24-wk omeprazole injection on the total, transcellular, and paracellular Mg2+ absorption in the duodenum, jejunum, ileum, and colon of male Sprague-Dawley rats.
METHODS
The rats received 20 mg/kg∙d subcutaneous omeprazole injection for 12 or 24 wk. Plasma and urinary Mg2+, Ca2+, and PO43− levels were measured. The plasma concentrations of 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), parathyroid hormone (PTH), fibroblast growth factor 23 (FGF-23), epidermal growth factor (EGF), and insulin were also observed. The duodenum, jejunum, ileum, and colon of each rat were mounted onto individual modified Using chamber setups to study the rates of total, transcellular, and paracellular Mg2+ absorption simultaneously. The expression of transient receptor potential melastatin 6 (TRPM6) and cyclin M4 (CNNM4) in the entire intestinal tract was also measured.
RESULTS
Single-dose omeprazole injection significantly increased the intraluminal pH of the stomach, duodenum, and jejunum. Omeprazole injection for 12 and 24 wk induced hypomagnesemia with reduced urinary Mg2+ excretion. The plasma Ca2+ was normal but the urinary Ca2+ excretion was reduced in rats with PPIH. The plasma and urinary PO43− levels increased in PPIH rats. The levels of 1α,25(OH)2D3 and FGF-23 increased, whereas that of plasma EGF decreased in the omeprazole-treated rats. The rates of the total, transcellular, and paracellular Mg2+ absorption was significantly lower in the duodenum, jejunum, ileum, and colon of the rats with PPIH than in those of the control rats. The percent suppression of Mg2+ absorption in the duodenum, jejunum, ileum, and colon of the rats with PPIH compared with the control rats was 81.86%, 70.59%, 69.45%, and 39.25%, respectively. Compared with the control rats, the rats with PPIH had significantly higher TRPM6 and CNNM4 expression levels throughout the intestinal tract.
CONCLUSION
Intestinal Mg2+ malabsorption was observed throughout the intestinal tract of rats with PPIH. PPIs mainly suppressed small intestinal Mg2+ absorption. Omeprazole exerted no effect on the intraluminal acidic pH in the colon. Thus, the lowest percent suppression of total Mg2+ absorption was found in the colon. The expression levels of TRPM6 and CNNM4 increased, indicating the presence of a compensatory response to Mg2+ malabsorption in rats with PPIH. Therefore, the small intestine is an appropriate segment that should be modulated to counteract PPIH.
Keywords: Adverse effect, Colon, Mg2+ absorption, Proton pump inhibitors-induced hypomagnesemia, Small intestine
Core tip: Proton pump inhibitors (PPIs) induced hypomagnesemia (PPIH) has attracted attention in the past decade. Previous studies proposed that PPIH is a consequence of intestinal Mg2+ malabsorption. However, the effect of prolonged PPI administration on duodenal, jejunal, ileal, and colonic Mg2+ absorption is largely unknown. In this study, the rats received 20 mg/ kg∙d subcutaneous omeprazole injection for 12 and 24 wk, which is comparable to 5 and 10 human years, respectively. Omeprazole injection induced hypomagnesemia with reduced urinary Mg2+ excretion. The rates of total, paracellular, and transcellular Mg2+ absorption reduced in the duodenum, jejunum, ilium, and colon were discovered in PPIH rats.
INTRODUCTION
Mg2+ is an essential ion that mediates several physiological functions in the brain, lung, heart and vessel, pancreas and liver, muscle, and bone[1]. Mg2+ disturbance has been implicated in the pathophysiological mechanisms of several diseases in those organs; therefore, Mg2+ supplement is a potential therapeutic regime in many of these diseases[1]. Over 99% of total body Mg2+ is stored in the bone, muscle, and soft tissues[1,2]. The remaining 1% is found in the plasma, which is tightly regulated by intestinal absorption and renal excretion[1,2]. Intestinal epithelium absorbs Mg2+ through paracellular passive and transcellular active mechanisms[2]. Transcellular Mg2+ absorption requires the activities of luminal transient receptor potential melastatin 6 (TRPM6) and basolateral cyclin M4 (CNNM4), whereas paracellular absorption is regulated by tight junction-associated claudin proteins. However, the study of transepithelial Mg2+ transport is limited by the poor understanding on the mechanism of intestinal Mg2+ absorption[2]. Previous studies proposed that paracellular and transcellular Mg2+ absorption exclusively proceeds in the small and large intestines, respectively[1,3,4]. In addition, the expression of TRPM6 mRNA is barely detected in mouse duodenum[4]. Conversely, another report demonstrated paracellular and transcellular Mg2+ absorption in the duodenum[5]. Immunofluore-scence imaging clearly showed the expression and localization of TRPM6 protein in the brush-border villi of the duodenum[6]. CNNM4 protein was also identified in small intestinal epithelium[7]. Therefore, the simultaneous study on the transcellular and paracellular Mg2+ absorption in the duodenum, jejunum, ileum, and colon will provide data on the mechanism of intestinal Mg2+ absorption.
Proton pump inhibitor (PPI)-induced hypomagnesemia (PPIH) has been reported since 2006[8-16]. Approximately 61% and 29% of patients with PPIH have PPIs prescription for at least 5 and 10 years, respectively[10]. However, PPIH was also diagnosed in patients who used PPIs for 2 months[17]. Clinical observations suggested that PPIH is a consequence of intestinal Mg2+ malabsorption[9,12,15], the mechanism of which is currently under debate. Previous studies proposed that PPIs suppress colonic Mg2+ absorption in normal and PPIH mice[4,13]. However, the effect of PPIs administration on colonic Mg2+ absorption had not been investigated[4,13]. Moreover, the stimulation of colonic Mg2+ absorption by using inulin fibers could not normalize plasma Mg2+ level in those PPIH mice[13]. A recent study has reported that omeprazole injection for 24 wk induces systemic Mg2+ deficiency and hypomagnesemia in male Sprague-Dawley rats[5]. The rate of transcellular and paracellular duodenal Mg2+ absorption is suppressed in rats with PPIH[5]. However, the effect of prolonged PPIs administration on jejunal, ileal, and colonic Mg2+ absorption in a PPIH model remains unknown.
The present study aimed to observe the paracellular and transcellular Mg2+ transport across the duodenum, jejunum, ileum, and colon in control and prolonged omeprazole-treated male Sprague-Dawley rats. The expression of TRPM6 and CNNM4 proteins in each intestinal segment was also studied. Plasma and urinary Mg2+, Ca2+, and PO43− levels were measured. The plasma concentrations of hormones that modulate Mg2+ homeostasis, such as 1α,25-dihydroxyvitamin D3 [1α,25(OH)2D3], parathyroid hormone (PTH), fibroblast growth factor 23 (FGF-23), epidermal growth factor (EGF), and insulin[3,18-21], were also determined. Given that 16.7 rat days are equivalent to 1 human year[22], the animals were treated with 20 mg/kg omeprazole daily for 12 and 24 wk which is comparable to 5 and 10 human years, respectively.
MATERIALS AND METHODS
Animals
This study was performed in strict compliance with the Animal for Scientific Purposes Act of Thailand and in accordance with Ethical Principles and Guidelines for the Use of Animals for Scientific Purposes, National Research Council of Thailand. All experimental procedures were approved by the Ethics Committee on Animal Experiment of Burapha University, Thailand. Male Sprague-Dawley rats (9 weeks old) were randomly divided into three experimental groups: control, 12-wk omeprazole, and 24-wk omeprazole treatments. The rats were acclimatized for 7 days and fed with standard pellet chow and reverse osmosis water given ad libitum. The health, body weight, and food intake were monitored and recorded daily.
The effect of single-dose subcutaneous omeprazole injection (20 mg/kg: Ocid® IV; Zydus Cadila, India) on intraluminal gastrointestinal pH was observed. The pellet chow was removed 4 h before and then retrieved 30 min after sham or omeprazole injection. At 2 and 24 h after administration, the stomach, duodenum, jejunum, ileum, cecum, and proximal colon were removed under thiopental anesthesia (70 mg/kg; Anesthal, Jagsonpal Pharmaceuticals Ltd., India). The intraluminal pH levels of the stomach, duodenum, jejunum, ileum, cecum, and proximal colon were measured by diagnostic test strips (MColorpHast™ pH-Indicator Strips, Merck-Millipore, German).
To study the effect of prolonging omeprazole injection, control and 24-wk-omeprazole-treated rats received subcutaneous sham or omeprazole (20 mg/kg) injection daily for 24 wk. The rats in the 12-wk-omeprazole-treated group received subcutaneous sham injected daily for 12 wk followed by subcutaneous omeprazole injection for 12 wk. At 24 h prior to the experimental endpoint, the rats were housed in a metabolic cage to collect food and water intake. Urinary and fecal output was also collected. The rats were anesthetized, blood was collected from the left ventricle, and the rats were subsequently sacrificed. The duodenum, jejunum, ileum, and colon were rapidly collected. The plasma concentrations of 1α,25(OH)2D3, PTH, FGF-23, EGF, and insulin were determined by Labhouse Chonburi Co. Ltd., Thailand. Plasma and urine Mg2+, Ca2+, and PO43− concentrations were also measured.
Magnesium flux measurement
The total, paracellular, and transcellular Mg2+ flux was studied in accordance with the method described by Thongon et al[5]. In brief, the duodenum, jejunum, ileum, and colon from individual rats were dissected into two pieces, which then were rapidly mounted onto individually modified Ussing chamber setups. Intestinal samples were bathed and equilibrated for 10 min with physiological bathing solution containing (in mmol/mL) 118 NaCl, 4.7 KCl, 1.1 MgCl2, 1.25 CaCl2, 23 NaHCO3, 12 D-glucose, 2.5 L-glutamine, and 2 D-mannitol (osmolality of 290-295 mmol/kg H2O and pH of 7.4). The solution was maintained at 37 °C and continuously gassed with 5% CO2 in 95% O2. The first piece of each intestinal segment was subjected to study the rate of total Mg2+ transport. The apical solution of each Ussing chamber setup was substituted with a Mg2+-containing bathing solution supplemented with (in mmol/L) 40 MgCl2, 2.5 CaCl2, 4.5 KCl, 12 D-glucose, 2.5 L-glutamine, 115 mannitol, and 10 HEPES (pH 7.4). Meanwhile, the basolateral solution was substituted with a Mg2+-free bathing solution containing (in mmol/L) 1.25 CaCl2, 4.5 KCl, 12 D-glucose, 2.5 L-glutamine, 250 D-mannitol, and 10 HEPES pH 7.4. To study the rate of paracellular Mg2+ transport, another piece of each intestinal segment was incubated with Mg2+ channel blocker Co(III)hexaammine (1 mmol/L; Sigma, St. Louis, MO, United States)[23] to inhibit transcellular Mg+ flux. The Mg2+-containing and Mg2+-free bathing solutions were also used to study paracellular Mg2+ transport. At 30, 60, and 120 min after solution replacements, a 100 µL solution was collected from the basolateral and apical sides. The Mg2+ concentration and Mg2+ flux rates were determined as previously described by Thongon and Krishnamra[24]. The rate of transcellular Mg2+ transport was calculated by subtracting the rate of total Mg2+ transport with the rate of paracellular Mg2+ transport from the same intestinal segment of the individual rats.
Western blot analysis
Western blot analysis was performed as previously described[5]. Epithelial cells of the duodenum, jejunum, ileum, and colon were collected and lysed in Piece® Ripa Buffer (Thermo Fisher Scientific Inc.) with 10% v/v protease inhibitor cocktail (Sigma). After being sonicated, centrifuged, and heated, 50 µg samples were loaded and separated on SDS-PAGE gel and then transferred to a polyvinylidene difluoride membrane. The membrane was blocked and probed with 1:1000 primary antibodies (Santa Cruz Biotechnology) raised against CNNM4 and TRPM6. The membranes were also reprobed with 1:5000 anti-β-actin monoclonal antibodies (Santa Cruz Biotechnology). Subsequently, the membrane was incubated with 1:10000 HRP-conjugated secondary antibodies (Santa Cruz Biotechnology), visualized by Thermo Scientific SuperSignal® West Pico Substrate (Thermo Fisher Scientific Inc.), and captured on CL-XPosure Film (Thermo Fisher Scientific Inc.). Densitometric analysis was performed using ImageJ for Mac Os X.
Statistical analysis
Results were expressed as mean ± SE. Two sets of data were compared using the unpaired Student's t-test. One-way ANOVA with Dunnett’s post-test was used for comparison of multiple sets of data. All data were analyzed by GraphPad Prism for Mac Os (GraphPad Software Inc., San Diego, CA, United States).
RESULTS
Single-dose omeprazole injection increased gastrointestinal pH
Our previous study showed that 20 mg/kg oral gavage and subcutaneous omeprazole administration markedly suppress gastric acid secretion[5]. In the present study, the rats were subjected to prolonged omeprazole administration (24 wk). Therefore, subcutaneous administration that often causes minimal pain or discomfort was chosen. The injection site was changed to avoid the repeated damage or irritation of the rat tissue.
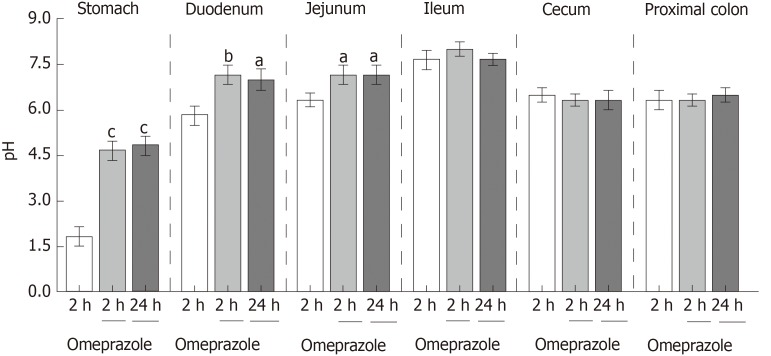
These experiments were performed to observe the effect of single-dose 20 mg/kg subcutaneous omeprazole injection on gastric acid secretion and gastrointestinal pH. Intraluminal pH of the stomach, duodenum, jejunum, ileum, cecum, and proximal colon were measured at 2 and 24 h after injection. In the vehicle-treated control group, the intraluminal pH levels of the stomach, duodenum, jejunum, ileum, cecum, and colon were 1.83 ± 0.31, 5.83 ± 0.30, 6.33 ± 0.21, 7.67 ± 0.42, 6.50 ± 0.22, and 6.67 ± 0.31, respectively. Omeprazole significantly increased gastric, duodenal, and jejunal pH at 24 h after injection (Figure 1). However, omeprazole did not affect the intraluminal acidic environment of the cecum and colon. These results indicated that 20 mg/kg omeprazole injection daily effectively suppressed the gastric acid secretion and increased the intraluminal pH of the stomach, duodenum, and jejunum through 24 wk of treatment.
Figure 1.
Effect of subcutaneous omeprazole injection on rat gastrointestinal pH. Intraluminal pH was measured by using test strips after 2 or 24 h after omeprazole administration. aP < 0.05, bP < 0.01, cP < 0.001,vs the control group (n = 6).
Metabolic characteristic of omeprazole-treated rats
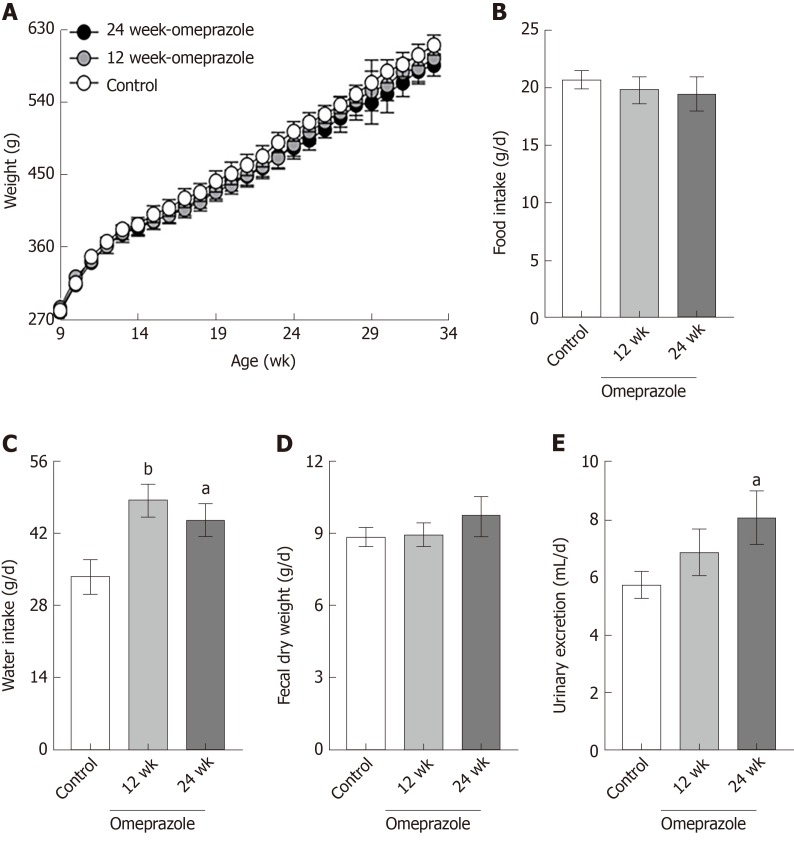
As demonstrated in Figure 2A, all rats showed equal growth after the 24 wk of the experiment (Figure 2A). Food intake (Figure 2B) and fecal excretion (Figure 2D) of all experimental groups were equal. Water intake (Figure 2C) and urine excretion (Figure 2E) significantly increased in the omeprazole-treated groups.
Figure 2.
Metabolic characteristics. A: Body weight; B: Food intake; C: Water intake; D: Fecal dry weight; E: Urinary excretion of control, 12 wk-omeprazole-treated, and 24 wk-omeprazole-treated groups. aP < 0.05, bP < 0.01, vs the control group (n = 6).
Omeprazole-induced hypomagnesemia
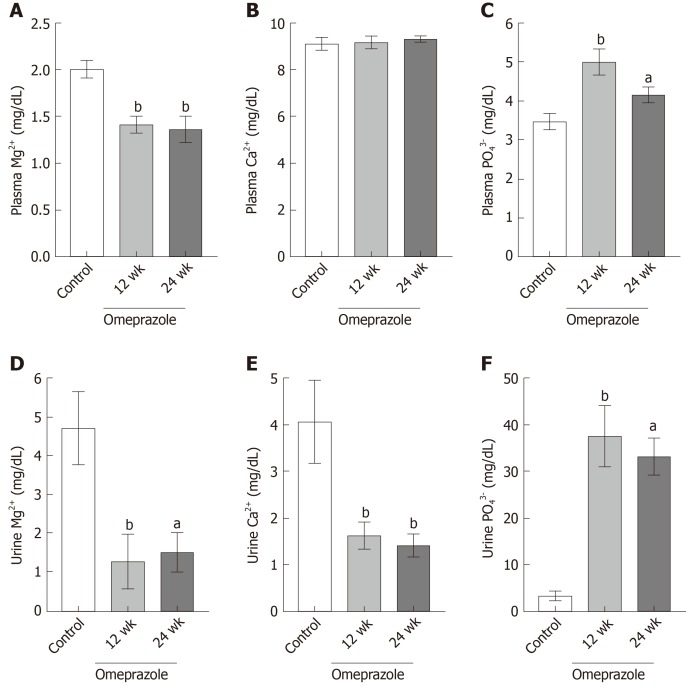
The 12- and 24-wk-omeprazole-treated rats had significantly reduced plasma (Figure 3A) and urinary Mg2+ concentration (Figure 3D). The plasma concentrations of the 12- and 24-omeprazole-treated groups were 1.41 ± 0.08 mg/dL and 1.37 ± 0.14 mg/dL respectively, which were lower than the reference rage of plasma Mg2+ concentration (1.7-2.4 mg/dL). Therefore, omeprazole induced hypomagnesemia in our rat model. In addition, the urinary Mg2+ concentrations of the 12- and 24-omeprazole-treated groups were 1.26 ± 0.72 mg/dL and 1.48 ± 0.52 mg/dL, respectively, which were also lower than the normal reference of 1.7-3.0 mg/dL. While the plasma Ca2+ concentration (Figure 3B) did not change, the urinary Ca2+ concentrations of the 12- (1.63 ± 0.28 mg/dL) and 24-wk-omeprazole-treated (1.42 ± 0.23 mg/dL) groups were significantly lower than those of the control group (4.06 ± 0.87 mg/dL) (Figure 3E). The plasma (Figure 3C) and urinary phosphate concentrations (Figure 3F) of the 12- and 24-omeprazole-treated groups significantly increased in comparison to its corresponding control group.
Figure 3.
Effect of omeprazole on plasma and urinary Mg2+, Ca2+, and PO43− levels. A: Plasma Mg2+; B: Plasma Ca2+; C: Plasma PO43−; D: Urinary Mg2+; E: Urinary Ca2+; F: Urinary PO43− levels of control, 12 wk-omeprazole-treated, and 24 wk-omeprazole-treated groups. aP < 0.05, bP < 0.01, vs the control group (n = 6).
Hormonal change in PPIH rats
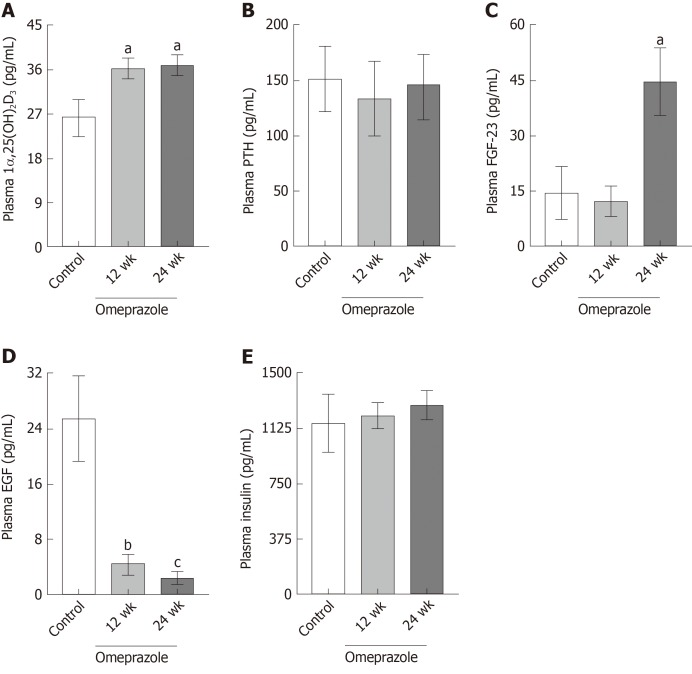
In consideration that 1α,25(OH)2D3, PTH, FGF-23, EGF, and insulin modulate Mg2+ homeostasis[3,18-21], their plasma concentrations in the rats with PPIH were determined. The plasma 1α,25(OH)2D3 (Figure 4A) and FGF-23 (Figure 4C) concentrations of the 24-wk-omeprazole-treated rats significantly increased compared with those of the control group. The plasma PTH (Figure 4B) and insulin (Figure 4E) of all experimental groups showed no difference. The 12- and 24-wk-omeprazole-treated groups had significantly lower plasma EGF levels than the control rats (Figure 4D).
Figure 4.
Effect of omeprazole on plasma 1α,25-dihydroxyvitamin D3, parathyroid hormone, fibroblast growth factor 23, epidermal growth factor, and insulin concentrations. A: Plasma 1α,25-dihydroxyvitamin D3; B: Plasma parathyroid hormone; C: Plasma fibroblast growth factor 23; D: Plasma epidermal growth factor; E: Plasma insulin of control, 12 wk-omeprazole-treated, and 24 wk-omeprazole-treated groups. aP < 0.05, bP < 0.01, cP < 0.001, vs the control group (n = 6). 1α,25(OH)2D3: 1α,25-dihydroxyvitamin D3; PTH: Parathyroid hormone; FGF-23: Fibroblast growth factor 23; EGF: Epidermal growth factor.
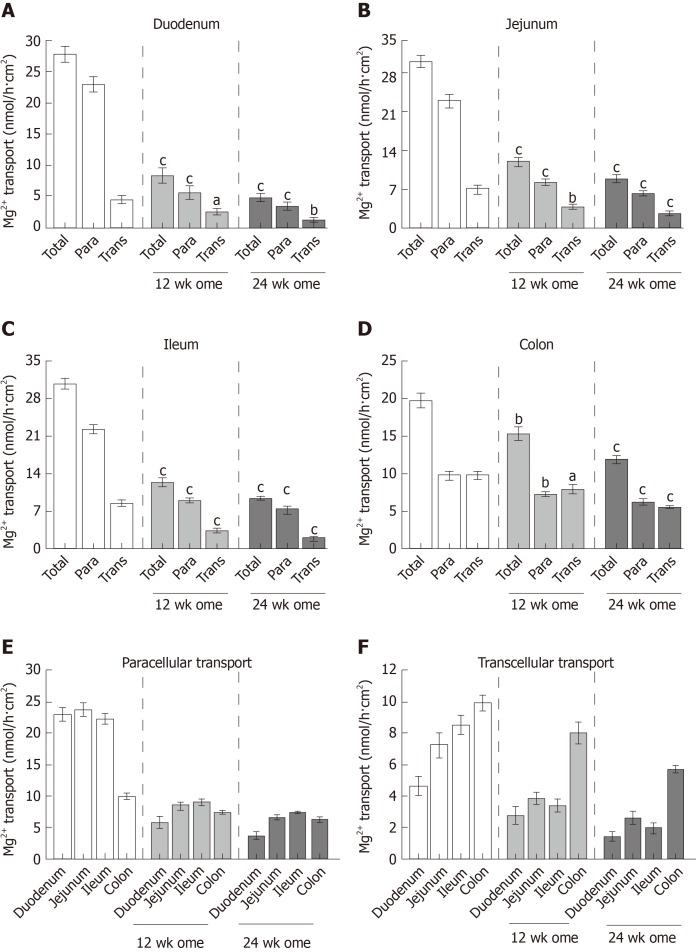
Segmental intestinal Mg2+ transport in PPIH rats
Previous research proposed that paracellular passive and transcellular active Mg2+ transport exclusively occur in the small and large intestines, respectively. In the present study, the total, paracellular, and transcellular Mg2+ transport rates of the vehicle-treated control group were 27.68 ± 1.36 nmol/h∙cm2, 23.04 ± 1.19 nmol/h∙cm2, and 4.65 ± 0.59 nmol/h∙cm2, respectively, in the duodenum (Figure 5A); 31.00 ± 1.19 nmol/h∙cm2, 23.73 ± 1.22 nmol/h∙cm2, and 7.27 ± 0.81 nmol/h∙cm2, respectively, in the jejunum (Figure 5B); 30.77 ± 0.94 nmol/h∙cm2, 22.23 ± 0.88 nmol/h∙cm2, and 8.53 ± 0.58 nmol/h∙cm2, respectively, in the ileum (Figure 5C); and 19.77 ± 0.99 nmol/h∙cm2, 9.83 ± 0.51 nmol/h∙cm2, and 9.93 ± 0.52 nmol/h∙cm2, respectively, in the colon (Figure 5D). In the 12-wk-omeprazole-treated rats, the total, paracellular, and transcellular Mg2+ transport rates were 8.55 ± 1.27 nmol/h∙cm2, 5.78 ± 1.03 nmol/h∙cm2, and 2.77 ± 0.53 nmol/h∙cm2, respectively, in the duodenum (Figure 5A); 12.36 ± 0.79 nmol/h∙cm2, 8.47 ± 0.57 nmol/h∙cm2, and 3.88 ± 0.42 nmol/h∙cm2, respectively, in the jejunum (Figure 5B); 12.39 ± 0.76 nmol/h∙cm2, 9.01 ± 0.45 nmol/h∙cm2, and 3.39 ± 0.44 nmol/h∙cm2, respectively, in the ileum (Figure 5C); and 15.41 ± 0.90 nmol/h∙cm2, 7.39 ± 0.33 nmol/h∙cm2, and 8.03 ± 0.67 nmol/h∙cm2, respectively, in the colon (Figure 5D). In the 24-wk-omeprazole-treated rats, the total, paracellular, and transcellular Mg2+ transport rates were 5.02 ± 0.51 nmol/h∙cm2, 3.59 ± 0.59 nmol/h∙cm2, and 1.43 ± 0.29 nmol/h∙cm2, respectively, in the duodenum (Figure 5A); 9.13 ± 0.75 nmol/h∙cm2, 6.48 ± 0.45 nmol/h∙cm2, and 2.65 ± 0.36 nmol/h∙cm2, respectively, in the jejunum (Figure 5B); 9.40 ± 0.40 nmol/h∙cm2, 7.43 ± 0.21 nmol/h∙cm2, and 1.97 ± 0.34 nmol/h∙cm2, respectively, in the ileum (Figure 5C); and 12.01 ± 0.56 nmol/h∙cm2, 6.29 ± 0.36 nmol/h∙cm2, and 5.71 ± 0.21 nmol/h∙cm2, respectively, in the colon (Figure 5D). In the small intestinal segment, Mg2+ was absorbed mainly through the paracellular route (Figure 5A-C and E). By contrast, the large intestine absorbed Mg2+ through the paracellular and transcellular routes in a comparable amount (Figure 5D). The rate of transcellular Mg2+ transport was the highest in the colon and the lowest in the duodenum (Figure 5F). In the 12- and 24-wk-omeprazole-treated rats, the total, paracellular, and transcellular Mg2+ transport rates were significantly lower in the duodenum, jejunum, ileum, and colon than in those of the corresponding vehicle-treated control rats (Figure 5A-D). These results suggest that prolonged omeprazole injection suppresses Mg2+ absorption throughout the intestinal tract.
Figure 5.
Effect of omeprazole on segmental intestinal Mg2+ absorption. A-D: Rate of total, paracellular, and transcellular Mg2+ transport of control, 12 wk-omeprazole-treated, and 24 wk-omeprazole-treated groups (A: Duodenum; B: Jejunum; C: Ileum; D: Colon); E: the rate paracellular; F: transcellular Mg2+ transport of control, 12 wk-omeprazole-treated, and 24 wk-omeprazole-treated groups. aP < 0.05, bP < 0.01, cP < 0.001, vs the corresponding control group (n = 6). Para: Paracellular; Trans: transcellular.
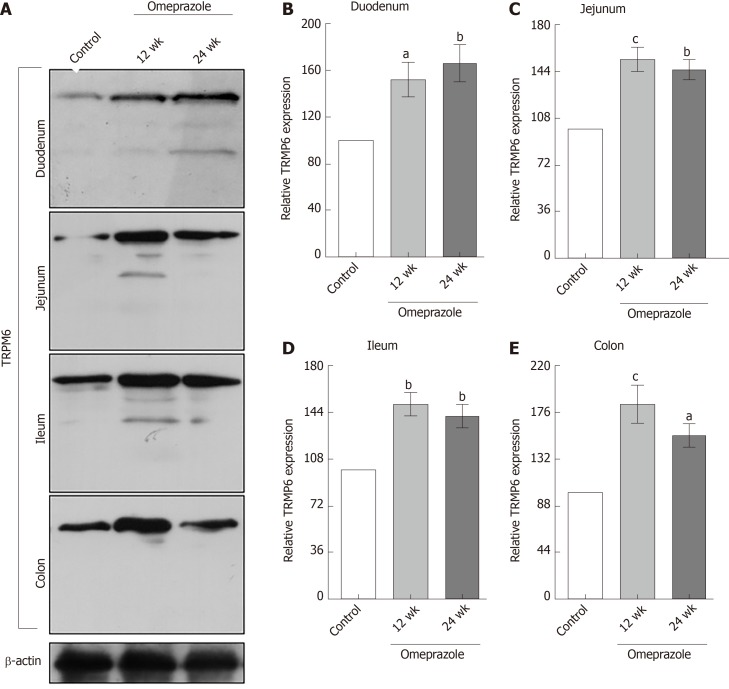
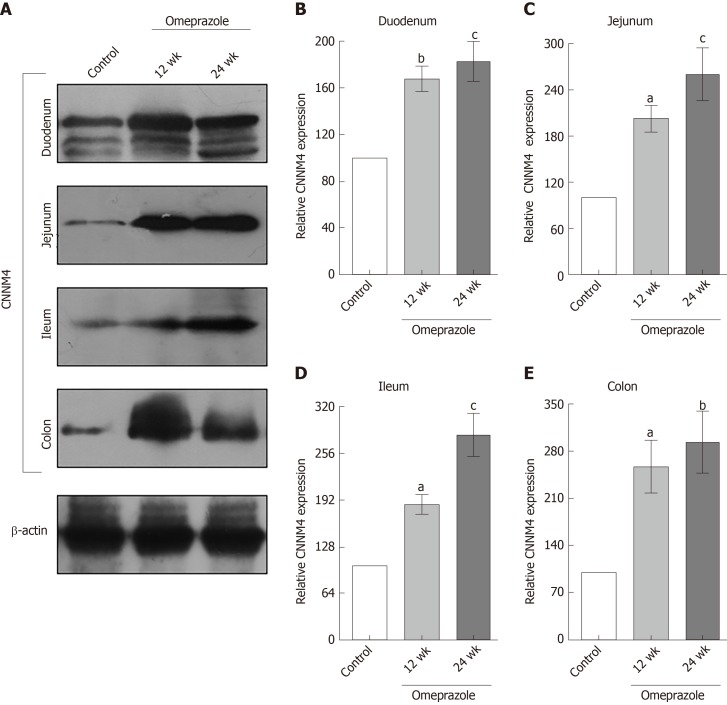
Segmental intestinal TRPM6 and CNNM4 expression in PPIH rats
Regarding our recent results on Mg2+ transport throughout the intestinal tract, these series of experiment aimed to study the expression of TRPM6 and CNNM4 in the duodenum, jejunum, ileum, and colon. As demonstrated in Figure 6, TRPM6 protein was detected in the duodenum, jejunum, ileum, and colon of all experimental groups. The level of TRPM6 expression was the lowest in the duodenum and the highest in the colon of the control (Figure 6A), 12-wk- (Figure 6B), and 24-wk-omeprazole (Figure 6C)-treated rats. The 12- and 24-wk-omeprazole-treated groups had significantly higher TRPM6 expression in the duodenum, jejunum, ileum, and colon than the vehicle-treated control group (Figure 7). Similar to TRPM6, CNNM4 protein was detected throughout the intestinal tract (Figure 8). The expression of CNNM4 protein significantly increased in the duodenum, jejunum, ileum, and colon of the 12- and 24-wk-omeprazole-treated groups compared with those of the control group.
Figure 6.
Transient receptor potential melastatin 6 expression in entire intestinal tract. A: Control; B: 12 wk-omeprazole-treated; C: 24 wk-omeprazole-treated groups. The quantitative immunobloting analysis and representative densitometric analysis of transient receptor potential melastatin 6 expression in duodenum, jejunum, ileum, and colon. aP < 0.05, bP < 0.01, cP < 0.001, vs the corresponding duodenal segment (n = 6). TRPM6: Transient receptor potential melastatin 6.
Figure 7.
The effect of omeprazole on transient receptor potential melastatin 6 expression in entire intestinal tract. A: Quantitative immunobloting analysis of transient receptor potential melastatin 6 expression in duodenum, jejunum, ileum, and colon. B: Duodenum; C: Jejunum; D: Ileum; E: Colon. Representative densitometric analysis of transient receptor potential melastatin 6 expression in duodenum, jejunum, ileum, and colon of control, 12 wk-omeprazole-treated, and 24 wk-omeprazole-treated groups. aP < 0.05, bP < 0.01, cP < 0.001, vs its corresponding vehicle-treated group (n = 5). TRPM6: Transient receptor potential melastatin 6.
Figure 8.
The effect of omeprazole on cyclin M4 expression in entire intestinal tract. A: Quantitative immunobloting analysis of cyclin M4 expression in duodenum, jejunum, ileum, and colon; B: Duodenum; C: Jejunum; D: Ileum; E: Colon. Representative densitometric analysis of cyclin M4 expression in duodenum, jejunum, ileum, and colon of control, 12 wk-omeprazole-treated, and 24 wk-omeprazole-treated groups. aP < 0.05, bP < 0.01, cP < 0.001, vs its corresponding vehicle-treated group (n = 5). CNNM4: Cyclin M4.
DISCUSSION
The present study showed paracellular and transcellular Mg2+ transport in the duodenum, jejunum, ileum, and colon. The rate of total Mg2+ transport was shown (in order of highest to lowest) in the jejunum, ileum, duodenum, and colon. Small intestinal epithelium absorbed Mg2+ mainly through the paracellular route. A comparable rate of paracellular and transcellular Mg2+ transport was shown in the colon.
The mechanism by which prolonged PPIs administration induce hypomagnesemia is currently unclear. In the present study, 12- and 24-wk omeprazole injection induced systemic Mg2+ depletion and hypomagnesemia. Similar to previous reports in PPIH patients[8,9,12-16], urinalysis of a recent PPIH rat model revealed reduced urinary Mg2+ excretion (less than 8.5 mg/dL), excluding urinary Mg2+ loss. The depletion of stored Mg2+ was also demonstrated in patients[9] and rats[5] with PPIH. Our results clearly showed that transcellular and paracellular Mg2+ transport mechanisms were markedly suppressed in the entire intestinal tract of rats with PPIH. Regarding the rate of total Mg2+ absorption, the length of intestinal segment, and diameter (Table 1)[25], the small intestine was the major intestinal segment for Mg2+ absorption. The percent suppression of total Mg2+ absorption in the duodenum, jejunum, ileum, and colon of the rats with PPIH rats was 81.86%, 70.59%, 69.45%, and 39.25% (Table 1), respectively. The percent suppression can be calculated using the following formula: 100 - [(total Mg2+ absorption of 24-wk-omeprazole group × 100) / total Mg2+ absorption of the control group]. Therefore, the small intestine is the major affected organs for the adverse effect of prolonged PPI administration. The stimulation of small intestinal Mg2+ absorption in PPIH is probably normalizing plasma Mg2+ level and requires further study. The present study demonstrated a higher expression level of TRPM6 protein in the colon compared with the small intestinal segment. The up-regulation of TRPM6 and CNNM4 expression in the rats with PPIH was also higher in the colon. The rate of transcellular Mg2+ absorption was the highest in the colon of the control and PPIH rats. However, the lowest percent suppression of total Mg2+ absorption was found in the colon of the rats with PPIH because single-dose omeprazole injection had no effect on intraluminal acidic environment in the colon. Our results could explain why the stimulation of colonic Mg2+ absorption could not normalize plasma Mg2+ level in the previous PPIH mouse model[13].
Table 1.
Rate of total Mg2+ absorption, segmental length, and diameter of the duodenum, jejunum, ileum, and colon of the rats
|
Rate of total Mg2+
transport |
Level of suppression | Length (mm)[25] | Diameter (mm)[25] | ||
| Control | 24-wk omeprazole | ||||
| Duodenum | 27.68 ± 1.36 | 5.02 ± 0.51 | 81.86% | 95-100 | 2.5-3 |
| Jejunum | 31.01 ± 1.19 | 9.12 ± 0.75 | 70.59% | 900-1350 | 4-5 |
| Ileum | 30.77 ± 0.94 | 9.40 ± 0.40 | 69.45% | 25-35 | 3-5 |
| Colon | 19.76 ± 0.98 | 12.01 ± 0.56 | 39.25% | 90-110 | 3-10 |
Level of suppression was a percentage decrement of total Mg2+ absorption in 24-wk omeprazole-treated rats compared with the corresponding control rats.
The overexpression of TRPM6 and CNNM4 proteins in the entire intestinal tract suggested the compensatory response in rats with PPIH. However, the rate of transcellular Mg2+ absorption was significantly suppressed in the duodenum, jejunum, ileum, and colon of the rats with PPIH. Hess et al[26], reported two common single-nucleotide polymorphisms (SNPs) in the TRPM6 gene that increase the risk for PPIH. These SNPs may explain why the overexpression of TRPM6 and CNNM4 could not increase transcellular Mg2+ absorption.
PPIH rats revealed normal plasma but reduced urinary Ca2+ concentration. Increment of plasma 1α,25(OH)2D3 in the rats with PPIH should stimulate intestinal Ca2+ absorption, renal tubular Ca2+ reabsorption, and bone resorption to regulate plasma Ca2+ concentration. Bone resorption not only increases plasma Ca2+ but also PO43− levels, which trigger FGF-23 release. Plasma 1α,25(OH)2D3 also stimulates FGF-23 release. FGF-23 further suppresses renal tubular PO43− reabsorption, which increases urinary PO43− excretion[27].
Plasma 1α,25(OH)2D3, FGF-23, and EGF levels are altered in rats with PPIH. However, the hormonal regulation of plasma Mg2+ level is largely unknown. In addition, the data from the study of hormonal control of intestinal Mg2+ absorption are often confusing and conflicting. Previous research proposed that 1α,25(OH)2D3 stimulates intestinal Mg2+ uptake[28]. In addition, 1α,25(OH)2D3 treatment for 7 d exerts no effect on intestinal Mg2+ absorption in male C57BL/6J mice[3]. 1α,25(OH)2D3 increases plasma and urinary Mg2+ levels[3] possibly through increasing bone resorption. In consideration that 1α,25(OH)2D3 increases urinary Mg2+ excretion[3], renal Mg2+ wasting is probably involved in the development of hypomagnesemia in prolonged PPI administration[11]. Hypomagnesemia increases plasma FGF-23 level[19]. Khuituan et al[29], demonstrated that FGF-23 markedly suppresses intestinal Ca2+ absorption. Magnesiotropic hormone EGF stimulates renal Mg2+ reabsorption to increase plasma Mg2+ level[20]. However, the direct effect of 1α,25(OH)2D3, FGF-23, and EGF on segmental intestinal Mg2+ absorption requires further study.
In conclusion, our recent study confirmed the adverse effect of prolonged PPI injection on plasma Mg2+ levels. In specific, PPIs can inhibit intestinal Mg2+ absorption. A higher level of suppression was shown in the small intestine than in the other organs. Therefore, the stimulation of small intestinal Mg2+ probably normalizes plasma Mg2+ in PPIH.
ARTICLE HIGHLIGHTS
Research background
Dietary intake is the sole source of Mg2+ in humans, and intestinal absorption plays a vital role in the regulation of normal Mg2+ balance. Previous case reports suggested that intestinal Mg2+ malabsorption is a major pathophysiological mechanism in proton pump inhibitor (PPI)-induced hypomagnesemia (PPIH).
Research motivation
The exact mechanism of PPI-inhibited intestinal Mg2+ absorption is still controversial. In addition, a simultaneous study on transcellular and paracellular Mg2+ absorption in the duodenum, jejunum, ileum, and colon of normal and PPIH had not been performed.
Research objectives
The present study aimed to observe the rate of paracellular and transcellular Mg2+ transport across the duodenum, jejunum, ileum, and colon in control and prolonged omeprazole-treated male Sprague-Dawley rats. Magnesiotropic hormones and proteins were measured.
Research methods
The rats received subcutaneous omeprazole injection for 12 or 24 wk. The duodenum, jejunum, ileum, and colon of each rat were mounted onto individual modified Ussing chamber setups to study the rates of total, transcellular, and paracellular Mg2+ absorption simultaneously. Magnesiotropic hormones and proteins were observed.
Research results
Hypomagnesemia and hypomagnesuria were demonstrated in the PPIs-treated rats. Plasma 1α,25-dihydroxyvitamin D3 and fibroblast growth factor 23 increased, whereas plasma epidermal growth factor level decreased in the omeprazole-treated rats. We clearly showed paracellular and transcellular Mg2+ absorption in the duodenum, jejunum, ileum, and colon of the control rats. Prolonged PPI treatment significantly inhibited transcellular and paracellular Mg2+ absorption in the duodenum, jejunum, ileum, and colon. High transient receptor potential melastatin 6 and cyclin M4 expression in the entire intestinal tract of the PPI-treated rats were demonstrated.
Research conclusions
Prolonged PPI administration markedly inhibits Mg2+ absorption throughout the entire length of intestinal tract and lead to systemic Mg2+ deficiency.
Research perspectives
PPIs mainly suppressed Mg2+ absorption in the small intestine. The stimulation of small intestinal Mg2+ absorption is probably nullifying the adverse effect of PPIs in chronic users.
ACKNOWLEDGEMENTS
We also thank Ms. Aroonphan Loturit, Ms. Kunlayanat Ruengwong, Ms. Narisara Tintawee, and Ms. Lamai Chaichandee of the Faculty of Allied Health Sciences, Burapha University for their excellent technical assistance.
Footnotes
Institutional review board statement: This study was reviewed and approved by Institutional Review Committee ( ID# 23/2559).
Institutional animal care and use committee statement: This study was approved by the Ethics Committee on Animal Experiment of Burapha University, Thailand.
Conflict-of-interest statement: The authors declare no conflict of interest.
ARRIVE guidelines statement: The authors have read the ARRIVE guidelines, and the manuscript was prepared and revised according to the ARRIVE guidelines.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: The Physiological Society of Thailand, No. 376.
Peer-review started: November 18, 2019
First decision: January 19, 2020
Article in press: March 5, 2020
Specialty type: Gastroenterology and hepatology
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Talamoni NGT, Huang LY S-Editor: Wang YQ L-Editor: A E-Editor: Ma YJ
Contributor Information
Nasisorn Suksridechacin, Division of Physiology, Department of Biomedical Sciences, Faculty of Allied Health Sciences, Burapha University, Chonburi 20131, Thailand.
Punnisa Kulwong, Division of Physiology, Department of Biomedical Sciences, Faculty of Allied Health Sciences, Burapha University, Chonburi 20131, Thailand.
Siriporn Chamniansawat, Division of Anatomy, Department of Biomedical Sciences, Faculty of Allied Health Sciences, Burapha University, Chonburi 20131, Thailand.
Narongrit Thongon, Division of Physiology, Department of Biomedical Sciences, Faculty of Allied Health Sciences, Burapha University, Chonburi 20131, Thailand. narongritt@buu.ac.th.
Data sharing statement
The data could be downloaded from the public databases, and no additional data are available.
References
- 1.de Baaij JH, Hoenderop JG, Bindels RJ. Magnesium in man: implications for health and disease. Physiol Rev. 2015;95:1–46. doi: 10.1152/physrev.00012.2014. [DOI] [PubMed] [Google Scholar]
- 2.Schuchardt JP, Hahn A. Intestinal Absorption and Factors Influencing Bioavailability of Magnesium-An Update. Curr Nutr Food Sci. 2017;13:260–278. doi: 10.2174/1573401313666170427162740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lameris AL, Nevalainen PI, Reijnen D, Simons E, Eygensteyn J, Monnens L, Bindels RJ, Hoenderop JG. Segmental transport of Ca²⁺ and Mg²⁺ along the gastrointestinal tract. Am J Physiol Gastrointest Liver Physiol. 2015;308:G206–G216. doi: 10.1152/ajpgi.00093.2014. [DOI] [PubMed] [Google Scholar]
- 4.Lameris AL, Hess MW, van Kruijsbergen I, Hoenderop JG, Bindels RJ. Omeprazole enhances the colonic expression of the Mg(2+) transporter TRPM6. Pflugers Arch. 2013;465:1613–1620. doi: 10.1007/s00424-013-1306-0. [DOI] [PubMed] [Google Scholar]
- 5.Thongon N, Penguy J, Kulwong S, Khongmueang K, Thongma M. Omeprazole suppressed plasma magnesium level and duodenal magnesium absorption in male Sprague-Dawley rats. Pflugers Arch. 2016;468:1809–1821. doi: 10.1007/s00424-016-1905-7. [DOI] [PubMed] [Google Scholar]
- 6.Voets T, Nilius B, Hoefs S, van der Kemp AW, Droogmans G, Bindels RJ, Hoenderop JG. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorption. J Biol Chem. 2004;279:19–25. doi: 10.1074/jbc.M311201200. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki D, Funato Y, Miura J, Sato S, Toyosawa S, Furutani K, Kurachi Y, Omori Y, Furukawa T, Tsuda T, Kuwabata S, Mizukami S, Kikuchi K, Miki H. Basolateral Mg2+ extrusion via CNNM4 mediates transcellular Mg2+ transport across epithelia: a mouse model. PLoS Genet. 2013;9:e1003983. doi: 10.1371/journal.pgen.1003983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Broeren MA, Geerdink EA, Vader HL, van den Wall Bake AW. Hypomagnesemia induced by several proton-pump inhibitors. Ann Intern Med. 2009;151:755–756. doi: 10.7326/0003-4819-151-10-200911170-00016. [DOI] [PubMed] [Google Scholar]
- 9.Cundy T, Dissanayake A. Severe hypomagnesaemia in long-term users of proton-pump inhibitors. Clin Endocrinol (Oxf) 2008;69:338–341. doi: 10.1111/j.1365-2265.2008.03194.x. [DOI] [PubMed] [Google Scholar]
- 10.Cundy T, Mackay J. Proton pump inhibitors and severe hypomagnesaemia. Curr Opin Gastroenterol. 2011;27:180–185. doi: 10.1097/MOG.0b013e32833ff5d6. [DOI] [PubMed] [Google Scholar]
- 11.Danziger J, William JH, Scott DJ, Lee J, Lehman LW, Mark RG, Howell MD, Celi LA, Mukamal KJ. Proton-pump inhibitor use is associated with low serum magnesium concentrations. Kidney Int. 2013;83:692–699. doi: 10.1038/ki.2012.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein M, McGrath S, Law F. Proton-pump inhibitors and hypomagnesemic hypoparathyroidism. N Engl J Med. 2006;355:1834–1836. doi: 10.1056/NEJMc066308. [DOI] [PubMed] [Google Scholar]
- 13.Hess MW, de Baaij JH, Gommers LM, Hoenderop JG, Bindels RJ. Dietary Inulin Fibers Prevent Proton-Pump Inhibitor (PPI)-Induced Hypocalcemia in Mice. PLoS One. 2015;10:e0138881. doi: 10.1371/journal.pone.0138881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quasdorff M, Mertens J, Dinter J, Steffen HM. Recurrent hypomagnesemia with proton-pump inhibitor rechallenge. Ann Intern Med. 2011;155:405–407. doi: 10.7326/0003-4819-155-6-201109200-00022. [DOI] [PubMed] [Google Scholar]
- 15.Shabajee N, Lamb EJ, Sturgess I, Sumathipala RW. Omeprazole and refractory hypomagnesaemia. BMJ. 2008;337:a425. doi: 10.1136/bmj.39505.738981.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Regolisti G, Cabassi A, Parenti E, Maggiore U, Fiaccadori E. Severe hypomagnesemia during long-term treatment with a proton pump inhibitor. Am J Kidney Dis. 2010;56:168–174. doi: 10.1053/j.ajkd.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 17.Metz DC, Sostek MB, Ruszniewski P, Forsmark CE, Monyak J, Pisegna JR. Effects of esomeprazole on acid output in patients with Zollinger-Ellison syndrome or idiopathic gastric acid hypersecretion. Am J Gastroenterol. 2007;102:2648–2654. doi: 10.1111/j.1572-0241.2007.01509.x. [DOI] [PubMed] [Google Scholar]
- 18.Vetter T, Lohse MJ. Magnesium and the parathyroid. Curr Opin Nephrol Hypertens. 2002;11:403–410. doi: 10.1097/00041552-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Matsuzaki H, Kajita Y, Miwa M. Magnesium deficiency increases serum fibroblast growth factor-23 levels in rats. Magnes Res. 2013;26:18–23. doi: 10.1684/mrh.2013.0331. [DOI] [PubMed] [Google Scholar]
- 20.Muallem S, Moe OW. When EGF is offside, magnesium is wasted. J Clin Invest. 2007;117:2086–2089. doi: 10.1172/JCI33004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alzaid AA, Dinneen SF, Moyer TP, Rizza RA. Effects of insulin on plasma magnesium in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. 1995;80:1376–1381. doi: 10.1210/jcem.80.4.7714113. [DOI] [PubMed] [Google Scholar]
- 22.Quinn R. Comparing rat's to human's age: how old is my rat in people years? Nutrition. 2005;21:775–777. doi: 10.1016/j.nut.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Wolf FI, Trapani V, Simonacci M, Mastrototaro L, Cittadini A, Schweigel M. Modulation of TRPM6 and Na(+)/Mg(2+) exchange in mammary epithelial cells in response to variations of magnesium availability. J Cell Physiol. 2010;222:374–381. doi: 10.1002/jcp.21961. [DOI] [PubMed] [Google Scholar]
- 24.Thongon N, Krishnamra N. Omeprazole decreases magnesium transport across Caco-2 monolayers. World J Gastroenterol. 2011;17:1574–1583. doi: 10.3748/wjg.v17.i12.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hebel R, Stromberg MW. Digestive system. In: Hebel R, Stromberg MW. Anatomy of the Laboratory Rat. Baltimore: Wiliams and Wilkins, 1976: 43-52 In: Hebel R, Stromberg MW, editors. [Google Scholar]
- 26.Hess MW, de Baaij JH, Broekman MM, Bisseling TM, Haarhuis BJ, Tan AC, Te Morsche RH, Hoenderop JG, Bindels RJ, Drenth JP. Common single nucleotide polymorphisms in transient receptor potential melastatin type 6 increase the risk for proton pump inhibitor-induced hypomagnesemia: a case-control study. Pharmacogenet Genomics. 2017;27:83–88. doi: 10.1097/FPC.0000000000000259. [DOI] [PubMed] [Google Scholar]
- 27.Jacquillet G, Unwin RJ. Physiological regulation of phosphate by vitamin D, parathyroid hormone (PTH) and phosphate (Pi) Pflugers Arch. 2019;471:83–98. doi: 10.1007/s00424-018-2231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hardwick LL, Jones MR, Brautbar N, Lee DB. Magnesium absorption: mechanisms and the influence of vitamin D, calcium and phosphate. J Nutr. 1991;121:13–23. doi: 10.1093/jn/121.1.13. [DOI] [PubMed] [Google Scholar]
- 29.Khuituan P, Teerapornpuntakit J, Wongdee K, Suntornsaratoon P, Konthapakdee N, Sangsaksri J, Sripong C, Krishnamra N, Charoenphandhu N. Fibroblast growth factor-23 abolishes 1,25-dihydroxyvitamin D₃-enhanced duodenal calcium transport in male mice. Am J Physiol Endocrinol Metab. 2012;302:E903–E913. doi: 10.1152/ajpendo.00620.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data could be downloaded from the public databases, and no additional data are available.