Key Points
Question
Could human exposure to magnetic field nonionizing radiation be associated with increased risk of attention-deficit/hyperactivity disorder in children?
Findings
This birth cohort study found a statistically significant association between a high level of maternal exposure to magnetic field nonionizing radiation, as captured with a monitoring meter, during pregnancy and an increased risk of attention-deficit/hyperactivity disorder in offspring. The association was stronger for children who received a diagnosis of attention-deficit/hyperactivity disorder in adolescence (≥12 years of age) than for those without such a diagnosis in adolescence, and primarily for children with attention-deficit/hyperactivity disorder with immune-related comorbidities.
Meaning
The findings should spur more research to examine the biological association of in utero magnetic field exposure with risk of attention-deficit/hyperactivity disorder in offspring.
Abstract
Importance
An association between maternal exposure to magnetic field (MF) nonionizing radiation during pregnancy and the risk of attention-deficit/hyperactivity disorder (ADHD) has been reported in both animal and human studies.
Objectives
To determine whether maternal exposure to high levels of MF nonionizing radiation is associated with an increased risk of ADHD in offspring by using more accurate measurements of MF nonionizing radiation levels and physician-diagnosed ADHD, rather than self-reports, and to determine whether the association differs for the subtypes of ADHD with or without immune-related comorbidities.
Design, Setting, and Participants
A longitudinal birth cohort study was conducted at Kaiser Permanente Northern California among 1482 mother-child pairs whose mothers were participants of an existing birth cohort and whose level of exposure to MF nonionizing radiation was captured during pregnancy in 2 studies conducted from October 1, 1996, to October 31, 1998, and from May 1, 2006, to February 29, 2012. The offspring were followed up from May 1, 1997, to December 31, 2017.
Exposure
All participating women wore a monitoring meter for 24 hours during pregnancy to capture the level of exposure to MF nonionizing radiation from any sources.
Main Outcomes and Measures
Physician-diagnosed ADHD and immune-related comorbidities of asthma or atopic dermatitis up to 20 years of age in offspring captured in the Kaiser Permanente Northern California electronic medical record from May 1, 1997, to December 31, 2017. Confounders were ascertained during in-person interviews during pregnancy.
Results
Among the 1454 mother-child pairs (548 white [37.7%], 110 African American [7.6%], 325 Hispanic [22.4%], 376 Asian or Pacific Islander [25.9%], and 95 other or unknown [6.5%]; mean [SD] maternal age, 31.4 [5.4] years]), 61 children (4.2%) had physician-diagnosed ADHD. Using Cox proportional hazards regression to account for follow-up time and confounders, compared with children whose mothers had a low level of exposure to MF nonionizing radiation during pregnancy, children whose mothers were exposed to higher levels of MF nonionizing radiation had more than twice the risk of ADHD (adjusted hazard ratio [aHR], 2.01; 95% CI, 1.06-3.81). The association was stronger for ADHD that persisted into adolescence (≥12 years of age), with an aHR of 3.38 (95% CI, 1.43-8.02). When the subtypes of ADHD were examined, the association existed primarily for ADHD with immune-related comorbidities (asthma or atopic dermatitis), with an aHR of 4.57 (95% CI, 1.61-12.99) for all ADHD cases and an aHR of 8.27 (95% CI, 1.96-34.79) for persistent cases of ADHD.
Conclusions and Relevance
Consistent with the emerging literature, this study suggests that in utero exposure to high levels of MF nonionizing radiation was associated with an increased risk of ADHD, especially ADHD with immune-related comorbidity. The findings should spur more research to examine the biological association of in utero MF exposure with risk of ADHD in offspring, given that almost everyone is exposed to it.
This cohort study examines whether maternal exposure to high levels of magnetic field nonionizing radiation is associated with an increased risk of attention-deficit/hyperactivity disorder (ADHD) in offspring.
Introduction
Approximately 11% of all children aged 4 to 17 years (>6.4 million children) in the United States receive a diagnosis of, or treatment for, attention-deficit/hyperactivity disorder (ADHD).1 Attention-deficit/hyperactivity disorder has been associated with poor school performance during childhood and with lifelong disabilities.2 According to the Centers for Disease Control and Prevention, the annual costs associated with ADHD treatment and care are estimated at $42 billion1,3,4 (and as high as $124 billion when burden to the family is included).5,6 The most troubling aspect of pediatric ADHD is that its prevalence has been steadily increasing during the last few decades, with acceleration since 2000.7 Without ruling out genetic susceptibility, such a secular increase points to the presence of important environmental risk factors.
One of the most relevant time windows for environmental risk factors to be associated with brain development is during pregnancy, when fetal brain development is susceptible to external insults that could have a long-lasting effect on brain function and neurobehavior. Among the limited research into the causes of ADHD associated with in utero environmental exposures, to our knowledge, the focus has thus far been on chemicals.8 One nonchemical factor that has not been examined is the ever-present nonionizing radiation, also known as magnetic fields (MFs), emitted from electric appliances, power lines, and wireless devices and networks including cell phone towers. Emerging human studies have begun to report that maternal exposure to MF nonionizing radiation during pregnancy is associated with an increased risk of several childhood illnesses, including immune-related conditions (such as asthma),9 obesity,10 and neurologic conditions (such as ADHD).11,12 An experimental study13 provided further evidence of (1) the direct link between in utero exposure to MF nonionizing radiation and ADHD in offspring and (2) a potential mechanism linking in utero exposure of MF nonionizing radiation with ADHD through altered neuronal developmental programming. Additional evidence from experimental animal studies has also recently been reported.14 Finally, a JAMA report showed that MF nonionizing radiation could affect human brain cell functions.15,16 The emerging evidence indicates that (1) there is a potentially adverse biological association between in utero exposure to MF nonionizing radiation and the health of offspring and (2) fetal brain development and programming is likely one of the vulnerable targets associated with in utero exposure to MF nonionizing radiation.
If research evidence shows that in utero MF nonionizing radiation exposure is a risk factor for ADHD, then this exposure would be a modifiable risk factor. Although almost everyone today is exposed to MF nonionizing radiation to some degree, prevention measures can be implemented to reduce the level of maternal MF nonionizing radiation exposure during pregnancy. Thus, understanding the association between in utero exposure to MF nonionizing radiation and the risk of ADHD would be an important first step. To further examine the association and improve on weaknesses in previous studies, we conducted a prospective birth cohort study with focuses on (1) an objective measurement of maternal exposure to MF nonionizing radiation during pregnancy and (2) a more accurate determination of ADHD cases through a physician’s diagnosis as opposed to maternal self-reporting.
Methods
Study Design and Participants
This study was based on the participants of an existing birth cohort of the Kaiser Permanente Northern California (KPNC) health care delivery system whose members have repeatedly been shown to be representative of the underlying community population.17,18 The mothers were pregnant women who participated in 2 previous studies (one conducted from October 1, 1996, to October 31, 1998, and the other from May 1, 2006, to February 29, 2012) that followed the same study protocol. The level of MF nonionizing radiation exposure was measured by asking participants to wear a monitoring meter throughout a 24-hour monitoring period during pregnancy. Their offspring were followed up from May 1, 1997, to December 31, 2017. The study was approved by the KPNC Institutional Review Board, and all participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Recruitment of Pregnant Women
All pregnant KPNC members aged 18 years or older who resided in the San Francisco Bay Area counties were identified through KPNC electronic medical record data. Those who intended to carry the pregnancy to term at the time of recruitment were eligible for participation in the study. All participants were asked to wear a meter that captured the level of MF nonionizing radiation exposure during a 24-hour monitoring period. An in-person interview was also conducted to ascertain risk factors and potential confounders at the same time. Of 2060 participating pregnant women who had valid measurements for MF nonionizing radiation levels during pregnancy, 1568 delivered live-born offspring, while the rest of the pregnancies ended mostly owing to miscarriage. After excluding those whose offspring did not receive pediatric care at KPNC after delivery, 1482 mother-child dyads were eligible for the present study (eFigure in the Supplement).
Measuring Levels of Exposure to MF Nonionizing Radiation During Pregnancy
Once a pregnant woman had given consent to participate in the study, she was asked to wear an EMDEX meter (Enertech Inc)9,10,19,20 to capture the level of MF nonionizing radiation exposure. During the 24-hour monitoring period, which occurred during the first or second trimester, the meters (EMDEX II and EMDEX Lite) captured levels of 40 to 800 Hz of MF nonionizing radiation encountered by the participating woman throughout her daily life. The MF nonionizing radiation level was measured in milligauss. To avoid potential measurement biases, the meters were calibrated before each use and programmed to show only the time of day, without displaying any MF nonionizing radiation exposure level, so that participants were not aware of their MF nonionizing radiation levels during the measurement period. This design was implemented to avoid changes in any routine daily activities owing to the MF nonionizing radiation level being displayed.
The level of MF exposure has been reported to be relatively stable when measured repeatedly over 12 to 26 months, and the study concluded that the measurement of the MF nonionizing radiation level on a single visit is a good indicator of personal exposure levels during a period of up to 26 months.21 In our study, we also conducted repeated measurements among a subset of 94 participants, and the correlation coefficient was 0.6 between 2 repeated measurements of MF nonionizing radiation, indicating a relatively stable exposure level during pregnancy.
To examine the association of high levels of MF nonionizing radiation with risk of ADHD, we used the 90th percentile of the 24-hour measurements as the MF index, which reflects the MF nonionizing radiation level at or above which a participant was exposed to for 10% of the time during the day. Given that everyone is exposed to MF nonionizing radiation at some levels, to classify participating women into low or high MF nonionizing radiation exposure groups, based on the experience of previous studies,9,10,19 we used a cutoff based on the 25th percentile of the MF index’s distribution, which corresponded to 1.3 mG. The participants whose MF nonionizing radiation level was lower than 1.3 mG were classified as having a low level of MF nonionizing radiation exposure during pregnancy, while the participants whose MF nonionizing radiation level was 1.3 mG or higher were classified as having a relatively higher daily level of MF nonionizing radiation exposure.
Outcome Measurement: ADHD Diagnosis and Immune-Related Comorbidities
All eligible participating children included in the study were followed up to 20 years of age until (1) they received a diagnosis of ADHD, (2) they left the KPNC system, or (3) the study period ended (at the end of 2017). Unlike many previous studies based on self-report, the determination of ADHD in this study was based on a physician’s diagnosis recorded in the KPNC electronic medical records. A diagnosis of ADHD was identified through International Classification of Diseases, Ninth Revision (ICD-9) code 314.x or International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) code F90.x. To avoid ambiguous cases that might have had temporary symptoms similar to ADHD, a child was considered to have had ADHD if there were 2 or more ADHD diagnoses that were at least 1 year apart. We excluded 28 children who had only 1 diagnosis. Thus, the final analyses included 1454 mother-child dyads. To enhance the accuracy of our ADHD case definition, we further identified children who had an ADHD diagnosis that persisted into adolescence (≥12 years of age; persistent cases). The persistent cases were more likely to be true and more severe cases of ADHD. If the exposure of MF nonionizing radiation during pregnancy was associated with ADHD and not with other factors (eg, confounders), one would expect to observe a stronger association with persistent (true) ADHD cases. Thus, confirmation of a stronger association with persistent cases of ADHD than with cases of children with ADHD who did not receive the diagnosis in adolescence could strengthen the observed association.
In addition, emerging literature has shown that some individuals with ADHD have a higher concurrence of immune-related comorbidities, such as asthma and atopic dermatitis (AD); thus, an immune-related cause for ADHD has been proposed.22,23,24,25,26,27,28 At the same time, MF nonionizing radiation exposure has been reported to have an association with the immune system.9,29,30,31,32,33 Thus, it is conceivable that in utero exposure to MF nonionizing radiation may specifically increase the risk of ADHD with an underlying immune cause. To examine this potential causal pathway, we divided children with ADHD into those with immune-related comorbidities (asthma or AD), as reported in the literature,23,24,25,26,27 and those without these comorbidities. Both asthma and AD were identified based on physicians’ diagnoses: ICD-9 codes of 493.x or ICD-10 codes of J45.x for asthma and ICD-9 codes of 691.8x or ICD-10 codes of L20.x for AD (excluding diaper or skin rash). A stronger association with ADHD cases with immune-related comorbidity, combined with a lack of association with ADHD cases without immune-related comorbidity, would strengthen the biological basis for the association between in utero exposure to MF nonionizing radiation and risk of ADHD because it would be consistent with the underlying mechanistic pathway.
Potential Confounders
In-person interviews were conducted with all participants during their pregnancy to ascertain information on many potential confounders. We evaluated several risk factors for ADHD as potential confounders, including maternal age, race/ethnicity, educational level, prenatal smoking and alcohol use, prepregnancy body mass index, sex of offspring, and sociodemographic characteristics.
Statistical Analysis
The Cox proportional hazards regression model was used to control for potential confounders. The Cox survival analysis also has the advantage of accounting for differing durations of follow-up for offspring. All children were followed up starting from birth until (1) they received a diagnosis of ADHD or (2) they were censored (ie, either left the KPNC system or did not have an ADHD diagnosis at the end of the study). We first examined the association between the level of maternal MF nonionizing radiation exposure (high vs low) and the risk of ADHD including all children. We then examined the association for persistent ADHD cases to assess the robustness of the association. Finally, we examined the association separately for ADHD cases with or without the immune-related comorbidities of asthma or AD.
We used the change-in-estimate criterion to identify confounders based on whether the ADHD hazard ratio for MF nonionizing radiation changed by 10% or more when the potential confounder was introduced into the model. A Kaplan-Meier survival curve was used to present the ADHD survival pattern separately for offspring with high or low levels of in utero exposure to MF nonionizing radiation. Consistent with previous studies examining the effect of MF nonionizing radiation,9,10,19,20,34 we did not identify any factors that met the definition for being a confounder using the change-in-estimate criterion. Nevertheless, we included in the model common sociodemographic characteristics, such as maternal age, educational level, and race/ethnicity, as well as some known risk factors for ADHD, such as maternal smoking and alcohol use during pregnancy, prepregnancy body mass index, and sex of offspring. All P values were from 2-sided tests and results were deemed statistically significant at P < .05.
Results
The mean (SD) maternal age of the study population was 31.4 (5.4) years; the racial/ethnic distribution was 548 white (37.7%), 110 African American (7.6%), 325 Hispanic (22.4%), 376 Asian or Pacific Islander (25.9%), and 95 other or unknown (6.5%); 137 (9.4%) reported smoking during pregnancy, and 620 (42.6%) reported alcohol use during pregnancy. Table 1 presents the characteristics of participants based on their high or low levels of MF nonionizing radiation exposure. As similarly shown in previous studies,9,10,19,20,34 the level of MF nonionizing radiation exposure was not associated with commonly known ADHD risk factors or socioeconomic characteristics, including maternal smoking and alcohol use during pregnancy, maternal history of ADHD, educational level, marital status, household income, sex of offspring, and breastfeeding history. Among all the factors examined in Table 1, only maternal age and prepregnancy body mass index showed a statistically significant difference between the 2 groups. Thus, the 2 MF nonionizing radiation exposure groups (the high level and low level) were comparable in all other examined factors that may be associated with the risk of ADHD. In addition, the amount of follow-up time (loss to follow-up) was comparable between the 2 exposure cohorts; by the end of the study (up to 20 years of follow-up), 73.6% (421 of 572) of the participants in the low-level exposure group and 65.1% (574 of 882) of the participants in the high-level exposure group remained within the KPNC system.
Table 1. Characteristics of the Study Population by Daily MF Exposure Level Among Pregnant Women Participants.
| Characteristic | Total No.a | MF at 90th percentile, No. (%) | P valued | |
|---|---|---|---|---|
| Low (n = 572)b | High (n = 882)c | |||
| Maternal age, y | ||||
| ≤25 | 201 | 75 (13.1) | 126 (14.3) | .005 |
| 26-30 | 420 | 145 (25.4) | 275 (31.2) | |
| 31-35 | 472 | 183 (32.0) | 289 (32.8) | |
| ≥36 | 361 | 169 (29.6) | 192 (21.8) | |
| Race/ethnicity | ||||
| White | 548 | 213 (37.2) | 335 (38.0) | .79 |
| African American | 110 | 48 (8.4) | 62 (7.0) | |
| Hispanic | 325 | 125 (21.9) | 200 (22.7) | |
| Asian or Pacific Islander | 376 | 145 (25.4) | 231 (26.2) | |
| Others or unknown | 95 | 41 (7.2) | 54 (6.1) | |
| Educational level | ||||
| <College | 762 | 283 (49.5) | 479 (54.3) | .18 |
| College | 424 | 180 (31.5) | 244 (27.7) | |
| Postgraduate | 268 | 109 (19.1) | 159 (18.0) | |
| Household income, $ | ||||
| <20 000 | 101 | 35 (6.1) | 66 (7.5) | .42 |
| 20 000-40 000 | 253 | 91 (15.9) | 162 (18.4) | |
| >40 000 | 1043 | 423 (74.0) | 620 (70.3) | |
| Marital status | ||||
| Single | 107 | 36 (6.3) | 71 (8.1) | .30 |
| Partner | 187 | 73 (12.8) | 114 (12.9) | |
| Married | 1157 | 463 (80.9) | 694 (78.7) | |
| Smoked since LMP | ||||
| Yes | 137 | 54 (9.4) | 83 (9.4) | .98 |
| No | 1317 | 518 (90.6) | 799 (90.6) | |
| Alcohol use since LMP | ||||
| Yes | 620 | 239 (41.8) | 381 (43.2) | .59 |
| No | 834 | 333 (58.2) | 501 (56.8) | |
| Maternal ADHD history | ||||
| Yes | 14 | 8 (1.4) | 6 (0.7) | .17 |
| No | 1440 | 564 (98.6) | 876 (99.3) | |
| Maternal prepregnancy BMI | ||||
| <25 | 858 | 309 (54.0) | 549 (62.2) | .002 |
| ≥25 | 596 | 263 (46.0) | 333 (37.8) | |
| Preterm delivery | ||||
| Yes | 112 | 41 (7.2) | 71 (8.1) | .56 |
| No | 1335 | 526 (92.3) | 809 (91.9) | |
| Breastfeeding | ||||
| No | 144 | 51 (9.0) | 93 (10.6) | .60 |
| Yes | 1280 | 509 (89.6) | 771 (88.0) | |
| Sex of offspring | ||||
| Male | 755 | 287 (50.2) | 468 (53.1) | .28 |
| Female | 699 | 285 (49.8) | 414 (46.9) | |
| Offspring still enrolled in KPNC at end of study | ||||
| Yes | 995 | 421 (73.6) | 574 (65.1) | .001 |
| No | 459 | 151 (26.4) | 308 (34.9) | |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); KPNC, Kaiser Permanente Northern California; LMP, last menstrual period; MF, magnetic field.
The number in individual categories may not match the total number owing to missing data.
The level of MF nonionizing radiation exposure is lower than 1.3 mG.
The level of MF nonionizing radiation exposure is 1.3 mG or higher.
From χ2 test.
A total of 61 children (4.2%) had physician-diagnosed ADHD. After adjustment for known risk factors for ADHD, including maternal age, educational level, race/ethnicity, maternal smoking and alcohol use during pregnancy, prepregnancy body mass index, and sex of offspring, Table 2 shows that, for all cases of ADHD, maternal daily exposure to higher levels of MF nonionizing radiation (≥1.3 mG) during pregnancy was associated with more than 2 times greater hazard of offspring receiving an ADHD diagnosis, with an adjusted hazard ratio of 2.01 (95% CI, 1.06-3.81). When examining the association separately for children with ADHD who had or had not received the diagnosis during adolescence, we found a stronger association for persistent ADHD, with an adjusted hazard ratio of 3.38 (95% CI, 1.43-8.02), whereas there was no association for children who no longer had an ADHD diagnosis in adolescence (Table 3).
Table 2. Maternal Exposure to MF Nonionizing Radiation During Pregnancy and Risk of ADHD in Offspring.
| MF at 90th percentile | ADHD, No. (%) | aHR (95% CI)a | |
|---|---|---|---|
| Yes | No | ||
| Low (n = 572)b | 12 (2.1) | 560 (97.9) | 1 [Reference] |
| High (n = 882)c | 49 (5.6) | 833 (94.4) | 2.01 (1.06-3.81) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; aHR, adjusted hazard ratio; MF, magnetic field.
Adjusted for maternal age, race/ethnicity, educational level, prenatal smoking and alcohol use, prepregnancy body mass index, and sex of offspring.
The level of MF nonionizing radiation exposure is lower than 1.3 mG.
The level of MF nonionizing radiation exposure is 1.3 mG or higher.
Table 3. Maternal Exposure to MF Nonionizing Radiation During Pregnancy and Risk of ADHD in Offspring With or Without Diagnosis Persisting Into Adolescence.
| MF at 90th percentile | ADHD, No. (%) | aHR (95% CI)a | |
|---|---|---|---|
| Yes | No | ||
| Persistent ADHDb | |||
| Low (n = 566)c | 6 (1.1) | 560 (98.9) | 1 [Reference] |
| High (n = 876)d | 43 (4.9) | 833 (95.1) | 3.38 (1.43-8.02) |
| No ADHD diagnosis in adolescence | |||
| Low (n = 566)c | 6 (1.1) | 560 (98.9) | 1 [Reference] |
| High (n = 839)d | 6 (0.7) | 833 (99.3) | 0.60 (0.19-1.91) |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; aHR, adjusted hazard ratio; MF, magnetic field.
Adjusted for maternal age, race/ethnicity, educational level, prenatal smoking and alcohol use, prepregnancy body mass index, and sex of offspring.
Defined as those who continued to have ADHD diagnoses at 12 years of age or older.
The level of MF nonionizing radiation exposure is lower than 1.3 mG.
The level of MF nonionizing radiation exposure is 1.3 mG or higher.
Given the previously reported associations of MF nonionizing radiation exposure with immune-related diseases and the existence of potential ADHD immune-related causes, we further examined the association of in utero exposure to MF nonionizing radiation with the risk of ADHD separately for children with ADHD and immune-related comorbidities (asthma or AD diagnosis) and children with ADHD without such comorbidities. Table 4 shows the results for all children with ADHD and for children with persistent ADHD. The results show that in utero exposure to MF nonionizing radiation was associated with an increased risk of ADHD primarily for children with immune-related comorbidities of asthma and/or AD, with more than 4 times the risk of ADHD with immune-related comorbidities (adjusted hazard ratio, 4.57; 95% CI, 1.61-12.99). The association was even stronger for persistent ADHD with immune-related comorbidities (adjusted hazard ratio, 8.27; 95% CI, 1.96-34.79). In contrast, no association was observed for children with ADHD without immune-related comorbidities. We also provided the results stratified by immune-related comorbidities in the eTable in the Supplement. The results are consistent with those in Table 4, although the interpretation of the stratified results could be different.
Table 4. Maternal Exposure to MF Nonionizing Radiation During Pregnancy and Risk of ADHD With or Without Immune-Related Comorbiditya.
| MF at 90th percentile | ADHD, No. (%) | aHR (95% CI)b | |
|---|---|---|---|
| Yes | No | ||
| Case definition of ADHDc | |||
| ADHD cases with asthma or AD | |||
| Low (n = 564)d | 4 (0.7) | 560 (99.3) | 1 [Reference] |
| High (n = 868)e | 35 (4.0) | 833 (96.0) | 4.57 (1.61-12.99) |
| ADHD cases without asthma and AD | |||
| Low (n = 568)d | 8 (1.4) | 560 (98.6) | 1 [Reference] |
| High (n = 847)e | 14 (1.7) | 833 (98.4) | 0.84 (0.34-2.04) |
| Persistent ADHDf | |||
| ADHD cases with asthma or AD | |||
| Low (n = 562)d | 2 (0.4) | 560 (99.6) | 1 [Reference] |
| High (n = 865)e | 32 (3.7) | 833 (96.3) | 8.27 (1.96-34.79) |
| ADHD cases without asthma and AD | |||
| Low (n = 564)d | 4 (0.7) | 560 (99.3) | 1 [Reference] |
| High (n = 844)e | 11 (1.3) | 833 (98.7) | 1.12 (0.34-3.64) |
Abbreviations: AD, atopic dermatitis; ADHD, attention-deficit/hyperactivity disorder; aHR, adjusted hazard ratio; MF, magnetic field.
Asthma or AD.
Adjusted for maternal age, race/ethnicity, educational level, prenatal smoking and alcohol use, prepregnancy body mass index, and sex of offspring.
Case definition of ADHD is defined as those who had 2 ADHD diagnoses at least 1 year apart.
The level of MF nonionizing radiation exposure is lower than 1.3 mG.
The level of MF nonionizing radiation exposure is 1.3 mG or higher.
Defined as those who continued to have ADHD diagnoses at 12 years of age or older.
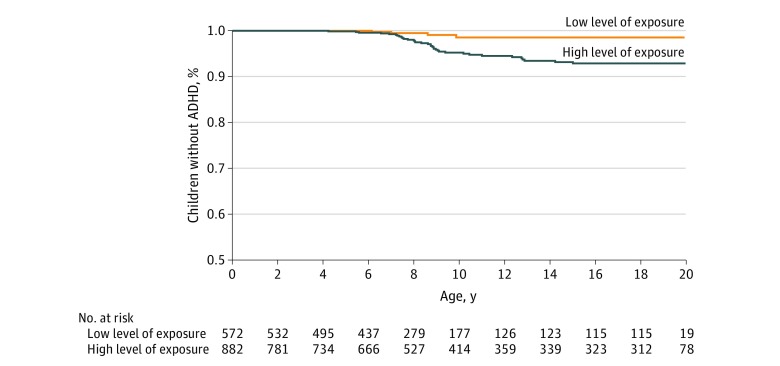
The Figure provides the Kaplan-Meier survival curve showing the proportion of offspring who remained free of ADHD with immune-related comorbidity throughout childhood, from birth up to 20 years of age. This graph shows that, starting at an early age when ADHD was normally diagnosed (around 5 years of age), there was a separation of the ADHD survival probability between the 2 MF nonionizing radiation exposure groups. Offspring whose mothers were exposed to high levels of MF nonionizing radiation during pregnancy had a consistently higher risk of ADHD (lower probability of remaining ADHD free) throughout childhood compared with offspring whose mothers were exposed to lower levels of MF nonionizing radiation during pregnancy.
Figure. Kaplan-Meier Survival Curve for Attention-Deficit/Hyperactivity Disorder (ADHD) With Immune-Related Comorbidities by Maternal Magnetic Field Nonionizing Radiation Exposure During Pregnancy.
Discussion
In this large birth cohort study of 1454 mother-child dyads with rarely available data on maternal MF nonionizing radiation exposure during pregnancy captured with a monitoring meter, we followed up the offspring of participating women for physician-diagnosed ADHD and immune-related comorbidities (asthma and AD) up to 20 years of age. We observed that in utero exposure to higher levels of MF nonionizing radiation was associated with more than 2 times the risk of ADHD. The association became stronger for children with persistent ADHD whose condition remained present into adolescence (Table 3). The observed association was mainly for children with ADHD and concurrent immune-related comorbidities (asthma and/or AD), which is consistent with the reported association between exposure to MF nonionizing radiation and the immune system.9,29,30,31,32 Thus, in addition to the overall association, the additional observations of a stronger association with persistent ADHD and ADHD with current immune-related comorbidities are consistent with the underlying biological plausibility and strengthen the observed association.
Given the limited understanding of the causes of ADHD, these findings could lead to a new research direction to elucidate environmental risk factors for ADHD during fetal development. These new findings would need to be replicated in future studies. If confirmed, the increasingly ubiquitous exposure to MF nonionizing radiation in human populations, including pregnant women, could be an important, nonetheless modifiable, risk factor for ADHD. Currently, the causes and the secular increase in incidence of ADHD remain poorly understood.
Although understanding its pathologic pathways remains limited at this point, in utero exposure to MF nonionizing radiation has been associated with neuropathologic changes in the brain,13 especially in the prefrontal cortex, including impaired glutamatergic synaptic transmission onto layer V pyramidal neurons of the prefrontal cortex. Thus, there is biological plausibility for in utero exposure to MF nonionizing radiation to increase the risk of ADHD in offspring through fetal development of both the brain and the immune system.
Given that the exposure of interest was maternal MF nonionizing radiation exposure during pregnancy, the factors that existed after birth, by definition, cannot act as confounders because they occurred after the exposure; thus, they are likely on the causal pathway. Other factors (eg, demographic characteristics and maternal smoking and alcohol use) did not meet the criteria of being a confounder to be associated with both the MF nonionizing radiation exposure and ADHD, as already described. Nevertheless, we included them in the regression models, and adjustment for those variables did not change the results.
Limitations and Strengths
This study has some limitations. Despite the significantly improved accuracy of our MF nonionizing radiation exposure measurements compared with previous studies, we were not able to ask women to carry the meter throughout pregnancy because it was not feasible. Thus, there may still be some inaccuracy in maternal MF nonionizing radiation exposure level. Given that this was a prospective study design, maternal MF nonionizing radiation exposure was ascertained during pregnancy before ADHD diagnosis in offspring. Any measurement inaccuracy, if it existed, would have been nondifferential (ie, not associated with the ADHD outcome). In principle, nondifferential measurement error would have attenuated the observed association. In other words, without the measurement error, the observed association would have been even stronger. In addition, the study measured MF nonionizing radiation at a low frequency (≤800 Hz); thus, the findings may not apply to MF nonionizing radiation at higher frequencies.
This study also has some strengths, including (1) a prospective design, thus reducing the likelihood of participation bias; (2) physician-diagnosed, rather than self-reported, ADHD, thus increasing the accuracy of outcome measurement; and (3) an objective measure of maternal MF nonionizing radiation level ascertained by a meter to reduce measurement error for the exposure.
Conclusions
Using a longitudinal birth cohort study design, we examined the association between maternal exposure to MF nonionizing radiation during pregnancy and risk of ADHD in offspring throughout childhood up to age 20 years. The study improved over previous studies by enhancing the accuracy of measuring MF nonionizing radiation exposure and of ADHD diagnosis. The findings provide new evidence that in utero exposure to a high level of MF nonionizing radiation is associated with an increased risk of ADHD in offspring. The association is primarily between MF nonionizing radiation exposure and ADHD with immune-related comorbidities and persistent cases of ADHD. The findings reveal a possible new risk factor that is ubiquitous in our modern day lives and should spur more research to examine this potential association.
eFigure. Flow Chart for the Study Population
eTable. Maternal Exposure to MF Nonionizing Radiation During Pregnancy and Risk of ADHD Stratified by the Presence or Absence of Immune-Related Comorbidity
References
- 1.Centers for Disease Control and Prevention Attention-deficit/hyperactivity disorder (ADHD). Accessed December 10, 2018. https://www.cdc.gov/ncbddd/adhd/research.html
- 2.García Murillo L, Ramos-Olazagasti MA, Mannuzza S, Castellanos FX, Klein RG. Childhood attention-deficit/hyperactivity disorder and homelessness: a 33-year follow-up study. J Am Acad Child Adolesc Psychiatry. 2016;55(11):-. doi: 10.1016/j.jaac.2016.07.772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Healthline. ADHD by the Numbers: Facts, Statistics, and You. Accessed January 8, 2020. https://www.healthline.com/health/adhd/facts-statistics-infographic
- 4.National Institute of Mental Health Attention-deficit/hyperactivity disorder (ADHD). Accessed March 3, 2019. https://www.nimh.nih.gov/health/statistics/attention-deficit-hyperactivity-disorder-adhd.shtml
- 5.Zhao X, Page TF, Altszuler AR, et al. . Family burden of raising a child with ADHD. J Abnorm Child Psychol. 2019;47(8):1327-1338. doi: 10.1007/s10802-019-00518-5 [DOI] [PubMed] [Google Scholar]
- 6.Gupte-Singh K, Singh RR, Lawson KA. Economic burden of attention-deficit/hyperactivity disorder among pediatric patients in the United States. Value Health. 2017;20(4):602-609. doi: 10.1016/j.jval.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 7.Visser SN, Danielson ML, Bitsko RH, et al. . Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry. 2014;53(1):34-46.e2. doi: 10.1016/j.jaac.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Froehlich TE, Anixt JS, Loe IM, Chirdkiatgumchai V, Kuan L, Gilman RC. Update on environmental risk factors for attention-deficit/hyperactivity disorder. Curr Psychiatry Rep. 2011;13(5):333-344. doi: 10.1007/s11920-011-0221-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li DK, Chen H, Odouli R. Maternal exposure to magnetic fields during pregnancy in relation to the risk of asthma in offspring. Arch Pediatr Adolesc Med. 2011;165(10):945-950. doi: 10.1001/archpediatrics.2011.135 [DOI] [PubMed] [Google Scholar]
- 10.Li DK, Ferber JR, Odouli R, Quesenberry CP Jr. A prospective study of in-utero exposure to magnetic fields and the risk of childhood obesity. Sci Rep. 2012;2:540. doi: 10.1038/srep00540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birks L, Guxens M, Papadopoulou E, et al. . Maternal cell phone use during pregnancy and child behavioral problems in five birth cohorts. Environ Int. 2017;104:122-131. doi: 10.1016/j.envint.2017.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lai H. Neurological effects of non-ionizing electromagnetic fields. Published March 2014. Accessed July 6, 2017. https://bioinitiative.org/wp-content/uploads/pdfs/sec09_2012_Evidence_Effects_Neurology_behavior.pdf
- 13.Aldad TS, Gan G, Gao XB, Taylor HS. Fetal radiofrequency radiation exposure from 800-1900 mHz-rated cellular telephones affects neurodevelopment and behavior in mice. Sci Rep. 2012;2:312. doi: 10.1038/srep00312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Othman H, Ammari M, Sakly M, Abdelmelek H. Effects of prenatal exposure to WIFI signal (2.45 GHz) on postnatal development and behavior in rat: Influence of maternal restraint. Behav Brain Res. 2017;326:291-302. doi: 10.1016/j.bbr.2017.03.011 [DOI] [PubMed] [Google Scholar]
- 15.Volkow ND, Tomasi D, Wang GJ, et al. . Effects of cell phone radiofrequency signal exposure on brain glucose metabolism. JAMA. 2011;305(8):808-813. doi: 10.1001/jama.2011.186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai H, Hardell L. Cell phone radiofrequency radiation exposure and brain glucose metabolism. JAMA. 2011;305(8):828-829. doi: 10.1001/jama.2011.201 [DOI] [PubMed] [Google Scholar]
- 17.Gordon NP. A Comparison of Sociodemographic and Health Characteristics of the Kaiser Permanente Northern California Membership Derived From Two Data Sources: The 2008 Member Health Survey and the 2007 California Health Interview Survey. Kaiser Permanente Division of Research; January 2012. [Google Scholar]
- 18.Gordon NP. Similarity of the Adult Kaiser Permanente Membership in Northern California to the Insured and General Population in Northern California: Statistics from the 2011-12 California Health Interview Survey. Kaiser Permanente Division of Research; June 2015. [Google Scholar]
- 19.Li DK, Odouli R, Wi S, et al. . A population-based prospective cohort study of personal exposure to magnetic fields during pregnancy and the risk of miscarriage. Epidemiology. 2002;13(1):9-20. doi: 10.1097/00001648-200201000-00004 [DOI] [PubMed] [Google Scholar]
- 20.Li DK, Chen H, Ferber JR, Odouli R, Quesenberry C. Exposure to magnetic field non-ionizing radiation and the risk of miscarriage: a prospective cohort study. Sci Rep. 2017;7(1):17541. doi: 10.1038/s41598-017-16623-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bracken TD, Rankin RF, Senior RS, Alldredge JR The EMDEX Project: Residential Study, Final Report. Electric Power Research Institute; 1994.
- 22.Zhou RY, Wang JJ, Sun JC, You Y, Ying JN, Han XM. Attention deficit hyperactivity disorder may be a highly inflammation and immune-associated disease (review). Mol Med Rep. 2017;16(4):5071-5077. doi: 10.3892/mmr.2017.7228 [DOI] [PubMed] [Google Scholar]
- 23.Lin YT, Chen YC, Gau SS, et al. . Associations between allergic diseases and attention deficit hyperactivity/oppositional defiant disorders in children. Pediatr Res. 2016;80(4):480-485. doi: 10.1038/pr.2016.111 [DOI] [PubMed] [Google Scholar]
- 24.Yang CF, Yang CC, Wang IJ. Association between allergic diseases, allergic sensitization and attention-deficit/hyperactivity disorder in children: a large-scale, population-based study. J Chin Med Assoc. 2018;81(3):277-283. doi: 10.1016/j.jcma.2017.07.016 [DOI] [PubMed] [Google Scholar]
- 25.Lee CY, Chen MH, Jeng MJ, et al. . Longitudinal association between early atopic dermatitis and subsequent attention-deficit or autistic disorder: a population-based case-control study. Medicine (Baltimore). 2016;95(39):e5005. doi: 10.1097/MD.0000000000005005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang LJ, Yu YH, Fu ML, et al. . Attention deficit-hyperactivity disorder is associated with allergic symptoms and low levels of hemoglobin and serotonin. Sci Rep. 2018;8(1):10229. doi: 10.1038/s41598-018-28702-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki C, Koyama M, Ota E, et al. . Allergic diseases in children with attention deficit hyperactivity disorder: a systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):120. doi: 10.1186/s12888-017-1281-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Instanes JT, Halmøy A, Engeland A, Haavik J, Furu K, Klungsøyr K. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry. 2017;81(5):452-459. doi: 10.1016/j.biopsych.2015.11.024 [DOI] [PubMed] [Google Scholar]
- 29.Boscolo P, Di Gioacchino M, Di Giampaolo L, Antonucci A, Di Luzio S. Combined effects of electromagnetic fields on immune and nervous responses. Int J Immunopathol Pharmacol. 2007;20(2)(suppl 2):59-63. doi: 10.1177/03946320070200S212 [DOI] [PubMed] [Google Scholar]
- 30.Misa-Agustiño MJ, Leiro-Vidal JM, Gomez-Amoza JL, et al. . EMF radiation at 2450 MHz triggers changes in the morphology and expression of heat shock proteins and glucocorticoid receptors in rat thymus. Life Sci. 2015;127:1-11. doi: 10.1016/j.lfs.2015.01.027 [DOI] [PubMed] [Google Scholar]
- 31.Salehi I, Sani KG, Zamani A. Exposure of rats to extremely low-frequency electromagnetic fields (ELF-EMF) alters cytokines production. Electromagn Biol Med. 2013;32(1):1-8. doi: 10.3109/15368378.2012.692343 [DOI] [PubMed] [Google Scholar]
- 32.Di Giampaolo L, Di Donato A, Antonucci A, et al. . Follow up study on the immune response to low frequency electromagnetic fields in men and women working in a museum. Int J Immunopathol Pharmacol. 2006;19(4)(suppl):37-42. [PubMed] [Google Scholar]
- 33.Yost MG, Burch JB. A recurring question: are there health effects of power-frequency magnetic fields? Arch Pediatr Adolesc Med. 2011;165(10):959-961. doi: 10.1001/archpediatrics.2011.169 [DOI] [PubMed] [Google Scholar]
- 34.Lee GM, Neutra RR, Hristova L, Yost M, Hiatt RA. A nested case-control study of residential and personal magnetic field measures and miscarriages. Epidemiology. 2002;13(1):21-31. doi: 10.1097/00001648-200201000-00005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Flow Chart for the Study Population
eTable. Maternal Exposure to MF Nonionizing Radiation During Pregnancy and Risk of ADHD Stratified by the Presence or Absence of Immune-Related Comorbidity