Abstract
In this study, we evaluated the efficacy of a micro-immunotherapy medicine (MIM), 2LALERG, in a preclinical model of allergic respiratory disease sensitized with birch pollen extract (BPE). BALB/c mice were immunized with BPE, or saline solution, and were then challenged. Micro-immunotherapy medicine pillules were diluted in water, and 3 doses (0.75; 1.5; 3 mg/mouse) were tested and compared to vehicle control (3 mg/mouse). Treatments and vehicle were orally administered by gavage for 10 days. Micro-immunotherapy medicine (0.75 mg/mouse) reduced the number of total cells as well as the levels of interleukin (IL)-13 in bronchoalveolar lavage fluid (BALF) compared to vehicle control. Eosinophils in BALF tended to be lower compared to vehicle group, and the difference is close to significance. Histological analysis in the lungs confirms a moderate effect of MIM (0.75 mg/mice) on inflammatory infiltration and mucus production. Serum levels of IL-5 in MIM (0.75 mg/mouse)-treated mice were lower compared to vehicle; IL-4 levels tended to be lower too. Total immunoglobulin E (IgE) decreased in serum of MIM (1.5 and 0.75 mg/mouse) groups compared to vehicle control. Micro-immunotherapy medicine exerted the highest effect at the lowest dose tested. Micro-immunotherapy medicine resolved the local and systemic inflammation, even if partially, in a model of pollen-induced, IgE-mediated inflammation.
Keywords: IgE-mediated inflammation, pollen-induced allergy, micro-immunotherapy, low doses, ultra-low doses
Introduction
Allergic diseases represent an increasing concern worldwide. Most prevalent in the Westernized countries, these diseases are now increasing in developing countries too in parallel to socioeconomic conditions.1 Environmental factors are mostly thought to be responsible. In particular, the prevalence of allergic airway diseases, such as allergic rhinitis (AR) and allergic asthma (AA), is increasing.2
Allergic rhinitis is a very common disease (between 10% and 40% of the population worldwide is affected) caused by environmental aeroallergen and characterized by an immunoglobulin E (IgE)–mediated inflammation of nasal mucosa resulting from introduction of allergen in sensitized individuals.3,4 Allergic rhinitis was defined in 1929 as a process that involved 3 cardinal symptoms occurring with allergen exposure: sneezing, nasal obstruction, and mucus discharge.3 The pathophysiology of AR is complex and includes activation and migration of effector cells to nasal mucosa, release of mediators (chemokines and cytokines) from inflammatory cells, and damage of the nasal epithelium. The AR reaction begins when the allergenic proteins are in contact with the nasal mucosa and are further processed by specialized cells of the immune system, initiating the production of IgE antibodies. IgE interacts with specific allergens and immune cells (mast cells and basophils) triggering a series of reactions: (1) The resident mucosal immune cells such as mast cells, eosinophils, and basophils start to release histamine as well as chemokines, cytokines, and adhesion molecules. (2) Those mediators set up the production of leukocytes in the bone marrow. (3) Circulating effector-activated neutrophils, Th2 lymphocytes, basophils, and eosinophils are attracted into the nasal epithelium resulting in mucosal inflammation.3 Each introduction of the allergen triggers the clinical manifestations of AR. Additionally, in patients with atopy, even in the absence of overt symptoms, levels of IgE remain high in the lymphoid tissue, causing persistent mucosal inflammation.3,5
Allergic asthma is a chronic airway inflammatory disease in which exposure to allergens causes intermittent attacks of breathlessness, airway hyperreactivity, wheezing, and cough.6 The pathogenesis of AA, as well as AR, involves an initial exposure to an allergen that triggers the Th2 cell-dependent stimulation of the immune system and the production of IgE and pro-inflammatory cytokines. The exposure to allergen(s) activates mast cells, which release mediators that facilitate recruitment of other immune cell types, including eosinophils, in the airways. Lung mast cells can initiate immediate hypersensitivity responses by releasing histamine, cysteinyl leukotrienes, and cytokines such as interleukin (IL)-1, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-16, transforming growth factor beta (TGF-β), and tumor necrosis factor alpha (TNF-α), in response to both IgE-mediated adaptive and innate immune responses.6-8
The management of allergic airway diseases is based on allergen avoidance, pharmacological treatment including antihistamines and intranasal corticosteroids, leukotriene receptor antagonists, mast cell stabilizers, and anticholinergics, but many patients seek complementary and alternative treatments to decrease inflammation and clinical manifestations of allergy.9-11 The inhalation of glucocorticoid is widely used; however, it is well-known that the long-term use of high dose can cause side effects, such as bone loss, insulin resistance, and predisposition to diabetes.11
Micro-immunotherapy (MI) is a therapeutic approach that can be used alone or in association with other therapies to offer clinical benefits while minimizing side effects.12 Micro-immunotherapy uses immunological regulators at low doses (LD) and ultra-low doses (ULD) to target the immune system and regulate immune responses in diseases without compromising immune responses nor body homeostasis. The active substances used in MI medicines are cytokines, hormones, growth factors, neuropeptides, nucleic acids, and specific nucleic acids (SNAs, Eurogentec). SNAs consist of single strands of DNA molecules, ranging from 16 to 34 bases, designed to target specifically 1 or more genes (European Patent: EP0670164B1).13-15
2LALERG (MIM) is notified as a homeopathic medicine and has been developed to decrease chronic IgE-mediated inflammation and symptoms of allergy. The medicine is notified by the Federal Agency for Medicines and Health Products in Belgium under notification number 1507CH119 F1. A double-blind versus placebo clinical study published in 2011, performed in a small cohort of patients affected by seasonal AR, indicates that the medicine, taken preventively 2 months before the pollinic season and during the pollen season, was well tolerated, and it could help in the treatment of AR symptoms. Micro-immunotherapy medicine significantly decreased the consumption of antihistamines and intranasal corticosteroids compared to placebo-treated patients.16
Several allergen challenge mouse models have been developed to study allergic reactions able to reproduce airway inflammatory features mimicking AR and AA.17-19 In this study, mice were immunized with birch pollen extract (BPE) by intraperitoneal injection; then, once sensitized, the mice were challenged for 4 consecutive days by intranasal route to set up an allergic reaction.
Micro-immunotherapy medicines are sublingually administered drugs; however, because rodents have their buccal mucosa keratinized, pillules were dissolved in pure water and administered orally by gavage. Previous in vivo study demonstrated that orally administered MIM is effective in reducing systemic and local inflammation in a model of rheumatoid arthritis.20 The hypothesized mode of action implies the involvement of the cells of innate and adaptive immune system (macrophages, monocytes, neutrophils, dendritic cells, and T and B lymphocytes) present in gastrointestinal tract, as well as the lymphoid nodes present in the upper small intestine, along with the Peyer patches in the ileum.21
In the current study, the authors aimed to perform a preclinical study to investigate about optimal dose and ability of MIM to inhibit pollen-induced allergic features as well as to reduce lung and systemic inflammation.
Materials and Method
Mice Model
For experiments, adult BALB/cByJ (BALB/c), 8-week-old female mice were purchased from Janvier, France, and housed at ArtImmune/CNRS (TAAM UPS44, Orleans, France) animal facility. All of the mice were kept in ventilated solid-bottom cages and housed in groups of 5 with chip bedding. All the animal experiments complied with the French Government animal experiment regulations and were approved by the “Ethics committee for animal experimentation of CNRS Campus Orleans” (CCO) under number CLE CCO 2015-1085.
Treatment
The tested MIM consists of lactose–sucrose pillules (also called globules) for oromucosal administration, impregnated with ethanolic preparations of immune mediators, and nucleic acids at LD and/or ULD. Low dose and ULD of active substances are obtained through a “serial kinetic process,” consisting of a 1/100 dilution process followed by vertical shaking, reproduced a defined number of times.
Micro-immunotherapy medicine composition is expressed as centesimal Hahnemannian (CH) dilutions, and it indicates the number of times by which the 2 proceedings are carried out for each active substance. The medicine is sequentially developed to transmit consecutive information to the body, the content of each capsule being specific, and the intake should follow the ascending order indicated on the blister. The composition of the tested MIM is as follows: human recombinant (hr)-IL-1: 17 CH; hr-IL-4: 17 or 27 CH; hr-IL-5: 17 CH; hr-IL-6: 17 CH; hr-IL-10: 17 CH; hr-IL-12: 9 CH; hr-IL-13: 17 CH; hr-TNF-α: 17 CH; hr-TGF-β: 5 CH; lung histaminum: 15 CH; and SNA targeting the human leukocyte antigen type II (HLA II) SNA-HLA II: 18 CH.
Vehicle pillules were prepared using same lactose–sucrose pillules but impregnated with only the vehicle solution in the absence of any active substance. The order of administration respected the MIM sequentiality and the order from 1 to 10 indicated on the blister. Three pillules doses were used daily to test MIM in vivo: 3 mg/mouse, 1.5 mg/mouse, and 0.75 mg/mouse. Vehicle control group received 3 mg/mouse of vehicle pillules daily.
To prepare the dose of 3 mg/mouse, the content of 1 capsule (0.38 g of lactose/sucrose pillules, MIM or vehicle) was dissolved in 25 mL of water (solution-1). The lowest doses were prepared making 1:2 dilution in water starting from solution-1. The concentrations of pillules were: 44 mM for solution-1, MIM (3 mg/mouse), and vehicle; 22 mM for solution-2 and MIM (1.5 mg/mouse); and 11 mM for solution-3 and MIM (0.75 mg/mouse).
In Vivo Animal Experiments
BALB/c (n = 10 mice/group) at 8 weeks of age were first immunized by intraperitoneal route with BPE (10 µg/mouse, Greer Laboratories) twice, on days 0 and 14. Then, mice were challenged with BPE (10 µg in phosphate-buffered saline) from day 21 until day 24 by intranasal route. Control mice received saline solution by nasal route.
The tested MIM (0.75, 1.5, and 3 mg/mouse) and vehicle (3 mg/mouse) were given orally by gavage (200 µL of diluted pillules to each mouse) for 10 days starting on day 14 until day 24. From day 21 to day 24, mice were treated 1 hour before challenge. Budesonide (3 mg/kg, # D1756; Sigma, Darmstadt, Germany), a potent glucocorticoid used as positive control, was administered by intranasal route (40 µL in saline buffer) 1 hour before antigen challenge, from day 21 to day 24. Mice body weight was measured daily starting from day 0 until day 25. Mice were killed on day 25 to analyze the severity of allergic airway and systemic inflammation. The experimental schema is shown in Figure 1A.
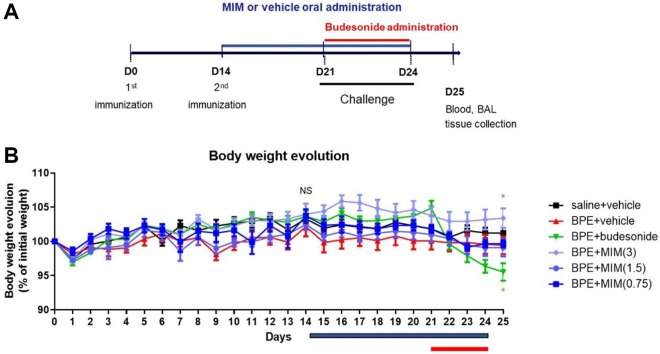
Figure 1.
Schema of the in vivo study and body weight evolution. A, Experimental schema of the in vivo study. Mice in the birch pollen extract (BPE) model were intraperitoneally injected with BPE on the 1st and 14th day and were challenged from 21st to 24th day. Micro-immunotherapy medicine (MIM) or vehicle was administered by gavage from day 14 to day 24. Budesonide was intranasally administered from the 21st until 24th day. Mice in the saline + vehicle control group were sensitized and stimulated in the same way as BPE model group except for using same volume of saline solution instead of pollen. B, Body weight evolution graph (mean expressed as % of the initial weight at day 0, and standard error of mean [SEM]). At day 14, there is no statistically significant difference between groups (NS). At day 25, BPE + budesonide mice showed a significant reduction in body weight compared to BPE + vehicle mice, while MIM (3 mg/mouse) induced an increase in body weight in BPE mice compared to BPE + vehicle control mice. *P < .05.
Bronchoalveolar Lavage
Bronchoalveolar lavage (BAL) was performed by washing the lungs 4 times with 0.5 mL of saline solution at room temperature. After centrifugation at 400g for 10 minutes at 4°C, the supernatant (cell-free bronchoalveolar lavage fluid [BALF]) of the first lavage was collected and stored at −80°C for cytokines analysis, while the cellular pellet was used to perform the cell counts.
Cell Counts in BALF
Cells were collected from BALF sedimentation after centrifugation at 400g for 10 minutes at 4°C. An aliquot of the cell pellets was stained with Turk’s solution and counted, and 200 000 cells were centrifuged onto microscopic slides (cytospin at 1000 rpm for 10 minutes, at room temperature). Air-dried preparations have been fixed and stained with Diff-Quik (Merz & Dade AG, Dudingen, Swizerland). Cell counts were made using oil immersion light microscopy.
Serum Collection and Storage
Blood was drawn from all animals by retro orbital puncture under light isoflurane (3%) anesthesia into tubes without anticoagulant. Blood tubes were centrifuged for 10 minutes at 5000 rpm (Eppendorf) 4°C, and serum obtained was frozen immediately without any further treatment and then stored at −80°C until further analysis.
Cytokine Evaluation in BALF and in Serum
Concentrations of Th2 cytokines (IL-4, IL-5 and IL-13) in BALF and serum were quantified by Multiplex immunoassay (# MCYTOMAG-70K-03; Millipore, Darmstadt, Germany) using MagPix reader (Bio Rad, Watford, United Kingdom).
Total IgE in Serum
Total IgE concentration in serum was evaluated by Multiplex immunoassay (Milliplex; Millipore) using MagPix reader (Bio Rad).
Histological Analysis
Mice were killed under isoflurane exposure at day 25, and then the lung tissues were collected. The left lung of each mice was removed and conserved in formaldehyde 4% and embedded in paraffin for histopathological analysis. Lung tissues from BPE + vehicle group (n = 10) and MIM (0.75 mg/mouse; n = 10) were cut into microslices (3 µm) and stained with periodic acid–Schiff (PAS). Slides were scanned with a Leica DM6000B microscope (Leica Microsystems, Milton Keynes, United Kingdom) (×40 magnifications, scale bar 100 µm). Three images per mouse and all 10 mice were analyzed.
Inflammatory and mucus scores were graded. Inflammatory score was calculated as follows: 0 = no inflammation, normal lung; 1 = moderate cells infiltration in total lung; 2 = moderate cells infiltration particularly around blood vessels and bronchi; 3 = increased cells infiltration around blood vessels and bronchi and infiltration in lung parenchyma; and 4 = increased cells infiltration in lung parenchyma and structure damage of lung.
The mucus secretion was assessed on PAS-stained sections and consists in general of mucopolysaccharides staining at the apical pole of goblets cells in the peribronchial area, which was graded using a semi-quantitative score from 0 to 5 with increasing grade of infiltration by 2 observers independently as follows: 1 = Normal lung; no mucus; 2 = Mucus in 1/3 of the bronchi; 3 = Mucus in 1/2 of the bronchi; 3 = Mucus in 2/3 of the bronchi; 4 = Mucus in 3/4 of the bronchi; and 5 = Mucus everywhere in the bronchi.
Statistical Analysis
Body weight evolution in the different experimental groups were analyzed graphically. Comparison between groups at day 14 and day 25 were performed with Kruskal-Wallis tests followed by pairwise comparisons, if Kruskal-Wallis test was statistically significant. Comparisons on lung inflammation, cytokines release in BALF, systemic inflammation, inflammatory infiltration, and mucus production were analyzed in the same way: Kruskal-Wallis test followed by pairwise comparisons. Pairwise comparisons were realized with Student t test or Wilcoxon test based on Shapiro-Wilk normality test results. For each end point, 5 comparisons of interest were performed: saline + vehicle versus BPE + vehicle, BPE + vehicle versus BPE + Budesonide, BPE + vehicle versus MIM,3 BPE + vehicle versus MIM (1.5), and BPE + vehicle versus MIM (0.75). A value of P < .05 was considered statistically significant. SAS software (version 9.4) was used for statistical analyses, while GraphPad Prism version 8 was used for preparing the graphs.
Results
Reduction of BPE-Induced Lung Inflammation by MIM
Weight values were expressed in percentage (%) of weight at day 0 (100%) to evaluate the body evolution during the experiment, before, and during treatment. At day 14, there were no statistical differences between the groups. Vehicle administration did not influence body weight in saline + vehicle mice, neither in BPE + vehicle mice: There was no difference in weight measured in mice before (from day 0 to day 13) and after (from day 14 to day 25) treatment (data not shown).
At day 25, BPE + budesonide mice showed a significant reduction in body weight compared to BPE + vehicle control mice, while MIM at the dose of 3 mg/mouse induced a slight increase in body weight. There is no statistically significant difference between saline + vehicle and BPE + vehicle groups, neither between MIM (0.75 or 1.5 mg/mouse)-treated BPE mice and vehicle-treated BPE mice (Figure 1B).
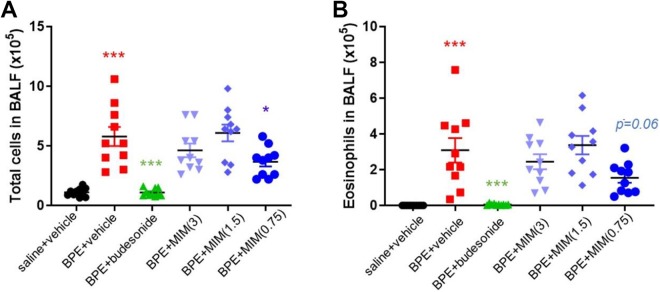
To evaluate the extent of airway inflammation, and determine the therapeutic effect of MIM on the inflammatory response, total cell and eosinophil counts in the BALF of mice were measured. Birch pollen extract + vehicle group had a clear increase in number of total cells compared to saline + vehicle group and an increase in eosinophils as well. As expected, in positive control group (BPE + budesonide), lower number of total cells and of eosinophils were observed in BALF than BPE + vehicle group. Treatment with MIM (0.75 mg/mouse) induced a significant reduction in the number of total cells compared to BPE + vehicle (Figure 2A).
Figure 2.
Effect of micro-immunotherapy medicine (MIM) on lung inflammation. The total number of inflammation-related BALF cells was counted (A), and the number of eosinophils (B) in BALF was measured too in all groups (n = 10 per group). Values are expressed as number × 105. Birch pollen extract (BPE) + vehicle–treated mice have higher number of cells and eosinophils in BALF than saline + vehicle mice; BPE + budesonide mice decreased their number of cells and eosinophils in BALF compared to BPE + vehicle mice; MIM (0.75 mg/mouse) decreased their number of cells in BALF compared to BPE + vehicle, while for eosinophil counts the difference is close to significance (P = .06). *P < .05; *** P ≤ .001.
Number of eosinophils in BALF tended to be lower in MIM (0.75 mg/mouse)-treated mice than BPE + vehicle mice, reaching almost significant difference (P = .06; Figure 2B).
Pulmonary Inflammation and Mucus Secretion Are Slightly Reduced by MIM
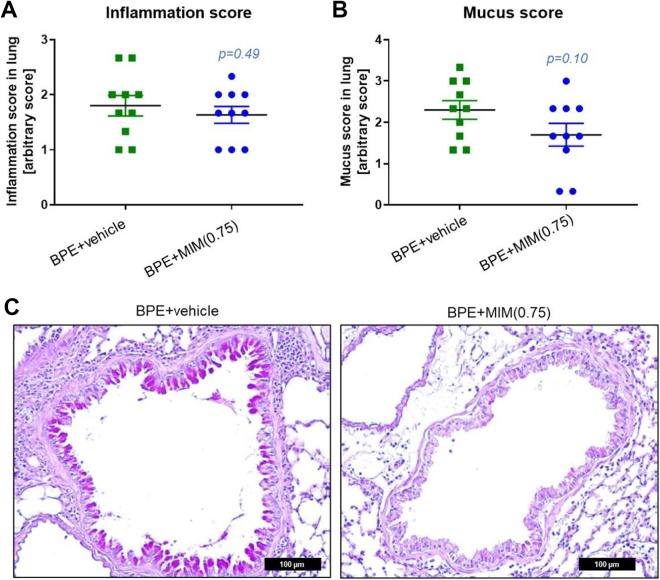
Histological analysis showed no effect on pulmonary inflammation or mucus production (Figure 3A and B).
Figure 3.
Effect of micro-immunotherapy medicine (MIM) in inflammatory infiltration and mucus production. Inflammatory score (A) and mucus production score (B) in birch pollen extract (BPE) nontreated mice and MIM (0.75 mg/mouse)-treated mice. (C) Representative images of AB-PAS staining. Mucus was found in the alveolar cavity of both the groups; however, in the MIM-treated mice, a tendency toward less mucus production has been observed. Scale bar = 100 µm.
Inhibition of BPE-Induced Th2 Cytokine Concentrations in BALF by MIM
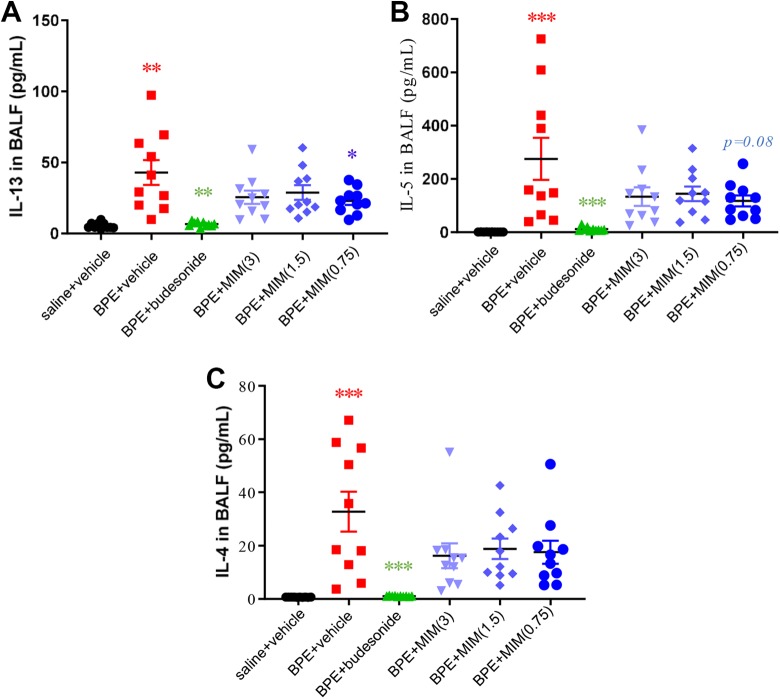
Pro-inflammatory Th2 cytokines IL-5, IL-4, and IL-13 increased in BALF of BPE + vehicle group compared to saline + vehicle group. Mice treated with budesonide reduced their level of cytokines in BALF compared to BPE + vehicle group (Figure 4A-C).
Figure 4.
Effect of micro-immunotherapy medicine (MIM) on cytokines release in BALF. Levels of IL-13 (A), IL-5 (B), and IL-4 (C) in BALF of all mice groups (n = 10 per group). Cytokines levels increased in BALF of birch pollen extract (BPE) + vehicle group compared to saline + vehicle group. Mice treated with budesonide reduced their level of cytokines in BALF compared to BPE + vehicle group. IL-13 levels were significantly lower in MIM (0.75 mg/mouse)-treated mice than in BPE + vehicle group. *P < .05; ** P ≤ .01; *** P ≤ .001.
Interleukin-13 levels were significantly lower in MIM (0.75 mg/mouse)-treated mice than in BPE + vehicle group (Figure 4A), while a trend toward lower levels in both MIM at 1.5 mg/mouse and MIM at 3 mg/mouse groups compared to BPE + vehicle was observed.
Interleukin-5 levels in MIM (0.75 mg/mouse)-treated mice were found to be lower than BPE + vehicle-treated mice, with a near-significant trend close to significance (P = .08; Figure 4B). Interleukin-4 levels have a tendency to be lower in MIM-treated groups compared to vehicle group but without significance (Figure 4C).
Inhibition of BPE-Induced Circulating Th2 Cytokine Levels by MIM
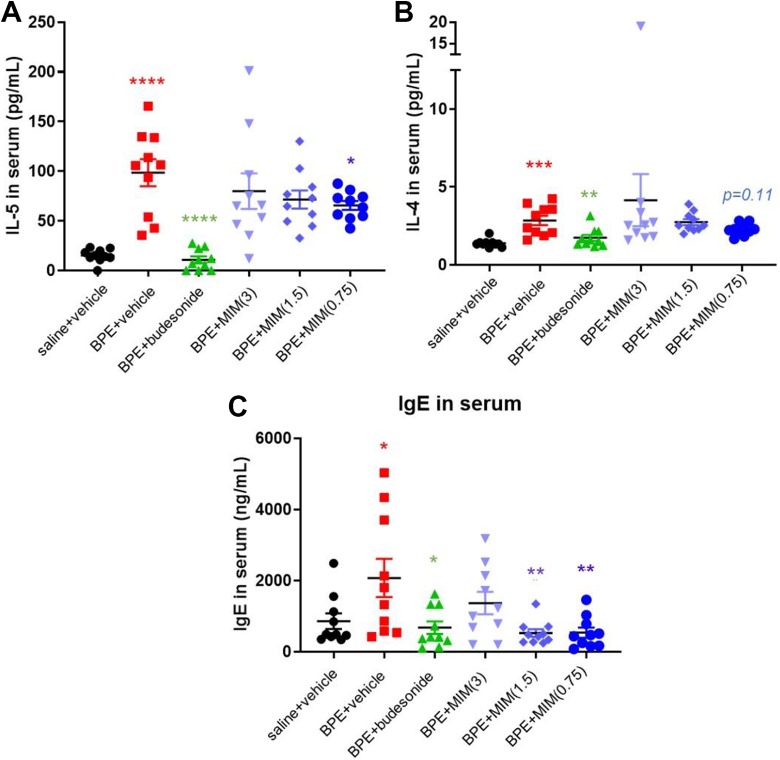
Birch pollen extract–immunized mice receiving vehicle showed a significant increase in circulating IL-4 and IL-5 in comparison to saline + vehicle control group, as expected. Budesonide treatment significantly decreased the levels of both cytokines compared to BPE + vehicle (Figure 5A and B). Interleukin-13 was measured but was not detectable in any groups (data not shown).
Figure 5.
Effect of micro-immunotherapy medicine (MIM) in systemic inflammation. Serum levels of interleukin (IL)-5 (A), IL-4 (B), and immunoglobulin E (IgE) (C) measured in all mice groups (n = 10 per group). Cytokines and total IgE were found increased in birch pollen extract (BPE)–immunized mice receiving vehicle compared to saline + vehicle control (A-C). Budesonide treatment significantly decreased the serum levels of cytokines and IgE compared to BPE + vehicle (A-C). The IL-5 levels were significantly lower in MIM (0.75 mg/mouse)-treated mice than in BPE + vehicle group (A). Two groups treated with MIM (1.5 and 0.75 mg/mouse) responded with a significant reduction in IgE compared to vehicle group (C). *P < .05; ** P ≤ .01; *** P ≤ .001; **** P ≤ .0001.
Micro-immunotherapy medicine (0.75 mg/mouse) treatment decreased serum levels of IL-5 compared to vehicle (Figure 5A). The levels of IL-4 tend to be lower in MIM (0.75 mg/mouse)-treated mice than in vehicle control mice, but no significant difference was found (P = .11; Figure 5B).
Inhibition of BPE-Induced Circulating Total IgE Levels by MIM
Total IgE were found increased in BPE-immunized mice receiving vehicle compared to saline + vehicle control. Budesonide treatment significantly decreased the serum levels of IgE compared to BPE+ vehicle (Figure 5C).
Two groups treated with MIM (1.5 and 0.75 mg/mouse) responded with a significant reduction in IgE compared to vehicle group, while the mice treated with the highest dose of MIM (3 mg/mouse) did not show a reduction in their IgE serum levels (Figure 5C).
Discussion
In the current study, authors wanted to examine optimal dose and in vivo effect of oral administered MIM on IgE-mediated inflammation in a mouse model of pollen-induced allergic inflammation.
To test the effect of MIM, a vehicle control was included in the experiment. It consisted of lactose–sucrose pillules without active substances that were administered in BPE-sensitized mice at the dose of 3 mg/mouse. The intake of that dose of sugar can be considered appropriate and low enough to avoid interference with sugar metabolism or sugar-induced inflammation since composition of low-sugar diet in mice contains about 50 g/kg of sucrose,22 corresponding to an intake of about 1 g/mouse. Body weight in saline + vehicle and BPE + vehicle groups was not impacted at the end of the study by the intake of the vehicle.
The mouse model sensitized and challenged with BPE, receiving vehicle, developed the common clinical features of AR and AA: The number of total cells and of eosinophils, as well as the levels of IL-5, IL-4, and IL-13, measured in BALF were significantly higher than in BALF of saline + vehicle control group. Furthermore, serum levels of IgE and Th2-related cytokines (IL-4 and IL-5) were increased in BPE + vehicle group compared to saline + vehicle control group.
Despite their adverse effects, glucocorticoids still remain the main anti-inflammatory treatment used to control and limit allergic symptoms.9-11,23 The in vivo study included a positive control group treated with a potent glucocorticoid, budesonide, which was administered in mice via the classical nasal route.23 Budesonide-treated mice reduced their levels of Th2 cytokines in BALF and in serum as well their levels of cells and eosinophils in BALF compared to BPE + vehicle; serum levels of IgE were also decreased in comparison with vehicle control group. Budesonide-treated mice exhibited a reduction in their body weight starting from the first day of treatment. At day 25, body weight in BPE + budesonide group was significantly lower than weight measured in BPE + vehicle group (Figure 1B). It has also been reported in clinical study that there is some association between a lower growth rate and a high dose use of inhaled glucocorticoids in children.24 On the other side, MIM (3 mg/mouse) treatment has slightly increased mice body weight, while the other 2 MIM doses tested have not influenced mice weight (Figure 1B).
Micro-immunotherapy medicine was formulated to help patients in the management of clinical manifestations of allergy and in the prevention of severe allergic reactions. Micro-immunotherapy medicine uses ULD of hr-pro-inflammatory cytokines involved in the establishment of the chronic IgE-mediated inflammation: IL-1β, IL-4, IL-5, IL-6, IL-10, IL-12, TNF-α, and IL-133,7,8 to counteract their overexpression and reduce allergic immune reactions and the associated symptoms. Micro-immunotherapy medicine uses as well ULD of SNA targeting HLA II molecules. Human leukocyte antigen II proteins are crucial to the functioning of the immune system and in the initial antigen presentation to T cells25; however, they are also implicated in the development of allergy because they are indispensable in the T cell–B cell interactions that drive IgE production.26 Ultra low dose of SNA-HLA II aims to control the overexpression of HLA II molecules and limits the IgE secretion and the IgE-mediated allergic responses. Ultra low dose of lung histaminum is used to inhibit the degranulation of basophils,27 while LD of TGF-β aimed to sustain TGF-β actions: suppression of T cells-mediated inflammation, activation of regulatory T cells, and promotion of tissue repair.28,29
The hypothesized mode of action of ULD and LD used in MIM might be explained with the concept of hormesis, also known as a biphasic dose–response relationship that differentiates dose-induced biological effects in 2 doses zones: the traditional and well-known effects due to high doses; the underestimated and often unknown effects induced by LDs.30 Hormesis, introduced for the first time 130 years ago by Schulz, has been studied and reintroduced by Calabrese, and it is becoming more and more familiar in several areas of toxicology and pharmacology.30-32 In spite of the large documentation that showed hormesis mechanisms in many fields (cellular biology, microbiology, medicine, nutrition, plant biology), there is still much to achieve about the therapeutic potentials of LDs.
The aim of the study was to perform a preclinical study to evaluate best lactose–sucrose pillules dose among the 3 tested (0.75; 1.5; 3 mg/mouse) and in vivo effects of the MIM on IgE-mediated inflammation. Authors are aware that more investigations are needed to understand molecular mechanisms and biological effects exerted by LD and ULD.
The tested MIM, at the dose of 0.75 mg/mouse (corresponding to a concentration of 11 mM), has reduced the number of total cells (Figure 2A) as well as the levels of the Th2 cytokine IL-13 in BALF compared to BPE + vehicle control (Figure 4A). Although not reaching significance, eosinophils in BALF tended to be lower, and the difference is close to be statistically significant (P = .06; Figure 2B).
Interleukin 13 is involved in the effector phase of allergic response, and it induces airway hyperresponsiveness, mucus production, and airway obstruction as well as smooth muscle hyperplasia.33 To evaluate the presence of inflammatory cells and mucus in lung and to confirm the local effect of the lowest tested dose of MIM, histological analysis was performed in BPE + vehicle and BPE + MIM (0.75 mg/mouse) groups. Histology results revealed no significant effect on pulmonary inflammation or mucus production, but the arbitrary visual score used was probably not appropriate (Figure 3A and B).
Levels of IL-5 in BALF of MIM-treated mice tended to be lower than in vehicle BPE-sensitized mice, and the difference between MIM (0.75 mg/mouse) and BPE + vehicle groups is close to significance (P = .08; Figure 4B). There is a trend toward lower level of IL-4 in BALF in MIM-treated mice compared to vehicle, but no statistical differences have been found (Figure 4C).
The allergic airway inflammation seems not to be completely resolved by the oral-administered MIM; however, the results suggest that the lowest dose tested has the higher local effects. The impact on systemic inflammation has been investigated by measuring Th2 cytokines and total IgE in serum. Birch pollen extract–immunized mice receiving vehicle showed a significant increase in circulating IL-4 and IL-5 compared to saline + vehicle control. Total IgE were increased too.
Micro-immunotherapy medicine (0.75 mg/mouse) treatment decreased IL-5 level in serum compared to vehicle, while just a trend was found in the other 2 MIM-treated groups (Figure 5A). Interleukin-5 plays a key role in mobilization of eosinophils from the bone marrow to the site of inflammation.34 Indeed, lower number of eosinophils in BALF has been observed in MIM (0.75 mg/mouse)-treated mice, and difference is at the very edge of significance (Figure 2B).
The serum levels of IL-4 tended to be lower in MIM (0.75 mg/mouse)-treated mice than BPE + saline controls, but no significant differences were found (Figure 5B). Immunoglobulin E is involved in the pathophysiological process of allergic reactions and play an important role in the development of airway type-1 hypersensitivity reactions and various allergic diseases, such as AR, AA, food allergies, and specific types of chronic urticaria and atopic dermatitis.35 Immunoglobulin E levels are higher in patients with allergic diseases.36,37
Birch pollen extract–sensitized mice, receiving MIM at 1.5 mg/mouse or 0.75 mg/mouse, responded to treatment with lower secretion of total IgE compared to BPE + vehicle control (Figure 5C). The highest MIM dose tested has not impacted IgE levels in a significant way, and just a trend has been observed.
Conclusions
In conclusion, the tested MIM appears to be effective in treatment of IgE-mediated inflammation, even if only partially, and might have a therapeutic potential as adjuvant in the treatment of allergic airway diseases. The lowest dose tested has exerted the major effect in vivo. Further investigations are needed to understand mode of action of MIM and all possible uses in the treatment of Th2-type and IgE-mediated inflammatory reactions.
Acknowledgments
The authors would like to thank Mrs Anne Naedts for having prepared and provided the vehicle used in the study and Mr Cedric Wolf and Mr. Michel Gangolf for having sent medicine and vehicle capsules to ArtImmune laboratory.
Authors’ Note: IF and DT designed the study. PC performed the experiments. IF, BL, and DT coordinated the overall undertaking of the study. CV performed statistical analyses. IF wrote the manuscript. BL revised the manuscript. All the authors read and approved the final manuscript. The data of the current study are available from the corresponding author on reasonable request.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BL, IF, and CV work for Labo’Life France, the company service provider of Labo’Life, specialized in pre-clinical, clinical development, and regulatory affairs. This professional relationship does not imply any misconduct on the part of the authors. DT and PC work for ArtImmune, a biotechnological company specialized in preclinical research.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was entirely funded by Labo’Life France.
ORCID iDs: Ilaria Floris  https://orcid.org/0000-0003-4089-3133
https://orcid.org/0000-0003-4089-3133
Beatrice Lejeune  https://orcid.org/0000-0001-9067-6116
https://orcid.org/0000-0001-9067-6116
References
- 1. Kadam K, Karbhal R, Jayaraman VK, Sawant S, Kulkarni-Kale U. AllerBase: a comprehensive allergen knowledgebase. Database (Oxford). 2017;2017:bax066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Togias A. Rhinitis and asthma: evidence for respiratory system integration. J Allergy Clin Immunol. 2003;111(6):1171–1183. [DOI] [PubMed] [Google Scholar]
- 3. Bernstein DI, Schwartz G, Bernstein JA. Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin North Am. 2016;36(2):261–278. [DOI] [PubMed] [Google Scholar]
- 4. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108(5):S147–S334. [DOI] [PubMed] [Google Scholar]
- 5. Bauchau V, Durham SR. Epidemiological characterization of the intermittent and persistent types of allergic rhinitis. Allergy. 2005;60(3):350–353. [DOI] [PubMed] [Google Scholar]
- 6. Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy. 2004;59(5):469–478. [DOI] [PubMed] [Google Scholar]
- 7. Barrett NA, Austen KF. Innate cells and T helper 2 cell immunity in airway inflammation. Immunity. 2009;31(3):425–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11(7):577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ridolo E, Montagni M, Melli V, Braido F, Incorvaia C, Canonica GW. Pharmacotherapy of allergic rhinitis: current options and future perspectives. Expert Opin Pharmacother 2014;15(1):73–83. [DOI] [PubMed] [Google Scholar]
- 10. Farnesi BC, Ducharme FM, Blais L, et al. Guided asthma self-management or patient self-adjustment? Using patients’ narratives to better understand adherence to asthma treatment. Patient Prefer Adherence. 2019;13:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lin SC, Shi LS, Ye YL. Advanced molecular knowledge of therapeutic drugs and natural products focusing on inflammatory cytokines in asthma. Cells. 2019;8(7):685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schwartz A. Ozonoterapia en la Infección por Virus Del papiloma humano (HPV). Ozone Ther Global J. 2017;7(1):5–16. [Google Scholar]
- 13. Thomas G, Cluzel H, Lafon J, Bruhwyler J, Lejeune B. Efficacy of 2LPAPI®, a micro-immunotherapy drug, in patients with high-risk papillomavirus genital infection. Adv Infect Dis. 2016;6(01):7–14. [Google Scholar]
- 14. Floris I, Appel K, Rose T, Lejeune B. 2LARTH®, a micro-immunotherapy medicine, exerts anti-inflammatory effects in vitro and reduces TNF-α and IL-1β secretion. J Inflamm Res. 2018;11:397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lilli NL, Révy D, Robelet S, Lejeune B. Effect of the micro-immunotherapy medicine 2LPARK® on rat primary dopaminergic neurons after 6-OHDA injury: oxidative stress and survival evaluation in an in vitro model of Parkinson’s disease. Degener Neurol Neuromuscul Dis. 2019;9:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van der Brempt X, Cumps J, Capieaux E. Efficacité clinique du 2L®ALERG, un nouveau traitement de type immunomodulateur par voie sublinguale dans le rhume des foins: une étude en double insu contre placebo. Revue Française d’Allergologie. 2011;51(4):430–436. [Google Scholar]
- 17. Xie Z, Yin J. Chinese birch pollen allergy and immunotherapy in mice. Inflammation. 2019;42(3):961–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tourdot S, Airouche S, Berjont N, et al. Efficacy of sublingual vectorized recombinant Bet v 1a in a mouse model of birch pollen allergic asthma. Vaccine. 2013;31(23):2628–2637. [DOI] [PubMed] [Google Scholar]
- 19. Spacova I, Petrova MI, Fremau A, et al. Intranasal administration of probiotic Lactobacillus rhamnosus GG prevents birch pollen-induced allergic asthma in a murine model. Allergy. 2019;74(1):100–110. [DOI] [PubMed] [Google Scholar]
- 20. Floris I, García-González V, Palomares B, Appel K, Lejeune B. The micro-immunotherapy medicine 2LARTH® reduces inflammation and symptoms of rheumatoid arthritis in vivo. Int J Rheumatol. 2020;2020:1594573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung C, Hugot JP, Barreau F. Peyer’s patches: the immune sensors of the intestine. Int J Inflam. 2010;2010:823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of high-fat and high-sucrose diets differentially affects glucose intolerance in mice. J Nutr. 2006;136(3):582–587. [DOI] [PubMed] [Google Scholar]
- 23. Becky Kelly EA, Busse WW, Jarjour NN. Inhaled budesonide decreases airway inflammatory response to allergen. Am J Respir Crit Care Med. 2000;162(3):883–890. [DOI] [PubMed] [Google Scholar]
- 24. Pruteanu AI, Chauhan BF, Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose-response effects on growth. Evid Based Child Health. 2014;9(4):931–1046. [DOI] [PubMed] [Google Scholar]
- 25. Van den Berg HA, Rand DA. Antigen presentation on MHC molecules as a diversity filter that enhances immune efficacy. J Theor Biol. 2003;224(2):249–267. [DOI] [PubMed] [Google Scholar]
- 26. Stephan V, Kuehr J, Seibt A, et al. Genetic linkage of HLA-class II locus to mite-specific IgE immune responsiveness. Clin Exp Allergy. 1999;29(8):1049–1054. [DOI] [PubMed] [Google Scholar]
- 27. Sainte-Laudy J, Sambucy JL, Belon P. Biological activity of ultra low doses I. Effect of ultra low doses of histamine on human basophil degranulation triggered by D. Pteronissinus extract In: Doutremepuich C. (ed) Ultra Low Doses. London, UK: Taylor and Francis; 1991: 127–138. [Google Scholar]
- 28. Schmidt-Weber CB, Blaser K. The role of TGF-β in allergic inflammation. Immunol Allergy Clin North Am. 2006;26(2):233–244. [DOI] [PubMed] [Google Scholar]
- 29. Salib RJ, Howarth PH. Transforming growth factor-β in allergic inflammatory disease of the upper airways: friend or foe? Clin Exp Allergy. 2009;39(8):1128–1135. [DOI] [PubMed] [Google Scholar]
- 30. Agathokleous E, Calabrese EJ. Hormesis: the dose response for the 21st century: the future has arrived. Toxicology. 2019;425:152249. [DOI] [PubMed] [Google Scholar]
- 31. Calabrese E. Hormesis: path and progression to significance. Int J Mol Sci. 2018;19(10):E2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang D, Calabrese EJ, Lian B, Lin Z, Calabrese V. Hormesis as a mechanistic approach to understanding herbal treatments in traditional Chinese medicine. Pharmacol Ther. 2018;184:42–50. [DOI] [PubMed] [Google Scholar]
- 33. Gour N, Wills-Karp M. IL-4 and IL-13 signaling in allergic airway disease. Cytokine. 2015;75(1):68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Palframan RT, Collins PD, Severs NJ, Rothery S, Williams TJ, Rankin SM. Mechanisms of acute eosinophil mobilization from the bone marrow stimulated by interleukin 5: the role of specific adhesion molecules and phosphatidylinositol 3-kinase. J Exp Med. 1998;188(9):1621–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hall S, Agrawal DK. Key mediators in the immunopathogenesis of allergic asthma. Int Immuno pharmacol. 2014;23(1):316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18(5):693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Platts-Mills TA, Schuyler AJ, Erwin EA, Commins SP, Woodfolk JA. IgE in the diagnosis and treatment of allergic disease. J Allergy Clin Immunol. 2016;137(6):1662–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]