Key Points
Question
What is the diagnostic yield of newborn screening for biliary atresia using direct or conjugated bilirubin measurements?
Findings
In this study that involved 124 385 newborns, a 2-stage screening approach based on direct or conjugated bilirubin measurements identified the 7 known infants with biliary atresia with a sensitivity of 100.0% and a specificity of 99.9%, although the 95% CI around the sensitivity was wide and the study design did not ensure complete ascertainment of false-negative results.
Meaning
These findings may help inform decision-making about newborn screening for biliary atresia, although further research is needed from larger populations to obtain more precise estimates of diagnostic yield and to better understand clinical outcomes and cost-effectiveness of this screening approach.
Abstract
Importance
Treating biliary atresia in newborns earlier can delay or prevent the need for liver transplant; however, treatment typically occurs later because biliary atresia is difficult to detect during its early stages.
Objective
To determine the diagnostic yield of newborn screening for biliary atresia with direct or conjugated bilirubin measurements and to evaluate the association of screening implementation with clinical outcomes.
Design, Setting, and Participants
A cross-sectional screening study of 124 385 infants born at 14 Texas hospitals between January 2015 and June 2018; and a pre-post study of 43 infants who underwent the Kasai portoenterostomy as treatment for biliary atresia at the region’s largest pediatric hepatology center before (January 2008-June 2011) or after (January 2015-June 2018) screening implementation. Final follow-up occurred on July 15, 2019.
Exposures
Two-stage screening with direct or conjugated bilirubin measurements. In stage 1, all newborns were tested within the first 60 hours of life, with a positive screening result defined as bilirubin levels exceeding derived 95th percentile reference intervals. In stage 2, infants who had a positive screening result in stage 1 were retested at or before the 2-week well-child visit, with a positive screening result defined as bilirubin levels greater than the stage 1 result or greater than 1 mg/dL.
Main Outcomes and Measures
The primary outcomes of the screening study were sensitivity, specificity, positive predictive value, and negative predictive value based on infants testing positive in both stages. The reference standard was biliary atresia diagnosed at the region’s pediatric hepatology centers. The primary outcome of the pre-post study was the age infants underwent the Kasai portoenterostomy for treatment of biliary atresia.
Results
Of 124 385 newborns in the screening study, 49.2% were female, 87.6% were of term gestational age, 70.0% were white, and 48.1% were Hispanic. Screening identified the 7 known infants with biliary atresia with a sensitivity of 100% (95% CI, 56.1%-100.0%), a specificity of 99.9% (95% CI, 99.9%-99.9%), a positive predictive value of 5.9% (95% CI, 2.6%-12.2%), and a negative predictive value of 100.0% (95% CI, 100.0%-100.0%). In the pre-post study, 24 infants were treated before screening implementation and 19 infants were treated after screening implementation (including 6 of 7 from the screening study, 7 from screening at nonstudy hospitals, and 6 from referrals because of clinical symptoms). The age infants underwent the Kasai portoenterostomy was significantly younger after screening was implemented (mean age, 56 days [SD, 19 days] before screening implementation vs 36 days [SD, 22 days] after screening implementation; between-group difference, 19 days [95% CI, 7-32 days]; P = .004).
Conclusions and Relevance
Newborn screening with direct or conjugated bilirubin measurements detected all known infants with biliary atresia in the study population, although the 95% CI around the sensitivity estimate was wide and the study design did not ensure complete ascertainment of false-negative results. Research is needed in larger populations to obtain more precise estimates of diagnostic yield and to better understand the clinical outcomes and cost-effectiveness of this screening approach.
This cross-sectional study characterizes the diagnostic accuracy of direct or conjugated bilirubin measurements for identifying newborns with biliary atresia and the associations between screening and age of therapeutic surgery.
Introduction
A critical challenge in pediatric hepatology is timely diagnosis of biliary atresia. Biliary atresia is the leading indication for pediatric liver transplant, affecting 1:8000 to 1:18 000 infants worldwide and progressing to end-stage liver disease by 2 years of life.1 The rapid course of biliary atresia can be slowed with the Kasai portoenterostomy, which is an operation that attempts to establish bile flow by removing atretic bile ducts and creating a liver-intestine anastomosis. Treatment by 30 days of life has the best chance of delaying or preventing the need for transplant.2,3 In the United States, however, treatment usually occurs later because biliary atresia is difficult to detect during its early stages.4
The American Academy of Pediatrics has recommended more studies to develop strategies to help detect biliary atresia earlier.5 One strategy, the stool color card program, screens infants for the pale stools that develop with biliary obstruction.6,7,8 Stool color card programs have improved treatment times and transplant-free survival in Japan and Taiwan, but they require infrastructure that is currently unavailable in the United States, such as a national call center to triage parents’ concerns and a standard 1-month well-child visit to review stool color. A second potential strategy uses newborn direct or conjugated bilirubin measurements, which are elevated in biliary atresia starting shortly after birth.9,10,11,12,13,14
This 2-part study included a screening study to determine the diagnostic yield of newborn direct or conjugated bilirubin measurements for biliary atresia and a pre-post study to evaluate the association of screening implementation with clinical outcomes.
Methods
This study was approved by the institutional review board at Baylor College of Medicine and all the participating study sites. For the screening study, a waiver of informed consent was approved because each site was already measuring total bilirubin (and accompanying direct or conjugated bilirubin) in the first 60 hours of life to assess the need for phototherapy based on guidelines from the American Academy of Pediatrics.15 In addition, the investigator (S.H.) reviewing the results was a credentialed physician at each hospital, and abnormal screening results were shared with clinicians or parents to potentially benefit the patient. For the pre-post study, a waiver of informed consent was approved because the data were collected retrospectively from electronic medical records.
Patient Selection for the Screening Study
Infants born at 14 south Texas hospitals between January 2015 and June 2018 were included (eTable 1 in the Supplement). Each hospital had a preexisting policy of measuring total bilirubin in the first 60 hours of life to assess the need for phototherapy.15 Direct or conjugated bilirubin levels (depending on the type of assay used at each site) were automatically reported alongside total bilirubin levels, although the significance of the values outside the reference intervals was not known and action was not routinely taken.
Screening Mechanics for the Screening Study
Screening was based on the observation that infants with biliary atresia have elevated direct or conjugated bilirubin levels starting at birth, and involved implementing a standardized clinical response to levels exceeding the reference intervals.9,10,11,12 The upper limit of normal for direct bilirubin was derived at each site using the 97.5th percentile value from 120 newborns consecutively measured.16 Bilirubin reference intervals were recalculated yearly with new machines to control for differences that occur with changes in the direct bilirubin assay’s reaction time, pH, or temperature.17 The upper limit of normal for conjugated bilirubin was set at 0.2 mg/dL based on established ranges and the consistency of conjugated bilirubin assays across sites.17,18
Screening followed a 2-stage prospective direct or conjugated bilirubin testing strategy. In stage 1, all infants were tested via heel stick within the first 60 hours of life as part of routine clinical assessment for hyperbilirubinemia.15 The infants who had values within their hospital’s reference interval were considered to have negative screening results in stage 1 and were not tested further. The infants who had values exceeding their hospital’s reference interval were considered to have positive screening results in stage 1, and underwent repeat testing in stage 2 at or before the 2-week well-child visit as part of standard clinical care. For infants no longer at the hospital, the study team called outpatient clinicians to confirm they knew about the abnormal screening results. In stage 2, infants were considered to have negative screening results if they had repeat direct or conjugated bilirubin levels less than or equal to their stage 1 levels and 1 mg/dL or less, which is the 2-week cutoff recommended by the North American and European pediatric gastroenterology societies.19 Infants with repeat bilirubin levels that were increasing or were greater than 1 mg/dL were considered to have positive screening results in stage 2. Infants with positive screening results in both stages 1 and 2 underwent further testing at the discretion of their clinical care team with consultation from the study team, specialists, or both.
Outcomes for the Screening Study
The primary outcomes were prespecified and included the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 2-stage screening. The reference standard was biliary atresia diagnosed by intraoperative cholangiogram and pathological examination of the biliary remnant.19 Infants meeting this reference standard were counted using 2 strategies. First, all infants with positive screening results in stage 1 (including those not undergoing stage 2 testing) were followed up until biliary atresia was diagnosed or excluded. Biliary atresia was excluded if direct or conjugated bilirubin levels had normalized, icterus was absent by 3 months per report from the parents or clinicians, or an alternative diagnosis was made. Second, infants with negative screening results in stage 1 were followed up indirectly by monitoring admissions at 3 pediatric hepatology centers in the region. These centers were the referral centers for the birth hospitals included in this study.
Patient Selection for the Pre-Post Screening Implementation Study
Infants who underwent the Kasai portoenterostomy by 90 days of life at the region’s largest pediatric hepatology center (Texas Children’s Hospital) were included. A 90-day cutoff was used because the Kasai portoenterostomy is seldom beneficial after 90 days.20 The prescreening group included patients treated between January 2008 and June 2011, which was before screening was implemented and before the value of newborn direct or conjugated bilirubin measurements for detecting biliary atresia was known.9 The postscreening group included patients treated between January 2015 and June 2018, which was after screening had been introduced to the region and implemented at various birth hospitals. Infants in the postscreening implementation group were detected by (1) the screening study, (2) clinicians who learned about the study and implemented a similar direct or conjugated bilirubin screening approach at nonstudy birth hospitals, or (3) clinical symptoms without screening.
Outcomes for the Pre-Post Screening Implementation Study
The prespecified primary outcome was the age infants underwent the Kasai portoenterostomy for treatment of biliary atresia. Additional outcomes were metrics used in biliary atresia studies, including time to conjugated bilirubin level normalization, transplant-free survival after undergoing the Kasai portoenterostomy, the proportion of infants treated by 30 days, the proportion normalizing their conjugated bilirubin level by 90 days after treatment, and the proportion surviving transplant-free 1 year after treatment.20,21,22,23,24 The post hoc analyses included time to specialist referral (defined as time from birth to first encounter with a specialist) and time to hepatology evaluation (defined as time from first encounter with a specialist to the Kasai portoenterostomy). Additional post hoc analyses included studying outcomes in subgroups of infants in the group after screening implementation (those detected by the screening study, by screening outside the study, or by clinical symptoms without screening).
Data Collection
Data were accrued until July 15, 2019. Data collected for the screening study appear in eTable 2 in the Supplement and those collected for the pre-post study appear in eTable 3 in the Supplement. Data on race and ethnicity were collected to detect correlations with the screening results (screening study) or to detect differences between the comparison groups (pre-post study). Race and ethnicity were selected by parents from fixed categories at each hospital and the data were combined across hospitals by the study team.
Statistical Analysis
For the screening study, the sample size was calculated using simulations with the bdpv package in R version 2.14.2 (R Foundation for Statistical Computing) to obtain a PPV comparable with newborn screening tests for other rare conditions (0.5%-6.0%).25 Inputs included a sensitivity of 99%, a specificity of 99%, no assumptions about stage 2 testing, and a North American birth prevalence for biliary atresia of 0.6 per 10 000.3,26 Based on these assumptions, screening 102 000 newborns was predicted to generate a PPV of 0.67% with a narrow 95% CI of 0.54% to 0.82%. Enrollment stopped at the end of the half-year period in which the calculated sample size was reached.
Birth prevalence calculations included all infants. Diagnostic yield calculations excluded infants not tested in stage 1 and those who had positive screening results in stage 1 but were not retested in stage 2, and used the efficient score method to determine the 95% CIs. Logistic regression was used to model testing status (negative vs positive screening results) in each stage as the dependent variable and patient characteristics (sex, gestational age, race, ethnicity, birth season, first bilirubin assay) as the independent variables. Observations without values were excluded from the respective analyses.
Bivariable analysis was first performed to generate unadjusted 2-sided P values. Multivariable analysis was then performed to adjust for effects of each characteristic. A 2-sided P value of <.008 was considered statistically significant based on a Bonferroni correction for the 6 characteristics evaluated (P = .05/6 = .008) to limit the potential for type I error due to multiple comparisons. Diagnostics on the final models were performed using the Hosmer-Lemeshow goodness-of-fit test.
For the pre-post study, comparisons were made using the unpaired t test (continuous variables), the log-rank test (time to event), and the Fisher exact or χ2 test (proportions). For time to normalization of conjugated bilirubin, infants were censored at the time of liver transplant if their conjugated bilirubin level was still elevated. For time to liver transplant, infants who did not receive a transplant were censored on July 15, 2019. Analyses for the pre-post study were 2-tailed, were not adjusted for multiple comparisons, and assumed a P value <.05 indicated statistical significance. For the screening and pre-post studies, all calculations were performed using the VassarStats website, Stata version 14.0 (StataCorp), Prism version 8 (GraphPad), or Excel version 2016 (Microsoft).
Results
Of 124 385 newborns in the screening study, 49.2% were female, 87.6% were of term gestational age, 70.0% were white, and 48.1% were Hispanic (eTable 4 in the Supplement). Data were not available on gestational age for 43.6% of infants; race, 12.5%; and ethnicity, 18.1%. Seven infants in the screened population were later diagnosed with biliary atresia (birth prevalence of 0.6 per 10 000).
Diagnostic Yield of the Screening Study
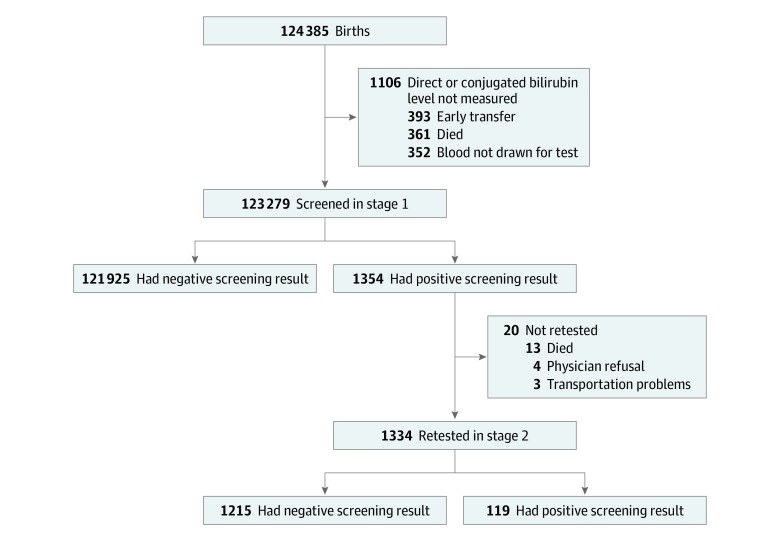
Stage 1 testing was performed in 99.1% of the study population (n = 123 279; Figure 1). Positive screening results were found in stage 1 testing for 1.1% of newborns (n = 1354). Newborns with positive vs negative screening results in stage 1 were significantly more likely to have been screened with direct bilirubin measurements (91.7% vs 67.3%, respectively; adjusted P < .001), have been born at a preterm gestational age (3.9% vs 1.3%; adjusted P < .001), have been born at an extremely preterm gestational age (2.2% vs 0.7%; adjusted P < .001), and have been identified as black (21.6% vs 13.9%; adjusted P < .001) (Table 1).
Figure 1. Patient Flow for the 2-Stage Screening Study for Biliary Atresia.
Stage 1 testing occurred within the first 60 hours of life. Stage 2 testing occurred at or before the 2-week well-child visit.
Table 1. Characteristics of Infants Testing Negative or Positive in Each Screening Stage.
| Characteristic | Stage 1 (n = 123 279)a,b | Stage 2 (n = 1334)a,c,d | ||||||
|---|---|---|---|---|---|---|---|---|
| No./total (%) | P valuee | P valuef | No./total (%) | P valuee | P valuef | |||
| Negative screening result | Positive screening result | Negative screening result | Positive screening result | |||||
| Sex | ||||||||
| Male | 61 835/121 924 (50.7) | 732/1354 (54.1) | .01 | .02 | 653/1215 (53.7) | 68/119 (57.1) | .48 | .07 |
| Female | 60 089/121 924 (49.3) | 622/1354 (45.9) | 562/1215 (46.3) | 51/119 (42.9) | ||||
| Gestational age | ||||||||
| Extremely preterm | 475/68 492 (0.7) | 18/826 (2.2) | <.001 | <.001 | 10/730 (1.4) | 3/82 (3.7) | .03 | .94 |
| Preterm | 871/68 492 (1.3) | 32/826 (3.9) | <.001 | <.001 | 15/730 (2.1) | 17/82 (20.7) | <.001 | .02 |
| Late preterm | 6744/68 492 (9.8) | 60/826 (7.3) | .03 | .20 | 42/730 (5.8) | 15/82 (18.3) | <.001 | .12 |
| Term | 60 402/68 492 (88.2) | 716/826 (86.7) | Reference | Reference | 663/730 (90.8) | 47/82 (57.3) | Reference | Reference |
| Race | ||||||||
| White | 74 871/106 798 (70.1) | 738/1099 (67.2) | Reference | Reference | 675/993 (68.0) | 53/87 (60.9) | Reference | Reference |
| Black | 14 847/106 798 (13.9) | 237/1099 (21.6) | <.001 | <.001 | 207/993 (20.8) | 22/87 (25.3) | .26 | .83 |
| Asian | 7913/106 798 (7.4) | 56/1099 (5.1) | .02 | .67 | 53/993 (5.3) | 3/87 (3.4) | .59 | .68 |
| Otherg | 9167/106 798 (8.6) | 68/1099 (6.2) | .03 | .05 | 58/993 (5.8) | 9/87 (10.3) | .08 | .48 |
| Hispanic | 48 122/99 904 (48.2) | 512/1061 (48.3) | .95 | .95 | 465/960 (48.4) | 40/84 (47.6) | .89 | .60 |
| Birth season | ||||||||
| Winter | 30 006/121 925 (24.6) | 305/1354 (22.5) | Reference | Reference | 272/1215 (22.4) | 29/119 (24.4) | Reference | Reference |
| Spring | 34 648/121 925 (28.4) | 385/1354 (28.4) | .25 | .04 | 344/1215 (28.3) | 33/119 (27.7) | .69 | .93 |
| Summer | 28 443/121 925 (23.3) | 314/1354 (23.2) | .31 | .05 | 287/1215 (23.6) | 22/119 (18.5) | .26 | .56 |
| Fall | 28 828/121 925 (23.6) | 350/1354 (25.8) | .02 | .67 | 312/1215 (25.7) | 35/119 (29.4) | .85 | .49 |
| First bilirubin test | ||||||||
| Direct | 82 013/121 925 (67.3) | 1242/1354 (91.7) | <.001 | <.001 | 1146/1215 (94.3) | 88/119 (74.0) | <.001 | <.001 |
| Conjugated | 39 912/121 925 (32.7) | 112/1354 (8.3) | 69/1215 (5.7) | 31/119 (26.1) | ||||
The differences during each stage of screening were modeled using logistic regression.
The Hosmer-Lemeshow goodness-of-fit test was used for the multivariable logistic regression model (P = .31).
The Hosmer-Lemeshow goodness-of-fit test was used for the multivariable logistic regression model (P = .49).
There were 20 infants with positive screening results in stage 1 but were not tested in stage 2.
Two-sided test evaluating the hypothesis that selected characteristics are associated with the screening result for both stages in the unadjusted bivariable analyses.
Two-sided test evaluating the hypothesis that selected characteristics are associated with the screening result for both stages in the multivariable analyses adjusted for sex, gestational age, race, ethnicity, birth season, and first bilirubin test.
Included American Indian, Alaskan Native, or self-reported as other.
Stage 2 testing was performed in 98.5% of infants (n = 1334) who had a positive screening result in stage 1 (Figure 1). Those not retested died before 2 weeks, had clinicians who refused further testing, or lacked transportation to the 2-week well-child visit (eTable 5 in the Supplement). There were 119 infants with positive screening results in stage 2, which comprised 8.9% of those retested and 0.1% of the screened population. Infants with positive vs negative screening results in stage 2 were significantly more likely to be screened initially with conjugated bilirubin measurements (26.1% vs 5.7%, respectively; adjusted P < .001; Table 1).
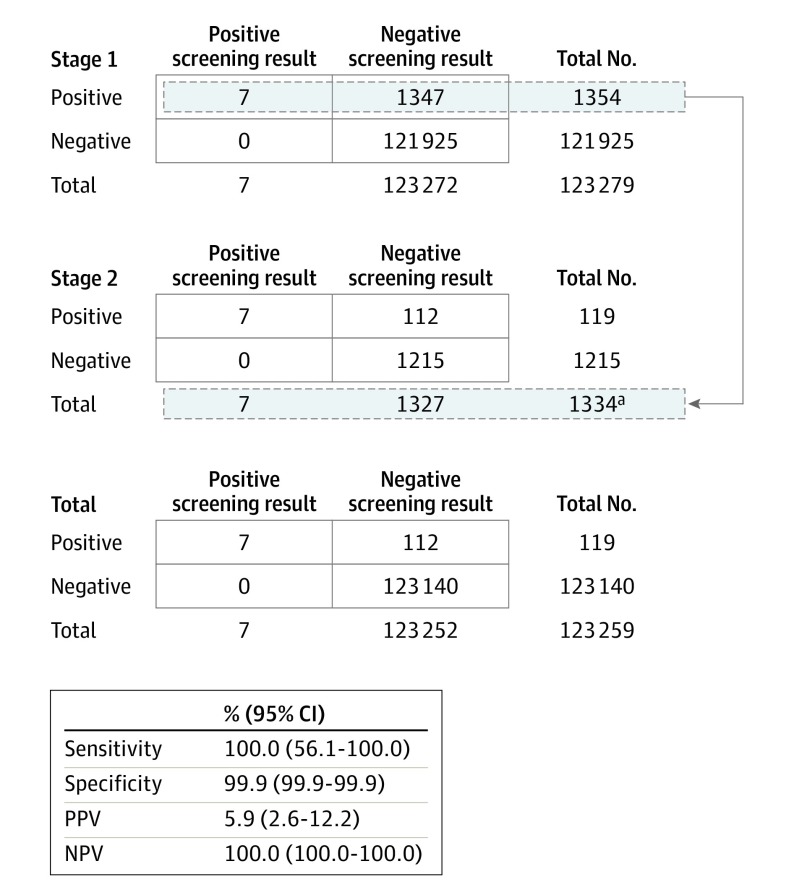
Stage 1 and 2 results were positive for the 7 known infants with biliary atresia in the study population, resulting in a screening sensitivity of 100.0% (95% CI, 56.1%-100.0%), a specificity of 99.9% (95% CI, 99.9%-99.9%), a PPV of 5.9% (95% CI, 2.6%-12.2%), and a NPV of 100.0% (95% CI, 100.0%-100.0%) (Figure 2). Infants with true positive screening results (n = 7) had initial newborn direct or conjugated bilirubin levels that exceeded site-specific reference intervals to varying degrees in stage 1 and were greater than 1 mg/dL in stage 2 (eTable 6 in the Supplement). Infants with false-positive screening results (n = 112) tested positive in both stages 1 and 2 but did not have biliary atresia (eTable 7 in the Supplement). Of these 112 infants, 9 (8.0%) had other cholestatic liver diseases, which included Alagille syndrome (n = 4), α1 antitrypsin deficiency (n = 3), ABCB11 deficiency (n = 1), and choledochal cyst (n = 1) (Table 2). Other false-positive screening results had no diagnosis determined (52.7%), cholestasis-associated conditions (15.2%), heterozygosity in cholestasis-related genes (10.7%), congenital infections (7.1%), and excessive red blood cell clearance (6.3%). To evaluate the false-positive screening results, additional direct or conjugated bilirubin measurements alone were needed for 25.0% (n = 28) of infants (Table 2), including 37.3% (n = 22) of infants who had no cause of cholestasis determined (eTable 8 in the Supplement).
Figure 2. Newborn Direct or Conjugated Bilirubin Screening for Biliary Atresia.
NPV indicates negative predictive value; PPV, positive predictive value.
aThere were 20 newborns who were not retested in stage 2 because 13 died, the physician refused to test in 4, and there were transportation problems for 3.
Table 2. Diagnoses and Evaluation for False-Positive Screening Results (n = 112).
| Description of diagnosis and evaluation | No. (%) |
|---|---|
| Type of diagnosis | |
| Not determined | 59 (52.7) |
| Cholestasis-associated conditionsa | 17 (15.2) |
| Heterozygosity in cholestasis-related genesb | 12 (10.7) |
| Cholestatic liver diseasesc | 9 (8.0) |
| Congenital infectionsd | 8 (7.1) |
| Excessive red blood cell clearance | 7 (6.3) |
| Type of evaluation performed | |
| Additional direct or conjugated bilirubin testing only | 28 (25.0) |
| Additional laboratory testing | 25 (22.3) |
| Additional noninvasive imaging | 38 (33.9) |
| Liver biopsy with or without percutaneous transhepatic cholangiogram | 20 (17.9) |
| Intraoperative cholangiogram | 1 (0.9) |
Included trisomy 21 (5 cases), gastroschisis (4 cases), trisomy 18 (3 cases), portosystemic shunt (2 cases), maternal lupus (1 case), omphalocele (1 case), and panhypopituitarism (1 case).
The gene names appear in eTable 7 in the Supplement.
Included Alagille syndrome (4 cases), α1 antitrypsin deficiency (3 cases), ABCB11 deficiency (1 case), and choledochal cyst (1 case).
Included cytomegalovirus (3 cases), syphilis (3 cases), coxsackievirus (1 case), and rubella (1 case).
Associations With Outcomes During the Pre-Post Screening Implementation Study
Infants who underwent the Kasai portoenterostomy at the region’s largest hepatology center before (n = 24) and after (n = 19) screening implementation were compared. The groups had similar demographic factors and similar complication rates after undergoing the Kasai portoenterostomy (eTable 9 in the Supplement). The group after screening implementation consisted of 6 infants detected by the screening study (1 infant with a true positive screening result underwent the Kasai portoenterostomy at another study site) and 7 infants detected by clinicians at nonstudy birth hospitals that had adopted 2-stage clinical intervention programs based on the screening study (eTable 10 in the Supplement). The group after screening implementation also included 6 infants born at hospitals not participating in the study who were referred because of concerning clinical symptoms.
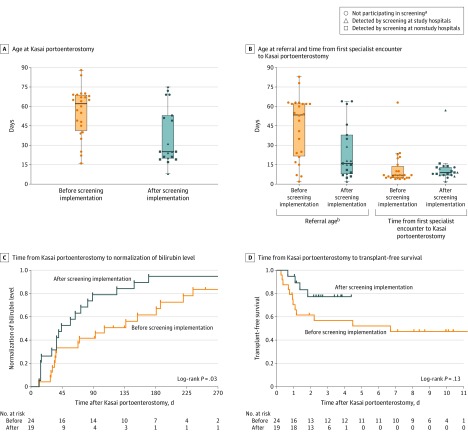
The age infants underwent the Kasai portoenterostomy was significantly younger after screening was implemented (mean age, 56 days [SD, 19 days] before implementation vs 36 days [SD, 22 days] after implementation; between-group difference, 19 days [95% CI, 7-32 days], P = .004; Figure 3A). After screening implementation, infants also were significantly more likely to undergo the Kasai portoenterostomy by 30 days (12.5% before implementation vs 57.9% after implementation; reciprocal of risk ratio, 4.6 [95% CI, 1.7-14.0], P = .003; Table 3).
Figure 3. Outcomes Associated With Implementation of Screening for Biliary Atresia.
In A and B, the box plot lines correspond from the bottom of box to the top: 25th percentile, median percentile, and 75th percentile. The whiskers extend to the upper and lower adjacent values. In C, the median follow-up time was 100 days (quartile 1-3, 36-186 days) in the group before screening implementation and 44 days (quartile 1-3, 15-89 days) in the group after screening implementation. In D, the median follow-up time was 3.3 years (quartile 1-3, 0.9-9.6 years) in the group before screening implementation and 2.5 years (quartile 1-3, 1.4-3.6 years) in the group after screening implementation.
aThe circles that represent infants in the group after screening implementation had biliary atresia detected by clinical symptoms rather than newborn direct or conjugated bilirubin tests.
bDefined as time from birth to first encounter with a specialist.
Table 3. Secondary Outcomes Associated With Implementation of Screening for Biliary Atresia.
| Outcome | No. (%) | Reciprocal of risk ratio (95% CI) |
P valuea | |
|---|---|---|---|---|
| Before screening implementation (n = 24) | After screening implementation (n = 19) | |||
| Underwent the Kasai portoenterostomy by 30 d of life | 3 (12.5) | 11 (57.9) | 4.6 (1.7-14.0) | .003 |
| Normalization of bilirubin level by 90 d after undergoing the Kasai portoenterostomyb | 10 (41.7) | 15 (78.9) | 1.9 (1.1-3.3) | .03 |
| Transplant-free survival 1 y after undergoing the Kasai portoenterostomy | 17 (70.8) | 18 (94.7) | 1.3 (1.0-1.9) | .06 |
Calculated using the Fisher exact test.
Determined by conjugated testing.
In the post hoc analyses, infants were referred to a specialist at significantly younger ages after screening implementation (mean age, 44 days [SD, 24 days] before implementation vs 25 [SD, 21] days after implementation; between-group difference, 20 days [95% CI, 6 to 34 days], P = .007; Figure 3B). However, after screening implementation, infants were not evaluated faster after referral (mean time for evaluation, 11 days [SD, 13 days] before implementation vs 12 days [SD, 12 days] after implementation; between-group difference, 0 days [95% CI, −8 to 7 days], P = .92; Figure 3B). Within the group after screening implementation, infants identified by clinical symptoms were referred and treated later, whereas infants screened either at study hospitals only or at study hospitals and nonstudy hospitals were more likely to undergo the Kasai portoenterostomy by 30 days (eTables 11 and 12 and eFigure in the Supplement).
For outcomes after undergoing the Kasai portoenterostomy, infants in the group after screening implementation compared with those in the group before screening implementation had significantly faster times to conjugated bilirubin normalization (log-rank P = .03; Figure 3C) but did not have a statistically significant difference in transplant-free survival (log-rank P = .13; Figure 3D). Infants in the group after screening implementation were significantly more likely to have normal conjugated bilirubin levels by 90 days after undergoing the Kasai portoenterostomy (41.7% before implementation vs 78.9% after implementation; reciprocal of risk ratio, 1.9 [95% CI, 1.1-3.3], P = .03; Table 3). Transplant-free survival rates 1 year after undergoing the Kasai portoenterostomy were higher but did not reach statistical significance (70.8% before screening implementation vs 94.7% after screening implementation; reciprocal of risk ratio, 1.3 [95% CI, 1.0-1.9], P = .06; Table 3). Post hoc comparisons showed that infants screened in study and nonstudy hospitals after screening implementation compared with those before screening implementation were more likely to survive transplant-free 1 year after undergoing the Kasai portoenterostomy (eTable 12 in the Supplement).
Discussion
This study evaluated newborn direct or conjugated bilirubin screening for biliary atresia using 2 approaches. First, in the screening study of a large population of newborns, direct or conjugated bilirubin testing had a sensitivity of 100%, a specificity of 99.9%, a PPV of 5.9%, and a NPV of 100.0%, but with a wide 95% CI for the sensitivity estimate because of the small number of true positive screening results. Second, in the pre-post study, screening was associated with earlier treatment; the mean age infants underwent the Kasai portoenterostomy was 56 days before screening implementation vs 36 days after screening implementation. After undergoing the Kasai portoenterostomy, infants in the group after screening implementation had significantly faster times to conjugated bilirubin normalization compared with infants in the group before screening implementation.
The association of screening with younger age when undergoing the Kasai portoenterostomy compares well with other biliary atresia screening methods currently in use. For example, in Taiwan, universal implementation of the stool color card program was associated with a mean age of 46 days when undergoing the Kasai portoenterostomy.7 In Japan, regional implementation of the stool color card program was associated with a mean age of 59.7 days when undergoing the Kasai portoenterostomy.6 One additional advantage of newborn direct or conjugated bilirubin screening is that it potentially allows infants to undergo the Kasai portoenterostomy before 30 days, the time interval correlating with the best chance of delaying or preventing the need for transplant.2,3 In the pre-post study, 57.9% of infants underwent the Kasai portoenterostomy by 30 days after screening implementation compared with 12.5% before screening implementation.
Screening also identified 112 infants without biliary atresia, resulting in a PPV of 5.9% that is comparable with the PPV range of 0.5% to 6.0% reported for other newborn screening tests.25 Some of the infants with false-positive screening results had conditions that could have benefitted from early detection and intervention. For example, infants with cholestatic liver diseases such as Alagille syndrome and α1 antitrypsin deficiency have been reported to present with life-threatening bleeding associated with vitamin K deficiency.27,28 Detection during the newborn period allowed for nutritional supplementation and vitamin K administration as needed. In addition, infants with congenital infections such as cytomegalovirus infection can be identified and treated quickly, which may help limit development of serious neurological sequelae.29
An important avenue of future research is the cost-effectiveness of screening. A recent Canadian study estimated an incremental cost-effectiveness ratio for bilirubin screening to be unacceptable at $473 840 per life-year gained.30 However, this calculation relied on assumptions not supported by data from this study, including a specificity of 98%, a full hepatology evaluation for all false-positive screening results, and 37% of infants undergoing the Kasai portoenterostomy by 30 days. The study also used life-years gained as a metric for effectiveness, whereas transplant-free life-years gained may also be informative because biliary atresia seldom results in death. Additional simulations using new information (including data from this study) can further assess the cost-effectiveness of screening.
One way to potentially improve cost-effectiveness is by reducing the number of infants who test positive in stage 1 or stage 2 but who are unlikely to have serious liver conditions. In stage 1, more than 90% of positive screening results were negative when retested in stage 2. The infants with positive screening results in stage 1 were more likely to have been tested with direct vs conjugated bilirubin, reflecting the inherent limitations of the direct bilirubin assay that also measures small amounts of unconjugated bilirubin.17 Even though some have suggested replacing the direct assay with the conjugated assay to avoid these limitations, 2 hospitals did the opposite during the study period and switched from conjugated assays to direct assays for cost and convenience reasons.18 In stage 2, 25% of infants who had positive screening results had subsequent normal direct or conjugated bilirubin levels and did not require further testing. Some of these false-positive results could have been avoided by performing stage 2 testing later; however, for practical reasons, stage 2 testing is best performed at the routine newborn follow-up well-child visit recommended by the American Academy of Pediatrics.
Even if studies with larger populations confirm the results from this study and screening is found to be cost-effective, there are still important implementation challenges that need to be addressed. First, birth hospitals would need to carefully derive and update site-specific reference intervals (techniques to screen newborn blood spots at central laboratories have not been developed). For example, 1 infant with a true positive screening result had a stage 1 bilirubin level of 0.4 mg/dL, which was abnormally high in the 98.4th to 99.6th percentile for that site but may have been overlooked if the reference intervals were inappropriately borrowed from another site. Second, stage 2 testing requires reliable access to medical centers willing to participate in screening. In this study, 0.2% of infants with positive screening results in stage 1 had parents or caregivers who could not travel to the well-child visit, and another 0.2% had clinicians who chose not to retest. Third, infants with a positive screening result must be quickly evaluated for biliary atresia, which can be difficult to detect during early stages of the disease when aminotransferase levels and liver stiffness may still be normal.31,32 The challenge specialists face was highlighted by an infant who had a true positive screening result in the study, but who underwent the Kasai portoenterostomy at 75 days despite identification at 2 weeks by screening.
Limitations
The screening study has several limitations. First, the small number of true positive screening results affected the 95% CI for sensitivity, which was wide at 56.1% to 100%, and lower limits may be unacceptable for a screening test. Second, many infants (43.6%) did not have gestational age recorded, preventing a complete analysis of the association between prematurity and the screening results. Third, screening only occurred at south Texas hospitals, potentially limiting the applicability of the study’s results to other populations. Fourth, a patient with biliary atresia could have been missed if an infant tested negative in stage 1, moved away from the region, and was then diagnosed with biliary atresia at a center other than one of the region’s 3 pediatric hepatology referral centers monitored during the study. However, normal direct or conjugated bilirubin levels in the newborn period would be very unlikely among those with biliary atresia.9,10,11,12 In addition, the study’s birth prevalence for biliary atresia of 0.6 per 10 000 newborns matches the birth prevalence reported in multiple North American and European studies; however, this prevalence is less than the birth prevalence of 1.6 per 10 000 reported in Taiwan.7,26
The pre-post study also has important limitations. First, the sample sizes in the groups before and after screening implementation were small. This may have prevented trends in outcomes such as 1-year transplant-free survival from reaching statistical significance. Second, the pre-post study design used historical controls that may have introduced bias because of temporal changes in care unrelated to screening. To limit biases with historical controls, infants in both groups were treated by the same hepatologists and surgeons at the same hospital. Furthermore, historical controls may be well-suited for biliary atresia studies because the types of care and outcomes have not changed significantly over the past 20 years. For example, the mean age infants undergo the Kasai portoenterostomy has remained the same, as has the proportion of infants who have normal bilirubin levels or survive transplant-free after undergoing the Kasai portoenterostomy.4,5,20,23,24,33
Conclusions
Newborn screening with direct or conjugated bilirubin measurements detected all known infants with biliary atresia in the study population, although the 95% CI around the sensitivity estimate was wide and the study design did not ensure complete ascertainment of false-negative results. Research is needed in larger populations to obtain more precise estimates of diagnostic yield and to better understand the clinical outcomes and cost-effectiveness of this screening approach.
eTable 1. Study Site Characteristics
eTable 2. Data Elements Collected in Screening Study
eTable 3. Data Elements Collected in Pre-Implementation/Post-Implementation of Screening Study
eTable 4. Characteristics of 124,385 Newborns in the Study Population
eTable 5. Screening Results Infants Testing Positive in Stage 1 who were not Tested in Stage 2 (N=20)
eTable 6. Two-Stage Screening Results for Infants with True Positive Screening Results (N=7)
eTable 7. Screening Results for Infants with False-Positive Screening Results who had a Cause of Cholestasis Identified (N=53)
eTable 8. Evaluation of Infants with False-Positive Screening Results who had no Cause of Cholestasis Identified (N=59)
eTable 9. Characteristics of Infants in Pre-Implementation and Post-Implementation of Screening
eTable 10. Screening Results for Infants Detected in Non-Study Site Birth Hospitals
eTable 11. Post-Hoc Analysis of Outcomes Associated with Infants in the Pre-Implementation of Screening Group and Screen Positive Infants
eTable 12. Post-Hoc Analysis of Outcomes Associated with Infants in the Pre-Implementation of Screening Group and Infants Detected by Screening in the Post-Implementation Period
eFigure. Post-Hoc Analysis of Age at Kasai Portoenterostomy, Age at Referral, and Time for Evaluation for Patients in the Pre-Implementation of Screening Group and Sub-groups of Patients in the Post-Implementation of Screening Group
References
- 1.Bezerra JA, Wells RG, Mack CL, et al. Biliary atresia: clinical and research challenges for the twenty-first century. Hepatology. 2018;68(3):1163-1173. doi: 10.1002/hep.29905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fanna M, Masson G, Capito C, et al. Management of biliary atresia in France 1986 to 2015: long-term results. J Pediatr Gastroenterol Nutr. 2019;69(4):416-424. doi: 10.1097/MPG.0000000000002446 [DOI] [PubMed] [Google Scholar]
- 3.Schreiber RA, Barker CC, Roberts EA, et al. ; Canadian Pediatric Hepatology Research Group . Biliary atresia: the Canadian experience. J Pediatr. 2007;151(6):659-665, 665.e1. doi: 10.1016/j.jpeds.2007.05.051 [DOI] [PubMed] [Google Scholar]
- 4.Wadhwani SI, Turmelle YP, Nagy R, Lowell J, Dillon P, Shepherd RW. Prolonged neonatal jaundice and the diagnosis of biliary atresia: a single-center analysis of trends in age at diagnosis and outcomes. Pediatrics. 2008;121(5):e1438-e1440. doi: 10.1542/peds.2007-2709 [DOI] [PubMed] [Google Scholar]
- 5.Wang KS; Section on Surgery; Committee on Fetus and Newborn; Childhood Liver Disease Research Network . Newborn screening for biliary atresia. Pediatrics. 2015;136(6):e1663-e1669. doi: 10.1542/peds.2015-3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu Y-H, Yokoyama K, Mizuta K, et al. Stool color card screening for early detection of biliary atresia and long-term native liver survival: a 19-year cohort study in Japan. J Pediatr. 2015;166(4):897-902.e1. doi: 10.1016/j.jpeds.2014.12.063 [DOI] [PubMed] [Google Scholar]
- 7.Lin J-S, Chen SC-C, Lu C-L, Lee H-C, Yeung C-Y, Chan W-T. Reduction of the ages at diagnosis and operation of biliary atresia in Taiwan: a 15-year population-based cohort study. World J Gastroenterol. 2015;21(46):13080-13086. doi: 10.3748/wjg.v21.i46.13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreiber RA, Masucci L, Kaczorowski J, et al. Home-based screening for biliary atresia using infant stool colour cards: a large-scale prospective cohort study and cost-effectiveness analysis. J Med Screen. 2014;21(3):126-132. doi: 10.1177/0969141314542115 [DOI] [PubMed] [Google Scholar]
- 9.Harpavat S, Finegold MJ, Karpen SJ. Patients with biliary atresia have elevated direct/conjugated bilirubin levels shortly after birth. Pediatrics. 2011;128(6):e1428-e1433. doi: 10.1542/peds.2011-1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong Z, Wu Y, Zheng L, Chen L, Lv Z. Can free carnitine or bilirubin in blood be used in neonatal screening for biliary atresia? Eur J Pediatr Surg. 2019. doi: 10.1055/s-0039-1698764 [DOI] [PubMed] [Google Scholar]
- 11.Noorulla F, Dedon R, Maisels MJ. Association of early direct bilirubin levels and biliary atresia among neonates. JAMA Netw Open. 2019;2(10):e1913321. doi: 10.1001/jamanetworkopen.2019.13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guthery SL, Deneau M, Christensen R, et al. Fractionated bilirubin in >250,000 Utah newborns confirms conjugated or direct bilirubin is elevated in the newborn period in biliary atresia: implications for newborn screening. Hepatology. 2019;70(S1):54A-55A. [Google Scholar]
- 13.Harpavat S, Ramraj R, Finegold MJ, et al. Newborn direct or conjugated bilirubin measurements as a potential screen for biliary atresia. J Pediatr Gastroenterol Nutr. 2016;62(6):799-803. doi: 10.1097/MPG.0000000000001097 [DOI] [PubMed] [Google Scholar]
- 14.Harpavat S, Garcia-Prats JA, Shneider BL. Newborn bilirubin screening for biliary atresia. N Engl J Med. 2016;375(6):605-606. doi: 10.1056/NEJMc1601230 [DOI] [PubMed] [Google Scholar]
- 15.American Academy of Pediatrics Subcommittee on Hyperbilirubinemia Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. 2004;114(1):297-316. doi: 10.1542/peds.114.1.297 [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute EP28-A3c: Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline. 3rd ed Clinical and Laboratory Standards Institute; 2008. [Google Scholar]
- 17.Doumas BT, Wu TW. The measurement of bilirubin fractions in serum. Crit Rev Clin Lab Sci. 1991;28(5-6):415-445. doi: 10.3109/10408369109106872 [DOI] [PubMed] [Google Scholar]
- 18.Davis AR, Rosenthal P, Escobar GJ, Newman TB. Interpreting conjugated bilirubin levels in newborns. J Pediatr. 2011;158(4):562-565.e1. doi: 10.1016/j.jpeds.2010.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fawaz R, Baumann U, Ekong U, et al. Guideline for the evaluation of cholestatic jaundice in infants: joint recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2017;64(1):154-168. doi: 10.1097/MPG.0000000000001334 [DOI] [PubMed] [Google Scholar]
- 20.Shneider BL, Brown MB, Haber B, et al. ; Biliary Atresia Research Consortium . A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr. 2006;148(4):467-474. doi: 10.1016/j.jpeds.2005.12.054 [DOI] [PubMed] [Google Scholar]
- 21.Serinet M-O, Wildhaber BE, Broué P, et al. Impact of age at Kasai operation on its results in late childhood and adolescence: a rational basis for biliary atresia screening. Pediatrics. 2009;123(5):1280-1286. doi: 10.1542/peds.2008-1949 [DOI] [PubMed] [Google Scholar]
- 22.Shneider BL, Magee JC, Karpen SJ, et al. ; Childhood Liver Disease Research Network (ChiLDReN) . Total serum bilirubin within 3 months of hepatoportoenterostomy predicts short-term outcomes in biliary atresia. J Pediatr. 2016;170:211-7.e1, 2. doi: 10.1016/j.jpeds.2015.11.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Superina R, Magee JC, Brandt ML, et al. ; Childhood Liver Disease Research and Education Network . The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg. 2011;254(4):577-585. doi: 10.1097/SLA.0b013e3182300950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bezerra JA, Spino C, Magee JC, et al. ; Childhood Liver Disease Research and Education Network (ChiLDREN) . Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA. 2014;311(17):1750-1759. doi: 10.1001/jama.2014.2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwon C, Farrell PM. The magnitude and challenge of false-positive newborn screening test results. Arch Pediatr Adolesc Med. 2000;154(7):714-718. doi: 10.1001/archpedi.154.7.714 [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Rivera C, Jolin-Dahel KS, Fortinsky KJ, Gozdyra P, Benchimol EI. International incidence and outcomes of biliary atresia. J Pediatr Gastroenterol Nutr. 2013;56(4):344-354. doi: 10.1097/MPG.0b013e318282a913 [DOI] [PubMed] [Google Scholar]
- 27.Per H, Arslan D, Gümüş H, Çoskun A, Kumandaş S. Intracranial hemorrhages and late hemorrhagic disease associated cholestatic liver disease. Neurol Sci. 2013;34(1):51-56. doi: 10.1007/s10072-012-0965-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Hasselt PM, Kok K, Vorselaars ADM, et al. Vitamin K deficiency bleeding in cholestatic infants with alpha-1-antitrypsin deficiency. Arch Dis Child Fetal Neonatal Ed. 2009;94(6):F456-F460. doi: 10.1136/adc.2008.148239 [DOI] [PubMed] [Google Scholar]
- 29.Liu G, Hai R, Liu F. Detection of congenital cytomegalovirus in newborns using nucleic acid amplification techniques and its public health implications. Virol Sin. 2017;32(5):376-386. doi: 10.1007/s12250-017-4055-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masucci L, Schreiber RA, Kaczorowski J, Collet JP, Bryan S. Universal screening of newborns for biliary atresia: cost-effectiveness of alternative strategies. J Med Screen. 2019;26(3):113-119. doi: 10.1177/0969141319832039 [DOI] [PubMed] [Google Scholar]
- 31.Harpavat S, Lupo PJ, Liwanag L, et al. Factors influencing time-to-diagnosis of biliary atresia. J Pediatr Gastroenterol Nutr. 2018;66(6):850-856. doi: 10.1097/MPG.0000000000001887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu J-F, Lee C-S, Lin W-H, et al. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. Hepatology. 2018;68(2):616-624. doi: 10.1002/hep.29856 [DOI] [PubMed] [Google Scholar]
- 33.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology. 2007;46(2):566-581. doi: 10.1002/hep.21790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Study Site Characteristics
eTable 2. Data Elements Collected in Screening Study
eTable 3. Data Elements Collected in Pre-Implementation/Post-Implementation of Screening Study
eTable 4. Characteristics of 124,385 Newborns in the Study Population
eTable 5. Screening Results Infants Testing Positive in Stage 1 who were not Tested in Stage 2 (N=20)
eTable 6. Two-Stage Screening Results for Infants with True Positive Screening Results (N=7)
eTable 7. Screening Results for Infants with False-Positive Screening Results who had a Cause of Cholestasis Identified (N=53)
eTable 8. Evaluation of Infants with False-Positive Screening Results who had no Cause of Cholestasis Identified (N=59)
eTable 9. Characteristics of Infants in Pre-Implementation and Post-Implementation of Screening
eTable 10. Screening Results for Infants Detected in Non-Study Site Birth Hospitals
eTable 11. Post-Hoc Analysis of Outcomes Associated with Infants in the Pre-Implementation of Screening Group and Screen Positive Infants
eTable 12. Post-Hoc Analysis of Outcomes Associated with Infants in the Pre-Implementation of Screening Group and Infants Detected by Screening in the Post-Implementation Period
eFigure. Post-Hoc Analysis of Age at Kasai Portoenterostomy, Age at Referral, and Time for Evaluation for Patients in the Pre-Implementation of Screening Group and Sub-groups of Patients in the Post-Implementation of Screening Group