Abstract
Background
The expression of programmed cell death ligand 1(PD-L1) is related to the efficacy of immune checkpoint inhibitors on patients with non-small cell lung cancer (NSCLC), but tumor tissue (TT) samples are difficult to obtain, and initial TT samples are difficult to reflect the spatial-temporal heterogeneity. Therefore, we explored the feasibility of separating circulating tumor cells (CTCs) and detecting PD-L1 expression on CTCs.
Patients and Methods
Peripheral blood specimens were sampled from 66 NSCLC patients, and CTCs were separated by membrane filtration based on size. For 59 patients with paired TT specimens, the expression of PD-L1 in their CTCs and TTs was determined using the immunohistochemistry and immunocytochemistry based on 28–8 antibody, respectively. The PD-L1 expression in TTs was set as a gold standard for calculation of sensitivity, specificity, consistency, positive predictive value (PPV), and negative predictive value (NPV), and the Cohen kappa coefficient for CTCs and paired TTs was calculated. In addition, the T-test, Chi-square test, and Mann–Whitney U-test were adopted to analyze the correlation of clinical pathological features and prognosis with PD-L1 expression.
Results
Sensitivity, specificity, concordance, PPV and NPV of detecting PD-L1 in CTCs of the 41 initial treated patients were 88.89%, 73.91%, 80%, 72.73% and 89.47%, respectively, and the Cohen kappa coefficient of CTC and paired TTs was 0.613. The univariate analysis of survival showed that the progression-free survival time of initial treated patients with positive PD-L1 expression was shorter than that of those with negative PD-L1 expression in CTCs or TTs (P>0.05), and the positive PD-L1 expression in CTCs or TTs had nothing to do with age, sex, smoking status, histological type, and stage (P > 0.05).
Conclusion
The study confirms the feasibility of CTC PD-L1 detection in peripheral blood and lays a foundation for exploring real-time and individualized immunotherapy molecular biomarkers.
Keywords: circulating tumor cell, non-small cell lung cancer, immunotherapy, PD-L1 level/expression
Introduction
Lung cancer is the most common cancer and a primary cause for patients died of cancer in the globe.1 Over the last few decades, major advances have been achieved in molecular targeted treatment for advanced lung cancer,2 which opens the era of individualized and precise medical treatment for lung cancer. In recent years, the discovery of immune checkpoint inhibitor (ICI) programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) and its successful application in the field of lung cancer have brought significant survival benefits for advanced non-small cell lung cancer (NSCLC), especially for people with negative driving mutations.3
ICIs are developed to target inhibitory checkpoint molecules.4 Thereby, increase of PD-L1 level in tumor tissues (TTs) enables the PD-L1 to escape immune surveillance due to its inhibition on immune cell activation. Clinical studies have shown that the expression of PD-L1 in TTs is related to the efficacy of PD-1/PD-L1 inhibitors, and ICI therapy on advanced NSCLC patients with high PD-L1 expression is more effective in the second-line and first-line treatment of them.5 Detection of PD-L1 expression in TTs has also been listed as routine recommendation for patients with advanced NSCLC in the National Comprehensive Cancer Network (NCCN) guidelines of the United States.6
However, with the continuous in-depth study on ICI therapy, a growing number of reports show that the expression of PD-L1 in TTs is affected by many factors, such as the spatial-temporal heterogeneity of TTs, the differences between biopsy specimens and surgical specimens, and treatment methods in the process of disease.7 Previous studies have found that a small proportion of patients with no PD-L1 expression in their TTs still benefit from PD-1/PD-L1 inhibitor, which might due to tumor heterogeneity and absence of real-time quantification of PD-L1.8 Moreover, most NSCLC patients are already at the advanced stage at the time of diagnosis, and the small biopsy specimens are insufficient to meet the needs of more and more molecular tests. The continuous development of precision medicine needs the enrichment of molecular detection based on other biological samples on the basis of tumor tissue samples.
With the characteristics of low invasiveness, specimen acquisition convenience, dynamic and real-time acquisition, liquid biopsy has captured extensive attention in recent years,9 and molecular detection of driver mutation based on liquid biopsy is also considered as an effective supplement before tissue detection.10 Circulating tumor cells (CTCs) analysis can be used for the diagnosis or prognosis analysis of patients. CTCs are likely to replace TTs as a material source for detecting genetic alterations and therapeutic targets.11 Moreover, they can offer effective survival-related prognostic information in lung cancer.12 The method for obtaining CTC should be selected under the consideration of the sensitivity of enrichment technology, and specificity of CTC-based diagnosis.13 CTC analysis by both cytopathological evaluation and size-based filtration is being studied as a biomarker for prediction of targeted therapy for NSCLC patients (NCT02372448, NCT03771404, NCT02951897)
In the era of immunotherapy, it is an urgent to explore molecular biomarkers based on other specimens to effectively supplement TT specimen detection. In this study, we have studied the feasibility of detecting the peripheral blood CTC PD-L1 with 28–8 antibody in NSCLC patients, analyzed the correlation of clinicopathological features, prognosis, and previous treatment status with CTC PD-L1 expression, with the goal of laying a foundation for the application of liquid biopsy in immunotherapy related molecular biomarkers.
Methods
Patients and Samples
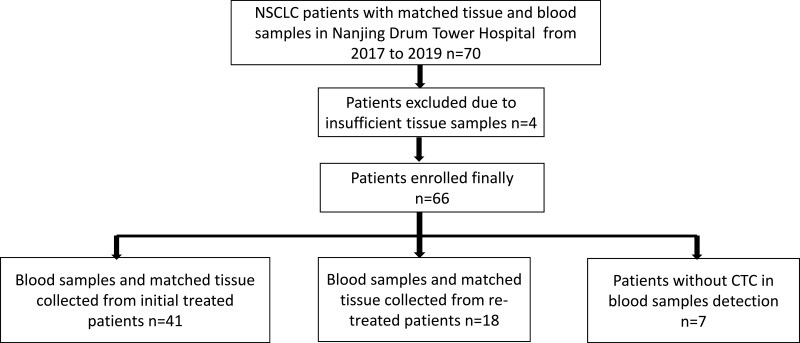
TTs and corresponding blood were sampled from 66 participants confirmed with NSCLC according to histology. After testing and counting CTCs, we further analyzed the expression of PD-L1 in CTCs of 59 patients with positive CTC PD-L1 expression and in paired TTs. The clinical and pathological characteristics of patients are shown in Table 1. Among them, TTs and peripheral blood were sampled from 41 initial treated NSCLC patients. Eighteen TT specimens were paraffin-embedded specimens retained by the pathology department at the time of initial diagnosis of 18 patients, and peripheral blood was sampled from them after at least first-line treatment (Figure 1). This study was conducted in accordance with the Helsinki Declaration and under the permission of the Ethics Committee of Nanjing Drum Tower Hospital Affiliated to Medical College of Nanjing University and informed written consent from each subject.
Table 1.
Basic Information of Patients
| The Number of Patients | 59 |
|---|---|
| Clinical information of patients (n = 59) |
n (%) |
| Age (Y) | |
| Median | 62 |
| Min-Max | 48–79 |
| Sex | |
| Male | 39 (66%) |
| Female | 20 (34%) |
| Smoking history | |
| With smoking history | 29 (49%) |
| Without smoking history | 30 (51%) |
| Histological type | |
| Adenocarcinoma | 49 (83%) |
| Squamous cell carcinoma | 10 (17%) |
| Staging | |
| II | 3 (5%) |
| III | |
| IIIA | 3 (5%) |
| IIIB | 5 (8%) |
| IIIC | 1 (2%) |
| IV | 47 (80%) |
Figure 1.
Flow chart of patient enrollment.
Separation and Identification of CTCs
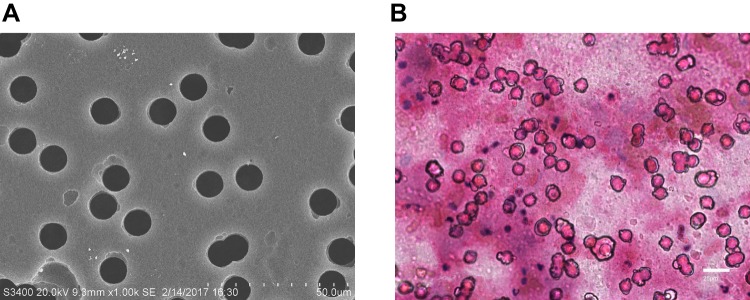
CTC analysis based on cell size was conducted with reference to previous studies.14 Peripheral blood (20 mL) was sampled, and stored in K2EDTA blood collection vessel (BD Vacutainer, Ref 367525, UK) at 4°C, and then treated for CTC enrichment within 1 hour of blood collection. Buffer containing 0.2% paraformaldehyde (Biosharp, BL539A) was used to dilute sampled blood,15 the blood was divided into two specimens. Each specimen was made to pass through a membrane with a pore size of 10 um, and two membranes were obtained. The pore size was set to 8 um using the isolation by size of epithelial tumor cells (ISET), and each membrane was randomly perforated. However, the diameter of major NSCLC cell lines was larger than 10 um,16 so we applied a physical separation method with pore size of 10 um according to the characteristics of lung cancer (Figure 2A). Filter (Ф25) was purchased from Beijing Haicheng Shijie Filter Equipment Co., Ltd, and the microfiltration membrane was constructed with polyethylene terephthalate (PET) by the Institute of Physics of Chinese Academy of Sciences. One of the membranes was stained by Eosin (HE), and then analyzed under a microscope. The isolated cells were identified as CTCs if they meet the morphological criteria:17–19 1) the nuclear-to-mass ratio was greater than 0.5; 2) the long diameter of the nucleus was larger than 10 um; 3) The shapes of nucleus and cell were irregular. Another membrane was used for subsequent PD-L1 expression analysis.
Figure 2.
CTC enrichment and identification. (A) SEM images of nuclear track membranes (original magnification 1000; bar, 50um). (B) HE staining of CTCs in patients’ blood samples: A representative example of CTC, characterized by a large and irregular nucleus and presence of nucleoli (original magnification 400; bar, 25um).
PD-L1 Determination
Detection of PD-L1 in TT Specimens
Representative 4 μm slices were prepared from FFPE tissue blocks. In short, all tissue slices were exposed to 3% hydrogen peroxide (OriGene; PV-6001) for 10 mins to inhibit the activity of endogenous peroxidase, and heat-mediated antigen retrieval was performed through Tris/EDTA buffer with 8 pH (OriGene; ZLI-9066). Afterwards, 200 μL PD-L1 antibody (Abcam, clone 28–8, monoclonal, 1:400, anti-rabbit) and tissue slices were incubated in a wet box at 4°C overnight, and then HRP-coupled goat anti-rabbit IgG secondary body (OriGene, PV-6001) was added. After addition, they were placed at 37°C for 30 mins, and incubated with 3, 3-diamino benzidine (DAB) (OriGene; ZLI-9017) at indoor temperature for 5 m. In the end, the slices were dried out and immobilized. Negative (no primary antibody) and positive (amniotic membrane roll) controls were adopted in each running. The staining results were interpreted by professional pathologists according to antibody specification. The specific positive PD-L1 was located in cell membrane and cytoplasm. Tumor cells with positive expression rate of PD-L1 ≥1% were considered as positive tumor cells, and recorded as TC1, and those with the rate <1% were considered as negative tumor cells, and recorded as TC0.20
Detection of PD-L1 in CTC Specimens
PD-L1 in samples with positive CTC examination results by immunocytochemistry (ICC) was quantified. The membrane was exposed to 3% H2O2 (OriGene; PV-6001) for 10 mins, and added with 200 μL PD-L1 antibody (Abcam, clone 28–8, monoclonal, 1:400, anti-rabbit) and incubated in a wet box at 4°C overnight. Afterwards, horseradish peroxidase (HRP)-coupled goat anti-rabbit immunoglobulin G (IgG) secondary body (OriGene, PV-6001) was added, let to stand at 37°C for 30 mins, and then incubated with 3,3-diamino benzidine (DAB) (OriGene; ZLI-9017) at room temperature for 5 mins. Finally, they were analyzed under a microscope. Cell lines with known PD-L1 expression (BL6 cell lines with high PD-L1 expression21 and A549 cell lines with negative PD-L1 expression22) were used as positive and negative control, respectively. BL6 and A549 cell lines were all purchased from the Cell Resource Center of Shanghai Institutes for Biological Science of the Chinese Academy of Sciences. They were cultured in RPMI 1640 culture medium (Corning, 10-040-CVR), with 10% fetal bovine serum (Gibco, 16000–044) in an incubator with 5%CO2 humidity at 37°C for subculture.
The expression of PD-L1 in CTCs was evaluated according to antibody specification, and the specific positive PD-L1 was located in cell membrane and cytoplasm. Tumor cells with positive expression rate of PD-L1 ≥1% were considered as positive tumor cells, and recorded as cTC1, and those with the rate <1% were considered as negative tumor cells, and recorded as cTC0.23 Immunohistochemical staining results were interpreted by professional pathologists who were ignorant of the PD-L1 results of blood samples and TTs until the study was completed.
Consistency Analysis
The sensitivity, specificity, consistency, positive predictive value (PPV), and negative predictive value (NPV) were figured as follows:24 Sensitivity = the number of cases with true positivity/(the number of cases with true positivity + the number of cases with false negativity); specificity = the number of cases with true negativity/(the number of cases with true negativity + the number of cases with false positivity); consistency = (the number of cases with true positivity + the number of cases s with true negativity)/(the total number of cases); PPV= The number of cases with true positivity/(the number of cases with true positivity + the number of cases with false positivity); NPV= the number of cases with true negativity/(the number of cases with true negativity + the number of cases with false negativity). The consistency between PD-L1 level in tissues and that in matched CTCs was verified by calculating Cohen’s kappa coefficient. If the coefficient ≤0.4, it was interpreted as poor to medium, and if it >0.4 or ≤0.6, it was interpreted as medium; If it >0.6 or ≤0.8, it was interpreted as sufficient, and if it >0.8, it was almost perfect.25
Statistical Analysis
The correlation of PD-L1 expression with clinical variables and survival time was analyzed in the 41 initial treated patients. Progression-free survival (PFS) is a period from the time of receiving treatment by a patient with tumor disease to the time when disease progression was found in the patient or the patient died of any reason. PD-L1 state in TT samples and blood samples were compared with clinicopathological variables of patients (smoking history, age, sex, histological stage, staging) using the Student’s t test, x2 test or the Mann–Whitney test. K-Mcurves and Cox regression analysis were employed for univariate analysis. All tests are presented as two-sided, with 95% CIs and relevant p values. Data analysis was carried out with SPSS Statistical Software Package 26.0.
Results
CTC Separation and Determination
Blood was sampled from 66 lung cancer patients, and CTCs were separated and enriched based on cell diameter, and identified by HE pathological staining. CTCs were found in blood samples from 59 patients (89%) (Figure 2B). Subsequently, the expression of PD-L1 in TTs and CTCs in 59 patients with detected CTCs was compared and analyzed, including 41 initial treated patients and 18 re-treated patients.
Expression of PD-L1 in TTs and CTCs
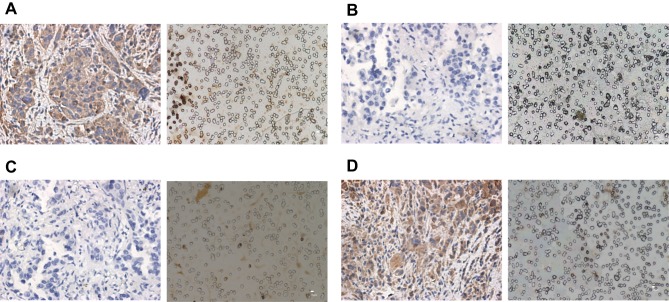
The PD-L1 staining of 59 samples showing more than 1 CTC was evaluated. Of 41 initial treated patients, 22 (54%) patients represent a positive expression rate of PD-L1 in CTCs over 1% (Figure 3), and 19 (46%) patients showed negative PD-L1 expression in CTCs. 18 patients (44%) showed the rate in TTs equal to 1% or more (Table 2), and 23 (56%) patients showed negative PD-L1 expression in TTs. Further analysis of the expression of PD-L1 in CTCs and its coincidence with that in corresponding TT specimens showed that the coincidence was related to whether the patients whose peripheral blood was sampled and CTCs were separated had received treatment.
Figure 3.
Staining of PD-L1 in TTs and corresponding CTCs of initial treated NSCLC patients. (A) Patient with PD-L1 expression rate ≥1%in TTs (left panel original magnification 400; bar, 25 um) and corresponding CTCs (Right panel, original magnification 400; bar, 10um). (B) Patient with PD-L1 expression rate <1% in TTs (left panel, original magnification 400; bar, 25um) and corresponding CTCs (Right panel, original magnification 400; bar, 10um). (C) Patient with PD-L1 expression rate <1% in TTs (left panel, original magnification 400; bar, 25um) and patient with PD-L1≥1% expression in CTCs (Right panel, original magnification 400; bar, 10um). (D) Patient with PD-L1 expression rate≥1% in TTs (left panel, original magnification 400; bar, 25um) and patient with PD-L1 expression rate <1% in CTCs (Right panel, original magnification 400; bar, 10um).
Table 2.
Consistency of PD-L1 Expression in TTs of Initial Treated Patients and Corresponding CTCs
| Patients N=41 | Blood Samples (CTCs) | ||
|---|---|---|---|
| ≥1% | <1% | ||
| Tissues | ≥1% | 16 | 2 |
| <1% | 6 | 17 | |
We compared the PD-L1 level in CTCs and matched TTs from the 41 patients, finding that the PD-L1 level in CTCs and matched TTs were consistent in 33 patients (80%) (Figure 3 and Table 3). The Cohen’s kappa coefficient was 0.613 (95%CI (0.378–0.848), P<0.001). The expression of PD-L1 in CTCs and matched TTs was highly consistent, but it was inconsistent in eight patients, among whom two patients showed negative PD-L1 expression in CTCs, but positive PD-L1 expression in TTs, and six patients showed opposite results. The sensitivity, specificity, consistency, PPV and NPV of PDL1 level in CTCs are listed in Table 4. Interestingly, in the 18 re-treated patients, there was no consistency between the expression of PD-L1 in CTCs and that in paired TT specimens (Table 3).
Table 3.
Consistency of PD-L1 Expression in TTs of Re-Treated Patients and Corresponding CTCs
| Patients N=18 | Blood Samples (CTCs) | ||
|---|---|---|---|
| ≥1% | <1% | ||
| Tissues | ≥1% | 0 | 9 |
| <1% | 9 | 0 | |
Table 4.
PD-L1 Status in CTCs
| Parameters | CTC PD-L1 |
|---|---|
| Sensitivity | 88.89% |
| Specificity | 73.91% |
| Concordance | 80% |
| PPV | 72.73% |
| NPV | 89.47% |
Correlation of the PD-L1 Expression with Survival Time and Clinicopathological Features of Patients
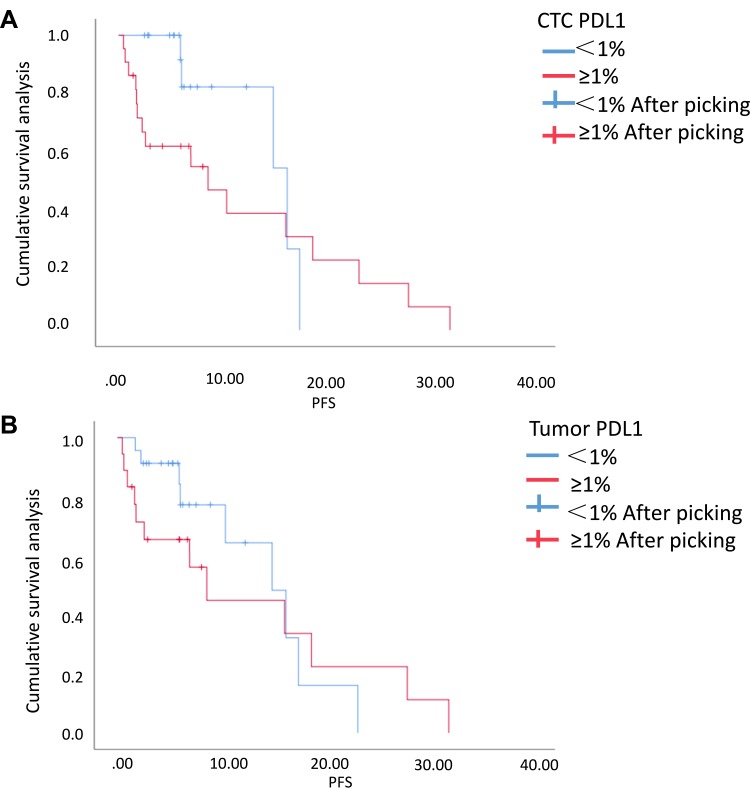
We analyzed the correlation of the PD-L1 expression with the progression-free survival time (PFS) in the 41 initial treated patients (Figure 4). Univariate analysis [HR: 1.298, 95% CI (0.517–3.259)] revealed that patients with positive PD-L1 expression in TTs experienced shorter PFS. The median PFS of patients with negative PD-L1 expression in TTs and those with positive expression were 6.07 and 5.96 months, respectively (P=0.579). Further analysis on the correlation of CTC PD-L1 expression with PFS also revealed similar phenomena: Patients with positive PD-L1 expression in CTCs also experienced shorter PFS [HR: 1.970, 95% CI (0.683–5.683)], and the median PFS of those with negative PD-L1 level in CTCs and those with positive level was 6.07 months, and 5.12 months, respectively (P=0.210). Univariate survival analysis showed that histological type was the factor affecting PFS of NSCLC patients (HR: 0.259, 95% CI (0.076–0.885), P=0.031). There was no significant difference in other variables (age: P: 0.496; sex: P: 0.478; smoking status: P: 0.860, and stage: P: 0.374).
Figure 4.
Survival curves of PD-L1. (A) The group with PD-L1 expression in CTCs ≥1% and the group with that <1% (P=0.210). (B) The group with PD-L1 expression in TTs ≥1% and the group with that <1% (P=0.579).
Further analysis on the relationship between PD-L1 expression and other clinical variables showed that 22/41 (54%) of initial treated patients had positive CTC PD-L1 expression and 19/41 (46%) initial treated patients had negative CTC PD-L1 expression, and the positive expression of CTC PD-L1 had nothing to do with age, sex, smoking status, histological type, and stage (all P> 0.05, Table 5). In addition, 18/41 (44%) patients showed a PD-L1 expression rate ≥1% in TTs, and 23/41 (56%) patients showed negative PD-L1 expression in TTs, and the positive expression of PD-L1 in TTs was not related to age, sex, smoking status, histological type, and stage (all P > 0.05, Table 5).
Table 5.
Correlation of PD-L1 Expression with Clinicopathological Variables
| The CTC Positive PD-L1 Group (n=22) | The CTC Negative PD-L1 Group (n=19) | t/χ2/Z value | P-value | The Tissue Positive PD-L1 Group (n=18) | The Tissue Negative PD-L1 group (n=23) | t/χ2/Z value | P-value | |
|---|---|---|---|---|---|---|---|---|
| Age | 61.7±5.9 | 64.4±9.9 | 1.081 | 0.287 | 62.2±5.8 | 63.6±9.4 | 0.530 | 0.599 |
| Sex | 0.141 | 0.757 | 0.437 | 0.540 | ||||
| Male | 14 | 11 | 12 | 13 | ||||
| Female | 8 | 8 | 6 | 10 | ||||
| Smoking | 12 (55%) | 7 (37%) | 1.285 | 0.350 | 9 (50%) | 10 (43%) | 0.173 | 0.758 |
| Histological type | 0.041 | 1.000 | 0.127 | 0.721 | ||||
| Adenocarcinoma | 18 | 16 | 14 | 20 | ||||
| Squamous cell carcinoma | 4 | 3 | 4 | 3 | ||||
| Staging | 0.967 | 0.334 | 0.381 | 0.703 | ||||
| II | 1 | 1 | 1 | 1 | ||||
| III | 2 | 4 | 3 | 3 | ||||
| IV | 19 | 14 | 14 | 19 |
Discussion
The successful application of immune checkpoint drugs in NSCLC has improved the treatment status of lung cancer and patients may obtain long-term clinical benefits based on immunotherapy. However, how to realize individualized immunotherapy and how to maximize the therapeutic effect and minimize the toxic reaction through the screening of molecular biomarkers are still the key exploration directions in immunotherapy. A number of Phase III clinical studies have confirmed that the expression of PD-L1 in TTs is related to the therapeutic effect of patients receiving corresponding immune checkpoint drugs.26,27 However, some studies have found that the expression of PD-L1 in TTs can be dynamically changed by the influence of radiotherapy, chemotherapy and targeted drugs in the process of disease progression. Moreover, the expression of PD-L1 is different between primary focus and metastatic focus of tumors, and between different metastatic focus, suggesting that PD-L1 expression has the spatial-temporal heterogeneity.28 With the continuous development of precision medicine, the detection of molecular biomarkers based on serum/plasma liquid biopsies has captured extensive attention due to its convenience, real-time and “point-to-surface” characteristics, which is conducive to overcoming the spatial-temporal heterogeneity of tumors, and opens up a new direction for further exploration of individualized treatment.
Recent studies have found that changes in plasma cfDNA concentration and bTMB in plasma cfDNA may be used as molecular biomarkers for predicting therapeutic effects in ICI therapy,29,30 all of which support the exploration of immunotherapy-related molecular biomarkers based on liquid biopsy.31 In the early study of small samples, it has been found that CTC PD-L1 expression can be detected in peripheral blood of tumor patients. However, previous studies are mostly limited to observation of PD-L1 expression on CTCs, lacking comparison with paired TTs, or the detection method of PD-L1 in TTs is inconsistent with that in CTCs, so it is difficult to judge the actual consistency.32,33 In this study, for the first time, we tried to isolate CTCs from peripheral blood of NSCLC patients based on pore size, further used 28–8 antibody to evaluate PD-L1 expression, and analyzed the correlation between PD-L1 expression in CLCs and that in paired TTs and its relationship with clinicopathological features.
CTC separation and enrichment based on size has been proved to be effective in separating and collecting CTCs in various solid tumors,34 and there is evidence that the CTC separation method based on pore size can provide a higher CTC collection rate than the CellSearch method.35 In our study, CTCs were successfully isolated from peripheral blood of 89% (59/66) of patients. Further comparison with the expression of PD-L1 in paired TTs showed that sensitivity, specificity, concordance, PPV and NPV of detecting PD-L1 in CTCs of the 41 initial treated patients was 88.89%, 73.91%, 80%, 72.73% and 89.47%), respectively. The Cohen kappa coefficient of CTC and paired TTs was 0.613. Those results revealed that PD-L1 detection based on CTCs has good consistency with PD-L1 detection based on TT specimens. Ilie M et al36 also used the size-based CTC separation and enrichment method to detect PD-L1 expression in CTCs and that in tissues, finding that the consistency between PD-L1 detection based on CTCs and tissues was 93%. Moreover, in this study, we adopted SP142 antibody to detect PD-L1, finding that only 10 patients (14%) showed a PD-L1 expression rate in TTs ≥1%, and only 6 patients showed positive PD-L1 expression in TTs and CTCs. Previous BLUEPRINT series studies have confirmed that in comparison with 22C3, SP263, 28–8, and SP142 antibodies, three antibodies except the SP142 antibody have a relatively high consistency and sensitivity.37,38 In our study, 18 (44%) of the 41 initial treated patients showed positive PD-L1 expression in tissues, and 22 patients (54%) showed positive PD-L1 expression in CTCs. Interestingly, PD-L1 expression in TTs and CTCs was not consistent in 18 re-treated patients. Considering the spatial-temporal heterogeneity of PD-L1 expression and the fact that PD-L1 expression may be affected by drug therapy previously received by patients and changes in tumor molecular biological characteristics during disease progression, it is more significant to explore the real-time PD-L1 detection based on peripheral blood CTC for molecular biomarkers in ICI.39
In addition to being related to the efficacy of ICI drug therapy, PD-L1 expression may also be related to the prognosis of NSCLC patients according to recent reports.40 In this study, we further analyzed the relationship between PFS of patients who received treatment and PD-L1 expression in them, finding that in the 41 initial treated NSCLC patients, patients with high PD-L1 expression according to tissue-based or CTC-based PD-L1 detection all experienced shorter PFS (5.96 vs 6.07 months; 5.12 vs 6.07 months), but there was no significant difference (P=0.579, 0.210), which may be due to insufficient specimen. The expression of PD-L1 was not related to age, sex, smoking status, histological type, and stage (all P > 0.05). Okita et al41 have found that PD-L1 expression in tissues was negatively correlated with relapse-free survival and overall survival of patients. However, another report found that patients with high PD-L1 expression experienced a longer survival time.42 At present, there is no definite conclusion about the relationship between PD-L1 expression and prognosis of patients, and whether the high expression of PD L1 in CTCs can be used to predict the overall poor prognosis of patients needs further study.
There are still some limitations in our study. For example, the sample size is small, and detailed stratification has not been used for re-treated patients, so further research is still needed based on expanded sample size.
In conclusion, it is urgent to explore relevant therapeutic effect/prognosis-related molecular biomarkers for individualized clinical application of immune checkpoint drugs. Based on the spatial-temporal heterogeneity of tumors and the dynamic changes of PD-L1 expression, this study has attempted to use 28–8 antibody to evaluate the expression of PD-L1 in CTCs of peripheral blood and in paired TTs of NSCLC patients for the first time,43 and has analyzed the consistency of the expression in initial treated patients/re-treated patients and its relationship with clinicopathological features in them. It has laid a foundation for providing more molecular biological information for clinical practice by adopting CTCs as an evaluation of PD-L1 expression in real time during the subsequent inaccessible TT specimens and dynamic changes of diseases.
Acknowledgment
This research was supported by Jiangsu Provincial Health Planning Commission Medical Research Project (NO.H2018111), Jiangsu Provincial Medical Youth Talent (No. QNRC2016043) and the Key Medical Science and Technology Development Project of Nanjing (No. ZKX16032).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi: 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125. doi: 10.1056/NEJMoa1713137 [DOI] [PubMed] [Google Scholar]
- 3.Santini FC, Hellmann MD. PD-1/PD-L1 axis in lung cancer. Cancer J. 2018;24(1):15–19. doi: 10.1097/PPO.0000000000000300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 5.Aguiar PN Jr., De Mello RA, Hall P, Tadokoro H, Lima Lopes G. PD-L1 expression as a predictive biomarker in advanced non-small-cell lung cancer: updated survival data. Immunotherapy. 2017;9(6):499–506. doi: 10.2217/imt-2016-0150 [DOI] [PubMed] [Google Scholar]
- 6.Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464–1472. doi: 10.6004/jnccn.2019.0059 [DOI] [PubMed] [Google Scholar]
- 7.Jilaveanu LB, Shuch B, Zito CR, et al. PD-L1 expression in clear cell renal cell carcinoma: an analysis of nephrectomy and sites of metastases. J Cancer. 2014;5(3):166–172. doi: 10.7150/jca.8167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horn L, Spigel DR, Vokes EE, et al. Nivolumab versus docetaxel in previously treated patients with advanced non-small-cell lung cancer: two-year outcomes from two randomized, open-label, Phase III trials (checkmate 017 and CHECKMATE 057). J Clin Oncol. 2017;35(35):3924–3933. doi: 10.1200/JCO.2017.74.3062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo W, Rao M, Qu J, Luo D. Applications of liquid biopsy in lung cancer-diagnosis, prognosis prediction, and disease monitoring. Am J Transl Res. 2018;10(12):3911–3923. [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YC, Zhou Q, Wu YL. The emerging roles of NGS-based liquid biopsy in non-small cell lung cancer. J Hematol Oncol. 2017;10(1):167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watanabe M, Kenmotsu H, Ko R, et al. Isolation and molecular analysis of circulating tumor cells from lung cancer patients using a microfluidic chip type cell sorter. Cancer Sci. 2018;109(8):2539–2548. doi: 10.1111/cas.2018.109.issue-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–6904. doi: 10.1158/1078-0432.CCR-04-0378 [DOI] [PubMed] [Google Scholar]
- 13.Ilie M, Hofman V, Long E, et al. Current challenges for detection of circulating tumor cells and cell-free circulating nucleic acids, and their characterization in non-small cell lung carcinoma patients. What is the best blood substrate for personalized medicine? Ann Transl Med. 2014;2(11):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156(1):57–63. doi: 10.1016/S0002-9440(10)64706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He C, Huang X, Su X, et al. The association between circulating tumor cells and Epstein-Barr virus activation in patients with nasopharyngeal carcinoma. Cancer Biol Ther. 2017;18(11):888–894. doi: 10.1080/15384047.2017.1281493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. 2012;23(1):30–38. doi: 10.1111/cyt.2012.23.issue-1 [DOI] [PubMed] [Google Scholar]
- 17.Buim ME, Fanelli MF, Souza VS, et al. Detection of KRAS mutations in circulating tumor cells from patients with metastatic colorectal cancer. Cancer Biol Ther. 2015;16(9):1289–1295. doi: 10.1080/15384047.2015.1070991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofman V, Ilie MI, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the cellsearch assay and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;129(7):1651–1660. doi: 10.1002/ijc.25819 [DOI] [PubMed] [Google Scholar]
- 19.Souza ESV, Chinen LT, Abdallah EA, et al. Early detection of poor outcome in patients with metastatic colorectal cancer: tumor kinetics evaluated by circulating tumor cells. Onco Targets Ther. 2016;9:7503–7513. doi: 10.2147/OTT.S115268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yanaki M, Kobayashi M, Aruga A, Nomura M, Ozaki M. In vivo antitumor effects of MK615 led by PD-L1 downregulation. Integr Cancer Ther. 2018;17(3):646–653. doi: 10.1177/1534735418766403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bo C, Wu Q, Zhao H, Li X, Zhou Q. Thymosin alpha1 suppresses migration and invasion of PD-L1 high-expressing non-small-cell lung cancer cells via inhibition of STAT3-MMP2 signaling. Onco Targets Ther. 2018;11:7255–7270. doi: 10.2147/OTT.S177943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoy SP, Rosen L, Mueller J, Murgu S. Programmed death-ligand 1 testing of lung cancer cytology specimens obtained with bronchoscopy. Cancer Cytopathol. 2018;126(2):122–128. doi: 10.1002/cncy.v126.2 [DOI] [PubMed] [Google Scholar]
- 24.Simon R. Sensitivity, specificity, PPV, and NPV for predictive biomarkers. J Natl Cancer Inst. 2015;107(8). doi: 10.1093/jnci/djv153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Becker G. Creating comparability among reliability coefficients: the case of cronbach alpha and Cohen kappa. Psychol Rep. 2000;87(3 Pt 2):1171–1182. doi: 10.2466/PR0.87.7.1171-1182 [DOI] [PubMed] [Google Scholar]
- 26.Reck M, Rodriguez-abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. doi: 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 27.Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255–265. doi: 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kythreotou A, Siddique A, Mauri FA, Bower M, Pinato DJ. PD-L1. J Clin Pathol. 2018;71(3):189–194. doi: 10.1136/jclinpath-2017-204853 [DOI] [PubMed] [Google Scholar]
- 29.Russo A, De Miguel Perez D, Gunasekaran M, et al. Liquid biopsy tracking of lung tumor evolutions over time. Expert Rev Mol Diagn. 2019;19(12):1099–1108. doi: 10.1080/14737159.2020.1680287 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Duan J, Cai S, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA oncol. 2019;5(5):696–702. doi: 10.1001/jamaoncol.2018.7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wojas-krawczyk K, Kalinka E, Grenda A, Krawczyk P, Milanowski J. Beyond PD-L1 markers for lung cancer immunotherapy. Int J Mol Sci. 2019;20(8):1915. doi: 10.3390/ijms20081915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schehr JL, Schultz ZD, Warrick JW, et al. High specificity in circulating tumor cell identification is required for accurate evaluation of programmed death-ligand 1. PLoS One. 2016;11(7):e0159397. doi: 10.1371/journal.pone.0159397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bao H, Bai T, Takata K, et al. High expression of carcinoembryonic antigen and telomerase reverse transcriptase in circulating tumor cells is associated with poor clinical response to the immune checkpoint inhibitor nivolumab. Oncol Lett. 2018;15(3):3061–3067. doi: 10.3892/ol.2017.7671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farace F, Massard C, Vimond N, et al. A direct comparison of CELLSEARCH and ISET for circulating tumour-cell detection in patients with metastatic carcinomas. Br J Cancer. 2011;105(6):847–853. doi: 10.1038/bjc.2011.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7(2):306–315. doi: 10.1097/JTO.0b013e31823c5c16 [DOI] [PubMed] [Google Scholar]
- 36.Ilie M, Szafer-glusman E, Hofman V, et al. Detection of PD-L1 in circulating tumor cells and white blood cells from patients with advanced non-small-cell lung cancer. Ann Oncol. 2018;29(1):193–199. doi: 10.1093/annonc/mdx636 [DOI] [PubMed] [Google Scholar]
- 37.Rimm DL, Han G, Taube JM, et al. A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA oncol. 2017;3(8):1051–1058. doi: 10.1001/jamaoncol.2017.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint Phase 2 project. J Thorac Oncol. 2018;13(9):1302–1311. doi: 10.1016/j.jtho.2018.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ancevski Hunter K, Socinski MA, Villaruz LC. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung cancer. Mol Diagn Ther. 2018;22(1):1–10. doi: 10.1007/s40291-017-0308-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan ZK, Ye F, Wu X, An HX, Wu JX. Clinicopathological and prognostic significance of programmed cell death ligand1 (PD-L1) expression in patients with non-small cell lung cancer: a meta-analysis. J Thorac Dis. 2015;7(3):462–470. doi: 10.3978/j.issn.2072-1439.2015.02.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okita R, Maeda A, Shimizu K, Nojima Y, Saisho S, Nakata M. PD-L1 overexpression is partially regulated by EGFR/HER2 signaling and associated with poor prognosis in patients with non-small-cell lung cancer. Cancer Immunol Immunother. 2017;66(7):865–876. doi: 10.1007/s00262-017-1986-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arrieta O, Cardona AF, Martin C, et al. Updated frequency of EGFR and KRAS mutations in nonsmall-cell lung cancer in Latin America: the Latin-American Consortium for the Investigation of Lung Cancer (CLICaP). J Thorac Oncol. 2015;10(5):838–843. doi: 10.1097/JTO.0000000000000481 [DOI] [PubMed] [Google Scholar]
- 43.Brunnstrom H, Johansson A, Westbom-fremer S, et al. PD-L1 immunohistochemistry in clinical diagnostics of lung cancer: inter-pathologist variability is higher than assay variability. Mod Pathol. 2017;30(10):1411–1421. doi: 10.1038/modpathol.2017.59 [DOI] [PubMed] [Google Scholar]