Abstract
Objective:
An important step in the development of new drugs for the treatment of bipolar disorder (BD) is the study of the extent to which novel lithium salts whose anionic component has an antioxidant effect can reduce oxidative DNA damage in human blood plasma in vitro. We investigated the effects of lithium salts containing different organic anionic components (lithium carbonate (Li-CAR), pyruvate (Li-PYR), succinate (Li-SUC), fumarate (Li-FUM) and ascorbate (Li-ASC)) on levels of the oxidative damage product of DNA—8-hydroxy-2’-deoxyguanosine (8-OH-dG) in blood plasma after incubation of blood samples from healthy individuals (healthy group) and patients with bipolar disorder (BD-group) with these chemical compounds.
Methods:
Blood incubation was carried out in the presence of lithium salts (1.2 mM) for 1 hour at 37°C. Measurement of 8-OH-dG concentrations in blood plasma was carried out by enzyme immunoassay using a DNA Damage Competitive Elisa Kit (Thermo Fisher Scientific, USA).
Results:
In samples without compounds (control), concentrations of 8-OH-dG in the BD-group did not differ from the group of healthy individuals. None of the tested compounds had a significant effect on 8-OH-dG in healthy individuals. In BD patients, Li-PYR significantly reduced levels of plasma 8-OH-dG, while other compounds did not have a noticeable effect.
Conclusion:
Lithium pyruvate reduces oxidative DNA damage in the blood of BD patients in vitro, demonstrating the potential of this compound to function not only as a mood stabilizer, but also as an antioxidant and cytoprotector.
Keywords: 8-hydroxy-2’-deoxyguanosine (8-OH-dG), blood plasma, lithium, pyruvate, bipolar disorder
Introduction
Bipolar disorder (BD) is one of the most common diseases in the world, especially in developed countries. Despite continuing scientific efforts, treatments available for BD are still problematic in terms of their efficacy and side effects.1 The drugs based on lithium salts (such as lithium carbonate) are widely used for BD treatment as mood stabilizers.2 However, the known toxic effects of lithium represent a significant problem. It is established that the degree of lithium accumulation and its toxicity vary depending of the dose, route and duration of its administration, as well as the anionic component of lithium salts. Selection of the anionic component in lithium preparations is important not only for reducing their toxicity but also for enhancing the positive therapeutic effect due to appearance of new pharmaceutic properties.
Given the important role of oxidative stress in the development of the pathological process,3 it makes sense to use an anionic component with an antioxidant effect. Lithium salts containing Krebs-cycle substrates as an anionic component have been shown to have high antioxidant potential using a voltammetric method in an artificial model system.4 It is important to test these promising new salts in a biological system; for example, to evaluate their effects on markers of oxidative damage of blood plasma biomacromolecules.
The studies of oxidative stress and markers of oxidative damage of biomacromolecules-proteins, lipids and DNA—has been carried out in various pathologies, including BD.3,5,6 We have previously evaluated the effects of organic lithium salts (Li-CAR, Li-PYR, Li-SUC, Li-FUM and Li-ASC) on the oxidative damage of proteins (marked by carbonylated proteins) and lipids (marked by lipid peroxidation products) in blood plasma samples from healthy individuals and individuals with alcohol use disorder.7 8-hydroxy-2-deoxyguanosine (8-OH-dG) is one of the most widely recognized biomarkers of oxidative DNA damage.8–10 Increases in blood concentration of 8-OH-dG occur against the background of oxidative stress as a result of cell death in body tissues due to apoptosis and/or necrosis, or from viruses, bacteria or blood cells (lymphocytes).10–12 Elevated levels of 8-OH-dG have been found in cardiovascular diseases,13 alcoholism,14 affective disorders,9,15 and other pathologies.
In this study, the effect of organic lithium salts (Li-CAR, Li-PYR, Li-SUC, Li-FUM and Li-ASC) on concentrations of 8-OH-dG in blood plasma was studied after incubation of blood samples from healthy individuals and BD patients with salts in vitro.
Materials and Methods
Blood samples from 20 healthy individuals (Healthy group), average age 34.5 (26.5–49.5) years, and 19 patients with bipolar disorder (BD group), average age 38.0 (22.0–51.0) years were used. The patients were treated in the clinic of the Mental Health Research Institute, Tomsk National Research Medical Center. They did not present with any active medical conditions and were not on any medications, apart from those prescribed for their psychiatric disorder. Diagnosis of bipolar disorder in all patients was carried out according to ICD-10, qualified as bipolar affective disorder (F31). Members of Healthy group were healthy individuals without chronic somatic diseases.
The study was conducted in compliance with all the principles of informed consent of the Helsinki Declaration of the World Medical Association and was approved by the local ethics committee of the Mental Health Research Institute TNRM (Protocol No 361 of 2017.10.23). All subjects provided informed consent before entering into the study.
Blood was taken from the ulnar vein in the morning on an empty stomach, using a sterile system of single application Vacutainer (“Becton Dickinson”, USA) with the anticoagulant sodium heparin. Li-CAR was from Sigma-Aldrich (Germany). Other lithium salts were synthesized for this experiment in the Research school of chemical and biomedical technologies of National Research Tomsk Polytechnic University as following. A calculated amount of the corresponding acid was dissolved in deionized water with moderate heating to 40°C and stirring. A stoichiometric amount of lithium carbonate was introduced into the solution with stirring; the course of the reaction was controlled by a change in pH. The resulting solution was cooled. The reaction product was filtered and isolated in crystalline form by the addition of ethanol. The crystalline precipitate was washed and dried during 24 hours, then elemental analysis was performed and the authenticity of the substances was checked. All compounds were dissolved in saline solution to conduct cell tests (sodium chloride 0.9%, JSC “ESKOM”, Russia). To assess the effects of the studied compounds on plasma concentrations of 8-OH-dG, fresh blood samples were incubated with different lithium salts for 1 hour at 37°C as described in.7 After incubation, plasma aliquots were obtained, then frozen and stored at −80°C until use. The final concentration of lithium compounds in the samples was 1.2 mM of lithium ions per liter. This concentration corresponded to a maximal therapeutic blood concentration of lithium in patients receiving treatment for affective disorders.16 The concentration of 8-OH-dG in thawed plasma aliquots was measured by an enzyme immunoassay protocol, using a DNA Damage Competitive Elisa Kit (# 19DD039A, Thermo Fisher Scientific, USA). Measurement of optical density of samples and calculation of concentrations of 8-OH-dG were carried out on an Epoch device (Biotech, USA).
Statistical analyses of the results were carried out using the program “Statistica-12” (TIBCO Inc, USA). To check for agreement with the normal distribution of quantitative indicators, the Shapiro-Wilk criterion was used. The data were represented as median and quartile range (QL–QU). The nonparametric Mann-Whitney test (U test) was used to assess group differences. Differences were considered to be statistically significant at p < 0.01.
Results and Discussion
Blood samples from healthy individuals and BD patients were incubated with different lithium salts. After incubation, plasma was separated from each blood sample and used for measurement of oxidative modification of DNA. Results are presented in the Table 1.
Table 1. The Effect of Lithium Salts (1.2 mM) on Blood Plasma 8-OH-dG Concentrations (ng/ml) of Healthy Persons and Bipolar Disorder Patients (BD) After Incubation with Blood Samples for 1 Hour (37°C), Me (QL–QU).
| SAMPLES (LITHIUM SALTS) |
HEALTHY N = 20 |
BD N = 19 |
||
| Control | 10.43 (8.57–13.30) | 10.70 (8.44–14.60) | ||
| Li-SUC | 10.46 (7.53–12.06) | 10.01 (6.95–11.94) | ||
| Li-ASC | 11.16 (9.06–13.25) | 14.32 (9.19–16.65) | ||
| Li-FUM | 9,95 (8,37–13,95) | 8,87 (4,75–13,84) | ||
| Li-PYR | 10.22 (8.10–12.61)* | 5,76 (3.19–8.91)*# | ||
| Li-CAR | 12.23 (8.37-14.23) | 9.52 (8.09-12.50) |
Notes: *(p < 0.01) indicates significant differences between groups (Healthy vs. BD); #(p < 0.01) indicates significant differences from controls within the group.
8-OH-dG blood plasma concentrations of healthy persons in all samples with the investigated compounds did not differ significantly from the control samples. That is, none of the lithium salts tested under the experimental conditions described above had a significant effect on 8-OH-dG plasma concentrations of healthy individuals. In BD patients, a statistically significant decrease in 8-OH-dG blood plasma concentrations was observed in the presence of Li-PYR as compared to the control samples that were not exposed to salt (p < 0.01). In addition, there was a significant difference between the Healthy and BD groups for samples exposed to Li-PYR salt (p < 0.01). (Fig. 1).
Figure 1.
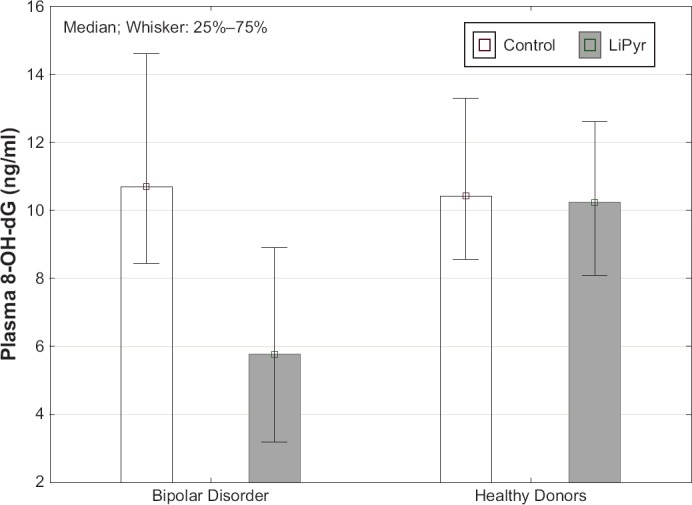
The Effect of Lithium Pyruvate (1.2 mM) on Blood Plasma 8-OH-dG Concentrations of Healthy Individuals and Bipolar Disorder Patients After Blood Incubation
Blood plasma 8-hydroxy-2’-deoxyguanosine (8-OH-dG) concentrations in ng/ml are presented as Me (QL–QU). Control (white columns) – results of measuring 8-OH-dG without lithium salts; LiPyr (grey columns) – results of measuring 8-OH-dG with Li-PYR. LiPyr – lithium pyruvate.
Other lithium compounds did not have a noticeable effect on 8-OH-dG blood plasma concentrations of BD patients (Table 1).
According to recent understanding of the role of oxidative stress in BD, oxidative stress can play a role as a nonspecific factor in the pathogenesis of affective disorders.5,8 Levels of oxidative stress in the body is often estimated by measuring products of oxidative modification in blood plasma.17 Our previous studies have shown elevated levels of oxidized (carbonylated) proteins in patients with depressive disorders. We have also demonstrated that there is an increase in the concentrations of oxidized proteins7 and oxidative DNA damage products in the blood plasma of alcoholic patients.6
However, even in the presence of oxidative stress in the body, plasma concentrations of some recognized markers of oxidative damage of biomacromolecules, in particular of DNA, can remain at normal levels, as our studies have shown. From the data presented in the table and in the figure, it can be seen that the 8-OH-dG concentration control samples of BD patients does not differ from healthy individuals. Ambiguous results of the search for an association of serum 8-OH-dG levels with depressive disorders have also been reported in the work of other authors,3 which presents the results of a meta-analysis of studies seeking to link depression with this marker of oxidative stress and analyzes the factors contributing to this ambiguity.
At the same time, some studies have found a significant increase in 8-OH-dG in BD.8,9,15 In addition, DNA damage in BD patients has been shown using the single cell gel electrophoresis comet assay.18
Higher levels of DNA oxidative damage in bipolar disorder patients could partly be explained by immune-inflammatory activation, with increased levels of cytokines IL-6, sTNFR2, IL-18, and consequent ROS activation.19 These processes could occur contaminantly with a defective DNA repair mechanism in bipolar patients. On the other hand, the higher levels of DNA oxidative damage seen in blood samples from bipolar disorder patients may reflect the failure of nonspecific and enzymatic antioxidant systems in BD. A similar depletion of antioxidant blood systems has been identified previously in alcoholism.20 However, lithium ions could inhibit pro-inflammatory pathways via inhibition of cyclooxygenase-2 (COX-2) and decrease of prostaglandins production.21,22
In the present study, a significant decrease in the concentration of 8-OH-dG was found in patients with BD in samples with Li-PYR compared to healthy individuals with a level of significance *p = 0.003, while other lithium salts did not show a noticeable effect (Table 1, Fig. 1).
The observed reduction in the product of oxidative DNA modification in the presence of Li-PYR may be due to the antioxidant and cytoprotective properties of this salt, which were detected on mononuclear cells. Lithium pyruvate significantly reduces apoptotic cell death due to oxidative damage in blood cells obtained from healthy volunteers and alcoholics.23 Notably, lithium itself can inhibit oxidative stress.24 Possible mechanisms of the antioxidant effect of lithium is to stimulate the expression of the transcription factor Nrf2. This protein activated by oxidative stress and pro-inflammatory stimuli.25 Opposite of that, lithium salts have a prooxidant effect. It has been shown, for example, that inorganic lithium chloride (LiCl) causes a significant increase in reactive oxygen species due to the activation of the PtkA-dependent pathway. It also stimulates NADPH oxidase (NOX) activity and mRNA expression of the NOX oxidase family under certain conditions.26 In general, clinical application of lithium is accompanied by a fear of side effects, making novel lithium salts with cytoprotective effects a highly attractive prospective treatment.
It should be noted that in the study of the effects of lithium salts on the oxidative damage of plasma proteins and lipids in healthy individuals and alcoholic patients, Li-PYR did not have a noticeable protective effect.7 That is, Li-PYR in vitro can differently affect the plasma biomacromolecules of healthy persons and patients with different pathologies. In the present study, we did not detect the effect of Li-PYR on the 8-OH-dG level in group of healthy individuals. The pronounced effect of Li-PYR reducing concentrations of 8-OH-dG was observed only in group BD (group of BD patients). Future studies should address the mechanistic basis for such differential sensitivity to Li-PYR.
Lithium pyruvate’s newly revealed effect of reducing 8-OH-dG in blood samples from BD patients substantiates the possibility of using this salt in the treatment of this pathology. Noteworthy, the number of manic episodes experienced by BD patients increases with higher levels of 8-OH-dG, suggesting that oxidative damage to 8-OHdG might be a potential marker of disease progression.27
The protective effect of lithium pyruvate on blood cell DNA can be associated with a general cytoprotective effect,23 making lithium pyruvate an attractive prospect as a novel treatment for bipolar patients. Lithium pyruvate could potentially act not only as a cytoprotective agent via GSK 3β inhibition,28 but also as a highly effective antioxidant. Attenuated effects of pyruvate on 8-OHdG plasma levels have been associated with a decrease in ability to repair damage to DNA bases in bipolar patients.29,30
Conclusion
Cytoprotective and antioxidative properties of pyruvate may become especially significant in the case of bipolar disorder, where DNA oxidation is correlated with manic episodes. Lithium pyruvate may act not only as a mood stabilizer; it may also reduce oxidative damage to DNA. The results of this study suggest that lithium pyruvate could prove to be a more effective treatment than lithium carbonate, which is in standard use, due to its antioxidative effects. Further study of this compound as a potential treatment for bipolar disorder is warranted.
Acknowledgments
This study was supported by the Russian Science Foundation, project No. 17-75-20045. This study was also partially supported by the Institute of Mental Health (AAAA-A19-119020690013-2).
The authors thank Dr. Alena Savonenko, Associate Professor in the Pathology Department of the Johns Hopkins University School of Medicine and Dr. Amy Huberman, Instructor in the Psychiatry Department of the Johns Hopkins University School of Medicine, Baltimore, MD, USA, for scientific and language editing.
Footnotes
Conflict of Interest
The authors declare no conflict of interests.
Author Contributions
All authors took part in experiments with blood samples. The design of experiments and preparation of tested lithium salts were done by Dr. Prokopieva, Prof. Bokhan and Dr. Plotnikov. Dr. Yarygina conducted experiments with blood plasma samples. Dr. Plotnikov conducted calculations of the results and statistical analyses. Writing the manuscript was completed by Dr. Prokopieva. All authors drafted the manuscript and interpreted results.
References
- 1.Bobo WV. The Diagnosis and Management of Bipolar I and II Disorders: Clinical Practice Update. Mayo Clinic Proceedings. 2017;92(10):1532–1551. doi: 10.1016/j.mayocp.2017.06.022. doi: [DOI] [PubMed] [Google Scholar]
- 2.Burgess SSA, Geddes J, Hawton KKE, Taylor MJ, Townsend E, Jamison K, Goodwin G. Lithium for maintenance treatment of mood disorders/Cochrane. [Retrieved 9 March 2016];Cochrane Database of Systematic Reviews. 2001 doi: 10.1002/14651858.CD003013. doi: [DOI] [Google Scholar]
- 3.Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology. 2015;51:164–175. doi: 10.1016/j.psyneuen.2014.09.025. doi: [DOI] [PubMed] [Google Scholar]
- 4.Plotnikov E, Voronova O, Linert W, Martemianov D, Korotkova E, Dorozhko E, Astashkina A, Martemianova I, Ivanova S, Bokhan N. Antioxidant and Immunotropic Properties of some Lithium Salts. J App Pharm Sci. 2016;6(1):086–089. doi: 10.7324/JAPS.2016.600115. [DOI] [Google Scholar]
- 5.Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro) degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676–692. doi: 10.1016/j.pnpbp.2010.05.004. doi: [DOI] [PubMed] [Google Scholar]
- 6.Prokopieva VD, Yarygina EG, Plotnikov EV, Vetlugina TP. Study of the effects of lithium salts in the presence of ethanol on the blood plasma product of oxidative DNA damage of healthy persons and alcoholic patients. Sibirskii Vestnik Psikhiatrii I Narkologii. 2019;1(102):5–11. doi: 10.26617/1810-3111-2019-1(102)-5-11. In Russ. [DOI] [Google Scholar]
- 7.Prokopieva VD, Plotnikov EV, Yarygina EG, Bokhan NA. The protective action of carnosine and organic lithium salts against ethanol-induced oxidative damage of blood plasma proteins and lipids of healthy persons and alcoholic patients. Biochemistry (Moscow) Supplement Series B Biomedical Chemistry. 2019;13(2):162–166. doi: 10.1134/S1990750819020082. doi: [DOI] [Google Scholar]
- 8.Brown N, Andreazza A, Young L. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014;218:61–68. doi: 10.1016/j.psychres.2014.04.005. doi: [DOI] [PubMed] [Google Scholar]
- 9.Forlenza MJ, Miller GE. Increased serum levels of 8-hydroxy-2’-deoxyguanosine in clinical depression. Psychosom Med. 2006;68(1):1–7. doi: 10.1097/01.psy.0000195780.37277.2a. [DOI] [PubMed] [Google Scholar]
- 10.Chernikov AV, Gudkov SV, Usacheva AM, Bruskov VI. Exogenous 8-Oxo-7,8-dihydro-2′-deoxyguanosine: Biomedical Properties, Mechanisms of Action, and Therapeutic Potential. Biochemistry (Moscow) 2017;82(13):1686–1701. doi: 10.1134/S0006297917130089. © Pleiades Publishing, Ltd., 2017. Original Russian Text © Chernikov AV, Gudkov SV, Usacheva AM, Bruskov VI. published in Uspekhi Biologicheskoi Khimii. 2017;57:267–302. doi: 10.1134/S0006297917130089. [DOI] [PubMed] [Google Scholar]
- 11.Vasil’eva IN, Podgornaya OI, Bespalov VG. Nucleosomal fraction of extracellular DNA as a marker of apoptosis. Tsitologiya. 2015;57:87–94. In Russ. PMID: 26. [PubMed] [Google Scholar]
- 12.Hirata J, Ohya M, Okuno T, Kumon K. Increased levels of circulating 8-oxodeoxyguanosine as a biomarker of sepsis severity in mice. J Intensive Care. 2014;2 doi: 10.1186/2052-0492-2-41. article 41. doi: [DOI] [Google Scholar]
- 13.Di Minno A, Turnu L, Porro B, Squellerio I, Cavalca V, Tremoli E, Di Minno MND. 8-Hydroxy-2-Deoxyguanosine Levels and Cardiovascular Disease: A Systematic Review and Meta-Analysis of the Literature. Antioxidants & Redox Signaling. 2016;24(10):548–555. doi: 10.1089/ars.2015.6508. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen CH, Pan CH, Chen CC, Huang MC. Increase oxidative DNA damage in patients with alcohol dependence and its correlation with alcohol withdrawal severety. Alcohol Clin Exp Res. 2011;35(2)(344):338. doi: 10.1111/j.1530-0277.2010.01349. doi: [DOI] [PubMed] [Google Scholar]
- 15.Maes M, Mihaylova I, Kubera M, Uytterhoeven M, Vrydags N, Bosmans E. Increased 8-hydroxy-deoxyguanosine, a marker of oxidative damage to DNA, in major depression and myalgic encephalomyelitis/chronic fatigue syndrome. Neuro Endocrinol Lett. 2009;30(6):715–722. PMID: 20035260 [Indexed for MEDLINE] [PubMed] [Google Scholar]
- 16.Tondo L, Alda M, Bauer M, Bergink V, Grof P, Hajek T, Lewitka U, Licht RW, Manchia M, Müller-Oerlinghausen B, Nielsen RE, Selo M, Simhandl C, Baldessarini RJ. Clinical use of lithium salts: guide for users and prescribers. International Journal of Bipolar Disorders. 2019;7(1):16. doi: 10.1186/s40345-019-0151-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bohan NA, Prokopieva VD, Ivanova SA, Vetlugina TP, Epimakhova EV, Plotnikov EV, Yarygina EG, Boiko AS. Oxidative stress and its correction in patients with alcohol dependence: results from research at the mental health research institute of the Tomsk national research medical center. Voprosy Narkologii. 2018;3:27–59. In Russ. https://elibrary.ru/item.asp?id=32849844. [Google Scholar]
- 18.Andreazza AC, Frey BN, Erdtmann B, Salvador M, Rombaldi F, Santin A, Gonçalves CA, Kapczinski F. DNA damage in bipolar disorder. Psychiatry Research. 2007;153(1):27–32. doi: 10.1016/j.psychres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 19.Brunoni AR, Supasitthumrong T, Teixeira AL, Vieira EL, Gattaz WF, Benseñor IM, Lotufo PA, Lafer B, Berk M, Carvalho AF, Maes M. Differences in the immune-inflammatory profiles of unipolar and bipolar depression. Journal of Affective Disorders. 2020;262:8–15. doi: 10.1016/j.jad.2019.10.037. [DOI] [PubMed] [Google Scholar]
- 20.Plotnikov E, Korotkova E, Voronova O, Sazhina N, Petrova E, Artamonov A, Chernyavskaya L, Dorozhko E. Comparative investigation of antioxidant activity of human serum blood by amperometric, voltammetric and chemiluminescent methods. Archives of medical science. 2016;12(5):1071–1076. doi: 10.5114/aoms.2015.50234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosetti F, Rintala J, Seemann R, Rosenberger TA, Contreras MA, Rapoport SI, Chang MC. Chronic lithium downregulates cyclooxygenase-2 activity and prostaglandin E2 concentration in rat brain. Molecular Psychiatry. 2002;7(8):845–850. doi: 10.1038/sj.mp.4001111. doi: [DOI] [PubMed] [Google Scholar]
- 22.Basselin M, Villacreses NE, Lee H-J, Bell JM, Rapoport SI. Chronic lithium administration attenuates up-regulated brain arachidonic acid metabolism in a rat model of neuroinflammation. J Neurochem. 2007;102:761–772. doi: 10.1111/j.1471-4159.2007.04593.x. doi: [DOI] [PubMed] [Google Scholar]
- 23.Plotnikov E, Losenkov I, Epimakhova E, Bohan N. Protective Effects of Pyruvic Acid Salt Against Lithium Toxicity and Oxidative Damage in Human Blood Mononuclear Cells. Adv Pharm Bull. 2019;9(2):302–306. doi: 10.15171/apb.2019.035. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan A, Jamwal S, Bijjem KR, Prakash A, Kumar P. Neuroprotective effect of hemeoxygenase-1/glycogen synthase kinase-3β modulators in 3-nitropropionic acid-induced neurotoxicity in rats. Neuroscience. 2015;287:66–77. doi: 10.1016/j.neuroscience.2014.12.018. [DOI] [PubMed] [Google Scholar]
- 25.Castillo-Quan JI, Li L, Kinghorn KJ, Ivanov DK, Tain LS, Slack C, Kerr F, Nespital T, Thornton J, Hardy J, Bjedov I, Partridge L. Lithium Promotes Longevity through GSK3/NRF2-Dependent Hormesis. Cell Rep. 2016;15(3):638–650. doi: 10.1016/j.celrep.2016.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee Y, Kim S-M, Jung E-H, Park J, Lee JW, Han I-O. Lithium chloride promotes lipid accumulation through increased reactive oxygen species generation. Biochim et Biophys Acta-Molecular and Cell Biology of Lipids. 2020;1865(2) doi: 10.1016/j.bbalip.2019.158552. article 158552. [DOI] [PubMed] [Google Scholar]
- 27.Soeiro-De-Souza MG, Andreazza AC, Carvalho AF, Machado-Vieira R, Young LT, Moreno RA. Number of manic episodes is associated with elevated DNA oxidation in bipolar disorder. International Journal of Neuropsychopharmacology. 2013;16(7):1505–1512. doi: 10.1017/S1461145713000047. [DOI] [PubMed] [Google Scholar]
- 28.Won E, Kim Y-K. An oldie but goodie: Lithium in the treatment of bipolar disorder through neuroprotective and neurotrophic mechanisms. International Journal of Molecular Sciences. 2017;18(12) doi: 10.3390/ijms18122679. article 2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ceylan D, Tuna G, Kirkali G, Tunca Z, Can G, Arat HE, Kant M, Dizdaroglu M, Özerdem A. Oxidatively-induced DNA damage and base excision repair in euthymic patients with bipolar disorder. DNA Repair. 2018;65:64–72. doi: 10.1016/j.dnarep.2018.03.006. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceylan D, Yılmaz S, Bora U, GamzeTuna, Ildız A, Akan P, Özerdem A. Increased plasma levels of 8-oxoguanine DNA glycosylase-1 in bipolar disorder. Psychiatry and Clinical Neurosciences. 2019 Oct 04; doi: 10.1111/pcn.12928. | journal-article. doi: [DOI] [PubMed] [Google Scholar]


