Abstract
During the past 50 years, the cellular and molecular mechanisms of synaptic plasticity have been studied in great detail. A plethora of signaling pathways have been identified that account for synaptic changes based on positive and negative feedback mechanisms. Yet, the biological significance of Hebbian synaptic plasticity (= positive feedback) and homeostatic synaptic plasticity (= negative feedback) remains a matter of debate. Specifically, it is unclear how these opposing forms of plasticity, which share common downstream mechanisms, operate in the same networks, neurons, and synapses. Based on the observation that rapid and input-specific homeostatic mechanisms exist, we here discuss a model that is based on signaling pathways that may adjust a balance between Hebbian and homeostatic synaptic plasticity. Hence, “alterations” in Hebbian plasticity may, in fact, resemble “enhanced” homeostasis, which rapidly returns synaptic strength to baseline. In turn, long-lasting experience-dependent synaptic changes may require attenuation of homeostatic mechanisms or the adjustment of homeostatic setpoints at the single-synapse level. In this context, we propose a role for the proteolytic processing of the amyloid precursor protein (APP) in setting a balance between the ability of neurons to express Hebbian and homeostatic synaptic plasticity.
Keywords: hebbian plasticity, homeostatic plasticity, synaptic scaling, amyloid precursor protein, BACE1, APPsα, amyloid-β
Introduction
The ability of neural tissue to adapt to specific stimuli through structural, functional and molecular changes plays a fundamental role in complex brain functions such as perception, decision-making, learning and memory (Citri and Malenka, 2008; Bailey et al., 2015). During the past 50 years, considerable effort has been spent to decipher and better understand the cellular and molecular mechanisms of Hebbian synaptic plasticity, which accounts for activity-dependent changes of synaptic weights based on positive feedback mechanisms (Hebb, 1949; Bliss and Lomo, 1973). It is now well-established that Hebbian plasticity resembles fast and lasting input-specific synaptic changes necessary for experience-dependent memory and learning (Bear, 1996; Chen and Tonegawa, 1997; Klintsova and Greenough, 1999). Experimentally, Hebbian mechanisms have been described in detail for excitatory pre- and postsynaptic sites (e.g., Petzoldt et al., 2016; Monday et al., 2018; Scheefhals and MacGillavry, 2018; Buonarati et al., 2019), where, for example, tetanic electrical stimulation at different frequencies results in the strengthening (long-term potentiation, LTP) or weakening (long-term depression, LTD) of neurotransmission (Bliss and Lomo, 1973; Dudek and Bear, 1992). Meanwhile, evidence has started to emerge for corresponding activity-dependent synaptic changes at GABAergic synapses (Bartos et al., 2011; Rozov et al., 2017; Chiu et al., 2019). Specifically, the plasticity of inhibitory neurotransmission seems to control the ability of neurons to express Hebbian plasticity of excitatory neurotransmission (Letzkus et al., 2015; Zhao et al., 2017).
While feedforward and feedback microcircuits dynamically match afferent excitation to recruited inhibition (Sprekeler, 2017), it has been recognized that, in the absence of physiological constraints, complex systems based solely on positive feedback mechanisms will experience instability—e.g., strong synapses will continue growing, while weakening of synapses will result in synapse elimination (Miller and Mackay, 1994). Indeed, during the past two decades, a plethora of cellular and molecular mechanisms have been identified that maintain neurons in a dynamic functional range by adjusting excitatory and inhibitory synaptic strength in a compensatory manner—i.e., based on negative feedback (Davis and Bezprozvanny, 2001; Marder and Prinz, 2003; Turrigiano and Nelson, 2004; Pozo and Goda, 2010; Keck et al., 2017). Yet, a major unresolved issue in the field concerns the interplay between Hebbian and compensatory—i.e., homeostatic—synaptic plasticity, which share common downstream mechanisms that change and/or adjust excitatory and inhibitory neurotransmission (Turrigiano et al., 1998; Feldman, 2002; Turrigiano and Nelson, 2004; Swanwick et al., 2006; Rannals and Kapur, 2011). Moreover, the biological significance of alterations in Hebbian and/or homeostatic plasticity for pathological brain states remains unclear.
In recent years, these questions have been discussed extensively by leading experts in the field (e.g., Vitureira and Goda, 2013; Fox and Stryker, 2017; Keck et al., 2017; Yee et al., 2017). It has been proposed, for example, that homeostatic plasticity operates on a longer time scale (Turrigiano, 2012; Tononi and Cirelli, 2014; Hengen et al., 2016)—thus not interfering with synaptic changes induced by Hebbian plasticity—and that all synapses of a neuron are adjusted by the same factor in the context of homeostatic “synaptic scaling” to preserve the relative differences between synapses (Turrigiano et al., 1998; Turrigiano, 2008; Vitureira and Goda, 2013). Meanwhile, theoretical modeling work has emphasized the importance of fast homeostatic mechanisms for network stability (Zenke et al., 2013), and robust experimental evidence has been provided for rapid homeostatic plasticity (Keck et al., 2011; Frank, 2014; Li et al., 2014). Furthermore, solid evidence suggests that homeostatic synaptic adaptation can occur locally, in subsets of synapses (e.g., Desai et al., 2002; Kim and Tsien, 2008; Vlachos et al., 2013). These findings indicate that Hebbian and homeostatic synaptic mechanisms may operate in parallel and could thus interfere with each other in the same subset of synapses.
In light of these considerations, it is interesting to note that the effects of classic Hebbian plasticity paradigms—e.g., local tetanic electrical stimulation (Bliss and Lomo, 1973)—have not yet been systematically evaluated for their effects on homeostatic synaptic plasticity induction. Therefore, in this article, we sought to present a “homeostatic view on classic LTP/LTD experiments” by highlighting mechanisms which may rapidly affect—and hence set a balance between—Hebbian and homeostatic synaptic plasticity (Figure 1). These considerations are put into clinical perspective by discussing the potential role of α- and β-secretase-mediated processing of the amyloid precursor protein (APP) in Hebbian and homeostatic synaptic plasticity (Figure 2).
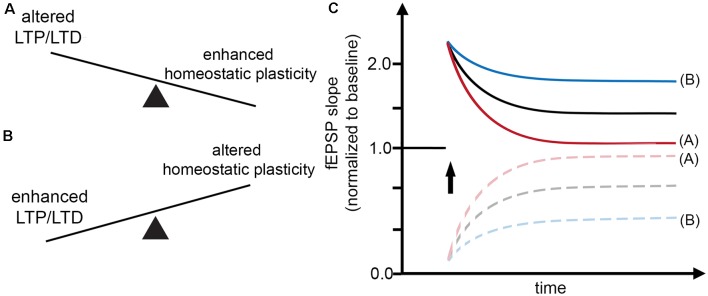
Figure 1.
Interaction between Hebbian and homeostatic synaptic plasticity. (A,B) Factors may exist which rapidly set a balance between Hebbian and homeostatic synaptic plasticity, thereby affecting the induction and persistence of experience-dependent synaptic changes. (C) Alterations in Hebbian plasticity—i.e., long-term potentiation (LTP) or depression (LTD) of evoked field excitatory postsynaptic potentials (fEPSPs; red curve)—may reflect enhanced homeostatic synaptic plasticity. In turn, alterations in homeostatic synaptic plasticity may account for enhanced LTP/LTD (blue curve).
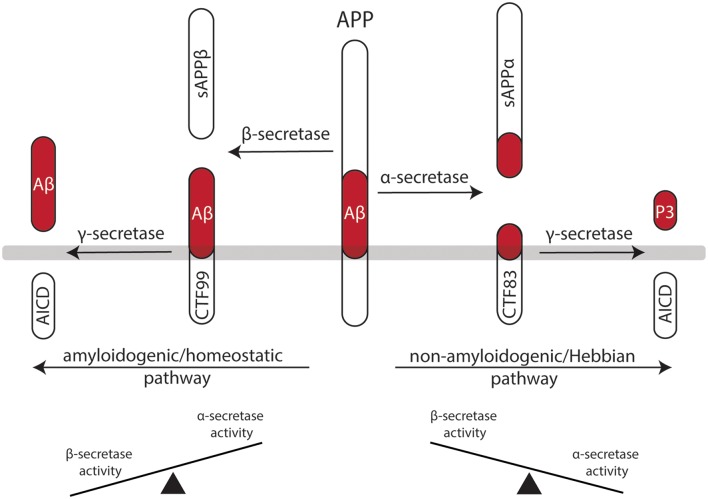
Figure 2.
Processing of the amyloid precursor protein (APP) may set a balance between Hebbian and homeostatic synaptic plasticity. Work in recent years has established a firm link between the non-amyloidogenic processing pathway—i.e., APP secreted ectodomain alpha (APPsα)—and the ability of neurons to express LTP of excitatory postsynapses. Likewise, evidence has started to emerge for the role of the amyloidogenic processing pathway—i.e., amyloid-β (Aβ)—in homeostatic synaptic plasticity. Hence, differential processing of APP via α- or β-secretases may set a balance between Hebbian and homeostatic synaptic plasticity in neural networks.
Opposing Roles of Ca2+ Signaling in Hebbian and Homeostatic Synaptic Plasticity
Central mechanisms that regulate the activity-dependent strengthening (or dampening) of excitatory neurotransmission are modification, trafficking and synthesis of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPA-Rs) at excitatory postsynapses (Malinow and Malenka, 2002; Diering and Huganir, 2018). Interestingly, both Hebbian and homeostatic synaptic plasticity recruit Ca2+-dependent signaling pathways which lead to characteristic changes in synaptic AMPA-R content and function (Malinow and Malenka, 2002; Song and Huganir, 2002; Derkach et al., 2007; Turrigiano, 2008). However, Ca2+ influx via N-methyl-D-aspartate receptors (NMDA-Rs) or voltage-gated Ca2+ channels (VGCCs) can have opposing effects on postsynaptic AMPA-R content in the context of Hebbian and homeostatic synaptic plasticity (Lee et al., 2000; Diering et al., 2014; Diering and Huganir, 2018).
In the case of LTP induction, for example, tetanic electrical stimulation, which triggers Ca2+ influx, can lead to an increase in postsynaptic AMPA-R content and hence potentiation of excitatory neurotransmission (= positive feedback mechanism). Conversely, increased intracellular Ca2+ levels are expected to trigger homeostatic synaptic down-scaling, which returns AMPA-R content to baseline (= negative feedback mechanism). Considering such rapid interactions between Hebbian and homeostatic plasticity mechanisms (Figure 1), a widely used interpretation of “alterations” in Hebbian plasticity—i.e., failure to persistently change the amplitude or the slope of evoked field excitatory postsynaptic potentials (fEPSPs)—may, in fact, resemble “enhanced” homeostasis, which effectively returns fEPSPs to baseline after the LTP- or LTD-inducing “network perturbation” (see Figures 1A,C). Conversely, signaling pathways that block homeostasis or change homeostatic setpoints will result in persisting changes of excitatory neurotransmission (Figures 1B,C). We have to concede, however, that molecular signaling pathways that attenuate or adjust local homeostatic plasticity at the level of individual synapses are not well-understood. It is also interesting to speculate in this context that changes in the ability of neurons to express homeostatic plasticity per se may suffice to generate Hebbian-like associative plasticity. Indeed, a recent study employed computational modeling to demonstrate associative properties of firing-rate homeostasis in recurrent neuronal networks (Gallinaro and Rotter, 2018).
Role of Dopamine in Homeostatic Synaptic Plasticity
Based on the above considerations, we recently tested for the role of dopamine in homeostatic synaptic plasticity (Strehl et al., 2018). We reasoned that neuromodulators which promote Hebbian plasticity (Otani et al., 2003; Mu et al., 2011; Sheynikhovich et al., 2013; Broussard et al., 2016) may also act by blocking the ability of neurons to express homeostatic synaptic plasticity. Indeed, we were able to demonstrate that dopamine blocks homeostatic plasticity of excitatory neurotransmission in entorhino-hippocampal tissue cultures (Strehl et al., 2018). Pharmacological activation of D1/5 receptors, but not D2/3 receptors, mimicked the effects of dopamine on homeostatic plasticity. These findings raise the intriguing possibility that dopamine may act as a permissive factor that promotes Hebbian plasticity, at least in part, by blocking homeostasis. Interestingly, the “anti-homeostatic” effects of dopamine were only observed in immature neurons during early postnatal development (Strehl et al., 2018). Hence, specific factors may exist which adjust homeostatic plasticity in specific cells depending on the state of the neural network. It remains to be shown, however, whether dopamine indeed promotes Hebbian plasticity by attenuating homeostatic plasticity at the level of individual synapses and whether dopamine acts on neurons or glia cells (or both) to assert its differential effects on plasticity. Regardless of these considerations, these results call for a re-evaluation of the available LTP/LTD literature and a systematic assessment of well-known “LTP-/LTD-promoting or -blocking factors” in homeostatic synaptic plasticity. As an example that is of considerable clinical relevance, we here discuss the potential role of APP processing in setting a balance between Hebbian and homeostatic synaptic plasticity.
The Role of The Amyloid Precursor Protein in Synaptic Plasticity
Work in recent years has established a firm link between APP and structural and functional plasticity (comprehensively reviewed in Müller et al., 2017). These studies are based on experiments using APP-deficient mice, or mice in which the APP gene has been genetically modified (Dawson et al., 1999; Magara et al., 1999; Seabrook et al., 1999; Turner et al., 2003; Herms et al., 2004). Historically, the majority of studies in the field have focused on addressing the role of APP and its cleavage products in Hebbian plasticity. More recently, some evidence has supported its involvement in homeostatic synaptic plasticity (Jang and Chung, 2016; Styr and Slutsky, 2018).
APP is a type I transmembrane protein ubiquitously expressed in all mammalian tissues (Müller-Hill and Beyreuther, 1989; Müller et al., 2017). It is differentially processed by secretases via two pathways (Figure 2): the amyloidogenic processing pathway generates amyloid-β (Aβ) peptides, which are implicated in the pathogenesis of Alzheimer’s disease (AD), while the non-amyloidogenic processing pathway produces the neuroprotective soluble ectodomain APPsα (Turner et al., 2003). In the amyloidogenic pathway, APP is cleaved by β-site APP cleaving enzyme (BACE1), which releases APP soluble fragment beta (APPsβ), followed by γ-secretase processing, which generates Aβ fragments and the APP intracellular domain (AICD; Vassar et al., 1999; Van Der Kant and Goldstein, 2015). In contrast, the non-amyloidogenic processing pathway recruits α-secretases releasing APPsα, again followed by γ-secretases that produce the P3 peptide and AICD (O’Brien and Wong, 2011; Van Der Kant and Goldstein, 2015).
Role of The Non-Amyloidogenic Pathway in Synaptic Plasticity
APP-deficient mice show alterations in dendritic morphologies and dendritic spine counts (Perez et al., 1997; Lee et al., 2010; Tyan et al., 2012; Weyer et al., 2014). These structural defects have been linked to alterations in LTP and deficits in learning and memory (Dawson et al., 1999; Hick et al., 2015). Interestingly, APPsα rescues several of the deficits of APP−/− animals, while APPsβ does not have such a positive effect on Hebbian plasticity (Ring et al., 2007; Hick et al., 2015). Consistent with this suggestion, enhanced LTP is observed in APPsα-treated acute brain slices prepared from rats (Ishida et al., 1997), and behavioral learning is augmented when mice are injected with APPsα (Meziane et al., 1998). Moreover, pharmacologic inhibition of α-secretase activity impairs LTP in rats, which can be rescued by APPsα (Taylor et al., 2008). This line of evidence suggests that APPsα secretion seems to be activity-dependent—that is, LTP-inducing protocols lead to an increase in APPsα (Nitsch et al., 1992; Fazeli et al., 1994). Therefore, it has been proposed that the non-amyloidogenic processing pathway plays an important role in mediating Hebbian synaptic plasticity (Figure 2). However, it should be clearly stated that APPsα has not yet been tested in the context of homeostatic synaptic plasticity. It thus remains to be shown whether some of the “positive” effects of APPsα on activity-dependent structural and functional plasticity are also mediated by its ability to modulate—i.e., to attenuate—homeostatic plasticity mechanisms.
Role of The Amyloidogenic Pathway in Synaptic Plasticity
The role of APP processing via the amyloidogenic pathway has been studied in detail for its pathogenic role in neurodegeneration (Goldsworthy and Vallence, 2013; Nieweg et al., 2015; Gupta and Goyal, 2016; Chen et al., 2017; Youn et al., 2019). What remains less understood is the physiological role of the amyloidogenic processing pathway and Aβ.
It seems well-established that elevated concentrations of Aβ are “synaptotoxic” by hindering the ability of neurons to express LTP, thereby having detrimental effects on learning and memory (Chiba et al., 2009; Jo et al., 2011; Samidurai et al., 2018). In this context, it has been shown that Aβ interferes with neural Ca2+ signaling—i.e., it blocks NMDA-Rs and Ca2+/calmodulin-dependent protein kinase II (CamKII; Zhao et al., 2004; Townsend et al., 2007; Gu et al., 2009; but see the work in Opazo et al., 2018, which suggests that Aβ activates CamKII). Similar to APPsα, an increase in synaptic activity and NMDA-R stimulation can also lead to an increase in Aβ production (Kamenetz et al., 2003; Lesné et al., 2005). Thus, it has been proposed that an increase in Aβ may act as a negative feedback mechanism by blocking Hebbian synaptic plasticity. In light of the herein proposed model (Figure 1), Aβ may also act by promoting homeostatic synaptic plasticity (see Figure 1).
Indeed, evidence has started to emerge for a physiological role of Aβ in homeostatic synaptic plasticity. For example, the AMPA-R scaffolding protein PICK1 mediates homeostatic synaptic plasticity (Anggono et al., 2011) and has been linked to Aβ-mediated “alterations” in synaptic plasticity (Alfonso et al., 2014). Similar evidence exists for interaction between Aβ and PSD-95 (Roselli et al., 2005; Sun and Turrigiano, 2011), GKAP (Roselli et al., 2011; Shin et al., 2012), calcineurin (D’Amelio et al., 2011; Kim and Ziff, 2014) and STEP61 (Kurup et al., 2010). Finally, BDNF and TNFα, which have been firmly linked to homeostatic synaptic plasticity (Rutherford et al., 1998; Stellwagen and Malenka, 2006; Becker et al., 2015), seem to be dysregulated in the AD brain (Fillit et al., 1991; Phillips et al., 1991). Along this line of evidence, a role for microglia in Aβ-mediated alterations in complex brain function has been suggested (Kitazawa et al., 2004; Hansen et al., 2018; Kinney et al., 2018; Hemonnot et al., 2019). However, it is important to note that the majority of these findings are based on experiments employing transgenic mouse models of AD or high concentrations of Aβ. Hence, direct experimental evidence for a physiological role of APP/Aβ in homeostatic synaptic plasticity is currently missing (Figure 2).
Clinical Implications and Perspective
Considering the detrimental effects of Aβ in Hebbian synaptic plasticity together with promising results in experiments employing a mouse model that expressed familial mutant APP in the absence of BACE1 (Cai et al., 2001; Luo et al., 2001; Roberds et al., 2001), pharmacologic inhibition of BACE1 has been tested as a potential treatment for the cognitive decline in AD (Yan and Vassar, 2014; Coimbra et al., 2018). Indeed, BACE1 inhibitors successfully lowered Aβ levels detected in the cerebrospinal fluid of AD patients (Kennedy et al., 2016; Egan et al., 2018). However, major clinical trials were discontinued due to a series of adverse effects or no improvement and even accelerated cognitive decline in patients (Coimbra et al., 2018; Egan et al., 2019). On the same note, mice lacking BACE1 showed increased neural excitability and spontaneous seizure activity (Hitt et al., 2010; Hu et al., 2010; Zhu et al., 2018; Vnencak et al., 2019), which have been linked to impaired homeostatic mechanisms (Wondolowski and Dickman, 2013; González et al., 2015). Although it is clear that BACE1 targets several other substrates in the nervous system (Barão et al., 2016), these observations support the notion that some of the adverse effects of clinically used BACE1 inhibitors could be explained by an impairment of Aβ-mediated homeostatic synaptic plasticity.
Hence, it will be important to evaluate the significance of APP processing via the amyloidogenic and non-amyloidogenic processing pathways in homeostatic synaptic plasticity. We are confident that a systematic assessment of “pro-homeostatic” effects of Aβ and possible “anti-homeostatic” effects of APPsα will provide new and important insights into the intricate interplay between Hebbian and homeostatic synaptic plasticity. These findings may also be of relevance for the development of new therapeutic strategies in neurological and psychiatric diseases associated with alterations in APP processing or increased Aβ levels.
Author Contributions
CG and AV wrote this manuscript and prepared the figures.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by Deutsche Forschungsgemeinschaft (CRC1080 and FOR1332 to AV).
References
- Alfonso S., Kessels H. W., Banos C. C., Chan T. R., Lin E. T., Kumaravel G., et al. (2014). Synapto-depressive effects of amyloid beta require PICK 1. Eur. J. Neurosci. 39, 1225–1233. 10.1111/ejn.12499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anggono V., Clem R. L., Huganir R. L. (2011). PICK1 loss of function occludes homeostatic synaptic scaling. J. Neurosci. 31, 2188–2196. 10.1523/jneurosci.5633-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey C. H., Kandel E. R., Harris K. M. (2015). Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb. Perspect. Biol. 7:a021758. 10.1101/cshperspect.a021758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barão S., Moechars D., Lichtenthaler S. F., De Strooper B. (2016). BACE1 physiological functions may limit its use as therapeutic target for Alzheimer’s disease. Trends Neurosci. 39, 158–169. 10.1016/j.tins.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Bartos M., Alle H., Vida I. (2011). Role of microcircuit structure and input integration in hippocampal interneuron recruitment and plasticity. Neuropharmacology 60, 730–739. 10.1016/j.neuropharm.2010.12.017 [DOI] [PubMed] [Google Scholar]
- Bear M. F. (1996). A synaptic basis for memory storage in the cerebral cortex. Proc. Natl. Acad. Sci. U S A 93, 13453–13459. 10.1073/pnas.93.24.13453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D., Deller T., Vlachos A. (2015). Tumor necrosis factor (TNF)-receptor 1 and 2 mediate homeostatic synaptic plasticity of denervated mouse dentate granule cells. Sci. Rep. 5:12726. 10.1038/srep12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. (1973). Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J. Physiology. 232, 331–356. 10.1113/jphysiol.1973.sp010273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard J. I., Yang K., Levine A. T., Tsetsenis T., Jenson D., Cao F., et al. (2016). Dopamine regulates aversive contextual learning and associated in vivo synaptic plasticity in the hippocampus. Cell Rep. 14, 1930–1939. 10.1016/j.celrep.2016.01.070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonarati O. R., Hammes E. A., Watson J. F., Greger I. H., Hell J. W. (2019). Mechanisms of postsynaptic localization of AMPA-type glutamate receptors and their regulation during long-term potentiation. Sci. Signal. 12:eaar6889. 10.1126/scisignal.aar6889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H., Wang Y., Mccarthy D., Wen H., Borchelt D. R., Price D. L., et al. (2001). BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 4, 233–234. 10.1038/85064 [DOI] [PubMed] [Google Scholar]
- Chen C., Tonegawa S. (1997). Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning and memory in the mammalian brain. Annu. Rev. Neurosci. 20, 157–184. 10.1146/annurev.neuro.20.1.157 [DOI] [PubMed] [Google Scholar]
- Chen G. F., Xu T. H., Yan Y., Zhou Y. R., Jiang Y., Melcher K., et al. (2017). Amyloid beta: structure, biology and structure-based therapeutic development. Acta Pharmacol. Sin. 38, 1205–1235. 10.1038/aps.2017.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T., Yamada M., Sasabe J., Terashita K., Shimoda M., Matsuoka M., et al. (2009). Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol. Psychiatry 14, 206–222. 10.1038/mp.2008.105 [DOI] [PubMed] [Google Scholar]
- Chiu C. Q., Barberis A., Higley M. J. (2019). Preserving the balance: diverse forms of long-term GABAergic synaptic plasticity. Nat. Rev. Neurosci. 20, 272–281. 10.1038/s41583-019-0141-5 [DOI] [PubMed] [Google Scholar]
- Citri A., Malenka R. C. (2008). Synaptic plasticity: multiple forms, functions and mechanisms. Neuropsychopharmacology 33, 18–41. 10.1038/sj.npp.1301559 [DOI] [PubMed] [Google Scholar]
- Coimbra J. R. M., Marques D. F. F., Baptista S. J., Pereira C. M. F., Moreira P. I., Dinis T. C. P., et al. (2018). Highlights in BACE1 inhibitors for Alzheimer’s disease treatment. Front. Chem. 6:178. 10.3389/fchem.2018.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio M., Cavallucci V., Middei S., Marchetti C., Pacioni S., Ferri A., et al. (2011). Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer’s disease. Nat. Neurosci. 14:69. 10.1038/nn.2709 [DOI] [PubMed] [Google Scholar]
- Davis G. W., Bezprozvanny I. (2001). Maintaining the stability of neural function: a homeostatic hypothesis. Annu. Rev Physiol 63, 847–869. 10.1146/annurev.physiol.63.1.847 [DOI] [PubMed] [Google Scholar]
- Dawson G. R., Seabrook G. R., Zheng H., Smith D. W., Graham S., O’dowd G., et al. (1999). Age-related cognitive deficits, impaired long-term potentiation and reduction in synaptic marker density in mice lacking the beta-amyloid precursor protein. Neuroscience 90, 1–13. 10.1016/s0306-4522(98)00410-2 [DOI] [PubMed] [Google Scholar]
- Derkach V. A., Oh M. C., Guire E. S., Soderling T. R. (2007). Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 8, 101–113. 10.1038/nrn2055 [DOI] [PubMed] [Google Scholar]
- Desai N. S., Cudmore R. H., Nelson S. B., Turrigiano G. G. (2002). Critical periods for experience-dependent synaptic scaling in visual cortex. Nat. Neurosci. 5, 783–789. 10.1038/nn878 [DOI] [PubMed] [Google Scholar]
- Diering G. H., Gustina A. S., Huganir R. L. (2014). PKA-GluA1 coupling via AKAP5 controls AMPA receptor phosphorylation and cell-surface targeting during bidirectional homeostatic plasticity. Neuron 84, 790–805. 10.1016/j.neuron.2014.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering G. H., Huganir R. L. (2018). The AMPA receptor code of synaptic plasticity. Neuron 100, 314–329. 10.1016/j.neuron.2018.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek S. M., Bear M. F. (1992). Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc. Natl. Acad. Sci. U S A 89, 4363–4367. 10.1073/pnas.89.10.4363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. F., Kost J., Tariot P. N., Aisen P. S., Cummings J. L., Vellas B., et al. (2018). Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 378, 1691–1703. 10.1056/NEJMoa1706441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M. F., Kost J., Voss T., Mukai Y., Aisen P. S., Cummings J. L., et al. (2019). Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med. 380, 1408–1420. 10.1056/NEJMoa1812840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli M. S., Breen K., Errington M. L., Bliss T. V. (1994). Increase in extracellular NCAM and amyloid precursor protein following induction of long-term potentiation in the dentate gyrus of anaesthetized rats. Neurosci. Lett. 169, 77–80. 10.1016/0304-3940(94)90360-3 [DOI] [PubMed] [Google Scholar]
- Feldman D. E. (2002). Synapses, scaling and homeostasis in vivo. Nat. Neurosci. 5, 712–714. 10.1038/nn0802-712 [DOI] [PubMed] [Google Scholar]
- Fillit H., Ding W., Buee L., Kalman J., Altstiel L., Lawlor B., et al. (1991). Elevated circulating tumor necrosis factor levels in Alzheimer’s disease. Neurosci. Lett. 129, 318–320. 10.1016/0304-3940(91)90490-k [DOI] [PubMed] [Google Scholar]
- Fox K., Stryker M. (2017). Integrating hebbian and homeostatic plasticity: introduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160413. 10.1098/rstb.2016.0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank C. A. (2014). Homeostatic plasticity at the Drosophila neuromuscular junction. Neuropharmacology 78, 63–74. 10.1016/j.neuropharm.2013.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinaro J. V., Rotter S. (2018). Associative properties of structural plasticity based on firing rate homeostasis in recurrent neuronal networks. Sci. Rep. 8:3754. 10.1038/s41598-018-22077-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsworthy M. R., Vallence A. M. (2013). The role of beta-amyloid in Alzheimer’s disease-related neurodegeneration. J. Neurosci. 33, 12910–12911. 10.1523/JNEUROSCI.2252-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González O. C., Krishnan G. P., Chauvette S., Timofeev I., Sejnowski T., Bazhenov M. (2015). Modeling of age-dependent epileptogenesis by differential homeostatic synaptic scaling. J. Neurosci. 35, 13448–13462. 10.1523/jneurosci.5038-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Z., Liu W., Yan Z. (2009). β-Amyloid impairs AMPA receptor trafficking and function by reducing Ca2+/calmodulin-dependent protein kinase II synaptic distribution. J. Biol. Chem. 284, 10639–10649. 10.1074/jbc.M806508200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A., Goyal R. (2016). Amyloid beta plaque: a culprit for neurodegeneration. Acta Neurol. Belg. 116, 445–450. 10.1007/s13760-016-0639-9 [DOI] [PubMed] [Google Scholar]
- Hansen D. V., Hanson J. E., Sheng M. (2018). Microglia in Alzheimer’s disease. J. Cell Biol. 217, 459–472. 10.1083/jcb.201709069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb D. O. (1949). The Organization of Behavior; A Neuropsychological Theory. Oxford, England: Wiley. [Google Scholar]
- Hemonnot A.-L., Hua J., Ulmann L., Hirbec H. (2019). Microglia in Alzheimer disease: well-known targets and new opportunities. Front. Aging Neurosci. 11:233. 10.3389/fnagi.2019.00233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengen K. B., Torrado Pacheco A., Mcgregor J. N., Van Hooser S. D., Turrigiano G. G. (2016). Neuronal firing rate homeostasis is inhibited by sleep and promoted by wake. Cell 165, 180–191. 10.1016/j.cell.2016.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herms J., Anliker B., Heber S., Ring S., Fuhrmann M., Kretzschmar H., et al. (2004). Cortical dysplasia resembling human type 2 lissencephaly in mice lacking all three APP family members. EMBO J. 23, 4106–4115. 10.1038/sj.emboj.7600390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hick M., Herrmann U., Weyer S. W., Mallm J. P., Tschape J. A., Borgers M., et al. (2015). Acute function of secreted amyloid precursor protein fragment APPsalpha in synaptic plasticity. Acta Neuropathol. 129, 21–37. 10.1007/s00401-014-1368-x [DOI] [PubMed] [Google Scholar]
- Hitt B. D., Jaramillo T. C., Chetkovich D. M., Vassar R. (2010). BACE1−/− mice exhibit seizure activity that does not correlate with sodium channel level or axonal localization. Adv. Exp. Med. Biol. 5:31. 10.1186/1750-1326-5-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Zhou X., He W., Yang J., Xiong W., Wong P., et al. (2010). BACE1 deficiency causes altered neuronal activity and neurodegeneration. J. Neurosci. 30, 8819–8829. 10.1523/jneurosci.1334-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida A., Furukawa K., Keller J. N., Mattson M. P. (1997). Secreted form of beta-amyloid precursor protein shifts the frequency dependency for induction of LTD and enhances LTP in hippocampal slices. Neuroreport 8, 2133–2137. 10.1097/00001756-199707070-00009 [DOI] [PubMed] [Google Scholar]
- Jang S. S., Chung H. J. (2016). Emerging link between Alzheimer’s disease and homeostatic synaptic plasticity. Neural Plast. 2016:7969272. 10.1155/2016/7969272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J., Whitcomb D. J., Olsen K. M., Kerrigan T. L., Lo S. C., Bru-Mercier G., et al. (2011). Aβ(1–42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3beta. Nat. Neurosci. 14, 545–547. 10.1038/nn.2785 [DOI] [PubMed] [Google Scholar]
- Kamenetz F., Tomita T., Hsieh H., Seabrook G., Borchelt D., Iwatsubo T., et al. (2003). APP processing and synaptic function. Neuron 37, 925–937. 10.1016/s0896-6273(03)00124-7 [DOI] [PubMed] [Google Scholar]
- Keck T., Scheuss V., Jacobsen R. I., Wierenga C. J., Eysel U. T., Bonhoeffer T., et al. (2011). Loss of sensory input causes rapid structural changes of inhibitory neurons in adult mouse visual cortex. Neuron 71, 869–882. 10.1016/j.neuron.2011.06.034 [DOI] [PubMed] [Google Scholar]
- Keck T., Toyoizumi T., Chen L., Doiron B., Feldman D. E., Fox K., et al. (2017). Integrating hebbian and homeostatic plasticity: the current state of the field and future research directions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160158. 10.1098/rstb.2016.0158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. E., Stamford A. W., Chen X., Cox K., Cumming J. N., Dockendorf M. F., et al. (2016). The BACE1 inhibitor verubecestat (MK-8931) reduces CNS β-amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl. Med. 8, 363ra150–363ra150. 10.1126/scitranslmed.aad9704 [DOI] [PubMed] [Google Scholar]
- Kim J., Tsien R. W. (2008). Synapse-specific adaptations to inactivity in hippocampal circuits achieve homeostatic gain control while dampening network reverberation. Neuron 58, 925–937. 10.1016/j.neuron.2008.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Ziff E. B. (2014). Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+-permeable AMPA receptors. PLoS Biol. 12:e1001900. 10.1371/journal.pbio.1001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney J. W., Bemiller S. M., Murtishaw A. S., Leisgang A. M., Salazar A. M., Lamb B. T. (2018). Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. 4, 575–590. 10.1016/j.trci.2018.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M., Yamasaki T. R., Laferla F. M. (2004). Microglia as a potential bridge between the amyloid beta-peptide and tau. Ann. N. Y. Acad. Sci. 1035, 85–103. 10.1196/annals.1332.006 [DOI] [PubMed] [Google Scholar]
- Klintsova A. Y., Greenough W. T. (1999). Synaptic plasticity in cortical systems. Curr. Opin. Neurobiol. 9, 203–208. 10.1016/s0959-4388(99)80028-2 [DOI] [PubMed] [Google Scholar]
- Kurup P., Zhang Y., Xu J., Venkitaramani D. V., Haroutunian V., Greengard P., et al. (2010). Aβ-mediated NMDA receptor endocytosis in Alzheimer’s disease involves ubiquitination of the tyrosine phosphatase STEP61. J. Neurosci. 30, 5948–5957. 10.1523/JNEUROSCI.0157-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. K., Barbarosie M., Kameyama K., Bear M. F., Huganir R. L. (2000). Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature 405, 955–959. 10.1038/35016089 [DOI] [PubMed] [Google Scholar]
- Lee K. J., Moussa C. E.-H., Lee Y., Sung Y., Howell B. W., Turner R. S., et al. (2010). Beta amyloid-independent role of amyloid precursor protein in generation and maintenance of dendritic spines. Neuroscience 169, 344–356. 10.1016/j.neuroscience.2010.04.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S., Ali C., Gabriel C., Croci N., Mackenzie E. T., Glabe C. G., et al. (2005). NMDA receptor activation inhibits alpha-secretase and promotes neuronal amyloid-beta production. J. Neurosci. 25, 9367–9377. 10.1523/jneurosci.0849-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letzkus J. J., Wolff S. B., Lüthi A. (2015). Disinhibition, a circuit mechanism for associative learning and memory. Neuron 88, 264–276. 10.1016/j.neuron.2015.09.024 [DOI] [PubMed] [Google Scholar]
- Li L., Gainey M. A., Goldbeck J. E., Feldman D. E. (2014). Rapid homeostasis by disinhibition during whisker map plasticity. Proc. Natl. Acad. Sci. U S A 111, 1616–1621. 10.1073/pnas.1312455111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y., Bolon B., Kahn S., Bennett B. D., Babu-Khan S., Denis P., et al. (2001). Mice deficient in BACE1, the Alzheimer’s beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 4, 231–232. 10.1038/85059 [DOI] [PubMed] [Google Scholar]
- Magara F., Müller U., Li Z.-W., Lipp H.-P., Weissmann C., Stagljar M., et al. (1999). Genetic background changes the pattern of forebrain commissure defects in transgenic mice underexpressing the β-amyloid-precursor protein. Proc. Natl. Acad. Sci. U S A 96, 4656–4661. 10.1073/pnas.96.8.4656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R., Malenka R. C. (2002). AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126. 10.1146/annurev.neuro.25.112701.142758 [DOI] [PubMed] [Google Scholar]
- Marder E., Prinz A. A. (2003). Current compensation in neuronal homeostasis. Neuron 37, 2–4. 10.1016/s0896-6273(02)01173-x [DOI] [PubMed] [Google Scholar]
- Meziane H., Dodart J. C., Mathis C., Little S., Clemens J., Paul S. M., et al. (1998). Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc. Natl. Acad. Sci. U S A 95, 12683–12688. 10.1073/pnas.95.21.12683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. D., Mackay D. J. (1994). The role of constraints in Hebbian learning. Neural Compu. 6, 100–126. 10.1162/neco.1994.6.1.100 [DOI] [Google Scholar]
- Monday H. R., Younts T. J., Castillo P. E. (2018). Long-term plasticity of neurotransmitter release: emerging mechanisms and contributions to brain function and disease. Ann. Rev. Neurosci. 41, 299–322. 10.1146/annurev-neuro-080317-062155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Zhao C., Gage F. H. (2011). Dopaminergic modulation of cortical inputs during maturation of adult-born dentate granule cells. J. Neurosci. 31, 4113–4123. 10.1523/jneurosci.4913-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller U. C., Deller T., Korte M. (2017). Not just amyloid: physiological functions of the amyloid precursor protein family. Nat. Rev. Neurosci. 18, 281–298. 10.1038/nrn.2017.29 [DOI] [PubMed] [Google Scholar]
- Müller-Hill B., Beyreuther K. (1989). Molecular biology of Alzheimer’s disease. Annu. Rev. Biochem. 58, 287–307. 10.1146/annurev.bi.58.070189.001443 [DOI] [PubMed] [Google Scholar]
- Nieweg K., Andreyeva A., Van Stegen B., Tanriöver G., Gottmann K. (2015). Alzheimer’s disease-related amyloid-β induces synaptotoxicity in human iPS cell-derived neurons. Cell Death Dis. 6, e1709–e1709. 10.1038/cddis.2015.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsch R. M., Slack B. E., Wurtman R. J., Growdon J. H. (1992). Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science 258, 304–307. 10.1126/science.1411529 [DOI] [PubMed] [Google Scholar]
- O’Brien R. J., Wong P. C. (2011). Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 34, 185–204. 10.1146/annurev-neuro-061010-113613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opazo P., Viana Da Silva S., Carta M., Breillat C., Coultrap S. J., Grillo-Bosch D., et al. (2018). CaMKII metaplasticity drives Aβ oligomer-mediated synaptotoxicity. Cell Rep. 23, 3137–3145. 10.1016/j.celrep.2018.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani S., Daniel H., Roisin M. P., Crepel F. (2003). Dopaminergic modulation of long-term synaptic plasticity in rat prefrontal neurons. Cereb. Cortex 13, 1251–1256. 10.1093/cercor/bhg092 [DOI] [PubMed] [Google Scholar]
- Perez R. G., Zheng H., Van Der Ploeg L. H., Koo E. H. (1997). The β-amyloid precursor protein of Alzheimer’s disease enhances neuron viability and modulates neuronal polarity. J. Neurosci. 17, 9407–9414. 10.1523/jneurosci.17-24-09407.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzoldt A. G., Lützkendorf J., Sigrist S. J. (2016). Mechanisms controlling assembly and plasticity of presynaptic active zone scaffolds. Curr. Opin. Neurobiol. 39, 69–76. 10.1016/j.conb.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Phillips H. S., Hains J. M., Armanini M., Laramee G. R., Johnson S. A., Winslow J. W. (1991). BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron 7, 695–702. 10.1016/0896-6273(91)90273-3 [DOI] [PubMed] [Google Scholar]
- Pozo K., Goda Y. (2010). Unraveling mechanisms of homeostatic synaptic plasticity. Neuron 66, 337–351. 10.1016/j.neuron.2010.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannals M. D., Kapur J. (2011). Homeostatic strengthening of inhibitory synapses is mediated by the accumulation of GABA(A) receptors. J. Neurosci. 31, 17701–17712. 10.1523/jneurosci.4476-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring S., Weyer S. W., Kilian S. B., Waldron E., Pietrzik C. U., Filippov M. A., et al. (2007). The secreted beta-amyloid precursor protein ectodomain APPs alpha is sufficient to rescue the anatomical, behavioral and electrophysiological abnormalities of APP-deficient mice. J. Neurosci. 27, 7817–7826. 10.1523/JNEUROSCI.1026-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberds S. L., Anderson J., Basi G., Bienkowski M. J., Branstetter D. G., Chen K. S., et al. (2001). BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 10, 1317–1324. 10.1093/hmg/10.12.1317 [DOI] [PubMed] [Google Scholar]
- Roselli F., Livrea P., Almeida O. F. (2011). CDK5 is essential for soluble amyloid β-induced degradation of GKAP and remodeling of the synaptic actin cytoskeleton. PLoS One 6:e23097. 10.1371/journal.pone.0023097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli F., Tirard M., Lu J., Hutzler P., Lamberti P., Livrea P., et al. (2005). Soluble β-amyloid1–40 induces NMDA-dependent degradation of postsynaptic density-95 at glutamatergic synapses. J. Neurosci. 25, 11061–11070. 10.1523/JNEUROSCI.3034-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozov A. V., Valiullina F. F., Bolshakov A. P. (2017). Mechanisms of long-term plasticity of hippocampal GABAergic synapses. Biochemistry 82, 257–263. 10.1134/S0006297917030038 [DOI] [PubMed] [Google Scholar]
- Rutherford L. C., Nelson S. B., Turrigiano G. G. (1998). BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron 21, 521–530. 10.1016/s0896-6273(00)80563-2 [DOI] [PubMed] [Google Scholar]
- Samidurai M., Ramasamy V. S., Jo J. (2018). β-amyloid inhibits hippocampal LTP through TNFR/IKK/NF-kappaB pathway. Neurol. Res. 40, 268–276. 10.1080/01616412.2018.1436872 [DOI] [PubMed] [Google Scholar]
- Scheefhals N., MacGillavry H. D. (2018). Functional organization of postsynaptic glutamate receptors. Mol. Cell. Neurosci. 91, 82–94. 10.1016/j.mcn.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook G. R., Smith D. W., Bowery B. J., Easter A., Reynolds T., Fitzjohn S. M., et al. (1999). Mechanisms contributing to the deficits in hippocampal synaptic plasticity in mice lacking amyloid precursor protein. Neuropharmacology 38, 349–359. 10.1016/s0028-3908(98)00204-4 [DOI] [PubMed] [Google Scholar]
- Sheynikhovich D., Otani S., Arleo A. (2013). Dopaminergic control of long-term depression/long-term potentiation threshold in prefrontal cortex. J. Neurosci. 33, 13914–13926. 10.1523/jneurosci.0466-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. M., Zhang N., Hansen J., Gerges N. Z., Pak D. T., Sheng M., et al. (2012). GKAP orchestrates activity-dependent postsynaptic protein remodeling and homeostatic scaling. Nat. Neurosci. 15:1655. 10.1038/nn.3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song I., Huganir R. L. (2002). Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 25, 578–588. 10.1016/s0166-2236(02)02270-1 [DOI] [PubMed] [Google Scholar]
- Sprekeler H. (2017). Functional consequences of inhibitory plasticity: homeostasis, the excitation-inhibition balance and beyond. Curr. Opin. Neurobiol. 43, 198–203. 10.1016/j.conb.2017.03.014 [DOI] [PubMed] [Google Scholar]
- Stellwagen D., Malenka R. C. (2006). Synaptic scaling mediated by glial TNF-α. Nature 440, 1054–1059. 10.1038/nature04671 [DOI] [PubMed] [Google Scholar]
- Strehl A., Galanis C., Radic T., Schwarzacher S. W., Deller T., Vlachos A. (2018). Dopamine modulates homeostatic excitatory synaptic plasticity of immature dentate granule cells in entorhino-hippocampal slice cultures. Front. Mol. Neurosci. 11:303. 10.3389/fnmol.2018.00303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styr B., Slutsky I. (2018). Imbalance between firing homeostasis and synaptic plasticity drives early-phase Alzheimer’s disease. Nat. Neurosci. 21, 463–473. 10.1038/s41593-018-0080-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Turrigiano G. G. (2011). PSD-95 and PSD-93 play critical but distinct roles in synaptic scaling up and down. J. Neurosci. 31, 6800–6808. 10.1523/jneurosci.5616-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanwick C. C., Murthy N. R., Kapur J. (2006). Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Mol. Cell. Neurosci. 31, 481–492. 10.1016/j.mcn.2005.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C. J., Ireland D. R., Ballagh I., Bourne K., Marechal N. M., Turner P. R., et al. (2008). Endogenous secreted amyloid precursor protein-alpha regulates hippocampal NMDA receptor function, long-term potentiation and spatial memory. Neurobiol. Dis. 31, 250–260. 10.1016/j.nbd.2008.04.011 [DOI] [PubMed] [Google Scholar]
- Tononi G., Cirelli C. (2014). Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron 81, 12–34. 10.1016/j.neuron.2013.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend M., Mehta T., Selkoe D. J. (2007). Soluble Abeta inhibits specific signal transduction cascades common to the insulin receptor pathway. J. Biol. Chem. 282, 33305–33312. 10.1074/jbc.m610390200 [DOI] [PubMed] [Google Scholar]
- Turner P. R., O’connor K., Tate W. P., Abraham W. C. (2003). Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog. Neurobiol. 70, 1–32. 10.1016/s0301-0082(03)00089-3 [DOI] [PubMed] [Google Scholar]
- Turrigiano G. (2012). Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb. Perspect. Biol. 4:a005736. 10.1101/cshperspect.a005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. G. (2008). The self-tuning neuron: synaptic scaling of excitatory synapses. Cell 135, 422–435. 10.1016/j.cell.2008.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. G., Nelson S. B. (2004). Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 5, 97–107. 10.1038/nrn1327 [DOI] [PubMed] [Google Scholar]
- Turrigiano G. G., Leslie K. R., Desai N. S., Rutherford L. C., Nelson S. B. (1998). Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391, 892–896. 10.1038/36103 [DOI] [PubMed] [Google Scholar]
- Tyan S. H., Shih A. Y., Walsh J. J., Maruyama H., Sarsoza F., Ku L., et al. (2012). Amyloid precursor protein (APP) regulates synaptic structure and function. Mol. Cell Neurosci. 51, 43–52. 10.1016/j.mcn.2012.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Kant R., Goldstein L. S. (2015). Cellular functions of the amyloid precursor protein from development to dementia. Dev. Cell 32, 502–515. 10.1016/j.devcel.2015.01.022 [DOI] [PubMed] [Google Scholar]
- Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., et al. (1999). β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741. 10.1126/science.286.5440.735 [DOI] [PubMed] [Google Scholar]
- Vitureira N., Goda Y. (2013). Cell biology in neuroscience: the interplay between Hebbian and homeostatic synaptic plasticity. J. Cell Biol. 203, 175–186. 10.1083/jcb.201306030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachos A., Ikenberg B., Lenz M., Becker D., Reifenberg K., Bas-Orth C., et al. (2013). Synaptopodin regulates denervation-induced homeostatic synaptic plasticity. Proc. Natl. Acad. Sci. U S A 110, 8242–8247. 10.1073/pnas.1213677110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vnencak M., Scholvinck M. L., Schwarzacher S. W., Deller T., Willem M., Jedlicka P. (2019). Lack of beta-amyloid cleaving enzyme-1 (BACE1) impairs long-term synaptic plasticity but enhances granule cell excitability and oscillatory activity in the dentate gyrus in vivo. Brain Struct. Funct. 224, 1279–1290. 10.1007/s00429-019-01836-6 [DOI] [PubMed] [Google Scholar]
- Weyer S. W., Zagrebelsky M., Herrmann U., Hick M., Ganss L., Gobbert J., et al. (2014). Comparative analysis of single and combined APP/APLP knockouts reveals reduced spine density in APP-KO mice that is prevented by APPsalpha expression. Acta Neuropathol. Commun. 2:36. 10.1186/2051-5960-2-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wondolowski J., Dickman D. (2013). Emerging links between homeostatic synaptic plasticity and neurological disease. Front. Cell. Neurosci. 7:223. 10.3389/fncel.2013.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R., Vassar R. (2014). Targeting the β secretase BACE1 for Alzheimer’s disease therapy. Lancet Neurol. 13, 319–329. 10.1016/S1474-4422(13)70276-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee A. X., Hsu Y. T., Chen L. (2017). A metaplasticity view of the interaction between homeostatic and Hebbian plasticity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 372:20160155. 10.1098/rstb.2016.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youn Y. C., Kang S., Suh J., Park Y. H., Kang M. J., Pyun J. M., et al. (2019). Blood amyloid-beta oligomerization associated with neurodegeneration of Alzheimer’s disease. Alzheimers. Res. Ther. 11:40. 10.1186/s13195-019-0499-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenke F., Hennequin G., Gerstner W. (2013). Synaptic plasticity in neural networks needs homeostasis with a fast rate detector. PLoS Comput. Biol. 9:e1003330. 10.1371/journal.pcbi.1003330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Watson J. B., Xie C.-W. (2004). Amyloid β prevents activation of calcium/calmodulin-dependent protein kinase II and AMPA receptor phosphorylation during hippocampal long-term potentiation. J. Neurophysiol. 92, 2853–2858. 10.1152/jn.00485.2004 [DOI] [PubMed] [Google Scholar]
- Zhao X., Huang L., Guo R., Liu Y., Zhao S., Guan S., et al. (2017). Coordinated plasticity among glutamatergic and GABAergic neurons and synapses in the barrel cortex is correlated to learning efficiency. Front. Cell Neurosci. 11:221. 10.3389/fncel.2017.00221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu K., Xiang X., Filser S., Marinkovic P., Dorostkar M. M., Crux S., et al. (2018). Beta-site amyloid precursor protein cleaving enzyme 1 inhibition impairs synaptic plasticity via seizure protein 6. Biol. Psychiatry 83, 428–437. 10.1016/j.biopsych.2016.12.023 [DOI] [PubMed] [Google Scholar]