Abstract
Background
Maternal polycystic ovary syndrome (PCOS) has been proposed as a model for investigating the role of prenatal androgen exposure in the development of neuropsychiatric disorders. However, women with PCOS are at higher risk of developing psychiatric conditions and previous studies are likely confounded by genetic influences.
Methods
A Swedish nationwide register-based cohort study was conducted to disentangle the influence of prenatal androgen exposure from familial confounding in the association between maternal PCOS and offspring attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorders (ASD), and Tourette's disorder and chronic tic disorders (TD/CTD). PCOS-exposed offspring (n = 21 280) were compared with unrelated PCOS-unexposed offspring (n = 200 816) and PCOS-unexposed cousins (n = 17 295). Associations were estimated with stratified Cox regression models.
Results
PCOS-exposed offspring had increased risk of being diagnosed with ADHD, ASD, and TD/CTD compared with unrelated PCOS-unexposed offspring. Associations were stronger in girls for ADHD and ASD but not TD/CTD [ADHD: adjusted hazard ratio (aHR) = 1.61 (95% confidence interval (CI) 1.31–1.99), ASD: aHR = 2.02 (95% CI 1.45–2.82)] than boys [ADHD: aHR = 1.37 (95% CI 1.19–1.57), ASD: aHR = 1.46 (95% CI 1.21–1.76)]. For ADHD and ASD, aHRs for girls were stronger when compared with PCOS-unexposed cousins, but slightly attenuated for boys.
Conclusions
Estimates were similar when accounting for familial confounding (i.e. genetics and environmental factors shared by cousins) and stronger in girls for ADHD and ASD, potentially indicating a differential influence of prenatal androgen exposure v. genetic factors. These results strengthen evidence for a potential causal influence of prenatal androgen exposure on the development of male-predominant neuropsychiatric disorders in female offspring of women with PCOS.
Key words: Androgens, epidemiology, familial confounding, neuropsychiatric disorders, polycystic ovary syndrome
Introduction
Prenatal androgen exposure has been hypothesized to be associated with the development of neuropsychiatric disorders including autism spectrum disorders (ASD) (Baron-Cohen et al., 2011), attention-deficit/hyperactivity disorder (ADHD) (de Bruin et al., 2006; Baron-Cohen et al., 2011), and Tourette's disorder and chronic tic disorders (TD/CTD) (Peterson et al., 1992; Alexander and Peterson, 2004; Martino et al., 2013). Additionally, some psychiatric disorders show a response to treatment with anti-androgens, such as TD/CTD (Peterson et al., 1994, 1998), potentially implicating altered androgenic pathways in their etiology.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder affecting women of reproductive age (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). The most prominent feature of PCOS is hyperandrogenism, characterized by elevated testosterone levels in serum and an increased testosterone to sex hormone-binding globulin ratio, which persists throughout a woman's reproductive years (Goodarzi et al., 2011). Previous studies have reported significantly higher levels of androgens throughout the pregnancies of women with PCOS, compared with pregnancies of women without PCOS (Sir-Petermann et al., 2002; Anderson et al., 2010; Barry et al., 2010; Maliqueo et al., 2013).
Studies have therefore utilized maternal PCOS as a model of offspring exposure to elevated levels of prenatal androgens, reporting that the children of women with PCOS are at increased risk of both ASD and ADHD (Palomba et al., 2012; Kosidou et al., 2016, 2017; Cherskov et al., 2018).
However, women with PCOS themselves have an elevated prevalence of a range of psychiatric disorders compared with women without PCOS, including ASD and ADHD (Ingudomnukul et al., 2007; Herguner et al., 2015; Cesta et al., 2016; Cherskov et al., 2018). Because these disorders are moderate to highly heritable (Posthuma and Polderman, 2013; Mataix-Cols et al., 2015), it is possible that the association between maternal PCOS and offspring neuropsychiatric disorders is confounded by shared genetic influences. Furthermore, some familial environmental factors associated with having received a diagnosis of maternal PCOS (e.g. access to health care, healthcare seeking behavior) may also influence whether the child receives a neuropsychiatric diagnosis and therefore may confound the association. Hence, further investigation is needed to disaggregate the influence of prenatal androgen exposure from familial confounding (i.e. genetic and/or environmental factors).
The first aim of this study was to measure, in the general population, the association between maternal PCOS and offspring neuropsychiatric disorders where prenatal androgen levels and/or altered androgen function have been implicated in their etiology. The second aim was to determine whether these associations were sensitive to familial confounding by comparing the risk of these same neuropsychiatric disorders in the offspring of siblings of women with PCOS. To this end, we compared cousins who differ in their prenatal androgen exposure, but share 12.5% of segregated genes and environmental factors shared by cousins that make them similar (D'Onofrio et al., 2013). If the increased risk for psychiatric disorders in the offspring of women with PCOS is mainly driven by familial factors, we would expect attenuated hazard ratios (HR) in the analysis which account for familial factors. However, if familial factors are not driving the findings (i.e. indicating a possible causal effect of prenatal androgen exposure), then the risk estimates for neuropsychiatric disorders should be similar when PCOS-exposed offspring are compared with PCOS-unexposed unrelated and related offspring.
Notably, rodent models of prenatal androgen exposure have measured anxiety-like behavior in female offspring, and to a lesser extent in the male offspring, attributable to changes in hormone receptor expression in the amygdala (Hu et al., 2015). Therefore, the third aim was to assess if the associations in the PCOS-exposed v. PCOS-unexposed offspring differed by sex.
Methods
Data sources
Using the unique personal identification number assigned to each individual in Sweden at birth or at immigration, several nationwide longitudinal registers containing health and sociodemographic data until 31 December 2013 were linked (see online Supplementary material for more details), including the Swedish Medical Birth Register (MBR), National Patient Register (NPR), Prescribed Drug Register (PDR), Multi-Generation Register (MGR), Total Population Register (TPR), Migration Register, and the Cause of Death Register (CDR).
Study population and exposure classification
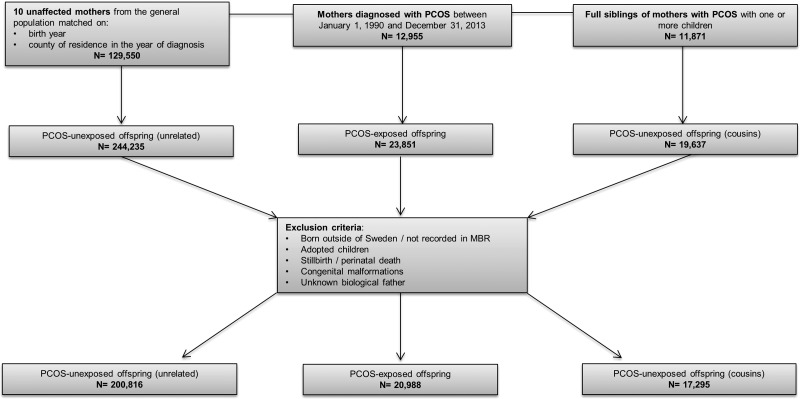
Women who had delivered at least one child were identified through the MGR. The first diagnostic criteria for PCOS were established in 1990 (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). Those women with PCOS were identified by having at least one PCOS International Classification of Diseases (ICD) code (ICD-9: 256E; ICD-10: E28.2) recorded in the MBR or in the NPR after the age of 13 and between 1990 and 2013. Women with a concurrent diagnosed condition that could cause symptoms similar to PCOS were excluded to ensure specificity (see online Supplementary Material for ICD codes). This yielded a total of 12 955 mothers with PCOS. Time of PCOS diagnosis (before or after delivery) was not taken into account, since elevated testosterone levels have been reported to be present throughout life in women with PCOS (Pinola et al., 2015). Each mother with PCOS was then matched on her birth year and county of residence within the year of diagnosis to 10 comparison mothers without a PCOS diagnosis randomly selected from the general population. Last, all full brothers (n = 4467) and sisters (n = 7404) of women with PCOS with children were identified from the MGR.
Using the MGR, offspring born to mothers with PCOS (PCOS-exposed), matched unaffected mothers (PCOS-unexposed), and full siblings were identified (PCOS-unexposed cousins). All offspring from each mother were included. Offspring were excluded if they were born outside of Sweden, adopted, stillborn or died on the day of birth, or had congenital malformations. This yielded a total of 20 988 PCOS-exposed offspring, 200 816 PCOS-unexposed offspring from the general population, and 17 295 PCOS-unexposed cousins (Fig. 1). Offspring were born between 1973 and 2013, leading to an age range of 0–40 years at the end of follow-up.
Fig. 1.
Flowchart of inclusion and exclusion criteria of study population. PCOS, polycystic ovary syndrome.
Outcome classification
ADHD – Individuals with either a diagnosis of ADHD in the NPR (ICD-9: 314; ICD-10: F90) or with a filled prescription of ADHD medication in the PDR [methylphenidate (Anatomical Therapeutic Chemical [ATC] classification = N06BA04); amphetamine [ATC = N06BA01]; dexamphetamine [ATC = N06BA02]; atomoxetine [ATC = N06BA09]; or lisdexamfetamine [ATC = N06BA12]] after age 3 were considered to have a diagnosis of ADHD. The date of diagnosis was defined as the date of the first diagnosis or first prescription of ADHD medication, whichever came first (Chen et al., 2017).
ASD – Individuals were identified as having ASD if they had at least one registered code in the NPR (ICD-9: 299 or ICD-10: F84), received after the age of 1.
TD/CTD – Individuals with a diagnosis of TD/CTD after 3 years of age were identified using a previously validated algorithm based on ICD codes (ICD-9: 306.2, 307C; ICD-10: F95), shown to have excellent positive predictive value when compared with clinical records (Ruck et al., 2015).
Covariates
Highest attained maternal education level was used as a proxy for offspring socioeconomic status. Maternal and paternal lifetime history of psychiatric disorders was determined by any recorded psychiatric diagnosis in the NPR. Region of birth (Nordic/non-Nordic) was extracted from the TPR. Offspring sex and year of birth, as well as maternal age at the offspring's birth, were extracted from the MBR.
Statistical analysis
Offspring were followed from the age when they were eligible to receive a diagnosis of the respective psychiatric conditions to the date of diagnosis of the neuropsychiatric disorder of interest, death, emigration, or end of follow-up (31 December 2013), whichever came first. Associations between maternal PCOS and offspring neuropsychiatric disorders in the general population were estimated as HR with 95% confidence intervals (CI) using stratified Cox regression models with attained age as the underlying time scale. Each psychiatric disorder was modeled separately in both crude and adjusted models. In addition to the maternal matching criteria (maternal birth year and county of residence within the year of PCOS diagnosis), potential confounding was addressed in the fully adjusted models by controlling for offspring sex and year of birth, maternal age at child's birth, maternal education, maternal region of birth, and maternal and paternal lifetime history of psychiatric disorders. Robust standard errors were used to account for dependence between observations since several children from the same family were included in the study population. In order to assess if the associations remained after adjusting for familial confounding, the analyses were repeated in the sample with PCOS-unexposed cousins in a similar manner. Cousins share on average 12.5% of the segregating genes. Furthermore, some environmental risk factors may be shared between cousins as well (e.g. family background characteristics such as socioeconomic position). Therefore, comparing differentially exposed cousins controls for both measured covariates included in the model, and reduces confounding from unmeasured factors (both genetic and environmental) shared between cousins (D'Onofrio et al., 2013).
The analysis was conducted first for all offspring combined, and then stratified by offspring sex. We performed Wald tests to formally test if the associations statistically differed by offspring sex.
Sensitivity analyses
Year of PCOS diagnosis – Diagnostic criteria for PCOS changed over the study period (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). Therefore, a stratified analysis of offspring born to women with their first PCOS diagnoses between 1990 and 2003 and between 2004 and 2013 was conducted.
Offspring from brothers v. sisters of women with PCOS – Depending on the diagnostic criteria used, the prevalence of PCOS varies from 5% to 15% in women of reproductive age (March et al., 2010). Given that the prevalence of a PCOS diagnosis in the NPR is lower than expected (approximately 2%),(Cesta et al., 2016) and the high heritability of PCOS (60–70%) (Vink et al., 2006; Cesta et al., 2017), it is highly likely that there are sisters of women with PCOS misclassified as ‘unaffected’ because they have not received a diagnosis of PCOS. The downstream implication of this is that their offspring would be misclassified as unexposed cousins in the primary analysis. However, the risk of misclassification is lower for the offspring of brothers of women with PCOS. Therefore, we performed the analysis for the unexposed cousins born to brothers and sisters of women with PCOS separately.
Outpatient coverage – The NPR includes only diagnoses made in inpatient care until 2001 when outpatient specialist care was added to the register. Therefore, if an individual received a diagnosis in outpatient care before 2001, they would only be identified as a case in this study if there was a subsequent diagnosis after 2001. This will lead to either a misclassification of the outcome, or left censoring of incident cases before 2001, thereby leading to a longer time to event. Hence, a sensitivity analysis was conducted where children were included only if their outpatient care was covered in the NPR. The minimum age for diagnosis for each of the outcomes varied and therefore different years of birth were used for each analysis: the analysis for ADHD included children born from 1998, for ASD children born from 2000, and for TD/CTD from 1998.
All analyses were performed using Stata statistical software version 14.0 (Stata Corps, Texas, USA).
Results
Descriptive characteristics
Descriptive characteristics of the study population by category of exposure are presented in Table 1. PCOS-exposed offspring were more often born to mothers who were slightly older and who had a lifetime history of psychiatric diagnoses, compared with both categories of PCOS-unexposed offspring. ASD, ADHD, and TD/CTD were more prevalent in PCOS-exposed offspring compared with PCOS-unexposed offspring born to unrelated mothers from the general population, but prevalence estimates were similar to their PCOS-unexposed cousins. The mean age (±standard deviation) of the offspring at the end of follow-up was 9.2 ± 7.6 years.
Table 1.
Descriptive characteristics of study population
| Characteristic | PCOS-exposed offspring (N = 20 988) | PCOS-unexposed offspring (unrelated) (N = 200 816) | PCOS-unexposed offspring (cousins) (N = 17 295) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Sex of offspring | ||||||
| Male | 10 779 | 51.36 | 102 528 | 51.06 | 8940 | 51.69 |
| Female | 10 209 | 48.64 | 98 288 | 48.94 | 8355 | 48.31 |
| Child's year of birth | ||||||
| 1973–1984 | 314 | 1.5 | 3317 | 1.65 | 817 | 4.72 |
| 1985–1989 | 539 | 2.57 | 6869 | 3.42 | 1151 | 6.66 |
| 1990–1994 | 1333 | 6.35 | 16 739 | 8.34 | 2059 | 11.91 |
| 1995–1999 | 2034 | 9.69 | 23 402 | 11.65 | 2213 | 12.8 |
| 2000–2004 | 3398 | 16.19 | 38 105 | 18.98 | 3242 | 18.75 |
| 2005–2009 | 6397 | 30.48 | 57 488 | 28.63 | 4284 | 24.77 |
| 2010–2013 | 6973 | 33.22 | 54 896 | 27.34 | 3529 | 20.4 |
| Maternal age at child's birth (years) | ||||||
| <20 | 540 | 2.57 | 6296 | 3.14 | 722 | 4.17 |
| 20–24 | 3847 | 18.33 | 43 570 | 21.70 | 4158 | 24.04 |
| 25–29 | 7108 | 33.87 | 74 121 | 36.91 | 6086 | 35.19 |
| 30–34 | 6486 | 30.90 | 57 995 | 28.88 | 4637 | 26.81 |
| 35+ | 3007 | 14.33 | 18 834 | 9.38 | 1692 | 9.78 |
| Mother's region of birth | ||||||
| The Nordic countries | 16 595 | 79.07 | 167 284 | 83.30 | 15 432 | 89.23 |
| Outside the Nordic countries | 4393 | 20.93 | 33 532 | 16.70 | 1863 | 10.77 |
| Maternal highest achieved educationa | ||||||
| Primary and secondary education | 2152 | 10.3 | 19 650 | 9.85 | 2019 | 11.72 |
| Upper secondary education | 9087 | 43.47 | 88 465 | 44.33 | 8267 | 47.98 |
| Post-secondary/post-graduate education | 9664 | 46.23 | 91 424 | 45.82 | 6945 | 40.31 |
| Maternal lifetime history of any psychiatric diagnosis | 4660 | 22.2 | 31 294 | 15.58 | 2914 | 16.85 |
| Paternal lifetime history of any psychiatric diagnosis | 2318 | 11.04 | 20 898 | 10.41 | 1845 | 10.67 |
| Offspring psychiatric disorders | ||||||
| ADHD | 681 | 3.24 | 5440 | 2.71 | 602 | 3.48 |
| ASD | 256 | 1.22 | 1765 | 0.88 | 202 | 1.17 |
| TD/CTD | 38 | 0.18 | 282 | 0.14 | 30 | 0.17 |
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorders; PCOS, polycystic ovary syndrome; TC/CTD, Tourette disorder/chronic tic disorder.
Categories do not add up to the total number because maternal education is missing for 0.6% of children.
Main analyses
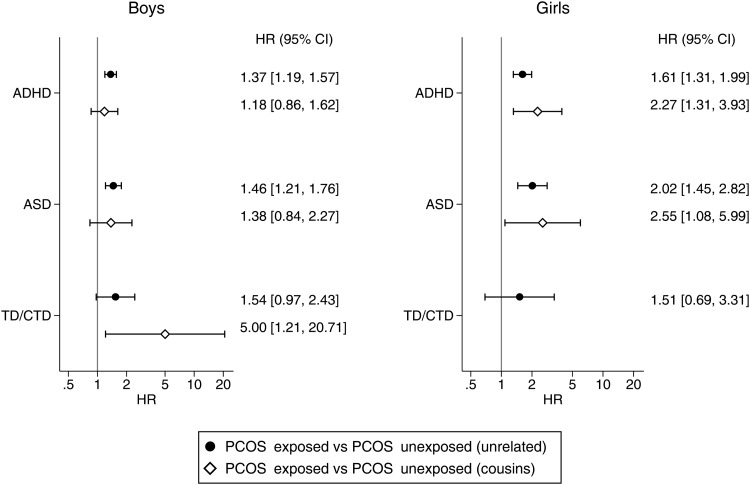
PCOS-exposed offspring had crude HRs ranging from 1.61 to 1.90 and adjusted HRs (aHR) from 1.46 to 1.60 for being diagnosed with ADHD, ASD, or TD/CTD (Table 2), compared with PCOS-unexposed unrelated offspring. Similar estimates were found when familial factors were controlled for by comparing PCOS-exposed offspring with their unexposed cousins, albeit with wider CIs due to the smaller sample sizes (Table 2). Figure 2 and Table 2 show the HRs of the analysis stratified by offspring sex. Associations were stronger in girls than in boys for both ADHD (aHR = 1.61 v. aHR = 1.37) and ASD (aHR = 2.02 v. aHR = 1.46) when compared with PCOS-unexposed unrelated offspring from the general population. Restricting the comparison group to PCOS-unexposed cousins led to a slight attenuation of the aHRs for ADHD and ASD in boys, but a slight increase in the aHR in girls. The difference between boys and girls was statistically significant for ADHD when PCOS-exposed offspring were compared with their unexposed cousins (p-value: 0.038), but not for any other association.
Table 2.
Crude and adjusted hazard ratios and 95% CIs for the risk of psychiatric disorders in PCOS-exposed and PCOS-unexposed offspring
| Outcome | All | Boys | Girls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of cases | Crude | Adjusteda | No. of cases | Crude | Adjustedb | No. of cases | Crude | Adjustedb | |
| Exposed/unexposed | HR (95% CI) | HR (95% CI) | Exposed/unexposed | HR (95% CI) | HR (95% CI) | Exposed/unexposed | HR (95% CI) | HR (95% CI) | |
| PCOS-exposed compared with PCOS-unexposed offspring (unrelated) | |||||||||
| ADHD | 681/5440 | 1.61 (1.45–1.79) | 1.46 (1.31–1.63) | 449/3758 | 1.52 (1.34–1.73) | 1.37 (1.19–1.57) | 232/1682 | 1.72 (1.42–2.09) | 1.61 (1.31–1.99) |
| ASD | 256/1765 | 1.79 (1.53–2.09) | 1.57 (1.34–1.84) | 177/1297 | 1.61 (1.33–1.95) | 1.46 (1.21–1.76) | 79/468 | 2.29 (1.68–3.11) | 2.02 (1.45–2.82) |
| TD/CTD | 38/282 | 1.90 (1.30–2.78) | 1.60 (1.08–2.37) | 30/220 | 1.76 (1.12–2.75) | 1.54 (0.97–2.43) | 8/62 | 1.69 (0.76–3.73) | 1.51 (0.69–3.31) |
| PCOS-exposed offspring compared with PCOS-unexposed offspring (cousins) | |||||||||
| ADHD | 681/602 | 1.50 (1.22–1.84) | 1.39 (1.10–1.76) | 449/418 | 1.27 (0.98–1.66) | 1.18 (0.86–1.62) | 232/184 | 2.40 (1.57–3.67) | 2.27 (1.31–3.93) |
| ASD | 256/202 | 1.76 (1.29–2.41) | 1.49 (1.06–2.10) | 177/156 | 1.81 (1.19–2.75) | 1.38 (0.84–2.27) | 79/46 | 2.64 (1.19–5.82) | 2.55 (1.08–5.99) |
| TD/CTD | 38/30 | 1.91 (0.98–3.73) | 1.61 (0.77–3.35) | 30/23 | 4.31 (1.52–12.22) | 5.00 (1.21–20.71) | 8/7 | 0.92 (0.12–7.26) | N/A |
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorders; CI, confidence interval; HR, hazard ratio; PCOS, polycystic ovary syndrome; TD/CTD, Tourette disorder/chronic tic disorders.
N/A = was not possible to fit the adjusted model due to too few cases.
Model adjusted for offspring sex and year of birth, maternal age at child's birth, maternal education, maternal region of birth, and maternal and paternal lifetime history of psychiatric disorders.
Model adjusted for offspring year of birth, maternal age at child's birth, maternal education, maternal region of birth, and maternal and paternal lifetime history of psychiatric disorders.
Fig. 2.
aHRs and 95% CIs for the risk of psychiatric disorders in PCOS-exposed and PCOS-unexposed offspring, stratified by sex. ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorders; CI, confidence interval; HR, hazard ratio; PCOS, polycystic ovary syndrome; TD/CTD, Tourette's disorder/chronic tic disorder. Note: For the TD/CTD analyses for girls, it was not possible to fit a fully adjusted model when comparing to PCOS-unexposed cousins as the number of cases was too few.
Risk of receiving a diagnosis for TD/CTD was similar between girls and boys (aHR = 1.51 v. aHR = 1.54) with overlapping CIs. When compared with PCOS-unexposed cousins, the aHR increased for boys, although with much wider CIs around the estimate. For girls, it was not possible to fit a fully adjusted model when comparing to PCOS-unexposed cousins as the number of TD/CTD cases was too few.
Kaplan–Meier curves are presented in online Supplementary Fig. S1, and Table S1 presents the follow-up time in person years among exposed and unexposed, shown separately for each outcome.
Sensitivity analyses
Year of PCOS diagnosis – Of the mothers with PCOS, 25% received their first PCOS diagnosis between 1990 and 2003, and they bore 28% of the PCOS-exposed offspring in this cohort. Table 3 shows the results of the analysis stratified by the years of PCOS diagnosis for ADHD and ASD only, as the number of cases in each of the strata for TD/CTD were too few. The estimates for both time periods were largely comparable with those of the primary analysis.
Table 3.
Adjusted hazard ratios and 95% CIs for the risk of ADHD and ASD in PCOS-exposed and PCOS-unexposed offspring, by year of maternal PCOS diagnoses
| Outcome | PCOS-unexposed offspring (unrelated) | PCOS-unexposed offspring (cousins) | ||
|---|---|---|---|---|
| n = exposed/unexposed | aHR (95% CI) | n = exposed/unexposed | aHR (95% CI) | |
| ADHD | ||||
| 1990–2003 | 291/1962 | 1.56 (1.32–1.84) | 291/234 | 1.35 (0.96–1.89) |
| 2004–2013 | 390/3479 | 1.39 (1.20–1.61) | 390/368 | 1.42 (1.03–1.95) |
| ASD | ||||
| 1990–2003 | 97/661 | 1.62 (1.28–2.05) | 97/73 | 1.39 (0.84–2.28) |
| 2004–2013 | 159/1105 | 1.54 (1.24–1.90) | 159/129 | 1.57 (1.00–2.47) |
Models adjusted for offspring sex and year of birth, maternal age at child's birth, maternal education, maternal region of birth, and maternal and paternal lifetime history of psychiatric disorders.
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorders; CI, confidence interval; HR, hazard ratio; PCOS, polycystic ovary syndrome.
Offspring of brothers v. sisters of women with PCOS – There was no clear difference in estimates calculated separately for offspring of brothers (n = 8584) and sisters (n = 9454) of women with PCOS (online Supplementary Table S2). These results were also similar to the estimates reported in the primary analysis.
Outpatient coverage – Estimates calculated for children with follow-up in outpatient care were similar to those in the main analysis (online Supplementary Table S3).
Discussion
In this national register-based study of mothers and their offspring, we found that children born to mothers with PCOS had a higher risk of being diagnosed with ADHD, ASD, and TD/CTD, compared with children born to mothers from the general population without PCOS. For ADHD and ASD, the comparison to PCOS-unexposed cousins attenuated the associations in boys and increased the estimates in girls. This supports the theory of prenatal androgen exposure influencing the development of some neuropsychiatric disorders, at least in female offspring, over and above shared familial factors.
The organizational/activational theory of steroid hormones posits that prenatal exposure to elevated androgens may cause permanent alterations (organizational effects) of neural systems leading to ‘hyper-masculine’ behavioral and cognitive traits, thereby increasing the risk for the development of neurodevelopmental and psychiatric disorders predominantly found in males such as ADHD, ASD, and TD/CTD (Arnold, 2009; Martino et al., 2013). For human female fetuses, levels of androgens are very low throughout gestation, whereas male fetuses have been shown to have higher levels of testosterone from approximately the 8th to 24th week of gestation. Evidence from both human and animal studies supports a larger effect of prenatal androgen exposure on cognitive and behavioral traits in females (Auyeung et al., 2013; Wang et al., 2017). Complementary to the results from our study, prenatal androgen exposure during late pregnancy has been found to increase anxiety-like behavior in female offspring, and to a lesser extent in the male offspring, in a rodent model (Hu et al., 2015).
Maternal PCOS has been utilized as a model of offspring exposure to elevated levels of prenatal androgens in previous studies. Consistent with the findings from our study, Kosidou and colleagues reported increased risk for ADHD and ASD in children of women with PCOS in their case–control studies [from similarly adjusted models: odds ratio (OR) = 1.42 (95% CI 1.26–1.58), OR = 1.59 (95% CI 1.34–1.88), respectively] (Kosidou et al., 2016, 2017). Further adjustment for obstetrical characteristics did not significantly influence their findings; however, accounting for obesity and cardiometabolic profiles in the mothers increased the estimates. It is relevant to note that our study population partially overlaps with those of Kosidou et al. However, the birth years of the children included in our study extend 11 years before and 2 years after their study period, yielding at least one-third more ADHD and ASD PCOS-exposed cases in our analysis. Further, while parental psychiatric history was accounted for in the studies by Kosidou et al., they did not attempt to further account for the influence of genetic confounding shared by PCOS and psychiatric disorders (Ingudomnukul et al., 2007; Herguner et al., 2015; Cesta et al., 2016), or by familial environmental factors such as health-seeking behavior. When stratified by offspring sex, the odds for having a diagnosis of ADHD was higher in females (OR = 1.53 v. 1.37 for male offspring), but the same for both sexes for ASD diagnoses (Kosidou et al., 2016, 2017).
In line with our findings, Palomba et al. (2012) reported a higher risk of ASD in daughters, but not in sons, of women with PCOS (Palomba et al., 2012). However, Cherskov et al. (2018) reported a higher adjusted risk of ASD in first born sons and but not daughters of women with PCOS (Cherskov et al., 2018).
It is difficult to draw causal conclusions based on observational studies due to the influence of unmeasured confounding. Genetically informative designs, such as family-based study designs, can be used to better account for unmeasured confounding of factors shared in families, such as genetic influences. Sibling comparison studies are often used, because comparing exposure discordant siblings allows for the adjustment for genetic and environmental factors shared between siblings (D'Onofrio et al., 2013). However, elevated testosterone levels are present throughout life in women with PCOS (Pinola et al., 2015), and therefore theoretically all of their offspring are exposed to elevated androgens in utero. This makes it impossible to conduct a sibling comparison study, where siblings need to be discordantly exposed. Therefore, we employed an ‘offspring of siblings’ study design where differentially exposed cousins were used as a comparison group to help account for unmeasured genetic and environmental factors. Our findings, based on these cousin comparisons, suggest that elevated androgens in utero influence the development of male-predominant neuropsychiatric disorders in female offspring over and above the genetic and familial factors associated with the maternal psychiatric illness that are comorbid with PCOS. This suggests that familial confounding does not play a large role in the findings of previous studies published on this topic.
However, there are some important limitations regarding the cousin design. First, the cousin comparison design requires assumptions about equal environmental influences and variability in measures across cousin types. Although this is the first study to account for familial factors in association between maternal PCOS and offspring neuropsychiatric disorders, in comparison with a sibling comparison design for example, the cousin comparison captures a smaller fraction of the potential confounding from genetic and environmental influences. The resulting residual genetic confounding could inflate our estimates of the association, and the true association between maternal PCOS and offspring psychiatric disorders might be weaker than what we observed.
On the other hand, compared with the prevalence of PCOS in the general population, PCOS is greatly underrepresented in the NPR. Therefore, misclassification of PCOS in the mothers is a possibility which consequently misclassifies the exposure status of some of the offspring, leading to a possible dilution of the measured associations. Further, this misclassification bias has the potential for a higher impact in the analysis between the PCOS-exposed offspring and their unexposed cousins, as misclassification of exposure is likely more frequent in analyses based on exposure-discordant cousins than in studies based on the general population. Due to the high heritability of PCOS, sisters of women with PCOS are expected to have higher levels of PCOS. When there is discordance between PCOS status in sisters, it is more likely to be due to misclassification than when considering women from the general population (Frisell et al., 2012). Therefore, we attempted to capture the degree of bias due to misclassification by analyzing cousins born to sisters of women with PCOS and cousins born to brothers of women with PCOS as two separate control groups. The results from the sensitivity analysis did not differ, indicating that it is unlikely that there is exaggerated misclassification bias in the cousin comparison analysis.
The diagnostic criteria for PCOS changed over the study period. The first diagnostic guidelines for PCOS established by the National Institute of Health in 1990 required the presence of both hyperandrogenemia and oligo- and/or anovulation for diagnosis. In 2003, the Rotterdam diagnostic guidelines were issued and introduced a third possible criterion – polycystic ovaries (Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group, 2004). As only two of the three criteria are required for PCOS diagnosis under the latter guidelines, there are PCOS phenotypes that do not include hyperandrogenemia, thereby creating a more heterogeneous group of women with PCOS in the later study years. Therefore, by stratifying the analysis based on year of PCOS diagnosis, it is more certain that children born to women with PCOS diagnosed between 1990 and 2003 were exposed to higher prenatal androgen levels. However, findings from this stratified analysis were similar to the main analysis. This may be due to significant referral bias for PCOS, where women with more complete phenotypes of PCOS (i.e. with hyperandrogenemia) and higher body mass index are more likely to seek and receive medical care (Lizneva et al., 2016), leading to a more homogeneous population of women diagnosed with PCOS in all years of our study.
Additionally, daughters of women with PCOS have a high likelihood of being diagnosed with PCOS, which could account for the higher rates of psychiatric disorders in this study (Xita and Tsatsoulis, 2006). However, symptoms of PCOS do not emerge before puberty and the appearance of a diagnosis in the Swedish National Registers occurs at a mean age of 28 years (Cesta et al., 2016). Therefore, due to the young age of the cohort, it is not possible to identify offspring with a potential PCOS diagnosis. Further, by comparing cousins we adjusted for some of the genetic factors influencing both PCOS and psychiatric disorders.
The main strengths of this study include the use of longitudinal population-based national register data to assess the association of maternal PCOS with neuropsychiatric outcomes in the offspring, and the use of a cousin comparison group to adjust for familial confounding. Further, this is the first study to assess the risk of TD/CTD in children of mothers with PCOS, and the first to include multiple psychiatric outcomes based on clinical diagnoses.
In addition to the limitations of the cousin-design discussed above and the change in the diagnostic criteria for PCOS, we did not have actual laboratory data on the androgen status during pregnancy of mothers with PCOS in this study. As a proxy, we performed a stratified analysis based on the diagnostic criteria of PCOS, identified by the year of PCOS diagnosis.
The role of the prenatal period in the etiology of neuropsychiatric disorders in children is of considerable research and clinical interest. Prenatal exposure to androgens has been the subject of many animal, clinical, and observational studies. Due to the elevated levels of androgens that are characteristic of PCOS, maternal PCOS has recently been used as a model for measuring the risk of elevated prenatal androgen exposure on the development of offspring psychiatric disorders. However, the results of these studies are at risk of being influenced by familial confounding due to the higher prevalence of psychiatric disorders in women with PCOS. We used a unique cousin-comparison design – which accounted for some degree of unmeasured familial confounding – made feasible by the comprehensive Swedish national registers. Our results add to a growing body of evidence supporting the influence of prenatal androgen exposure on the development of male-predominant neuropsychiatric disorders in children born to women with PCOS, with potentially differential influence of the prenatal environment v. genetics depending on the sex of the offspring. However, due to the limitations of the cousin design, these findings should be confirmed using other approaches and study designs.
Author ORCIDs
Carolyn E. Cesta, 0000-0001-5759-9366
Financial support
This work was supported by the Swedish Research Council (340-2013-5867 CA, 538-2013-8864 CMB, K2014-62X-14647-12-51 ML), the Swedish Research Council for Health, Working Life and Welfare (2015-00569 LFC, 2015-00075 MR), and the Swedish foundation for Strategic Research (KF10-0039 ML).
Conflict of interest
Prof. Larsson has served as a speaker for Eli-Lilly and Shire, and has received research grants from Shire, all outside the scope of this submitted work. Prof. Bulik reports: Shire (grant recipient, Scientific Advisory Board member) and Pearson and Walker (author, royalty recipient). Prof. Mataix-Cols and Dr Fernández de la Cruz receive royalties for contributing articles to UpToDate, Wolters Kluwer Health (unrelated to the present work). Prof. Landén declares that he has, over the past 36 months, received lecture honoraria from Lundbeck and served as scientific consultant for EPID Research Oy. The other authors have no conflict of interest to declare.
Ethical approval
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. This study was approved by the regional ethical review board in Stockholm, Sweden (diary number 2013/862-31/5; 2016/1214-32). The requirement for informed consent was waived because the study was register-based and the included individuals were not identifiable at any time.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291719000424.
click here to view supplementary material
References
- Alexander GM and Peterson BS (2004) Testing the prenatal hormone hypothesis of tic-related disorders: gender identity and gender role behavior. Development and Psychopathology 16, 407–420. [DOI] [PubMed] [Google Scholar]
- Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL and Dunaif A (2010) Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. The Journal of Clinical Endocrinology and Metabolism 95, 2180–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP (2009) The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and Behavior 55, 570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auyeung B, Lombardo MV and Baron-Cohen S (2013) Prenatal and postnatal hormone effects on the human brain and cognition. Pflügers Archiv. European Journal of Physiology 465, 557–571. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B and Knickmeyer R (2011) Why are autism spectrum conditions more prevalent in males? PLoS Biology 9, e1001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M and Hardiman PJ (2010) Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. Journal of Obstetrics and Gynaecology 30, 444–446. [DOI] [PubMed] [Google Scholar]
- Cesta CE, Mansson M, Palm C, Lichtenstein P, Iliadou AN and Landen M (2016) Polycystic ovary syndrome and psychiatric disorders: Co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology 73, 196–203. [DOI] [PubMed] [Google Scholar]
- Cesta CE, Kuja-Halkola R, Lehto K, Iliadou AN and Landen M (2017) Polycystic ovary syndrome, personality, and depression: a twin study. Psychoneuroendocrinology 85, 63–68. [DOI] [PubMed] [Google Scholar]
- Chen Q, Brikell I, Lichtenstein P, Serlachius E, Kuja-Halkola R, Sandin S and Larsson H (2017) Familial aggregation of attention-deficit/hyperactivity disorder. Journal of Child Psychology and Psychiatry 58, 231–239. [DOI] [PubMed] [Google Scholar]
- Cherskov A, Pohl A, Allison C, Zhang H, Payne RA and Baron-Cohen S (2018) Polycystic ovary syndrome and autism: a test of the prenatal sex steroid theory. Translational Psychiatry 8, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin EI, Verheij F, Wiegman T and Ferdinand RF (2006) Differences in finger length ratio between males with autism, pervasive developmental disorder-not otherwise specified, ADHD, and anxiety disorders. Developmental Medicine and Child Neurology 48, 962–965. [DOI] [PubMed] [Google Scholar]
- D'Onofrio BM, Lahey BB, Turkheimer E and Lichtenstein P (2013) Critical need for family-based, quasi-experimental designs in integrating genetic and social science research. American Journal of Public Health 103(Suppl 1), S46–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisell T, Oberg S, Kuja-Halkola R and Sjolander A (2012) Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology 23, 713–720. [DOI] [PubMed] [Google Scholar]
- Goodarzi MO, Dumesic DA, Chazenbalk G and Azziz R (2011) Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nature Reviews: Endocrinology 7, 219–231. [DOI] [PubMed] [Google Scholar]
- Herguner S, Harmanci H and Toy H (2015) Attention deficit-hyperactivity disorder symptoms in women with polycystic ovary syndrome. International Journal of Psychiatry in Medicine 50, 317–325. [DOI] [PubMed] [Google Scholar]
- Hu M, Richard JE, Maliqueo M, Kokosar M, Fornes R, Benrick A, Jansson T, Ohlsson C, Wu X, Skibicka KP and Stener-Victorin E (2015) Maternal testosterone exposure increases anxiety-like behavior and impacts the limbic system in the offspring. Proceedings of the National Academy of Sciences of the United States of America 112, 14348–14353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingudomnukul E, Baron-Cohen S, Wheelwright S and Knickmeyer R (2007) Elevated rates of testosterone-related disorders in women with autism spectrum conditions. Hormones and Behavior 51, 597–604. [DOI] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C and Gardner RM (2016) Maternal polycystic ovary syndrome and the risk of autism spectrum disorders in the offspring: a population-based nationwide study in Sweden. Molecular Psychiatry 21, 1441–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosidou K, Dalman C, Widman L, Arver S, Lee BK, Magnusson C and Gardner RM (2017) Maternal polycystic ovary syndrome and risk for attention-deficit/hyperactivity disorder in the offspring. Biological Psychiatry 82, 651–659. [DOI] [PubMed] [Google Scholar]
- Lizneva D, Kirubakaran R, Mykhalchenko K, Suturina L, Chernukha G, Diamond MP and Azziz R (2016) Phenotypes and body mass in women with polycystic ovary syndrome identified in referral versus unselected populations: systematic review and meta-analysis. Fertility and Sterility 106, 1510–1520. e1512. [DOI] [PubMed] [Google Scholar]
- Maliqueo M, Lara HE, Sanchez F, Echiburu B, Crisosto N and Sir-Petermann T (2013) Placental steroidogenesis in pregnant women with polycystic ovary syndrome. European Journal of Obstetrics, Gynecology, and Reproductive Biology 166, 151–155. [DOI] [PubMed] [Google Scholar]
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ and Davies MJ (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Human Reproduction 25, 544–551. [DOI] [PubMed] [Google Scholar]
- Martino D, Macerollo A and Leckman JF (2013) Neuroendocrine aspects of Tourette syndrome. International Review of Neurobiology 112, 239–279. [DOI] [PubMed] [Google Scholar]
- Mataix-Cols D, Isomura K, Perez-Vigil A, Chang Z, Ruck C, Larsson KJ, Leckman JF, Serlachius E, Larsson H and Lichtenstein P (2015) Familial risks of Tourette syndrome and chronic tic disorders. A population-based cohort study. JAMA Psychiatry 72, 787–793. [DOI] [PubMed] [Google Scholar]
- Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R and La Sala GB (2012) Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clinical Endocrinology 77, 898–904. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Scahill L, Naftolin F, Keefe D, Charest NJ and Cohen DJ (1992) Steroid hormones and CNS sexual dimorphisms modulate symptom expression in Tourette's syndrome. Psychoneuroendocrinology 17, 553–563. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Scahill L, Naftolin F, Keefe D, Charest NJ, King RA, Hardin MT and Cohen DJ (1994) Steroid hormones and Tourette's syndrome: early experience with antiandrogen therapy. Journal of Clinical Psychopharmacology 14, 131–135. [PubMed] [Google Scholar]
- Peterson BS, Zhang H, Anderson GM and Leckman JF (1998) A double-blind, placebo-controlled, crossover trial of an antiandrogen in the treatment of Tourette's syndrome. Journal of Clinical Psychopharmacology 18, 324–331. [DOI] [PubMed] [Google Scholar]
- Pinola P, Piltonen TT, Puurunen J, Vanky E, Sundstrom-Poromaa I, Stener-Victorin E, Ruokonen A, Puukka K, Tapanainen JS and Morin-Papunen LC (2015) Androgen profile through life in women with polycystic ovary syndrome: a Nordic multicenter collaboration study. The Journal of Clinical Endocrinology and Metabolism 100, 3400–3407. [DOI] [PubMed] [Google Scholar]
- Posthuma D and Polderman TJ (2013) What have we learned from recent twin studies about the etiology of neurodevelopmental disorders? Current Opinion in Neurology 26, 111–121. [DOI] [PubMed] [Google Scholar]
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertility and Sterility 81, 19–25. [DOI] [PubMed] [Google Scholar]
- Ruck C, Larsson KJ, Lind K, Perez-Vigil A, Isomura K, Sariaslan A, Lichtenstein P and Mataix-Cols D (2015) Validity and reliability of chronic tic disorder and obsessive-compulsive disorder diagnoses in the Swedish National Patient Register. BMJ Open 5, e007520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F and Recabarren SE (2002) Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Human Reproduction 17, 2573–2579. [DOI] [PubMed] [Google Scholar]
- Vink JM, Sadrzadeh S, Lambalk CB and Boomsma DI (2006) Heritability of polycystic ovary syndrome in a Dutch twin-family study. The Journal of Clinical Endocrinology and Metabolism 91, 2100–2104. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Chou MC, Chou WJ, Lee MJ, Lee SY, Lin PY, Lee YH, Yang YH and Yen CF (2017) Potential role of pre- and postnatal testosterone levels in attention-deficit/hyperactivity disorder: is there a sex difference? Neuropsychiatric Disease and Treatment 13, 1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xita N and Tsatsoulis A (2006) Review: fetal programming of polycystic ovary syndrome by androgen excess: evidence from experimental, clinical, and genetic association studies. The Journal of Clinical Endocrinology and Metabolism 91, 1660–1666. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291719000424.
click here to view supplementary material