Abstract
Objectives:
Asenapine, a potent serotonin 7 (5-HT7) receptor antagonist, was examined for efficacy as an antidepressant in depressed bipolar subjects. It was predicted that subjects with the genetic variant of the short form of the serotonin transporter (5HTTR) would be more likely to respond.
Experimental Design:
A subset of patients participating in a randomized, placebo-controlled study of the efficacy of asenapine in bipolar I depression also underwent genetic testing for the 5HTTR. Montgomery Åsberg Depression Rating Scale (MADRS) score was ≥ 26 prior to randomization to asenapine or placebo for 8 weeks. Gene testing was performed before breaking the blind.
Principal Observations:
Nine patients completing the study also underwent gene testing. At study end, the average MADRS improvement was −19.80 ± SD 8.59 for the 4 people randomized to asenapine and −3.80 ± 9.01 for the 5 people receiving placebo (P = 0.021, t = 2.88). Anxiety, as measured by the Hamilton Anxiety Rating Scale (HAM-A), also improved in asenapine-treated patients (−15.40 ± 6.15 vs. −2.80 ± 7.95, P = 0.023, t = 2.803). Six participants had the short form of the 5HTTR, and it is believed they influenced the significant outcome in this small sample.
Conclusions:
While this is a very small sample, asenapine appears to have a beneficial effect on both depression and anxiety in depressed bipolar I patients compared to treatment with placebo. Due to the large fraction of subjects with the short form, the hypothesis that the SF-5HTTR might increase asenapine response could not be adequately tested.
Keywords: anxiety, asenapine, bipolar disorder, bipolar depression, depression, serotonin transporter
Depressive symptoms are responsible for the majority of symptomatic time in bipolar illness.1,2 In type I bipolar patients, depression may occupy over 65% of symptomatic time, or nearly one third of their lives,2,3 and underlies high rates of functional disability and suicide.4,5 Treatment of bipolar depression can be problematic since antidepressants may be associated with destabilization of the illness.6,7 There remains a critical need for agents that are both safe and effective in the treatment of bipolar depression.
Asenapine is a dibenzo-oxepino-pyrrole second-generation antipsychotic medication that can interact with a wide variety of serotonin (5HT) and dopamine receptors.8 Importantly, it is an antagonist with reasonably high affinity to the dopamine D2 receptor (Ki = 1.3 nM) and 5HT2A (Ki = 0.06 nM) receptors,8 through which it presumably mediates some of its antipsychotic and anti-manic properties.9–11 Additionally, it also has high affinity to several 5HT receptors (5-HT2C, Ki 0.03 nM; 5-HT2A, Ki 0.06 nM; 5-HT7, Ki 0.13 nM; 5-HT2B, Ki 0.16 nM; 5-HT6, Ki 0.25 nM) where it is also antagonistic. In preclinical studies, blockade of some of these receptors has demonstrated anti-depressive or anxiolytic efficacy (e.g., 12 [for 5HT6 and 5HT7]; 13 [5HT2c]; 14 [5HT2c]). In particular, several 5HT7 antagonist agents have demonstrated antidepressant properties (e.g., vorioxetine,15,16 or LuAA2100417), specifically in bipolar disorder (e.g., lurasidone18). The utility of asenapine in ameliorating the depressive symptoms in mixed states is suggestive of potential efficacy in bipolar depression.19,20 Consequently, asenapine appears to have a possible role in meeting an unmet clinical need.
The serotonin transporter (5HTTR) has been the focus of extensive investigation.21 The serotonin transporter gene, solute carrier family 6 (neurotransmitter transporter), member 4 (SLC6A4), which encodes the protein that mediates the reuptake of synaptic serotonin back into the presynaptic neuron, has polymorphisms which alter its expression.22,23 Variants in this gene, collectively known as the ‘short form’, reduce the number of 5HTTR pump units expressed in the synapse.22,23 Despite some controversy, research generally supports a relationship between the short form of 5HTTR and depression.22–28 Since subjects with the short form are expected to have elevated levels of synaptic 5HT, the short form provided a theoretical framework of how serotonin reuptake inhibiting antidepressants might lead to loss of antidepressant efficacy or destabilization of bipolar illness.29,30 These same data also lead us to predict that individuals with the short form of the 5HTTR would be more likely to respond to a medication that blocks post-synaptic 5HT receptors.
Pharmacogenomics as an aspect of personalized medicine, is rapidly emerging as the new standard of care. In psychiatry, pharmacogenomics is still in its infancy.31 Most data regarding pharmacogenomics in psychiatry relate to potential for side effects. There are emerging data that are beginning to identify predictors of response.
In this report, we present data regarding a subset of patients involved in a study designed to determine if asenapine added to unchanged ongoing treatment might be effective in treating bipolar I depression. The parent study was a double-blind, randomized, placebo-controlled study of asenapine in bipolar I depression. The subset of patients presented in this report underwent pharmacogenetic testing for short-form variants of SLC6A4.
Methods
Study Design
The larger study was a randomized, placebo-controlled, 8-weeks asenapine monotherapy efficacy trial in depressed type I bipolar patients. Nine subjects underwent gene testing for the short form of the serotonin transporter. Subjects entered the study if they had type I bipolar illness (confirmed with Mini International Neuropsychiatric Interview (MINI).32 They had to be ≥ 18 or ≤ 55 years of age and be able to understand and sign an informed consent. Depressive symptoms were quantified with the Montgomery Åsberg Depression Rating Scale (MADRS),33 manic symptoms were measured with the Young Mania Rating Scale (YMRS),34 anxiety was measured with the Hamilton Anxiety Scale (Ham-A),35 the overall clinical impression with the bipolar version of the Clinical Global Impression (CGI-BD),36 and suicidality was measured with the Columbia-Suicide Severity Rating Scale (C-SSRS).37 A MADRS score ≥ 26 was required for study entry. Patients were evaluated weekly for the first 2 weeks and biweekly thereafter for a total of 8 weeks.
After treatment, subjects were offered participation in the genetic aspect of the study. After signing a separate informed consent, they provided cheek swabs which were sent for gene analysis. Nine subjects underwent genetic testing. The samples had DNA extracted and were tested specifically for both the short form of the promoter of the 5HTTR and the LG genotype of the 5HHTR-linked polymorphic region (rs25531). Both of these variants are associated with reduced protein expression of the serotonin transporter.22,23
Treatment
Only one patient in the asenapine group was off all other medications. Concomitant medications, which were unchanged for the duration of the entire study and at least 2 weeks prior to study entry, included for the asenapine group: three on lamotrigine, and one each on bupropion, venlafaxine, vortioxetine, methylphenidate, and gabapentin; and for the placebo group: one each on lamotrigine, lithium, fluoxetine, quetiapine, ziprasidone, methylphenidate, alprazolam, lorazepam, and methadone. They were given either unflavored sublingual asenapine or placebo that was started at 5 mg twice daily. The dose could be increased to 10 mg twice daily or decreased to 5 mg once daily based on tolerance or clinical response. Rescue lorazepam was available for the first 4 weeks of the study, but none of the subjects presented here used any rescue doses.
Statistical Analysis
The primary outcome measure was the difference in improvement from baseline to study end in subjects that had a low expressing genotype of the 5HTTR versus the long form. Inadequate numbers of patients with the long form prevented that analysis. Instead, we examined the effects of having a short form of 5HTTR on change from baseline to days 7, 14, 28, 42 and 56 (study end) in active and placebo treated patients for both depression and anxiety with an unpaired, 2-tailed, Student’s T-test. Missing data were remedied with last observation carried forward. Statistical significance was set at probability (P) < 0.05. All statistical analysis was performed by Prism version 7.00.
Results
Effect on Depression
Improvement in MADRS was statistically significantly greater in the asenapine group than the placebo group across genotypes, measured as changes from baseline to endpoint (mean −19.80 ± SD 8.59 vs. −3.80 ± 9.01, respectively; t = 2.875, P = 0.021) (Figure 1). Similarly, all other time points were also significant (day 7 [−22.00 ± 8.63 vs. −2.60 ± 5.81; t = 4.168, P = 0.0031]; day 14 [−28.40 ± 6.03 vs. −4.60 ± 5.68; t = 6.425, P = 0.0002]; day 28 [−26.40 ± 6.99 vs. −5.80 ± 7.16; t = 4.606, P = 0.0017]; day 42 [−24.40 ± 9.24 vs. −6.20 ± 8.17; t = 3.248, P = 0.0117]) (Figure 1). Comparing only 6 subjects with the short form of the 5HTTR (3 received placebo and 3 received asenapine [Table 1]), we found that differences in depression were no longer significant (−19.67 ± 7.64 vs. −9.33 ± 6.43, t = 1.79, P = 0.15, Power = 0.44). The effect size for subjects with the short form was 0.6. While many of the ongoing evaluations throughout the study are significant (Figure 1), it is important to note that the study was not intended to examine those points, and the significance would vanish if we corrected for multiple t-tests.
Figure 1.
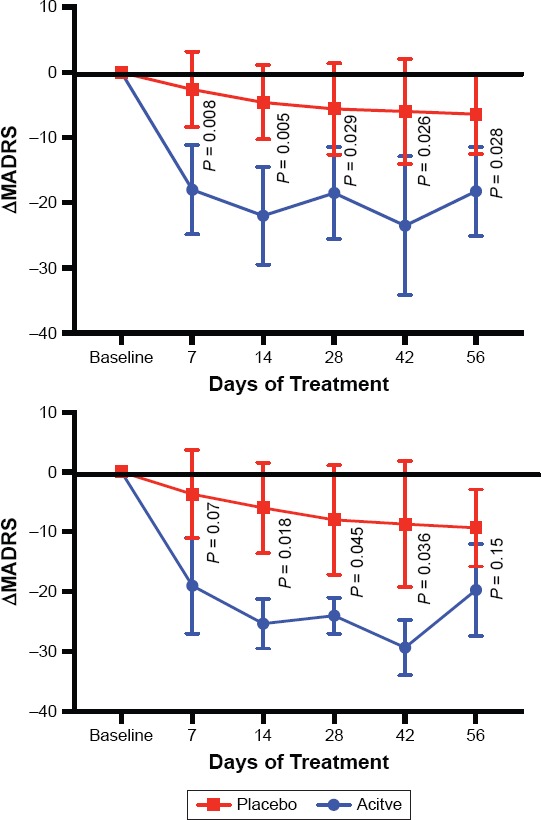
The Mean Change from Baseline in MADRS Total Scores Throughout the Study
Top is the entire sample, bottom is the 6 individuals who have the short form of the serotonin transporter. Graphed numbers are means +/− standard deviation.
Table 1. The Genotype of the 5HTTR Gene (SLC6A4) in Whom Genomic Testing was Completed.
| Asenapine Treated | Placebo Treated | |||||||
| Patient ID | Short Form | Long Form | Short Form | Long Form | ||||
| 2002 | LA/LA | |||||||
| 2004 | S/LA | |||||||
| 2007 | LA/LG | |||||||
| 2008 | LA/LA | |||||||
| 2009 | S/LA | |||||||
| 2010 | LA/LA | |||||||
| 2011 | S/LA | |||||||
| 2013 | LA/LG | |||||||
| 2014 | S/S | |||||||
(Note the missing numbers represent either screen failures [n = 3] or did not undergo genetic testing, [n = 1].) S and LG are the genotypes associated with lower expression of the serotonin transporter protein.
The change in the Clinical Global Impression for Depression (CGI-D) was significantly greater for asenapine-treated subjects (−2.8 ± 0.5) versus placebo (−0.2 ± 0.37; t = 3.75, P = 0.006). When only subjects with the short form were examined, the CGI-D at end of study was not significant (−2.0 ± 1.0 vs. −0.67 ± 0.58; t = 2.0, P = 0.12, Power = 0.8) (Figure 1). The overall effect size of the asenapine treatment as measured by the MADRS was 1.82, which means that the difference between the means is nearly 2 standard deviations. For the subjects with the short form, the effect size was 0.63.
Effect on Anxiety
Anxiety also improved significantly in the asenapine-treated subjects compared to placebo-treatment on day 14 (−19.40 ± 9.84 vs. −4.40 ± 5.23; t = 3.011, P = 0.0168), day 28 (−19.00 ± 3.32 vs. −4.60 ± 7.44; t = 3.954, P = 0.0042), day 42 (−16.80 ± 5.76 vs. −4.00 ± 5.70; t = 3.531, P = 0.0077) and endpoint day 56 (−15.40 ± 6.15 vs. −2.80 ± 7.95; t = 2.803, P = 0.0231) (Figure 2). The overall effect size of the asenapine treatment was 1.9.
Figure 2.
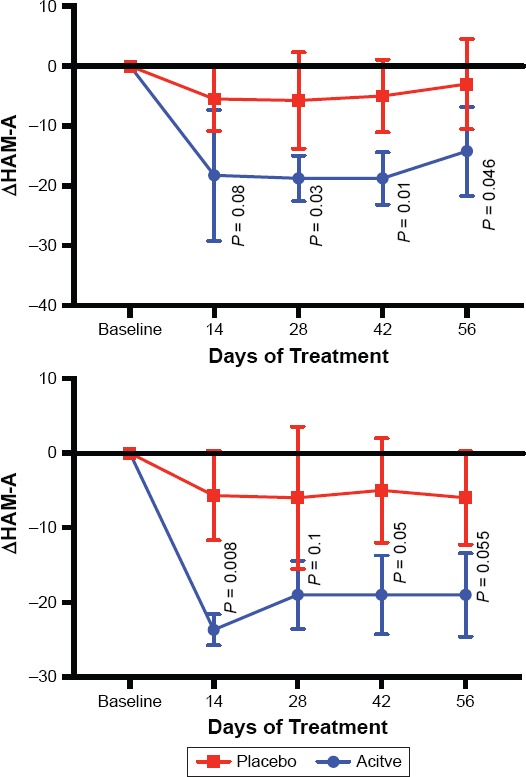
The Mean Change from Baseline in HAM-A Total Scores Throughout the Study
Top is the entire sample, bottom is the 6 individuals who have the short form of the serotonin transporter. Graphed numbers are means +/− standard deviation.
For the 6 subjects who had the short form and sufficient data, the change in anxiety was −19.0 ± 5.57 for asenapine-treated individuals, compared to −6.0 ± 6.25 for placebo-treated subjects (Figure 2). P was equal to 0.055 (t = 2.69, Power = 0.77). The effect size was less than half of the entire sample at 0.74.
As with the analysis of the depressive symptoms, many of the ongoing evaluations throughout the study are significant (Figure 2), so it is important to note that the study was not intended to examine those points, and the significance would vanish if we corrected for multiple t-tests.
Safety
Effect on Mania
Manic symptoms did not significantly change from the low baseline (asenapine group and placebo group were 8.60 ± 2.41 and 5.00 ± 4.53, respectively) on day 7 (0.200 ± 6.870 vs. −1.000 ± 2.828; P = 0.7273), day 14 (−4.400 ± 3.507 vs. −3.400 ± 5.128; P = 0.7282), day 28 (−2.800 ± 7.918 vs. −2.400 ± 4.159; P = 0.92), day 42 (−5.600 ± 4.037 vs. −2.200 ± 3.962; P = 0.2158) or endpoint day 56 (−4.600 ± 3.715 vs. −1.800 ± 5.167; P = 0.35).
Effect on Suicide
Suicidal ideation as measured by the C-SSRS was not significant at any point in the study. There were no discontinuations due to suicidal ideation, no suicide attempts, and no self-injury by anyone at any point in the study. C-SSRS suicide risk scores were low at baseline (Placebo 1.2, asenapine 0.2; ns), and changed minimally at study end (0 ± 0.3 vs. −0.6 ± 0.6; ns).
Side Effects
All patients experienced at least one adverse event (AE). Patients receiving asenapine experienced dysgusia (60%), gastrointestinal (GI) upset (60%), weight gain (40%), pain of some sort (40%), and increased cholesterol (20%). Patients receiving placebo experienced dysgusia (40%), GI upset (40%), fatigue (40%), akathisia (20%), and increased triglycerides (20%). There did not appear to be a difference in the incidence of AEs in patients with or without the short form (mean ± SD).
Genomic Testing
Three subjects receiving active medication, and three subjects receiving placebo were found to have a short form of 5HTTR. Table 1 lists the genotype of the patients in whom genomic testing was completed.
Discussion
Asenapine has been approved in the United States and the European Union for the treatment of acute mania with or without mixed features in bipolar I disordered patients and specifically, it reduces depressive symptoms in these manic states.1,38,39 In this study, we attempted to preliminarily determine whether the genetic variant of the short form of the 5HTTR might predict response. The larger study was performed at two sites, but gene testing was done in only one site. The results of the entire sample will be presented elsewhere, in this report we focus on the subset of patients in whom genetic testing was completed. Unfortunately, the study was terminated early by the sponsor for non-safety related reasons, significantly limiting sample size.
Despite the very small sample, the results across genotype were highly significant for both depressive (Figure 1) and anxiety symptoms (Figure 2). However, when only the subjects with the short form of the 5HTTR gene (SLC6A4) are included in the analysis, the results do not reach statistical significance at end point for either depression (Figure 1) or anxiety (Figure 2). The effect size for the 6 subjects with the short form was around half of the effect size for the entire sample. For the subjects with just the short form, the power for depression was moderate (0.44), but high for anxiety (0.77). This subanalysis does not support the hypothesis that individuals with depression and the short form of 5HTTR may respond better to serotoninergic blockade. However, there is a notable difference in the power of the sample between anxiety and depression (0.77 vs. 0.44, respectively), and power increases with reduced variance. This may be indirect evidence that subjects with the short form of 5HTTR and anxiety may preferentially benefit from post-synaptic 5HT blockade. Larger studies will be needed to confirm this interpretation, and it may be more reasonable to focus on anxiety.
The safety profile of asenapine in the current study does not appear to depart from previous placebo-controlled reports.38,39 Additionally, we did not note any difference in the prevalence of AEs in asenapine-treated patients with or without the short form. However, this final point is tentative given the small number of subjects with the long form of the 5HTTR gene.
There are significant limitations to this study, most prominently the small sample size. Additionally, the asymmetrical distribution of the short form of 5HTTR (74% with the short form) in our sample further limited testing of the effect of the short form in predicting outcome. Furthermore, this exploratory study suggests that response of anxiety to asenapine may be better predicted by the short form of 5HTTR, but subjects were recruited for presence of depression, not anxiety. Despite these limitations, these data at least suggest that the short form of the 5HTTR may not be driving positive effects of asenapine on depression and anxiety.
Conclusions
These results provide provisional evidence that asenapine may be effective in treating bipolar depression and anxiety, but suggest that these findings may not be related to the short form of the 5HTTR. There is indirect evidence that the short form may be a better predictor of response to anxiety. Future studies examining the effects of genetic variation in bipolar depression are indicated.
References
- 1.Fourth. Washington, DC: American Psychiatric Association; 2000. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Text Revision. [Google Scholar]
- 2.Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 3.Altshuler LL, Kupka RW, Hellemann G, Frye MA, Sugar CA, McElroy SL, Nolen WA, Grunze H, Leverich GS, Keck PE, Zermeno M, Post RM, Suppes T. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation bipolar treatment outcome network. Am J Psychiatry. 2010;167(6):708–715. doi: 10.1176/appi.ajp.2009.09010105. doi: [DOI] [PubMed] [Google Scholar]
- 4.Simon GE, Bauer MS, Ludman EJ, Operskalski BH, Unutzer J. Mood symptoms, functional impairment, and disability in people with bipolar disorder: specific effects of mania and depression. J Clin Psychiatry. 2007;68(8):1237–1245. doi: 10.4088/jcp.v68n0811. [DOI] [PubMed] [Google Scholar]
- 5.Baldessarini RJ, Vieta E, Calabrese JR, Tohen M, Bowden CL. Bipolar depression: overview and commentary. Harv Rev Psychiatry. 2010;18(3):143–157. doi: 10.3109/10673221003747955. [DOI] [PubMed] [Google Scholar]
- 6.El-Mallakh RS, Karippot A. Use of antidepressants to treat depression in bipolar disorder. Psychiatr Serv. 2002;53(5):580–584. doi: 10.1176/appi.ps.53.5.580. [DOI] [PubMed] [Google Scholar]
- 7.El-Mallakh RS, Karippot A. Chronic depression in bipolar disorder. Am J Psychiatry. 2006;163(8):1337–1341. doi: 10.1176/ajp.2006.163.8.1337. quiz 1478. [DOI] [PubMed] [Google Scholar]
- 8.Citrome L. Asenapine review, part I: Chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10(6):893–903. doi: 10.1517/17425255.2014.908185. doi: [DOI] [PubMed] [Google Scholar]
- 9.Reynolds GP. Receptor mechanisms of antipsychotic drug action in bipolar disorder – focus on asenapine. Ther Adv Psychopharmacol. 2011;1(6):197–204. doi: 10.1177/2045125311430112. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinoshita T, Bai YM, Kim JH, Miyake M, Oshima N. Efficacy and safety of asenapine in Asian patients with an acute exacerbation of schizophrenia: a multicentre, randomized, double-blind, 6-week, placebo-controlled study. Psychopharmacology (Berl) 2016;233(14):2663–2674. doi: 10.1007/s00213-016-4295-9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szegedi A, Durgam S, Mackle M, Yu SY, Wu X, Mathews M, Landbloom RP. Randomized, Double-Blind, Placebo-Controlled Trial of Asenapine Maintenance Therapy in Adults with an Acute Manic or Mixed Episode Associated with Bipolar I Disorder. Am J Psychiatry. 2018;175(1):71–79. doi: 10.1176/appi.ajp.2017.16040419. doi: [DOI] [PubMed] [Google Scholar]
- 12.Partyka A, Jastrzębska-Więsek M, Antkiewicz-Michaluk L, Michaluk J, Wąsik A, Canale V, Zajdel P, Kołaczkowski M, Wesołowska A. Novel antagonists of 5-HT6 and/or 5-HT7 receptors affect the brain monoamines metabolism and enhance the anti-immobility activity of different antidepressants in rats. Behav Brain Res. 2018;359:9–16. doi: 10.1016/j.bbr.2018.10.004. doi: [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Marcinkiewcz CA, Dorrier CE, Lopez AJ, Kash TL. Ethanol induced adaptations in 5-HT2c receptor signaling in the bed nucleus of the stria terminalis: implications for anxiety during ethanol withdrawal. Neuropharmacology. 2015;89:157–167. doi: 10.1016/j.neuropharm.2014.09.003. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuznetsova EG, Amstislavskaya TG, Bulygina VV, Tibeikina MA, Popova NK. Effect of 5HT2C-receptor agonist MK-212 on blood corticosterone level and behavior in mice. Bull Exp Biol Med. 2006;142(5):594–597. doi: 10.1007/s10517-006-0427-2. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura A, Aritomi Y, Sasai K, Kitagawa T, Mahableshwarkar AR. Randomized, double-blind, placebo-controlled 8-week trial of the efficacy, safety, and tolerability of 5, 10, and 20mg/day vortioxetine in adults with major depressive disorder. Psychiatry Clin Neurosci. 2018;72(2):64–72. doi: 10.1111/pcn.12565. doi: [DOI] [PubMed] [Google Scholar]
- 16.Tegin C, Tegin G, El-Mallakh RS. Effective Vortioxetine Dose Varies with Extent of Antidepressant Use Across Countries. Psychopharmnacol Bull. 2018;48(1):26–39. [PMC free article] [PubMed] [Google Scholar]
- 17.Alvarez E, Perez V, Dragheim M, Loft H, Artigas F. A double-blind, randomized, placebo-controlled, active reference study of Lu AA21004 in patients with major depressive disorder. Int J Neuropsychopharmacol. 2012;15(5):589–600. doi: 10.1017/S1461145711001027. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suppes T, Kroger H, Pikalov A, Loebel A. Lurasidone adjunctive with lithium or valproate for bipolar depression: A placebo-controlled trial utilizing prospective and retrospective enrolment cohorts. J Psychiatr Res. 2016;78:86–93. doi: 10.1016/j.jpsychires.2016.03.012. doi: [DOI] [PubMed] [Google Scholar]
- 19.Szegedi A, Zhao J, van Willigenburg A, Nations KR, Mackle M, Panagides J. Effects of asenapine on depressive symptoms in patients with bipolar I disorder experiencing acute manic or mixed episodes: a post hoc analysis of two 3-week clinical trials. BMC Psychiatry. 2011;11:101–117. doi: 10.1186/1471-244X-11-101. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berk M, Tiller JW, Zhao J, Yatham LN, Malhi GS, Weiller E. Effects of Asenapine in Bipolar I Patients Meeting Proxy Criteria for Moderate-to-Severe Mixed Major Depressive Episodes: A Post Hoc Analysis. J Clin Psychiatry. 2015;76(6):728–734. doi: 10.4088/JCP.13m08827. [DOI] [PubMed] [Google Scholar]
- 21.Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry. 2010;167(5):509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luddington NS, Mandadapu A, Husk M, El-Mallakh RS. Clinical implications of genetic variation in the serotonin transporter promoter region: a review. Prim Care Companion J Clin Psychiatry. 2009;11(3):93–102. doi: 10.4088/pcc.08r00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78(5):815–826. doi: 10.1086/50385. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 25.Hammen C, Brennan PA, Keenan-Miller D, Hazel NA, Najman JM. Chronic and acute stress, gender, and serotonin transporter gene-environment interactions predicting depression symptoms in youth. J Child Psychol Psychiatry. 2010;51(2):180–187. doi: 10.1111/j.1469-7610.2009.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MH, Sanders E, Howe M, Singh M, Hallmayer J, Kim E, Chang K. Association of Anxiety Symptoms in Offspring of Bipolar Parents with Serotonin Transporter-Linked Polymorphic Region (5-HTTLPR) Genotype. J Child Adolesc Psychopharmacol. 2015;25(6):458–466. doi: 10.1089/cap.2014.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li D, He L. Meta-analysis supports association between serotonin transporter (5-HTT) and suicidal behavior. Mol Psychiatry. 2007;12(1):47–54. doi: 10.1038/sj.mp.4001890. [DOI] [PubMed] [Google Scholar]
- 29.El-Mallakh RS, Ghaemi SN, Sagduyu K, Thase ME, Wisniewski SR, Nierenberg AA, Zhang HW, Pardo TA, Sachs G. Antidepressant-Associated Chronic Irritable Dysphoria (ACID) in STEP-BD Patients. J Affect Disord. 2008;111(2–3):372–377. doi: 10.1016/j.jad.2008.03.025. for the STEP-BD Investigators. [DOI] [PubMed] [Google Scholar]
- 30.Ghaemi SN, Ostacher MM, El-Mallakh RS, Borrelli D, Baldassano CF, Kelley ME, Filkowski MM, Hennen J, Sachs G, Goodwin FK, Baldessarini RJ. Antidepressant discontinuation in bipolar depression: a Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry. 2010;71(4):372–380. doi: 10.4088/JCP.08m04909gre. [DOI] [PubMed] [Google Scholar]
- 31.El-Mallakh RS, Roberts RJ, El-Mallakh PL, Findlay LJ, Reynolds KK. Pharmacogenomics in Psychiatric Practice. Clin Lab Med. 2016;36(3):507–523. doi: 10.1016/j.cll.2016.05.00. doi: [DOI] [PubMed] [Google Scholar]
- 32.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 33.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 34.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 35.Hamilton M. The Assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 36.Spearing MK, Post RM, Leverich GS, Brandt D, NBolen W. Modification of the Clinical Global Impressions (CGI) scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73(3):159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 37.Madan A, Frueh BC, Allen JG, Ellis TE, Rufino KA, Oldham JM, Fowler JC. Psychometric Reevaluation of the Columbia-Suicide Severity Rating Scale: Findings from a Prospective, Inpatient Cohort of Severely Mentally Ill Adults. J Clin Psychiatry. 2016;77(7):e867–e873. doi: 10.4088/JCP.15m10069. doi: [DOI] [PubMed] [Google Scholar]
- 38.Kinoshita T, Bai YM, Kim JH, Miyake M, Oshima N. Efficacy and safety of asenapine in Asian patients with an acute exacerbation of schizophrenia: a multicentre, randomized, double-blind, 6-week, placebo-controlled study. Psychopharmacology (Berl) 2016;233(14):2663–2674. doi: 10.1007/s00213-016-4295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szegedi A, Durgam S, Mackle M, Yu SY, Wu X, Mathews M, Landbloom RP. Randomized, Double-Blind, Placebo-Controlled Trial of Asenapine Maintenance Therapy in Adults with an Acute Manic or Mixed Episode Associated with Bipolar I Disorder. Am J Psychiatry. 2018;175(1):71–79. doi: 10.1176/appi.ajp.2017.16040419. [DOI] [PubMed] [Google Scholar]


