Abstract
Multiple intramuscular (IM) injections of vitamin A have been shown to decrease bronchopulmonary dysplasia in very low birth weight (VLBW) neonates. However, this regime is neither practical nor popular. Oral vitamin A has failed to achieve adequate plasma levels. We aimed to investigate if a schedule of initial IM followed by oral supplementation can reduce vitamin A deficiency. This was a blinded, randomized controlled trial, conducted in a level III neonatal unit. Neonates with birth weight from 750 to 1250 g, were enrolled at the age of 24–96 h of life. They were randomly allocated to vitamin A supplementation (VAS) (n = 61) or placebo group (n = 59). VAS group received vitamin A 5000 IU IM on alternate days till establishment of adequate enteral feeds, followed by oral 10,000 IU daily for 28 days. The primary outcome was incidence of vitamin A deficiency (plasma retinol < 200 μg/L) on day 28. A total of 120 neonates with mean (SD) gestation age and birth weight of 31 (2.4) weeks and 1065 (141) g, respectively were enrolled. More than 90% of cases were vitamin A deficient at the baseline. The proportion of vitamin A deficient infants on day 28 of study was significantly lower in VAS group compared to placebo group (4% vs. 61%, p < 0.001). The median (1st–3rd quartile) plasma retinol levels (μg/L) were significantly higher in VAS group compared to placebo [489 (295,627) vs. 184 (156,240), p < 0.001]. We conclude that the IM followed by oral VAS significantly reduced the incidence of vitamin A deficiency in VLBW infants.
Keywords: Bronchopulmonary dysplasia, Neonate, Very low birth weight, Vitamin A
Introduction
Preterm and very low birth weight (VLBW) infants have lower plasma concentration as well as hepatic stores of vitamin A compared to term infants [1, 2]. Vitamin A is necessary for regulation of normal lung growth, epithelial differentiation and maintaining integrity of respiratory tract epithelium [2–4]. Its deficiency predisposes to recurrent infections, squamous metaplasia, and increased risk of bronchopulmonary dysplasia (BPD) [2–5]. Many preventive and therapeutic strategies including postnatal steroids, caffeine, and gentle ventilation demonstrate variable success in reducing BPD; and vitamin A supplementation (VAS) seems to have promising effects [2, 6, 7]. Initial trials [8, 9] demonstrated inconsistent effects of VAS on prevention of BPD until Tyson et al. [3] reported increased vitamin A levels and reduced incidence of BPD after intramuscular (IM) supplementation. However, 25% of the infants had low serum retinol concentrations even after receiving vitamin A. Ambalavanan et al. tried higher doses, but did not find any increase in retinol levels or improvement in clinical outcomes as compared to standard regimen [4]. The major difficulty with these regimens is that they necessitate 12 IM injections and hence are not in widespread use [10]. The oral route of administration of vitamin A offers an attractive alternative. However, in extremely low birth weight (ELBW) infants, oral VAS was associated with suboptimal plasma levels in 50% of the study population and did not improve outcomes [1]. An alternative method, combining an initial parenteral and then enteral supplementation of vitamin A, has been suggested but has not been evaluated in controlled trials [11]. We conducted this study to determine the efficacy of IM followed by oral VAS, in reducing vitamin A deficiency in very low birth weight (VLBW) babies. We hypothesized that this modified schedule, will lead to higher plasma retinol concentrations, decreased incidence of vitamin A deficiency and improved health outcomes.
Materials and methods
This was a randomized placebo-controlled trial conducted in a level III neonatal unit in PGIMER, Chandigarh from August 2007 to August 2008. All inborn neonates with birth weight 750–1250 g and between 24 and 96 h of life were eligible. Babies with lethal congenital malformations or shock were excluded. Eligible neonates were enrolled after getting informed written consent from one of the parents. The trial was approved by Institute Ethics Committee of PGIMER Chandigarh and was registered with Clinical Trials Registry of India (CTRI/2009/091/000010). The enrolled neonates underwent stratified block randomization according to birth weight (750–999 g and 1000–1250 g). Each stratum had permuted, even-numbered, randomly varying block sizes. The random sequence was generated online from the website www.randomizer.org. The investigators, supervisors, caregivers, laboratory personnel, and statistician were blinded to the intervention. The randomization sequence code was opened only after completion of biochemical and statistical analysis.
Intervention
The enrolled infants received vitamin A (5000 IU IM or 10,000 IU orally) or placebo. The vitamin A preparations used in this trial were Aquasol A (retinol palmitate; 40,000 IU/mL; USV Pharmaceuticals, Mumbai, India) for IM administration and retinol palmitate 10,000 IU/mL (BASF, Germany) for oral administration. Normal saline was used as IM placebo. Hospital pharmacy prepared a placebo appearing identical to oral vitamin A solution using approved ingredients. Pre-filled syringes (0.5 mL, 30G 5/16″ needle-Becton–Dickinson, New Jersey, USA) containing 0.125 mL (5000 IU) of vitamin A or normal saline; and amber colored glass bottles, containing 50 mL of oral vitamin A or placebo, were prepared in the pharmacy. The body of the syringes was covered completely by an opaque tape. A non-study person, who generated the randomization sequence prepared serially pre-numbered plastic study boxes, each containing a set of either 14 prefilled syringes of vitamin A and 1 bottle of oral vitamin A, or 14 prefilled syringes of normal saline and one bottle of oral placebo. One complete box was allocated to one infant. A nurse administered the drug at 9 AM on alternate days. Once enteral intake exceeded 50% of daily fluid requirement, IM administration was switched to oral. One mL of the drug mixed in feeds was given either through orogastric tube or spoon. The drug was administered till 28 days from start of study or death, whichever was earlier. If feeds were stopped or decreased to less than 50% of total fluid intake for any reason, IM administration was restarted in place of oral.
The treating physicians retained responsibility for decisions regarding use of therapies other than vitamin A. They also decided on the need to start or stop feeds, advancement of feeds and volume of fluids.
Evaluation
Plasma retinol was estimated before administration of first dose and on day 14 ± 2 and 28 ± 2 of the study. On study day 28 ± 2, apart from plasma retinol estimation, retinol binding protein (RBP) was also estimated. Subsequently, the infant was administered 2000 IU/kg of vitamin A IM and a second venous blood sample was taken 3 h later for repeat RBP estimation and determination of ∆-RBP, which was calculated as percent increase in RBP levels from baseline value after IM administration of vitamin A. Plasma retinol concentration was estimated by high performance liquid chromatography, by using a quaternary gradient pump (Series 200, Perkin Elmer, Massachusetts, USA) with a 100 μL fixed volume injector coupled with an auto-injector and a photodiode array-detector, with two fixed wavelengths, using tocopherol acetate as the internal standard. Plasma RBP was estimated by two-site ELISA by using a human retinol-binding protein kit (Immunology Consultants Laboratory Inc., USA). We calculated daily vitamin A intake from routine sources using reference values [12].
Infants were assessed weekly for potential signs of vitamin A toxicity. In presence of any sign, detailed evaluation including liver function tests, hematologic indices and plasma retinol estimation was planned. The decision of further administration of study drug was left to the treating physicians without breaking the code.
Outcome
The primary outcome was proportion of infants with plasma retinol levels < 200 μg/L on study day 28 ± 2. Other biochemical outcomes were proportion of infants with plasma retinol levels < 200 μg/L on study day 14 ± 2, RBP < 25 mg/L and ∆-RBP > 10% on study day 28 ± 2. Secondary clinical outcomes evaluated were incidence of BPD (National Institutes of Health consensus definition) [13], sepsis, pneumonia [14], intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), death and duration of ventilation and oxygen.
Sample size
We recruited 120 infants to detect a 54% relative reduction (from 54 to 25%) in the proportion of infants with plasma retinol < 200 μg/L on study day 28, with 80% power and α level of 0.05. The margin of 54% was chosen as it was associated with a statistically significant reduction in incidence of BPD in NICHD trial [3].
Statistical analysis
We described categorical variables as percentages; normally distributed numerical variables as mean (SD) and those with skewed distributions as median (1st, 3rd quartile). Numerical variables were compared by Student’s t test or Mann–Whitney U test as applicable. Whereas, Chi square test or Fisher’s exact test, as appropriate was used for categorical. SPSS version 15 (IBM-SPSS, Armonk, NY) used for statistical analysis and p < 0.05 was considered significant. We analyzed by intention-to-treat.
Results
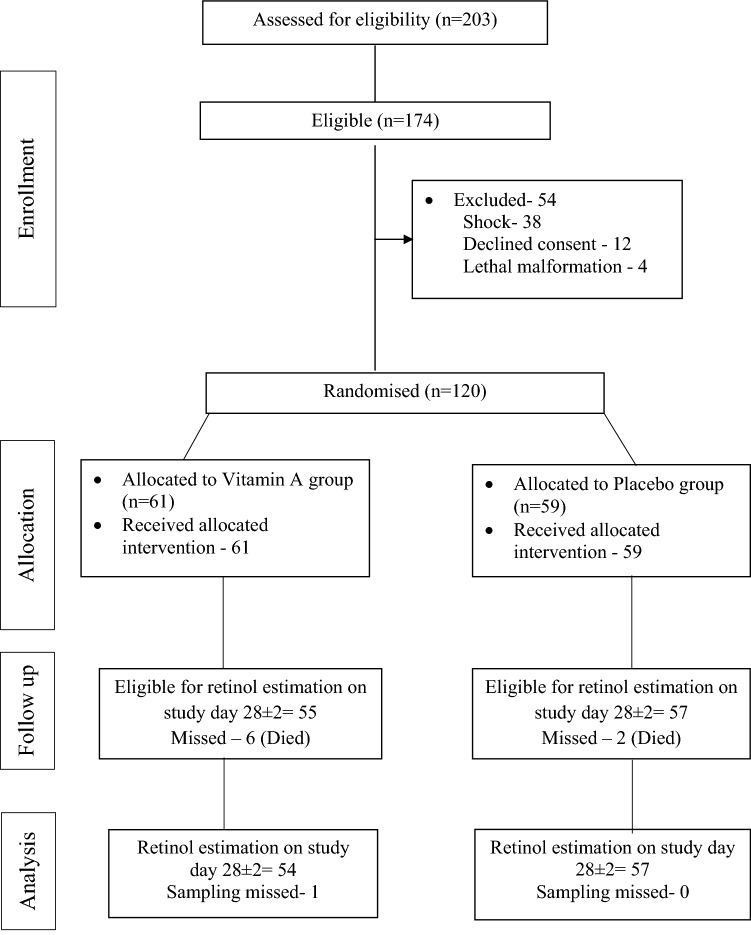
A total of 120 infants were enrolled, of which 61 and 59 were allocated to VAS and placebo groups respectively (Fig. 1). Baseline demographic characteristics and morbidity profile were similar in both groups (Table 1). Surfactant administration rates were similar in both groups (30% vs. 27%). The median (1st, 3rd quartile) number of IM drug doses received were 1 (0, 9) and 1 (0, 7) in VAS and placebo groups respectively. Eleven infants received postnatal steroids (vitamin A—4 (7%) vs. placebo—7 (12%), p = 0.36). The mean daily vitamin A intake from sources other than study drug increased with age (Fig. 2) but was not different between two groups.
Fig. 1.
Flow of the study
Table 1.
Baseline characteristics of vitamin A and placebo groups at enrollment
| Characteristic | Vitamin A group (n = 61) | Placebo group (n = 59) |
|---|---|---|
| Gestational age (weeks) | 31 (2.4) | 31 (2.5) |
| Mean (SD) | ||
| Birth weight (g) | 1047 (143) | 1084 (137) |
| Mean (SD) | ||
| Male sex (%) | 36 (59) | 32 (54) |
| Caesarean delivery (%) | 26 (43) | 28 (47) |
| Antenatal steroids (%) | 53 (87) | 50 (85) |
| Antenatal risk factors for sepsis (%) | 11 (18) | 8 (14) |
| Small for gestational age n (%) | 23 (38) | 17 (29) |
| Apgar score at 1 min | 7 (6, 8) | 7 (5, 8) |
| Median (1st, 3rd quartile) | ||
| Apgar score at 5 min | 8 (8, 9) | 8 (8, 9) |
| Median (1st, 3rd quartile) | ||
| On partial feeds (%) | 42 (69) | 40 (68) |
| On antibiotics (%) | 23 (38) | 26 (44) |
| Intraventricular hemorrhage (%) | 5 (8) | 6 (10) |
| On respiratory support (%) | 15 (25) | 13 (22) |
| On invasive ventilation (%) | 6 (10) | 3 (5) |
| FiO2 (%) | 25 (25, 30) | 25 (25, 30) |
| Median (1st, 3rd quartile) |
CPAP, continuous positive airway pressure; NIPPV, non invasive positive pressure ventilation; FiO2, fraction of inspired oxygen
Fig. 2.
Weekly vitamin A intake from routine sources. *RMAnova for between-groups comparison (p > 0.05)
Biochemical outcomes
The baseline plasma retinol was low but similar in both groups (Table 2). During the study period, it increased significantly in both groups, but the increase was much more in VAS group resulting in significant differences on both study days. In ELBW infants also, similar findings were seen [median (IQR) plasma retinol—vitamin A group: 91 (81,121) µg/L at study entry, 282 (217,343) µg/L on study day 14 ± 2 and 317 (281,589) µg/L on study day 28 ± 2, RM Anova p < 0.001; placebo group: 82 (71, 96) µg/L at study entry, 110 (82,155) µg/L on study day 14 ± 2 and 147 (122,190) µg/L on study day 28 ± 2, RM Anova p 0.01].
Table 2.
Biochemical outcomes: plasma retinol, RBP and ∆-RBP
| Parameter | Time point of assessment | Vitamin A group (n = 55) | Placebo group (n = 59) | p value |
|---|---|---|---|---|
| Plasma retinol levels (µg/L) | At study entry | 94 (81,147) | 94 (76,110) | 0.15 |
| Median (1st, 3rd quartile) | On day 14 ± 2 | 273 (180,454) | 119 (87,161) | < 0.001 |
| On day 28 ± 2 | n = 54 | n = 57 | < 0.001 | |
| 489 (295,627) | 184 (156,240) | |||
| Vitamin A deficiency (plasma retinol < 200 µg/L) n (%) | At study entry | 52 (94.5) | 58 (98.3) | 0.35 |
| On day 14 ± 2 | 16 (29.1) | 56 (94.9) | < 0.001 | |
| On day 28 ± 2 | n = 54 | n = 57 | < 0.001 | |
| 2 (3.7) | 35 (61.4) | |||
| Severe vitamin A deficiency (plasma retinol < 100 µg/L) n (%) | At study entry | 29 (52.7) | 36 (61.0) | 0.37 |
| On day 14 ± 2 | 0 | 21 (35.6) | < 0.001 | |
| On day 28 ± 2 | n = 54 | n = 57 | 0.50a | |
| 0 | 2 (3.5) | |||
| RBP < 25 mg/L at study end n (%) | n = 41 | n = 53 | < 0.001 | |
| 5 (12.2) | 32 (60.4) | |||
| ∆-RBP > 10% at study end n (%) | n = 41 | n = 53 | 0.03 | |
| 13 (31.7) | 29 (54.7) |
Analysis of all categorical variables was by Chi square with continuity correction and all numerical variables was by Mann–Whitney test unless specified
RBP, retinol binding protein; ∆-RBP, change in retinol binding protein
aFisher’s exact test
At enrollment, majority of infants were vitamin A deficient in both groups. However, on days 14 and 28, proportion of deficient infants was significantly lower in VAS group compared to placebo. Similar trend was also observed in ELBW infants (proportion of vitamin A deficient infants at study entry, day 14 ± 2 and 28 ± 2 was 100%, 20%, and 0% in vitamin A group vs. 100%, 100% and 78% respectively in placebo group, p < 0.001). The proportion of infants with plasma RBP levels < 25 mg/L and ∆-RBP > 10% at study day 28 ± 2 was significantly lower in vitamin A group. The plasma retinol levels at study conclusion had a good correlation with plasma RBP levels obtained concurrently (Spearman’s ρ = 0.913, p < 0.001).
Clinical outcomes
There was no significant difference between two groups in various respiratory and non-respiratory outcomes (Table 3). There was a trend towards decreased need for ventilation in the intervention group (7% vs. 0%, p = 0.05). BPD rates were similar but a higher proportion had moderate to severe BPD in placebo group.
Table 3.
Secondary outcomes (respiratory and non-respiratory)
| Parameter | Vitamin A group (n = 55) | Placebo group (n = 59) | p value |
|---|---|---|---|
| Duration of any ventilation (h) | n = 20 | n = 21 | 0.39 |
| Median (1st–3rd quartile) | 96 (72,120) | 120 (72,168) | |
| Duration of invasive ventilation (h) | n = 14 | n = 8 | 0.07 |
| Median (1st–3rd quartile) | 72 (66,102) | 108 (96,138) | |
| Duration of oxygen therapy (h) | n = 35 | n = 29 | 0.22 |
| Median (1st–3rd quartile) | 120 (72,216) | 144 (96,282) | |
| Infants requiring oxygen at end of the study n (%) | 8 (13) | 11 (19) | 0.41 |
| Infants requiring invasive ventilation at the end of the study n (%) | 0 (0) | 4 (7) | 0.055a |
| BPD n (%) | 5 (8) | 7 (12) | 0.55 |
| Severity of BPD n (%) | 0.23a | ||
| Mild | 4 (80) | 3 (42) | |
| Moderate | 1 (20) | 2 (29) | |
| Severe | 0 | 2 (29) | |
| Duration of hospitalization (days) | 31 (18, 43) | 27 (20, 46) | 0.71 |
| Median (1st–3rd quartile) | |||
| Intraventricular hemorrhage n (%) | 5 (8) | 6 (10) | 0.71 |
| All-cause mortality n (%) | 8 (13) | 4 (7) | 0.36 |
| Periventricular leukomalacia n (%) | 2 (3.3) | 4 (6.8) | 0.43a |
| Blood stream infection n (%) | 22 (36) | 18 (30) | 0.52 |
| Pneumonia n (%) | 18 (30) | 20 (34) | 0.60 |
Analysis of all categorical variables was by Chi square and all numerical variables was by Mann–Whitney test unless specified
BPD, bronchopulmonary dysplasia
aFisher’s exact test
Safety
None of the study subjects had vitamin A toxicity. Two (1.6%) infants in the vitamin A group had plasma retinol > 800 µg/L (maximum 853 µg/L) on day 28 ± 2, compared to none in placebo group but there were no clinical signs of toxicity.
Discussion
A combination of IM plus oral VAS regime significantly increased plasma retinol levels and decreased the proportion of vitamin A deficient infants on study days 14 ± 2 and 28 ± 2. The proposed VAS regime also replete hepatic vitamin A stores as indicated by ∆-RBP response at study completion. Similar results were seen in ELBW infants. Unlike other studies [3, 10, 15, 16] we recruited all infants irrespective of their respiratory status. This is because BPD also occurs in a significant proportion of VLBW infants who have no or minimal respiratory distress in immediate postnatal period [17].
Previously published trials also looked at oral VAS in preterm infants [1, 18–21]. Coutsoudis et al. reported oral supplementation of 25,000 IU vitamin A on day 1, 4 and 8 in preterm infants resulted in higher mean serum retinol concentrations than placebo at 5 h after last dose of vitamin A [18]. We collected blood samples prior to administration of subsequent drug dose to ensure that plasma values do not reflect peak levels. Wardle et al. observed that oral VAS in ELBW infants in a dose of 5000 IU/day for 28 days did not result in a significant increase in plasma retinol levels, except at 24 h after the first dose [1]. Calisici et al. used oral vitamin A 30,000 IU/kg weekly and didn’t find any reduction in BPD or mortality [21]. The results of the largest placebo controlled ongoing trial (NeoVitaA Trial) to assess the role of postnatal high-dose oral VAS (5000 IU/kg/day) in reducing BPD or death in ELBW infants are still awaited [22]. We found a very high incidence of vitamin A deficiency at enrollment, consistent with other studies [23–25]. Similar findings were reported by Shah et al. [26] but Mishra et al. [27] found higher cord blood retinol levels (172 ± 99 µg/L) in 38 VLBW infants, though 80% of infants were vitamin A deficient. The study population of Mishra et al. [27] had a higher gestation (32 ± 2.7 wk) and birth weight (1157 ± 267 g). Also, retinol levels in cord blood are likely to be higher than in immediate postnatal period because of influence of antenatal steroids [28]. Hence, values obtained after 24 h of age are more representative of the neonate’s vitamin A status than cord blood.
None of the ELBW infants in our study were vitamin A deficient after supplementation. In the NICHD trial and trial by Ambalavanan et al. [3, 4], 25% and 21% of infants were vitamin A deficient despite IM supplementation of 5000 IU/day and 10,000 IU/day for 28 days, respectively. The differences could be because ELBW infants in our study had a higher gestation and birth weight when compared to these two trials. We estimated RBP and ∆-RBP at study completion as measures of vitamin A stores although retinol has also been used by others [3, 4]. This was because RBP is more stable than retinol with respect to light and temperature and a smaller volume of blood is required for RBP measurement. RBP has been validated for measurement of hepatic vitamin A stores in preterm infants [29]. Others have also reported good correlation between retinol levels and RBP [30]. A significantly lesser proportion of infants with RBP < 25 mg/L and ∆-RBP > 10% in vitamin A group shows that modified VAS regimen not only increased blood concentration of vitamin A but also liver stores. The modified VAS regimen appears to be safe. None of the trials in preterms reported any major adverse effects [2]. Also, toxic level in neonates is not well defined as levels up to 1140 µg/L have been well tolerated [4].
The current study was mainly aimed for the biochemical parameters and did not have adequate statistical power to evaluate clinical outcomes. The proportion of infants who developed BPD was 8% and 12% in VAS and placebo groups respectively. A sample size of 931 infants would be required in each group in order to demonstrate a 33% reduction in BPD rate, with 80% power at α level of 0.05. This would require a large multicenter trial.
Routine oral vitamin A supplementation is not recommended in the healthy term, normal weight infants. In settings where vitamin A deficiency is a public health problem, high-dose vitamin A supplementation is recommended in children between 6 and 59 months of age. At present, there is insufficient evidence to recommend routine oral vitamin A supplementation in low birth weight infants. Trials of intramuscular vitamin A have been shown to decrease the incidence of BPD in VLBW babies, but giving alternate day intramuscular injections in small sized infants with poor muscle mass have precluded its acceptance.
The modified VAS regimen achieved adequate vitamin A levels and stores, and significantly reduced the incidence of vitamin A deficiency in VLBW infants. This regime strives to utilize the potential benefit of IM route, yet avoids the disadvantages of frequent IM injections. Adequately powered studies are required to demonstrate the clinical benefits of improved vitamin A status by this regimen.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedure performed in study were in accordance with the ethical standards of the Institutional Ethics Committee of PGIMER Chandigarh and with the 1964 Helsinki declaration and its later amendments.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Arch Dis Child Fetal Neonatal Ed. 2001;84:F9–F13. doi: 10.1136/fn.84.1.F9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Darlow BA, Graham PJ, Rojas-Reyes MX. Vitamin A supplementation to prevent mortality and short- and long-term morbidity in very low birth weight infants. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD000501.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tyson JE, Wright LL, Oh W, et al. Vitamin A supplementation for extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. 1999;340:1962–1968. doi: 10.1056/NEJM199906243402505. [DOI] [PubMed] [Google Scholar]
- 4.Ambalavanan N, Wu T-J, Tyson JE, Kennedy KA, Roane C, Carlo WA. A comparison of three vitamin a dosing regimens in extremely-low-birth-weight infants. J Pediatr. 2003;142:656–661. doi: 10.1067/mpd.2003.214. [DOI] [PubMed] [Google Scholar]
- 5.Gawronski CA, Gawronski KM. Vitamin A supplementation for prevention of bronchopulmonary dysplasia: cornerstone of care or futile therapy? Ann Pharmacother. 2016;50:680–684. doi: 10.1177/1060028016647066. [DOI] [PubMed] [Google Scholar]
- 6.Baveja R, Christou H. Pharmacological strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:209–218. doi: 10.1053/j.semperi.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Ambalavanan N, Carlo WA. Ventilatory strategies in the prevention and management of bronchopulmonary dysplasia. Semin Perinatol. 2006;30:192–199. doi: 10.1053/j.semperi.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. J Pediatr. 1987;111:269–277. doi: 10.1016/S0022-3476(87)80086-0. [DOI] [PubMed] [Google Scholar]
- 9.Pearson E, Bose C, Snidow T, et al. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. J Pediatr. 1992;121:420–427. doi: 10.1016/S0022-3476(05)81800-1. [DOI] [PubMed] [Google Scholar]
- 10.Ambalavanan N, Kennedy K, Tyson J, Carlo WA. Survey of vitamin a supplementation for extremely-low-birth-weight infants: is clinical practice consistent with the evidence? J Pediatr. 2004;145:304–307. doi: 10.1016/j.jpeds.2004.04.046. [DOI] [PubMed] [Google Scholar]
- 11.Shenai JP. Vitamin A supplementation in very low birth weight neonates: rationale and evidence. Pediatrics. 1999;104:1369–1374. doi: 10.1542/peds.104.6.1369. [DOI] [PubMed] [Google Scholar]
- 12.Walsh MC. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004;114:1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 13.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 14.Gastmeier P, Geffers C, Schwab F, Fitzner J, Obladen M, Ruden H. Development of a surveillance system for nosocomial infections: the component for neonatal intensive care units in Germany. J Hosp Infect. 2004;57:126–131. doi: 10.1016/j.jhin.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Ravishankar C, Nafday S, Green RS, et al. A trial of vitamin A therapy to facilitate ductal closure in premature infants. J Pediatr. 2003;143:644–648. doi: 10.1067/S0022-3476(03)00501-8. [DOI] [PubMed] [Google Scholar]
- 16.Coutsoudis A, Adhikari M, Coovadia HM. Serum vitamin A (retinol) concentrations and association with respiratory disease in premature infants. J Trop Pediatr. 1995;41:230–233. doi: 10.1093/tropej/41.4.230. [DOI] [PubMed] [Google Scholar]
- 17.Charafeddine L, D’Angio CT, Phelps DL. Atypical chronic lung disease patterns in neonates. Pediatrics. 1999;103:759–765. doi: 10.1542/peds.103.4.759. [DOI] [PubMed] [Google Scholar]
- 18.Coutsoudis A, Adhikari M, Pillay K, Kuhn L, Coovadia HM. Effect of vitamin A supplementation on morbidity of low-birth-weight neonates. S Afr Med J. 2000;90:730–736. [PubMed] [Google Scholar]
- 19.Meyer S, Gortner L. Early postnatal additional high-dose oral vitamin a supplementation versus placebo for 28 days for preventing bronchopulmonary dysplasia or death in extremely low birth weight infants. Neonatology. 2014;105:182–188. doi: 10.1159/000357212. [DOI] [PubMed] [Google Scholar]
- 20.Rakshasbhuvankar A, Patole S, Simmer K, Pillow JJ. Enteral vitamin A for reducing severity of bronchopulmonary dysplasia in extremely preterm infants: a randomised controlled trial. BMC Pediatr. 2017;17:204. doi: 10.1186/s12887-017-0958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calisici E, Yarci E, Degirmencioglu H, et al. PO-0731 the effects of early oral vitamin A treatment on the prevention of bronchopulmonary displasia in the low birth weight Infants. Arch Dis Child. 2014;99(Suppl 2):A494. [Google Scholar]
- 22.Meyer S, Gortner L, NeoVitaA Trial investigators Up-date on the NeoVitaA Trial: obstacles, challenges, perspectives, and local experiences. Wien Med Wochenschr. 2017;167:264–270. doi: 10.1007/s10354-016-0500-z. [DOI] [PubMed] [Google Scholar]
- 23.Brandt RB, Mueller DG, Schroeder JR, et al. Serum vitamin A in premature and term neonates. J Pediatr. 1978;92:101–104. doi: 10.1016/S0022-3476(78)80086-9. [DOI] [PubMed] [Google Scholar]
- 24.Tolba AM, Hewedy FM, Al-Senaidy AM, Al-Othman AA. Neonates’ vitamin A status in relation to birth weight, gestational age, and sex. J Trop Pediatr. 1998;44:174–177. doi: 10.1093/tropej/44.3.174. [DOI] [PubMed] [Google Scholar]
- 25.Mazumder S, Taneja S, Bhatia K, Neovita India Study Group et al. Efficacy of early neonatal supplementation with vitamin A to reduce mortality in infancy in Haryana, India (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385:1333–1342. doi: 10.1016/S0140-6736(14)60891-6. [DOI] [PubMed] [Google Scholar]
- 26.Shah RS, Rajalakshmi R. Vitamin A status of the newborn in relation to gestational age, body weight, and maternal nutritional status. Am J Clin Nutr. 1984;40:794–800. doi: 10.1093/ajcn/40.4.794. [DOI] [PubMed] [Google Scholar]
- 27.Mishra S, Thomas K, Agarwal R, Gupta YK, Deorari AK, Paul VK. Correlation between umbilical cord and maternal intrapartum serum retinol in very low birth weight neonates. Indian J Pharmacol. 2008;40(Suppl 2):S37–S56. [Google Scholar]
- 28.Georgieff MK, Chockalingam UM, Sasanow SR, Gunter EW, Murphy E, Ophoven JJ. The effect of antenatal betamethasone on cord blood concentrations of retinol-binding protein, transthyretin, transferrin, retinol, and vitamin E. J Pediatr Gastroenterol Nutr. 1988;7:713–717. doi: 10.1097/00005176-198809000-00016. [DOI] [PubMed] [Google Scholar]
- 29.Weinman AR, Jorge SM, Martins AR, de Assis Md, Martinez FE, Camelo JS., Jr Assessment of vitamin A nutritional status in newborn preterm infants. Nutrition. 2007;23:454–460. doi: 10.1016/j.nut.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Jain MK, Mehta NJ, Fonseca M, Pai NV. Correlation of serum vitamin A and its transport protein (RBP) in malnourished and vitamin A deficient children. J Postgrad Med. 1990;36:119–123. [PubMed] [Google Scholar]