Abstract
The kidney is comprised of highly complex structures that rely on self-maintenance for their functions, and tissue repair and regeneration in renal diseases. We devised a proteomics assay to measure the turnover of individual proteins in mouse kidney. Mice were metabolically labeled with a specially formulated chow containing nitrogen-15 (15N) with the absence of normal 14N atoms. Newly synthesized proteins with 15N contents were distinguished from their 14N counterparts by mass spectrometry. In total, we identified over 4,000 proteins from the renal cortex with a majority of them contained only 15N. About 100 proteins had both 14N- and 15N-contents. Notably, the long-lived proteins that had large 14N/15N ratios were mostly matrix proteins. These included proteins such as type IV and type VI collagen, laminin, nidogen and perlecan/HSPG2 that constitute the axial core of the glomerular basement membrane (GBM). In contrast, the surface lamina rara proteins such as agrin and integrin had much shorter longevity, suggesting their faster regeneration cycle. The data illustrated matrix proteins that constitute the basement membranes in the renal cortex are constantly renewed in an ordered fashion. In perspective, the global profile of protein turnover is usefully in understanding the protein-basis of GBM maintenance and repair.
Subject terms: Biochemistry, Biological techniques
Introduction
The basement membrane matrices have important roles in the kidney. The glomerular basement membrane (GBM) is an integral part of the filtration barrier between the layers of fenestrated endothelial cells and the interdigitated podocyte foot processes1,2. Characterized as a finely interwoven network of collagen and laminin fibers, its long-term integrity is maintained by the adjacent cells through an incompletely understood process3,4. It is known that the matrix proteins are produced, and presumably also recycled, by these two cell types. Genetic mutations of the genes encoding the GBM proteins are associated with kidney diseases, such as Alport syndrome and Pierson syndrome5–12. In a number of kidney diseases caused by systemic conditions such as diabetes, autoimmune including Goodpasture disease, membranous nephropathy and dense deposit disease of C3 glomerulopathy, among others, thickening and irregularity of the GBM are the characteristic features of the respective diseases13–16. Although the mechanisms for the pathological transformation remain unknown, it can be postulated that the normal turnover needed for maintaining the balance of matrix proteins is altered.
In order to gain insight into the natural dynamics of protein turnover in the kidney, particularly in extracellular matrices, we conducted a proteomic study following 12 weeks of feeding mice with stable isotope nitrogen-15 (15N). During this period of time, newly synthesized proteins had incorporated 15N in their proteins. By performing mass spectrometry to compare the 14N versus 15N content of each protein through a proteomic method known as stable isotope labeling of mammals (SILAM)17,18, we measured the turnover dynamics of thousands of proteins in the mouse kidney.
Results
Extremely long-lived proteins in the urea-insoluble fraction of kidney lysate
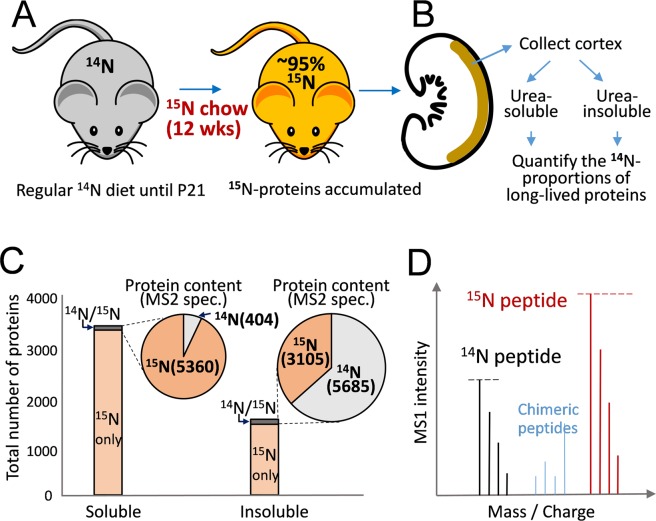
Individual proteins have a wide range of in vivo turnover rate19,20, and the balance between protein synthesis and catabolism is tightly regulated in health and disease conditions. As we intended to address the long-standing question as to whether mature GBM is actively renewed4,21, we had chosen a 12-week labeling schedule. Starting from postnatal age of P21 for a consecutive 12 weeks, C57BL/6 J mice were subjected to dietary treatment of exclusive blue-green algae-based chow with all nitrogen atoms being 15N with the absence of 14N (Fig. 1A and Methods). These animals developed normally22. From the kidney cortex, we extracted most cellular proteins with 8 M urea solution, whereas matrix proteins were enriched in the urea-insoluble fraction (Fig. 1B). By performing liquid chromatography-tandem mass spectrometry (LC-MS/MS), 3482 proteins were identified in the urea-soluble fraction and 1562 proteins in the urea-insoluble fraction (Fig. 1C). Among these proteins, only 87 and 109 proteins in the soluble and insoluble fractions respectively were also detectable with their 14N-peptides that had existed for 12 weeks or longer. When the relative amounts between older 14N and newer 15N proteins were evaluated by MS2 spectral count values, the insoluble fraction contains a larger quantity of the older proteins than the soluble fraction (measured by total spectral count: 5685 vs. 404 as in Fig. 1C insets: pie charts), suggesting matrix proteins in general live longer.
Figure 1.
The 15N labeling workflow in conjunction with mass spectrometry identified long-lived proteins in the kidney cortex. (A) C57BL/6 J mice were subjected to an exclusive 15N chow starting on P21 for 12 consecutive weeks. 15N was incorporated into newly synthesized proteins (represented by red color). (B) Three kidneys from three mice were used to collect the cortex tissues (marked with brown color), which were then combined and extracted for proteins using 8 M urea solution. The samples were fractionated into urea-soluble and urea-insoluble fractions and separately digested with Trypsin/Lys-C endopeptidases. The resulting peptides were detected as either fully 14N- or fully 15N-peptides by LC-MS/MS and the remaining 14N-content of each individual protein as the proportion of the total protein amount was calculated. (C) In the bar graph, the total number of individual proteins detected either through their 14N- or 15N-peptides were counted. In total, 3482 individual proteins were detected from the urea-soluble fraction and 1562 from the urea-insoluble fraction, of which the majority proteins with the exceptions of 87 and 109 proteins respectively were identified only by their labeled 15N peptides. As shown in the pie-chart insets, between the 87 and 109 protein groups we evaluated their 14N versus 15N contents as a whole based on the sum of MS2 spectral counts of all proteins in each group (value in parenthesis). Clearly, the soluble fraction contained more than 10 times less remaining 14N proteins in total quantity, 404 versus 5685 spectra. (D) In order to calculate the proportions between 14N and 15N contents of an individual protein, we relied on the MS1 intensity values of its peptide spectra (represented by the black and red spikes respectively), and collectively calculated the composite ratios of respective under-curve-areas based on the reconstructed chromatographs of corresponding 14N versus 15N peptide pairs (not shown, details in Methods). Chimeric peptides shown in blue represent partially labeled peptides that contained both 14N and 15N, which were not accounted for in the calculation of 14N proportion of individual proteins. The figures were created in Adobe Illustrator v24.0 (https://www.adobe.com/products/illustrator.html).
Long-lived collagen and laminin of the glomerular basement membrane
Beside the proteins that had been completely replaced by their new 15N counterparts, those detected with both 14N- and 15N-peptides had let us estimate their old versus new proportions based on MS1 signal intensities of the corresponding 14N and 15N spectral pairs (Fig. 1D). We note that proteins with high abundance of remaining 14N were the extracellular matrix proteins and certain histone protein isoforms (Supplementary File 1). The longevity of histone isoforms such as H3.1 and H3.2, and some H2 variants were likely due to their expression associated with DNA replication, and that they are not degraded as cells divide. Under these circumstances, their abundance in 14N contents suggests these histone variants were mainly from slow- or non-dividing cells in the kidney.
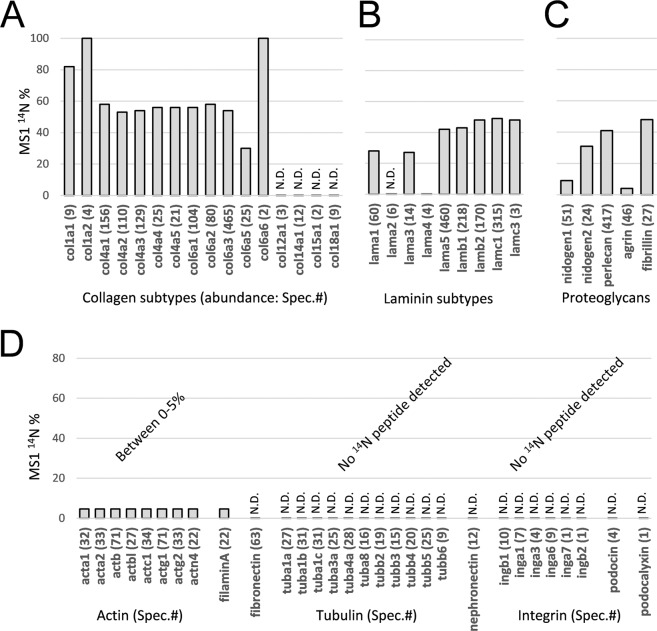
Matrix proteins such as type IV and VI Collagens, Laminins and Perlecan (basement membrane-specific heparan sulfate proteoglycan/HSPG/HSPG2) all had high 14N contents. In total, sixteen distinct collagen isoforms were detected. Collagen-XII, XIV, XV and XVIII were only detected in the fully 15N channel, indicating complete turnover. Meanwhile, collagen-I, IV and VI, which abundance levels were higher, retained 30–83% 14N in the urea-insoluble sample (Fig. 2A). Collagen 4α1–5 that are the main components of the renal basement membrane structures were detected at varying levels, from 21 spectra for Collagen 4α5 to 156 spectra for Collagen 4α1 (Supplementary File 1), however, all had similar 14N contents between 54% and 59% that reflected their individual turnover dynamics (Fig. 2A). Collagen 4α1 and 4α2 are components of the tubular basement membrane (TBM) and Collagen 4α3–5 form the key fibrous meshwork of the GBM. Their maintenance, regeneration and remodeling have been extensively studied in normal kidney physiology as well as renal diseases, such as X-linked and autosomal recessive Alport syndrome carrying genetic mutations in COL4A3, COL4A4 or COL4A5 gene.
Figure 2.
Collagen, laminin and other components of the GBM have the most long-lived proteins with high remaining 14N contents. (A–C) The matrix proteins such as collagen, laminin and proteoglycan proteins were analyzed for their remaining 14N proportions in the urea-insoluble fraction. The y-axis is the percentage of 14N content and the x-axis lists all identified proteins in the group. (D) In contrast, intracellular proteins had more complete turnover to 15N. Actin, tubulin cytoskeletal proteins and membrane proteins connecting to the GBM such as integrin and podocin all had low (between 0–5%) or no 14N detected (N.D.: not detected). Note that artificial upper and lower limits of the 14N/15N ratios were set at 20 and 0.05, and therefore any measured 14N contents between 0–5% had been artificially roundup to 5% as in actin and filamin-A.
Similarly to collagen, laminin retained 24–49% of their 14N contents across their nine identified isoforms of α1–5, β1 and 2, and γ1 and 3 (Fig. 2B)(Mutations of LAMB2 that encodes Laminin β2 are associated with Pierson syndrome). As it appeared, only Laminin α5, β1, β2 and γ1 were detected at high levels (Fig. 2B), suggesting α5/β1/γ1 (referred to as LM-511) and α5/β2/γ1 (LM-521) being the dominant configurations of the triple helices. Among them 42% to 49% of remaining 14N contents detected, which was slightly lower than of percentages of type IV collagen, possibly indicating faster turnover of laminin α5/β1,2/γ1 than collagen IV.
With respect to the linker proteoglycan proteins of the GBM meshwork, namely nidogen and perlecan that bind collagen-IV, as well as nidogen and agrin that bind the laminin matrix23, they had varying percentage contents of 14N (Fig. 2C). While Perlecan and Nidogen-2 (also known as osteonidogen) had 41% and 31% 14N remaining with 417 and 24 total spectra identified respectively, Agrin and Nidogen-1 (also known as entactin) had only 4.7% and 9% 14N remaining represented by 46 and 51 total spectra respectively. It should be noted that Agrin and Nidogen-2 form the lamina rara between the GBM and the cell layers24,25 that are expected to turnover more completely than the GBM core proteins. Therefore it was unexpected that Nidogen-2 out-lived Nidogen-1. Transiently expressed ECM protein such as nephronectin (Npnt) had reached complete turnover, as well as fibronectin that forms direct contact with cell surface proteins (Fig. 2D). Moving further towards the cell layers, transmembrane proteins such as integrin and podycalyxin that anchor the cells to the GBM had all reached their complete turnover from 14N to 15N (Fig. 2D). Also as expected, intracellular proteins such as actin, tubulin, podocin were completely or almost completely reconstituted with the newer 15N atoms (Fig. 2D).
Urea-insoluble GBM proteins lived longer than their soluble counterparts – suggesting the surface layers more frequently renewed
Although extraction with 8 M urea was a crude method to fractionate cellular versus matrix proteins, at a global level, it provided an approximate estimation of how tightly each protein was associated to the matrices based on solubility. In this regard, for any individual proteins as being expressed by cells and integrated into matrix, we sought to explore whether they initially adopted a relatively “loose” association with the matrix (likely through non-covalent binding that did not withstand 8 M urea). To this end, we directly compared the 14N proportions of the same protein in urea-soluble versus insoluble fractions (Fig. 3A).
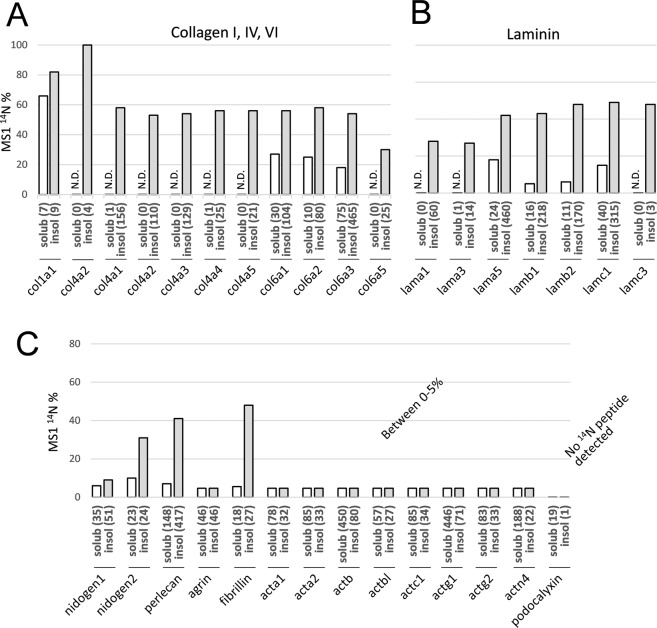
Figure 3.
Comparison of remaining 14N percentage among individual proteins in urea-soluble versus insoluble fractions. (A,B) Individual collagen and laminin proteins with their total levels (value as MS2 spectral count in parenthesis) and remaining 14N percentage (y-axis value) separately compared between their urea-soluble (open bar) and insoluble isolates (filled bar). Note that proteins in the soluble fraction had either no or less 14N proportions than their insoluble counterparts. (C) Other GBM proteins such as nidogen, perlecan and fibrillin all had the similar profile, whereas cytoskeletal actin, although more abundantly harvested in the soluble fraction (spectral count value in parenthesis), with its insoluble contents completely turned over to 15N.
Remarkably, collagen-IV was nearly absent with only 2 spectra recorded in the soluble fraction, as compared to 441 spectral counts recorded in the insoluble fraction, suggesting covalent association of collagen-IV to the matrix immediately after being assembled into the GBM. As for soluble laminin, there were 24, 16, 11 and 40 spectra recorded for α5, β1, β2 and γ1 respectively. The 14N percentages were 18%, 5%, 6% and 15%, respectively, in contrast to 42%, 43%, 48% and 49% in urea-insoluble fraction (Fig. 3B). These data indicate that newly expressed (15N) and assembled laminin was more loosely associated with the matrix core. Similarly, soluble perlecan had 7.4% remaining 14N compared to 41% 14N in the insoluble form; and soluble nidogen had 6% and 10% (for isoforms 1 and 2) remaining 14N compared to 9% and 31% of the same proteins in the insoluble fractions. By contrast, agrin had only 4.7% remaining 14N in both fractions (Fig. 3C), consistent with its association with the lamina rara that is constantly renewed by the podocytes and endothelia23,25,26.
Discussion
By metabolic labeling of whole mice with exclusively heavy 15N diet for 12 consecutive weeks, we measured the remaining proportions of the 14N-proteins in the kidney by mass spectrometry. Most 14N contents were in the urea-insoluble fraction of mainly matrix proteins. It should be noted that our choice of labeling between 3 and 15 weeks was mainly based on the weaning schedule, and that 15N was provided conveniently at the transition to solid food. This choice of timeline would have complicated the outcome of 15N/14N ratios in a number of ways beyond regular protein turnover. For instance, when the pups grew in physical size, newer 15N proteins in the kidney might have swamped their 14N counterparts in quantity, and the calculated ratios no longer accurately reflect the rate of turnover. In addition, proteins that were not expressed before 3 weeks of age might have been inadvertently attributed to rapid turnover. Therefore, depending on the specific purpose of a study, we stress the need to design labeling schedule accordingly.
Although our global analysis does not distinguish glomerular versus tubular basement membrane matrices, known components of the GBM generally had higher proportions of long-lived 14N proteins. As collagen-IV α3/4/5 form the key fibrous meshwork of the GBM9, their maintenance, regeneration and remodeling have been extensively studied in the context of Alport syndrome caused by mutations of Collagen IV proteins of the GBM5,7,9. Collagen IV and VI had the highest 14N percentage of over 50%, followed by laminin that together forms the core structure of the GBM, and then by linker proteoglycan agrin in proximity to the cell layers27,28. Lastly, the cell surface proteins that interact with the GBM such as integrin all had no remaining 14N. In general, these percentage values are reversely correlated with the turnover rates of these proteins, which to a certain degree reflect the natural course of GBM renewal. As expected, our protein longevity results correlate with the locations of these proteins within the GBM, with collagen IV and laminin concentrated in the lamina densa, agrin enriched in the outer layer of lamina rara, and integrin forming the anchors for the adjacent cells27. However, it is important to note that perlecan had a much longer lifetime as compared to agrin, and similarly, linker proteins Nidogen-1 and -2 also had contrasting levels of 14N percentages, at 9% and 31% respectively. Our data from measuring protein turnover strongly suggest the GBM meshwork is renewed in an ordered fashion.
Overall, the study validated the general workflow of measuring molecular turnover in the renal cortex, particularly among matrix proteins that form important basement membrane structures in the kidney. It can be postulated that in human diseases that affect the histologic appearance of the GBM, such as GBM thickening and irregularity in diabetic nephropathy, ribbon-like GBM in dense-deposit disease and other immune-mediated membranous nephropathy types, there involve an altered pattern among the matrix proteins in their turnover kinetics. Testing these disease conditions with the 15N-labeling workflow may yield molecular insights into the underlying etiology and pathophysiology.
Materials and Methods
Stable isotope labeling in mouse (SILAM)
The method of 15N label of mice was described previously17,22. In brief, starting at postnatal day 21 for a consecutive 12 weeks, C57BL/6 J mice were fed exclusively with a 15N-raised spirulina diet (from Cambridge Isotopes and Harlan Laboratories). At the end, the 15N-proteins in the serum was determined to be greater than 99%22. All animal procedures were approved by Institutional Animal Care and Use Committee of the Northwestern University (approved protocol number IS00000429 and IS00000862), and carried out in accordance with the guidelines and regulations for the Care and Use of Laboratory Animals.
Lysis and fractionation of the kidney tissue
Freshly collected kidneys were flash-frozen and then stored in liquid nitrogen. The tissue was harvested by surgical dissection of the renal cortex with a small portion of the outer medulla, avoiding the papilla and the renal sinus. The collected tissue was minced into pieces with a scalpel and then submerged into 4x volume of 8 M urea solution. The mixture was homogenized by sonication, followed by centrifugation at 18,000 g for 10 min. The supernatant was collected as the urea-soluble fraction. The resulting pellet was washed three times with 500 μL of 8 M urea and subjected to repeated sonication followed by centrifugation in each round. At the end, SDS sample buffer containing 4% SDS, 20% glycerol, 10% 2-mercaptoethanol, 0.004% bromphenol blue and 0.125 M Tris HCl, pH = 6.8 was added to the final pellet. After an additional sonication round, there was no visible pellet remaining and the solution was considered the urea-insoluble fraction.
Proteomic preparation
The urea-soluble fraction was subjected to a standard in-solution digestion protocol. In brief, the 8 M solution that contained the soluble lysate was first diluted 4 times to a final solution containing 2 M urea. The proteins were subjected to standard reduction (with TCEP), alkylation (with iodoacetamide) before digested with Trypsin/Lys-C Mix (purchased from Promega Corp.) following the manufacturer’s standard digestion protocol for overnight at 37 °C. On the next day, the solution was loaded to a C18 column (Millipore) and tryptic peptides were recovered in acetyl nitrate (ACN) and then dried by vacuum. Separately, the urea insoluble fraction in SDS sample buffer was resolved by running 12% SDS PAGE. The sample was allowed to run ~1 cm into the gel, then the gel was stained with GelCode Blue (Thermo Fisher Scientific). The stained gel lane was excised into ~1 mm3-sized gel cubes. These gel cubes were subsequently subjected to a standard in-gel reduction, alkylation and Trypsin/Lys-C digestion (Promega) procedure.
Mass spectrometry
The procedures for 15N-based proteomics were described previously22,29. Briefly, after trypsin digestion 3 μg of the peptides was dissolved in 94.785% H2O, 5% acetonitrile (ACN), 0.125% formic acid (FA) solution, and then loaded onto a nanoViper C18 trap column. Following a two hour gradient to 99.875% ACN and 0.125% FA solution the peptides were separated, and then electrosprayed from the Nanospray Flex Ion Source and analyzed on the Orbitrap Fusion Tribrid mass spectrometer. MS parameters were as follows: ion transfer tube temp was 300 °C with Easy-IC internal mass calibration, and the default charge state was 2. Detector type was set to Orbitrap at 60 K resolution, wide quad isolation, normal scan mass range between 300 and 1500 m/z. Max injection time was 50 ms, AGC target was at 200,000, microscans was at 1, S-lens RF level was 60. Without source fragmentation, datatype for positive and centroid and MIPS was on, included charge states of 2–6 (reject unassigned). Dynamic exclusion enabled with n = 1. Precursor selection decision was based on the most intense, top 20, isolation window = 1.6, scan range = auto normal, first mass = 110, collision energy 30%, CID, Detector type = ion trap, max injection time = 75 ms, AGC target – 10,000, inject ions for all available parallelizable time.
Spectra analysis and protein quantification
Spectra analysis was done using Integrated Proteomics Pipeline (IP2), including running ProLuCID searches against the RefSeq mouse dataset. Basic parameters of 50 ppm precursor mass tolerance and 600 ppm for fragmented ions were used. Searches were filtered with DTAselect containing one peptide per protein, at least one tryptic end and unlimited missed cleavages of a minimum of 6 amino acid, with a false discovery rate (FDR) <0.001, fixed modification of +57.02146 Da on cysteine residues, and all precursor mass within 10 ppm of expected. To estimate peptide FDRs accurately (set at <1%), target/decoy database was used containing the reversed sequences of all the proteins appended to the target database30. Searches were done for combined light and heavy peptides and Census quantified31. Only fully 14N- or 15N-containing peptides were considered.
To calculate the 14N/15N peptide ion intensity, the ProLuCID results were used to reconstruct MS1 ion chromatograms in the m/z range that included both the heavy and light peptide22,31. The intensity ratios were then calculated per peptide using the reconstructed chromatogram. Census also allows for filtration of poor-quality peptide ratio measurements. With QuantCompare, the final peptide ratios were generated. For each protein, its heavy versus light ratios were represented by the composite of all peptide ratios identified by MS that are assigned to the protein. In cases of extremely low signals in one of the two channels, which will render extremely high or low ratio values mathematically, we arbitrarily set lower limit of the protein ratios at 0.05. The final list of RefSeq protein entries were searched against the UniProtKB database to obtain a non-redundant set of proteins based on their unique gene identifiers (listed in Supplementary File 1).
Supplementary information
Acknowledgements
We thank Laith Ali and Jeffery Savas for performing mass spectrometry and proteomic data analysis. This study was partly supported by National Institutes of Health (R21AI131087, to J.J.). We are grateful to Northwestern University George M. O’Brien Kidney Core Center (NU-GoKIDNEY, P30DK114857-01A1) for technical assistance.
Author contributions
P.L. performed experiments and analyzed data. J.J., P.L. and X.F.X. wrote the main manuscript text and prepared Figs. 1–3. All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62348-6.
References
- 1.Miner JH. Developmental biology of glomerular basement membrane components. Curr. Opin. Nephrol. Hypertens. 1998;7:13–19. doi: 10.1097/00041552-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Suh JH, Miner JH. The glomerular basement membrane as a barrier to albumin. Nat. Rev. Nephrol. 2013;9:470–477. doi: 10.1038/nrneph.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedchenko V, Kitching AR, Hudson BG. Goodpasture’s autoimmune disease - A collagen IV disorder. Matrix Biol. 2018;71-72:240–249. doi: 10.1016/j.matbio.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abrahamson DR. Role of the podocyte (and glomerular endothelium) in building the GBM. Semin. Nephrol. 2012;32:342–349. doi: 10.1016/j.semnephrol.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kashtan CE, et al. Alport syndrome: a unified classification of genetic disorders of collagen IV alpha345: a position paper of the Alport Syndrome Classification Working Group. Kidney Int. 2018;93:1045–1051. doi: 10.1016/j.kint.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 6.Savige J, et al. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J. Am. Soc. Nephrol. 2013;24:364–375. doi: 10.1681/ASN.2012020148. [DOI] [PubMed] [Google Scholar]
- 7.Kruegel J, Rubel D, Gross O. Alport syndrome–insights from basic and clinical research. Nat. Rev. Nephrol. 2013;9:170–178. doi: 10.1038/nrneph.2012.259. [DOI] [PubMed] [Google Scholar]
- 8.Perkoff, G. T. The hereditary renal diseases. N Engl J Med277, 79–85 contd, 10.1056/NEJM196707132770206 (1967). [DOI] [PubMed]
- 9.Funk SD, Lin MH, Miner JH. Alport syndrome and Pierson syndrome: Diseases of the glomerular basement membrane. Matrix Biol. 2018;71-72:250–261. doi: 10.1016/j.matbio.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chew C, Lennon R. Basement Membrane Defects in Genetic Kidney Diseases. Front. Pediatr. 2018;6:11. doi: 10.3389/fped.2018.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kashtan CE, Segal Y. Genetic disorders of glomerular basement membranes. Nephron Clin. Pract. 2011;118:c9–c18. doi: 10.1159/000320876. [DOI] [PubMed] [Google Scholar]
- 12.Hudson BG. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J. Am. Soc. Nephrol. 2004;15:2514–2527. doi: 10.1097/01.ASN.0000141462.00630.76. [DOI] [PubMed] [Google Scholar]
- 13.Pedchenko V, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N. Engl. J. Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith RJH, et al. C3 glomerulopathy - understanding a rare complement-driven renal disease. Nat. Rev. Nephrol. 2019;15:129–143. doi: 10.1038/s41581-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas M. Thin glomerular basement membrane nephropathy: incidence in 3471 consecutive renal biopsies examined by electron microscopy. Arch. Pathol. Lab. Med. 2006;130:699–706. doi: 10.1043/1543-2165(2006)130[699:TGBMNI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Tervaert TW, et al. Pathologic classification of diabetic nephropathy. J. Am. Soc. Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 17.Savas JN, Toyama BH, Xu T, Yates JR, 3rd, Hetzer MW. Extremely long-lived nuclear pore proteins in the rat brain. Science. 2012;335:942. doi: 10.1126/science.1217421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savas JN, Park SK, Yates JR., 3rd Proteomic Analysis of Protein Turnover by Metabolic Whole Rodent Pulse-Chase Isotopic Labeling and Shotgun Mass Spectrometry Analysis. Methods Mol. Biol. 2016;1410:293–304. doi: 10.1007/978-1-4939-3524-6_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toyama BH, et al. Identification of long-lived proteins reveals exceptional stability of essential cellular structures. Cell. 2013;154:971–982. doi: 10.1016/j.cell.2013.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verzijl N, et al. Effect of collagen turnover on the accumulation of advanced glycation end products. J. Biol. Chem. 2000;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- 21.Miner JH. Organogenesis of the kidney glomerulus: focus on the glomerular basement membrane. Organogenesis. 2011;7:75–82. doi: 10.4161/org.7.2.15275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu P, et al. Selective permeability of mouse blood-aqueous barrier as determined by (15)N-heavy isotope tracing and mass spectrometry. Proc. Natl Acad. Sci. USA. 2018;115:9032–9037. doi: 10.1073/pnas.1807982115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groffen AJ, Veerkamp JH, Monnens LA, van den Heuvel LP. Recent insights into the structure and functions of heparan sulfate proteoglycans in the human glomerular basement membrane. Nephrol. Dial. Transpl. 1999;14:2119–2129. doi: 10.1093/ndt/14.9.2119. [DOI] [PubMed] [Google Scholar]
- 24.Bader BL, et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell Biol. 2005;25:6846–6856. doi: 10.1128/MCB.25.15.6846-6856.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groffen AJ, et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J. Histochem. Cytochem. 1998;46:19–27. doi: 10.1177/002215549804600104. [DOI] [PubMed] [Google Scholar]
- 26.Borza DB. Glomerular basement membrane heparan sulfate in health and disease: A regulator of local complement activation. Matrix Biol. 2017;57-58:299–310. doi: 10.1016/j.matbio.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suleiman H, et al. Nanoscale protein architecture of the kidney glomerular basement membrane. Elife. 2013;2:e01149. doi: 10.7554/eLife.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morita H, et al. Heparan Sulfate of Perlecan Is Involved in Glomerular Filtration. J. Am. Soc. Nephrology. 2005;16:1703–1710. doi: 10.1681/asn.2004050387. [DOI] [PubMed] [Google Scholar]
- 29.Hickox AE, et al. Global Analysis of Protein Expression of Inner Ear Hair Cells. J. Neurosci. 2017;37:1320–1339. doi: 10.1523/jneurosci.2267-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias JE, Gygi SP. Target-decoy search strategy for mass spectrometry-based proteomics. Methods Mol. Biol. 2010;604:55–71. doi: 10.1007/978-1-60761-444-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park SK, Venable JD, Xu T, Yates JR., 3rd A quantitative analysis software tool for mass spectrometry-based proteomics. Nat. Methods. 2008;5:319–322. doi: 10.1038/nmeth.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.