Abstract
Functional dyspepsia (FD) and gastroparesis (GP) are common disorders of the upper gastrointestinal tract. The pathophysiology of these conditions is likely to be heterogenous, and factors such as altered motility, sensitivity and response to nutrition have been identified as putative underlying mechanisms. Motility, sensitivity as well as responses to nutrition can be influenced or mediated by peptide hormones and serotonin released from the gastrointestinal mucosa. This review summarizes the role of GI peptides in functional dyspepsia and gastroparesis. In most studies, the levels of somatostatin, ghrelin, and motilin did not differ between healthy volunteers and FD or GP patients, but higher symptom burden was often correlated with higher peptide levels. Ghrelin and motilin receptor agonists showed promising results in improvement of the gastric emptying, but the link with improvement of symptoms is less predictable. Serotonin agonists have a potential to improve symptoms in both FD and idiopathic gastroparesis. Drugs acting on the GLP-1 and on the PYY receptors deserve further investigation. There is a need for systematic large scale studies.
Keywords: functional dyspepsia, gastroparesis, gastrointestinal peptides, cholecystokinin, glucagonlike peptide 1, peptide YY, motilin, ghrelin
Introduction
Functional Dyspepsia
Functional dyspepsia (FD), defined as “epigastric symptoms affecting daily life, such as postprandial fullness, early satiation, epigastric pain and burning, in the absence of underlying organic abnormalities” (1), is an extremely common functional gastrointestinal disorder. In the general population, the prevalence of FD is found to be up to 21% (2, 3). Although only a minority of H. pylori infected patients remain asymptomatic after successful eradication therapy, patients reporting helicobacter pylori-associated dyspeptic symptoms are now being recognized as a separate entity referred to as H. pylori associated dyspepsia (1, 4, 5).
To facilitate the management of FD, the Rome Consensus subdivided FD into two subtypes: Postprandial Distress Syndrome (PDS) (60%) characterized by meal-related symptoms, such as postprandial fullness, early satiation, postprandial epigastric pain and other symptoms triggered by food ingestion, and Epigastric Pain Syndrome (EPS) (20%) characterized by epigastric pain and burning (4, 6). Approximately 20% of FD patients overlaps between PDS and EPS.
FD is extremely common, with estimates of 10–30% prevalence in the general population, and is associated with substantial medical care costs and a considerable health economic impact (7–9). A proportion of 20–25% of the patients with severe and refractory GI symptoms also have psychosocial co-morbidities such as anxiety, depression or somatization and severely impaired daily functioning (about 10% of these patients have work disability). Somatization, namely multiple stress-related symptoms of unknown origin resulted to be the most important risk factor for impaired QOL in patients with severe functional dyspepsia (10). This FD subgroup is often referred to advanced care, which may be associated with even higher health economic costs (11).
Finally, FD patients also show an important degree of overlap with gastro-esophageal reflux disease (GERD) (12, 13) and irritable bowel syndrome (IBS), and are, thus, often misclassified.
Gastroparesis
Gastroparesis is characterized by delayed gastric emptying and by upper gastrointestinal symptoms (nausea, vomiting, abdominal pain, early satiety, bloating) in the absence of mechanical obstruction (14). Two of the most common types of gastropareses are idiopathic gastroparesis and diabetic gastroparesis (15). Gastroparesis can also be a complication of upper gastrointestinal surgery, neurological disease, collagen vascular disorders, viral infections, or drugs use (16). It is associated with a major impact on the patients' quality of life and substantial social and health economic costs (17).
Gastrointestinal Peptides
In the classical pathophysiological model, functional gastrointestinal disorders (FGIDs) are considered heterogeneous conditions, and symptoms are attributed to a combination of motility disturbances, visceral hypersensitivity, low grade mucosal immune activation, and altered processing of gut-brain signals (18). This is based on the presence of impaired gastric storage and emptying function in FD and gastroparesis, as well as findings of visceral hypersensitivity and increased levels of depression, somatization and anxiety, which are considered markers of altered gut-brain interaction (19–21).
Recent research has focused on visceral hypersensitivity as a common mechanism determining symptom severity and impact across several functional gastrointestinal disorders (19). To date, the focus of research has mainly been on hypersensitivity to mechanical stimuli, studied by balloon distention (22). However, there is increasing evidence for a role for visceral hypersensitivity to specific nutrients as well, suggested amongst other by the observation that FODMAPs induce symptoms and the observation that specific nutrients induce local immune activation in irritable bowel syndrome (IBS) patients but not in health (23, 24).
The gastrointestinal mucosa expresses a wide range of chemosensing receptors, which detect the presence and nature of nutrients in the lumen (25, 26). Nutrients are mainly sensed in the duodenum and jejunum, and initiate an avalanche-effect by releasing gut peptides from entero-endocrine cells into the blood stream. The brain receives these signals through activation of the vagus nerve or directly via the fenestrated blood brain region, the area postrema (25, 26).
There is recent evidence of nutrient-specific enhanced release of gut peptide hormones [motilin, ghrelin, peptide YY (PYY), cholecystokinin (CCK), and glucagon-like peptide 1 (GLP-1)] in FD, which was correlated to intensities of the provoked symptoms. However, most studies are somewhat artificial as they used intraduodenal tube administration of selected nutrients, rather than ingestion of a true meal (27).
The aim of this review was to describe the current evidence on the role of gastrointestinal (GI) peptides in FGID, especially in FD and gastroparesis. We will also address implications for future applications or modulations of gastrointestinal peptides for FD and idiopathic and diabetic gastroparesis treatment.
Methods
We conducted a Pubmed and Medline search for papers, reviews, metanalyses, case series, and RCTs using the following keywords and their associations: functional dyspepsia, gastroparesis, gastrointestinal peptides, CCK, GLP-1, PYY, motilin, ghrelin, and dipeptidyl peptidase (Figure 1). We included also included preliminary evidence from abstracts belonging to main national and international gastroenterological meetings (e.g., United European Gastroenterology Week, Digestive Disease Week, Neurogastroenterology and Motility meetings, and the Belgian Gastroenterology week).
Figure 1.
PRISMA flow chart of included studies in the systematic review.
Results
Preliminary Consideration
Both in FD-PDS and in gastroparesis, symptoms are triggered by ingestion of a meal (28, 29). The release of gut peptides in response to nutrient intake is expected to be triggered sequentially, driven by the location of the entero-endocrine cells that are expressing them. Thus, nutrient arrival in the stomach is thought to affect the release of gastrin, ghrelin and potentially somatostatin, while duodenal exposure to nutrients may impact on the release of CCK, motilin, PYY, and GLP-1, among others (25–27). In addition, serotonin release is expected to occur when nutrients enter the duodenum (25) (Figure 2). The association between peptide levels and symptoms in FD and gastroparesis is summarized in Table 1.
Figure 2.
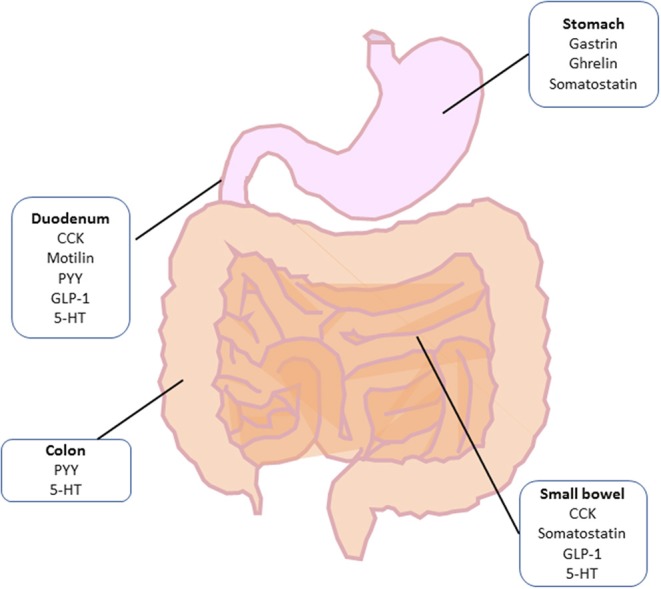
Overview of gastrointestinal peptides and their site of release.
Table 1.
Summary findings on the link between gut peptides, functional dyspepsia, and gastroparesis.
| References | Peptide | Method | Subjects | Major findings |
|---|---|---|---|---|
| Jonnson et al. (30) | Gastrin | Gastrin dosage | FD patients | No altered gastrin levels. |
| He et al. (31) | Gastrin | Gastrin dosage | FD patients with delayed gastric emptying and HV | Higher gastrin levels |
| Walecka-Kapica et al. (32) | Gastrin | Gastrin dosage | PDS and EPS patients and HV | Higher gastrin levels |
| Yoshikawa et al. (33) | Gastrin | Gastrin dosage | FD patients on H2-blockers | Gastrin levels do not predict H2-blockers response |
| He et al. (29) | Somatostatin | Plasma somatostatin dosage and mucosal expression | FD with normal/delayed gastric emptying and HV | No differences between FD and HV |
| Jonnson et al. (30) | Somatostatin | Plasma somatostatin dosage | FD with normal/delayed gastric emptying and HV | Higher somatostatin levels associated with higher symptoms' burden and higher heartburn severity scores; rapid, transient, somatostatin peak during a stress interview |
| Russo et al. (34) | Somatostatin | Plasma somatostatin dosage | 42 PDS and 12 EPS patients | Somatostatin levels tendency to be lower in PDS vs. EPS, without reaching statistical significance |
| Katagiri et al. (35) | Somatostatin | Plasma somatostatin dosage | HV administered with Itopride | Acute increase of somatostatin levels |
| Foxx-Orenstein et al. (36) | Somatostatin | Somatostatin analog Octreotide administration | HV | Slowed gastric emptying, enhanced fasting gastric volumes and suppressed meal-induced volume increments |
| Yagi et al. (37) | Ghrelin | Ghrelin dosage | Gastroparesis and HV (multiple studies) | No significant difference between patients and HV |
| Kim et al. (38) | Ghrelin | Ghrelin dosage | PDS and EPS patients vs. HV | Significant correlation between ghrelin levels and symptom severity, namely epigastric pain in EPS, early satiation in PDS patients |
| Shindo et al. (39) | Ghrelin | Ghrelin dosage | PDS and EPS patients with NERD | Negative correlation between plasma ghrelin levels and gastric emptying rate in PDS but not with EPS patients |
| Takamori et al. (40) | Ghrelin | Ghrelin dosage | Dismotility-like dyspepsia patients (Rome II criteria) vs. HV | Lower Ghrelin levels vs. HV |
| Nishizawa et al. (41) | Ghrelin | Ghrelin dosage | FD patients vs. HV | Higher Ghrelin levels vs. HV |
| Pilichiewicz et al. (42) | Ghrelin | Ghrelin dosage | FD patients vs. HV with high-fat meal ingestion | Ingestion of the meal did not affect plasma ghrelin levels in FD vs. HV |
| Akamizu et al. (43) | Ghrelin | Ghrelin i.v. administration b.i.d. for 2 weeks | FD patients with loss of appetite | Significantly increased appetite and tendency to increased daily food intake in FD patients with loss of appetite |
| Arai et al. (44) | Ghrelin | Rikkunshito administration | FD patients | Improved upper gastrointestinal symptoms, correlating with increased plasm ghrelin levels |
| Suzuki et al. (45) | Ghrelin | Rikkunshito administration | Helicobacter pylori-infected participants with increased plasma ghrelin levels | Improved upper gastrointestinal symptoms |
| Gaddipati et al. (46) | Ghrelin | Ghrelin dosage | Idiopathic, diabetic and post-surgical Gastroparesis patients vs. HV gastroparesis | Increased ghrelin levels after sham feeding in HV and IG patients vs. diabetic and postsurgical gastroparesis |
| Tack et al. (47) | Ghrelin | Gastric emptying and meal-related symptoms evaluation | IG patients | Increased gastric emptying and improved symptoms |
| Murray et al. (48) | Ghrelin | Gastric emptying and meal-related symptoms evaluation | Diabetic gastroparesis patients | Increased gastric emptying |
| Binn et al. (49) | Ghrelin | Gastric emptying evaluation | Neurogenic Gastroparesis patients | Increased gastric emptying |
| Ejskjaer et al. (50) | Ghrelin | Ulimorelin (ghrelin agonist) i.v. administration | Diabetic gastroparesis patients | Increased gastric emptying |
| Heyland et al. (51) | Ghrelin | Ulimorelin (ghrelin agonist) i.v. administration vs. Metoclopramide | Critical ill patients with enteral feeding intolerance | Increased gastric emptying for both treatments, impossible differentiation |
| Ejskjaer et al. (52) | Ghrelin | TZP-102 (ghrelin agonist) Phase 2a study, 12 weeks study | Diabetic gastroparesis patients | Increased gastric emptying |
| Mc Callum et al. (53) | Ghrelin | TZP-102 ghrelin agonist) Phase 2b study, 12 weeks study | Diabetic gastroparesis patients | Failed to confirm Increased gastric emptying |
| Lembo et al. (54) | Ghrelin | Relamorelin injections | Diabetic gastroparesis patients | Reduced vomiting frequency/severity; accelerated gastric emptying |
| Camilleri et al. (55) | Ghrelin | Relamorelin injections | Diabetic gastroparesis patients | Accelerated gastric emptying |
| Russo et al. (34) | Motilin | Motilin dosage | PDS and EPS patients | Higher motilin levels in EPS vs. PDS patients |
| Labo et al. (56) | Motilin | Motilin dosage | FD patients with delayed gastric emptying | Absence of motilin levels fluctuations during the interdigestive state; gastric phase III contractions absence |
| Achem-Karam et al. (57) | Motilin | Motilin dosage | Diabetic gaastroparesis patients | Elevated and fluctuating motilin plasma levels during the interdigestive state; antral phase III activity is absent |
| Talley et al. (58) | Motilin | ABT-229 administration | FD patients with and without delayed gastric emptying | No significant symptoms improvement |
| Talley et al. (59) | Motilin | ABT-229 administration | Type 1 diabetes mellitus patients | No significant symptoms improvement |
| McCallum et al. (60) | Motilin | Mitemcinal | Patients with idiopathic and diabetic gastroparesis | Accelerates gastric empying |
| Mccallum et al. (61) | Motilin | Mitemcinal | Diabetic patients with gastroparesis symptoms | Symptoms relief vs. placebo |
| Cuomo et al. (62) | Motilin | Motilin dosage | HV | Contraction of proximal stomach, increases satiety |
| Deloose et al. (63) | Motilin | Camicinal | HV | Stimulates MMC and gastric emptying |
| Hellstrom et al. (64) | Motilin | Camicinal single dose administration (25, 50, or 125 mg) | Type 1 Diabetic patients with gastroparesis symptoms | significantly accelerated gastric emptying of solids by 125 mg dose |
| Barton et al. (65) | Motilin | Camicinal | Diabetic patients with gastroparesis symptoms | Significantly accelerated gastric emptying |
| Chapman et al. (66) | Motilin | Camicinal | Critical ill patients with enteral feeding intolerance | Camicinal single dose (50 mg) acceleratedgastric emptying and increased glucose absorption |
| Chiloiro et al. (67) | CCK | Standard solid-liquid meal (gastric emptying) | H. pylori associated dyspepsia patients | Significantly lower basal values compared to H. pylori negative patients |
| Bharucha et al. (27) | CCK | Intraduodenal dextrose and lipid adminstration | FD patients vs. HV | Correlation between plasma concentrations of CCK and provoked symptoms Early increase of CCK plasma levels |
| Barbera et al. (68) | CCK | Intraduodenal administration of lipids | FD patients | Increases sensitivity to gastric distention |
| Feinle et al. (69) | CCK | Duodenal lipid infusion + CCK-A antagonist dexloxiglumide | FD patients | Lipid increased plasma CCK levels Dexloxiglumide reduced gastric compliance Gastric distention relieved by dexloxiglumide |
| van Boxel et al. (70) | CCK | Duodenal perfusion | FD patients vs. HV | Mean mucosal CCK concentration was lower in FD patients |
| Feinle-Bisset et al. (71) | CCK | High (HF) and low (LF) fat yogurt | FD patients | Plasma CCK was higher after HF compared to LF |
| Rotondo et al. (72) | GLP-1 | Dipeptidyl peptidase-4 inhibitor (vildagliptin) | HV | Inhibition of gastric accommodation and increased GLP-1 plasma levels |
| Mano et al. (73) | GLP-1 | Hot water and broth (with rice) | HV | Rise in GLP-1 after ingestion of synthesized broth |
| Witte et al. (74) | GLP-1 | Liquid meal | FD patients (EPS) | Similar GLP-1 levels to HV, correlation with nausea |
| Pilichiewicz et al. (42) | PYY | PYY dosage | FD patients vs. HV with high-fat meal ingestion | Lower postprandial PYY levels compared to HV |
| Witte et al. (74) | PYY | Liquid meal | FD patients (EPS) | PYY3-36 is correlated with the sensation of fullness |
| Tack et al. (75) | 5-HT | Cisapride (5-HT4 agonist) | HV | Enhances gastric distension and accommodation |
| Kessing et al. (76) | 5-HT | Prucalopride (5-HT4 agonist) | HV after a standardized meal | Accelerates gastric emptying in male volunteers |
| Carbone et al. (77) | 5-HT | Prucalopride (5-HT4 agonist) | Patients with gastroparesis | Enhances gastric emptying |
| Netzer et al. (78) | 5-HT | Ondansetron (5-HT3 antagonist) | HV | No effect on gastric emptying |
| Janssen et al. (79) | 5-HT | Ondansetron (5-HT3 antagonist) | HV | No effect on gastric compliance, gastric tone |
| Van Oudenhove et al. (80) | 5-HT | Busprione (5-HT1A agonist) | HV | Relaxation of the proximal stomach + decreases gastric emptying |
| Tack et al. (81) | 5-HT | Busprione (5-HT1A agonist) | FD patients | Decreased symptoms + increased gastric accommodation |
| Geeraerts et al. (82) | 5-HT | Acute tryptophan depletion | HV | Reduction in 5-HT levels in duodenum |
| Tack et al. (83) | 5-HT | Paroxetine | HV | Enhances gastric accommodation |
| Janssen et al. (84) | 5-HT | Citalopram (5-HT reuptake inhibitor) | HV | Preprandial gastric relaxation, lower postprandial volume increase + enhances liquid emptying |
| Jannsen et al. (85) | 5-HT | Citalopram (5-HT reuptake inhibitor) | HV | Suppresses gastric phase 2 Stimulates intestinal phase 3 |
| Wilmer et al. (86) | 5-HT | Ondansetron (5-HT3 antagonist) | HV | Suppresses gastric component of phase 3 |
| Chueng et al. (87) | 5-HT | 5-HT postprandial levels | FD patients | Decreased levels of 5-HT |
Gastrin
Gastrin is released by G-cells in the stomach and is a major stimulus for gastric acid secretion (25). As a group, FD patients do not seem to have altered gastrin levels according to a study of Jonsson et al. (30). However, in a study by He et al., FD patients with delayed gastric emptying had significantly higher gastrin levels (31). A recent study from Poland confirmed these findings, with elevated gastrin levels in both PDS and EPS (32). Use of acid suppressive therapy, often applied in FD as first-line therapy, may increase gastrin levels and it remains unclear to which extent the studies could rigorously exclude such confounder. In a relatively small study from Japan, gastrin serum level did not predict the response to H2 blocker therapy in FD (33).
Somatostatin
Somatostatin is released in the stomach but also in the small bowel, and has a strong inhibitory effect on gastrointestinal motility and secretion (25). In the study by He et al., plasma somatostatin levels and mucosal expression of somatostatin in the antrum and the duodenum did not differ between health and FD, with normal or delayed emptying (31). The same was found in FD patients as a group in the study by Jonsson et al., but higher symptom burden was associated with higher fasting somatostatin levels in FD, and somatostatin levels were also correlated with heartburn severity scores (30). FD patients displayed a rapid, transient, somatostatin peak during a stress interview compared to matched controls (30). In a study by Russo et al., comparing gut peptide levels between 42 PDS and 12 EPS patients, somatostatin levels tended to be lower in PDS compared to EPS but this did not reach statistical significance (34). Itopride, a prokinetic agent with mixed dopamine-2 receptor and cholinesterase inhibitory actions, was reported to acutely increase somatostatin plasma levels (35). The somatostatin analog octreotide was reported to slow gastric emptying, enhance fasting gastric volumes and suppress meal-induced volume increments in healthy subjects (36). Clinical reports with somatostatin analogs in FD patients are lacking.
Ghrelin
Ghrelin is produced by endocrine P/D1 cells in the stomach, with plasma levels that increase during fasting and decrease after food intake (25, 88). Ghrelin a 28 amino acid peptide which needs to have an octanoyl group attached to its third serine residue to be biologically active (25). Ghrelin levels are inversely related to body weight (89, 90) and decrease with increasing extent of gastric mucosal atrophy (91, 92). Several studies have investigated ghrelin release in FD and gastroparesis, and the findings are conflicting (38). Most studies found no difference in fasting ghrelin levels in FD compared to health (37, 39–41, 93, 94). However, in a small group of EPS and PDS patients compared to health, correlations were reported between ghrelin levels and symptom severity, in particular epigastric pain in EPS and early satiation in PDS (38). Shindo et al. found a negative correlation between plasma acylated ghrelin levels and gastric emptying rate in patients with PDS but not with EPS (39). Takamori et al. reported lower levels of des-acyl ghrelin (the inactive form after hydrolysis of the octanoyl group), in dysmotility-like dyspepsia according to the Rome II criteria (40) while Nishizawa et al. reported higher ghrelin levels in FD patients as a group (41). The ingestion of a high fat meal in FD patients did not differently affect the plasma ghrelin levels in FD compared to healthy subjects (42). Intravenous administration of ghrelin, twice daily for 2 weeks, significantly increased appetite and tended to increase daily food intake in FD patients with loss of appetite (43). Furthermore, Arai et al. observed a clear improvement in upper gastrointestinal symptoms in FD patients after administration of the Japanese Kampo medicine Rikkunshito, which increased plasma ghrelin levels (44). Suzuki et al. also showed Rikkunshito was effective among H. pylori-infected participants with increased plasma ghrelin levels (45).
Plasma ghrelin levels increased with sham feeding in healthy controls and patients with idiopathic gastroparesis but not in patients with diabetic or postsurgical gastroparesis, indicative of a role for intact vagal signaling in the control of ghrelin release (46). In pilot studies, acute intravenous administration of ghrelin enhanced gastric emptying rate in idiopathic and diabetic gastroparesis (47–49). In idiopathic gastroparesis patients, symptoms were also improved.
Subsequently, several ghrelin agonists have been studied, with a major focus on diabetic gastroparesis. The intravenously administered macrocylic peptidomimetic molecule ulimorelin, enhanced gastric emptying, and was subsequently mainly studied in critical care patients, with lack of differentiation from metoclopramide (50, 51). The orally administered TZP-102 showed promising results in phase 2a, but this was not confirmed in phase 2b (52, 53). Relamorelin, an injectable ghrelin receptor agonist, showed efficacy in diabetic gastroparesis patients with active vomiting symptoms in two placebo-controlled phase 2 studies and is being evaluated in phase 3 studies (54, 55).
Motilin
Motilin is released from M-cells situated in the proximal duodenum during the fasted state, is a stimulus for strong antral contractions and has a hunger signaling function (25, 95). Several studies evaluated plasma motilin levels in FD and gastroparesis (34, 56, 96–98). FD patients as a group have comparable fasting plasma levels to those in health (95). Russo et al. reported higher fasting motilin plasma levels in EPS compared to PDS (34). In the same study, elevated CRF levels were also reported in PDS. The relevance of this finding is unclear. It is well-known that motilin plasma levels fluctuate with interdigestive motility and are maximal during gastric phase III (95). The study by Russo et al. did not correct for migrating motor complex (MMC) cycle, which could be a major confounder, as it is conceivable that PDS patients have less occurrence of gastric phase III (96).
In patients with FD and delayed gastric emptying, motilin plasma levels did not display the normal fluctuations during the interdigestive state and gastric phase III contractions were absent (56). In patients with diabetic gastroparesis, motilin plasma levels were elevated but still fluctuating during the interdigestive state, although antral phase III activity was absent (57, 98). In FD patients with unexplained loss of appetite, gastric phase III contractions are suppressed, suggesting low plasma levels, but these were not measured in this study (99).
Several macrolide antibiotics such as erythromycin and azithromycin have motilin receptor agonistic effects, and have a stimulatory effect on gastric emptying rate (100–102). The impact on symptoms, however, was often disappointing (101). A number of macrolides without antibiotics but with motilin receptor agonistic properties were developed for the treatment of FD and diabetic gastroparesis (103). However, invariably, they failed to provide significant symptomatic benefit in phase 2 studies and no agent progressed into phase 3 studies (58–61, 104). The main reasons that have been put forward to explain the lack of success in trials with motilin agonist drugs for gastroparesis have been the use of too high doses, which impact on gastric accommodation, and the use of long-acting agents which are prone to desensitization (62, 103).
Camicinal is a novel small molecule motilin receptor agonist with short half-life, which was shown to induce gastric phase III contractions during the fasting state and dose-dependently enhance gastric emptying rate (63, 64). In a phase 2 study, the lowest dose of camicinal significantly improved symptoms, confirming the therapeutic potential of this class of agents, whereas only the highest dose studied enhanced gastric emptying. Indicating that enhanced emptying rate does not underlie the symptom improvement (65). Camicinal was also studied in critical care patients, but the drug has not advanced to phase 3 in any indication (66).
Cholecystokinin (CCK)
CCK is a brain-gut peptide released from I-cells in the upper small intestine upon food intake, especially after meals containing high fat or protein amounts (25). In H. pylori associated dyspepsia patients, significantly lower CCK basal values were demonstrated in comparison to H. pylori negative patients (67). Hyper responsiveness to CCK can be one of the pathophysiological pathways for the occurrence of symptoms in FD patients (105). A recent study showed a correlation between the release of gut peptide hormones as CCK and provoked symptoms after infusion of nutrients into the duodenum (27). However, in this study, intraduodenal tube administration of selected nutrients was used, rather than ingestion of a true meal. An early increase of CCK plasma levels was found, followed later by a rise of other peptides such as GLP-1 and PYY. Previously, it has also been shown that the intraduodenal infusion of fat may trigger symptoms as fullness and discomfort and to sensitize the stomach to gastric distension (68, 105). Duodenal lipids induce higher CCK levels in patients with FD compared to health, and the CCK-A receptor antagonist dexloxiglumide, was able to reduced sensitivity to gastric distension after lipid administration (69, 70, 106). However, ingestion of a low fat meal when patients perceived intake of a high fat meal (cognitive factors) did not significantly change the CCK level but was associated with higher symptom scores (71).
In addition, a CCK antagonist accelerated the gastric emptying rate which could lead to a benefit in both functional dyspepsia as gastroparesis patients (107). The improvement in gastric emptying probably involves an effect of CCK on capsaicin-sensitive vagal pathways (107). Infusion of CCK in healthy volunteers resulted in an increase in gastric compliance, but this was not confirmed in a study with FD patients (108). Unfortunately, in spite of a number of positive mechanistic observations, CCK-receptor antagonists were not further developed for the treatment of FD.
Glucagon-Like Peptide 1 (GLP-1)
GLP-1, secreted by intestinal endocrine L-cells upon food intake, slows the gastric emptying in diabetes with a decrease in glycemia (108). In healthy controls, elevated GLP-1 plasma levels after administration of the Dipeptidyl peptidase-4 inhibitor vildagliptin, were associated with impaired gastric accommodation (72). In Japan, gastric emptying was measured in healthy subjects and increased significantly after ingestion of a broth with rice, which was accompanied by a significantly more rapid rise in plasma GLP-1 and glucose levels compared to rice with water (73). In an earlier study, it was shown that GLP-1 was correlated with nausea in a single meal experiment in FD patients subtype EPS as well as in healthy volunteers (74). This would be an interesting fact for the use of medication acting on the GLP-1 receptor for the treatment of gastroparesis patients with nausea as one of their main symptoms.
Peptide YY (PYY)
PYY is a gut hormone secreted from endocrine L-cells in the gut mucosa, most prominently present in the ileum and the colon, and released into the circulation after ingestion of food (25, 109). As mentioned above, the intake of lipids is often a trigger for symptoms in FD. In FD patients, ingestion of a high fat meal was associated with lower postprandial PYY levels compared to healthy volunteers (42). In addition, PYY was found to be correlated with symptoms such as a sensation of fullness in EPS patients after a single drink test and a satiety test (74). However, based on the literature, little is known about the effect of PYY in FD patients.
Serotonin (5-HT)
5-HT is also released by entero-endocrine cells in the gastrointestinal tract, in response to mechanical stimulation or the presence of nutrients or toxins (25, 110, 111). It has its effect via 14 known serotonin receptors, but we will focus on the most relevant ligands in this review. The role of 5-HT in upper gastrointestinal physiology remains unclear, due to a lack of suitable agonists and antagonists for human application (110). While 5-HT4 agonists enhance gastric accommodation and gastric emptying, 5-HT3 antagonists had no significant effect on these functions, and 5-HT1A agonists enhance gastric accommodation and tend to slow gastric emptying (75–81). Alternative approaches to unravel a role for 5-HT in gastric sensorimotor function has been the use of tryptophan depletion (82) and the administration of selective serotonin reuptake inhibitors (SSRIs) (83, 84). Acute tryptophan depletion enhanced gastric accommodation, which was also observed with short-term SSRI use, while acute intravenous SSRI administration inhibited accommodation, suggesting that endogenous serotonin release serves to limit gastric accommodation (82–84). In terms of interdigestive gastric motility, acute intravenous SSRI administration suppresses gastric phase 3 while stimulating intestinal phase 3, and ondansetron also inhibited the occurrence of gastric phase 3 (85, 86).
Studies focusing on IBS have shown that circulating 5-HT levels rise after a meal, and that this rise is exaggerated in IBS with diarrhea and suppressed in IBS with constipation (112, 113). These studies used platelet-depleted plasma to measure circulating plasma levels of gastrointestinal origin, thereby eliminating the confounding effect of storage in thrombocytes. Similar studies in FD are lacking. One study measured plasma 5-HT in FD and found decreased basal and postprandial plasma compared to health (87). This is in agreement with a recent study reporting a decreased number of duodenal serotonin containing endocrine cells in FD patients (74).
Several 5-HT receptor agonists/antagonists, such as cisapride (5-HT4 agonist, 5-HT2, and 5-HT3 antagonist), tegaserod (5-HT4 and 5-HT1 agonist, 5-HT2a/b antagonist), mosapride (5-HT4 agonist, 5-HT3 antagonist) and revexepride (5-HT4 agonist) have been evaluated for the treatment of dyspepsia and gastroparesis, although not all studies show efficacy (114–116). A recent metanalysis showed that FD patients treated with serotonin receptor agonists have a significantly better symptom response compared to placebo (117) and the most recently published evidence indicates efficacy for prucalopride in idiopathic gastroparesis and emerging efficacy for velusetrag in gastroparesis (118, 119). Case series suggest potential benefit of the 5-HT3 antagonists granisetron or ondansetron for symptoms of nausea and vomiting in gastroparesis, but formal studies are lacking (120, 121).
Summary and Conclusions
FD and gastroparesis, two of the most common FGIDs, are both characterized by upper GI symptoms. FD patients are subdivided in PDS and EPS patients, defined by symptoms as postprandial fullness, early satiety, epigastric pain, and epigastric burning. Patients with gastroparesis are characterized by nausea with or without vomiting, and often also similar symptoms as in FD, with a significantly delayed gastric emptying in the absence of mechanical obstruction. The most common subgroups are idiopathic and diabetic gastroparesis. The pathophysiology of both FGIDs is based on a combination of motility disturbances, visceral hypersensitivity, low grade mucosal immune activation, and altered processing of gut-brain-signals. Recent observations support a new pathophysiological model in at least subsets of patients with FD and gastroparesis, which involves visceral hypersensitivity to nutrients. Nutrient sensing occurs in the stomach and duodenum and is signaled to the brain through neural pathways, but especially through the release of gut peptides, which was shown in some studies to be correlated with symptoms in FD and gastroparesis.
In this review, the effect of peptides as gastrin, somatostatin, and ghrelin, all released by endocrine cells in the stomach, and of motilin, CCK, GLP-1, PYY and 5-HT, secreted in the duodenum, was summarized. Previous studies showed contradictory results regarding an increase in peptide levels in FD patients compared to health, but the impact of confounders, as the use of acid suppressive therapy for gastrin, the impact of MMC cycle for motilin, and the accumulation of 5-HT in thrombocytes, was not taken into account (28–30, 50). In most studies, the levels of somatostatin, ghrelin, and motilin did not differ between healthy volunteers and FD patients, however higher symptom burden was often correlated with higher peptide levels (28, 29, 36, 48). Nevertheless, most of these studies are limited by small sample sizes. Furthermore, a study by Russo et al. showed a trend toward higher somatostatin and motilin levels in EPS patients compared to PDS patients (32). However, the effect of gut peptides was mainly analyzed in FD patients as a group compared to healthy controls and only rarely in terms of EPS vs. PDS subgroups. In addition, little is known about the relation of gut peptides in FD patients fulfilling Rome IV criteria. H. pylori associated dyspepsia patients were shown to have lower CCK levels compared to H.pylori negative patients (67).
In patients with FD and gastroparesis, the correlation of gut peptides and gastric emptying was studied. Previously, a negative correlation was found between acylated ghrelin and gastric emptying (39). Intravenous administration of ghrelin increased the appetite in FD and enhanced gastric emptying and symptoms in idiopathic gastroparesis (43, 47–49). In addition, intraduodenal administration lipid administration provoked FD symptoms whose severity was correlated with CCK levels (27). Nevertheless, studies in which gut peptides are examined after eating a standard meal with an analysis on symptoms and motility disturbances, are lacking.
Based on the literature, low grade inflammation with increased mast cell and eosinophil count would underlie in the pathophysiological mechanisms of FGIDs and lead to an impaired barrier function. Duodenal factors, such as nutrients, may play a role in the activation of those eosinophils and mast cells. Therefore, it would be interesting to further investigate the effect of nutrients or diets on the release of GI peptides and evaluate this as a potential treatment option for FD or gastroparesis. Drugs acting on peptide receptors have already been tested in both groups, but is the scope of the available data is limited. Ghrelin agonists such as ulimorelin, relamorelin, and TZP-102, as well as 5-HT4 agonists and CCK antagonists all showed promising results in terms of improvement of the gastric emptying (50, 52–55, 66, 117–119). In addition, the use of motilin receptor agonists (macrolide antibiotics and camicinal) enhanced the gastric emptying, but there the link with improvement of symptoms is less predictable (66, 101). Serotonin agonists have a potential to improve symptoms in both FD and idiopathic gastroparesis (114, 117–119). Drugs acting on the GLP-1 and on the PYY receptors deserve further investigation, because of the link between GLP-1 release and nausea, and the link between PYY release and postprandial fullness (74).
In summary, there is a clear need for in-depth evaluation of release of GI peptides after a standard meal in larger sample sizes of Rome IV PDS and EPS and gastroparesis patients. This should be complemented with detailed studies of drugs altering the level of GI peptides or their effect on their receptors.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
KV, ES, and JT drafted the manuscript. All authors made edits and corrections and reviewed and approved the final version of the test.
Conflict of Interest
JT has given Scientific advice to AlfaWassermann, Allergan, Christian Hansen, Danone, Grünenthal, Ironwood, Janssen, Kiowa Kirin, Menarini, Mylan, Neutec, Novartis, Noventure, Nutricia, Shionogi, Shire, Takeda, Theravance, Tramedico, Truvion, Tsumura, Zealand, and Zeria pharmaceuticals and has served on the Speaker bureau for Abbott, Allergan, AstraZeneca, Janssen, Kyowa Kirin, Menarini, Mylan, Novartis, Shire, Takeda, Truvion, and Zeria. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This research was supported by a Methusalem grant from Leuven University to JT.
References
- 1.Stanghellini V, Chan FK, Hasler WL, Malagelada JR, Suzuki H, Tack J, et al. Gastroduodenal disorders. Gastroenterology. (2016) 150:1380–92. 10.1053/j.gastro.2016.02.011 [DOI] [PubMed] [Google Scholar]
- 2.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. (2006) 12:2661–6. 10.3748/wjg.v12.i17.2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piessevaux H, De Winter B, Louis E, Muls V, De Looze D, Pelckmans P, et al. Dyspeptic symptoms in the general population: a factor and cluster analysis of symptom groupings. Neurogastroenterol Motil. (2009) 21:378–88. 10.1111/j.1365-2982.2009.01262.x [DOI] [PubMed] [Google Scholar]
- 4.Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. (2015) 64:1353–67. 10.1136/gutjnl-2015-309252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford AC, Moayyedi P, Jarbol DE, Logan RF, Delaney BC. Meta-analysis: Helicobacter pylori 'test and treat' compared with empirical acid suppression for managing dyspepsia. Aliment Pharmacol Ther. (2008) 28:534–44. 10.1111/j.1365-2036.2008.03784.x [DOI] [PubMed] [Google Scholar]
- 6.Tack J, Talley NJ. Functional dyspepsia–symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. (2013) 10:134–41. 10.1038/nrgastro.2013.14 [DOI] [PubMed] [Google Scholar]
- 7.Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. (2013) 38:170–7. 10.1111/apt.12355 [DOI] [PubMed] [Google Scholar]
- 8.Aro P, Talley NJ, Agréus L, Johansson SE, Bolling-Sternevald E, Storskrubb T, et al. Functional dyspepsia impairs quality of life in the adult population. Aliment Pharmacol Ther. (2011) 33:1215–24. 10.1111/j.1365-2036.2011.04640.x [DOI] [PubMed] [Google Scholar]
- 9.El-Serag HB, Talley NJ. Health-related quality of life in functional dyspepsia. Aliment Pharmacol Ther. (2003) 18:387–93. 10.1046/j.1365-2036.2003.01706.x [DOI] [PubMed] [Google Scholar]
- 10.Van Oudenhove L, Vandenberghe J, Vos R, Fischler B, Demyttenaere K, Tack J. Abuse history, depression, and somatization are associated with gastric sensitivity and gastric emptying in functional dyspepsia. Psychosom Med. (2011) 73:648–55. 10.1097/PSY.0b013e31822f32bf [DOI] [PubMed] [Google Scholar]
- 11.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. (2016) 150:1262–79. 10.1053/j.gastro.2016.02.032 [DOI] [PubMed] [Google Scholar]
- 12.Quigley EM, Lacy BE. Overlap of functional dyspepsia and GERD–diagnostic and treatment implications. Nat Rev Gastroenterol Hepatol. (2013) 10:175–86. 10.1038/nrgastro.2012.253 [DOI] [PubMed] [Google Scholar]
- 13.Pleyer C, Bittner H, Locke GR, Choung RS, Zinsmeister AR, Schleck CD, et al. Overdiagnosis of gastro-esophageal reflux disease and underdiagnosis of functional dyspepsia in a USA community. Neurogastroenterol Motil. (2014) 26:1163–71. 10.1111/nmo.12377 [DOI] [PubMed] [Google Scholar]
- 14.Tack J, Camilleri M. New developments in the treatment of gastroparesis and functional dyspepsia. Curr Opin Pharmacol. (2018) 43:111–7. 10.1016/j.coph.2018.08.015 [DOI] [PubMed] [Google Scholar]
- 15.Stanghellini V, Tack J. Gastroparesis: separate entity or just a part of dyspepsia? Gut. (2014) 63:1972–8. 10.1136/gutjnl-2013-306084 [DOI] [PubMed] [Google Scholar]
- 16.Hasler WL. Gastroparesis: pathogenesis, diagnosis and management. Nat Rev Gastroenterol Hepatol. (2011) 8:438–53. 10.1038/nrgastro.2011.116 [DOI] [PubMed] [Google Scholar]
- 17.Hirsch W, Nee J, Ballou S, Petersen T, Friedlander D, Lee HN, et al. Emergency Department Burden of Gastroparesis in the United States, 2006 to 2013. J Clin Gastroenterol. (2019) 53:109–13. 10.1097/MCG.0000000000000972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. (2016) 150:1257–61. 10.1053/j.gastro.2016.03.035 [DOI] [PubMed] [Google Scholar]
- 19.Simrén M, Törnblom H, Palsson OS, van Tilburg MAL, Van Oudenhove L, Tack J, et al. Visceral hypersensitivity is associated with GI symptom severity in functional GI disorders: consistent findings from five different patient cohorts. Gut. (2018) 67:255–62. 10.1136/gutjnl-2016-312361 [DOI] [PubMed] [Google Scholar]
- 20.Jones MP, Tack J, Van Oudenhove L, Walker MM, Holtmann G, Koloski NA, et al. Mood and anxiety disorders precede development of functional gastrointestinal disorders in patients but not in the population. Clin Gastroenterol Hepatol. (2017) 15:1014–20.e4. 10.1016/j.cgh.2016.12.032 [DOI] [PubMed] [Google Scholar]
- 21.Van Den Houte K, Carbone F, Tack J. Postprandial distress syndrome: stratification and management. Expert Rev Gastroenterol Hepatol. (2019) 13:37–46. 10.1080/17474124.2019.1543586 [DOI] [PubMed] [Google Scholar]
- 22.Farré R, Vanheel H, Vanuytsel T, Masaoka T, Törnblom H, Simrén M, et al. In functional dyspepsia, hypersensitivity to postprandial distention correlates with meal-related symptom severity. Gastroenterology. (2013) 145:566–73. 10.1053/j.gastro.2013.05.018 [DOI] [PubMed] [Google Scholar]
- 23.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. (2014) 146:67–75.e5. 10.1053/j.gastro.2013.09.046 [DOI] [PubMed] [Google Scholar]
- 24.Fritscher-Ravens A, Pflaum T, Mösinger M, Ruchay Z, Röcken C, Milla PJ, et al. Many patients with irritable bowel syndrome have atypical food allergies not associated with immunoglobulin E. Gastroenterology. (2019) 157:109–18.e5. 10.1053/j.gastro.2019.03.046 [DOI] [PubMed] [Google Scholar]
- 25.Farré R, Tack J. Food and symptom generation in functional gastrointestinal disorders: physiological aspects. Am J Gastroenterol. (2013) 108:698–706. 10.1038/ajg.2013.24 [DOI] [PubMed] [Google Scholar]
- 26.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. (2014) 63:179–90. 10.1136/gutjnl-2013-305112 [DOI] [PubMed] [Google Scholar]
- 27.Bharucha AE, Camilleri M, Burton DD, Thieke SL, Feuerhak KJ, Basu A, et al. Increased nutrient sensitivity and plasma concentrations of enteral hormones during duodenal nutrient infusion in functional dyspepsia. Am J Gastroenterol. (2014) 109:1910–20; quiz 09:21. 10.1038/ajg.2014.330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bisschops R, Karamanolis G, Arts J, Caenepeel P, Verbeke K, Janssens J, et al. Relationship between symptoms and ingestion of a meal in functional dyspepsia. Gut. (2008) 57:1495–503. 10.1136/gut.2007.137125 [DOI] [PubMed] [Google Scholar]
- 29.Karamanolis G, Caenepeel P, Arts J, Tack J. Determinants of symptom pattern in idiopathic severely delayed gastric emptying: gastric emptying rate or proximal stomach dysfunction? Gut. (2007) 56:29–36. 10.1136/gut.2005.089508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson BH, Uvnäs-Moberg K, Theorell T, Gotthard R. Gastrin, cholecystokinin, and somatostatin in a laboratory experiment of patients with functional dyspepsia. Psychosom Med. (1998) 60:331–7. 10.1097/00006842-199805000-00020 [DOI] [PubMed] [Google Scholar]
- 31.He MR, Song YG, Zhi FC. Gastrointestinal hormone abnormalities and G and D cells in functional dyspepsia patients with gastric dysmotility. World J Gastroenterol. (2005) 11:443–6. 10.3748/wjg.v11.i3.443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walecka-Kapica E, Klupinska G, Stec-Michalska K, Olszowiec K, Pawłowicz M, Chojnacki C. [Gastrin secretion in patients with functional dyspepsia]. Pol Merkur Lekarski. (2009) 26:362−5. [PubMed] [Google Scholar]
- 33.Yoshikawa I, Murata I, Kume K, Kanagawa K, Hirohata Y, Nakamura H, et al. Serum pepsinogen can predict response to H2-receptor antagonist in patients with functional dyspepsia. Aliment Pharmacol Ther. (2002) 16:1805–9. 10.1046/j.1365-2036.2002.01352.x [DOI] [PubMed] [Google Scholar]
- 34.Russo F, Chimienti G, Clemente C, Riezzo G, D'Attoma B, Martulli M. Gastric activity and gut peptides in patients with functional dyspepsia: postprandial distress syndrome versus epigastric pain syndrome. J Clin Gastroenterol. (2017) 51:136–44. 10.1097/MCG.0000000000000531 [DOI] [PubMed] [Google Scholar]
- 35.Katagiri F, Shiga T, Inoue S, Sato Y, Itoh H, Takeyama M. Effects of itopride hydrochloride on plasma gut-regulatory peptide and stress-related hormone levels in healthy human subjects. Pharmacology. (2006) 77:115–21. 10.1159/000093485 [DOI] [PubMed] [Google Scholar]
- 36.Foxx-Orenstein A, Camilleri M, Stephens D, Burton D. Effect of a somatostatin analogue on gastric motor and sensory functions in healthy humans. Gut. (2003) 52:1555–61. 10.1136/gut.52.11.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yagi T, Asakawa A, Ueda H, Miyawaki S, Inui A. The role of ghrelin in patients with functional dyspepsia and its potential clinical relevance (Review). Int J Mol Med. (2013) 32:523–31. 10.3892/ijmm.2013.1418 [DOI] [PubMed] [Google Scholar]
- 38.Kim YS, Lee JS, Lee TH, Cho JY, Kim JO, Kim WJ, et al. Plasma levels of acylated ghrelin in patients with functional dyspepsia. World J Gastroenterol. (2012) 18:2231–7. 10.3748/wjg.v18.i18.2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shindo T, Futagami S, Hiratsuka T, Horie A, Hamamoto T, Ueki N, et al. Comparison of gastric emptying and plasma ghrelin levels in patients with functional dyspepsia and non-erosive reflux disease. Digestion. (2009) 79:65–72. 10.1159/000205740 [DOI] [PubMed] [Google Scholar]
- 40.Takamori K, Mizuta Y, Takeshima F, Akazawa Y, Isomoto H, Ohnita K, et al. Relation among plasma ghrelin level, gastric emptying, and psychologic condition in patients with functional dyspepsia. J Clin Gastroenterol. (2007) 41:477–83. 10.1097/01.mcg.0000225614.94470.47 [DOI] [PubMed] [Google Scholar]
- 41.Nishizawa T, Suzuki H, Nomoto Y, Masaokas T, Hosoda H, Mori M, et al. Enhanced plasma ghrelin levels in patients with functional dyspepsia. Aliment Pharmacol Ther. (2006) 24(Suppl. 4):S104–10. 10.1111/j.1365-2036.2006.00032.x [DOI] [Google Scholar]
- 42.Pilichiewicz AN, Feltrin KL, Horowitz M, Holtmann G, Wishart JM, Jones KL, et al. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. (2008) 103:2613–23. 10.1111/j.1572-0241.2008.02041.x [DOI] [PubMed] [Google Scholar]
- 43.Akamizu T, Iwakura H, Ariyasu H, Hosoda H, Murayama T, Yokode M, et al. Repeated administration of ghrelin to patients with functional dyspepsia: its effects on food intake and appetite. Eur J Endocrinol. (2008) 158:491–8. 10.1530/EJE-07-0768 [DOI] [PubMed] [Google Scholar]
- 44.Arai M, Matsumura T, Tsuchiya N, Sadakane C, Inami R, Suzuki T, et al. Rikkunshito improves the symptoms in patients with functional dyspepsia, accompanied by an increase in the level of plasma ghrelin. Hepatogastroenterology. (2012) 59:62–6. 10.5754/hge11246 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki H, Matsuzaki J, Fukushima Y, Suzaki F, Kasugai K, Nishizawa T, et al. Randomized clinical trial: rikkunshito in the treatment of functional dyspepsia–a multicenter, double-blind, randomized, placebo-controlled study. Neurogastroenterol Motil. (2014) 26:950–61. 10.1111/nmo.12348 [DOI] [PubMed] [Google Scholar]
- 46.Gaddipati KV, Simonian HP, Kresge KM, Boden GH, Parkman HP. Abnormal ghrelin and pancreatic polypeptide responses in gastroparesis. Dig Dis Sci. (2006) 51:1339–46. 10.1007/s10620-005-9022-z [DOI] [PubMed] [Google Scholar]
- 47.Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. (2005) 22:847–53. 10.1111/j.1365-2036.2005.02658.x [DOI] [PubMed] [Google Scholar]
- 48.Murray CD, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, et al. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. (2005) 54:1693–8. 10.1136/gut.2005.069088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Binn M, Albert C, Gougeon A, Maerki H, Coulie B, Lemoyne M, et al. Ghrelin gastrokinetic action in patients with neurogenic gastroparesis. Peptides. (2006) 27:1603–6. 10.1016/j.peptides.2005.12.008 [DOI] [PubMed] [Google Scholar]
- 50.Ejskjaer N, Vestergaard ET, Hellström PM, Gormsen LC, Madsbad S, Madsen JL, et al. Ghrelin receptor agonist (TZP-101) accelerates gastric emptying in adults with diabetes and symptomatic gastroparesis. Aliment Pharmacol Ther. (2009) 29:1179–87. 10.1111/j.1365-2036.2009.03986.x [DOI] [PubMed] [Google Scholar]
- 51.Heyland DK, van Zanten ARH, Grau-Carmona T, Evans D, Beishuizen A, Schouten J, et al. A multicenter, randomized, double-blind study of ulimorelin and metoclopramide in the treatment of critically ill patients with enteral feeding intolerance: PROMOTE trial. Intensive Care Med. (2019) 45:647–56. 10.1007/s00134-019-05593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ejskjaer N, Wo JM, Esfandyari T, Mazen Jamal M, Dimcevski G, Tarnow L, et al. A phase 2a, randomized, double-blind 28-day study of TZP-102 a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. (2013) 25:e140–50. 10.1111/nmo.12064 [DOI] [PubMed] [Google Scholar]
- 53.McCallum RW, Lembo A, Esfandyari T, Bhandari BR, Ejskjaer N, Cosentino C, et al. Phase 2b, randomized, double-blind 12-week studies of TZP-102, a ghrelin receptor agonist for diabetic gastroparesis. Neurogastroenterol Motil. (2013) 25:e705–17. 10.1111/nmo.12184 [DOI] [PubMed] [Google Scholar]
- 54.Lembo A, Camilleri M, McCallum R, Sastre R, Breton C, Spence S, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. (2016) 151:87–96.e6. 10.1053/j.gastro.2016.03.038 [DOI] [PubMed] [Google Scholar]
- 55.Camilleri M, McCallum RW, Tack J, Spence SC, Gottesdiener K, Fiedorek FT. Efficacy and safety of relamorelin in diabetics with symptoms of gastroparesis: a randomized, Placebo-Controlled Study. Gastroenterology. (2017) 153:1240–50.e2. 10.1053/j.gastro.2017.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labo G, Bortolotti M, Vezzadini P, Bonora G, Bersani G. Interdigestive gastroduodenal motility and serum motilin levels in patients with idiopathic delay in gastric emptying. Gastroenterology. (1986) 90:20–6. 10.1016/0016-5085(86)90069-7 [DOI] [PubMed] [Google Scholar]
- 57.Achem-Karam SR, Funakoshi A, Vinik AI, Owyang C. Plasma motilin concentration and interdigestive migrating motor complex in diabetic gastroparesis: effect of metoclopramide. Gastroenterology. (1985) 88:492–9. 10.1016/0016-5085(85)90512-8 [DOI] [PubMed] [Google Scholar]
- 58.Talley NJ, Verlinden M, Snape W, Beker JA, Ducrotte P, Dettmer A, et al. Failure of a motilin receptor agonist (ABT-229) to relieve the symptoms of functional dyspepsia in patients with and without delayed gastric emptying: a randomized double-blind placebo-controlled trial. Aliment Pharmacol Ther. (2000) 14:1653–61. 10.1046/j.1365-2036.2000.00868.x [DOI] [PubMed] [Google Scholar]
- 59.Talley NJ, Verlinden M, Geenen DJ, Hogan RB, Riff D, McCallum RW, et al. Effects of a motilin receptor agonist (ABT-229) on upper gastrointestinal symptoms in type 1 diabetes mellitus: a randomised, double blind, placebo controlled trial. Gut. (2001) 49:395–401. 10.1136/gut.49.3.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McCallum RW, Cynshi O, Investigative Team . Clinical trial: effect of mitemcinal (a motilin agonist) on gastric emptying in patients with gastroparesis - a randomized, multicentre, placebo-controlled study. Aliment Pharmacol Ther. (2007) 26:1121–30. 10.1111/j.1365-2036.2007.03461.x [DOI] [PubMed] [Google Scholar]
- 61.McCallum RW, Cynshi O, US investigative team . Efficacy of mitemcinal, a motilin agonist, on gastrointestinal symptoms in patients with symptoms suggesting diabetic gastropathy: a randomized, multi-center, placebo-controlled trial. Aliment Pharmacol Ther. (2007) 26:107–16. 10.1111/j.1365-2036.2007.03346.x [DOI] [PubMed] [Google Scholar]
- 62.Cuomo R, Vandaele P, Coulie B, Peeters T, Depoortere I, Janssens J, et al. Influence of motilin on gastric fundus tone and on meal-induced satiety in man: role of cholinergic pathways. Am J Gastroenterol. (2006) 101:804–11. 10.1111/j.1572-0241.2005.00339.x [DOI] [PubMed] [Google Scholar]
- 63.Deloose E, Depoortere I, de Hoon J, Van Hecken A, Dewit OE, Vasist Johnson LS, et al. Manometric evaluation of the motilin receptor agonist camicinal (GSK962040) in humans. Neurogastroenterol Motil. (2018) 30:e13173. 10.1111/nmo.13173 [DOI] [PubMed] [Google Scholar]
- 64.Hellström P, Tack J, Johnson L, Hacqouil K, Barton M, Richards D, et al. The pharmacodynamics, safety, and pharmacokinetics of single doses of the motilin agonist, camicinal, in type 1 diabetes mellitus with slow gastric emptying. Br J Pharmacol. (2016) 173:1768–77. 10.1111/bph.13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barton ME, Otiker T, Johnson LV, Robertson DC, Dobbins RL, Parkman HP, et al. A randomized, double-blind, placebo-controlled phase II study (MOT114479) to evaluate the safety and efficacy and dose response of 28 days of orally administered camicinal, a motilin receptor agonist, in diabetics with gastroparesis. Gastroenterology. (2014) 146:S20 10.1016/S0016-5085(14)60070-6 [DOI] [Google Scholar]
- 66.Chapman MJ, Deane AM, O'Connor SL, Nguyen NQ, Fraser RJ, Richards DB, et al. The effect of camicinal (GSK962040), a motilin agonist, on gastric emptying and glucose absorption in feed-intolerant critically ill patients: a randomized, blinded, placebo-controlled, clinical trial. Crit Care. (2016) 20:232. 10.1186/s13054-016-1420-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiloiro M, Russo F, Riezzo G, Leoci C, Clemente C, Messa C, et al. Effect of Helicobacter pylori infection on gastric emptying and gastrointestinal hormones in dyspeptic and healthy subjects. Dig Dis Sci. (2001) 46:46–53. 10.1023/A:1005601623363 [DOI] [PubMed] [Google Scholar]
- 68.Barbera R, Feinle C, Read NW. Abnormal sensitivity to duodenal lipid infusion in patients with functional dyspepsia. Eur J Gastroenterol Hepatol. (1995) 7:1051–7. 10.1097/00042737-199511000-00007 [DOI] [PubMed] [Google Scholar]
- 69.Feinle C, Meier O, Otto B, D'Amato M, Fried M. Role of duodenal lipid and cholecystokinin A receptors in the pathophysiology of functional dyspepsia. Gut. (2001) 48:347–55. 10.1136/gut.48.3.347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Boxel OS, ter Linde JJ, Oors J, Otto B, Weusten BL, Feinle-Bisset C, et al. Functional dyspepsia patients have lower mucosal cholecystokinin concentrations in response to duodenal lipid. Eur J Gastroenterol Hepatol. (2014) 26:205–12. 10.1097/MEG.0000000000000001 [DOI] [PubMed] [Google Scholar]
- 71.Feinle-Bisset C, Meier B, Fried M, Beglinger C. Role of cognitive factors in symptom induction following high and low fat meals in patients with functional dyspepsia. Gut. (2003) 52:1414–8. 10.1136/gut.52.10.1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rotondo A, Masuy I, Verbeure W, Biesiekierski JR, Deloose E, Tack J. Randomised clinical trial: the DPP-4 inhibitor, vildagliptin, inhibits gastric accommodation and increases glucagon-like peptide-1 plasma levels in healthy volunteers. Ent Pharmacol Ther. (2019) 49:997–1004. 10.1111/apt.15195 [DOI] [PubMed] [Google Scholar]
- 73.Mano F, Ikeda K, Joo E, Yamane S, Harada N, Inagaki N. Effects of three major amino acids found in Japanese broth on glucose metabolism and gastric emptying. Nutrition. (2018) 46:153–8.e1. 10.1016/j.nut.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 74.Witte AB, Hilsted L, Holst JJ, Schmidt PT. Peptide YY3-36 and glucagon-like peptide-1 in functional dyspepsia. Secretion and role in symptom generation. Scand J Gastroenterol. (2016) 51:400–9. 10.3109/00365521.2015.1101780 [DOI] [PubMed] [Google Scholar]
- 75.Tack J, Broeckaert D, Coulie B, Janssens J. Influence of cisapride on gastric tone and on the perception of gastric distension. Alim Pharmacol Ther. (1998) 12:761–6. 10.1046/j.1365-2036.1998.00366.x [DOI] [PubMed] [Google Scholar]
- 76.Kessing BF, Smout AJ, Bennink RJ, Kraaijpoel N, Oors JM, Bredenoord AJ. Prucalopride decreases esophageal acid exposure and accelerates gastric emptying in healthy subjects. Neurogastroenterol Motil. (2014) 26:1079–86. 10.1111/nmo.12359 [DOI] [PubMed] [Google Scholar]
- 77.Carbone F, Tack J. The effect of prucalopride on gastric accommodation in healthy volunteers. Neurogastroenterol Motil. (2014) 26(Suppl. 1):3–4. 10.1111/nmo.12411 [DOI] [PubMed] [Google Scholar]
- 78.Netzer P, Gaia C, Lourens ST, Reber P, Wildi S, Noelpp U, et al. Does intravenous ondansetron affect gastric emptying of a solid meal, gastric electrical activity or blood hormone levels in healthy volunteers? Aliment Pharmacol Ther. (2002) 16:119–27. 10.1046/j.1365-2036.2002.01152.x [DOI] [PubMed] [Google Scholar]
- 79.Janssen P, Vos R, Van Oudenhove L, Tack J. Influence of the 5-HT(3) receptor antagonist ondansetron on gastric sensorimotor function and nutrient tolerance in healthy volunteers. Neurogastroenterol Motil. (2011) 23:444–9.e175. 10.1111/j.1365-2982.2010.01655.x [DOI] [PubMed] [Google Scholar]
- 80.Van Oudenhove L, Kindt S, Vos R, Coulie B, Tack J. Influence of buspirone on gastric sensorimotor function in man. Aliment Pharmacol Ther. (2008) 28:1326–33. 10.1111/j.1365-2036.2008.03849.x [DOI] [PubMed] [Google Scholar]
- 81.Tack J, Janssen P, Masaoka T, Farré R, Van Oudenhove L. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. (2012) 10:1239–45. 10.1016/j.cgh.2012.06.036 [DOI] [PubMed] [Google Scholar]
- 82.Geeraerts B, Van Oudenhove L, Boesmans W, Vos R, Vanden Berghe P, Tack J. Influence of acute tryptophan depletion on gastric sensorimotor function in humans. Am J Physiol Gastrointest Liver Physiol. (2011) 300:G228–35. 10.1152/ajpgi.00020.2010 [DOI] [PubMed] [Google Scholar]
- 83.Tack J, Broekaert D, Coulie B, Fischler B, Janssens J. Influence of the selective serotonin reuptake inhibitor paroxetine on gastric sensorimotor function in man. Alim Pharmacol Ther. (2003) 17:603–8. 10.1046/j.1365-2036.2003.01469.x [DOI] [PubMed] [Google Scholar]
- 84.Janssen P, Van Oudenhove L, Casteels C, Vos R, Verbeke K, Tack J. The effects of acute citalopram dosing on gastric motor function and nutrient tolerance in healthy volunteers. Aliment Pharmacol Ther. (2011) 33:395–402. 10.1111/j.1365-2036.2010.04522.x [DOI] [PubMed] [Google Scholar]
- 85.Janssen P, Vos R, Tack J. The influence of citalopram on interdigestive gastrointestinal motility in man. Aliment Pharmacol Ther. (2010) 32:289–95. 10.1111/j.1365-2036.2010.04351.x [DOI] [PubMed] [Google Scholar]
- 86.Wilmer A, Tack J, Coremans G, Janssens J, Peeters T, Vantrappen G. 5-Hydroxytryptamine3 receptors are involved in the initiation of gastric phase 3 motor activity in man. Gastroenterology. (1993) 105:773–80. 10.1016/0016-5085(93)90895-J [DOI] [PubMed] [Google Scholar]
- 87.Cheung CK, Lee YY, Chan Y, Cheong PK, Law WT, Lee SF, et al. Decreased Basal and postprandial plasma serotonin levels in patients with functional dyspepsia. Clin Gastroenterol Hepatol. (2013) 11:1125–9. 10.1016/j.cgh.2013.03.026 [DOI] [PubMed] [Google Scholar]
- 88.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. (2001) 50:1714–9. 10.2337/diabetes.50.8.1714 [DOI] [PubMed] [Google Scholar]
- 89.Shiiya T, Nakazato M, Mizuta M, Date Y, Mondal MS, Tanaka M, et al. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J Clin Endocrinol Metab. (2002) 87:240–4. 10.1210/jcem.87.1.8129 [DOI] [PubMed] [Google Scholar]
- 90.Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. (2001) 50:707–9. 10.2337/diabetes.50.4.707 [DOI] [PubMed] [Google Scholar]
- 91.Suzuki H, Masaoka T, Hosoda H, Nomura S, Ohara T, Kangawa K, et al. Plasma ghrelin concentration correlates with the levels of serum pepsinogen I and pepsinogen I/II ratio–a possible novel and non-invasive marker for gastric atrophy. Hepatogastroenterology. (2004) 51:1249–54. [PubMed] [Google Scholar]
- 92.Kawashima J, Ohno S, Sakurada T, Takabayashi H, Kudo M, Ro S, et al. Circulating acylated ghrelin level decreases in accordance with the extent of atrophic gastritis. J Gastroenterol. (2009) 44:1046–54. 10.1007/s00535-009-0120-0 [DOI] [PubMed] [Google Scholar]
- 93.Lee KJ, Cha DY, Cheon SJ, Yeo M, Cho SW. Plasma ghrelin levels and their relationship with gastric emptying in patients with dysmotility-like functional dyspepsia. Digestion. (2009) 80:58–63. 10.1159/000215389 [DOI] [PubMed] [Google Scholar]
- 94.Shinomiya T, Fukunaga M, Akamizu T, Irako T, Yokode M, Kangawa K, et al. Plasma acylated ghrelin levels correlate with subjective symptoms of functional dyspepsia in female patients. Scand J Gastroenterol. (2005) 40:648–53. 10.1080/00365520510015403 [DOI] [PubMed] [Google Scholar]
- 95.Deloose E, Janssen P, Depoortere I, Tack J. The migrating motor complex: control mechanisms and its role in health and disease. Nat Rev Gastroenterol Hepatol. (2012) 9:271–85. 10.1038/nrgastro.2012.57 [DOI] [PubMed] [Google Scholar]
- 96.Wilmer A, Van Cutsem E, Andrioli A, Tack J, Coremans G, Janssens J. Prolonged ambulatory gastrojejunal manometry in severe motility-like dyspepsia: lack of correlation between dysmotility, symptoms and gastric emptying. Gut. (1998) 42:235–42. 10.1136/gut.42.2.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kamerling IM, Van Haarst AD, Burggraaf J, Schoemaker RC, Biemond I, Heinzerling H, et al. Motilin effects on the proximal stomach in patients with functional dyspepsia and healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 284:G776–81. 10.1152/ajpgi.00456.2002 [DOI] [PubMed] [Google Scholar]
- 98.Imura H, Seino Y, Mori K, Itoh Z, Yanaihara N. Plasma motilin levels in normal subjects and patients with diabetes mellitus and certain other diseases. Fasting levels and responses to food and glucose. Endocrinol Jpn. (1980) 27(Suppl. 1):151–5. 10.1507/endocrj1954.27.Supplement_151 [DOI] [PubMed] [Google Scholar]
- 99.Tack J, Deloose E, Ang D, Scarpellini E, Vanuytsel T, Van Oudenhove L, et al. Motilin-induced gastric contractions signal hunger in man. Gut. (2016) 65:214–24. 10.1136/gutjnl-2014-308472 [DOI] [PubMed] [Google Scholar]
- 100.Janssens J, Peeters TL, Vantrappen G, Tack J, Urbain JL, De Roo M, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. (1990) 322:1028–31. 10.1056/NEJM199004123221502 [DOI] [PubMed] [Google Scholar]
- 101.Arts J, Caenepeel P, Verbeke K, Tack J. Influence of erythromycin on gastric emptying and meal related symptoms in functional dyspepsia with delayed gastric emptying. Gut. (2005) 54:455–60. 10.1136/gut.2003.035279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Larson JM, Tavakkoli A, Drane WE, Toskes PP, Moshiree B. Advantages of azithromycin over erythromycin in improving the gastric emptying half-time in adult patients with gastroparesis. J Neurogastroenterol Motil. (2010) 16:407–13. 10.5056/jnm.2010.16.4.407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tack J, Peeters TL. What comes after macrolides and other motilin stimulants? Gut. (2001) 49:317–8. 10.1136/gut.49.3.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Russo A, Stevens JE, Giles N, Krause G, O'Donovan DG, Horowitz M, et al. Effect of the motilin agonist KC 11458 on gastric emptying in diabetic gastroparesis. Aliment Pharmacol Ther. (2004) 20:333–8. 10.1111/j.1365-2036.2004.02066.x [DOI] [PubMed] [Google Scholar]
- 105.Chua AS, Keeling PW, Dinan TG. Role of cholecystokinin and central serotonergic receptors in functional dyspepsia. World J Gastroenterol. (2006) 12:1329–35. 10.3748/wjg.v12.i9.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fried M, Feinle C. The role of fat and cholecystokinin in functional dyspepsia. Gut. (2002) 51(Suppl. 1):i54–7. 10.1136/gut.51.suppl_1.i54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scarpignato C, Varga G, Corradi C. Effect of CCK and its antagonists on gastric emptying. J Physiol. (1993) 87:291–300. 10.1016/0928-4257(93)90035-R [DOI] [PubMed] [Google Scholar]
- 108.Chua AS, Keeling PW. Cholecystokinin hyperresponsiveness in functional dyspepsia. World J Gastroenterol. (2006) 12:2688–93. 10.3748/wjg.v12.i17.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. (1985) 89:1070–7. 10.1016/0016-5085(85)90211-2 [DOI] [PubMed] [Google Scholar]
- 110.Gershon MD, Tack J. The serotonin signalling system: from basic understanding to drug development for functional GI disorders. Gastroenterology. (2007) 219:172–80. 10.1053/j.gastro.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 111.Kidd M, Modlin IM, Gustafsson BI, Drozdov I, Hauso O, Pfragner R. Luminal regulation of normal and neoplastic human EC cell serotonin release is mediated by bile salts, amines, tastants, and olfactants. Am J Physiol Gastrointest Liver Physiol. (2008) 295:G260–72. 10.1152/ajpgi.00056.2008 [DOI] [PubMed] [Google Scholar]
- 112.Atkinson W, Lockhart S, Whorwell PJ, Keevil B, Houghton LA. Altered 5-hydroxytryptamine signaling in patients with constipation- and diarrhea-predominant irritable bowel syndrome. Gastroenterology. (2006) 130:34–43. 10.1053/j.gastro.2005.09.031 [DOI] [PubMed] [Google Scholar]
- 113.Dunlop SP, Coleman NS, Blackshaw E, Perkins AC, Singh G, Marsden CA, et al. Abnormalities of 5-hydroxytryptamine metabolism in irritable bowel syndrome. Clin Gastroenterol Hepatol. (2005) 3:349–57. 10.1016/S1542-3565(04)00726-8 [DOI] [PubMed] [Google Scholar]
- 114.Tack J, Van den Houte K, Carbone F. The unfulfilled promise of prokinetics for functional dyspepsia/postprandial distress syndrome. Am J Gastroenterol. (2019) 114:204–6. 10.14309/ajg.0000000000000072 [DOI] [PubMed] [Google Scholar]
- 115.Hallerback BI, Bommelaer G, Bredberg E, Campbell M, Hellblom M, Lauritsen K, et al. Dose finding study of mosapride in functional dyspepsia: a placebo-controlled, randomized study. Aliment Pharmacol Ther. (2002) 16:959–67. 10.1046/j.1365-2036.2002.01236.x [DOI] [PubMed] [Google Scholar]
- 116.Tack J, Rotondo A, Meulemans A, Thielemans L, Cools M. Randomized clinical trial: a controlled pilot trial of the 5-HT4 receptor agonist revexepride in patients with symptoms suggestive of gastroparesis. Neurogastroenterol Motil. (2016) 28:487–97. 10.1111/nmo.12736 [DOI] [PubMed] [Google Scholar]
- 117.Jin M, Mo Y, Ye K, Chen M, Liu Y, He C. Efficacy of serotonin receptor agonists in the treatment of functional dyspepsia: a meta-analysis. Arch Med Sci. (2019) 15:23–32. 10.5114/aoms.2017.69234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Carbone F, Van den Houte K, Clevers E, Andrews CN, Papathanasopoulos A, Holvoet L, et al. Prucalopride in gastroparesis: a randomized placebo-controlled crossover study. Am J Gastroenterol. (2019) 114:1265–74. 10.14309/ajg.0000000000000304 [DOI] [PubMed] [Google Scholar]
- 119.Abell T, Kuo B, Esfandyari T, Canafax D, Camerini R, Grimaldi M, et al. Velusetrag improves gastroparesis both in symptoms and gastric emptying in patients with diabetic or idiopathic gastroparesis in a 12-week global phase 2B study. Gastroenterology. (2019) 156:S164 10.1016/S0016-5085(19)37201-4 [DOI] [Google Scholar]
- 120.Midani D, Parkman HP. Granisetron transdermal system for treatment of symptoms of gastroparesis: a prescription registry study. J Neurogastroenterol Motil. (2016) 22:650–5. 10.5056/jnm15203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Simmons K, Parkman HP. Granisetron transdermal system improves refractory nausea and vomiting in gastroparesis. Dig Dis Sci. (2014) 59:1231–4. 10.1007/s10620-014-3097-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.