Abstract
In this study, we investigate the associations of objectively measured waking (sedentary, light physical activity [LPA] and moderate-to-vigorous physical activity [MVPA]) and sleep duration and quality characteristics with cardiometabolic risk among older women. Participants from the Healthy Women Study 2010–11 follow-up visit (n = 136, age = 73 ± 2 years, white = 91.9%) concurrently wore an ActiGraph GT1M accelerometer and Actiwatch-2 for seven days. A composite cardiometabolic risk score was calculated by transforming metabolic syndrome (MetS) components and summing z-scores. Multivariable regression models were fitted to relate waking and sleep estimates with the MetS z-score after adjustment for covariates. Compositional data analysis was used to predict the MetS z-score when fixed durations of time were reallocated from one characteristic to another. MVPA (per 10 min/day increase; β = −7.80, P < 0.01), LPA (per 30 min/day increase; β = −0.29, P = 0.04), and sleep efficiency (β = −0.10, P = 0.04) were inversely associated with MetS z-score, while sedentary time (per 30 min/day increase; β = 0.34, P = 0.01) was positively associated with MetS z-score. Reallocation of 5 min from MVPA to sleep, sedentary, or LPA resulted in the greatest predicted change in MetS z-score. On average, the reallocation of 5 min from MVPA to other characteristics predicted an 11% increase in triglycerides, 6% decrease in HDL-C, and 5% increase in waist circumference. Lastly, reallocating 30 min of sedentary time to LPA was associated with a modestly lower predicted MetS z-score. This study suggests that MVPA is the most important contributor of MetS and that maintaining MVPA and increasing LPA may be beneficial for reducing cardiometabolic risk among older women.
Keywords: Aerobic physical activity, Sleep quality, Metabolic syndrome, Cardiometabolic health
1. Introduction
Presence of metabolic syndrome (MetS) is closely associated with the development of type 2 diabetes mellitus and CVD (Meigs, 2000). Evidence suggests that MetS disproportionality affects older adults; with rates estimated to be three times higher than that of young adults in the U.S. population (Ford et al., 2002). In particular, MetS prevalence is higher among older women, compared to older men in the U.S. (58% and 47%, respectively) (Ford et al., 2002). Yet, limited evidence exists examining how changes in modifiable risk factors may influence MetS risk in older women.
Low levels of (or inadequate) physical activity is a known modifiable risk factor for MetS (Laaksonen et al., 2002, Healy et al., 2008). Age and sex disparities in physical activity have been reported among U.S. adults, with approximately 50.2% of women (53.1% for men) (Centers for Disease Control and Prevention, 2011) and only 21.9% of women aged ≥65 years (33.9% for men aged ≥65 years) meeting physical activity guidelines (Keadle et al., 2016). Sleep duration and quality have also emerged as modifiable risk factors for MetS (Jennings et al., 2007, Hall et al., 2008, Okubo et al., 2014). Evidence suggests that metrics of both sleep duration and quality are directly associated with the prevalence of MetS in middle-aged adults (Jennings et al., 2007, Hall et al., 2008, Okubo et al., 2014). Prior studies have reported that older women are susceptible to inadequate sleep duration, poor sleep quality, and sleep disorders including sleep apnea, restless leg syndrome, and circadian rhythm sleep disorders (Vitiello et al., 2004).
A number of studies have shown independent associations of waking (i.e., sedentary behavior and physical activity) and sleep duration and quality characteristics with cardiometabolic risk factors in middle-aged populations (Jennings et al., 2007, Hall et al., 2012, Coughlin et al., 2004). However, few studies have examined the associations of both waking and sleep characteristics with cardiometabolic risk factors in older women. Further, studies reporting on the duration of sleep and waking behaviors, including sedentary, light intensity (LPA), and moderate to vigorous intensity physical activity (MVPA), typically report results using traditional models that assume time spent in one behavior is independent of time spent in other behaviors, when in fact these behaviors are co-dependent when conceptualized as behaviors comprising the 24-hour sleep-wake or activity cycle (Chastin et al., 2015). Hence, analysis of these behaviors requires models that can incorporate these dependencies. Lastly, most studies use self-reported questionnaires that are subject to considerable recall and response bias (Sallis and Saelens, 2000). Without objectively determined measures of waking and sleep characteristics, it is likely that associations with metabolic risk reported in the literature are biased towards the null.
The present study sought to 1) examine the independent associations of objectively measured waking and sleep characteristics with MetS among older women using traditional multivariable regression analysis and 2) to determine the combined association of time (minutes) spent in daily behaviors (sleep, sedentary, LPA, and MVPA) with MetS among older women, using compositional data analysis.
2. Methods
2.1. Study participants
A detailed overview of the Healthy Women Study is provided in the Supplementary Methods section. For this study, participants were eligible if they participated in an ancillary study during the 2010–2011 cycle to collect objectively measured physical activity and sleep data. A total of 166 participants participated in this ancillary study during the EBT4 cycle, and 144 participants reported valid ActiGraph and Actiwatch accelerometer data. Of these, 8 participants who had missing values on any components of MetS were excluded from the analysis leaving a final analytical sample of 136 participants included in this study. All participants provided written informed consent and the protocol was approved by the University of Pittsburgh institutional review board.
2.2. Objective assessment of waking and sleep behaviors and characteristics
Assessment of waking and sleep behaviors in the Healthy Women Study has been previously described (Lambiase et al., 2013, Gabriel et al., 2013) and is described in detail in the Supplementary Methods. In brief, participants concurrently wore an ActiGraph GT1M accelerometer (Physical activity; Pensacola, FL) on the dominant hip and Actiwatch-2 (Sleep; Mini Mitter Division of Respironics Inc.) for seven consecutive days. During this time, participants also completed a comprehensive sleep diary, including recording clock times corresponding to time in and out of bed. Once the devices were returned to study personnel, data from the ActiGraph and Actiwatch were downloaded using proprietary software. Data files from both devices were expressed as 60-second epochs (or intervals) and summary variables were obtained using standard algorithms for the waking and sleep periods.
2.3. Cardiometabolic risk factors and MetS z-score
Waist circumference was measured in the standing position at the navel using a fiberglass retractable tape measure. Blood pressure was measured using the Multiple Risk Factor Intervention Trial protocol (MRFIT) (Sherwin et al., 1996). Total cholesterol, high-density lipoprotein cholesterol (HDL-C), triglycerides, and fasting serum glucose concentrations were measured by conventional enzymatic methods from a 12-hour fasting blood sample. We categorized cardiometabolic risk factors based on the National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) guidelines for women (Cholesterol, 2001). The presence of more than three of the following five criteria defined presence of MetS: (1) waist circumference ≥88 cm, (2) blood pressure ≥130 mm Hg systolic blood pressure (SBP), ≥85 mm Hg diastolic blood pressure (DBP); (3) fasting serum glucose ≥100 mg/dL; (4) serum triglycerides ≥150 mg/dL; and (5) HDL-C ≤50 mg/dL. To examine the association of waking and sleep characteristics and cardiometabolic risk factors, a composite cardiometabolic risk score was calculated by transforming cardiometabolic risk factors into z-scores. Each component of MetS was standardized by subtracting the sample mean from the individual value and dividing by the standard deviation (Henry et al., 2009, Grundy et al., 2005). The HDL-C z-score was inverted to indicate increasing risk with increasing value. Mid-blood pressure, defined as (SBP + DBP)/2, was calculated for BP measures and then, we summed z-scores to create a continuous MetS z-score (Yang et al., 2008, Lau et al., 2015). It has been suggested that continuous scores would be more sensitive to both small and large changes and less susceptible to errors than dichotomous approaches (Ragland, 1992).
2.4. Covariate measures
Data on potential confounding factors was collected using self-reported questionnaires at EBT4 visit. Potential confounders included age, race, educational attainment, use of anti-hypertensive medications, use of lipid-lowering medications, and accelerometer wear time. Wear time was extracted from ActiGraph data. As most participants were White (91.9%), race status was classified as White or non-White. Participants were classified into one of three education attainment groups based on the highest grade level completed (high school degree or less, some college/vocational training, or college or higher) for analysis. We did not adjust for smoking because all participants included in the analytic sample were classified as non-smokers via self-report at the EBT4 visit.
2.5. Statistical analysis
Categorical variables are reported as frequencies and proportions and continuous variables are reported as means ± (SD). T-values and Cohen’s d effect sizes ([Mi-Mj]/SDpooled) were reported for the significant differences to indicate the magnitude of the differences (Cohen et al., 2003). Multivariable linear regression was used to relate objective waking and sleep characteristics (independent variables) to MetS z-score (dependent variable). Initial multivariable models examined independent associations of waking and sleep characteristics with MetS, after adjusting for age, race, educational attainment, accelerometer wear time, use of anti-hypertensive, and use of lipid-lowering medications. We additionally examined the association of waking and sleep characteristics with odds of MetS, and individual components meeting MetS criteria, using logistic regression models adjusting for the same covariates. Prior to analysis, MVPA was log-transformed to normalize its distributions. Residuals were tested for homoscedasticity, linearity, and independence. Standardized beta coefficients (std. β), and 95% confidence intervals (CI) for each characteristic were derived from the models and used to evaluate the relative importance of each waking and sleep characteristics in the models.
Compositional data analysis was conducted using the R package ‘compositions’ (van den Boogaart and Tolosana-Delgado, 2008). Compositional behaviors included minutes per day of sleep, sedentary, LPA, and MVPA and were scaled to the proportion of total minutes in a day. Compositional data were subject to an isometric log-ratio transformation and represented as isometric log-ratio co-ordinates. The log-ratio methodology allows the use of standard statistical methods on transformed data and subsequent translation of results back into the original units (Chastin et al., 2015). Geometric means of each composition element were calculated and adjusted to a sum of 1440 min to determine the average minutes per day engaged in each respective behavior (Fairclough et al., 2017). A variation matrix was calculated to determine the variation between composition elements. Lower values in the variation matrix imply greater proportionality (e.g. co-dependence) of two elements. Multivariable linear regression model parameters were generated to determine the significance of the association between the isometric log-ratio co-ordinates (single composition as the independent variable) with MetS z-score and MetS components (dependent variables) in a model adjusting for age, race, education, use of anti-hypertensive medications, and use of lipid-lowering medications. Isotemporal substitution analyses were then used to predict the difference in MetS z-score, and each MetS z-score component when a fixed duration of time spent in one behavior was reallocated to another behavior (separate models for each hypothetical time replacement scenario). All models were adjusted for the covariates described above. The difference in the predicted outcomes was calculated by simple subtraction of the new composition (time reallocation) from the mean composition (mean time spent in respective behaviors). We conducted a sensitivity analysis by combining the duration of LPA and MVPA into a total physical activity estimate to examine the association of total physical activity, regardless of intensity level, on overall metabolic health in our sample.
An alpha level of 0.05 was used for all analyses. A 2-sided value of P ≤ 0.05 was considered statistically significant for all models. All analyses were performed using SAS software version 9.4 (SAS Institute Inc, Cary, NC) and R version 3.5.1 run on RStudio (version 1.0.153, RStudio: Integrated Development for R. RStudio, Inc., Boston, MA).
3. Results
3.1. Participant characteristics
Of the 245 women who attended the EBT4 visit, 166 participants (67.8%) completed an accelerometer ancillary study. No statistically significant differences were observed between participants who completed the accelerometer ancillary study compared to those who did not participate (n = 79, Supplement Table 1). Participant characteristics of the entire analytic sample, and by MetS status (No MetS vs. MetS), are displayed in Table 1. Of the 136 participants included in the analytic sample, 42 participants (30.9%) had MetS and the remaining 94 participants (69.1%) were classified as no MetS. The average age of the analytic sample was 73 ± 2 years and 91.9% were White. Compared to participants with MetS, those classified as no MetS had a lower BMI (T-value:-7.08, Cohen’s d effect size:1.24), lower insulin levels (T-value:-7.82, Cohen’s d effect size:1.35), were more physically active (T-value:2.89, Cohen’s d effect size:0.59), and spent less time being sedentary (T-value:-1.99, Cohen’s d effect size:0.36). Additionally, participants classified as no MetS had a higher sleep efficiency (T-value:2.32, Cohen’s d effect size:0.43); although this difference may not be clinically relevant (83.0% versus 85.6%). Lastly, an earlier WASO (T-value:-2.12, Cohen’s d effect size:0.39) was observed in participants classified as no MetS, compared with those with MetS.
Table 1.
Characteristics of the analytic sample by metabolic syndrome status in the Healthy Women Study.
| Variable | Total (n = 136) | MetS (n = 42) | No MetS (n = 94) | P-value |
|---|---|---|---|---|
| Age, y | 73.3 ± 1.7 | 73.2 ± 1.7 | 73.4 ± 1.6 | 0.55 |
| White, n (%) | 124 (91.9) | 39 (95.1) | 85 (90.4) | 0.50 |
| Education, n (%) | ||||
| High school or less | 30 (22.2) | 11 (26.8) | 19 (20.2) | |
| Some college | 30 (22.2) | 11 (26.8) | 19 (20.2) | |
| 4-y degree or higher | 75 (55.6) | 19 (46.3) | 56 (59.6) | 0.36 |
| CVD risk factors | ||||
| BMI, kg/m2 | 27.6 ± 5.1 | 31.4 ± 5.2 | 25.7 ± 3.9 | <0.01* |
| Total cholesterol, mg/dL | 216.3 ± 45.2 | 212.9 ± 54.1 | 217.2 ± 41.3 | 0.61 |
| LDL-c, mg/dL | 124.6 ± 39.5 | 122.5 ± 46.2 | 124.8 ± 36.4 | 0.76 |
| Insulin, mU/dL | 13.3 ± 5.2 | 17.6 ± 5.3 | 11.3 ± 3.9 | <0.01* |
| Medication use, n (%) | ||||
| Lipid-lowering medication | 67 (49.3) | 28 (41.8) | 39 (58.2) | <0.01* |
| Antihypertensive medication | 65 (47.8) | 36 (55.4) | 29 (44.6) | <0.01* |
| Antidiabetic medication | 8 (5.9) | 4 (2.9) | 4 (2.9) | 0.25 |
| MetS components | ||||
| WC (cm) | 90.3 ± 13.1 | 101.9 ± 11.4 | 85.1 ± 10.3 | <0.01* |
| HDL-c (mg/dL) | 68.0 ± 14.8 | 58.8 ± 13.8 | 72.1 ± 13.3 | <0.01* |
| Triglycerides (mg/dL) | 118.6 ± 51.0 | 158.4 ± 52.6 | 100.7 ± 38.9 | <0.01* |
| Glucose (mg/dL) | 103.5 ± 14.5 | 113.3 ± 17.1 | 99.1 ± 10.6 | <0.01* |
| SBP (mm Hg) | 122.9 ± 20.6 | 130.0 ± 20.1 | 119.7 ± 20.1 | <0.01* |
| DBP (mm Hg) | 65.4 ± 11.0 | 66.1 ± 11.4 | 65.0 ± 10.8 | 0.58 |
| MetS Z-score (sum of Z-scores) | −0.01 ± 3.2 | 3.3 ± 2.6 | −1.5 ± 2.3 | <0.01* |
| Physical Activity | ||||
| Wear time, min/day | 1044.2 ± 114.2 | 1060.1 ± 131.5 | 1037.1 ± 105.6 | 0.28 |
| Sedentary, min/day | 746.3 ± 108.2 | 773.7 ± 121.8 | 734.1 ± 99.8 | 0.05* |
| LPA, min/day | 284.9 ± 67.2 | 232.0 ± 49.9 | 287.4 ± 67.5 | 0.52 |
| MVPA, min/day | 12.9 ± 16.5 | 7.0 ± 9.5 | 15.6 ± 18.2 | <0.01* |
| Sleep | ||||
| Total sleep time (min) | 401.1 ± 53.9 | 396.3 ± 51.7 | 403.3 ± 55.0 | 0.49 |
| Efficiency (%) | 84.8 ± 6.1 | 83.0 ± 6.0 | 85.6 ± 6.0 | 0.02* |
| WASO (min) | 46.2 ± 23.6 | 52.5 ± 23.2 | 43.4 ± 23.3 | 0.04* |
| Onset latency (min) | 12.6 ± 9.8 | 13.8 ± 9.6 | 12.1 ± 9.9 | 0.36 |
| Fragmentation Index | 29.9 ± 11.5 | 32.6 ± 11.7 | 28.6 ± 11.3 | 0.06 |
Abbreviations: MetS, metabolic syndrome; CVD, cardiovascular disease; BMI, body mass index; LDL-c, low-density lipoprotein cholesterol; WC, waist circumference; HDL-c, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure; LPA, light intensity physical activity; MVPA, moderate to vigorous physical Activity; WASO, wake after sleep onset.
Note: Values are mean ± standard deviation (SD) unless otherwise indicated; Freedson’s cut point was used to define the accelerometer-based physical activity estimates; We used a t-test to compare the means of continuous variables between groups and the chi-square test or Fisher’s exact test for comparisons of categorical variables between groups; *P < 0.05.
3.2. Independent associations of waking and sleep characteristics with MetS z-score
Table 2 displays the independent associations of all waking and sleep characteristics with MetS z-score. After adjusting for age, race, educational attainment, accelerometer wear time, use of anti-hypertensive, and use of lipid-lowering medications, 10 min per day increase in MVPA (log-transformed) was associated with 7.80 unit decrease in MetS z-score (P < 0.01). Additionally, 30 min per day increase in LPA was associated with a 0.29 unit decrease in MetS z-score (all P < 0.05). In contrast, 30 min per day increase in sedentary time was associated with a 0.34 unit increase in MetS z-score (P = 0.01). Following adjustment for the same covariates, sleep efficiency was the only sleep measure that had a statistically significant (inverse) association with MetS z-score (P = 0.04). In multivariable logistic regression models (MetS vs. no MetS), a 10 min/day increase in MVPA and higher sleep efficiency were associated with a lower odds of MetS (Supplementary Table 2). MVPA (10 min/day increase) and sleep efficiency (1% increase) were inversely associated with odds of having waist circumference above 88 cm. Additionally, LPA (30 min/day increase) was inversely associated with odds of hypertension while sedentary time (30 min/day increase) was positively associated with odds of hypertension (see Table, Supplementary Tables 3–7). Additionally, results from the sensitivity analysis were similar when we combined LPA and MVPA to examine the relations using total physical activity as the independent variable (Supplementary Table 8).
Table 2.
Association of waking and sleep characteristics with metabolic syndrome z-score.
| β (std. β) | 95% CI | P-value | |
|---|---|---|---|
| PA | |||
| Log transformed MVPA (per 10 min/day) | −7.80 (−0.33) | −11.87, −3.75 | <0.01* |
| LPA (per 30 min/day) | −0.29 (−0.20) | −0.56, −0.02 | 0.04* |
| Sedentary Time (per 30 min/day) | 0.34 (0.39) | 0.09, 0.59 | 0.01* |
| Sleep | |||
| Total sleep time (min) | 0.01 (0.11) | 0.00, 0.02 | 0.26 |
| Efficiency (%) | −0.10 (−0.19) | −0.20, −0.01 | 0.04* |
| SOL (min) | 0.05 (0.16) | −0.01, 0.11 | 0.08 |
| WASO (min) | 0.02 (0.18) | 0.00, 0.05 | 0.18 |
| Fragmentation index | 0.05 (0.17) | 0.00, 0.10 | 0.06 |
Abbreviations: std., standardized; CI, confidence interval; PA, physical activity; MVPA, moderate-to-vigorous physical activity; LPA, light intensity physical activity; SOL, sleep onset latency; WASO, Wake After Sleep Onset.
Note: Age, race, education, accelerometer wear time, use of antihypertensive and lipid-lowering medications were adjusted in the model; *P < 0.05.
3.3. Isotemporal substitution compositional data analysis
Compositional means of time spent in each behavior are displayed in Table 3. On average participants were sedentary for just over 50% of a 24-hour period and spent approximately 28% of the time sleeping. While participants spent approximately 20% of time engaged in LPA, on average they were only engaged in MVPA approximately 0.5% of the time. Time spent sleeping and sedentary exhibited a high (<0.08) co-dependence with each other (Table 4). Whereas, time spent in MVPA exhibited the least co-dependence with other behaviors. The model parameters from multivariable linear regression indicated that the isometric log-ratio co-ordinates (single variable) and use of lipid-lowering medications, had the strongest associations with MetS z-score (Supplementary Table 9). The isometric co-ordinates were additionally significantly associated with waist circumference, HDL-C, and triglycerides (Supplementary Table 9).
Table 3.
Geometric means of activity behaviors by metabolic syndrome status.
| All (n = 136) | MetS (n = 55) | No MetS (n = 81) | |
|---|---|---|---|
| Sleep (min/day) | 402.62 | 396.30 | 407.23 |
| Sedentary (min/day) | 748.66 | 768.27 | 735.98 |
| LPA (min/day) | 282.24 | 271.79 | 287.28 |
| MVPA (min/day) | 6.48 | 3.63 | 9.50 |
Abbreviations: MetS, metabolic syndrome; min, minutes; LPA, light intensity physical activity; MVPA, moderate to vigorous physical activity.
Note: Compositional mean was adjusted to sum to 1440 min/day.
Table 4.
Compositional variation matrix of time spent in individual activity behaviors.
| Sedentary | LPA | MVPA | |
|---|---|---|---|
| Sleep | 0.05 | 0.09 | 1.86 |
| Sedentary | 0.00 | 0.09 | 1.93 |
| LPA | – | 0.00 | 1.69 |
Abbreviations: LPA, light physical activity; MVPA, moderate to vigorous physical activity.
Note: Values closer to zero indicated that the times spent in the two behaviors included in the ratio were highly co-dependent.
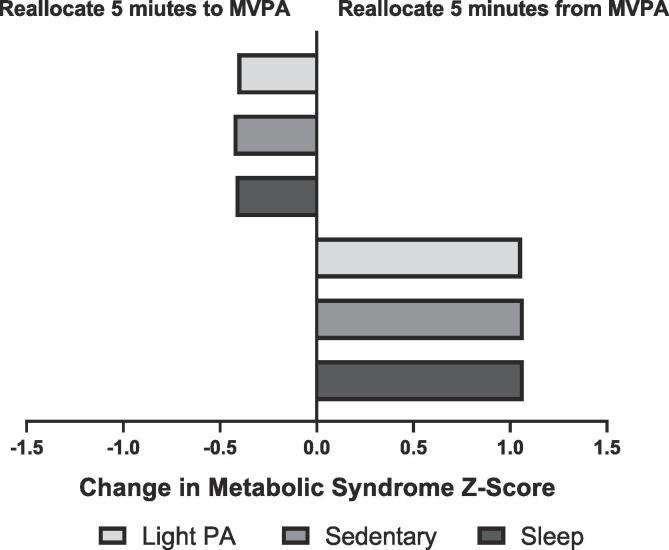
The mean proportion of time spent in MVPA was less than 0.005 (7.2 min). Therefore, we evaluated 5 min of time reallocation to and from MVPA to other behaviors. For sedentary time, LPA, and sleep, we evaluated 30 min of time reallocation among these behaviors. The predicted differences in MetS z-score following hypothetical time reallocation from one characteristic to another are displayed in Table 5. Overall, the largest predicted difference in MetS z-score was observed when 5 min of MVPA was replaced by 5 min of other behaviors (LPA, sedentary, or sleep). This resulted in a predicted increase of approximately 1-unit in the MetS z-score (Table 5). The association was asymmetrical showing a change of lower magnitude when 5 min of other behaviors were reallocated to MVPA (Fig. 1). Reallocation of 30 min of LPA and sleep to the sedentary time predicted increases in MetS z-score by 0.11 units and 0.05 units respectively. These associations were symmetrical with the opposite reallocations predicting similar decreases in MetS z-score (−0.11 and −0.05, respectively). Additionally, we observed similar results when LPA and MVPA combined for the sensitivity analysis (Supplementary Table 10).
Table 5.
Predicted differences in metabolic syndrome z-score and individual metabolic syndrome components.
| MetS z-score | Sleep | Sedentary | LPA | MVPA |
|---|---|---|---|---|
| Sleep | – | 0.05 | −0.05 | −0.42 |
| Sedentary time | −0.05 | – | −0.10 | −0.43 |
| LPA | 0.05 | 0.11 | – | −0.41 |
| MVPA | 1.07 | 1.07 | 1.06 | – |
| WC (cm) | ||||
| Sleep | – | 0.77 | 0.99 | −1.78 |
| Sedentary time | −0.74 | – | 0.20 | −1.90 |
| LPA | −0.99 | −0.26 | – | −1.94 |
| MVPA | 4.65 | 4.78 | 4.81 | – |
| HDL-C (mg/dL) | ||||
| Sleep | – | 0.36 | 0.33 | 1.78 |
| Sedentary time | −0.34 | – | −0.03 | 1.71 |
| LPA | −0.31 | 0.03 | – | 1.72 |
| MVPA | −4.43 | −4.37 | −4.37 | – |
| Triglycerides (mg/dL) | ||||
| Sleep | – | −0.20 | −0.39 | −5.08 |
| Sedentary time | 0.37 | – | −0.36 | −5.01 |
| LPA | 0.76 | 0.38 | – | −4.95 |
| MVPA | 12.78 | 12.71 | 12.65 | – |
| Glucose (mg/dL) | ||||
| Sleep | – | −0.07 | −0.30 | −0.74 |
| Sedentary time | 0.06 | – | −0.20 | −0.73 |
| LPA | 0.39 | 0.32 | – | −0.67 |
| MVPA | 1.84 | 1.83 | 1.78 | – |
| SBP (mm Hg) | ||||
| Sleep | – | 0.32 | −1.42 | −0.59 |
| Sedentary time | −0.34 | – | −1.76 | −0.73 |
| LPA | 1.55 | 1.88 | – | −0.67 |
| MVPA | 1.46 | 1.51 | 1.21 | – |
| DBP (mm Hg) | ||||
| Sleep | – | 0.52 | −0.27 | 0.30 |
| Sedentary time | −0.52 | – | −0.81 | 0.21 |
| LPA | 0.35 | 0.85 | – | 0.35 |
| MVPA | −0.71 | −0.62 | −0.76 | – |
Abbreviations: MetS, metabolic syndrome; LPA, light intensity physical activity; MVPA, moderate to vigorous physical activity; WC, waist circumference; HDL-C, high-density lipoprotein cholesterol; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Note: Reallocation of time from the behavior in rows to behaviors in columns. Reallocation between MVPA and other behaviors (sleep, sedentary, and LPA) is 5 min. The reallocation among sleep, sedentary, and LPA is 30 min. Models adjusted for age, race, education, accelerometer wear time, use of antihypertensive and lipid-lowering medications were adjusted in the model.
Fig. 1.
Predicted difference in metabolic syndrome z-score following 5 min of time reallocation to or from MVPA. Abbreviations: MVPA, moderate to vigorous physical activity, PA, physical activity. Note: Models are adjusted for age, race, education, use of antihypertensive and lipid-lowering medications were adjusted.
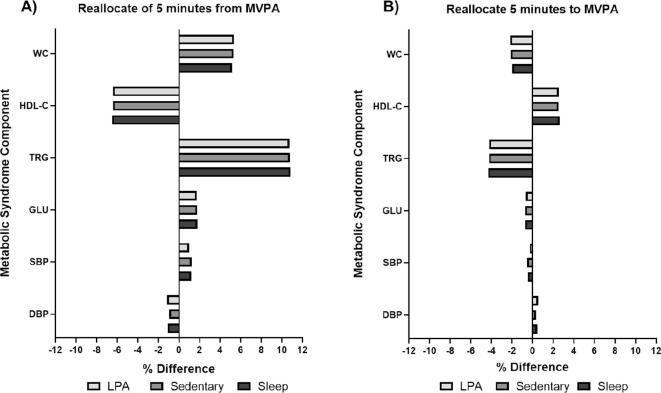
Similar to our results with MetS z-score, the largest predicted differences of the individual MetS components was largely with the reallocation of 5 min from MVPA to other behaviors (Table 5). The hypothetical time reallocation of MVPA suggested the largest changes for concentrations of blood lipids (triglycerides and HDL-C) and waist circumference. On average the reallocation of 5 min from MVPA to other behaviors predicted an approximately 11% higher blood triglyceride concentrations, a 6% lower HDL-C concentrations, and a 5% higher waist circumference (Supplementary Fig. 1). These associations were again asymmetrical with the reallocation of 5 min from other behaviors to MVPA having smaller associations (approximately 4% lower triglycerides, 3% higher HDL, and 2% lower waist circumference). The reallocation of 30 min from time spent sedentary or time sleeping to LPA predicted the largest decrease in SBP (approximately 1.5% lower). This association was symmetrical with the opposite reallocation predicting similar increases in SBP.
4. Discussion
4.1. Principal findings
In the present study, we examined the associations of objectively measured waking and sleep characteristics with cardiometabolic risk using traditional regression modeling and isotemporal substitution compositional data analysis in a sample of older women. In our traditional multivariable regression analysis, we found that a 10 min/day increase in MVPA had the strongest inverse association with MetS z-score. Our isotemporal substitution compositional data analysis added to this finding by indicating that the hypothetical reallocation of time away from MVPA, to other waking behaviors, had the largest impact on change in MetS z-score. This association was the most pronounced for triglycerides, HDL-C, and waist circumference. Whereas, time spent in LPA was associated with the largest predicted difference in SBP. Together these data indicate that MVPA has the strongest association with metabolic health. Also, while increasing MVPA time is likely most beneficial for metabolic health in older women, targeted messages encouraging the maintenance of current MVPA levels may be a more feasible intervention goal as these older women continue to age.
4.2. Comparison with the literature
The 2018 Physical Activity Guidelines Scientific Advisory Committee identified evaluating LPA, alone or in combination with MVPA, as an overarching recommendation for future research studies (Physical Activity Guidelines Advisory Committee, 2018). Our study helps to narrow this gap in the literature on older women. Consistent with prior studies, the results from our multivariable linear regression analysis support the notion that MVPA has the strongest association with MetS (Peterson et al., 2014, LaMonte et al., 2017). Our findings are in line with the findings from prior studies that report a beneficial association of LPA (Buman et al., 2010 Sep 15) and the detrimental association of sedentary time (Gennuso et al., 2013, Inoue et al., 2012, Stamatakis et al., 2012) with cardiometabolic health among older women. Additionally, the present study is in agreement with prior work indicating that higher sleep efficiency is inversely associated with cardiometabolic risk in older adults (Strand et al., 2015).
In agreement with the current study, prior isotemporal substitution analyses found that replacing MVPA with other behaviors including sleep, sedentary, and LPA was associated with higher cardiometabolic risk among older adults (Hamer et al., 2014). We observed that the opposite replacement (reallocating 5 min of sleep, sedentary, or LPA with MVPA) showed favorable impacts, of a smaller magnitude. The asymmetry of these results suggests that maintaining even a small amount of daily MVPA, not reallocating to other behaviors, may be more important than increasing MVPA time for the maintenance of cardiometabolic health among older women. Nonetheless, data from several longitudinal cohort studies have documented age-related declines in MVPA, particularly during the mid to late life transition (Pettee Gabriel et al., 2018). This may in part be due to functional limitations, pain, or chronic disease, including multi-morbidity. For this reason, health promotion messaging focused on replacing sedentary time with LPA is considered more clinically relevant in this population. In the present study, women spent approximately 7 min per day engaged in MVPA compared to more than 280 min per day engaged in LPA. However, in our isotemporal substitution compositional data analysis, we found that the reallocation of 30 min to or from LPA only predicted a modest favorable change in MetS z-score.
Results from our traditional linear regression analysis and isotemporal substitution compositional data analysis indicated that MVPA and LPA differentially associated with individual MetS components. In our traditional multivariable regression analyses, an increase of 10 min per day in MVPA specifically had an inverse association with waist circumference. Adding to this, our isotemporal substitution compositional data analysis found that reallocating MVPA to other behaviors was associated with the largest changes in waist circumference, triglycerides, and HDL-C. While these changes were hypothetical, they were of clinically relevant magnitudes (Lemes, 2018). These findings are supported by prior studies that have reported an inverse association of MVPA with waist circumference using traditional multivariable regression analysis (Cooper, 2014, Shibata, 2016) and the unfavorable change of waist circumference, triglycerides, and HDL-C when 10 or 30 min of MVPA is reallocated to other behaviors (Chastin et al., 2015, Buman, 2013, Boyle, 2017). Interestingly, LPA was associated with reduced risk of hypertension and isotemporal substitution compositional data analysis found that the reallocation of 30 min spent sedentary or sleeping to LPA was particularly associated with lower SBP. Consistent with the present investigation, several cross-sectional studies documented the inverse association between LPA and blood pressure in middle-aged adults (Khoja, 2016, Howard, 2015). Hence, our results suggest that focusing on maintaining MVPA and increasing LPA may have the greatest overall benefit for the prevention of MetS.
4.3. Strengths and limitations
Traditional regression methods are not well suited for the analysis of co-dependent variables constrained to the sum of a whole (e.g. 100%). Compositional data analysis is a novel analytical method that allows for the meaningful inference of each composition element with respect to the reaming elements. This approach provides better insight into how co-dependent behaviors within a fixed 24-hour activity cycle may influence cardiometabolic risk. For example, results from both our traditional multivariable regression and compositional analyses confirmed that time spent in MVPA had the strongest association with MetS. However, the compositional data analysis method additionally allowed us to infer that the maintenance of MVPA, not reallocating it to other behaviors, is more importing than increasing time spent in MVPA. A second major strength of the present study is the use of objectively measured physical activity and sleep characteristics. This provides more accurate exposure estimates and is less prone to biases often associated with self-reported data.
However, there are several limitations to consider when interpreting the results of the current study. A relatively small sample size of the present study may affect the reliability of our results with low statistical power. The majority of participants in the present study were White older women; therefore, future studies are needed in diverse studies to examine potential differences in these associations by race/ethnicity and other important individual-level factors. While this increases the internal validity of our study, our results and related inferences may be limited to this population. Additionally, the reallocation of 5 min away from MVPA reflects only 2 min of MVPA per day since the mean time spent per day in MVPA was 7 min in this analytic sample of older women. Therefore, while our methodological approach to define reallocation periods by intensity category is applicable to future studies, the reallocation of 5 min and this relation with MetS may not be generalizable to other study samples with a higher mean MVPA. Freedson count cut-point threshold values were used to classify physical activity and sedentary time, which may not be applicable to older adults. The potential misclassification that can result when threshold values are applied to accelerometer count data to deriving more meaningful summary estimates may underestimate the association of physical activity and sedentary time with MetS. The use of waist-worn, uni-axial accelerometers is unable to detect upper body movement (e.g., resistance training) that may also contribute to improved health especially in older adults. Although the participants were relatively healthy and ambulatory, we could not adjust for self-rated health or functional status that may lead to reverse causality. Similarly, while sleep actigraphy is considered a valid field-based method to estimate sleep duration and quality compared to polysomnography (Weiss et al., 2010), the actigraphy-based estimates provide indirect estimates of sleep. Lastly, given the cross-sectional nature of the current study, we cannot discern causality between waking and, sleep characteristics and cardiometabolic health. The findings of the current study need to be confirmed in longitudinal cohorts of men and women and in multi-ethnic groups.
5. Conclusion
In this cross-sectional study of older women, we found that MVPA and LPA were inversely associated with MetS z-score while sedentary time was positively associated with MetS z-score. Isotemporal substitution compositional data analysis further indicated that maintaining time spent in MVPA and the replacement of time spent in sedentary to LPA may be a beneficial strategy for improved cardiometabolic health in older women.
Clinical trial registraion number: NCT00005160.
CRediT authorship contribution statement
Joowon Lee: Conceptualization, Formal analysis, Writing - original draft. Maura E. Walker: Formal analysis, Writing - original draft, Writing - review & editing. Karen A. Matthews: Writing - review & editing. Lewis H. Kuller: Writing - review & editing. Nalini Ranjit: Writing - review & editing. Kelley Pettee Gabriel: Conceptualization, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the dedicated Healthy Women Study (HWS) participants and the contributions of the HWS study staff. This study was funded by the National Heart, Lung, and Blood contract R01-HL-028226. Additionally, this work was supported by 5T32-HL-125232 (JWL and MEW) from the National Heart, Lung, and Blood Institute. The results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2020.101071.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Meigs J.B. Invited commentary: insulin resistance syndrome? syndrome X? Multiple metabolic syndrome? a syndrome at all? factor analysis reveals patterns in the fabric of correlated metabolic risk factors. Am. J. Epidemiol. 2000;152(10):908–911. doi: 10.1093/aje/152.10.908. [DOI] [PubMed] [Google Scholar]
- Ford E.S., Giles W.H., Dietz W.H. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287(3):356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- Laaksonen D.E., Lakka H.M., Salonen J.T., Niskanen L.K., Rauramaa R., Lakka T.A. Low levels of leisure-time physical activity and cardiorespiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25(9):1612–1618. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- Healy G.N., Wijndaele K., Dunstan D.W. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31(2):369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Adult Participation in Aerobic and Muscle-Strengthening Physical Activities—United States, 2011. Morb Mortal Wkly Rep. 2013;62(No.RR-17):326-30. [PMC free article] [PubMed]
- Keadle S.K., McKinnon R., Graubard B.I., Troiano R.P. Prevalence and trends in physical activity among older adults in the United States: a comparison across three national surveys. Prev. Med. 2016;1(89):37–43. doi: 10.1016/j.ypmed.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings J.R., Muldoon M.F., Hall M., Buysse D.J., Manuck S.B. Self-reported sleep quality is associated with the metabolic syndrome. Sleep. 2007;30(2):219–223. doi: 10.1093/sleep/30.2.219. [DOI] [PubMed] [Google Scholar]
- Hall M.H., Muldoon M.F., Jennings J.R., Buysse D.J., Flory J.D., Manuck S.B. Self-reported sleep duration is associated with the metabolic syndrome in midlife adults. Sleep. 2008;31(5):635–643. doi: 10.1093/sleep/31.5.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo N., Matsuzaka M., Takahashi I. Relationship between self-reported sleep quality and metabolic syndrome in general population. BMC public health. 2014;14(1):562. doi: 10.1186/1471-2458-14-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitiello M.V., Larsen L.H., Moe K.E. Age-related sleep change: gender and estrogen effects on the subjective–objective sleep quality relationships of healthy, noncomplaining older men and women. J. Psychosom. Res. 2004;56(5):503–510. doi: 10.1016/S0022-3999(04)00023-6. [DOI] [PubMed] [Google Scholar]
- Hall M.H., Okun M.L., Sowers M. Sleep is associated with the metabolic syndrome in a multi-ethnic cohort of midlife women: the SWAN Sleep Study. Sleep. 2012;35(6):783–790. doi: 10.5665/sleep.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin S.R., Mawdsley L., Mugarza J.A., Calverley P.M., Wilding J.P. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. Eur. Heart J. 2004;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Chastin S.F., Palarea-Albaladejo J., Dontje M.L., Skelton D.A. Combined effects of time spent in physical activity, sedentary behaviors and sleep on obesity and cardio-metabolic health markers: a novel compositional data analysis approach. PLoS One. 2015;10(10) doi: 10.1371/journal.pone.0139984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallis J.F., Saelens B.E. Assessment of physical activity by self-report: status, limitations, and future directions. Res. Q. Exerc. Sport. 2000;71(sup2):1–4. doi: 10.1080/02701367.2000.11082780. [DOI] [PubMed] [Google Scholar]
- Lambiase MJ, Gabriel KP, Kuller LH, Matthews KA. Temporal relationships between physical activity and sleep in older women. Medicine and science in sports and exercise. 2013 Dec;45(12). [DOI] [PMC free article] [PubMed]
- Gabriel K.P., Matthews K.A., Pérez A., Edmundowicz D., Kohl H.W., III, Hawkins M.S., Janak J.C., Kriska A.M., Kuller L.H. Self-reported and accelerometer physical activity levels and coronary artery calcification progression in older women: results from the Healthy Women Study. Menopause (New York, NY) 2013;20(2):152. doi: 10.1097/gme.0b013e31826115af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin R., Sengupta A., Havas S. Blood pressure in minorities screened for the Multiple Risk Factor Intervention Trial (MRFIT) Public Health Rep. 1996;111(Suppl 2):68. [PMC free article] [PubMed] [Google Scholar]
- National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285:2486–97. [DOI] [PubMed]
- Henry R.M., Ferreira I., Dekker J.M., Nijpels G., Scheffer P.G., Stehouwer C.D. The metabolic syndrome in elderly individuals is associated with greater muscular, but not elastic arterial stiffness, independent of low-grade inflammation, endothelial dysfunction or insulin resistance—the Hoorn Study. J. Hum. Hypertens. 2009;23(11):718. doi: 10.1038/jhh.2009.8. [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Jr, Spertus J.A. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Yang X., Telama R., Hirvensalo M., Mattsson N., Viikari J., Raitakari O. The longitudinal effects of physical activity history on metabolic syndrome. Med. Sci. Sports Exerc. 2008;40(8):1424. doi: 10.1249/MSS.0b013e318172ced4. [DOI] [PubMed] [Google Scholar]
- Lau C., Yu R., Woo J. Effects of a 12-week hatha yoga intervention on cardiorespiratory endurance, muscular strength and endurance, and flexibility in Hong Kong Chinese adults: a controlled clinical trial. Evid.-Based Complem. Alternative Med. 2015;2015 doi: 10.1155/2015/958727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland D.R. Dichotomizing continuous outcome variables: dependence of the magnitude of association and statistical power on the cutpoint. Epidemiology (Cambridge, Mass.) 1992;3(5):434–440. doi: 10.1097/00001648-199209000-00009. [DOI] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S.G., Aiken L.S. third ed. Erlbaum Associates; NJ: Lawrence: 2003. Applied MultipleRegression/Correlation Analysis for the Behavioral Sciences. [Google Scholar]
- van den Boogaart K.G., Tolosana-Delgado R. “Compositions”: a unified R package to analyze compositional data. Comput. Geosci. 2008;34(4):320–338. [Google Scholar]
- Fairclough S.J., Dumuid D., Taylor S. Fitness, fatness and the reallocation of time between children’s daily movement behaviours: an analysis of compositional data. Int. J. Behav. Nutrit. Phys. Activity. 2017;14(1):64. doi: 10.1186/s12966-017-0521-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee . US Department of Health and Human Services; Washington, DC: 2018. Physical activity guidelines advisory committee scientific report. [Google Scholar]
- Peterson M.D., Al Snih S., Stoddard J., McClain J., Lee I. Adiposity and insufficient MVPA predict cardiometabolic abnormalities in adults. Med. Sci. Sports Exerc. 2014;46(6):1133. doi: 10.1249/MSS.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaMonte M.J., Lewis C.E., Buchner D.M. Both light intensity and moderate-to-vigorous physical activity measured by accelerometry are favorably associated with cardiometabolic risk factors in older women: the Objective Physical Activity and Cardiovascular Health (OPACH) study. J. Am. Heart Assoc. 2017;6(10) doi: 10.1161/JAHA.117.007064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman M.P., Hekler E.B., Haskell W.L. Objective light-intensity physical activity associations with rated health in older adults. Am. J. Epidemiol. 2010;172(10):1155–1165. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennuso K.P., Gangnon R.E., Matthews C.E., Thraen-Borowski K.M., Colbert L.H. Sedentary behavior, physical activity, and markers of health in older adults. Med. Sci. Sports Exerc. 2013;45(8):1493. doi: 10.1249/MSS.0b013e318288a1e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Sugiyama T., Takamiya T. Television viewing time is associated with overweight/obesity among older adults, independent of meeting physical activity and health guidelines. J. Epidemiol. 2012;22(1):50–56. doi: 10.2188/jea.JE20110054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis E., Davis M., Stathi A., Hamer M. Associations between multiple indicators of objectively-measured and self-reported sedentary behaviour and cardiometabolic risk in older adults. Prev. Med. 2012;54(1):82–87. doi: 10.1016/j.ypmed.2011.10.009. [DOI] [PubMed] [Google Scholar]
- Strand L.B., Carnethon M., Biggs M.L. Sleep disturbances and glucose metabolism in older adults: the cardiovascular health study. Diabetes Care. 2015;38(11):2050–2058. doi: 10.2337/dc15-0137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M., Stamatakis E., Steptoe A. Effects of substituting sedentary time with physical activity on metabolic risk. Med. Sci. Sports Exerc. 2014;46(10):194. doi: 10.1249/MSS.0000000000000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettee Gabriel K., Sidney S., Jacobs D.R., Jr Ten-year changes in accelerometer-based physical activity and sedentary time during midlife: the CARDIA study. Am. J. Epidemiol. 2018;187(10):2145–2150. doi: 10.1093/aje/kwy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemes Ítalo Ribeiro. Aerobic training reduces blood pressure and waist circumference and increases HDL-c in metabolic syndrome: a systematic review and meta-analysis of randomized controlled trials. J. Am. Soc. Hypertens. 2018;12(8):580–588. doi: 10.1016/j.jash.2018.06.007. [DOI] [PubMed] [Google Scholar]
- Cooper, Andrew J.M. Association between objectively assessed sedentary time and physical activity with metabolic risk factors among people with recently diagnosed type 2 diabetes. Diabetologia. 2014;57(1):73–82. doi: 10.1007/s00125-013-3069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A.I. Physical activity, television viewing time, and 12-year changes in waist circumference. Med. Sci. Sports Exerc. 2016;48(4):633. doi: 10.1249/MSS.0000000000000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman Matthew P. Reallocating time to sleep, sedentary behaviors, or active behaviors: associations with cardiovascular disease risk biomarkers, NHANES 2005–2006. Am. J. Epidemiol. 2013;179(3):323–334. doi: 10.1093/aje/kwt292. [DOI] [PubMed] [Google Scholar]
- Boyle Terry. Reallocating time to sleep, sedentary time, or physical activity: associations with waist circumference and body mass index in breast cancer survivors. Cancer Epidemiol. Prevent. Biomarkers. 2017;26(2):254–260. doi: 10.1158/1055-9965.EPI-16-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoja Samannaaz S. Association of light-intensity physical activity with lower cardiovascular disease risk burden in rheumatoid arthritis. Arthritis Care Res. 2016;68(4):424–431. doi: 10.1002/acr.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard Bethany. Associations of low-and high-intensity light activity with cardiometabolic biomarkers. Med. Sci. Sports Exerc. 2015;47(10):2093–2101. doi: 10.1249/MSS.0000000000000631. [DOI] [PubMed] [Google Scholar]
- Weiss A.R., Johnson N.L., Berger N.A., Redline S. Validity of activity-based devices to estimate sleep. J. Clin. Sleep Med. 2010;6(04):336–342. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.