Summary
Background
Estimates of incidence of switching to second-line antiretroviral therapy (ART) among children with HIV are necessary to inform the need for paediatric second-line formulations. We aimed to quantify the cumulative incidence of switching to second-line ART among children in an international cohort collaboration.
Methods
In this international cohort collaboration study, we pooled individual patient-level data for children younger than 18 years who initiated ART (two or more nucleoside reverse-transcriptase inhibitors [NRTI] plus a non-NRTI [NNRTI] or boosted protease inhibitor) between 1993 and 2015 from 12 observational cohort networks in the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global Cohort Collaboration. Patients who were reported to be horizontally infected with HIV and those who were enrolled in trials of treatment monitoring, switching, or interruption strategies were excluded. Switch to second-line ART was defined as change of one or more NRTI plus either change in drug class (NNRTI to protease inhibitor or vice versa) or protease inhibitor change, change from single to dual protease inhibitor, or addition of a new drug class. We used cumulative incidence curves to assess time to switching, and multivariable proportional hazards models to explore patient-level and cohort-level factors associated with switching, with death and loss to follow-up as competing risks.
Findings
At the data cutoff of Sept 16, 2015, 182 747 children with HIV were included in the CIPHER dataset, of whom 93 351 were eligible, with 83 984 (90·0%) from sub-Saharan Africa. At ART initiation, the median patient age was 3·9 years (IQR 1·6–6·9) and 82 885 (88·8%) patients initiated NNRTI-based and 10 466 (11·2%) initiated protease inhibitor-based regimens. Median duration of follow-up after ART initiation was 26 months (IQR 9–52). 3883 (4·2%) patients switched to second-line ART after a median of 35 months (IQR 20–57) of ART. The cumulative incidence of switching at 3 years was 3·1% (95% CI 3·0–3·2), but this estimate varied widely depending on the cohort monitoring strategy, from 6·8% (6·5–7·2) in settings with routine monitoring of CD4 (CD4% or CD4 count) and viral load to 0·8% (0·6–1·0) in settings with clinical only monitoring. In multivariable analyses, patient-level factors associated with an increased likelihood of switching were male sex, older age at ART initiation, and initial NNRTI-based regimen (p<0·0001). Cohort-level factors that increased the likelihood of switching were higher-income country (p=0·0017) and routine or targeted monitoring of CD4 and viral load (p<0·0001), which was associated with a 166% increase in likelihood of switching compared with CD4 only monitoring (subdistributional hazard ratio 2·66, 95% CI 2·22–3·19).
Interpretation
Our global paediatric analysis found wide variations in the incidence of switching to second-line ART across monitoring strategies. These findings suggest the scale-up of viral load monitoring would probably increase demand for paediatric second-line ART formulations.
Introduction
In 2017, an estimated 1·8 million children (younger than 15 years) were living with HIV worldwide, of whom 52% had access to antiretroviral therapy (ART).1 A concerted effort will be needed to achieve the ambitious UNAIDS 90–90-90 goals to end AIDS by 2020 among children: ensuring that 90% of children living with HIV are diagnosed, 90% of those diagnosed are on ART, and 90% of those on ART attain and maintain viral suppression.2 Children and adolescents have persistently lagged behind adults in their progress towards the first two 90% targets,3 leading to increased efforts to expand access to HIV diagnosis and ART for children across a variety of clinical settings in several countries.4 As more children receive ART and treatment programmes mature, development of strategies to meet the third 90% target of sustained viral suppression will be the long-term challenge. Achievement of this goal requires a comprehensive understanding of the durability of first-line ART regimens and patterns of switching to second-line ART across geographical regions and different country-income settings to ensure future treatment needs are met.5
The short-term effectiveness of ART in children is undisputed, with high survival, immune and growth recovery, and the proportion of patients with a suppressed viral load at 12 months after initiation of ART ranging from 70% to 95%.6–8 Comparatively less data are available on the durability of first-line ART and the use of second-line treatment in children. The PENPACT trial,9 which comprised patients from predominately high-income countries, reported that 71% (188 of 266) of children remained on their first-line regimen 5 years after starting ART, compared with 95% or more of children in the CHER8 and ARROW7 trials that comprised children from Africa. Observational cohorts have reported wide variations in the probability of switching to second-line ART after treatment failure, with the definition of treatment failure varying between studies. One large South African observational cohort10 reported that 19% of children (Kaplan-Meier estimate, 95% CI 18–21) had virological failure 3 years after initiation of ART. Among the 252 children with 1 year or more of follow-up after virological failure, 38% (95% CI 32–45) switched to second-line ART. In a west African cohort,11 322 (12%) of 2676 children with HIV had clinical-immunological failure after 24 months of ART, of whom 21 (7%) switched to second-line ART. Other cohort studies in Asia12 and Europe13 have reported a 17–23% probability of children switching to second-line ART by 5 years after initiation of ART. Comparison across these studies is difficult because of the heterogeneity of patients’ characteristics, initial ART regimens, monitoring strategies, and the varying definitions of treatment failure and switching.
We aimed to provide the first global estimates of the incidence of switching to second-line ART among children with HIV using a uniform definition of switching, and to assess associated factors (ie, patient-level and cohort-level factors). This analysis is a key step in understanding the use of second-line regimens globally and across programmes that operate under various strategies for treatment monitoring and guidelines for switching to second-line ART.
Methods
Study design and population
In this international cohort collaboration study, we pooled data from the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) network. CIPHER is a global network of observational paediatric HIV cohorts. The collaboration includes 12 international networks: Baylor International Pediatric AIDS Initiative (BIPAI); European Pregnancy and Paediatric HIV Cohort Collaboration (EPPICC); the International Epidemiology Databases to Evaluate AIDS (IeDEA) Consortium, comprising IeDEA Asia-Pacific, IeDEA Central Africa, IeDEA East Africa, IeDEA Southern Africa, IeDEA West Africa, and Caribbean, Central and South America network (CCASAnet); International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) 219C and P1074; Médecins Sans Frontières (MSF); Optimal Models (ICAP at Columbia University); and Pediatric HIV/AIDS Cohort Study (PHACS). The network covers 52 countries and most networks comprised multiple cohorts and each cohort included data from one or more clinics (primary care clinic, primary community clinic, or hospital).
We pooled individual participant-level data from the CIPHER dataset for patients younger than 10 years at enrolment (a proxy for perinatal HIV infection), younger than 18 years at initiation of a standard combination ART regimen (ie, at least three antiretroviral drugs, including at least two nucleoside reverse-transcriptase inhibitors [NRTIs] plus either a non-NRTI [NNRTI] or a ritonavir-boosted protease inhibitor) between Jan 1, 1993, and Sept 1, 2015, and with 1 day or more of follow-up after initiation of ART. Patients who were reported to be horizontally infected and those enrolled in clinical trials of treatment monitoring, switching, or interruption strategies were excluded.
Individual patient-level data were sent to the University of Cape Town (Cape Town, South Africa) for data cleaning and data management using a standardised protocol based on the HIV Cohorts Data Exchange Protocol. The dataset was then sent to the University College London (London, UK) for analysis.
All participating networks received local ethics approvals to transfer anonymised data for this study. The pooling of data at the University of Cape Town was approved by the University of Cape Town Health Research Ethics Committee (UCT HREC [reference 264/2014]).
Outcomes
The main study outcome was cumulative incidence of all-cause switching to second-line ART from first-line ART for any reason (hereafter referred to as switching). Switching was defined as change of one or more NRTI plus either change in drug class (NNRTI to protease inhibitor or vice versa) or protease inhibitor change, change from single to dual protease inhibitor, or addition of a new drug class. With this definition we endeavoured to capture major treatment changes because of treatment failure or major toxic side-effects of drugs and allow for comparisons with previous analyses of switching in children that used similar approaches.12,13
Statistical analysis
We explored cohort-level and patient-level potential predictors for switching. We generated cohort-level factors, which were geographical region, treatment monitoring strategy, and country-income group. The geographical regions with eligible data were categorised as Europe, the USA, Asia, Latin America (ie, the Caribbean, and Central and South America), southern Africa, and the rest of sub-Saharan Africa. Southern Africa was defined as Botswana and South Africa and was considered separate from the rest of sub-Saharan Africa because lopinavir-based regimens were introduced in 2010 as first-line ART for children younger than 3 years in Botswana and South Africa and are not part of the standardised first-line regimen in the rest of sub-Saharan Africa, and Botswana and South Africa also had early roll-out of routine viral load monitoring in 2007–08,14,15 unlike the rest of sub-Saharan Africa.16
Strategies for treatment monitoring were assigned at the cohort level according to the presence and frequency of measurements of CD4 (CD4% or CD4 count) and viral load. Cohorts were classified as having routine monitoring of CD4 or viral load if more than 60% of children had one or more measurement of CD4 or viral load after initiation of ART and the median time between consecutive measurements was fewer than 60 weeks. Cohorts were classified as having targeted monitoring of CD4 or viral load if 5–60% of children had one or more measurements of CD4 or viral load after initiation of ART, or if more than 60% of children had one or more measurements but consecutive measures were more than 60 weeks apart. On the basis of these definitions, cohorts were classified into four groups: routine monitoring of CD4 and viral load, routine monitoring of CD4 and targeted monitoring of viral load, routine monitoring of CD4 only (<5% of children with viral load measurements), or clinical monitoring only (ie, targeted monitoring of CD4 only, or <5% of participants have measurements of CD4 and viral load). Country-income groups were assigned by use of the World Bank classification17 (high-income and upper-middle-income, lower-middle-income, or low-income economies) at the median year of ART initiation in the cohort.
For the HIV Cohorts Data Exchange Protocol website see http://www.hicdep.org
We investigated the following patient-level independent variables measured at ART initiation: sex, age (<3, 3–5, 6–9, and ≥10 years), known previous AIDS diagnosis (WHO stage 3–4 or US Centres for Disease Control and Prevention stage C; yes or no), initial ART regimen (protease inhibitor or NNRTI based), and calendar year (≤2004, 2005–2007, 2008–2010, and ≥2011). We also collected data on bodyweight and CD4% or CD4 count at initiation of ART where available.
We calculated the weight-for-age Z scores relative to the 1990 British Growth Reference values in Stata18 (≤−2, >−2 to 0, and >0) and immunodeficiency for age with the WHO standard definition19 based on CD4% or CD4 cell count. Conventionally, CD4% is reported for children younger than 5 years and CD4 count is reported for children aged 5 years and older. No immunodeficiency is defined as CD4% over 35% for those younger than 12 months, over 30% for those aged 12–35 months, or over 25% for those aged 36–59 months, and a CD4 count of over 500 cells per μL for those aged 5 years or older; mild immunodeficiency is defined as CD4% of 30–35% for those younger than 12 months, 25–30% for those aged 12–35 months, or 20–25% for those aged 36–59 months, and a CD4 count of 350–499 cells per μL for those aged 5 years or older; advanced immunodeficiency is defined as CD4% of 25–29% for those aged 12 months or younger, 20–24% for those aged 12–35 months, or 15–19% for those aged 36–59 months, and a CD4 count of 200–349 cells per μL for those aged 5 years or older; and severe immunodeficiency is defined as CD4% less than 25% for those younger than 12 months, less than 20% for those aged 12–35 months, or less than 15% for those aged 35–59 months, and a CD4 count of less than 200 cells per μL for those aged 5 years or older.
We used descriptive statistics to illustrate the patient-level and cohort-level characteristics at ART initiation.
Patients were censored at the earliest of the following: switching to second-line ART, death, last visit, or 21st birthday. We summarised the cumulative incidence of switching allowing for the competing risks of death and loss to follow-up.20 Cumulative incidence of switching at 3 years after initiation of ART was stratified by geographical region, initial ART regimen, and cohort monitoring strategy.
Patients were considered as lost to follow-up if they had no visit data for 1 year or more before our study data inclusion cutoff (Sept 16, 2015), except for cohorts in the EPPICC, PHACS, and IMPAACT networks, for which a cutoff of 2 years or more was used because data collection for these cohorts is done annually and to account for time lags in reporting. We administratively censored follow-up of children at the date of last clinic visit. Additionally, we administratively censored patients who transferred to a different clinical site not part of a participating cohort during their follow-up or if they were transferred to adult care.
We summarised the independent associations between cumulative incidence of switching and patient characteristics at initiation of ART and cohort characteristics by subdistribution hazard ratios calculated using multivariable competing-risks proportional hazards regression.21
Additionally, the number of children in southern Africa younger than 3 years at the time of initiating a lopinavir-based regimen who switched to an NNRTI-based second-line regimen aged 3 years or older is reported and their viral load at time of switching summarised.
We did two sensitivity analyses. First, to assess the potential association between low weight-for-age Z score, immunosuppression at initiation of ART, and likelihood of switching. We repeated the regression models using patient-level data from a subset of cohorts in which more than 60% of children had bodyweight and CD4 measurements at ART initiation, and did the multivariable analysis with and without weight-for-age Z score and immunodeficiency for age.
In the second sensitivity analysis, we repeated all analyses redefining switching to second-line ART by removing the requirement for a simultaneous change of one or more NRTI when changing across drug class (NNRTI to protease inhibitor or vice versa) or within the protease inhibitor drug class.
We did all analyses using Stata version 14.2.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
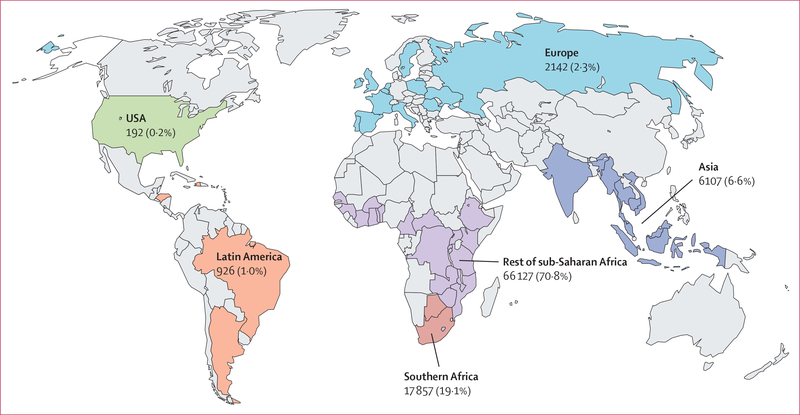
At the time of data cutoff, Sept 16, 2015, the CIPHER dataset comprised patient-level data on 182 747 children living with HIV, of whom 93 351 (51%) met our inclusion criteria (appendix p 20). 12 networks covering 52 countries in the CIPHER database had eligible children, and most children were in the rest of sub-Saharan Africa (71%), with 19·1% in southern Africa, 6·6% in Asia, and the remaining 3·5% in Europe, Latin America, and the USA (figure 1). The calendar year of ART initiation ranged from 1993 to 2015, with over 70% of children initiating ART in 2008 or later (table 1). Half of patients were male, and the median age at ART initiation was 3·9 years (IQR 1·6–6·9), with two-thirds aged 5 years or younger. The median age at ART initiation was similar across all regions, except for in the USA where the median age was younger than 1 year. 40 261 (43·1%) children had known AIDS diagnosis at ART initiation, and among 50 892 (54·5%) children with available CD4 data, 37 962 (74·6%) had advanced or severe immunodeficiency, with Asia and southern Africa having the highest proportions of patients with severe immunodeficiency.
Figure 1:
Geographical distribution of children with HIV included in study
Table 1:
Patient characteristics at time of ART initiation
| USA (n=192) | Latin America (n=926) | Europe (n=2142) | Asia (n=6107) | Southern Africa (n=17 857) | Rest of sub-Saharan Africa (n=66 127) | Total (n=93 351) | |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Male | 89 (46·4%) | 441 (47·6%) | 1016 (47·4%) | 3213 (52·6%) | 9070 (50·8%) | 33 236 (50·3%) | 47 065 (50·4%) |
| Female | 103 (53·7%) | 485 (52·4%) | 1126 (52·6%) | 2894 (47·4%) | 8784 (49·2%) | 32 859 (49·7%) | 46 251 (49·6%) |
| Missing data | 0 | 0 | 0 | 0 | 3 (<0·1%) | 32 (<0·1%) | 35 (<0·1%) |
| Age, years | |||||||
| Median | 0·7 (0·2–3·4) | 4·2 (1·6–7·3) | 3·2 (0·7–7·0) | 4·5 (2·3–7·0) | 3·6 (1·1–6·8) | 3·8 (1·7–6·9) | 3·9 (1·6–6·9) |
| <3 | 138 (71·9%) | 362 (39·1%) | 1028 (48·0%) | 1927 (31·6%) | 8082 (45·3%) | 27 856 (42·1%) | 39 393 (42·2%) |
| 3–5 | 34 (17·7%) | 242 (26·1%) | 447 (20·9%) | 2068 (33·9%) | 4127 (23·1%) | 16 876 (25·5%) | 23 794 (25·5%) |
| 6–9 | 20 (10·4%) | 244 (26·4%) | 450 (21·0%) | 1910 (31·3%) | 4934 (27·6%) | 19 042 (28·8%) | 26 600 (28·5%) |
| ≥10 | 0 | 78 (8·4%) | 217 (10·1%) | 202 (3·3%) | 714 (4·0%) | 2353 (3·6%) | 3564 (3·8%) |
| CD4% (<5 years)* | |||||||
| n (%) | 92 (57·1%) | 323 (61·5%) | 995 (75·0%) | 2208 (65·9%) | 7315 (67·6%) | 14 643 (37·0%) | 25 576 (45·9%) |
| Median | 32 (24–38) | 19 (13–28) | 23 (16–33) | 15 (8–21) | 17 (11–24) | 16 (11–21) | 16 (11–23) |
| CD4 cell count (≥5 years)* | |||||||
| n (%) | 21 (67·7%) | 391 (97·5%) | 718 (88·1%) | 2381 (86·5%) | 4753 (67·5%) | 16 262 (61·2%) | 24 526 (65·2%) |
| Median | 409 (218–631) | 335 (165–568) | 330 (204–525) | 195 (59–332) | 308 (147–537) | 306 (162–522) | 297 (148–507) |
| Immunodeficiency for age† | 113 (58·9%) | 714 (77·1%) | 1718 (80·2%) | 4693 (76·9%) | 12 202 (68·3%) | 31 452 (47·6%) | 50 892 (54·5%) |
| None | 45 (39·8%) | 175 (24·5%) | 463 (27·0%) | 402 (8·6%) | 1944 (15·9%) | 5100 (16·2%) | 8129 (16·0%) |
| Mild | 26 (23·0%) | 76 (10·6%) | 238 (13·9%) | 375 (8·0%) | 1081 (8·9%) | 3005 (9·6%) | 4801 (9·4%) |
| Advanced | 16 (14·2%) | 126 (17·7%) | 280 (16·3%) | 803 (17·1%) | 1474 (12·1%) | 5303 (16·9%) | 8002 (15·7%) |
| Severe | 26 (23·0%) | 337 (47·2%) | 737 (42·9%) | 3113 (66·3%) | 7703 (63·1%) | 18 044 (57·4%) | 29 960 (58·9%) |
| Known AIDS diagnosis | 20 (10·4%) | 45 (4·9%) | 368 (17·2%) | 3526 (57·7%) | 9910 (55·5%) | 26 392 (39·9%) | 40 261 (43·1%) |
| Weight-for-age Z score† | 95 (49·5%) | 841 (90·8%) | 1051 (49·1%) | 5575 (91·3%) | 11 610 (65·0%) | 49 090 (74·2%) | 68 262 (73·1%) |
| ≤−2 | 19 (20·0%) | 368 (43·8%) | 168 (16·0%) | 3664 (65·7%) | 5498 (47·4%) | 27 257 (55·5%) | 36 974 (54·2%) |
| >−2 to 0 | 52 (54·7%) | 394 (46·9%) | 486 (46·2%) | 1644 (29·5%) | 4911 (42·3%) | 17 785 (36·2%) | 25 272 (37·0%) |
| >0 | 24 (25·3%) | 79 (9·4%) | 397 (37·8%) | 267 (4·8%) | 1201 (10·3%) | 4048 (8·3%) | 6016 (8·8%) |
| Initial ART regimen | |||||||
| NNRTI based | 114 (59·4%) | 827 (89·3%) | 1194 (55·7%) | 5850 (95·8%) | 10 368 (58·1%) | 64 532 (97·6%) | 82 885 (88·8%) |
| Nevirapine | 85 (44·3%) | 277 (29·9%) | 680 (31·8%) | 4383 (71·8%) | 885 (5·0%) | 55 354 (83·7%) | 61 664 (66·1%) |
| Efavirenz | 28 (14·6%) | 550 (59·4%) | 514 (24·0%) | 1467 (24·0%) | 9483 (53·1%) | 9178 (13·9%) | 21 220 (22·7%) |
| Other NNRTI | 1 (0·5%) | 0 | 0 | 0 | 0 | 0 | 1 (<0·1%) |
| Protease inhibitor based | 78 (40·6%) | 99 (10·7%) | 948 (44·3%) | 257 (4·2%) | 7489 (41·9%) | 1595 (2·4%) | 10 466 (11·2%) |
| Ritonavir-boosted lopinavir | 66 (34·4%) | 94 (10·2%) | 895 (41·8%) | 253 (4·1%) | 7486 (41·9%) | 1595 (2·4%) | 10 389 (11·1%) |
| Other protease inhibitor | 12 (6·3%) | 5 (0·5%) | 53 (2·5%) | 4 (0·1%) | 3 (<0·1%) | 0 | 77 (0·1%) |
| Calendar year of ART initiation | |||||||
| ≤2004 | 152 (79·2%) | 321 (34·7%) | 643 (30·0%) | 535 (8·8%) | 1600 (9·0%) | 1448 (2·2%) | 4699 (5·0%) |
| 2005–07 | 28 (14·6%) | 207 (22·4%) | 529 (24·7%) | 1733 (28·4%) | 5071 (28·4%) | 15 639 (23·7%) | 23 207 (24·9%) |
| 2008–10 | 8 (4·2%) | 218 (23·5%) | 630 (29·4%) | 1891 (31·0%) | 7440 (41·7%) | 26 433 (40·0%) | 36 620 (39·2%) |
| ≥2011 | 4 (2·1%) | 180 (19·4%) | 340 (15·9%) | 1948 (31·9%) | 3746 (21·0%) | 22 607 (34·2%) | 28 825 (30·9%) |
| Monitoring strategy | |||||||
| Routine CD4 and viral load | 192 (100%) | 402 (43·4%) | 2123 (99·1%) | 3404 (55·7%) | 17 857 (100%) | 2005 (3·0%) | 25 983 (27·8%) |
| Routine CD4 and targeted viral load | 0 | 0 | 19 (0·9%) | 2442 (40·0%) | 0 | 14 246 (21·5%) | 16 707 (17·9%) |
| Routine CD4 only | 0 | 524 (56·6%) | 0 | 260 (4·3%) | 0 | 35 748 (54·1%) | 36 532 (39·1%) |
| Clinical only | 0 | 0 | 0 | 1 (<0·1%) | 0 | 14 128 (21·4%) | 14 129 (15·1%) |
| Country-income group | |||||||
| Low | NA | 524 (56·6%) | NA | 2947 (48·3%) | NA | 36 780 (55·6%) | 40 251 (43·1%) |
| Lower-middle | NA | 169 (18·3%) | 390 (18·2%) | 507 (8·3%) | NA | 29 347 (44·4%) | 30 413 (32·6%) |
| High and upper-middle | 192 (100%) | 233 (25·2%) | 1752 (81·8%) | 2653 (43·4%) | 17 857 (100%) | 0 | 22 687 (24·3%) |
Data are n (%) or median (IQR), unless otherwise indicated. ART=antiretroviral therapy. NA=not applicable. NNRTI=non-nucleoside reverse-transcriptase inhibitor.
CD4%6 is reported in children younger than 5 years and CD4 count is reported for children 5 years and older. The denominators for calculations of the proportion with a measurement are therefore in the subgroups younger than 5 years and aged 5 years and older.
CD4 data and bodyweight data only recorded in a subset of cohorts, and so the total number of participants, used as the denominator for the proportion calculation in each subcategory, for each region is supplied. The WHO immunosuppression-for-age measure uses all data available, both CD4% and CD4 cell count. Here we only report the median CD4% among those younger than 5 years and the median CD4 count in those aged 5 years or older, but many patients had both markers recorded.
82 885 (88·8%) children initiated an NNRTI-based regimen (61 664 [66·1%] on nevirapine), although regional variations were observed (table 1; appendix p 21). In southern Africa, 6803 (84%) of 8082 patients younger than 3 years at ART initiation were initiated on a ritonavir-boosted lopinavir-based regimen compared with 1262 (4·5%) of 27 856 in the rest of sub-Saharan Africa (appendix p 22).
Strategies for treatment monitoring also varied between the geographical regions. In the USA, Europe, and southern Africa, almost all patients were in cohorts with routine monitoring of CD4 and viral load, whereas in Asia 56% of patients were in cohorts with routine monitoring of CD4 and viral load and 40% were in cohorts with routine monitoring of CD4 and targeted monitoring of viral load. In the rest of sub-Saharan Africa, only 3·0% of patients were in cohorts with routine monitoring of CD4 and viral load, equal proportions of patients (21·4%) were in cohorts with routine monitoring of CD4 and targeted monitoring of viral load and in cohorts with clinical monitoring only, and 54·1% were in cohorts with only CD4 monitoring.
Median duration of follow-up after ART initiation was 26 months (IQR 9–52), with longer follow-up in regions outside of Africa (table 2). At data cutoff (without use of competing risks and ignoring switching to second-line ART) 5417 (5·8%) patients had died, 13 846 (14·8%) were lost to follow-up and not known to have died, 19 888 (21·3%) had transferred to another clinic or to adult care, and 54 200 (58·1%) were still in follow-up.
Table 2:
Follow-up status and cumulative incidence of switch by 3 years after start of ART by geographical region
| USA (n=192) | Latin America (n=926) | Europe (n=2142) | Asia (n=6107) | Southern Africa (n=17 857) | Rest of sub-Saharan Africa (n=66 127) | Overall (n=93 351) | |
|---|---|---|---|---|---|---|---|
| Follow-up* | |||||||
| Median (IQR), months | 41 (23–79) | 52 (24–93) | 49 (23–81) | 38 (17–69) | 29 (12–58) | 24 (8–47) | 26 (9–52) |
| Switched to second-line ART | 72 (37·5%) | 123 (13·3%) | 464 (21·7%) | 587 (9·6%) | 1255 (7·0%) | 1382 (2·1%) | 3883 (4·2%) |
| Died | 0 | 4 (0·4%) | 3 (0·1%) | 31 (0·5%) | 17 (0·1%) | 394 (0·6%) | 449 (0·5%) |
| Lost to follow-up | 15 (7·8%) | 214 (23·1%) | 235 (11·0%) | 674 (11·0%) | 3446 (19·3%) | 13 688 (20·7%) | 18 272 (19·6%) |
| Administrative censoring | 105 (54·7%) | 585 (63·2%) | 1440 (67·2%) | 4815 (78·8%) | 13 139 (73·6%) | 50 663 (76·6%) | 70 747 (75·8%) |
| Cumulative incidence switched by 3 years after start of ART | |||||||
| Overall | 26·1% (20·0–32·7) | 6·5% (4·9–8·3) | 12·2% (10·8–13·7) | 6·6% (5·9–7·3) | 5·4% (5·1–5·9) | 1·5% (1·4–1·6) | 3·1% (3·0–3·2) |
| Age at initiation of ART, years | |||||||
| <3 years | 25·7% (18·5–33·4) | 4·9% (2·9–7·6) | 11·7% (9·8–13·9) | 7·0% (5·8–8·3) | 3·7% (3·2–4·2) | 1·1% (0·9–1·2) | 2·5% (2·3–2·7) |
| 3–5 | 27·6% (13·7–43·3) | 5·0% (2·7–8·5) | 8·9% (6·4–12·0) | 7·0% (5·8–8·4) | 6·5% (5·7–7·4) | 1·4% (1·2–1·6) | 3·1% (2·9–3·4) |
| 6–9 | 27·1% (9·8–48·0) | 8·3% (5·1–12·4) | 14·8% (11·5–18·4) | 5·6% (4·5–6·9) | 7·2% (6·3–8·1) | 2·0% (1·8–2·3) | 3·7% (3·4–4·0) |
| ≥10 | ·· | 12·3% (5·7–21·6) | 16·6% (11·5–22·5) | 6·7% (2·7–13·5) | 6·7% (4·5–9·5) | 2·4% (1·7–3·4) | 4·9% (4·0–5·9) |
| Initial ART regimen | |||||||
| Protease inhibitor based | 10·1% (4·4–18·5) | 5 ·1% (1·6–11·6) | 7·0% (5·4–8·8) | 3·6% (1·6–7·1) | 3·2% (2·7–3·7) | 4·3% (3·2–5·7) | 3·9% (3·4–4·3) |
| NNRTI based | 37·0% (28·1–45·9) | 6·6% (5·0–8·5) | 16·1% (14·0–18·4) | 6·6% (5·9–7·4) | 7·0% (6·4–7·6) | 1·4% (1·3–1·5) | 3·0% (2·9–3·2) |
| Monitoring strategy | |||||||
| Routine CD4 and viral load | 26·1% (20·0–32·7) | 7·7% (5·3–10·7) | 12·3% (10·8–13·8) | 8·7% (7·8–9·8) | 5·4% (5·1–5·9) | 6·1% (5·0–7·4) | 6·8% (6·5–7·2) |
| Routine CD4 and targeted viral load | ·· | ·· | 5·9% (0·4–23·5) | 2·8% (2·0–3·7) | ·· | 2·1% (1·8–2·4) | 2·2% (1·9–2·4) |
| Routine CD4 only | ·· | 5·5% (3·6–7·9) | ·· | 3·5% (1·1–8·3) | ·· | 1·1% (1·0–1·3) | 1·2% (1·1–1·4) |
| Clinical only | ·· | ·· | ·· | ·· | ·· | 0·8% (0·6–1·0) | 0·8% (0·6–1·0) |
Data are n (%) or cumulative incidence (95% CI), unless otherwise indicated. ART=antiretroviral therapy. NNRTI=non-nucleoside reverse-transcriptase inhibitor.
Competing risk analysis, censored at the first of the following events: switched to second-line, death, loss to follow-up or date of last clinic visit (before transfer out or data cutoff).
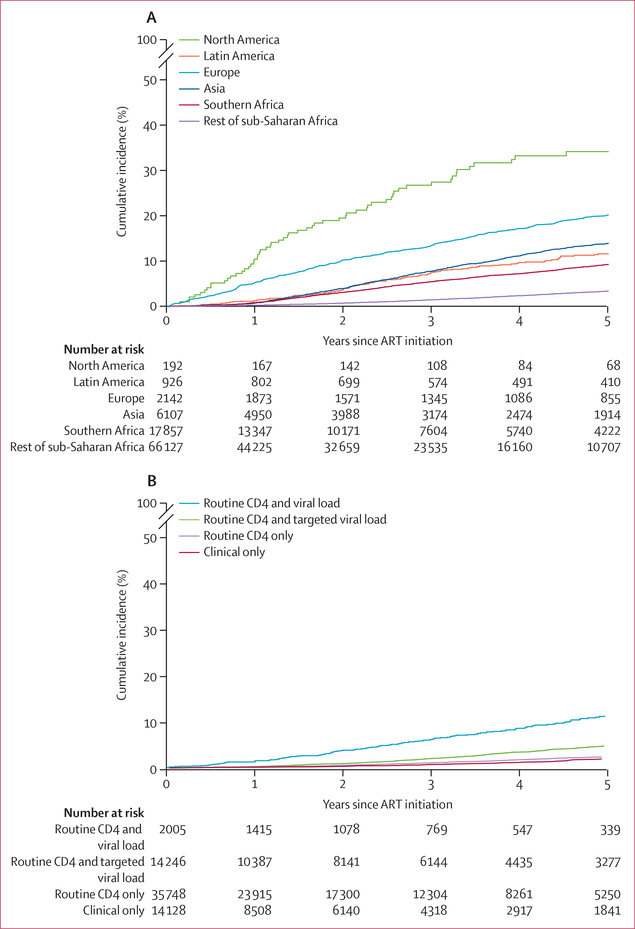
Over 265 942 person-years of follow-up, 4·2% of patients met our definition of switching to second-line ART, and on the basis of our competing-risk analysis 0·5% died, 19·6% were lost to follow-up, and 20·7% transferred before switching. The crude rate of switching was 14·6 switches per 1000 person-years (95% CI 14·1–15·1). The cumulative incidence of switching by 3 years after initiation of ART was 3·1% (95% CI 3·0–3·2), with wide variation between geographical region, initial regimen, and monitoring strategies (table 2, figure 2A). The cumulative incidences of switching by 1, 2, and 3 years after initiation of ART stratified by these variables are shown in the appendix (pp 14–15). We found the highest incidence of switching to be among children who initiated ART on NNRTI-based regimens in the USA and the lowest incidence among children on NNRTI-based regimens in sub-Saharan Africa with CD4 or clinical only monitoring. Among children who started ART in 2011 or later, similar variations in the incidence of switching across treatment monitoring strategies was observed (appendix p 15). Because the rest of sub-Saharan Africa was the only region that used all four types of monitoring strategy, we further explored the cumulative incidence of switching within this region (figure 2B) and found the cumulative incidence of switching at 3 years to be 6·1% (95% CI 5·0–7·4) in cohorts with routine monitoring of CD4 and viral load compared with less than 2% (table 2) in cohorts with no viral load monitoring.
Figure 2: Cumulative incidence of switching to second-line ART among children at 3 years after ART initiation.
(A) Incidence by region. (B) Incidence by monitoring strategy for sub-Saharan Africa (excluding Botswana and South Africa) only at 3 years after ART initiation. ART=antiretroviral therapy.
Among the 3883 children who switched to second-line ART, the median time to switch was 35 months (IQR 20–57; appendix pp 18–19). The median age at switch was 8·6 years (IQR 5·5–11·5), and 3329 (85·7%) switches were from an NNRTI-based to a protease inhibitor-based regimen, 419 (10·8%) were from a protease inhibitor-based to an NNRTI-based regimen, and 135 (3·5%) were other switches. Among children with recorded CD4% or CD4 cell counts at the time of switch (n=3016), 1265 (41·9%) had severe immunodeficiency and 359 (11·9%) had advanced immunodeficiency. Among the 2419 (62·3%) patients with measurements of viral load at the time of switching, 2013 (83·2%) had a viral load of more than 1000 copies per mL. 203 (5%) patients had a tuberculosis diagnosis at the time of switching. 75 children younger than 3 years at the start of lopinavir-based first-line ART switched to an NNRTI-based second-line ART when older than 3 years and while virally suppressed to under 1000 copies per mL. Among the 2219 (57%) patients with a reported reason for switching, 1132 (51%) switched because of treatment failure, 67 (3%) because of toxic side-effects of drugs, and 1020 (46%) because of other (unspecified) reasons.
In our multivariable analyses, individual patient-level factors associated with an increased likelihood of switching were male sex, older age at ART initiation, initiation of ART on an NNRTI-based regimen, and earlier calendar year of initiation of ART (table 3). In the multivariable analysis of cohort-level factors associated with switching, treatment monitoring strategy was indentified as a factor. Compared with monitoring of CD4 only, routine CD4 and viral load monitoring was associated with a 166% increase in the likelihood of switching, whereas clinical only monitoring was associated with a 32% decrease in likelihood (table 3). High-income and upper-middle-income countries were associated with an increased likelihood of switching compared with low-income countries. All geographical regions outside of Africa had increased likelihoods of switching compared with southern Africa, whereas we saw no difference between southern Africa and the rest of sub-Saharan Africa.
Table 3:
Factors associated with switching to second-line ART
| All children (main analysis; n=93 351) |
Subset of cohorts that recorded CD4% or CD4 cell count and bodyweight at ART initiation (sensitivity analyses; n=39 724) |
||||
|---|---|---|---|---|---|
| Univariable analysis | Multivariable analysis | Univariable analysis | Multivariable analysis, not including immunodeficiency for age and weight-for-age Z score | Multivariable analysis, including immunodeficiency for age and weight-for-age Z score | |
| Patient-level factors | |||||
| Sex | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 |
| Female vs male | 0·79 (0·74–0·84) | 0·78 (0·73–0·83) | 0·80 (0·75–0·86) | 0·79 (0·74–0·84) | 0·81 (0·74–0·88) |
| Age, years | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 |
| <3 | 0·61 (0·56–0·66) | 0·63 (0·58–0·68) | 0·58 (0·54–0·63) | 0·68 (0·62–0·74) | 0·62 (0·54–0·70) |
| 3–5 | 0·77 (0·71–0·84) | 0·73 (0·67–0·79) | 0·78 (0·71–0·85) | 0·75 (0·69–0·82) | 0·71 (0·64–0·79) |
| 6–9 | 1 | 1 | 1 | 1 | 1 |
| ≥10 | 1·24 (1·11–1·51) | 1·15 (0·98–1·35) | 1·17 (1·00–1·37) | 1·17 (0·99–1·38) | 1·26 (1·05–1·51) |
| Known AIDS diagnosis | p=0·16 | p=0·10 | p=0·16 | p=0·87 | p=0·96 |
| Yes vs no | 0·96 (0·90–1·02) | 0·95 (0·88–1·01) | 1·05 (0·98–1·12) | 1·01 (0·94–1·08) | 1·00 (0·88–1·16) |
| Immunodeficiency for age | ·· | ·· | p<0·0001 | ·· | p<0·0001 |
| None | ·· | ·· | 1·07 (0·90–1·27) | ·· | 0·91 (0·75–1·10) |
| Mild | ·· | ·· | 1·21 (1·00–1·46) | ·· | 1·16 (0·85–1·43) |
| Advanced | ·· | ·· | 1 | ·· | 1 |
| Severe | ·· | ·· | 1·39 (1·21–1·58) | ·· | 1·40 (1·21–1·62) |
| Weight-for-age Z score | ·· | ·· | p=0·38 | ·· | p=0·17 |
| ≤−2 | ·· | ·· | 0·95 (0·87–1·03) | ·· | 1·07 (0·97–1·17) |
| >−2 to 0 | ·· | ·· | 1 | ·· | 1 |
| >0 | ·· | ·· | 1·00 (0·87–1·16) | ·· | 0·93 (0·80–1·09) |
| Initial ART regimen | p=0·004 | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 |
| Protease inhibitor based vs NNRTI based | 1·15 (1·05–1·26) | 0·70 (0·63–0·79) | 0·68 (0·61–0·76) | 0·51 (0·45–0·58) | 0·58 (0·49–0·68) |
| Calendar year | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 |
| ≤2004 | 2·96 (2·70–3·24) | 1·39 (1·26–1·53) | 2·28 (2·08–2·51) | 1·41 (1·27–1·56) | 1·41 (1·24–1·61) |
| 2005–07 | 1·45 (1·35–1·57) | 1·26 (1·17–1·36) | 1·27 (1·17–1·38) | 1·18 (1·08–1·28) | 1·18 (1·07–1·30) |
| 2008–10 | 1 | 1 | 1 | 1 | 1 |
| ≥2011 | 0·55 (0·47–0·63) | 0·61 (0·53–0·71) | 0·49 (0·41–0·58) | 0·53 (0·44–0·62) | 0·59 (0·48–0·73) |
| Cohort-level factors | |||||
| Cohort monitoring strategy | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 |
| Routine CD4 and viral load | 4·57 (4·18–5·00) | 2·66 (2·22–3·19) | 2·81 (2·55–3·10) | 1·90 (1·60–2·26) | 2·37 (1·94–2·89) |
| Routine CD4 and targeted viral load | 2·18 (1·95–2·43) | 1·95 (1·75–2·18) | 1·29 (1·15–1·45) | 1·29 (1·13–1·46) | 1·43 (1·24–1·66) |
| Routine CD4 only | 1 | 1 | 1 | 1 | 1 |
| Clinical only | 0·75 (0·62–0·91) | 0·68 (0·56–0·83) | ·· | ·· | ·· |
| Country-income group | p<0·0001 | p=0·0017 | p<0·0001 | p=0·22 | p=0·44 |
| Low | 1 | 1 | 1 | 1 | 1 |
| Lower-middle | 1·04 (0·94–1·14) | 1·09 (0·98–1·21) | 1·01 (0·90–1·12) | 1·09 (0·96–1·20) | 1·01 (0·88–1·16) |
| High and upper-middle | 3·21 (2·98–3·47) | 1·33 (1·13–1·56) | 2·26 (2·09–2·46) | 1·14 (0·97–1·33) | 0·87 (0·71–1·08) |
| Geographical region | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 |
| USA | 4·16 (3·20–5·42) | 4·04 (3·07–5·32) | 3·01 (1·84–4·93) | 2·73 (1·63–4·58) | 4·39 (2·28–8·46) |
| Europe | 2·30 (2·07–2·56) | 2·22 (1·97–2·49) | 2·05 (1·83–2·29) | 1·84 (1·63–2·09) | 2·22 (1·87–2·63) |
| Latin America | 1·23 (1·03–1·49) | 1·73 (1·40–2·15) | 1·28 (1·05–1·55) | 1·59 (1·27–2·00) | 1·88 (1·46–2·42) |
| Asia | 1·27 (1·15–1·40) | 1·38 (1·23–1·54) | 1·18 (1·07–1·31) | 1·06 (0·94–1·18) | 0·86 (0·74–1·01) |
| Southern Africa | 1 | 1 | 1 | 1 | 1 |
| Rest of sub-Saharan Africa | 0·35 (0·33–0·38) | 0·89 (0·74–1·07) | 0·47 (0·44–0·52) | 0·69 (0·57–0·83) | 0·66 (0·52–0·84) |
Data are subdistributional hazard ratios (95% CIs) unless otherwise stated. CD4 means CD4% or CD4 cell count. ART=antiretroviral therapy. NNRTI=non-nucleoside reverse transcriptase inhibitor.
In the first sensitivity analysis, which was restricted to patient-level data from a subset of cohorts that recorded CD4% or CD4 cell count and bodyweight at ART initiation in over 60% of participants (n=39 724), the risk factors for switching remained consistent with the main analyses, except for some differences in the effect of the geo graphical region. Patients in the rest of sub-Saharan Africa had a decreased likelihood of switching compared with those in southern Africa. The association between country-level incomes was no longer present (table 3). Additionally, patients with severe immunodeficiency had an increased likelihood of switching compared with those with advanced immunodeficiency, but we saw no association between switching and weight-for-age Z score at initiation of ART.
In the second sensitivity analysis that broadened the definition of switching, the number of patients who met the definition increased from 3883 (4·2%) to 4035 (4·3%). Most of the additional switches were from an NNRTI-based to a protease inhibitor-based regimen. Factors associated with switching and hazard estimates were broadly similar to those identified in the main analyses (appendix pp 16–17).
Discussion
The incidence of switching to second-line ART among children with HIV≠≠ was 14·6 switches per 1000 person-years with a cumulative incidence of 3·1% by 3 years after initiation of ART. However, we identified large variations between individual patient characteristics, geographical regions, and by cohort monitoring strategies.
3 years after initiation of ART, the cumulative incidence of switching was lowest among patients in cohorts in the rest of sub-Saharan Africa with clinical only monitoring, and was only slightly increased in this region when monitoring of CD4 was available. These estimates are lower than the median proportion of patients who switched after 4 years of follow-up reported in the ARROW trial (63 [5%] of 1206),7 which was conducted in the rest of sub-Saharan Africa where all patients initiated NNRTI-based regimens and were managed with only clinical or CD4 monitoring. However, because ARROW is a clinical trial, patients were followed up more closely than they might be in routine care, and the median duration of follow-up in ARROW (4 years [IQR 3·7–4·4]) was longer than in our cohort. WHO forecasting models estimate that the proportion of children with HIV on ART globally who are receiving second-line regimens, irrespective of duration on ART, was 4·1% in 2013 and increased to 6·1% in 2015.22 However, these estimates are cross-sectional and based on extrapolations from historical trends in global antiretroviral procurement data and projections from assumptions regarding ART coverage. Therefore, the estimates cannot be directly compared with our estimates of cumulative incidence of switching to second-line ART at 3 years after initiation of ART.
In our analysis, patients who were managed in settings that monitored their viral load were twice as likely to switch to second-line ART compared with children in settings that only had access to CD4 or clinical monitoring, or both. This finding is consistent with findings from adult HIV modelling studies23 that estimate that the number of patients receiving second-line ART in settings with rapid scale-up of viral load monitoring will increase two to three times compared with slow or no scale-up of viral load monitoring.
Studies24,25 have reported that 20–40% of children with only clinical or CD4 monitoring had evidence of virological failure (viral load ≥1000 copies per mL) at 3–4 years after initiation of ART, highlighting the poor sensitivity of these monitoring strategies in detecting virological failure. This issue is particularly important in sub-Saharan Africa where most children initiate ART on NNRTI-based regimens with low genetic thresholds for resistance.26 Although the PENPACT-1 trial9 reported no difference in clinical outcomes of children on NNRTI-based or protease inhibitor-based regimens who were assigned to switch to second-line ART after virological failure at over 1000 copies per mL (early switch) or at over 10 000 copies per mL (delayed switch), adult studies27,28 in sub-Saharan Africa have shown increased risks of morbidity and mortality in patients with delayed switching to second-line ART. A comparison of the clinical outcomes of children managed under a variety of monitoring strategies and time between treatment failure and switching is warranted to determine the best use of resources to obtain optimal outcomes in this population.
In our study, we estimated that most regions had a higher cumulative incidence of switching to second-line ART among children who initiated an NNRTI-based regimen than among those who initiated a protease inhibitor-based regimen. However, in the rest of sub-Saharan Africa we estimated a higher cumulative incidence of switching among children who initiated protease inhibitor-based regimens than those who initiated NNRTI-based regimens, although this estimate was based on a small proportion of children starting on protease inhibitors in that region (2·4%, all ritonavir-boosted lopinavir). Review of these data indicates that our finding might be partly because of incident tuberculosis and the need to avoid protease inhibitors when initiating a tuberculosis treatment regimen containing rifampin. Because the tuberculosis data were incompletely reported, we could not completely explore this hypothesis. The finding that older age at initiation of ART is associated with an increased likelihood of switching has been previously reported11,13 and could be partly due to the lack of available paediatric formulations for young children and poorer adherence among adolescents than among younger children. The increased incidence of switching among male patients has been previously reported in paediatric and adult cohorts,11,12,29 and warrents further study.
Our analysis suggested that even after adjusting for monitoring strategy and patient-level characteristics, being in the rest of sub-Saharan Africa and in low-income countries remained independently associated with a decreased likelihood of switching to second-line therapy. The comparatively less frequent use of second-line ART in such settings, even when viral load monitoring was available, could partly be because of the higher thresholds for virological failure recommended by WHO for low-income and middle-income countries than for high-income and upper-middle-income countries.30 This finding could also reflect the paucity of access to second-line drugs and clinicians’ fears about availability of subsequent third-line therapy, although these factors were not measured in our study.
The low global cumulative incidence of switching to second-line ART reported in our analysis, which was dominated by a large number of children in southern Africa and the rest of sub-Saharan Africa, reflects standard practice for those who intitated ART between 1993 and 2015 in participating programmes. Since 2015, scale-up of viral load monitoring has been ongoing and is likely to substantially increase the early detection of treatment failure and demand for second-line ART. However, the extent of the increase in demand for second-line ART across settings remains unclear and will still be subject to local resources and guidance. Although less guidence and data exist on the optimal use of the low-cost integrase inhibitor dolutegravir in second-line ART in children, its roll-out as first-line and second-line ART in adults will probably lead to increased call for its use in children.31 Because our study spans a large age spectrum and time period, it provides crucial insight into how clinicians have assessed and responded to first-line treatment failure in children on ART to date. These insights can be of use both to forecast future paediatric ART needs and to identify settings in which the system might be failing children and potential points of intervention. Future assessments of the durability of first-line regimens in-line with when new drugs are rolled out will be crucial to ensure sufficient availability of paediatric formulations in the future.
This study had several limitations. First, few cohorts reported data on the reasons for ART switch, and among those that did report reasons almost half of the reasons were unspecified as other reasons. Furthermore, few cohorts had data on viral load at the time of switching, and so we could not elucidate whether the switch was because of treatment failure. However, because of our conservative definition of switching to second-line ART, we feel that most of the switches were true switches to second-line ART rather than minor treatment modifications or simplifications. Since 2010 in South Africa, guidelines have recommended to switch children aged 3 years and older to an NNRTI-based regimen if they were younger than 3 years at initiation of a lopinavir-based ART regimen and if they were virally suppressed.14 We considered that this recommendation might lead to overestimation of switches among this population; however, only 75 children were switched in this manner while virally suppressed, and thus their potential misclassification would not substantially affect our findings. Second, this is an observational study with sources of potential bias such as the high proportion of children lost to follow-up, which has probably resulted in incomplete ascertainment of switching and deaths. This limitation has been addressed in part by our use of a competing-risk analyses. Third, AIDS diagnoses might have been under-reported at initiation of ART in some low-income country settings because of restricted capacity for clinical diagnostics. Data are also incomplete on co-infections (eg, tuberculosis) and the availability of alternative antiretroviral drugs restricted our ability to explore possible reasons for the geographical variations in switching patterns. Finally, although this is a large global cohort collaboration, we are still extrapolating data from a finite number of cohorts to a global estimate.
For CIPHER website see http://www.iasociety.org/CIPHER
In conclusion, we found that the cumulative incidence of switching to second-line ART varied widely between both geographical regions and by monitoring strategies. Given the maturing cohorts and expanding roll-out of viral load testing and new drugs, we anticipate that the use of second-line regimens will increase, although geographical variation will most likely persist for the foreseeable future. The effect of delayed versus fast switching to second-line ART after treatment failure on longer-term clinical outcomes and treatment options among children remain unclear and warrant further exploration.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for studies published in English from database inception to Nov 16, 2017, that assessed the probability of switching to second-line antiretroviral therapy (ART) in children with HIV across geographical regions and under different monitoring strategies using the search terms “child”, “children”, OR “adolescent”; “HIV”, “antiretroviral therapy”, “switch”, AND “second-line”. We identified several clinical trials and several cohort studies that reported on the probability of switching that used various different definitions of switching. Few studies estimated the incidence of switching to second-line ART in children across several countries with different strategies of treatment monitoring. To our knowledge, no published global-level analysis of switching to second-line ART among children exists that uses a uniform definition of switching.
Added value of this study
This study provides the first global estimates of the incidence of switching to second-line ART, using individual patient-level data for over 93 000 children across 52 countries. We found a low cumulative incidence of switching of 3·1% by 3 years after initiation of ART globally, but with significant variations across geographical regions and by treatment monitoring strategies. Compared with CD4 (CD4% or CD4 cell count) or clinical only monitoring, children in settings with routine or targeted monitoring of viral load were twice as likely to switch to second-line ART.
Implications of all the available evidence
As HIV treatment programmes mature, understanding patterns in the use of second-line ART is crucial to ensure future needs of paediatric treatment are met. The wide variations in the incidence of switching to second-line ART across regions and monitoring strategies highlight the need to assess the effect of low rates of switching and prolonged treatment failure before switching on clinical outcomes in children and the potential implications of expanding access to viral load testing on future use of second-line ART in resource-limited settings.
Acknowledgments
We thank all participating networks, clinics, clinic personnel, and patients who contributed data and made the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global Cohort Collaboration Time on First Line Project possible; a full list is in the appendix (pp 1–13). We also thank Martina Penazzato and Rohan Hazra for their guidance and support as part of the CIPHER Executive Committee, and Samantha Hodgetts for her administrative support. The study was sponsored by the International AIDS Society-CIPHER. CIPHER is made possible by support from founding sponsor ViiV Healthcare and Janssen. Funding details of the individual cohort networks are listed in the appendix (pp 2–3).
Funding International AIDS Society-CIPHER, UK Medical Research Council.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
Data are accessible in principle by applying to the Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global Cohort Collaboration Data Centres. The CIPHER Global Cohort Collaboration is a multinetwork, multisite collaboration and this study combined data from different sites. The data do not belong to the CIPHER Global Cohort Collaboration itself; data ownership remains with the participating sites. Each site has approval from its own local Institutional Review Board to collect routine data on patients and to transfer those data anonymously to the CIPHER Global Cohort Collaboration Project University of Cape Town Research Centre (Cape Town, South Africa). For some sites and networks, Institutional Review Board approval for use of this data is restricted to the specific protocols approved to protect patient identities. Requests for access to data can be directed to the International AIDS Society CIPHER contact Samantha Hodgetts (samantha.hodgetts@iasociety.org).
See Online for appendix
References
- 1.UNAIDS. Global HIV statistics. Fact sheet July 2017. Joint United Nations Programme on HIV/AIDS, 2018. http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_en.pdf (accessed Dec 11, 2018). [Google Scholar]
- 2.UNAIDS. 90–90-90 an ambitious treatment target to help end the AIDS epidemic 2014. Joint United Nations Programme on HIV/AIDS, March 3, 2017. http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf (accessed Dec 11, 2018). [Google Scholar]
- 3.Davies M-A, Pinto J, Bras M. Getting to 90–90-90 in paediatric HIV: what is needed? J Int AIDS Soc 2015; 18 (suppl 6): 20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Essajee S, Vojnov L, Penazzato M, et al. Reducing mortality in HIV-infected infants and achieving the 90–90-90 target through innovative diagnosis approaches. J Int AIDS Soc 2015; 18 (suppl 6): 20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Penazzato M, Palladino C, Sugandhi N. Prioritizing the most needed formulations to accelerate paediatric antiretroviral therapy scale-up. Curr Opin HIV AIDS 2017; 12: 369–76. [DOI] [PubMed] [Google Scholar]
- 6.Ciaranello AL, Chang Y, Margulis AV, et al. Effectiveness of pediatric antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Clin Infect Dis 2009; 49: 1915–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ARROW Trial Team. Routine versus clinically driven laboratory monitoring and first-line antiretroviral therapy strategies in African children with HIV (ARROW): a 5-year open-label randomised factorial trial. Lancet 2013; 381: 1391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotton MF, Violari A, Otwombe K, et al. Early time-limited antiretroviral therapy versus deferred therapy in South African infants infected with HIV: results from the children with HIV early antiretroviral (CHER) randomised trial. Lancet 2013; 382: 1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The PENPACT-1 (PENTA 9/PACTG 390) Study Team. First-line antiretroviral therapy with a protease inhibitor versus non-nucleoside reverse transcriptase inhibitor and switch at higher versus low viral load in HIV-infected children: an open-label, randomised phase 2/3 trial. Lancet Infect Dis 2011; 11: 273–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies MA, Moultrie H, Eley B, et al. Virologic failure and second-line antiretroviral therapy in children in South Africa—the IeDEA Southern Africa collaboration. J Acquir Immune Defic Syndr 2011; 56: 270–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desmonde S, Eboua FT, Malateste K, et al. Determinants of durability of first-line antiretroviral therapy regimen and time from first-line failure to second-line antiretroviral therapy initiation. AIDS 2015; 29: 1527–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed TJ, Teeraananchai S, Kerr S, et al. Short communication: impact of viral load use on treatment switch in perinatally HIV-infected children in Asia. AIDS Res Hum Retroviruses 2016; 33: 230–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.European Pregnancyand Paediatric HIV Cohort Collaboration (EPPICC) study group in EuroCoord. Time to switch to second-line antiretroviral therapy in children with HIV in Europe and Thailand. Clin Infect Dis 2018; 66: 594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Department of Health, Republic of South Africa. The South African antiretroviral treatment guidelines 2010. South African National AIDS Council; http://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf (accessed Dec 11, 2018). [Google Scholar]
- 15.Ministry of Health, Republic of Botswana. 2012 Botswana National HIV & AIDS treatment guidelines. Ministry of Health, Republic of Botswana, 2012. https://aidsfree.usaid.gov/sites/default/files/tx_botswana_2012.pdf (accessed Jan 15, 2019). [Google Scholar]
- 16.Stevens WS, Marshall TM. Challenges in implementing HIV load testing in South Africa. J Infect Dis 2010; 20 (suppl 1): S78–84. [DOI] [PubMed] [Google Scholar]
- 17.The World Bank. World Bank Country and Lending groups. World Bank Group; https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lendinggroups (accessed Dec 11, 2018). [Google Scholar]
- 18.Vidmar SI, Cole TJ, Pan H. Standardizing anthropometric measures in children and adolescents with functions for egen: update. Stata J 2013; 13: 366–78. [Google Scholar]
- 19.WHO. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: World Health Organization, 2007. http://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf (accessed Dec 11, 2018). [Google Scholar]
- 20.Geng EH, Odeny TA, Lyamuya RE, et al. Estimation of mortality among HIV-infected people on antiretroviral therapy treatment in east Africa: a sampling based approach in an observational, multisite, cohort study. Lancet HIV 2015; 2: e107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509. [Google Scholar]
- 22.WHO. AIDS medicines and diagnostics service. Combined global demand forecasts for antiretroviral medicines and HIV diagnostics in low- and middle-income countries from 2015 to 2020. Geneva: World Health Organization, 2016. http://apps.who.int/iris/bitstream/10665/250088/1/9789241511322-eng.pdf?ua=1 (accessed Dec 11, 2018). [Google Scholar]
- 23.Estill J, Ford N, Salazar-Vizcaya L, et al. The need for second-line antiretroviral therapy in adults in sub-Saharan Africa up to 2030: a mathematical modelling study. Lancet HIV 2016; 3: e132–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mossoro-Kpinde CD, Gody JC, Mboumba Bouassa RS, et al. High levels of virological failure with major genotypic resistance mutations in HIV-1-infected children after 5 years of care according to WHO-recommended 1st-line and 2nd-line antiretroviral regimens in the Central African Republic: a cross-sectional study. Medicine (Baltimore) 2017; 96: e6282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szubert AJ, Prendergast AJ, Spyer MJ, et al. Virological response and resistance among HIV-infected children receiving long-term antiretroviral therapy without virological monitoring in Uganda and Zimbabwe: observational analyses within the randomised ARROW trial. PLoS Med 2017; 14: e1002432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison L, Melvin A, Fiscus S, et al. HIV-1 drug resistance and second-line treatment in children randomized to switch at low versus higher RNA thresholds. J Acquir Immune Defic Syndr 2015; 70: 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohr JK, Ive P, Horsburgh CR, et al. Marginal structural models to assess delays in second-line HIV treatment initiation in South Africa. PLoS One 2016; 11: e0161469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramadhani HO, Bartlett JA, Thielman NM, et al. The effect of switching to second-line antiretroviral therapy on the risk of opportunistic infections among patients infected with Human Immunodeficiency Virus in Northern Tanzania. Open Forum Infect Dis 2016; 3: ofw018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Haas AD, Keiser O, Balestre E, et al. Monitoring and switching of first-line antiretroviral therapy in sub-Saharan Africa: collaborative analysis of adult treatment cohorts. Lancet HIV 2015; 2: e271–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WHO. Antiretroviral therapy for HIV infection in infants and children. Recommendations for a public health approach: 2010 revision. Geneva: World Health Organization, 2010. https://www.who.int/hiv/pub/paediatric/infants2010/en/ (accessed Dec 11, 2018). [Google Scholar]
- 31.Vitoria M, Hill A, Ford N, et al. The transition to dolutegravir and other new antiretrovirals in low-income and middle-income countries: what are the issues? AIDS 2018; 32: 1551–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.