Abstract
The utility of C-reactive protein (CRP) as a marker of disease severity, therapeutic response and prognosis in tuberculosis has been suggested. This study aims to determine the levels of high sensitivity CRP (hs CRP) among the pediatric tuberculosis cases. A case control study was conducted on 60 clinically diagnosed (clinical findings and radiography and/or contact history and/or Mantoux test) or microbiologically confirmed (smear and/or culture and/or Cartridge based Nucleic Acid Amplification test positive) pediatric tuberculosis cases ≤ 12 years. hs CRP levels were estimated in the cases and healthy controls using ELISA. Median levels of serum hs CRP were significantly higher in pediatric tuberculosis cases (25 mg/l) as compared to controls (0.530 mg/l). No significant correlation was found with age, gender, site of tuberculosis or presence of dissemination. Lower levels were found with palpable lymphadenopathy. Levels were not significantly different between microbiologically confirmed cases and those who were negative by one or more of the microbiological tests of staining, culture and cartridge based nucleic acid amplification test. hs CRP can be used in diagnostic algorithms of pediatric tuberculosis to rule out tuberculosis. Further studies could help in determining the prognostic and therapeutic response of hs CRP among children leading to better management.
Keywords: hs CRP, Pediatric, Tuberculosis, Diagnosis
Introduction
Despite the discovery of effective anti tubercular drugs more than 5 decades back, tuberculosis (TB) still remains one of the leading causes of deaths worldwide. World Health Organization (WHO) estimated a global burden of 10.4 million TB patients in 2016 of which only 61% was reported. The worst affected was the South-East Asian region, constituting around 45% of the estimated new cases, with India contributing maximum (25%) to the gap in the incidence and the reported cases globally [1]. In endemic countries, pediatric TB remains an important but sometimes unrecognized cause of disease and even mortality. Childhood TB constitutes 10–20% of all TB cases in the high burden countries [2]. Globally, 2,39,000 children died from tuberculosis in 2015. The incidence of TB in India in 2016 has been estimated to be around 27,90,000 of which new pediatric TB cases were approximately 2,27,000 [3]. The children are more likely to progress to disease after exposure to Mycobacterium tuberculosis as compared to the adults due to the differences in the immune mechanisms [4]. As childhood TB is an indicator of new exposure rather than reactivation, it may act as a measure of continuing transmission of infection [5].
The diagnosis of TB remains challenging in resource limited high burden settings, particularly due to continued dependence on smear microscopy and empirical clinical diagnosis due to the lack of proper laboratory diagnostic facilities. TB in children remains difficult to diagnose both for the clinicians as well as the microbiologists. The children may present with vague clinical symptoms which may mimic other common childhood diseases and lead to delay in clinical diagnosis. Microbiological diagnosis is rarely achieved due to paucibacillary disease in children and inadequate sample volumes. The positivity of Ziehl–Neelsen (ZN) staining and conventional culture on Lowenstein Jensen’s (LJ) medium remains low from suboptimal samples in children [6]. Despite the introduction of molecular diagnostic techniques like Cartridge based Nucleic Acid Amplification test (CBNAAT), limited availability and affordability prevent people with TB symptoms from accessing these services. So an intensified research in the field of development of innovative diagnostic tools has been identified as the need of the hour for an effective TB control strategy.
Acute phase reactant like serum C reactive protein (CRP) has been found to be increased in TB, the levels being highest in advanced disease. The levels have been found to fall with the therapy and also correlate with the clinical response [7]. CRP participates in the innate immune response by binding to the ligands such as phosphocholine on the dead or dying cells and bacteria, activates the complement C1q and the classical complement pathway and promotes phagocytosis [8]. It is rapidly increased in response to peptide regulating factors in inflammatory conditions and has a short half life which makes it useful as a clinical marker [9]. Rapid CRP test has been found to be useful in excluding pulmonary tuberculosis as point of care test in clinical settings [10]. It was found in a study that as the mycobacterial load increases, CRP levels increase in response to the inflammatory process. It may not be of diagnostic value per se but may have a role in determining the prognosis and categorizing the patients. The data also revealed that the rise in CRP level was related to the extent of damage to the lung parenchyma. Thus CRP levels can be indirectly used as a marker to identify advanced stage of the disease, thereby helping the health workers to put an extra effort on these patients and monitor them closely [11]. In a study done on pediatric TB patients, it was found that a positive CRP test can be a sensitive indicator of an active disease while its return to normal indicates a good therapeutic response and it also appears to be a sign of good prognosis [12]. Serial CRP measurement may also prove to be a valuable biomarker to evaluate treatment efficacy [10]. In some studies, an elevated CRP in active TB has been suggested to have higher specificity than symptom based screening algorithms. Their findings also suggest that screening algorithms using Point-of-care (POC)-CRP can be utilized in ruling out active TB in people living with HIV thus facilitating timely initiation of isoniazid preventive therapy [13].
This study was conducted with the aim to determine the levels of high sensitivity C-reactive protein (hs CRP) among the pediatric TB patients and evaluate its utility in ruling out TB in the pediatric age group.
Materials and Methods
A prospective case control study was conducted from November 2016 to October 2017 after approval from Institutional Research Ethics Committee in the Departments of Microbiology and Pediatrics, University college of Medical Sciences and Guru Teg Bahadur Hospital, Delhi, a tertiary care hospital. Sixty clinically diagnosed (on the basis of clinical findings and radiography and/or contact history and/or Mantoux test) or microbiologically confirmed (smear and/or culture and/or Cartridge based Nucleic Acid Amplification test positive) pediatric TB cases ≤ 12 years of age were recruited in the study as per Revised National Tuberculosis Control Programme definitions [14]. Demographic, clinical and other relevant details of the patients were recorded in a predesigned proforma. Written informed consent was taken from the guardian of the participants and assent was obtained from the cases wherever applicable.
Pulmonary TB Clinically diagnosed/microbiologically confirmed case involving lung paren-chyma/tracheal bronchial tree, miliary TB and patients with both pulmonary and extrapulmonary features [14].
Extrapulmonary TB Clinically diagnosed/microbiologically confirmed case involving organs other than lungs [14].
Clinical features of pulmonary TB: Fever and cough of 2 weeks or more, recent unexplained weight loss, history of contact with an infectious person and/or reactive tuberculin skin test and/or suggestive chest radiograph features.
Clinical features of extrapulmonary TB: Non-specific symptoms like fever, weight loss, anorexia along with the specific symptoms as per the site involved with/without radiography suggestive of TB and/or contact history and/or reactive tuberculin skin test [15].
Exclusion Criteria
Immunocompromised state and previous history of TB (no previous history of TB implied no history of intake of anti tubercular drugs too).
Relevant specimens according to the site of TB for microbiological tests and 1 ml blood specimens for hs CRP estimation were collected from all the cases. 30 age and sex matched apparently healthy children who had come for routine vaccinations were enrolled as controls. Pre vaccination blood specimen was also collected from each of the healthy controls for estimation of levels of serum hs CRP. Relevant specimens from all the cases were subjected to Ziehl–Neelsen (ZN) staining for acid fast bacilli (AFB), culture on Lowenstein–Jensen (LJ) medium for M. tuberculosis and cartridge based nucleic acid amplification test (CBNAAT). All specimens were handled in biosafety cabinet II B2 [16]. Non sterile specimens were processed using N-acetyl-l-cysteine-NaOH method. Inoculated LJ slopes were incubated for up to 8 weeks at 37 °C. Preliminary identification of cultures was done by morphology and growth rate and further confirmed by niacin, p-nitrobenzoic and MPT64 antigen detection tests [17]. CBNAAT was performed on all specimens as per SOP developed by Revised National Tuberculosis control Programme (RNTCP) at the DOTS center [14].
Serum was separated for all the blood specimens collected for the estimation of hs CRP and stored at − 20 °C till further processing. The test was performed using commercially available high sensitivity C-reactive protein assay (Xema, CRP Ultra EIA, Moscow, Russia) based on the principle of solid phase two-site sandwich ELISA. The mean absorbance values (OD450) for each pair of calibrators and samples were calculated. A calibration curve on graph paper: OD versus C-reactive protein concentration was plotted. Since the ELISA reader could read OD values only up to 3.5 which corresponded to the highest concentration of the standard (25 mg/l) that was provided with the test kit, the concentrations corresponding to the OD obtained as > 3.5 were considered as > 25 mg/l.
Statistical Analysis
Data was analyzed using SPSS 20 software. Median levels and interquartile range (IQR) of hs CRP were calculated for various groups among cases and controls. Non parametric tests were applied to report the statistical significance. p value < 0.05 was considered significant.
Results
The median levels of serum hs CRP were found to be higher in pediatric TB cases (25 mg/l, IQR 24.30–25.00) as compared to healthy controls (0.530 mg/l, IQR 0.00–2.79) and the difference was statistically significant (p = 0.000). Figure 1 shows the Receiver-operating-characteristic (ROC) curve analysis for serum levels of hs CRP among the pediatric TB patients and healthy controls. AUC for hs CRP was found to be 0.987 (95% confidence interval of 0.970–1.004). Best cut-off value of 6.32 mg/l was obtained by giving equal weightage to sensitivity and specificity. The optimum cut-off point was calculated where sum of sensitivity and specificity was maximum (sensitivity 96.7%; specificity 90%). Thus AUC of serum hs CRP levels was found to significantly discriminate between the pediatric TB and healthy children (p = 0.00).
Fig. 1.
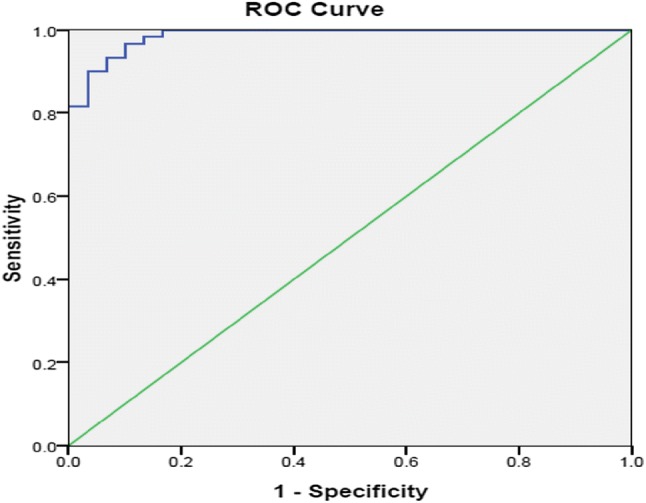
Receiver-operating-characteristic (ROC) curve for serum levels of hs CRP among pediatric TB cases and healthy controls
The age of the children in this study ranged from 2 months to 12 years, mean age being 6.8 years. The maximum number of children in this study was above 5 years of age. Out of total 60 cases, number of males in the study was 33 (55%) while the number of females was 27 (45%), male to female ratio being 1.2. Serum levels of hs CRP did not vary significantly with age and gender.
Of total 60 patients, 35 (58%) were diagnosed to be pulmonary TB and 25 (42%) were extrapulmonary TB according to the latest RNTCP guidelines. Tubercular meningitis was the predominant form constituting 48% of the extrapulmonary TB followed by skeletal TB (20%) (Table 1).
Table 1.
Sites of pediatric extrapulmonary TB (n = 25)
| Sites of extrapulmonary TB | Number (%) |
|---|---|
| Tubercular meningitis | 12 (48%) |
| Skeletal TB | 5 (20%) |
| Pleural TB | 3 (12%) |
| Tubercular empyema | 1 (4%) |
| Tubercular liver abscess | 1 (4%) |
| Tubercular lymphadenopathy | 1 (4%) |
| Abdominal TB | 1 (4%) |
| Tubercular psoas abscess | 1 (4%) |
The median level of serum hs CRP among pulmonary TB cases was 25.00 mg/l (IQR 24.23–25.00) while the level in extrapulmonary TB was 25.00 mg/l (IQR 24.35–25.00), the difference in the levels being statistically insignificant (p = 1.000).
Fever, loss of appetite, weight loss and pallor were the most common symptoms at presentation in pediatric TB patients. Apart from the non specific symptoms and signs like fever, loss of appetite, weight loss and pallor; cough was a predominant symptom in pulmonary TB cases and was present in 80% of the patients as compared to 24% of the patients with the extrapulmonary TB. Headache, vomiting, seizure and altered sensorium were more common in extrapulmonary TB patients. Table 2 shows comparison of median serum levels of hs CRP with various clinical features. Only 8/60 (13%) children presented with chills along with fever but the median serum levels of hs CRP were significantly lower in those children 24.02 mg/l (IQR 21.27–25.00) as compared to those who did not have chills 25 mg/l (24.68–25.00) (p = 0.047). Palpable lymphadenopathy was present in 10/60 patients who had significantly lower levels of hs CRP 24.35 mg/l (IQR 17.50–25.00) as compared to patients without lymphadenopathy 25 mg/l (IQR 24.90–25.00) (p = 0.020). There was no significant difference in the levels with respect to the presence or absence of other features.
Table 2.
Median serum levels of hs CRP associated with various clinical features in pediatric TB
| Clinical features | Median levels of serum hs CRP in mg/l (IQR) | p value |
|---|---|---|
| Fever | ||
| Positive group (n = 52) | 25 (24.30–25.00) | 1.000 |
| Negative group (n = 8) | 25 (13.69–25.00) | |
| Evening rise of temperature | ||
| Positive group (n = 13) | 25 (22.69–25.00) | 0.184 |
| Negative group (n = 47) | 25 (24.58–25.00) | |
| Chills | ||
| Positive group (n = 8) | 24.02 (21.27–25.00) | 0.047 |
| Negative group (n = 52) | 25 (24.68–25.00) | |
| Cough | ||
| Positive group (n = 34) | 25 (24.81–25.00) | 0.316 |
| Negative group (n = 26) | 25 (24.24–25.00) | |
| Dyspnea | ||
| Positive group (n = 15) | 25 (22.06–25.00) | 0.705 |
| Negative group (n = 45) | 25 (24.35–25.00) | |
| H/o recurrent pneumonia | ||
| Positive group (n = 4) | 25 (22.79–25.00) | 0.830 |
| Negative group (n = 56) | 25 (24.30–25.00) | |
| Loss of appetite | ||
| Positive group (n = 49) | 25 (23.90–25.00) | 0.525 |
| Negative group (n = 11) | 25 (24.58–25.00) | |
| Weight loss | ||
| Positive group (n = 46) | 25 (24.06–25.00) | 0.371 |
| Negative group (n = 14) | 25 (24.87–25.00) | |
| Abdominal pain | ||
| Positive group (n = 7) | 25 (22.06–25.00) | 0.668 |
| Negative group (n = 53) | 25 (24.35–25.00) | |
| Lymphadenopathy | ||
| Positive group (n = 10) | 24.35 (17.50–25.00) | 0.020 |
| Negative group (n = 50) | 25 (24.90–25.00) | |
| Vomiting | ||
| Positive group (n = 18) | 25 (24.24–25.00) | 0.662 |
| Negative group (n = 42) | 25 (24.41–25.00) | |
| Headache | ||
| Positive group (n = 8) | 25 (18.07–25.00) | 0.564 |
| Negative group (n = 52) | 25 (24.30–25.00) | |
| Seizure | ||
| Positive group (n = 15) | 25 (24.24–25.00) | 0.899 |
| Negative group (n = 45) | 25 (24.35–25.00) | |
Bold values indicates statistically significant values
Fifty-one patients (85%) had findings on radiograph suggestive of tuberculosis. 12 of 60 (20%) cases had normal chest X-ray while the predominant finding of lung consolidation was present in 25 of 60 (42%), pleural effusion in 9/60 (15%), miliary mottling in 6/60 (10%) cases. Lymphadenopathy was found in 12 (20%) cases. Hydrocephalus and enlarged ventricles were also important findings in tubercular meningitis patients and was present in 8 of 60 (13%) cases. Median serum levels of hs CRP were compared with the important radiological features in the pediatric TB cases (Table 3). There was no significant difference in the serum levels of hs CRP on the basis of presence (denoted by positive group) or absence (denoted by negative group) of a particular radiological feature.
Table 3.
Median serum levels of hs CRP associated with important radiological features in pediatric TB
| Radiological feature | Median levels of serum hs CRP in mg/l (IQR) | p value |
|---|---|---|
| Lung consolidation | ||
| Negative group (n = 35) | 25 (24.47–25.00) | 0.500 |
| Positive group (n = 25) | 25 (23.90–25.00) | |
| Miliary mottling | ||
| Negative group (n = 54) | 25 (24.23–25.00) | 0.193 |
| Positive group (n = 6) | 25 (25.00–25.00) | |
| Pleural effusion | ||
| Negative group (n = 51) | 25 (24.23–25.00) | 0.499 |
| Positive group (n = 9) | 25 (24.73–25.00) | |
| Normal chest x ray | ||
| Negative group (n = 48) | 25 (24.3–25.00) | 0.561 |
| Positive group (n = 12) | 25 (18.04–25.00) | |
| Lymphadenopathy on radiology | ||
| Negative group (n = 48) | 25 (24.47–25.00) | 0.715 |
| Positive group (n = 12) | 25 (22.24–25.00) | |
| Hydrocephalus and enlarged ventricles | ||
| Negative group (n = 52) | 25 (24.47–25.00) | 0.167 |
| Positive group (n = 8) | 24.79 (9.52–25.00) | |
Tuberculin skin test was done in 54 cases and an induration diameter of ≥ 10 mm was considered as reactive. In the study, 30 (56%) cases tested reactive.
Acid fast bacilli were demonstrated by ZN staining in only 12 out of 60 (20%) cases. M. tuberculosis was isolated on culture in 17 out of 60 (28%) cases. Among 60 cases, 30 (50%) were CBNAAT positive. 33 of 60 (55%) cases were microbiologically confirmed (by ≥ 1 of smear microscopy/culture/CBNAAT). The median levels of hs CRP were compared with respect to positivity by 3 microbiological techniques (Table 4) but were not significantly different among AFB positive and negative cases (p = 0.322), culture positive and negative cases (p = 0.345) and CBNAAT positive and negative groups (p = 0.121).
Table 4.
Median serum levels of hs CRP associated with microbiological diagnosis
| Diagnostic techniques | Median serum levels of hs CRP in mg/l (IQR) | p value |
|---|---|---|
| Direct demonstration of acid fast bacilli | ||
| Positive group (n = 12) | 25 (25.00–25.00) | 0.322 |
| Negative group (n = 48) | 25 (24.23–25.00) | |
| Isolation of M. tuberculosis on LJ media | ||
| Positive group (n = 17) | 25 (25.00–25.00) | 0.345 |
| Negative group (n = 43) | 25 (23.57–25.00) | |
| Direct demonstration and/or isolation | ||
| Positive group (n = 19) | 25 (25.00–25.00) | 0.272 |
| Negative group (n = 41) | 25 (23.00–25.00) | |
| CBNAAT | ||
| Positive group (n = 30) | 25 (24.89–25.00) | 0.121 |
| Negative group (n = 30) | 25 (21.61–25.00) | |
Spearman’s rho correlation of hs CRP was calculated with the hemoglobin levels, total leucocyte count (TLC) and packed cell volume (PCV) among the pediatric TB cases. Significant inverse correlation was found with PCV (− 0.316, p value = 0.021) while the correlation with hemoglobin and TLC was not found to be significant.
Discussion
Pediatric TB remains highly under diagnosed. Conventional microbiological tests have low sensitivity for the diagnosis of tuberculosis, especially in the pediatric population. A number of circulating biomarkers are under study which may prove to be useful. Recently, CRP has attracted attention as a candidate biomarker for active tuberculosis.
In this study, the majority of children were males and above 5 years of age as reported by other studies from Delhi [2, 5]. While another Indian study showed a female preponderance and the majority of the children were below 5 years of age [18]. Age and gender distribution of pediatric TB may vary from place to place.
Pulmonary TB has been reported as the predominant form in the pediatric age group from previous studies as has been documented in our study too [19, 20]. Few studies, however have shown extrapulmonary form of TB to be more common among the pediatric patients [5, 21]. Tubercular meningitis was the commonest presentation amounting to 48% of extrapulmonary TB cases in this study in concordance with other studies [18, 20]. This is in contrast to recent studies that have reported lymphadenopathy as the most common extrapulmonary form of TB in the pediatric age group [5, 19]. Being a tertiary care hospital in Delhi catering to the patients of the adjoining states, our hospital might be receiving increased referrals of severe forms of TB from the peripheral health centers.
In many studies, the role of CRP as a point of care test to differentiate between tubercular and non tubercular infection has been suggested [10, 13, 22]. In this study, hs CRP levels in the pediatric TB cases were found to be significantly higher as compared to the healthy controls. So hs CRP has a power to discriminate between TB case and healthy control. Similar results were reported by another study done on children where the levels of CRP among both pulmonary and extrapulmonary TB were found to be raised as compared to the healthy children, though no significant difference in the level of hs CRP among the children with pulmonary and extrapulmonary TB was reported similar to our study [22].
Though hs CRP could not differentiate between the types of TB, the above findings suggest that it could help in ruling out TB in pediatric age group where the microbiological confirmation is difficult to achieve especially in the extrapulmonary types.
The levels of hs CRP in this study did not show any significant difference between the patients with radiological evidence of TB and those without it. But a previous study reported significantly higher levels of CRP among pulmonary TB patients having evidence of parenchymal involvement [11].
Its utility as point-of-care test among adults especially among sputum smear negative suspected pulmonary TB cases has been studied [23]. Smear microscopy was positive for acid fast bacilli in only 20% and culture was positive in only 28% of the pediatric TB cases in this study which is similar to microbiological confirmation by conventional methods in the previous studies [6, 24]. The overall microbiological diagnosis remains difficult in children due to insufficient specimens and paucibacillary nature of the disease. In our study, the median serum levels of hs CRP were not found to be significantly different among the cases that were microbiologically positive from those who were microbiologically negative, though in a previous study serum CRP levels were significantly lower in sputum smear-negative group when compared to the sputum smear positive patients. Moreover, they also reported an increase in the level with increase in the number of bacilli in the smear and thus, also correlating higher CRP levels with extent of severity of disease, more chances of initial drug resistance, delayed sputum conversion and delayed response [11]. Also in another study done on variety of specimens, CRP levels were found to be significantly higher in culture positive and smear positive cases [25]. Higher CRP reflected increased inflammation which may be due to higher bacillary load as suggested by positive microbiological tests. In both the studies the population under study was mainly adults. Pediatric TB being paucibacillary, these studies may not truly reflect the correlation of the parameters with CRP in children. Almost all the cases except very few in our study had hs CRP levels much above the cut off value i.e. > 20 mg/l. Relatively higher values of hs CRP was found in our pediatric TB cases; further studies could be done to know the difference in the levels of hs CRP between the tubercular or other inflammatory causes, so as to better utilize this biomarker in the diagnosis of pediatric TB. This becomes even more crucial in pediatric TB where a lot of variations in clinical and radiological presentations have been reported with not so well defined microbiological correlation [26].
Conclusion
In a developing country like India where significant numbers of pediatric TB cases remain undiagnosed due to lack of gold standard, tuberculosis is many times diagnosed based on clinical and radiological criteria along with history. It is a diagnosis of exclusion in cases where microbiological diagnosis of TB can’t be established and other common etiologies like malaria have been ruled out by common laboratory tests. Although a non specific marker of inflammation, hs CRP could significantly differentiate between pediatric TB cases and healthy controls. It may be incorporated in the algorithms for diagnosing pediatric TB along with clinical history, microbiology, radiology and tuberculin skin test and it may prove to be an important tool in ruling out TB in children who are mostly not confirmed microbiologically due to paucibacillary disease especially the extrapulmonary forms.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. Global tuberculosis report 2017. Geneva: World Health Organisation; 2017. Available from: http://www.who.int/tb/publications/global_report/MainText_13Nov2017.pdf?ua=1. Accessed 6 Mar 2018.
- 2.Raizada N, Sachdeva KS, Swaminathan S, Kulsange S, Khaparde SD, Nair SA, et al. Piloting Upfront Xpert MTB/RIF testing on various specimens under programmatic conditions for diagnosis of TB & DR TB in paediatric population. PLoS ONE. 2015;10(10):e0140375. doi: 10.1371/journal.pone.0140375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of Tuberculosis mortality in children: a mathematical modeling study. Lancet Glob Health. 2017;5(9):e898–e906. doi: 10.1016/S2214-109X(17)30289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sandgren A, Cuevas LE, Dara M, Gie RP, Grzemska M, Hawkridge A, et al. Childhood tuberculosis: progress requires an advocacy strategy now. Eur Respir J. 2012;40(2):294–297. doi: 10.1183/09031936.00187711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalra A. Care seeking and treatment related delay among childhood tuberculosis patients in Delhi, India. Int J Tuberc Lung Dis. 2017;21(6):645–650. doi: 10.5588/ijtld.16.0563. [DOI] [PubMed] [Google Scholar]
- 6.Singh S, Singh A, Prajapati S, Kabra SK, Lodha R, Mukherjee A, et al. Xpert MTB/RIF assay can be used on archived gastric aspirate and induced sputum samples for sensitive diagnosis of paediatric tuberculosis. BMC Microbiol. 2015;15:191. doi: 10.1186/s12866-015-0528-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zumla A, Wallis R, Doherty M, Klein N, Parida S, Olesen O, et al. Joint TDR/EC expert consultation on biomarkers in tuberculosis. Geneva: World Health Organization; 2008. [Google Scholar]
- 8.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Investig. 2003;111(12):1805–1812. doi: 10.1172/JCI200318921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Young B, Gleeson M, Cripps AW. C-reactive protein: a critical review. Pathology. 1991;23(2):118–124. doi: 10.3109/00313029109060809. [DOI] [PubMed] [Google Scholar]
- 10.Drain PK, Mayeza L, Bartman P, Hurtadol R, Moodley P, Varghese S, et al. Diagnostic accuracy and clinical role of rapid C-reactive protein testing in HIV-infected TB suspects in South Africa. Int J Tuberc Lung Dis. 2014;18(1):20–26. doi: 10.5588/ijtld.13.0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rao S, Bernhardt V. Serum C-reactive protein in pulmonary tuberculosis: correlation with bacteriological load and extent of disease. Infect Dis Clin Pract. 2009;17(5):314–316. doi: 10.1097/IPC.0b013e3181a4c73d. [DOI] [Google Scholar]
- 12.Kumar M, Swati, Verma M. Prognostic value of C-reactive protein in pediatric tubercular patients on ATT. IOSR J Dent Med Sci. 2016;15(6):52–57. [Google Scholar]
- 13.Yoon C, Davis JL, Huang L, Muzoora C, Byakwaga H, Scibetta C, et al. Point of care C-reactive protein testing to facilitate implementation of Isoniazid preventive therapy for people living with HIV. J Acquir Immune Defic Syndr. 2014;65(5):551–556. doi: 10.1097/QAI.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Central TB Division, Government of India. Revised national tuberculosis control programme: technical and operational guidelines for TB control in India. New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; 2016. p. 1–269.
- 15.Singh V, Mahto D. Antitubercular drugs and RNTCP guidelines for childhood tuberculosis. In: Gupta P, Menon PSN, Ramji S, Lodha R, editors. PG Textbook of pediatrics: infections and systemic disorders. 2. New Delhi: Jaypee Brothers Medical Publishers; 2018. p. 1348. [Google Scholar]
- 16.Forbes BA, Sahm DF, Weissfeld AS. Mycobacteria and other bacteria with unusual growth requirements. In: Forbes BA, Sahn DF, Weissfeld AS, editors. Bailey & Scott’s diagnostic microbiology. 13. Missouri: Mosby Elsevier Inc; 2014. pp. 484–512. [Google Scholar]
- 17.Central TB Division, Government of India. Revised National TB Control Programme Training Manual for Mycobacterium tuberculosis culture and drug susceptibility testing. New Delhi: Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare; 2009. Available from: http://www.tbcindia.nic.in/showfile.php?lid=2991. Accessed 3 Oct 2017.
- 18.Gupta R, Garg A, Venkateshwar V, Kanitkar M. Spectrum of childhood tuberculosis in BCG vaccinated and unvaccinated children. Med J Armed Forces India. 2009;65(4):305–307. doi: 10.1016/S0377-1237(09)80088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devrim I, Aktürk H, Bayram N, Apa H, Tulumoğlu S, Devrim F, et al. Differences between pediatric extra-pulmonary and pulmonary tuberculosis: a warning sign for the future. Mediterr J Hematol Infect Dis. 2014;6(1):e2014058. doi: 10.4084/mjhid.2014.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain SK, Ordonez A, Kinikar A, Gupte N, Thakar M, Mave V, et al. Pediatric tuberculosis in young children in India: a prospective study. Biomed Res Int. 2013;2013:783698. doi: 10.1155/2013/783698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Satyanarayana S, Shivashankar R, Vashist RP, Chauhan LS, Chadha SS, Dewan PK, et al. Characteristics and programme-defined treatment outcomes among childhood tuberculosis (TB) patients under the national TB programme in Delhi. PLoS ONE. 2010;5(10):e13338. doi: 10.1371/journal.pone.0013338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar NP, Anuradha R, Andrade BB, Suresh N, Ganesh R, Shankar J, et al. Circulating biomarkers of pulmonary and extrapulmonary tuberculosis in children. Clin Vaccine Immunol. 2013;20(5):704–711. doi: 10.1128/CVI.00038-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson D, Badri M, Maartens G. Performance of serum C-reactive protein as a screening test for smear-negative tuberculosis in an ambulatory high HIV prevalence population. PLoS ONE. 2011;6(1):e15248. doi: 10.1371/journal.pone.0015248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rebecca B, Chacko A, Verghese V, Rose W. Spectrum of pediatric tuberculosis in a tertiary care setting in South India. J Trop Pediatr. 2018 doi: 10.1093/tropej/fmy007. [DOI] [PubMed] [Google Scholar]
- 25.Brown J, Clark K, Smith C, Hopwood J, Lynard O, Toolan M, et al. Variation in C-reactive protein response according to host and mycobacterial characteristics in active tuberculosis. BMC Infect Dis. 2016;16:265. doi: 10.1186/s12879-016-1612-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta N, Kashyap B, Dewan P, Hyanki P, Singh NP. Clinical spectrum of pediatric tuberculosis: a microbiological correlation from a tertiary care center. J Trop Pediatr. 2018 doi: 10.1093/tropej/fmy026. [DOI] [PubMed] [Google Scholar]


