Abstract
Reactive oxygen species (ROS) are essential in regulating various physiological functions. However, overproduction of ROS is implicated in the pathogenesis of various inflammatory diseases. Antioxidant therapy has thus represented an effective strategy for the treatment of oxidative stress relevant inflammatory diseases. Conventional anti-oxidative agents showed limited in vivo effects owing to their non-specific distribution and low retention in disease sites. Over the past decades, significant achievements have been made in the development of antioxidant nanotherapies that exhibit multiple advantages such as excellent pharmacokinetics, stable anti-oxidative activity, and intrinsic ROS-scavenging properties. This review provides a comprehensive overview on recent advances in antioxidant nanotherapies, including ROS-scavenging inorganic nanoparticles, organic nanoparticles with intrinsic antioxidant activity, and drug-loaded anti-oxidant nanoparticles. We highlight the biomedical applications of antioxidant nanotherapies in the treatment of different inflammatory diseases, with an emphasis on inflammatory bowel disease, cardiovascular disease, and brain diseases. Current challenges and future perspectives to promote clinical translation of antioxidant nanotherapies are also briefly discussed.
Keywords: oxidative stress, nanoparticles, antioxidant nanotherapies, ROS, inflammatory diseases
Introduction
Reactive oxygen species (ROS) are chemical species containing unpaired electrons of oxygen and oxidizing agents that are readily turned to free radicals, such as hydroxyl radical (•OH), superoxide anion radical (•), singlet oxygen (1O2), hydrogen peroxide (H2O2), and hypochlorous acid (HOCl) (Bayir, 2006; D'Autréaux and Toledano, 2007; Nosaka and Nosaka, 2017). Living organisms systematically keep a balance between ROS and antioxidant defenses including exogenous and endogenous antioxidants, such as vitamin C, vitamin E, glutathione, superoxide dismutase (SOD), catalase (CAT), and peroxiredoxins (PRXs). Basic levels of ROS play an essential role in the cellular signal transduction processes and maintain oxygen homeostasis in the physiological environment. However, excessive ROS can lead to oxidative stress, cause DNA fragmentation, protein oxidation, and lipid peroxidation, which are associated with various inflammatory diseases, such as cardiovascular diseases, inflammatory bowel disease (IBD), neurodegenerative diseases, asthma, diabetes, and arthritis (Andersen, 2004; Fraisl et al., 2009). Accordingly, antioxidant therapy has been recognized as a potentially plausible approach for treating ROS-related inflammatory diseases (Apostolova and Victor, 2015).
Over the past decades, a number of natural and synthetic anti-oxidative compounds have been reported. For example, Edaravone is the only clinically approved low molecular weight radical scavenger used for the treatment of cerebral ischemic stroke, which has been proved to exert its therapeutic effects through scavenging free radicals and upregulating endothelial NOS expression in the cerebral tissue, thus preventing lipid oxidation and reducing neuronal damage (Yamamoto et al., 1997; Edaravone Acute Infarction Study Group, 2003). Nevertheless, the clinical use of Edaravone has been limited due to its multiple adverse effects such as hepatic and renal toxicity (Watanabe et al., 2008). Various adverse effects of low molecular weight ROS scavengers are mainly due to their non-specific distribution, high renal clearance, and low delivery efficiency. The emergence of nanotechnology has considerably overcome these limitations and inspires the next wave of technological innovation in antioxidant therapy. Nanomedicines can remarkably improve the pharmacokinetics properties of antioxidative compounds and simultaneously decrease their side effects. More excitingly, some types of nanomaterials, such as inorganic nanoparticles, possess inherent antioxidant activities, by directly reacting with ROS and/or mimicking the natural antioxidant enzymes, thereby showing powerful ROS-scavenging capability. Various ROS-scavenging and/or ROS-responsive antioxidative nanotherapies derived from organic materials, or inorganic/organic hybrid materials have also attracted extensive attention recently (Lu et al., 2016; Saravanakumar et al., 2017), yielding promising results to decrease oxidative damages in various animal models of diverse diseases. In addition, gas-generating (e.g., hydrogen) nanomaterials showed beneficial therapeutic effects in inflammatory diseases, due to their anti-inflammatory and anti-oxidative properties (He et al., 2017; Zhang B. et al., 2019). In this context, different types of antioxidant nanotherapies have been constructed based on various materials, such as carbon, metal oxides, nanocrystals, lipids, and polymers, as well as a variety of newly developed materials (Petros and DeSimone, 2010).
Herein we aim to provide a comprehensive review on the recent progress in antioxidant nanoparticles for the treatment of inflammatory diseases. Different antioxidant nanoparticles based on inorganic and/or organic materials as well as drug-loaded nanoparticles are first introduced. Then we highlight biomedical applications of these nanoparticles in anti-inflammatory therapy, with emphasis on the treatment of IBD, cardiovascular disease, brain diseases, and other inflammatory diseases. Finally, major challenges regarding the clinical translation of these antioxidant nanotherapies are discussed.
Different Antioxidant Nanoparticles
ROS-Scavenging Inorganic Nanoparticles
Over the past decades, a variety of inorganic nanoparticles based on carbon, metal, and metal-organic frameworks (MOFs) with natural enzyme-like activities have been developed to decrease the damage induced by ROS in biomedical areas (Table 1). These enzyme-like antioxidative nanomaterials have been defined as nanozymes by Yan in 2007 (Gao et al., 2007). ROS-scavenging inorganic nanoparticles show outstanding biological stability, versatile functionality, and regulatory activity.
Table 1.
Representative ROS-scavenging inorganic nanoparticles.
| Nanoparticles | Applications | Evaluation models | Administration routes | References |
|---|---|---|---|---|
| Fullerenes | Cortical infarction | In vivo in rats | Intravenous or intracerebroventricular injection | Lin et al., 2002 |
| Amyotrophic lateral sclerosis | In vivo in mice | Loaded into mini-osmotic pumps and implanted into the peritoneum | Dugan et al., 1997 | |
| Alzheimer's disease | In vivo in rats | Intrahippocampal injection | Gordon et al., 2017 | |
| Asthma | In vitro in human lung mast cells; in vivo in mice | Intranasal administration | Norton et al., 2012 | |
| Ceria oxide nanoparticles | Liver fibrosis | In vivo in rats | Intravenous injection | Oró et al., 2016 |
| Photoreceptor degeneration | In vivo in mice/rats | Intracardial injection; Intravitreal injection | Kong et al., 2011; Cai et al., 2012; Wong et al., 2013, 2015 | |
| Alzheimer's disease | In vivo in mice | Unilateral subicular injection | Kwon et al., 2016 | |
| Multiple sclerosis | In vivo in mice | Intravenous injection | Heckman et al., 2013 | |
| Platinum nanoparticles | Cerebral cavernous malformation disease | In vitro in HeLa, MCF-7, and Caco-2 cells | N/A | Moglianetti et al., 2016 |
| Cerebral ischemia | In vivo in mice | Intravenous injection | Takamiya et al., 2012 | |
| Manganese-based nanoparticles | Parkinson's Disease | In vitro in SH-SY5Y cells | N/A | Singh et al., 2017 |
| Ear-inflammation | In vitro in HeLa cells; in vivo in mice | Subcutaneous injection | Yao et al., 2018 | |
| Prussian blue nanoparticles | Liver inflammation | In vitro in HUVECs, RAW264.7, HBZY-1, NIH-3T3, and HT cells; in vivo in mice | Intravenous injection | Zhang W. et al., 2016 |
| Molybdenum nanoparticles | Acute kidney injury | In vitro in HEK293 cells; in vivo in mice | Intravenous injection | Ni et al., 2018 |
| V2O5@pDA@ MnO2 nanoparticles |
Ear inflammation | In vitro in HEK293T cells; in vivo in mice | Intravenous injection | Huang et al., 2016 |
| Ceria-zirconia nanoparticles | Sepsis | In vitro in RAW264.7 cells; in vivo in mice/rats | Intravenous injection; intraperitoneal administration | Soh et al., 2017 |
Fullerenes
Fullerenes are the first reported SOD-mimicking inorganic nanomaterials, representing the landmark in the development of ROS-scavenging inorganic nanoparticles. Fullerenes are three-dimensional carbon networks, and the most common form of fullerenes is buckminsterfullerene (C60) with 60 carbon atoms arranged in a sphere. C60 fullerene can behave as a free radical “sponge,” with each C60 molecule capable of quenching multiple radical species (Krusic et al., 1991; Markovic and Trajkovic, 2008). The molecular mechanism underlying antioxidant reactions of C60 compounds is that electron-deficient regions of C60 cooperates with the attached malonyl groups to electrostatically guide and stabilize superoxide, thereby promoting its decomposition (Markovic and Trajkovic, 2008). C60(C(COOH)2)2 nanoparticles can be selectively internalized by oxidation-damaged cerebral endothelial cells, and greatly inhibit their apoptosis induced by ROS, which is related to the modulation of the c-Jun NH2-terminal kinase (JNK) signaling pathway (Lao et al., 2009). Several studies demonstrated that fullerenes can significantly alleviate ROS-dependent neuronal injury induced by N-methyl-D-aspartate or K+ deficiency in vitro (Dugan et al., 1997; Ali et al., 2008). The neuroprotective effects of fullerenes were also validated in cortical infarction induced by ischemia-reperfusion (Lin et al., 2002), amyotrophic lateral sclerosis in SOD mutant mice (Dugan et al., 1997), and Alzheimer's disease in rats induced by amyloid-β25−35 peptide (Gordon et al., 2017). Treatment with fullerenes also inhibited mast cell function in vitro, showing potent therapeutic effects on allergic asthma (Norton et al., 2012) and CCl4-mediated acute liver toxicity (Gharbi et al., 2005).
Ceria Oxide Nanoparticles
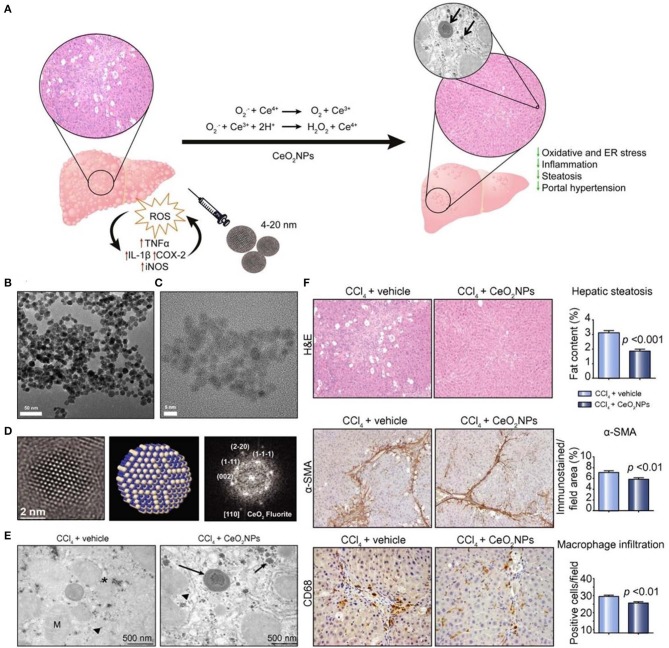
Ceria oxide nanoparticles have been demonstrated to possess SOD-mimetic activity, resulting from the mixed valence states of Ce3+ and Ce4+ (Celardo et al., 2011; Bryant et al., 2016). It has become the most widely used inorganic nanomedicine for antioxidant therapy. At the nanoscale, Ce3+ and Ce4+ coexist on the surface of the Ce oxide lattice, and Ce4+ can be reduced to Ce3+ due to the presence of surface oxygen vacancies, therefore effectively decreasing ROS levels. Previous studies suggested that the Ce3+/Ce4+ ratio has an important effect on the antioxidant activity of ceria oxide nanoparticles, which is dominated by the microenvironment (Das et al., 2013). Li et al. reported a novel strategy to significantly improve the superoxide-scavenging activity of ceria oxide nanoparticles (> 5 nm) with different shapes and a negligible Ce3+/Ce4+ ratio through electron transfer (Li et al., 2015). Cerium oxide nanoparticles exhibited tissue protective actions in inflammatory diseases (Hirst et al., 2009), wound healing (Davan et al., 2012), photoreceptor degeneration (Cai et al., 2012), and neurodegenerative diseases (Kwon et al., 2016). Also, cerium oxide nanoparticles can accumulate in the liver and spleen following intravenous (i.v.) injection, showing effectiveness in therapy of liver fibrosis in rats (Oró et al., 2016). Mechanistically, cerium oxide nanoparticles are able to reduce ROS levels in HepG2 cells and protect CCl4-treated rats against chronic liver injury by inhibiting liver steatosis, reducing portal hypertension, and decreasing overexpression of inflammatory genes (Figure 1).
Figure 1.
Schematic, characterization, and in vivo evaluation of CeO2 nanoparticles (NPs). (A) Schematic illustration of therapeutic mechanisms of CeO2NPs. (B) High-resolution transmission electron microscopy (HR-TEM) photomicrograph at low (43,000×) magnification revealing loose CeO2NPs agglomerates. (C) High magnification image (400,000×) of individual 4 nm CeO2NPs with spherical shape. (D) (Left) The HR-TEM image of single CeO2 NP showing the spherical morphology; (middle) atomistic simulation of CeO2NP with a characteristic fluorite-like electronic structure; (right) FFT of the selected nanoparticle with indicated atomic planes. (E) TEM image of liver tissue obtained from a CCl4-treated rat receiving vehicle and a CCl4-treated rat receiving CeO2NPs. Hepatocyte intracellular space containing mitochondria (M), organelles, fat droplets (asterisk), glycogen inclusions (arrow head), and aggregates of CeO2NPs (arrows). (F) Effect of CeO2NPs on hepatic steatosis, fibrogenic and infiltrating cells. Reproduced with permission from Oró et al. (2016).
Platinum Nanoparticles
Platinum is mainly used as a chemotherapeutic agent in clinical practice. Platinum nanoparticles were proven to have multiple activities mimicking SOD, catalase (CAT), and NADPH oxidoreductase (Hikosaka et al., 2008; Zhang et al., 2010; Oró et al., 2016; Pedone et al., 2017; Francesca et al., 2018), and they can catalyze the reduction of H2O2 to water and molecular oxygen, suggesting that platinum nanoparticles are potential medicinal candidates for oxidative stress diseases. Platinum nanoparticles possess strong and broad antioxidant properties, and they could significantly decrease the lipopolysaccharide (LPS)-induced production of intracellular ROS and inflammatory cytokines in vitro (Rehman et al., 2012). As radical scavenging materials, platinum nanoparticles showed potent activity in a cellular model of human cerebral cavernous malformation disease (Moglianetti et al., 2016), exhibited significant neuroprotective effects on ischemic mouse brains (Takamiya et al., 2012), effectively protected keratinocytes against UV-induced inflammation (Yoshihisa et al., 2010), and inhibited pulmonary inflammation induced by acute cigarette smoking (Onizawa et al., 2009). Kim et al. conjugated platinum nanoparticles with a fusion protein based on a platinum binding peptide and a HIV-1 TAT-derived peptide. Of note, the TAT peptide is a cell-penetrating peptide capable of enhancing translocation and internalization of platinum nanoparticles into the cytoplasm. Compared with unconjugated platinum nanoparticles, the fusion protein-conjugated platinum nanoparticles drastically improved the bioavailability of platinum nanoparticles in Caenorhabditis elegans, leading to similar antioxidant effects at only one hundredth dose (Kim et al., 2010).
Manganese-Based Nanoparticles
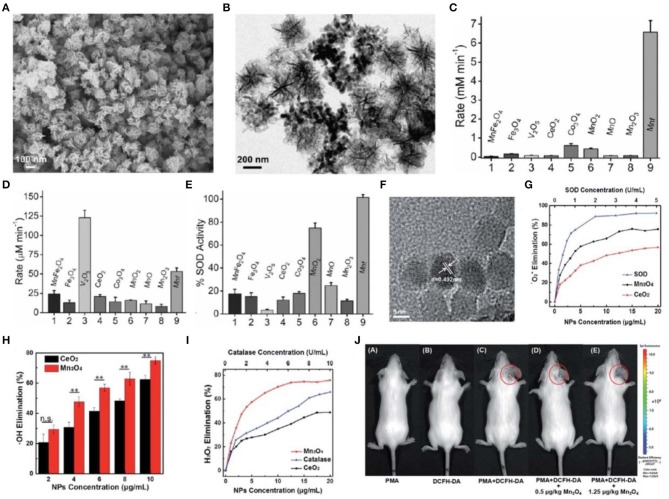
A previous study demonstrated that natural MnSOD is superior to FeSOD and Cu/Zn SOD for the treatment of oxidative stress-related chronic diseases (Miriyala et al., 2012). This implicated that manganese-based nanoparticles may possess excellent ROS-scavenging activities (Singh et al., 2018; Singh N. et al., 2019). A recent study reported the flower-like Mn3O4 nanoparticles that functionally mimic three major antioxidant enzymes including SOD, CAT, and glutathione peroxidase (GPx). Mn3O4 nanoparticles exhibited excellent antioxidant activities and played a crucial role in protecting Parkinson disease-like cells from cytotoxicity induced by a neurotoxin 1-methyl-4-phenylpyridinium (MPP+) in vitro (Singh et al., 2017) (Figures 2A–E). In another study, Mn3O4 nanoparticles were synthesized via a hydrothermal method (Sun et al., 2019). These nanoparticles scavenged nearly 75% • due to the two oxidation states of Mn2+ and Mn3+, which is superior to CeO2 nanoparticles. In addition, Mn3O4 nanoparticles can eliminate H2O2 and •OH. They demonstrated intracellular ROS-scavenging activities in HeLa cells in vitro and effectively protected mice from ROS-induced ear-inflammation in vivo (Figures 2F–J).
Figure 2.
Characterization and antioxidative activity of manganese-based nanoparticles. (A,B) Scanning electron microscopy (SEM) (A) and TEM (B) images of Mn3O4 nanoparticles with flower-like morphology (Mnf). (C–E) A comparison of CAT (C), glutathione peroxidase (D), and SOD (E)-like activity of Mnf with other metal oxide nanoparticles. (F) TEM image of Mn3O4 nanoparticles synthesized via a hydrothermal method. (G–I) Dependence between the elimination of •O2− (G), •OH (H), H2O2 (I) and concentrations of Mn3O4 and CeO2 nanoparticles. (J) In vivo fluorescence imaging of mice with PMA-induced ear inflammation after treatment with Mn3O4 nanoparticles. Images in (A–E) are reproduced with permission from Singh et al. (2017). Images in (F–J) are reproduced with permission from Yao et al. (2018).
Other ROS-Scavenging Inorganic Nanoparticles
In addition to the above mentioned nanoparticles, some other inorganic nanoparticles have been examined for ROS-scavenging, such as nanoparticles derived from Prussian blue (Zhang W. et al., 2016; Chen et al., 2018), gold/platinum and diamond (Martín et al., 2010), ruthenium (Cao et al., 2017), molybdenum (Ni et al., 2018), and vanadium (Vernekar et al., 2014). In particular, Zhang et al. discovered that Prussian blue nanoparticles possess CAT- and SOD-like activities, thereby effectively scavenging ROS, protecting cells from oxidative stress induced by cisplatin, diallyl trisulfide, LPS, phorbol 12-myristate 13-acetate (PMA), UV irradiation, high-level glucose, oxidized low density lipoprotein (OxLDL), and hypoxia/hypoglycemia and reoxygenation in vitro (Zhang W. et al., 2016). Prussian blue nanoparticles also exerted desirable anti-inflammatory effects in LPS-induced liver inflammation in mice. The peroxidase-like properties of Prussian blue nanoparticles are mainly ascribed to the FeNx units (Chen et al., 2018). Ruthenium nanoparticles could also decompose H2O2 and scavenge •OH, •, 1O2, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid-derived free radicals (ABTS•+), and 1,1-diphenyl-2-picrylhydrazyl radical (•DPPH), and therefore exert cytoprotective effects against H2O2-induced oxidative stress in vitro (Cao et al., 2017). However, in vivo efficacies of ruthenium nanoparticles remain to be explored. In another study, Mugesh et al. found that V2O5 nanowires displayed notable GPx-like antioxidant activity to protect cells from oxidative damage (Vernekar et al., 2014). Ceria-zirconia composite nanozymes (Soh et al., 2017), Gd@C82-(ethylenediamine)8 nanoparticles (Li et al., 2016), and V2O5@pDA@MnO2 nanocomposites (Huang et al., 2016) with multiple antioxidant activities were also investigated.
Organic Nanoparticles With Intrinsic Antioxidant Activities
Besides ROS-scavenging inorganic nanoparticles, organic nanoparticles with intrinsic antioxidant activities have been emerging as a potential platform for the treatment of numerous diseases associated with inflammatory disorders (Kim K. S. et al., 2015; Zhu and Su, 2017; Kwon et al., 2019). Several types of organic nanoparticles have shown higher antioxidative advantages over conventional antioxidants and radical scavengers, owing to their predominant properties in terms of pharmacokinetics, biodistribution, delivery efficiency, and robust scavenging capabilities of multiple radicals (Lu et al., 2016; Ferreira et al., 2018; Ye et al., 2019; Zafar et al., 2019). In this section, these organic nanoparticles will be briefly introduced (Table 2).
Table 2.
Different organic nanoparticles with intrinsic antioxidant activities.
| Types of organic nanoparticles | Applications | Evaluation models | Administration routes | References | |
|---|---|---|---|---|---|
| Bilirubin-derived nanoparticles | BRNPs | Hepatic ischemia-reperfusion injury | In vitro in primary mouse hepatocytes; in vivo in mice | Intravenous injection | Kim J. Y. et al., 2017 |
| Asthma | In vivo in mice | Intravenous injection; intraperitoneal administration | Kim D. E. et al., 2017 | ||
| Inflammatory bowel disease | In vivo in mice | Intravenous injection | Lee et al., 2016a | ||
| Pancreatic islet xenotransplantation | In vivo in mice | Intraperitoneal administration | Kim M. J. et al., 2017 | ||
| DOX@BRNPs Dox@bt-BRNPs cisPt@BRNPs |
Tumor | In vivo in mice | Intravenous injection | Lee et al., 2016b, 2017, 2018 | |
| HABN | Colitis | In vivo in mice | Oral administration | Lee et al., 2020 | |
| Melanin-like nanoparticles | MMPP | Acute kidney injury | In vitro in HEK293 cells; in vivo in mice | Intravenous injection | Sun et al., 2019 |
| PEG-MeNPs | Ischemic stroke | In vitro in Neuro 2A cells; in vivo in mice | Intravenous injection | Liu et al., 2017 | |
| PDA-PEG/CP | Tumor | In vitro in HeLa cells | N/A | Zhu and Su, 2017 | |
| Liposome-BSM | Tumor | In vivo in mice | Subcutaneous injection | Chu et al., 2016 | |
| PDA NPs | Periodontal disease | In vivo in mice | Subgingival administration | Bao et al., 2018 | |
| PDA | Acute peritonitis and acute lung injury | In vivo in mice | Intravenous injection; intraperitoneal administration | Zhao et al., 2018 | |
| Free radical-containing organic nanoparticles | RNPN and RNPO | Breast cancer | In vitro in MDA-MB-231 cells; in vivo in mice | Intravenous injection | Shashni and Nagasaki, 2018 |
| RNPO | Inflammatory bowel disease | In vivo in mice | Oral administration | Vong et al., 2012, 2014, 2015 | |
| RNPN and RNPO | Colon cancer | In vitro in C-26 cells; in vivo in mice | Intravenous injection | Vong and Nagasaki, 2016 | |
| RNPN and RNPO | Epidermoid cancers | In vitro in KB-3-1 and KB/MRP cells | N/A | Shashni et al., 2017 | |
| RNPN | Radiation-induced organ dysfunctions | In vitro in RAW264.7 cells; in vivo in mice | Subcutaneous injection | Feliciano et al., 2017 | |
| RNPN | Ischemia reperfusion injury | In vivo in mice | Intravenous injection | Yoshitomi et al., 2011 | |
| RNPs | Nonalcoholic steatohepatitis | In vivo in mice | Intravenous injection | Eguchi et al., 2015 | |
| RNPs | Neurological deficits | In vivo in mice | Intravenous injection | Chonpathompikunlert et al., 2012 | |
| Phenolic nanoparticles | HPOX | Asthma | In vitro in RAW264.7 cells; In vivo in mice | Intratracheal administration | Yoo et al., 2013 |
| Hindlimb ischemia | In vivo in mice | Intramuscular injection | Cho et al., 2015 | ||
| H2O2-associated inflammatory diseases | In vitro in RAW264.7 cells | N/A | Cho et al., 2012 | ||
| PVAX | Acute liver failure | In vitro in RAW264.7 cells; in vivo in mice | Intravenous injection | Ko et al., 2014 | |
| PVO | Hepatic ischemia-reperfusion injure | In vivo in mice | Intravenous injection | Kang et al., 2016 | |
| Nanoparticles derived from functional cyclodextrin materials | Nanoparticles based on PBAP-conjugated cyclodextrin | Tumor | In vitro in B16F10 and RAW264.7 cells; in vivo in mice | Intravenous injection | Zhang et al., 2015 |
| Arterial restenosis | In vitro in rat VSMCs; in vivo in rats | Intravenous injection | Feng et al., 2016 | ||
| Inflammatory bowel disease | In vivo in mice | Oral administration | Zhang Q. et al., 2016 | ||
| Atherosclerosis | In vitro in RAW264.7 cells; in vivo in mice | Intravenous injection | Dou et al., 2017 | ||
| Abdominal aortic aneurysm | In vivo in mice | Intravenous injection | Cheng et al., 2018 | ||
| Peritonitis | In vitro in RAW264.7 cells; in vivo in mice | Intraperitoneal administration | Zhang et al., 2017 | ||
| Colitis-associated colon cancer | In vitro in C26 murine colon carcinoma cells; in vivo in mice | Oral administration | Zhang Q. et al., 2019 | ||
| LCD nanoparticles | Peritonitis, acute lung injury, and atherosclerosis | In vitro in neutrophils and macrophages; in vivo in mice | Intraperitoneal administration; intravenous injection | Guo et al., 2019 | |
| TPCD nanoparticles | Peritonitis, acute lung injury, drug-induced organ toxicity, and atherosclerosis | In vivo in mice | Intraperitoneal administration; intravenous injection | Li et al., 2018; Wang et al., 2018 | |
| Other organic nanoparticles | PVA-C | Air pouch model | In vitro in BMSCs; in vivo in rats | Subcutaneous injection | Wu et al., 2019 |
Bilirubin-Derived Nanoparticles
Bilirubin, a natural metabolite of hemoglobin, has been suggested as a primary physiological antioxidant for scavenging various ROS and protecting cells/tissues from oxidative damage. However, clinical applications of bilirubin have been restricted due to its poor water-solubility and toxicity. To overcome the drawbacks of natural bilirubin, Jon and coworkers developed polyethylene glycol (PEG)-conjugated bilirubin nanoparticles through a combined covalent conjugation and self-assembly method, which were defined as BRNPs (Lee et al., 2016a; Kim D. E. et al., 2017; Kim J. Y. et al., 2017). Compared to pristine bilirubin, BRNPs have more excellent water dispersibility, better pharmacokinetic properties, and higher accumulation in oxidative stress-induced inflammatory lesions. More importantly, BRNPs inherited the intrinsic powerful antioxidant capability of bilirubin, as evidenced by their efficacies in the treatment of a variety of oxidative stress-associated diseases, such as hepatic ischemia-reperfusion injury (Kim J. Y. et al., 2017), asthma (Kim D. E. et al., 2017), IBD (Lee et al., 2016a), and pancreatic islet xenotransplantation (Kim M. J. et al., 2017). In addition, BRNPs protected different cells against cytotoxicity caused by H2O2 and decreased inflammatory responses resulting from activated macrophages. Moreover, other bilirubin-derived nanoparticles can be used as a companion medicine for effective treatment of cancers and colitis (Lee et al., 2017, 2018, 2020). As a typical paradigm, Lee et al. constructed a hyaluronic acid-bilirubin nanomedicine (HABN) for inhibition of acute colitis (Lee et al., 2020). In vivo experiments demonstrated that HABN could target the inflamed colonic epithelium, restore the dysregulated intestinal barriers, and simultaneously modulate the gut microbiota and immune responses in mice bearing colitis.
Melanin-Like Nanoparticles
Melanins are well-known endogenous biopolymers that are widely distributed in most living organisms. They have attracted much attention because of their fascinating biological characteristics, such as free radical quenching, photoprotection, and photosensitization (Ju et al., 2011; Solano, 2017; Wang X. et al., 2019). However, melanins can only be dissolved in strongly alkaline aqueous solutions, which largely limits their biomedical applications (Wang X. et al., 2019). Bioinspired polymerization of dopamine can generate melanin-like nanoparticles with similar properties to natural melanins. These melanin-like nanoparticles, often known as polydopamine (PDA) nanoparticles, contain a population of antioxidant groups, thereby exhibiting strong free radical-scavenging ability. PDA nanoparticles have been widely studied in recent years (Chu et al., 2016; Liu et al., 2017; Zhu and Su, 2017; Bao et al., 2018; Zhao et al., 2018; Sun et al., 2019). Cai et al. designed a melanin-based antioxidant defense nanosystem for acute kidney injury (Sun et al., 2019). The ultrasmall Mn2+-chelated and PEG-decorated melanin (MMPP) nanoparticles exhibited good physiological stability and excellent anti-oxidative activities toward various toxic ROS. In vivo results indicated that MMPP nanoparticles can serve as a multifunctional nanotheranostic system for the treatment of acute kidney injury. Notably, Shi's proved that bioinspired melanin nanoparticles (MeNPs) have excellent scavenging activity for multiple reactive oxygen and nitrogen species (RONS), such as •, H2O2, •OH, •NO, and ONOO− (Liu et al., 2017).
Free Radical-Containing Organic Nanoparticles
Spurred by the development of enzymology, a series of ROS-scavenging organic nanoparticles with enzyme-mimicking functions have been engineered as new antioxidant candidates for the biomedical applications. As a representative example, antioxidative nanotherapies self-assembled by integrating a stable nitroxide radical-containing ROS trapper, 4-hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (Tempol) into the side chain of amphiphilic copolymers have been intensively investigated by Nagasaki's group (Yoshitomi and Nagasaki, 2014; Ikeda and Nagasaki, 2018). Two types of redox nanoparticles (RNPs), pH-sensitive RNPN and pH-insensitive RNPO have been synthesized and demonstrated remarkable protective and therapeutic effects in diverse diseases, such as breast or colon cancers (Vong and Nagasaki, 2016; Shashni and Nagasaki, 2018), IBD (Vong et al., 2012, 2014, 2015), radiation-induced organ dysfunctions (Feliciano et al., 2017), ischemia reperfusion injury (Yoshitomi et al., 2011), non-alcoholic steatohepatitis (Eguchi et al., 2015), and neurological deficit (Chonpathompikunlert et al., 2012). These studies have unambiguously substantiated the antioxidative effects of RNPN and RNPO without discernible adverse side effects.
Phenolic Nanoparticles
Phenolic compounds, such as hydroxybenzyl alcohol (HBA) and vanillyl alcohol have long been widely used for treating ischemic brain injury and coronary heart disease, because of their antioxidant, anti-inflammatory, and anti-nociceptive activities (Berwin Singh et al., 2018). Recently, HBA-containing copolyoxalate (HPOX) was designed and synthesized, which can be degraded completely to release therapeutic HBA and 1,4-cyclohexendimethanol in the presence of H2O2 (Yoo et al., 2013). The biocompatible HPOX nanoparticles exerted highly potent antioxidant and anti-inflammatory effects by reducing the generation of ROS and suppressing the expression of pro-inflammatory mediators in stimulated macrophages. Given their beneficial effects in a mouse model of asthma by suppressing the recruitment of eosinophils and neutrophils as well as the expression of iNOS, HPOX nanoparticles exhibited tremendous potential as an anti-asthmatic agent. Likewise, HPOX nanoparticles also significantly promoted angiogenesis and blood flow perfusion in a mouse mode of hindlimb ischemia (Cho et al., 2015). Through similar procedures, vanillyl alcohol-containing copolyoxalate (PVAX) and poly (vanillin oxalate) (PVO) were synthesized by a simple one-step polymerization, and both of them are able to scavenge H2O2 (Ko et al., 2014; Jeong et al., 2016; Jung et al., 2019). PVAX nanoparticles and manganese porphyrin showed synergistic antioxidant and anti-inflammatory activities in mice with acetaminophen-induced acute liver failure (Ko et al., 2014). Kang et al. developed H2O2-triggered bubble-generating antioxidant PVO polymeric nanomaterials that can significantly suppress liver damages due to ischemia/reperfusion injury by inhibiting inflammation and apoptosis (Kang et al., 2016). In view of the advantages of excellent biodegradability, antioxidant activity, anti-inflammatory property, and low cytotoxicity, these phenolic nanoparticles have great potential as therapeutics for inflammatory diseases.
Nanoparticles Derived From Functional Cyclodextrin Materials
Most recently, our group has developed a series of ROS-responsive materials by conjugating phenylboronic acid pinacol ester (PBAP) onto β-cyclodextrin (β-CD), a cyclic oligosaccharide with excellent in vivo safety (Zhang and Ma, 2013). The obtained ROS-responsive materials can be used to construct nanoparticles for site-specific delivery and inflammation-responsive release of different therapeutics (Juni et al., 2013; Lamprecht, 2015; Zhang Q. et al., 2016; Alaarg et al., 2017; Kotla et al., 2019). Interestingly, nanoparticles based on PBAP-conjugated cyclodextrin materials can effectively eliminate H2O2, thereby inhibiting inflammatory responses and oxidative stress in stimulated macrophages (Zhang et al., 2017). These H2O2-eliminating cyclodextrin nanoparticles efficaciously alleviated the symptoms of peritonitis and colitis in mice, by reducing the counts of neutrophils and macrophages as well as inhibiting the secretion of pro-inflammatory cytokines, chemokines, and oxidative mediators (Zhang et al., 2017; Zhang Q. et al., 2019). Also, we found that nanoparticles prepared from a luminol-conjugated β-CD material (LCD) can inhibit inflammatory response, oxidative stress, and recruitment of neutrophils and macrophages (Guo et al., 2019). In addition, LCD nanoparticles effectively reduced the levels of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, myeloperoxidase (MPO), and ROS in neutrophils stimulated with PMA.
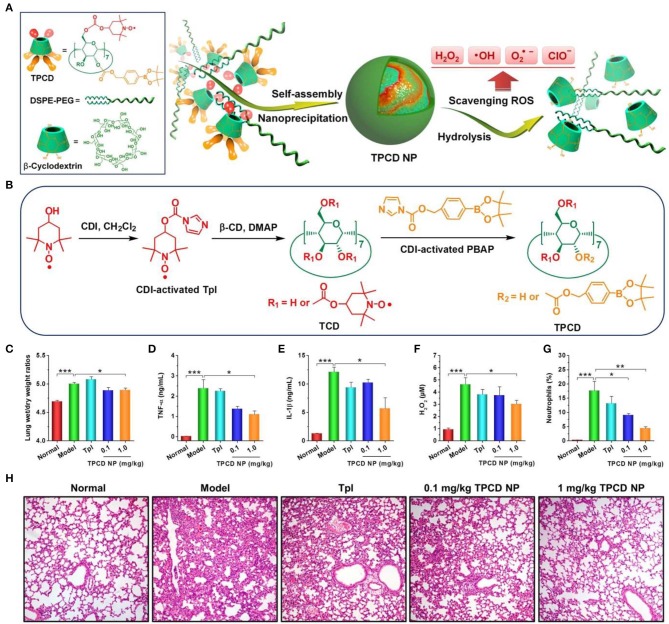
Nevertheless, almost all available organic nanoparticles can only eliminate limited oxygen species in ROS. To circumvent this shortcoming, our group synthesized a broad-spectrum ROS-eliminating material (i.e., TPCD), by simultaneously conjugating Tempol and phenylboronic acid pinacol ester onto β-CD (Li et al., 2018; Wang et al., 2018). TPCD can be easily produced into SOD/CAT-mimetic and size-controlled nanoparticles. The resulting TPCD nanoparticles can effectively scavenge multiple reactive species, including •, H2O2, radicals, and hypochlorite (Figures 3A,B). Compared with the corresponding nanotherapies with relative narrow-spectrum ROS-eliminating capability, TPCD nanoparticles more effectively protected macrophages from H2O2-induced apoptosis and decreased inflammatory responses in vitro. Consistently, TPCD nanoparticles showed superior efficacies in the animal models of acute and chronic inflammation, such as peritonitis, acute lung injury, drug-induced organ toxicity, and atherosclerosis. Importantly, preliminary in vitro and in vivo tests demonstrated the good safety profile of TPCD nanoparticles.
Figure 3.
Design and preparation broad-spectrum ROS-scavenging nanoparticles for treatment of acute lung injury (ALI). (A) Schematic illustration of engineering of a broad-spectrum ROS-scavenging material and its nanoparticles based on functionalized β-CD. (B) The synthetic route of β-CD conjugated with Tempol (Tpl) and PBAP units (TPCD). CDI, 1,1-carbonyldiimidazole; DMAP, 4-dimethylaminopyridine; TCD, Tpl-conjugated β-CD; PBAP, 4-(hydroxymethyl) phenylboronic acid pinacol ester. (C–H) Treatment of ALI with TPCD nanoparticles (i.e., TPCD NP) in mice. (C) The lung wet-to-dry weight ratios after different treatments. (D–G) The expression levels of TNF-α (D), IL-1β (E), and H2O2 (F) in bronchoalveolar lavage fluid from mice with LPS-induced ALI and subjected to different treatments. (G) Quantified neutrophil counts in pulmonary tissues of ALI mice. (H) H&E-stained pathological sections of lung tissues. Data are mean ± standard error (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001. Reproduced with permission from Li et al. (2018).
Other Organic Nanoparticles
Other nanoparticles derived from small molecules such as ascorbic acid (Bhatia et al., 2019), citric acid (Wu et al., 2019), and trolox (Wattamwar et al., 2010), in combination with polymeric scaffolds, have also provided us with a powerful arsenal for antioxidant and anti-inflammatory treatment. For instance, a chemically stable antioxidant molecule, citric acid was grafted with polyvinyl alcohol (Wu et al., 2019). The obtained polymer PVA-C showed anti-apoptotic ability in stem cells, and it also upregulated the nuclear peroxisome proliferator-activated receptor γ and MnSOD by releasing citric acid.
Drug-Loaded Nanoparticles With Antioxidant Activity
Both natural and synthetic antioxidants of small molecules have been broadly used or studied for anti-inflammatory therapies of numerous diseases associated with oxidative stress. The generally used natural antioxidants include vitamin C, vitamin E, glutathione, β-carotene, flavonoids, and curcumin, while Edaravone, lipoic acid, N-acetylcysteine, and Tempol are frequently examined synthetic compounds. The main limitations of small molecule antioxidants lie in their systemic distribution, rapid metabolism, and low retention at the diseased site, thereby resulting in low bioavailability in target tissues/cells. In this aspect, nanoparticles can protect the loaded small molecule antioxidants from hydrolysis, achieve site-specific delivery and controlled release of antioxidants at disease sites, and therefore improve their bioavailability (Table 3) (Wang et al., 2015; Kang et al., 2016; Jung et al., 2018; Larrañaga et al., 2018; Gou et al., 2019). For example, our group fabricated a SOD/CAT- mimetic nanomedicine comprising H2O2-eliminating nanoparticles derived from a PBAP-conjugated β-CD material and a free radical scavenger Tempol (Zhang Q. et al., 2016). After oral administration, this nanotherapy was able to efficiently target the inflamed colon in colitic mice, and remarkably decrease non-specific distribution, thus notably alleviating manifestations relevant to colitis, with the efficacy superior over free Tempol.
Table 3.
Typical drug-loaded nanoparticles with antioxidant activity.
| Carrier materials | Loaded drugs | Applications | Evaluation models | Administration routes | References |
|---|---|---|---|---|---|
| OxbCD | Tempol | Inflammatory bowel disease | In vivo in mice | Oral administration | Zhang Q. et al., 2016 |
| Lipids | Curcumin | Sepsis | In vitro in RAW264.7 cells; in vivo in mice | Intraperitoneal injection | Wang et al., 2015 |
| Silk fibroin | Curcumin | Inflammatory bowel disease | In vitro in RAW264.7 cells; in vivo in mice | Oral or intravenous administration | Gou et al., 2019 |
| Vanillyl alcohol-incorporated copolyoxalate | Curcumin | Peripheral artery disease | In vitro in RAW264.7 cells, NIH3T3 fibroblasts, and vascular endothelial cells; in vivo in mice | Intramuscular injection | Jung et al., 2018 |
| PVO | Vanillin | Ischemia/reperfusion injury | In vitro in RAW264.7 cells; in vivo in mice | Intravenous injection | Kang et al., 2016 |
| β-Galactosidase-conjugated with anti-PECAM | CAT | Acute lung transplantation injury | In vitro in HUVECs; in vivo in rats | Intravenous injection | Kozower et al., 2003 |
| Polyketal | SOD | Myocardial ischemia-reperfusion injury | In vitro in RAW264.7 cells; in vivo in rats | Myocardial injection | Seshadri et al., 2010 |
| Engineered exosomes | CAT mRNA | Parkinson's Disease | In vitro in Neuro2A cells; in vivo in mice | Subcutaneous implantation with Matrigel | Kojima et al., 2018 |
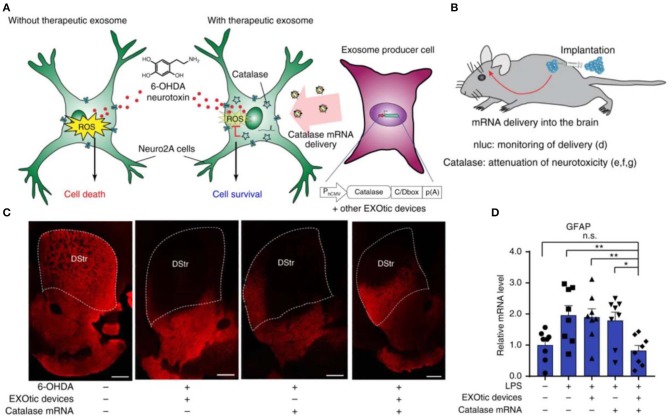
In addition to the conventional small-molecule ROS scavengers, other biological agents, such as natural antioxidant enzymes SOD, CAT, and nucleic acids have been loaded into nanoparticles for anti-oxidative treatment (Kozower et al., 2003; Seshadri et al., 2010; Chen et al., 2013). Petro et al. constructed poly (D,L-lactide co-glycolide) (PLGA) nanoparticles separately loaded with SOD, CAT, or their combination (Petro et al., 2016). In a thromboembolic rat model, sequential administration of CAT/PLGA and SOD/PLGA nanoparticles effectively attenuated inflammatory responses, inhibited neuronal cell apoptosis, and suppressed edema formation, by protecting the blood-brain barrier (BBB) from ROS-mediated reperfusion injury. In a recent study, Kojima et al. constructed designer exosomes that can deliver CAT mRNA into target cells with high efficiency, thereby attenuating neurotoxicity and neuroinflammation in both in vitro and in vivo models of Parkinson's disease (Figure 4) (Kojima et al., 2018).
Figure 4.
Applications of the engineered exosomes loaded with CAT mRNA in Parkinson's disease. (A) Schematic illustration of protection against neurotoxicity in an in vitro experimental model of Parkinson's disease by CAT mRNA delivery. (B) Engineered exosome producer cells were subcutaneously implanted with Matrigel in living mice. (C) Immunostaining result of tyrosine hydroxylase (TH)-positive neurons. Scale bars, 500 μm. (D) Attenuation of neuroinflammation caused by systemic LPS injection with catalase mRNA delivery in vivo. Reproduced with permission from Kojima et al. (2018). *p < 0.05, **p < 0.01; n.s., no significance.
Therapeutic Applications of Antioxidant Nanotherapies in Inflammatory Diseases
Inflammation is a protective response of the innate immune system against pathogens or irritants (Karin and Clevers, 2016). However, uncontrolled inflammation plays a fundamental role in the progress of a large number of human diseases, such as asthma, pneumonia, rheumatoid arthritis, IBD, as well as cardiovascular and neurodegenerative diseases. It has been well-documented that excessive accumulation of ROS contributes to the pathogenesis of both acute and chronic inflammation (Zhang and Kaufman, 2008). In this section, we will provide an overview of the applications of antioxidant nanomedicines in the treatment of different inflammatory diseases.
Inflammatory Bowel Disease
Inflammatory bowel disease (IBD), including Crohn's disease and ulcerative colitis (UC), is a chronic inflammatory disorder in the gastrointestinal tract. The intestinal mucosa of patients suffering from IBD is characterized by overproduction of ROS and an imbalance of antioxidants, resulting in oxidative damage. It has been found that the mucosal ROS concentrations are 10–100 times higher in patients with IBD (Simmonds et al., 1992; Sedghi et al., 1993). Treatment with antioxidant agents can mitigate IBD in both animal models and patients (Moura et al., 2015). Unfortunately, non-specific distribution and low retention of these compounds often results in multiple side effects. So far, a number of studies have reported the construction of orally available antioxidant nanoparticles for IBD therapy with high inflamed colon retention and therapeutic effects (Vong et al., 2012; Lamprecht, 2015; Alaarg et al., 2017; Bak et al., 2018; Gou et al., 2019; Kotla et al., 2019; Li S. et al., 2019; Schilrreff et al., 2019).
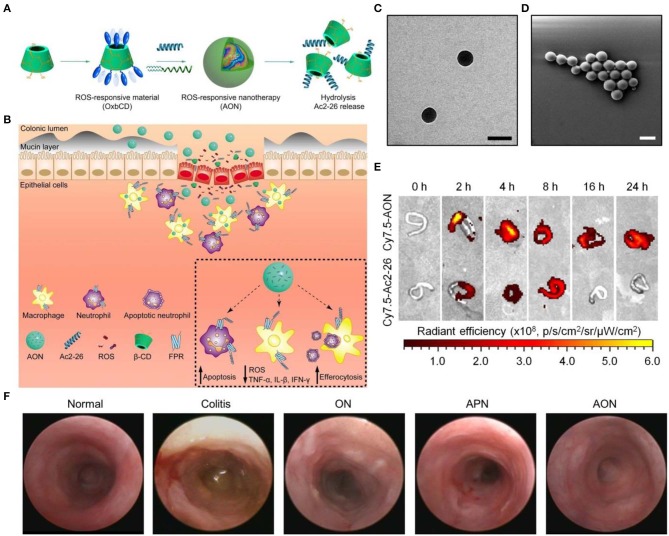
For IBD treatment by site-specific delivery of a small-molecule antioxidant Tempol, our group fabricated a ROS-responsive nanoplatform based on a PBAP-conjugated β-CD material. The resulting SOD/CAT-mimetic nanotherapy was able to efficiently accumulate in the inflamed colon in mice and notably mitigated manifestations relevant to colitis, showing remarkable efficacies in three mouse colitis models including DSS-induced acute and chronic colitis as well as TNBS-induced colitis (Zhang Q. et al., 2016). Also, we constructed affinity nanoparticles via host-guest interactions between cyclodextrin-containing hydrophilic copolymers and Tempol. This host-guest nanotherapy exhibited desirable antioxidant and anti-inflammatory effects in macrophages and in mice with DSS-induced colitis (Xue et al., 2018). Most recently, we packaged a proresolving peptide Ac2-26 into nanoparticles derived from the aforementioned ROS-responsive material (i.e., PBAP-conjugated β-CD) to obtain a multifunctional nanotherapy, defined as AON (Figure 5) (Li C. et al., 2019). AON effectively protected Ac2-26 from degradation under gastrointestinal conditions. By oral delivery of this nanotherapy to mice with DSS-induced colitis, site-specific accumulation of AON and triggerable release of Ac2-26 in response to high levels of ROS at the inflammatory colons were successfully achieved. In mice with DSS-induced acute and chronic colitis as well as in IL-10-deficient mice that spontaneously develop colitis, oral administration of AON notably attenuated manifestations related to colitis, and significantly decreased expression of proinflammatory cytokines and oxidative stress-related molecular mediators, which was more potent than free Ac2-26 or a non-responsive control nanotherapy based on PLGA. Mechanistically, AON can effectively inhibit inflammatory responses and oxidative stress in stimulated macrophages, notably attenuate neutrophil trafficking, promote efferocytosis of apoptotic neutrophils, as well as regulate phenotypic switching of macrophages in vitro. In vivo efficacies of AON are most likely achieved by accelerating intestinal mucosal wound healing, expediting the resolution of inflammation, reshaping gut microbiota, and increasing the production of short-chain fatty acids. Moreover, oral delivery of AON showed excellent safety profile in mice.
Figure 5.
A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease. (A,B) Schematic illustration of engineering of a ROS-responsive peptide nanotherapy (AON) (A) and targeted treatment of colitis (B). (C,D) TEM (C) and SEM (D) images of AON. Scale bars, 200 nm. (E) Selective accumulation of AON in the inflamed colons of mice with acute colitis. (F) Representative mini-endoscopic images of colons from colitic mice at day 7 after different treatments. ON, blank nanoparticles based on the ROS-responsive material of PBAP-conjugated β-CD (i.e., OxbCD); APN, Ac2-26-containing nanoparticles based on a non-responsive polymer PLGA. Reproduced with permission from Li C. et al. (2019).
Cardiovascular Diseases
Cardiovascular disease is a leading cause of mortality worldwide. Increasing evidence has demonstrated that excessive ROS production is closely linked to the mitochondrial dysfunction, cell apoptosis, endoplasmic reticulum stress, and autophagy, which facilitates the occurrence and development of cardiovascular disease (Brown and Griendling, 2015). The intracellular ROS have a critical role in the pathogenesis of various cardiovascular diseases, such as atherosclerosis, myocardial hypertrophy, myocardial infarction, myocardial ischemia-reperfusion injury, and heart failure (Harrison et al., 2003). ROS in the cardiovascular system are predominantly produced by activated NADPH oxidase (NOX) which is the primary oxidase system underlying oxidative stress. NOX-derived ROS production induces activation of endothelial nitric oxide synthase (eNOS), leading to eNOS uncoupling and mitochondrial dysfunction, eventually causing sustained oxidative damage and the development of cardiovascular diseases (Zhang Y. et al., 2020).
ROS-scavenging nanotherapies have demonstrated great potential in the prevention and treatment of cardiovascular disease. They can protect cardiac progenitor cells from oxidative stress (Pagliari et al., 2012), prevent ROS-induced death of implanted mesenchymal stem cells for myocardial infarction treatment (Park et al., 2015), and eliminate excessive ROS to mitigate atherosclerosis (Chmielowski et al., 2017; Wang et al., 2018). As a typical example, our previously developed TPCD nanoparticles significantly attenuated ROS-induced inflammation and cell apoptosis in macrophages, by eliminating broad-spectrum ROS in cells (Li et al., 2018; Wang et al., 2018). In addition, TPCD nanoparticles could effectively inhibit foam cell formation in macrophages and vascular smooth muscle cells (VSMCs), by decreasing cellular internalization of oxLDL. After i.v. delivery, TPCD nanoparticles accumulated in atherosclerotic lesions of apolipoprotein E-deficient (ApoE−/−) mice, which was mainly achieved by passive targeting through the dysfunctional endothelium and translocation via inflammatory cells. In ApoE−/− mice subjected to high-fat diets, treatment with TPCD nanoparticles by i.v. administration efficaciously inhibited the development of atherosclerosis and stabilized atherosclerotic plaques, resulting less cholesterol crystals, smaller necrotic cores, thicker fibrous caps, and lower levels of macrophages and matrix metalloproteinase-9, as compared to control drugs previously developed for antiatherosclerosis. These beneficial effects of TPCD nanoparticles were considered to be relevant to reduced systemic and local oxidative stress and inflammation. In view of the good safety profile of TPCD nanoparticles in preliminary in vivo tests based on different animal models (Li et al., 2018; Wang et al., 2018), this type of nanoparticles deserve further development as a potential antiatherosclerotic nanotherapy. In another study, Hao et al. developed injectable fullerenol nanoparticle-loaded alginate hydrogel, which showed excellent ROS-scavenging activity (Hao et al., 2017). This fullerenol/alginate hydrogel could serve as a cell delivery vehicle and suppress oxidative stress-mediated cell apoptosis in brown adipose-derived stem cells (BADSCs) in vitro, by attenuating JNK signaling pathways and activating p38 MAPK signaling pathways. Moreover, this functional hydrogel remarkably enhanced the retention and survival of implanted BADSCs in myocardial infarction zone via regulating the ROS microenvironment, thereby reinforcing therapeutic efficacy for cardiac repair.
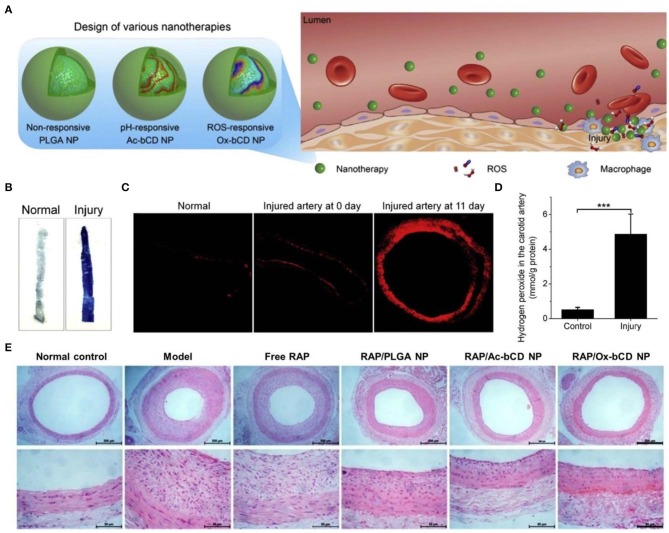
On the other hand, ROS-scavenging nanoparticles can serve as an effective nanoplatform for site-specific delivery of therapeutics to the sites of vascular inflammation (Seshadri et al., 2010; Wu et al., 2018; Zhang R. et al., 2020). As well-documented, substantially increased ROS levels are positively related to endothelial dysfunction and pathogenesis of restenosis after percutaneous coronary interventions (Juni et al., 2013). To develop targeting nanotherapies for restenosis, our group engineered ROS-responsive nanotherapies by loading rapamycin (RAP) into nanoparticles derived from a PBAP-conjugated β-CD material (Figure 6) (Feng et al., 2016). Thus, obtained RAP-containing nanoparticles exhibited ROS-triggerable drug release, showing significantly enhanced anti-migration and anti-proliferative effects as compared to free RAP and a non-responsive RAP nanotherapy. In addition, the ROS-responsive nanoparticles can accumulate at the injured site in the carotid artery of rats subjected to balloon angioplasty injury. In a rat model of arterial restenosis, treatment with the ROS-responsive RAP nanotherapy by i.v. injection more effectively attenuated neointimal hyperplasia than free RAP and a non-responsive nanotherapy. Further studies demonstrated that in vivo efficacy of the ROS-responsive RAP nanotherapy can be additionally improved by integrating with a pH-responsive β-CD material to afford a pH/ROS dual-responsive nanotherapy (Zhang R. et al., 2020). Of note, surface engineering of the dual-responsive nanoparticles via a peptide (KLWVLPKGGGC) targeting type IV collagen can notably increase their accumulation at injured carotid arteries, thereby potentiating in vivo efficacy of the dual-responsive RAP nanotherapy. Similarly, therapeutic advantages of the ROS-responsive RAP nanotherapy was demonstrated in a mouse model of atherosclerosis in ApoE−/− mice (Dou et al., 2017) and an animal model of abdominal aortic aneurysm in rats (Cheng et al., 2018). In both cases, treatment with ROS-responsive nanotherapies can significantly reduce oxidative stress in diseased aortas. Consequently, ROS-responsive nanotherapies hold great potential for precision therapy of different vascular inflammatory diseases.
Figure 6.
Engineering of different inflammation-responsive nanotherapies for targeted treatment of restenosis. (A) Design of inflammation-triggerable nanoparticles. (B) Evans Blue staining indicates successful establishment of injury in the carotid artery. (C) DHE-stained cryosections showing oxidative stress in injured carotid arteries. The image at day 0 was acquired from the sample immediately collected after injury. (D) The levels of hydrogen peroxide in the carotid artery with or without injury. (E) H&E stained histological sections of carotid arteries isolated from rats subjected to various treatments. Scale bars, 200 μm (the upper panel), 50 μm (the lower panel). PLGA NP, Ac-bCD NP, and Ox-bCD NP represents nanoparticles based on a non-responsive polymer PLGA, a pH-responsive material of acetelated β-CD, and a ROS-responsive material of PBAP-conjugated β-CD, respectively. Data are mean ± standard deviation (n = 3). ***P < 0.001. Reproduced with permission from Feng et al. (2016).
Brain Diseases
Brain is an extremely metabolically active organ with low levels of antioxidant enzymes and high levels of redox-active substrates (e.g., Cu and Fe), and therefore it is especially vulnerable to oxidative stress. A growing body of evidence has substantiated that the excessive production of ROS plays a critical role in a common pathophysiology of brain diseases, such as cerebral infarction, Alzheimer's disease (AD), and Parkinson's disease (PD) (Barnham et al., 2004; Chen et al., 2011; Kim G. H. et al., 2015). Conventional antioxidant therapies cannot effectively inhibit ROS-amplified brain injury for their limited ability to cross the BBB and target the disease sites.
Antioxidant nanotherapies have been emerging as an effective strategy to overcome the BBB and to provide targeted release of different therapeutics in the impaired brain (Hu et al., 2015; Kwon et al., 2016, 2018; Liu et al., 2017). For example, Liu et al. fabricated PEG-coated melanin nanoparticles MeNPs (Liu et al., 2017). Neuroprotective and anti-inflammatory activities of MeNPs were evaluated by in vitro studies in Neuro 2A cells. It was found that MeNPs could decrease oxidative stress damage and inhibit CoCl2-induced ischemic injury, without notable effects on mitochondrial function. Further in vivo studies in a rat model of ischemic stroke indicated that rats pretreated with MeNPs showed less infarct area in the ischemic brain (~14%) compared with the control group (~32%). Also, MeNPs efficiently suppressed the generation of • in brains. Consequently, MeNPs could attenuate RONS-induced inflammatory responses through suppressing the expression of typical mediators related to oxidative stress and inflammation in vitro and in vivo.
In addition, ROS-scavenging inorganic nanoparticles were used for treatment of neurodegenerative diseases. In this aspect, Kwon and coworkers constructed triphenylphosphonium (TPP)-conjugated CeO2 nanoparticles, for targeting the mitochondria and potential therapy of AD (Kwon et al., 2016). Such nanoparticles significantly mitigated reactive gliosis and mitochondrial morphological damage in an AD model of 5XFAD transgenic mice. In a recent study, the same group designed three different types of ceria nanoparticles, i.e., ceria, TPP-ceria, and cluster-ceria nanoparticles, to selectively scavenge intracellular, mitochondrial, and extracellular ROS, respectively (Kwon et al., 2018). Therapeutic effects of three ceria nanoparticles were compared in mice with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD. Mice treated with either ceria nanoparticles or TPP-ceria nanoparticles exhibited significantly higher levels of tyrosine hydroxylase (a hallmark of PD) and lower levels of lipid peroxidation, compared to those treated with cluster-ceria nanoparticles. This finding indicated that decreasing mitochondrial and/or intracellular oxidative stress is critical to treat PD, while elimination of extracellular ROS is not an effective strategy to prevent neurodegeneration.
Other Inflammatory Diseases
Asthma is a chronic pulmonary disease, characterized by recurrent airflow limitation, airway remodeling, and airway hyperreactivity. Inhaling corticosteroids is the most common treatment for asthma. However, adverse effects associated with the frequent steroid administration precipitate the need for alternative therapeutics or novel delivery routes (Vij, 2011; Lim et al., 2016; Wang L. et al., 2019). Oxidative stress and inflammation play an important role in the pathogenesis of asthma. Growing evidence has indicated that nanoparticle-based anti-inflammation and antioxidant strategies are promising for the treatment of allergic airway inflammation and asthma (Fatani, 2014; Sahiner et al., 2018; Alexescu et al., 2019; Singh A. P. et al., 2019). The anti-asthmatic effects of two organic nanoparticles, i.e., BRNPs and HPOX nanoparticles with intrinsic antioxidant activity were evaluated using a murine model of asthma (Yoo et al., 2013; Kim D. E. et al., 2017). Both of them could reduce the recruitment of inflammatory cells and expression of inflammatory cytokines, thus may be used as potential nanotherapies for asthma. On the other hand, corticosteroids encapsulated in organic nanoparticles afforded more sustained therapeutic effects than free drugs. For example, a biodegradable polymer PVAX was modified to prepare dexamethasone (DEX)-loaded porous PVAX microparticles by a double emulsion method (Jeong et al., 2016). PVAX microparticles themselves remarkably reduced oxidative stress and down-regulated the expression of proinflammatory mediators such as TNF-α and iNOS. Notably, therapeutic effects of DEX-loaded porous PVAX microparticles were much better than PVAX microparticles alone, indicating a significant synergistic effect.
Acute lung injury (ALI), a heterogeneous pulmonary disease with the severe manifestation of acute respiratory distress syndrome, continues to cause high morbidity and mortality in critically ill patients (Rubenfeld et al., 2005; Sadikot et al., 2017). ALI is closely related to the systemic inflammatory response and the increased cellular ROS (Chabot et al., 1998). Cerium oxide nanoparticles were found able to reduce oxidative stress in vitro and in vivo (Arvapalli et al., 2015; Xu et al., 2016). They also exhibited protective effects against sepsis-induced ALI and radiation-induced lung injury. In mice with paraquat-induced ALI, the levels of ROS, malondialdehyde, NF-κB, phosphorylated NF-κB, TNF-α, and IL-1β were significantly reduced by porous Se@SiO2 nanospheres that contain Se to scavenge intracellular free radicals (Zhu et al., 2017). Also, resveratrol-loaded lipid-core nanocapsules (RSV-LNCs) were studied to ameliorate ALI via the ERK and PI3K/Akt pathways (de Oliveira et al., 2019). In addition, intrinsically bioactive nanoparticles derived from functional cyclodextrin materials TPCD and LCD that were developed by our group, were able to accumulate in the injured lungs, suppress oxidative stress, and significantly reduce the infiltration of inflammatory cells in a mouse model of ALI (Figures 3C–H) (Li et al., 2018; Guo et al., 2019). Consequently, both inorganic and organic ROS-scavenging nanoparticles can serve as a potent remedy for the treatment of ALI.
Recently, different antioxidant nanotherapies have been applied in the treatment of peritonitis, an inflammation of the peritoneum. Porfire and coauthors evaluated efficacy of three different Cu/Zn-SOD formulations in LPS-induced peritonitis (Porfire et al., 2014). PEGylated liposomes showed the most significant antioxidant and anti-inflammatory properties in this case. Ac2-26-containing nanoparticles, developed using an anti-inflammatory peptide Ac2-26 as well as diblock copolymers PLGA-PEG and collagen IV-targeting PLGA-PEG, could significantly inhibit the recruitment of polymononuclear neutrophils in a zymosan-induced peritonitis model (Kamaly et al., 2013). Our group developed H2O2-eliminating nanoparticles, based on PBAP-conjugated β-CD materials, also displayed desirable therapeutic effects in mice with peritonitis, by reducing ROS production, inhibiting neutrophil infiltration and neutrophil-induced macrophage recruitment, as well as down-regulating the expression of inflammatory cytokines and chemokines (Zhang et al., 2017).
Concluding Remarks
Oxidative stress is at the basis of a variety of inflammatory pathologies, and therefore antioxidant nanotherapies have been extensively investigated as a new therapeutic strategy. Significant advances have been achieved in the field of antioxidant nanotherapies for different inflammatory diseases, in which ROS-scavenging inorganic nanoparticles, organic nanoparticles with intrinsic antioxidant activity, and drug-loaded nanoparticles with antioxidant activity are generally used. Indeed, extensive preclinical studies have demonstrated desirable performances of ROS-scavenging nanotherapies in different in vitro and in vivo inflammatory models. However, for the currently developed antioxidant nanotherapies, only a few of them have been intensively and systemically examined in diverse animal models. Even few translation studies have been conducted for antioxidant nanotherapies thus far.
There are still some critical challenges need to be addressed for further clinical translation of these anti-inflammatory nanotherapies. First, synthesis processes for currently developed antioxidant nanoparticles need to be optimized to produce nanotherapies with good quality control, such as highly defined structures and physicochemical characters as well as good batch-to-batch reproducibility, from the view point of preparation. The cost-efficient mass production is also a critical factor that should be carefully considered according to the benefits of different antioxidant nanoparticles and their potential applications in clinical practice. Second, both acute and long-term chronic toxicities of various antioxidant nanotherapies must be comprehensively tested, particularly for ROS-scavenging inorganic nanoparticles. The physicochemical and biological properties of nanoparticles greatly influence their interactions with the living organisms. The developed antioxidant nanotherapies or nanocarriers should be biocompatible and easy to clear by the body. Thirdly, the ROS level varies among different inflammatory disorders as well as throughout the different stages of the same inflammatory disease, which largely decide the dose and dosing frequency of antioxidant nanotherapies. The appropriate dose should control the intracellular ROS toward the beneficial therapeutic effects without causing pathological ones. These issues unfortunately remain elusive. Also, a thorough assessment of risks and benefits of antioxidative nanoparticles is a main ethical issue. The ideal antioxidative nanoparticles should maximize the well-being of patients and reduce or avoid therapy-induced side effects, thereby affording patients with the benefits outweighing the risks. With the aforementioned challenges to be resolved, we are confident that the clinical applications of antioxidant nanotherapies for the treatment of inflammatory diseases will be realize in the foreseeable future, resulting in improved safety and individualized healthcare.
Author Contributions
C-WL and L-LL drafted the manuscript. C-WL, L-LL, and SC created the tables and figures and performed literature searches. J-XZ and W-LL revised the manuscript and edited the final draft.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the National Natural Science Foundation of China (Nos. 81971727 and 81901866) and the Program for Distinguished Young Scholars of TMMU.
References
- Alaarg A., Perez-Medina C., Metselaar J. M., Nahrendorf M., Fayad Z. A., Storm G., et al. (2017). Applying nanomedicine in maladaptive inflammation and angiogenesis. Adv. Drug Deliv. Rev. 119, 143–158. 10.1016/j.addr.2017.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexescu T., Tarmure S., Negrean V., Cosnarovici M., Ruta V., Popovici I., et al. (2019). Nanoparticles in the treatment of chronic lung diseases. J. Mind Med. Sci. 6, 224–231. 10.22543/7674.62.P224231 [DOI] [Google Scholar]
- Ali S. S., Hardt J. I., Dugan L. L. (2008). Sod activity of carboxyfullerenes predicts their neuroprotective efficacy: a structure-activity study. Nanomed. Nanotechnol. 4, 283–294. 10.1016/j.nano.2008.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen J. K. (2004). Oxidative stress in neurodegeneration: Cause or consequence? Nat. Med. 10, S18–S25. 10.1038/nrn1434 [DOI] [PubMed] [Google Scholar]
- Apostolova N., Victor V. M. (2015). Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid. Redox Signal. 22, 686–729. 10.1089/ars.2014.5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvapalli R., Manne N., Rice K., Blough E. (2015). Therapeutic efficacy of cerium oxide nanoparticles against sepsis induced acute lung injury. FASEB J. 29, 620–614. [Google Scholar]
- Bak A., Ashford M., Brayden D. J. (2018). Local delivery of macromolecules to treat diseases associated with the colon. Adv. Drug Deliv. Rev. 136–137, 2–27. 10.1016/j.addr.2018.10.009 [DOI] [PubMed] [Google Scholar]
- Bao X., Zhao J., Sun J., Hu M., Yang X. (2018). Polydopamine nanoparticles as efficient scavengers for reactive oxygen species in periodontal disease. ACS Nano 12, 8882–8892. 10.1021/acsnano.8b04022 [DOI] [PubMed] [Google Scholar]
- Barnham K. J., Masters C. L., Bush A. I. (2004). Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3, 205–214. 10.1038/nrd1330 [DOI] [PubMed] [Google Scholar]
- Bayir H. (2006). Reactive oxygen species. Crit. Care Med. 33, S498–S501. 10.1097/01.CCM.0000186787.64500.12 [DOI] [PubMed] [Google Scholar]
- Berwin Singh S. V., Adam A. G., Tripathy N., Lee D., Khang G. (2018). Reactive oxygen species responsive naturally occurring phenolic-based polymeric prodrug. Adv. Exp. Med. Biol. 1078, 291–301. 10.1007/978-981-13-0950-2_15 [DOI] [PubMed] [Google Scholar]
- Bhatia S. K., Wadhwa P., Hong J. W., Hong Y. G., Jeon J. M., Lee E. S., et al. (2019). Lipase mediated functionalization of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) with ascorbic acid into an antioxidant active biomaterial. Int. J. Biol. Macromol. 123, 117–123. 10.1016/j.ijbiomac.2018.11.052 [DOI] [PubMed] [Google Scholar]
- Brown D. I., Griendling K. K. (2015). Regulation of signal transduction by reactive oxygen species in the cardiovascular system. Circ. Res. 116, 531–549. 10.1161/CIRCRESAHA.116.303584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant N., Johnson M., Walker M., Riley K., Sims C. (2016). Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 5:E15 10.3390/antiox5020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X., Sezate S. A., Seal S., McGinnis J. F. (2012). Sustained protection against photoreceptor degeneration in tubby mice by intravitreal injection of nanoceria. Biomaterials 33, 8771–8781. 10.1016/j.biomaterials.2012.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G.-J., Jiang X., Zhang H., Zheng J., Croley T. R., Yin J.-J. (2017). Exploring the activities of ruthenium nanomaterials as reactive oxygen species scavengers. J. Environ. Sci. Health C 35, 223–238. 10.1080/10590501.2017.1391516 [DOI] [PubMed] [Google Scholar]
- Celardo I., Pedersen J. Z., Traversa E., Ghibelli L. (2011). Pharmacological potential of cerium oxide nanoparticles. Nanoscale 3, 1411–1420. 10.1039/c0nr00875c [DOI] [PubMed] [Google Scholar]
- Chabot F., Mitchell J. A., Gutteridge J. M., Evans T. W. (1998). Reactive oxygen species in acute lung injury. Eur. Respir. J. 11, 745–757. [PubMed] [Google Scholar]
- Chen H., Yoshioka H., Kim G. S., Jung J. E., Okami N., Sakata H., et al. (2011). Oxidative stress in ischemic brain damage: Mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid. Redox Signal. 14, 1505–1517. 10.1089/ars.2010.3576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Wang Q., Huang L., Zhang H., Rong K., Zhang H., et al. (2018). Prussian blue with intrinsic heme-like structure as peroxidase mimic. Nano Res. 11, 4905–4913. 10.1007/s12274-018-2079-8 [DOI] [Google Scholar]
- Chen Y.-P., Chen C.-T., Hung Y., Chou C.-M., Liu T.-P., Liang M.-R., et al. (2013). A new strategy for intracellular delivery of enzyme using mesoporous silica nanoparticles: Superoxide dismutase. J. Am. Chem. Soc. 135, 1516–1523. 10.1021/ja3105208 [DOI] [PubMed] [Google Scholar]
- Cheng J., Zhang R., Li C., Tao H., Dou Y., Wang Y., et al. (2018). A targeting nanotherapy for abdominal aortic aneurysms. J. Am. Coll. Cardiol. 72, 2591–2605. 10.1016/j.jacc.2018.08.2188 [DOI] [PubMed] [Google Scholar]
- Chmielowski R. A., Abdelhamid D. S., Faig J. J., Petersen L. K., Gardner C. R., Uhrich K. E., et al. (2017). Athero-inflammatory nanotherapeutics: Ferulic acid-based poly(anhydride-ester) nanoparticles attenuate foam cell formation by regulating macrophage lipogenesis and reactive oxygen species generation. Acta Biomater. 57, 85–94. 10.1016/j.actbio.2017.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho B. R., Ryu D. R., Lee K. S., Lee D. K., Bae S., Kang D. G., et al. (2015). P-hydroxybenzyl alcohol-containing biodegradable nanoparticle improves functional blood flow through angiogenesis in a mouse model of hindlimb ischemia. Biomaterials 53, 679–687. 10.1016/j.biomaterials.2015.02.107 [DOI] [PubMed] [Google Scholar]
- Cho S., Hwang O., Lee I., Lee G., Yoo D., Khang G., et al. (2012). Chemiluminescent and antioxidant micelles as theranostic agents for hydrogen peroxide associated-inflammatory diseases. Adv. Funct. Mater. 22, 4038–4043. 10.1002/adfm.201200773 [DOI] [Google Scholar]
- Chonpathompikunlert P., Fan C.-H., Ozaki Y., Yoshitomi T., Yeh C.-K., Nagasaki Y. (2012). Redox nanoparticle treatment protects against neurological deficit in focused ultrasound-induced intracerebral hemorrhage. Nanomedicine 7, 1029–1043. 10.2217/nnm.12.2 [DOI] [PubMed] [Google Scholar]
- Chu M., Hai W., Zhang Z., Wo F., Wu Q., Zhang Z., et al. (2016). Melanin nanoparticles derived from a homology of medicine and food for sentinel lymph node mapping and photothermal in vivo cancer therapy. Biomaterials 91, 182–199. 10.1016/j.biomaterials.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Das S., Dowding J. M., Klump K. E., McGinnis J. F., Self W., Seal S. (2013). Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine 8, 1483–1508. 10.2217/nnm.13.133 [DOI] [PubMed] [Google Scholar]
- D'Autréaux B., Toledano M. B. (2007). ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 8, 813–824. 10.1038/nrm2256 [DOI] [PubMed] [Google Scholar]
- Davan R., Prasad R. G. S. V., Jakka V. S., Aparna R. S. L., Phani A. R., Jacob B., et al. (2012). Cerium oxide nanoparticles promotes wound healing activity in in-vivo animal model. J. Bionanosci. 6, 78–83. 10.1166/jbns.2012.1074 [DOI] [Google Scholar]
- de Oliveira M. T. P., de Sa Coutinho D., Tenorio de Souza E., Staniscuaski Guterres S., Pohlmann A. R., Silva P. M. R., et al. (2019). Orally delivered resveratrol-loaded lipid-core nanocapsules ameliorate lps-induced acute lung injury via the erk and pi3k/akt pathways. Int. J. Nanomed. 14, 5215–5228. 10.2147/IJN.S200666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Y., Chen Y., Zhang X., Xu X., Chen Y., Guo J., et al. (2017). Non-proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials 143, 93–108. 10.1016/j.biomaterials.2017.07.035 [DOI] [PubMed] [Google Scholar]
- Dugan L. L., Turetsky D. M., Du C., Lobner D., Wheeler M., Almli C. R., et al. (1997). Carboxyfullerenes as neuroprotective agents. Proc. Natl. Acad. Sci. U.S.A. 94, 9434–9439. 10.1073/pnas.94.17.9434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edaravone Acute Infarction Study Group (2003). Effect of a novel free radical scavenger, edaravone (mci-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc. Dis. 15, 222–229. 10.1159/000069318 [DOI] [PubMed] [Google Scholar]
- Eguchi A., Yoshitomi T., Lazic M., Johnson C. D., Vong L. B., Wree A., et al. (2015). Redox nanoparticles as a novel treatment approach for inflammation and fibrosis associated with nonalcoholic steatohepatitis. Nanomedicine 10, 2697–2708. 10.2217/nnm.15.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatani S. H. (2014). Biomarkers of oxidative stress in acute and chronic bronchial asthma. J. Asthma 51, 578–584. 10.3109/02770903.2014.892965 [DOI] [PubMed] [Google Scholar]
- Feliciano C. P., Tsuboi K., Suzuki K., Kimura H., Nagasaki Y. (2017). Long-term bioavailability of redox nanoparticles effectively reduces organ dysfunctions and death in whole-body irradiated mice. Biomaterials 129, 68–82. 10.1016/j.biomaterials.2017.03.011 [DOI] [PubMed] [Google Scholar]
- Feng S., Hu Y., Peng S., Han S., Tao H., Zhang Q., et al. (2016). Nanoparticles responsive to the inflammatory microenvironment for targeted treatment of arterial restenosis. Biomaterials 105, 167–184. 10.1016/j.biomaterials.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Ferreira C. A., Ni D., Rosenkrans Z. T., Cai W. (2018). Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 11, 4955–4984. 10.1007/s12274-018-2092-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraisl P., Aragonés J., Carmeliet P. (2009). Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat. Rev. Drug Discov. 8, 139–152. 10.1038/nrd2761 [DOI] [PubMed] [Google Scholar]
- Francesca G., Moglianetti M., Pompa P., Bardi G. (2018). Platinum nanoparticles decrease reactive oxygen species and modulate gene expression without alteration of immune responses in thp-1 monocytes. Nanomaterials 8:392 10.3390/nano8060392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L., Zhuang J., Nie L., Zhang J., Zhang Y., Gu N., et al. (2007). Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2, 577–583. 10.1038/nnano.2007.260 [DOI] [PubMed] [Google Scholar]
- Gharbi N., Pressac M., Hadchouel M., Szwarc H., Wilson S. R., Moussa F. (2005). [60]fullerene is a powerful antioxidant in vivo with no acute or subacute toxicity. Nano Lett. 5, 2578–2585. 10.1021/nl051866b [DOI] [PubMed] [Google Scholar]
- Gordon R., Podolski I., Makarova E., Deev A., Mugantseva E., Khutsyan S., et al. (2017). Intrahippocampal pathways involved in learning/memory mechanisms are affected by intracerebral infusions of amyloid-β25-35 peptide and hydrated fullerene c60 in rats. J. Alzheimers. Dis. 58, 711–724. 10.3233/JAD-161182 [DOI] [PubMed] [Google Scholar]
- Gou S., Huang Y., Wan Y., Ma Y., Zhou X., Tong X., et al. (2019). Multi-bioresponsive silk fibroin-based nanoparticles with on-demand cytoplasmic drug release capacity for CD44-targeted alleviation of ulcerative colitis. Biomaterials 212, 39–54. 10.1016/j.biomaterials.2019.05.012 [DOI] [PubMed] [Google Scholar]
- Guo J., Li D., Tao H., Li G., Liu R., Dou Y., et al. (2019). Cyclodextrin–derived intrinsically bioactive nanoparticles for treatment of acute and chronic inflammatory diseases. Adv. Mater. 31:e1904607. 10.1002/adma.201904607 [DOI] [PubMed] [Google Scholar]
- Hao T., Li J., Yao F., Dong D., Wang Y., Yang B., et al. (2017). Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano 11, 5474–5488. 10.1021/acsnano.7b00221 [DOI] [PubMed] [Google Scholar]
- Harrison D., Griendling K. K., Landmesser U., Hornig B., Drexler H. (2003). Role of oxidative stress in atherosclerosis. Am. J. Cardiol. 91, 7–11. 10.1016/S0002-9149(02)03144-2 [DOI] [PubMed] [Google Scholar]
- He Y., Zhang B., Chen Y., Jin Q., Wu J., Yan F., et al. (2017). Image-guided hydrogen gas delivery for protection from myocardial ischemia-reperfusion injury via microbubbles. ACS Appl. Mater. Interfaces 9, 21190–21199. 10.1021/acsami.7b05346 [DOI] [PubMed] [Google Scholar]
- Heckman K. L., DeCoteau W., Estevez A., Reed K. J., Costanzo W., Sanford D., et al. (2013). Custom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brain. ACS Nano 7, 10582–10596. 10.1021/nn403743b [DOI] [PubMed] [Google Scholar]
- Hikosaka K., Kim J., Kajita M., Kanayama A., Miyamoto Y. (2008). Platinum nanoparticles have an activity similar to mitochondrial nadh:Ubiquinone oxidoreductase. Colloids Surf. B Biointerfaces 66, 195–200. 10.1016/j.colsurfb.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Hirst S. M., Karakoti A. S., Tyler R. D., Sriranganathan N., Seal S., Reilly C. M. (2009). Anti-inflammatory properties of cerium oxide nanoparticles. Small 5, 2848–2856. 10.1002/smll.200901048 [DOI] [PubMed] [Google Scholar]
- Hu B., Dai F., Fan Z., Ma G., Tang Q., Zhang X. (2015). Nanotheranostics: Congo red/rutin-mnps with enhanced magnetic resonance imaging and h2o2-responsive therapy of alzheimer's disease in appswe/ps1de9 transgenic mice. Adv. Mater. 27, 5499–5505. 10.1002/adma.201502227 [DOI] [PubMed] [Google Scholar]
- Huang Y., Liu Z., Liu C., Ju E., Zhang Y., Ren J., et al. (2016). Self-assembly of multi-nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. Int. Ed. 55, 6646–6650. 10.1002/anie.201600868 [DOI] [PubMed] [Google Scholar]
- Ikeda Y., Nagasaki Y. (2018). Antioxidative biointerface: Biocompatible materials scavenging reactive oxygen species. Biomed. Mater. 13:044103. 10.1088/1748-605X/aab720 [DOI] [PubMed] [Google Scholar]
- Jeong D., Kang C., Jung E., Yoo D., Wu D., Lee D. (2016). Porous antioxidant polymer microparticles as therapeutic systems for the airway inflammatory diseases. J. Control. Release 233, 72–80. 10.1016/j.jconrel.2016.04.039 [DOI] [PubMed] [Google Scholar]
- Ju K. Y., Lee Y., Lee S., Park S. B., Lee J. K. (2011). Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 12, 625–632. 10.1021/bm101281b [DOI] [PubMed] [Google Scholar]
- Jung E., Lee J., Jeong L., Park S., Lee M., Song C., et al. (2019). Stimulus-activatable echogenic maltodextrin nanoparticles as nanotheranostic agents for peripheral arterial disease. Biomaterials 192, 282–291. 10.1016/j.biomaterials.2018.11.022 [DOI] [PubMed] [Google Scholar]
- Jung E., Noh J., Kang C., Yoo D., Song C., Lee D. (2018). Ultrasound imaging and on-demand therapy of peripheral arterial diseases using H2O2-activated bubble generating anti-inflammatory polymer particles. Biomaterials 179, 175–185. 10.1016/j.biomaterials.2018.07.003 [DOI] [PubMed] [Google Scholar]
- Juni R. P., Duckers H. J., Vanhoutte P. M., Virmani R., Moens A. L. (2013). Oxidative stress and pathological changes after coronary artery interventions. J. Am. Coll. Cardiol. 61, 1471–1481. 10.1016/j.jacc.2012.11.068 [DOI] [PubMed] [Google Scholar]
- Kamaly N., Fredman G., Subramanian M., Gadde S., Pesic A., Cheung L., et al. (2013). Development and in vivo efficacy of targeted polymeric inflammation-resolving nanoparticles. Proc. Natl. Acad. Sci. U.S.A. 110, 6506–6511. 10.1073/pnas.1303377110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C., Cho W., Park M., Kim J., Park S., Shin D., et al. (2016). H2O2-triggered bubble generating antioxidant polymeric nanoparticles as ischemia/reperfusion targeted nanotheranostics. Biomaterials 85, 195–203. 10.1016/j.biomaterials.2016.01.070 [DOI] [PubMed] [Google Scholar]
- Karin M., Clevers H. (2016). Reparative inflammation takes charge of tissue regeneration. Nature 529, 307–315. 10.1038/nature17039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. E., Lee Y., Kim M., Lee S., Jon S., Lee S. H. (2017). Bilirubin nanoparticles ameliorate allergic lung inflammation in a mouse model of asthma. Biomaterials 140, 37–44. 10.1016/j.biomaterials.2017.06.014 [DOI] [PubMed] [Google Scholar]
- Kim G. H., Kim J. E., Rhie S. J., Yoon S. (2015). The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 24, 325–340. 10.5607/en.2015.24.4.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Takuji S., Yusei M. (2010). The effect of tat conjugated platinum nanoparticles on lifespan in a nematode caenorhabditis elegans model. Biomaterials 31, 5849–5854. 10.1016/j.biomaterials.2010.03.077 [DOI] [PubMed] [Google Scholar]
- Kim J. Y., Lee D. Y., Kang S., Miao W., Kim H., Lee Y., et al. (2017). Bilirubin nanoparticle preconditioning protects against hepatic ischemia-reperfusion injury. Biomaterials 133, 1–10. 10.1016/j.biomaterials.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Kim K. S., Lee D., Song C. G., Kang P. M. (2015). Reactive oxygen species-activated nanomaterials as theranostic agents. Nanomedicine 10, 2709–2723. 10.2217/nnm.15.108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M. J., Lee Y., Jon S., Lee D. Y. (2017). Pegylated bilirubin nanoparticle as an anti-oxidative and anti-inflammatory demulcent in pancreatic islet xenotransplantation. Biomaterials 133, 242–252. 10.1016/j.biomaterials.2017.04.029 [DOI] [PubMed] [Google Scholar]
- Ko E., Jeong D., Kim J., Park S., Khang G., Lee D. (2014). Antioxidant polymeric prodrug microparticles as a therapeutic system for acute liver failure. Biomaterials 35, 3895–3902. 10.1016/j.biomaterials.2014.01.048 [DOI] [PubMed] [Google Scholar]
- Kojima R., Bojar D., Rizzi G., Hamri G. C.-E., El-Baba M. D., Saxena P., et al. (2018). Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for parkinson's disease treatment. Nat. Commun. 9:1305. 10.1038/s41467-018-03733-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong L., Cai X., Zhou X., Wong L. L., Karakoti A. S., Seal S., et al. (2011). Nanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathways. Neurobiol. Dis. 42, 514–523. 10.1016/j.nbd.2011.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotla N. G., Rana S., Sivaraman G., Sunnapu O., Vemula P. K., Pandit A., et al. (2019). Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 146, 248–266. 10.1016/j.addr.2018.06.021 [DOI] [PubMed] [Google Scholar]
- Kozower B. D., Christofidou-Solomidou M., Sweitzer T. D., Muro S., Buerk D. G., Solomides C. C., et al. (2003). Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 21, 392–398. 10.1038/nbt806 [DOI] [PubMed] [Google Scholar]
- Krusic P. J., Wasserman E., Keizer P. N., Morton J. R., Preston K. F. (1991). Radical reactions of C60. Science 254, 1183–1185. 10.1126/science.254.5035.1183 [DOI] [PubMed] [Google Scholar]
- Kwon H. J., Cha M.-Y., Kim D., Kim D. K., Soh M., Shin K., et al. (2016). Mitochondria-targeting ceria nanoparticles as antioxidants for alzheimer's disease. ACS Nano 10, 2860–2870. 10.1021/acsnano.5b08045 [DOI] [PubMed] [Google Scholar]
- Kwon H. J., Kim D., Seo K., Kim Y. G., Han S. I., Kang T., et al. (2018). Ceria nanoparticle systems for selective scavenging of mitochondrial, intracellular, and extracellular reactive oxygen species in parkinson's disease. Angew. Chem. Int. Ed. 130, 9552–9556. 10.1002/ange.201805052 [DOI] [PubMed] [Google Scholar]
- Kwon S., Ko H., You D. G., Kataoka K., Park J. H. (2019). Nanomedicines for reactive oxygen species mediated approach: An emerging paradigm for cancer treatment. Acc. Chem. Res. 52, 1771–1782. 10.1021/acs.accounts.9b00136 [DOI] [PubMed] [Google Scholar]
- Lamprecht A. (2015). Nanomedicines in gastroenterology and hepatology. Nat. Rev. Gastroenterol. Hepatol. 12, 195–204. 10.1038/nrgastro.2015.37 [DOI] [PubMed] [Google Scholar]
- Lao F., Chen L., Li W., Ge C., Qu Y., Sun Q., et al. (2009). Fullerene nanoparticles selectively enter oxidation-damaged cerebral microvessel endothelial cells and inhibit jnk-related apoptosis. ACS Nano 3, 3358–3368. 10.1021/nn900912n [DOI] [PubMed] [Google Scholar]
- Larrañaga A., Isa I. L. M., Patil V., Thamboo S., Lomora M., Fernández-Yague M. A., et al. (2018). Antioxidant functionalized polymer capsules to prevent oxidative stress. Acta Biomater. 67, 21–31. 10.1016/j.actbio.2017.12.014 [DOI] [PubMed] [Google Scholar]
- Lee D. Y., Kim J. Y., Lee Y., Lee S., Miao W., Kim H. S., et al. (2017). Black pigment gallstone inspired platinum-chelated bilirubin nanoparticles for combined photoacoustic imaging and photothermal therapy of cancers. Angew. Chem. Int. Ed. 56, 13684–13688. 10.1002/anie.201707137 [DOI] [PubMed] [Google Scholar]
- Lee Y., Kim H., Kang S., Lee J., Park J., Jon S. (2016a). Bilirubin nanoparticles as a nanomedicine for anti-inflammation therapy. Angew. Chem. Int. Ed. 55, 7460–7463. 10.1002/anie.201602525 [DOI] [PubMed] [Google Scholar]
- Lee Y., Lee S., Jon S. (2018). Biotinylated bilirubin nanoparticles as a tumor microenvironment–responsive drug delivery system for targeted cancer therapy. Adv. Sci. 5:1800017. 10.1002/advs.201800017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Lee S., Lee D. Y., Yu B., Miao W., Jon S. (2016b). Multistimuli-responsive bilirubin nanoparticles for anticancer therapy. Angew. Chem. Int. Ed. 55, 10676–10680. 10.1002/anie.201604858 [DOI] [PubMed] [Google Scholar]
- Lee Y., Sugihara K., Gillilland M. G., Jon S., Kamada N., Moon J. J. (2020). Hyaluronic acid–bilirubin nanomedicine for targeted modulation of dysregulated intestinal barrier, microbiome and immune responses in colitis. Nat. Mater. 19, 118–126. 10.1038/s41563-019-0462-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Zhao Y., Cheng J., Guo J., Zhang Q., Zhang X., et al. (2019). A proresolving peptide nanotherapy for site-specific treatment of inflammatory bowel disease by regulating proinflammatory microenvironment and gut microbiota. Adv. Sci. 6:1900610. 10.1002/advs.201900610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Guan M., Wang T., Zhen M., Zhao F., Shu C., et al. (2016). Gd@C82-(ethylenediamine) 8 nanoparticle: A new high-efficiency water-soluble ros scavenger. ACS Appl. Mater. Interfaces 8, 25770–25776. 10.1021/acsami.6b08659 [DOI] [PubMed] [Google Scholar]
- Li L., Guo J., Wang Y., Xiong X., Tao H., Li J., et al. (2018). A broad-spectrum ros-eliminating material for prevention of inflammation and drug-induced organ toxicity. Adv. Sci. 5:1800781. 10.1002/advs.201800781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Xie A., Li H., Zou X., Zhang Q. (2019). A self-assembled, ros-responsive janus-prodrug for targeted therapy of inflammatory bowel disease. J. Control. Release 316, 66–78. 10.1016/j.jconrel.2019.10.054 [DOI] [PubMed] [Google Scholar]
- Li Y., He X., Yin J.-J., Ma Y., Zhang P., Li J., et al. (2015). Acquired superoxide-scavenging ability of ceria nanoparticles. Angew. Chem. Int. Ed. 54, 1832–1835. 10.1002/anie.201410398 [DOI] [PubMed] [Google Scholar]
- Lim Y. H., Tiemann K. M., Hunstad D. A., Elsabahy M., Wooley K. L. (2016). Polymeric nanoparticles in development for treatment of pulmonary infectious diseases. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 8, 842–871. 10.1002/wnan.1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. Y., Su-Feng F., Shin-Zong L., Cheng-Kong C., Tieng-Yau L., Low-Tone H. (2002). Local carboxyfullerene protects cortical infarction in rat brain. Neurosci. Res. 43, 317–321. 10.1016/S0168-0102(02)00056-1 [DOI] [PubMed] [Google Scholar]
- Liu Y., Ai K., Ji X., Askhatova D., Du R., Lu L., et al. (2017). Comprehensive insights into the multi-antioxidative mechanisms of melanin nanoparticles and their application to protect brain from injury in ischemic stroke. J. Am. Chem. Soc. 139, 856–862. 10.1021/jacs.6b11013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Aimetti A. A., Langer R., Gu Z. (2016). Bioresponsive materials. Nat. Rev. Mater. 2, 1–17. 10.1038/natrevmats.2016.75 [DOI] [Google Scholar]
- Markovic Z., Trajkovic V. (2008). Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 29, 3561–3573. 10.1016/j.biomaterials.2008.05.005 [DOI] [PubMed] [Google Scholar]
- Martín R., Menchon C., Apostolova N., Victor V. M., Alvaro M., Herance J. R., et al. (2010). Nano-jewels in biology. Gold and platinum on diamond nanoparticles as antioxidant systems against cellular oxidative stress. ACS Nano 4, 6957–6965. 10.1021/nn1019412 [DOI] [PubMed] [Google Scholar]
- Miriyala S., Spasojevic I., Tovmasyan A., Salvemini D., Vujaskovic Z., St. Clair D., et al. (2012). Manganese superoxide dismutase, MnSOD and its mimics. Biochim. Biophys. Acta 1822, 794–814. 10.1016/j.bbadis.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moglianetti M., Luca E. D., Pedone D., Marotta R., Pompa P. P. (2016). Platinum nanozymes recover cellular ros homeostasis in oxidative stress-mediated disease model. Nanoscale 8, 3739–3752. 10.1039/C5NR08358C [DOI] [PubMed] [Google Scholar]
- Moura F. A., de Andrade K. Q., Dos Santos J. C. F., Araujo O. R. P., Goulart M. O. F. (2015). Antioxidant therapy for treatment of inflammatory bowel disease: does it work? Redox Biol. 6, 617–639. 10.1016/j.redox.2015.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni D., Jiang D., Kutyreff C. J., Lai J., Yan Y., Barnhart T. E., et al. (2018). Molybdenum-based nanoclusters act as antioxidants and ameliorate acute kidney injury in mice. Nat. Commun. 9:5421. 10.1038/s41467-018-07890-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S. K., Wijesinghe D. S., Dellinger A., Sturgill J., Zhou Z., Barbour S., et al. (2012). Epoxyeicosatrienoic acids are involved in the C70 fullerene derivative–induced control of allergic asthma. J. Allergy Clin. Immunol. 130, 761–769. 10.1016/j.jaci.2012.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosaka Y., Nosaka A. Y. (2017). Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 117, 11302–11336. 10.1021/acs.chemrev.7b00161 [DOI] [PubMed] [Google Scholar]
- Onizawa S., Aoshiba K., Kajita M., Miyamoto Y., Nagai A. (2009). Platinum nanoparticle antioxidants inhibit pulmonary inflammation in mice exposed to cigarette smoke. Pulm. Pharmacol. Ther. 22, 340–349. 10.1016/j.pupt.2008.12.015 [DOI] [PubMed] [Google Scholar]
- Oró D., Yudina T., Fernández-Varo G., Casals E., Reichenbach V., Casals G., et al. (2016). Cerium oxide nanoparticles reduce steatosis, portal hypertension and display anti-inflammatory properties in rats with liver fibrosis. J. Hepatol. 64, 691–698. 10.1016/j.jhep.2015.10.020 [DOI] [PubMed] [Google Scholar]
- Pagliari F., Mandoli C., Forte G., Magnani E., Pagliari S., Nardone G., et al. (2012). Cerium oxide nanoparticles protect cardiac progenitor cells from oxidative stress. ACS Nano 6, 3767–3775. 10.1021/nn2048069 [DOI] [PubMed] [Google Scholar]
- Park J., Kim B., Han J., Oh J., Park S., Ryu S., et al. (2015). Graphene oxide flakes as a cellular adhesive: Prevention of reactive oxygen species mediated death of implanted cells for cardiac repair. ACS Nano 9, 4987–4999. 10.1021/nn507149w [DOI] [PubMed] [Google Scholar]
- Pedone D., Moglianetti M., Luca E. D., Bardi G., Pompa P. P. (2017). Platinum nanoparticles in nanobiomedicine. Chem. Soc. Rev. 46, 4951–4975. 10.1039/C7CS00152E [DOI] [PubMed] [Google Scholar]
- Petro M., Jaffer H., Yang J., Kabu S., Morris V. B., Labhasetwar V. (2016). Tissue plasminogen activator followed by antioxidant-loaded nanoparticle delivery promotes activation/mobilization of progenitor cells in infarcted rat brain. Biomaterials 81, 169–180. 10.1016/j.biomaterials.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros R. A., DeSimone J. M. (2010). Strategies in the design of nanoparticles for therapeutic applications. Nat. Rev. Drug Discov. 9, 615–627. 10.1038/nrd2591 [DOI] [PubMed] [Google Scholar]
- Porfire A. S., Leucuta S. E., Kiss B., Loghin F., Parvu A. E. (2014). Investigation into the role of Cu/Zn-SOD delivery system on its antioxidant and antiinflammatory activity in rat model of peritonitis. Pharmacol. Rep. 66, 670–676. 10.1016/j.pharep.2014.03.011 [DOI] [PubMed] [Google Scholar]
- Rehman M. U., Yoko Y., Yusei M., Tadamichi S. (2012). The anti-inflammatory effects of platinum nanoparticles on the lipopolysaccharide-induced inflammatory response in raw 264.7 macrophages. Inflamm. Res. 61, 1177–1185. 10.1007/s00011-012-0512-0 [DOI] [PubMed] [Google Scholar]
- Rubenfeld G. D., Caldwell E., Peabody E., Weaver J., Martin D. P., Neff M., et al. (2005). Incidence and outcomes of acute lung injury. N. Engl. J. Med. 353, 1685–1693. 10.1056/NEJMoa050333 [DOI] [PubMed] [Google Scholar]
- Sadikot R. T., Kolanjiyil A. V., Kleinstreuer C., Rubinstein I. (2017). Nanomedicine for treatment of acute lung injury and acute respiratory distress syndrome. Biomedicine Hub. 2, 1–12. 10.1159/000477086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahiner U. M., Birben E., Erzurum S., Sackesen C., Kalayci O. (2018). Oxidative stress in asthma: Part of the puzzle. Pediatr. Allergy Immunol. 29, 789–800. 10.1111/pai.12965 [DOI] [PubMed] [Google Scholar]
- Saravanakumar G., Kim J., Kim W. J. (2017). Reactive oxygen species-responsive drug delivery systems: Promises and challenges. Adv. Sci. 4:1600124. 10.1002/advs.201600124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilrreff P., Simioni Y. R., Jerez H. E., Caimi A. T., de Farias M. A., Villares Portugal R., et al. (2019). Superoxide dismutase in nanoarchaeosomes for targeted delivery to inflammatory macrophages. Colloids Surf. B Biointerfaces 179, 479–487. 10.1016/j.colsurfb.2019.03.061 [DOI] [PubMed] [Google Scholar]
- Sedghi S., Fields J. Z., Klamut M., Urban G., Durkin M., Winship D., et al. (1993). Increased production of luminol enhanced chemiluminescence by the inflamed colonic mucosa in patients with ulcerative colitis. Gut 34, 1191–1197. 10.1136/gut.34.9.1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri G., Sy J. C., Brown M., Dikalov S., Yang S. C., Murthy N., et al. (2010). The delivery of superoxide dismutase encapsulated in polyketal microparticles to rat myocardium and protection from myocardial ischemia-reperfusion injury. Biomaterials 31, 1372–1379. 10.1016/j.biomaterials.2009.10.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashni B., Alshwimi A., Minami K., Furukawa T., Nagasaki Y. (2017). Nitroxide radical-containing nanoparticles as potential candidates for overcoming drug resistance in epidermoid cancers. Polymer. 116, 429–438. 10.1016/j.polymer.2017.02.052 [DOI] [Google Scholar]
- Shashni B., Nagasaki Y. (2018). Nitroxide radical-containing nanoparticles attenuate tumorigenic potential of triple negative breast cancer. Biomaterials 178, 48–62. 10.1016/j.biomaterials.2018.05.042 [DOI] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R. J., Niall R., Van Someren M., Blake D. R., et al. (1992). Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology 103, 186–196. 10.1016/0016-5085(92)91112-H [DOI] [PubMed] [Google Scholar]
- Singh A. P., Biswas A., Shukla A., Maiti P. (2019). Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target Ther. 4:33. 10.1038/s41392-019-0068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Geethika M., Eswarappa S. M., Mugesh G. (2018). Manganese-based nanozymes: Multienzyme redox activity and effect on the nitric oxide produced by endothelial nitric oxide synthase. Chem. Eur. J. 24, 8393–8403. 10.1002/chem.201800770 [DOI] [PubMed] [Google Scholar]
- Singh N., Savanur M. A., Srivastava S., D'Silva P., Mugesh G. (2017). A redox modulatory Mn3 O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a parkinson's disease model. Angew. Chem. Int. Ed. 56, 14267–14271. 10.1002/anie.201708573 [DOI] [PubMed] [Google Scholar]
- Singh N., Savanur M. A., Srivastava S., D'Silva P., Mugesh G. (2019). A manganese oxide nanozyme prevents the oxidative damage of biomolecules without affecting the endogenous antioxidant system. Nanoscale 11, 3855–3863. 10.1039/C8NR09397K [DOI] [PubMed] [Google Scholar]
- Soh M., Kang D. W., Jeong H. G., Kim D., Kim D. Y., Yang W., et al. (2017). Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. Int. Ed Engl. 56, 11399–11403. 10.1002/anie.201704904 [DOI] [PubMed] [Google Scholar]
- Solano F. (2017). Melanin and Melanin-Related polymers as materials with biomedical and biotechnological Applications-Cuttlefish Ink and mussel foot proteins as inspired biomolecules. Int. J. Mol. Sci. 18:E1561. 10.3390/ijms18071561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T., Jiang D., Rosenkrans Z. T., Ehlerding E. B., Ni D., Qi C., et al. (2019). A Melanin-Based natural antioxidant defense nanosystem for theranostic application in acute kidney injury. Adv. Funct. Mater. 29:1904833. 10.1002/adfm.201904833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamiya M., Miyamoto Y., Yamashita T., Deguchi K., Ohta Y., Abe K. (2012). Strong neuroprotection with a novel platinum nanoparticle against ischemic Stroke- and tissue plasminogen activator-related brain damages in mice. Neuroscience 221, 47–55. 10.1016/j.neuroscience.2012.06.060 [DOI] [PubMed] [Google Scholar]
- Vernekar A. A., Sinha D., Srivastava S., Paramasivam P. U., D'Silva P., Mugesh G. (2014). An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Commun. 5:5301. 10.1038/ncomms6301 [DOI] [PubMed] [Google Scholar]
- Vij N. (2011). Nano-based theranostics for chronic obstructive lung diseases: Challenges and therapeutic potential. Expert Opin. Drug Deliv. 8, 1105–1109. 10.1517/17425247.2011.597381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L. B., Mo J., Abrahamsson B., Nagasaki Y. (2015). Specific accumulation of orally administered redox nanotherapeutics in the inflamed colon reducing inflammation with dose-response efficacy. J. Control. Release 210, 19–25. 10.1016/j.jconrel.2015.05.275 [DOI] [PubMed] [Google Scholar]
- Vong L. B., Nagasaki Y. (2016). Combination treatment of murine colon cancer with doxorubicin and redox nanoparticles. Mol. Pharm. 13, 449–455. 10.1021/acs.molpharmaceut.5b00676 [DOI] [PubMed] [Google Scholar]
- Vong L. B., Tomita T., Yoshitomi T., Matsui H., Nagasaki Y. (2012). An orally administered redox nanoparticle that accumulates in the colonic mucosa and reduces colitis in mice. Gastroenterology 143, 1027–1036.e3. 10.1053/j.gastro.2012.06.043 [DOI] [PubMed] [Google Scholar]
- Vong L. B., Yoshitomi T., Morikawa K., Saito S., Matsui H., Nagasaki Y. (2014). Oral nanotherapeutics: Effect of redox nanoparticle on microflora in mice with dextran sodium sulfate-induced colitis. J. Gastroenterol. 49, 806–813. 10.1007/s00535-013-0836-8 [DOI] [PubMed] [Google Scholar]
- Wang J., Wang H., Zhu R., Liu Q., Fei J., Wang S. (2015). Anti-inflammatory activity of curcumin-loaded solid lipid nanoparticles in il-1β transgenic mice subjected to the lipopolysaccharide-induced sepsis. Biomaterials 53, 475–483. 10.1016/j.biomaterials.2015.02.116 [DOI] [PubMed] [Google Scholar]
- Wang L., Feng M., Li Q., Qiu C., Chen R. (2019). Advances in nanotechnology and asthma. Ann. Transl. Med. 7:180. 10.21037/atm.2019.04.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Sheng J., Yang M. (2019). Melanin-based nanoparticles in biomedical applications: From molecular imaging to treatment of diseases. Chin. Chem. Lett. 30, 533–540. 10.1016/j.cclet.2018.10.010 [DOI] [Google Scholar]
- Wang Y., Li L., Zhao W., Dou Y., An H., Tao H., et al. (2018). Targeted therapy of atherosclerosis by a broad-spectrum reactive oxygen species scavenging nanoparticle with intrinsic anti-inflammatory activity. ACS Nano 12, 8943–8960. 10.1021/acsnano.8b02037 [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tahara M., Todo S. (2008). The novel antioxidant edaravone: From bench to bedside. Cardiovas. Ther. 26, 101–114. 10.1111/j.1527-3466.2008.00041.x [DOI] [PubMed] [Google Scholar]
- Wattamwar P. P., Mo Y., Wan R., Palli R., Zhang Q., Dziubla T. D. (2010). Antioxidant activity of degradable polymer poly (trolox ester) to suppress oxidative stress injury in the cells. Adv. Funct. Mater. 20, 147–154. 10.1002/adfm.200900839 [DOI] [Google Scholar]
- Wong L. L., Hirst S. M., Pye Q. N., Reilly C. M., Seal S., McGinnis J. F. (2013). Catalytic nanoceria are preferentially retained in the rat retina and are not cytotoxic after intravitreal injection. PLoS ONE 8:e58431 10.1371/journal.pone.0058431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L. L., Pye Q. N., Chen L., Seal S., McGinnis J. F. (2015). Defining the catalytic activity of nanoceria in the P23H-1 rat, a photoreceptor degeneration model. PLoS ONE 10:e0121977. 10.1371/journal.pone.0121977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Chen X., Wang Y., Xiao H., Peng Y., Lin L., et al. (2018). Aortic Plaque-Targeted andrographolide delivery with Oxidation-Sensitive micelle effectively treats atherosclerosis via simultaneous ROS capture and Anti-Inflammation. Nanomed. Nanotechnol. 14, 2215–2226. 10.1016/j.nano.2018.06.010 [DOI] [PubMed] [Google Scholar]
- Wu X., Dai H., Xu C., Liu L., Li S. (2019). Citric acid modification of a polymer exhibits antioxidant and anti-inflammatory properties in stem cells and tissues. J. Biomed. Mater. Res. A 107, 2414–2424. 10.1002/jbm.a.36748 [DOI] [PubMed] [Google Scholar]
- Xu P. T., Maidment B. W., III., Antonic V., Jackson I. L., Das S., Zodda A., et al. (2016). Cerium oxide nanoparticles: A potential medical countermeasure to mitigate radiation-induced lung injury in CBA/J mice. Radiat. Res. 185, 516–526. 10.1667/RR14261.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F., Wang Y., Zhang Q., Han S., Zhang F., Jin T., et al. (2018). Self-assembly of Affinity-controlled nanoparticles via Host–guest interactions for drug delivery. Nanoscale 10, 12364–12377. 10.1039/C8NR01518J [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Yuki S., Watanabe T., Mitsuka M., Saito K.-I., Kogure K. (1997). Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Res. 762, 240–242. 10.1016/S0006-8993(97)00490-3 [DOI] [PubMed] [Google Scholar]
- Yao J., Cheng Y., Zhou M., Zhao S., Lin S., Wang X., et al. (2018). ROS scavenging mn3o4 nanozymes for in vivo anti-inflammation. Chem. Sci. 9, 2927–2933. 10.1039/C7SC05476A [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H., Zhou Y., Liu X., Chen Y., Duan S., Zhu R., et al. (2019). Recent advances on reactive oxygen Species-responsive delivery and diagnosis system. Biomacromolecules 20, 2441–2463. 10.1021/acs.biomac.9b00628 [DOI] [PubMed] [Google Scholar]
- Yoo D., Guk K., Kim H., Khang G., Wu D., Lee D. (2013). Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int. J. Pharm. 450, 87–94. 10.1016/j.ijpharm.2013.04.028 [DOI] [PubMed] [Google Scholar]
- Yoshihisa Y., Honda A., Zhao Q.-L., Makino T., Abe R., Matsui K., et al. (2010). Protective effects of platinum nanoparticles against UV-light-induced epidermal inflammation. Exp. Dermatol. 19, 1000–1006. 10.1111/j.1600-0625.2010.01128.x [DOI] [PubMed] [Google Scholar]
- Yoshitomi T., Hirayama A., Nagasaki Y. (2011). The ROS scavenging and renal protective effects of pH-responsive nitroxide Radical-containing nanoparticles. Biomaterials 32, 8021–8028. 10.1016/j.biomaterials.2011.07.014 [DOI] [PubMed] [Google Scholar]
- Yoshitomi T., Nagasaki Y. (2014). Reactive oxygen species-scavenging nanomedicines for the treatment of oxidative stress injuries. Adv. Healthc. Mater. 3, 1149–1161. 10.1002/adhm.201300576 [DOI] [PubMed] [Google Scholar]
- Zafar M. S., Quarta A., Marradi M., Ragusa A. (2019). Recent developments in the reduction of oxidative stress through antioxidant polymeric formulations. Pharmaceutics 11:505. 10.3390/pharmaceutics11100505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Wang F., Zhou H., Gao D., Yuan Z., Wu C., et al. (2019). Polymer dots compartmentalized in liposomes as a photocatalyst for in situ hydrogen therapy. Angew. Chem. Int. Ed. 58, 2744–2748. 10.1002/anie.201813066 [DOI] [PubMed] [Google Scholar]
- Zhang D., Wei Y., Chen K., Zhang X., Xu X., Shi Q., et al. (2015). Biocompatible reactive oxygen species (ROS)-responsive nanoparticles as superior drug delivery vehicles. Adv. Healthc. Mater. 4, 69–76. 10.1002/adhm.201400299 [DOI] [PubMed] [Google Scholar]
- Zhang J. X., Ma P. X. (2013). Cyclodextrin-based supramolecular systems for drug Delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 65, 1215–1233. 10.1016/j.addr.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Kaufman R. J. (2008). From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462. 10.1038/nature07203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Laug L., Munchgesang W., Pippel E., Gosele U., Brandsch M., et al. (2010). Reducing stress on cells with Apoferritin-encapsulated platinum nanoparticles. Nano Lett. 10, 219–223. 10.1021/nl903313r [DOI] [PubMed] [Google Scholar]
- Zhang Q., Tao H., Lin Y., Hu Y., An H., Zhang D., et al. (2016). A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials 105, 206–221. 10.1016/j.biomaterials.2016.08.010 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Zhang F., Chen Y., Dou Y., Tao H., Zhang D., et al. (2017). Structure–property correlations of reactive oxygen species-responsive and hydrogen peroxide-eliminating materials with anti-oxidant and anti-inflammatory activities. Chem. Mater. 29, 8221–8238. 10.1021/acs.chemmater.7b02412 [DOI] [Google Scholar]
- Zhang Q., Zhang F., Li S., Liu R., Jin T., Dou Y., et al. (2019). A multifunctional nanotherapy for targeted treatment of colon cancer by simultaneously regulating tumor microenvironment. Theranostics 9, 3732–3753. 10.7150/thno.34377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Liu R., Liu C., Pan L., Qi Y., Cheng J., et al. (2020). A pH/ROS Dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials 230, 119605. 10.1016/j.biomaterials.2019.119605 [DOI] [PubMed] [Google Scholar]
- Zhang W., Hu S., Yin J.-J., He W., Lu W., Ma M., et al. (2016). Prussian blue nanoparticles as multienzyme mimetics and reactive oxygen species scavengers. J. Am. Chem. Soc. 138, 5860–5865. 10.1021/jacs.5b12070 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Murugesan P., Huang K., Cai H. (2020). NADPH oxidases and oxidase crosstalk in cardiovascular diseases: Novel therapeutic targets. Nat. Rev. Cardiol. 17, 170–194. 10.1038/s41569-019-0260-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Zeng Z., Liu L., Chen J., Zhou H., Huang L., et al. (2018). Polydopamine nanoparticles for the treatment of acute Inflammation-induced injury. Nanoscale 10, 6981–6991. 10.1039/C8NR00838H [DOI] [PubMed] [Google Scholar]
- Zhu Y., Deng G., Ji A., Yao J., Meng X., Wang J., et al. (2017). Porous Se@Sio2 nanospheres treated Paraquat-induced acute lung injury by resisting oxidative stress. Int. J. Nanomedicine 12, 7143–7152. 10.2147/IJN.S143192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Su M. (2017). Polydopamine nanoparticles for combined Chemo- and photothermal cancer therapy. Nanomaterials 7:160. 10.3390/nano7070160 [DOI] [PMC free article] [PubMed] [Google Scholar]