Abstract
Nocardiopsis species are aerobic, gram-positive, non-acid fast rods isolated from soil, waters, and animals. They are opportunistic human pathogens, but very few cases have been published so far. We report the first case of fatal pulmonary infection related to Nocardiopsis dassonvillei in an immunocompetent patient with chronic obstructive pulmonary disease.
Keywords: 16S rDNA sequencing, Chronic obstructive, pulmonary disease, Emerging pathogen, Nocardiopsis dassonvillei, Pulmonary infection
In February 2019, a 85-year-old man with chronic obstructive pulmonary disease (COPD) was admitted to the pneumology department at Fréjus Hospital (France) for worsening of chronic dyspnoea, asthenia and fever. COPD exacerbation was suspected by his general practitioner 10 days before, but the patient had unfavourable evolution despite oral receipt of 7 days of amoxicillin 1 g three times a day and levofloxacin 500 mg two times a day. Noteworthy medical history consisted of COPD (GOLD 2019 stage 3D) and emphysema treated by inhaled salmeterol and fluticasone 1000/100 μg/day, tiotropium, clarithromycin 3 days a week and long-term oxygen therapy (3 L/min). In August 2014, he was diagnosed with chronic pulmonary aspergillosis and received voriconazole for a period of 6 months. At admission, the patient's haemodynamic status was stable. The pulse rate was 68 beats/min, blood pressure 123/65 mm Hg and peripheral oxygen saturation 93% on oxygen 3 L/min. Body temperature was 38.5°C and Quick Sequential Organ Failure Assessment (qSOFA) score was 2. Chest auscultation revealed diffuse rhonchi and bibasilar crackles. The rest of the physical examination was unremarkable.
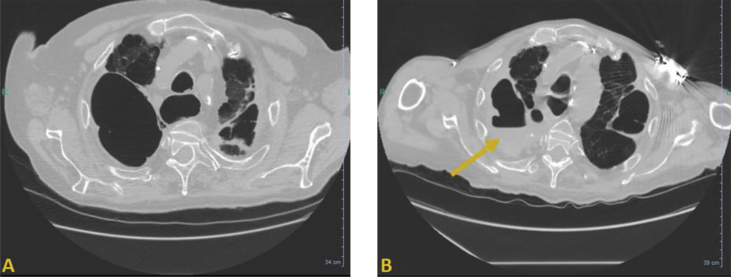
Laboratory investigations revealed inflammatory markers such as elevated C-reactive protein (73 mg/L), normocytic anaemia (9.0 g/dL) and neutrophilia (10.5 × 109/L). Urinary antigen test for Legionella pneumophila was negative. Blood cultures were performed and remained sterile. Chest computed tomographic scan revealed known emphysema in the right upper lobe and revealed a hydroaeric level suggesting a pulmonary infection (Fig. 1). Empirical intravenous antibiotic therapy was initiated consisting of cefotaxime, amikacin and metronidazole. After 48 hours of treatment, the patient was persistently febrile and a bronchoalveolar lavage was performed. Gram staining performed directly on the purulent fluid yielded numerous neutrophils and a few yeast. Culture grew numerous dry dissimilar crinkled colonies displaying a white pigmentation with a soil-like odor on sheep's blood and chocolate agar plates after 4 days of incubation under aerobic atmosphere. Gram staining performed on colonies revealed Gram-positive branched rods.
Fig. 1.
Chest computed tomographic scan (axial plane) (A) in 2016 showing severe emphysema in right upper lobe and (B) in 2019 showing appearance of hydroaeric level (arrow), suggesting pulmonary infection.
The strain was sent to our laboratory at Nice University Hospital for identification. MALDI-TOF MS (Bruker, Germany) analysis failed to identify the isolate even after a sample preparation method using an ethanol treatment followed by extraction by formic acid and acetonitrile. Unfortunately, the very slow bacterial growth delayed the time to accurate identification. Species-level identification was finally achieved 11 days after the initiation of antibiotic therapy using 16S recombinant DNA (rDNA) gene sequencing performed from colonies, as previously described [1]. 16S rDNA nucleotide sequence alignment showed that there was 99.92% homology between the sequence of the clinical isolate and the 16S rDNA sequence of Nocardiopsis dassonvillei type strain (DSM 43111, accession no. NR_074635). Antibiotic susceptibility testing was performed using Etest strips (bioMérieux, France) on Müller-Hinton medium and Clinical and Laboratory Standards Institute interpretative standards for Nocardia spp [2]. We tested the primary recommended antibiotic list [2]. MIC values were determined after 72 hours of incubation at 35°C under aerobic conditions (Table 1). Despite a brief improvement of clinical and biological parameters such as fever and C-reactive protein level, the patient's neurologic and pulmonary status continued to deteriorate, and he died 11 days after admission and initiation of the antibiotic treatment.
Table 1.
Antimicrobial susceptibility testing of clinical isolated determined by Etest method
| Antimicrobial agent | MIC (μg/mL) | Clinical categorization |
|---|---|---|
| Amikacin | 0.064 | S |
| Amoxicillin/clavulanic acid | 1 | S |
| Ceftriaxone | 4 | S |
| Ciprofloxacin | 1 | S |
| Clarithromycin | 4 | R |
| Imipenem | 0.19 | S |
| Linezolid | 0.25 | S |
| Minocycline | 0.032 | S |
| Trimethoprim/sulfamethoxazole | 0.032 | S |
| Tobramycin | 0.016 | S |
Interpretations of MICs in terms of susceptibility for Nocardia made according to Clinical and Laboratory Standards Institute criteria [2]. R, resistant; S, susceptible.
The genus Nocardiopsis was first described by Meyer in 1976 [3] and is now part of the Nocardiopsaceae family. Nocardiopsis species are Gram-positive, non–acid-fast, catalase-positive actinomycetes with nocardioform substrate mycelia. These strictly aerobic bacteria were first isolated from marine forms, terrestrial forms and soil [4,5]. To date, two species—Nocardiopsis synnemataformans and N. dassonvillei—are considered to be potential opportunistic pathogens in human. N. dassonvillei has been involved in rare cases of infection, especially in patients with cutaneous [6], subcutaneous [7] and respiratory [8,9] diseases. Only a single case of bloodstream infection has been described with a favourable outcome [10].
To our knowledge, this is the first report of a case of fatal infection caused by N. dassonvillei, but it confirms the invasive potential of this pathogen. Our patient manifested an acute exacerbation of his severe underlying respiratory disease due to N. dassonvillei infection. This microorganism is likely the main cause of the patient's death. Indeed, N. dassonvillei was the only bacterium isolated in culture of the bronchoalveolar lavage sample (105 CFU/mL). A low number of Candida albicans colonies were also isolated on the agar plate (<103 CFU/mL), but this microorganism is not commonly considered to be a pulmonary pathogen. Furthermore, the patient showed an unfavourable response to initial nonoptimal empiric antibiotic therapy (ceftriaxone MIC, 4 mg/L). Finally, local factors may have promoted lung colonization and infection such as emphysema, sequelae of Aspergillosis infection and inhaled corticoids for COPD. Concerning the source of contamination, cutaneous infections caused by N. dassonvillei are known to be acquired through traumatic inoculation of the organism from soil or organic matter [6,7]. However, little is known about the exact source of contamination in N. dassonvillei respiratory infections. In our patient with COPD, airborne contamination through environmental exposure and pulmonary inhalation was likely—a route which has been previously suggested [9].
N. dassonvillei should be considered as a true emerging pathogen when isolated in respiratory specimens in patients with underlying respiratory disease. Hygiene control teams should look for an environmental reservoir as the source of contamination. 16S rDNA sequencing is a reliable tool to accurately identify this pathogen and to increase our knowledge concerning N. dassonvillei infections.
Conflict of Interest
None declared.
Acknowledgements
We thank C. Besancon, C. Blanc, S. Courtial, S. Esposito and S. Carpentier from the technician's team of the bacteriology laboratory of Nice academic hospital and the entire technician's team of the laboratory for their technical assistance.
References
- 1.Ruimy R., Breittmayer V., Elbaze P., Lafay B., Boussemart O., Gauthier M. Phylogenetic analysis and assessment of the genera Vibrio, photobacterium, aeromonas, and plesiomonas deduced from small-subunit rRNA sequences. Int J Syst Bacteriol. 1994;44:416e26. doi: 10.1099/00207713-44-3-416. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute . Susceptibility testing of mycobacteria, nocardiae, and other aerobic Actinomycetes; approved standard. CLSI document M24-A2. 2nd ed. Clinical and Laboratory Standards Institute; Wayne, PA: 2011. [PubMed] [Google Scholar]
- 3.Meyer J. Nocardiopsis, a new genus of the order Actinomycetales. Int J Syst Bacteriol. 1976;26:487–493. [Google Scholar]
- 4.Bennur T., Kumar A.R., Zinjarde S., Javdekar V. Nocardiopsis species: incidence, ecological roles and adaptations. Microbiol Res. 2015;174:33–47. doi: 10.1016/j.micres.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Ibrahim A.H., Desoukey S.Y., Fouad M.A., Kamel M.S., Gulder T.A.M., Abdelmohsen U.R. Natural product potential of the genus Nocardiopsis. Mar Drugs. 2018;16:147. doi: 10.3390/md16050147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.González-López M.A., González-Vela M.C., Salas-Venero C.A., Conde R., Val-Bernal J.F. Cutaneous infection caused by Nocardiopsis dassonvillei presenting with sporotrichoid spread. J Am Acad Dermatol. 2011;65:e90–e91. doi: 10.1016/j.jaad.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Sindhuphak W., Macdonald E., Head E. Actinomycetoma caused by Nocardiopsis dassonvillei. Arch Dermatol. 1985;121:1332–1334. [PubMed] [Google Scholar]
- 8.Rudramurthy M., Sumangala B, Honnavar P, Madhav YB, Munegowda KC, Ravi D. Nasal vestibulitis due to Nocardiopsis dassonvillei in a diabetic patient. J Med Microbiol. 2012;61(pt 8):1168–1173. doi: 10.1099/jmm.0.038240-0. [DOI] [PubMed] [Google Scholar]
- 9.Mordarska H., Zakrzewska-Czerwiñska J., Paściak M., Szponar B., Rowiñski S. Rare, suppurative pulmonary infection caused by Nocardiopsis dassonvillei recognized by glycolipid markers. FEMS Immunol Med Microbiol. 1998;21:47–55. doi: 10.1111/j.1574-695X.1998.tb01148.x. [DOI] [PubMed] [Google Scholar]
- 10.Beau F., Bollet C., Coton T., Garnotel E., Drancourt M. Molecular identification of a Nocardiopsis dassonvillei blood isolate. J Clin Microbiol. 1999;37:3366–3368. doi: 10.1128/jcm.37.10.3366-3368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]